Abstract
Declining estrogen production after menopause causes osteoporosis in which the resorption of bone exceeds the increase in bone formation. We recently found that mice deficient in the β-subunit of follicle-stimulating hormone (FSHβ) are protected from bone loss despite severe estrogen deficiency. Here we show that FSHβ-deficient mice have lowered TNFα levels. However, TNFα-deficient mice are resistant to hypogonadal bone loss despite having elevated FSH, suggesting that TNFα is critical to the effect of FSH on bone mass. We find that FSH directly stimulates TNFα production from bone marrow granulocytes and macrophages. We also explore how TNFα up-regulation induces bone loss. By modeling the known actions of TNFα, we attribute the high-turnover bone loss to an expanded osteoclast precursor pool, together with enhanced osteoblast formation. TNFα inhibits osteoblastogenesis in the presence of ascorbic acid in culture medium, but in its absence this effect becomes stimulatory; thus, ascorbic acid reverses the true action of TNFα. Likewise, ascorbic acid blunts the effects of TNFα in stimulating osteoclast formation. We propose that hypogonadal bone loss is caused, at least in part, by enhanced FSH secretion, which in turn increases TNFα production to expand the number of bone marrow osteoclast precursors. Ascorbic acid may prevent FSH-induced hypogonadal bone loss by modulating the catabolic actions of TNFα.
Keywords: postmenopausal osteoporosis, TNFα, bone, ascorbic acid, hypogonadal
Postmenopausal osteoporosis is a leading cause of morbidity and mortality in the increasingly aging population, with fracture rates exceeding the combined incidence of breast cancer, stroke, and heart attacks in postmenopausal women (1). Traditionally, this bone loss has been attributed solely to declining estrogen levels. However, we recently showed that the pituitary hormone follicle-stimulating hormone (FSH), the secretion of which is under estrogenic feedback, directly enhances osteoclast formation and function. The deletion of its β-subunit (FSHβ) protects against bone loss despite severe hypogonadism (2). This finding indicates that FSH is a requirement for hypogonadal bone loss and, although awaiting definitive proof, suggests strongly that elevated FSH contributes to the genesis of postmenopausal osteoporosis.
However, enhanced osteoclastogenesis, a consequence of the direct action of FSH on its Gi-coupled receptor on osteoclast precursors, does not fully explain hypogonadal bone loss. There are accompanying alterations in bone and bone marrow, notably enhanced bone formation, increased T lymphocyte production, and macrophage activation. The alterations in immune function have been attributed to an increase in TNFα production that is thought to arise solely from estrogen deficiency. However, because TNF inhibits osteoblast differentiation in vitro, the increased bone formation has not been attributed to TNFα. Thus, the genesis of enhanced bone formation, an essential component of the high-turnover bone loss, has remained unclear.
Ablation of the TNFα gene in mice abrogates gonadectomy-induced bone loss, osteoclastic and osteoblastic activation, and the accompanying immune cell alterations (3). That gonadectomy elevates FSH levels in these animals suggests that TNFα is essential for, and downstream of, FSH action on bone (3). Consistent with this hypothesis, TNFα does not modulate FSH secretion (4). In fact, there is direct evidence in Sertoli cells and testicular macrophages, respectively, that FSH enhances TNF receptor and TNFα expression (5, 6). Together, these findings prompted us to explore whether FSH mediates the production of TNFα and whether the abrogation of bone loss in FSHβ-deficient mice arises in part from decreased TNFα production.
We show that FSHβ-deficient mice have low circulating TNFα, that FSH directly stimulates TNFα production from bone marrow granulocytes and macrophages, and that TNFα stimulates osteoclast precursor expansion and osteoblast differentiation. We also find that ascorbic acid, which is required for posttranslational modification of proline to hydroxyproline in collagen and is thus used as a differentiation inducer in culture studies, acts to reverse the stimulatory effects of TNFα on osteoblast and osteoclast formation. Confirmatory evidence that high-turnover bone loss is due to enhanced osteoclast precursor expansion, and that TNFα is proosteoblastogenic rather than antiosteoblastogenic, comes from further mathematical modeling of the known action of TNFα in TNFα-overexpressing mice. We propose that the effects of FSH on bone mass identified earlier (2) are, at least in part, exerted via the modulation of TNFα production by bone marrow macrophages and granulocytes.
Results
FSH Regulates TNFα Production.
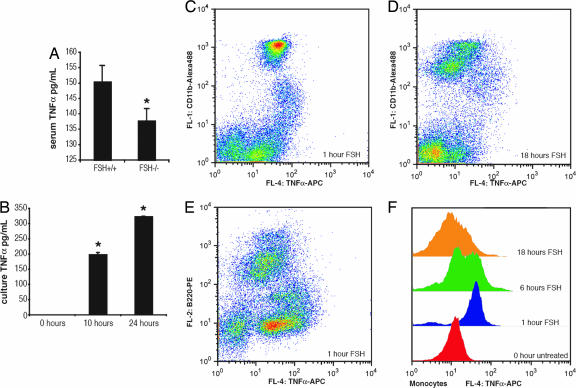
Although estrogen is known to suppress TNFα production from immune cells (7), whether FSH or its lack thereof alters TNFα expression has never been investigated. We find that, despite severe estrogen deficiency, FSHβ-deficient mice have lower serum levels of TNFα compared with littermate controls (Fig. 1A). This finding suggests that impaired FSH signaling attenuates the otherwise stimulatory effect of estrogen deficiency on TNFα production. In parallel experiments, the exposure of bone marrow cultures to recombinant FSH caused an increase in supernatant TNFα levels measured using an ELISA (Fig. 1B).
Fig. 1.
FSH regulates TNFα production. (A) FSHβ−/− mice display reduced levels of serum TNFα despite estrogen deficiency (P = 0.03; n = 4; sampled twice). (B) Secretion of TNFα into cell culture supernatants was analyzed at 10 and 24 h after FSH (100 ng/ml) addition (P = 0.03 for 10 h and 0.01 for 24 h). (C–E) Effect of FSH on TNFα production in primary CD11b+ macrophages/granulocytes or B220+ cells. Bone marrow from C57BL mice was flushed and resuspended in OPTI-MEM containing 5% FBS. After 2 h of incubation, FSH (100 ng/ml) was added, and the cells were sampled at 1 h (C and E), 6 h (data not shown), and 18 h (D) after addition. The cells were fixed in PhosphoFix, permeabilized in 90% MEOH, and stained with either of two antibody combinations: CD11b-Alexa Fluor 488 and TNFα-allophycocyanin (C and D) or B220-phycoerythrin and TNFα-allophycocyanin (E). (F) A display of the time course of TNFα staining after the addition of FSH (100 ng/ml) in gated macrophages/granulocytes.
Both macrophages, which together with granulocytes make up half of the cells present in bone marrow, and T lymphocytes, which account for 1–3% of cells in the bone marrow, have been suggested as sources of TNFα production in estrogen deficiency (8, 9). To elucidate which cell(s) were producing TNFα, we doubly stained cells from bone marrow with antibodies to TNFα, as well as to markers for macrophages/granulocytes (CD11b), B lymphocytes (B220), and T lymphocytes (CD3). We found that macrophages and granulocytes expressed high levels of TNFα after FSH stimulation but that other cell types did not (Fig. 1 C–F). These results suggest that TNFα up-regulation is likely downstream of FSH and is, at least in part, mediated by granulocytes and macrophages present in the bone marrow.
TNFα Induces High-Turnover Bone Loss Through Osteoclast Precursor Expansion.
We next sought to understand how elevations in TNFα contribute to the observed high-turnover bone loss seen in hypogonadal states. Prior investigations suggest that there are three major actions of TNFα on bone. TNFα strongly augments osteoclast differentiation and resorptive function by stimulating the same signal transduction pathways induced by RANK-L (10). TNFα also increases the number of CD11b+ osteoclast precursors by enhancing macrophage colony-stimulating factor (M-CSF)-mediated proliferation (11, 12). Additionally, TNFα potently inhibits osteoblast differentiation through negatively regulating the lifespan of osteoblast progenitors (13). To determine which of these actions was critical in bringing about high-turnover bone loss, we adapted a mathematical model of bone metabolism to allow for the individual application of each of the three actions of TNFα (14). Specifically, we examined how each of these actions contributed to the bone loss phenotype observed in animals overexpressing TNFα (Table 1; see also Table 2, which is published as supporting information on the PNAS web site).
Table 1.
Bone changes in transgenic (Tg) TNF-overexpressing mice
| Mouse | Osteoclast precursors, % of CD11bhi splenocytes | Total bone mineral density, mg/cm3 | Osteoclasts per surface/bone surface | Osteoblasts per surface/bone surface | Osteoblasts per paw after 4 weeks of treatment |
|---|---|---|---|---|---|
| Wild type | 1.8–2.6(11, 34) | 452 (35) | 3.75 (35) | 5 (35) | |
| TNF-Tg | 9.1–15.5(11, 34) | 330 (35) | 6 (35) | 12 (35) | |
| TNF-Tg + RANK-Fc injection | 13.2 (34) | ||||
| TNF-Tg × RANK−/− | 20.7 (34) | ||||
| TNF-Tg + etanercept injection | 3.2 (11) | ||||
| Wild type + OPG injection | 535 (35) | 2 (35) | 1 (35) | ||
| TNF-Tg + OPG injection | 623 (35) | 1.5 (35) | 4 (35) | ||
| TNF-Tg at 10 weeks of age | 25 (36) | ||||
| TNF-Tg at 14 weeks; untreated | 65 (36) | ||||
| TNF-Tg at 14 weeks; OPG injection | 20 (36) | ||||
| TNF-Tg at 14 weeks; OPG and etanercept | 1 (36) | ||||
| TNF-Tg at 14 weeks; etanercept injection | 30 (36) |
References are shown in parentheses.
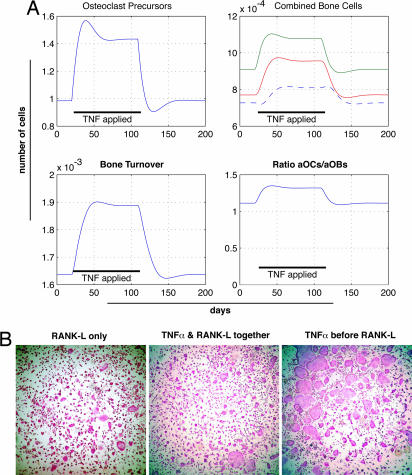
Using this model, we found that simulating TNFα-induced increases in the number of osteoclast precursors correctly accounted for most of the observed phenotype of TNFα-transgenic mice (Fig. 2A). Furthermore, this model suggested that, if TNFα served solely to augment osteoclast differentiation, the number of osteoclast precursors would fall, not rise as was observed in vivo. Moreover, if TNFα inhibited osteoblast differentiation (as has been shown in vitro; see ref. 13), there would be concomitant declines in osteoclast numbers, not increases as noted in vivo. In agreement with the in silico finding that TNFα's main action is to augment osteoclast precursor numbers, TNFα added to osteoclast precursors 24 h before the addition of the differentiation-inducing cytokine RANK-L led to significantly greater increases in osteoclast formation than when TNFα and RANK-L were added simultaneously (Fig. 2B).
Fig. 2.
Modeling of TNFα action suggests that it induces osteoclast precursor expansion. A mathematical model of bone metabolism (14) was adapted such that osteoclast differentiation proceeded from a pool of osteoclast precursors that could be varied independent of changes in the differentiation rate of osteoclasts. (A) The pool of osteoclast precursors was increased, as indicated by the black bar, and the effects on the number of osteoclast precursors (Upper Left), the number of osteoclasts, osteoblast precursors, and osteoblasts (Upper Right), the bone turnover (Lower Left), and net bone formation/loss (Lower Left) are shown. Note that disturbing the system by increasing the number of osteoclast precursors caused a rise in the number of active osteoclasts, responding osteoblasts, and active osteoblasts, as well as an increase in bone turnover and bone loss. (B) The effects of TNFα on expanding the osteoclast precursor pool were tested experimentally by stimulating osteoclast precursors with TNFα either 24 h before the addition of the differentiation signal RANK-L (Right) or by adding TNFα at the same time RANK-L was added (Center). The number of osteoclasts formed when TNFα was allowed to expand the osteoclast precursor pool was greater than when TNFα was given at the same time as the differentiation signal RANK-L. (Left) Control, RANK-L only.
Ascorbic Acid Reverses the Effects of TNFα on Osteoblasts and Osteoclasts.
Elevations in osteoblast function accompany the high-turnover bone loss induced by estrogen deficiency. However, FSH does not impact osteoblast differentiation (2), and TNFα is an established potent inhibitor of osteoblast differentiation in vitro (15). However, TNFα-overexpressing transgenic mice have elevated osteoblast activity (Table 1 and 2). This incongruence prompted us to reexamine the function of TNFα on osteoblast differentiation. Although changes in osteoclast precursor numbers led to reactive increases in osteoblast numbers in our mathematical model, we attempted to isolate the function of TNFα on osteoblast differentiation by recreating the phenotype of the TNFα-transgenic mouse treated with the RANK-L inhibitor osteoprotegrin (OPG), which blocks osteoclastogenesis. Interestingly, the bone mineral density of TNFα-transgenics treated with OPG is higher than that of wild-type mice treated with OPG (Table 1). Attempts to reproduce that in vivo finding with our in silico model suggested that TNFα does not inhibit osteoblast differentiation; in contrast, they suggested that TNFα may increase osteoblast formation or decrease osteoblast apoptosis (Fig. 5, which is published as supporting information on the PNAS web site).
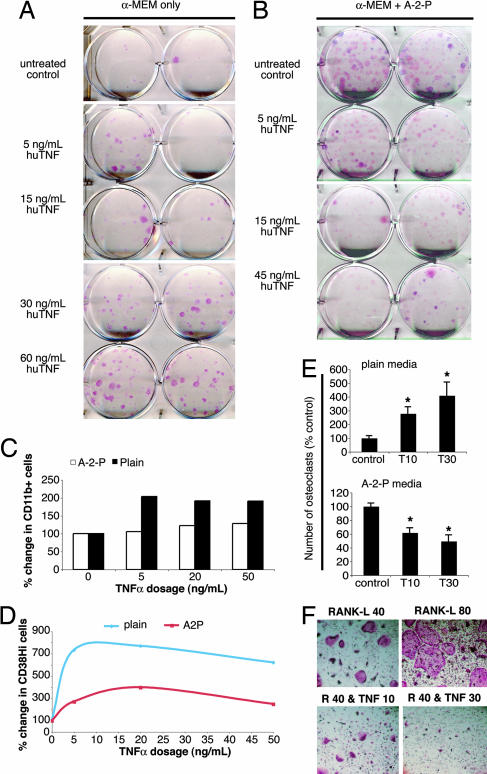
Consistent with previous reports of TNFα action on osteoblast differentiation, we found that TNFα dose-dependently decreased the number of osteoblast-like colonies using the established protocol for CFU-F colony formation (Fig. 3B). The established osteoblast differentiation method uses ascorbic acid (vitamin C) to induce the expression of osteoblast-specific genes. Although ascorbic acid is believed to boost the immune system, ironically recent findings suggest that it may inhibit inflammation. Specifically, the ascorbic acid derivative dehydroascorbic acid (DHA) noncompetitively inhibits IκB kinases and thus prevents the activation of the downstream transcription factor NF-κB (16). When NF-κB activation is blocked, TNFα signaling leads to either apoptosis or replicative senescence (17, 18). We hypothesized that ascorbic acid in the culture media might account for the inhibitory effects of TNFα on osteoblast differentiation. When we cultured total bone marrow without ascorbic acid, we found that TNFα dose-dependently increased the number of alkaline phosphatase-positive CFU-F colonies (Fig. 3A).
Fig. 3.
Ascorbic acid reverses the actions of TNFα on bone metabolism. A and B examine the role of ascorbic acid in modulating TNFα action on osteoblast formation. (A) TNFα dose-dependently increases CFU-F colony formation from total bone marrow in the absence of the ascorbic acid derivative ascorbate-2-phosphate. (B) In the presence of ascorbate-2-phosphate, TNFα dose-dependently decreases CFU-F colony formation from total bone marrow. C and D examine the role of ascorbic acid in modulating TNFα action on osteoclast precursor expansion and osteoclast formation. (C) Treatment of total bone marrow with TNFα leads to an expansion in the number of CD11b+ cells, as assessed by flow cytometry; this effect is blunted in media containing ascorbate-2-phosphate (A-2-P). The y axis denotes the percentage of change in the percentage of all bone marrow cells that were CD11b+. (D) Blunting of the effect of TNFα on the number of cells displaying CD38, a marker of TNFα action on murine macrophages. Total bone marrow from C57BL mice was plated in media either with ascorbate-2-phosphate (A-2-P) or without it in the presence of various doses of TNFα (0–50 ng/ml). After washing to remove nonadherent cells, EDTA was used to lift the adherent cells; these cells were stained with antibodies to CD11b and CD38 after which 30,000 cells were analyzed by flow cytometry. The frequency of CD38+ cells was calculated and plotted as a percentage change from the non-TNFα-treated group. (E) The effects of TNFα on increasing osteoclast formation (Upper) are reversed in the presence of ascorbate-2-phosphate, such that TNFα decreases RANK-L-induced osteoclast formation (Lower). P = 0.02 for 10 ng/ml TNFα and 0.03 for 30 ng/ml in Upper; P = 0.008 for 10 ng/ml and 0.007 for 30 ng/ml in Lower. (F) The appearance of osteoclasts formed in ascorbate-2-phosphate- (A-2-P)-containing medium. Murine bone marrow was flushed and plated with M-CSF (5 ng/ml) for 24 h. Nonadherent cells were used for purification on a Ficoll column. The interface layer was then plated at 3 × 104 cells per well in media containing A-2-P or in media without A-2-P (data not shown). M-CSF (30 ng/ml) was added with RANK-L at 80 ng/ml (Upper Right) or 40 ng/ml (Upper Left and Lower). To some wells, murine TNFα was added at 10 ng/ml (Lower Left) or 30 ng/ml (Lower Right). After 5 days of culture, the cells were fixed and stained for tartrate-resistant acid phosphatase (TRAP).
More nonalkaline phosphatase-positive cells, such as macrophages, were observed in TNFα-stimulated cultures without ascorbic acid compared with when it was added. We thus speculated that ascorbic acid might also inhibit TNFα-induced macrophage differentiation. To test this hypothesis, we analyzed by flow cytometry the number of CD11b+ cells in total bone marrow cultures after the addition of increasing amounts of TNFα. We found that TNFα application increased the number of CD11b+ cells but that the addition of ascorbic acid largely prevented this increase (Fig. 3C). Likewise, the percentage of cells expressing the TNFα-induced differentiation marker CD38 was attenuated by the inclusion of ascorbic acid (Fig. 3D). Because CD11b+ cells serve as osteoclast precursors, we examined whether the loss of their expansion in the presence of ascorbic acid was able to prevent TNFα-induced increases in osteoclast formation. We found that inclusion of ascorbic acid in the culture medium caused TNFα to inhibit osteoclast formation instead of stimulating it (Fig. 3 E and F). Overall, we found that TNFα increases both osteoclast and osteoblast formation and that both of these actions are reversed by ascorbic acid. A comprehensive diagram showing the actions of FSH and TNF on the skeleton is shown in Fig. 4.
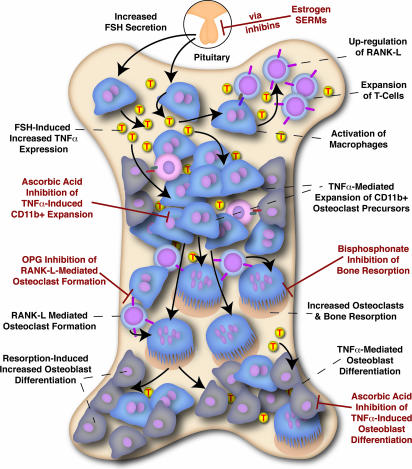
Fig. 4.
Integrated hypothesis for hypogonadal bone loss. Ovarian dysfunction and the loss of estrogen lead to decreased inhibin levels and dramatic increases in FSH levels. FSH, in turn, directly stimulates osteoclast differentiation and TNFα production from bone marrow macrophages/granulocytes. TNFα (shown as T) acts to increase M-CSF levels and/or M-CSF receptor expression, resulting in an expansion of the number of osteoclast precursors. Additionally, TNFα may prime macrophages to induce the proliferation of activated T lymphocytes, which highly express RANK-L and further contribute to TNFα production. The overabundance of osteoclast precursors, coupled with the osteoclastic differentiation agents FSH and RANK-L, which is expressed on T lymphocytes and stromal cells, likely compose the proosteoclastic component of high-turnover bone loss. TNFα-induced increases in the number of osteoblasts, as well as resorption-induced osteoblast formation, likely compose the proosteoblastic component of high-turnover bone loss. TNFα action can be blocked by treatment with etanercept or analogs, or, as we have found in this report, through supplementation with ascorbic acid (vitamin C). The proosteoclastogenic actions of RANK-L can be blocked by RANK-Fc or OPG. The resorptive function of osteoclasts can be blocked by bisphosphonates, which are taken up by resorbing osteoclasts and modulate their sensitivity to apoptotic stimuli. Estrogen replacement therapy or selective estrogen receptor modulator (SERM) therapy, per our hypothesis, may decrease FSH levels to reduce TNFα expression.
Discussion
Severely estrogen-deficient FSHβ-null mice with normal testosterone levels (19) are resistant to bone loss that normally accompanies hypogonadism (2). In contrast, mice deficient in the enzyme aromatase, which are equally severely hypogonadal, but with increased FSH and testosterone levels lose bone profoundly (19, 20). Haploinsufficiency of FSHβ increases bone mass but does not reduce estrogen or elevate testosterone (2, 19). The latter argues for a direct, sex steroid-independent action of FSH on the skeleton. The premise that the osteoclast is the primary target for FSH is strengthened by our demonstration that FSHβ-null mice have suppressed osteoclast formation in vivo and in ex vivo cultures (2). This osteoclast-specific action noted in vivo is consistent with data showing a direct action of FSH in vitro on osteoclastogenesis and bone resorption in both mice and humans (2).
A potential direct stimulatory action of FSH on bone mass in vivo, although awaiting definitive proof, is clinically meaningful in view of the close correlation between bone loss and changes in serum FSH, rather than sex steroids measured across the menopausal transition (21). In another study, plasma levels of inhibin correlate with bone mass more strongly than with FSH (22). The relationship between hormone levels and bone mass might simply be correlative rather than causal. However, the observation that amenorrheic women with high FSH levels are osteoporotic whereas amenorrheic women with normal or low FSH levels are not (23) highlights the potential importance of FSH in mediating, at least in part, hypogonadal bone loss in humans. Thus, a therapeutic goal could well be to suppress serum FSH levels, for example, with an antibody, in an effort to prevent bone loss, while sparing the ovaries. Genetic evidence in mice that an ≈50% reduction in plasma FSH enhances bone mass without affecting ovarian function (2) attests to the conceptual feasibility of this approach.
Although FSH stimulates bone resorption in vitro (2), this osteoclast-stimulatory action of FSH cannot fully explain the increased bone formation and immune cell alterations that accompany hypogonadal osteoporosis. There is strong evidence showing that T lymphocytes and inflammatory cytokines, such as TNFα and IL-7, play essential roles in hypogonadal bone loss (3, 24). Evidence that FSH triggers TNFα production from macrophages and granulocytes, we believe, bridges the estrogen–FSH axis to the previously proven immune-mediated changes in bone cells (24). Thus, we may have identified the physiological trigger, FSH, for the enhanced TNFα production that accompanies estrogen deficiency, which was not identified in previous studies (5), likely representing differences in experimental protocol.
That FSH stimulates TNFα production is complemented by loss-of-function studies demonstrating lowered TNFα levels in FSHβ-deficient mice, despite severe hypogonadism. Thus, although estrogen deficiency, for example after ovariectomy, elevates TNFα expression (24), it appears from our study that FSH is required for this action, as it is for the accompanying bone loss (2). Interestingly, mice deficient in the thyroid-stimulating hormone (TSH) receptor (TSHR) lose bone and have elevated TNFα levels; recombinant TSH expectedly inhibits TNFα expression (25). Thus, the deletion of the TNFα gene on a TSHR-deficient background rescues the bone loss, suggesting that the osteopenia from TSHR deficiency arises mainly from elevated TNFα levels (25–27). Taken together, the data indicate that (i) glycoprotein hormone receptors in bone regulate TNFα production and (ii) modulation of TNF may represent a key mechanism through which the pituitary gland affects bone mass.
Remaining unclear, however, is how TNFα causes high-turnover osteoporosis when in fact it suppresses osteoblast differentiation in vitro (13, 15). By adopting a mathematical model, we found that all of the actions of TNFα in causing a high-turnover bone loss, for which the TNFα-transgenic is a valid model, can be ascribed to an increase in the osteoclast precursor pool. Modeling the phenotype of TNFα-transgenic mice treated with OPG suggested that TNFα was proosteoblastic. Consistent with this notion, there is a dissociation between the osteoclast and osteoblast phenotypes in TNFα-Tg/CD44−/− mice (28). Note, however, that FSH does not affect the osteoblast, although osteoblast precursors do express FSH receptors (2). To understand the discrepancy between the modeling/in vivo data and previously documented in vitro suppression of osteoblastogenesis, we determined whether ascorbic acid, an adjunct used in culture experiments, affected TNFα-induced osteoblastogenesis. Indeed, we found that TNFα enhanced osteoblast differentiation in the absence of added ascorbic acid, whereas it inhibited osteoblastogenesis in cultures containing ascorbic acid. In fact, we also noted that the increase in the osteoclast precursor pool is reversed with ascorbic acid.
Prior studies have implicated ascorbic acid in the control of bone metabolism. Low dietary intake of ascorbic acid is associated with decreases in bone mass despite greater rates of bone formation and increases in mineralizing surfaces (29). In postmenopausal women, a high intake of ascorbic acid lowers the levels of the bone turnover marker c-telopeptide (30), whereas a low intake of ascorbic acid increases the rate of bone loss (31). Our data provide a plausible explanation of these findings: decreased levels of ascorbic acid would elevate bone turnover causing increases in both osteoclastic and osteoblastic function, but with a net bone loss. Indeed, sfx mice deficient in an enzyme essential for the production of ascorbic acid undergo spontaneous fractures at an early age (32). Thus, we speculate that the treatment of postmenopausal osteoporosis might benefit from supplementation with ascorbic acid. The Women's Health Initiative recently found, in a retrospective analysis, that women taking ascorbic acid in addition to estrogen had significantly greater bone mineral density gains at all sites compared with those on estrogen alone (33). Further studies are urgently required to evaluate the beneficial effects of ascorbic acid in the treatment and prevention of hypogonadal bone loss in prospectively designed clinical trials.
Methods
Cell Preparation.
Information regarding C57BL6J and FSHβ mice is available elsewhere (2). To obtain total bone marrow, mice were killed by an institutionally approved protocol, both femurs and tibiae were surgically extracted, and their bone marrow was flushed. The bone marrow pellet was resuspended in Opti-MEM I (Invitrogen, Carlsbad, CA) containing 5% FBS (Select USA stock; Invitrogen) and 1% penicillin/streptomycin.
Osteoclast Formation Assays.
Recombinant murine M-CSF (5 ng/ml; R & D Systems, Minneapolis, MN) was added, and the cells were incubated for 1 day. Nonadherent cells were collected and layered over 6 ml of Ficoll–Hypaque (Amersham/GE Health Sciences, Piscataway, NJ) for density centrifugation. The cells from the interface layer were collected and resuspended in α-MEM containing 10% FBS and 1% penicillin/streptomycin. Cells were plated at 3 × 104 per well in a 96-well plate with M-CSF (30 ng/ml) and various concentrations of RANK-L (R & D Systems). After 5 days, the cells were stained according to Technical Bulletin 445 from BD Biosciences (San Jose, CA).
CFU-F Colony Formation.
Total bone marrow was diluted in α-MEM with 10% FBS and 1% penicillin/streptomycin with or without 1 mM ascorbate-2-phosphate (phosphorylated form of ascorbic acid) and plated at 3 × 106 cells per well in six-well plates. After 3 weeks (with ascorbate-2-phosphate) or 6 weeks (without ascorbate-2-phosphate) the cells were stained as described previously (28).
Flow Cytometry.
Total bone marrow was diluted in Opti-MEM (see Cell Preparation) and allowed to acclimatize in an incubator for 2 h. A 0-h sample was taken, FSH (100 ng/ml) was added, and the cells were sampled at 1, 6, and 18 h after addition. The cells were fixed by using PhosphoFix (BD Biosciences,) and permeabilized by using 90% methanol at −20°C. Cells were stained for TNFα-allophycocyanin alone or in combination with CD11b-Alexa Fluor 488, B220-phycoerythrin, or CD3-FITC (antibodies from BD Biosciences). Stained cells were washed, and 30,000 events were collected on a FACSCalibur as described previously (28). FACS data were analyzed with Flow-Jo software (Tree Star, Ashland, OR) for Macintosh.
Serum ELISAs.
Serum from FSHβ-null mice and control littermates was taken as previously described (2). A commercial kit for murine TNFα (KMC3011; Invitrogen) was used according to the manufacturer's directions. Serum was diluted 1:1 in diluent provided by the manufacturer. Four animals for each group were assayed two times; Student's paired t tests were performed from the sum of all measurements for each group.
Mathematical Modeling.
The mathematical model of bone metabolism first reported by Lemaire et al. (14) was adapted such that the one-component osteoclast system was divided to include a preosteoclast component and an osteoclast component. All other parameters were set as previously described (14). Calculations and graph plotting were carried out by using MatLab (Release 14; MathWorks, Natick, MA) with a three-file program consisting of separate files for parameters, model, and execution/graphing.
Supplementary Material
Acknowledgments
We gratefully acknowledge support from the National Institutes of Health through Grants AG14917 (to M.Z.), DK70526 (to M.Z.), AG23176 (to M.Z.), AG12951 (to H.C.B.), AR47700 (to H.C.B.), and HD043945 (to T.R.K.); an Endocrinology Training Grant from the National Institute of Diabetes and Digestive and Kidney Diseases (supporting J.I.); and a Medical Scientist Training Program institutional grant (to Mount Sinai School of Medicine). J.I. won the Anthony Means Award from the Endocrine Society, and L.S. won the Award for Outstanding Research in the Pathophysiology of Osteoporosis from the American Society for Bone and Mineral Research.
Abbreviations
- OPG
osteoprotegrin
- FSH
follicle-stimulating hormone
- M-CSF
macrophage colony-stimulating factor.
Footnotes
Cnflict of interest statement: M.Z. is on the speaker bureau for Roche, GlaxoSmithKline, Aventis, Procter & Gamble, and Merck. M.Z. has research grant support from Procter & Gamble.
References
- 1.US Department of Health and Human Services. Bone Health and Osteoporosis: A Report of the Surgeon General. Rockville, MD: US Department of Health and Human Services, Office of the Surgeon General; 2004. pp. 68–87. [Google Scholar]
- 2.Sun L, Peng Y, Sharrow AC, Iqbal J, Zhang Z, Papachristou DJ, Zaidi S, Zhu LL, Yaroslavskiy BB, Zhou H, et al. Cell. 2006;125:247–260. doi: 10.1016/j.cell.2006.01.051. [DOI] [PubMed] [Google Scholar]
- 3.Roggia C, Gao Y, Cenci S, Weitzmann MN, Toraldo G, Isaia G, Pacifici R. Proc Natl Acad Sci USA. 2001;98:13960–13965. doi: 10.1073/pnas.251534698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van der Poll T, Romijn JA, Endert E, Sauerwein HP. Metabolism. 1993;42:303–307. doi: 10.1016/0026-0495(93)90078-3. [DOI] [PubMed] [Google Scholar]
- 5.Hutson JC. J Reprod Immunol. 1993;23:63–72. doi: 10.1016/0165-0378(93)90027-f. [DOI] [PubMed] [Google Scholar]
- 6.Mauduit C, Besset V, Caussanel V, Benahmed M. Biochem Biophys Res Commun. 1996;224:631–637. doi: 10.1006/bbrc.1996.1077. [DOI] [PubMed] [Google Scholar]
- 7.Ralston SH, Russell RG, Gowen M. J Bone Miner Res. 1990;5:983–988. doi: 10.1002/jbmr.5650050912. [DOI] [PubMed] [Google Scholar]
- 8.Cenci S, Weitzmann MN, Roggia C, Namba N, Novack D, Woodring J, Pacifici R. J Clin Invest. 2000;106:1229–1237. doi: 10.1172/JCI11066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pacifici R, Brown C, Puscheck E, Friedrich E, Slatopolsky E, Maggio D, McCracken R, Avioli LV. Proc Natl Acad Sci USA. 1991;88:5134–5138. doi: 10.1073/pnas.88.12.5134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lam J, Takeshita S, Barker JE, Kanagawa O, Ross FP, Teitelbaum SL. J Clin Invest. 2000;106:1481–1488. doi: 10.1172/JCI11176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li P, Schwarz EM, O'Keefe RJ, Ma L, Looney RJ, Ritchlin CT, Boyce BF, Xing L. Arthritis Rheum. 2004;50:265–276. doi: 10.1002/art.11419. [DOI] [PubMed] [Google Scholar]
- 12.Yao Z, Li P, Zhang Q, Schwarz EM, Keng P, Arbini A, Boyce BF, Xing L. J Biol Chem. 2006;281:11846–55. doi: 10.1074/jbc.M512624200. [DOI] [PubMed] [Google Scholar]
- 13.Gilbert LC, Rubin J, Nanes MS. Am J Physiol. 2005;288:E1011–E1018. doi: 10.1152/ajpendo.00534.2004. [DOI] [PubMed] [Google Scholar]
- 14.Lemaire V, Tobin FL, Greller LD, Cho CR, Suva LJ. J Theor Biol. 2004;229:293–309. doi: 10.1016/j.jtbi.2004.03.023. [DOI] [PubMed] [Google Scholar]
- 15.Bertolini DR, Nedwin GE, Bringman TS, Smith DD, Mundy GR. Nature. 1986;319:516–518. doi: 10.1038/319516a0. [DOI] [PubMed] [Google Scholar]
- 16.Carcamo JM, Pedraza A, Borquez-Ojeda O, Golde DW. Biochemistry. 2002;41:12995–13002. doi: 10.1021/bi0263210. [DOI] [PubMed] [Google Scholar]
- 17.Beg AA, Baltimore D. Science. 1996;274:782–784. doi: 10.1126/science.274.5288.782. [DOI] [PubMed] [Google Scholar]
- 18.Ponnappan U. Front Biosci. 1998;3:152–168. doi: 10.2741/a271. [DOI] [PubMed] [Google Scholar]
- 19.Abel MH, Huhtaniemi I, Pakarinen P, Kumar TR, Charlton HM. Reproduction. 2003;125:165–173. doi: 10.1530/rep.0.1250165. [DOI] [PubMed] [Google Scholar]
- 20.Oz OK, Hirasawa G, Lawson J, Nanu L, Constantinescu A, Antich PP, Mason RP, Tsyganov E, Parkey RW, Zerwekh JE, Simpson ER. J Steroid Biochem Mol Biol. 2001;79:49–59. doi: 10.1016/s0960-0760(01)00130-3. [DOI] [PubMed] [Google Scholar]
- 21.Sowers MR, Jannausch M, McConnell D, Little R, Greendale GA, Finkelstein JS, Neer RM, Johnston J, Ettinger B. J Clin Endocrinol Metab. 2006;91:1261–1267. doi: 10.1210/jc.2005-1836. [DOI] [PubMed] [Google Scholar]
- 22.Perrien DS, Achenbach SJ, Bledsoe SE, Walser B, Suva LJ, Khosla S, Gaddy D. J Clin Endocrinol Metab. 2006;91:1848–1854. doi: 10.1210/jc.2005-2423. [DOI] [PubMed] [Google Scholar]
- 23.Devleta B, Adem B, Senada S. J Bone Miner Metab. 2004;22:360–364. doi: 10.1007/s00774-004-0495-1. [DOI] [PubMed] [Google Scholar]
- 24.Toraldo G, Roggia C, Qian WP, Pacifici R, Weitzmann MN. Proc Natl Acad Sci USA. 2003;100:125–130. doi: 10.1073/pnas.0136772100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hase H, Ando T, Eldeiry L, Brebene A, Peng Y, Liu L, Amano H, Davies TF, Sun L, Zaidi M, Abe E. Proc Natl Acad Sci USA. 2006;103:12849–12854. doi: 10.1073/pnas.0600427103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abe E, Marians RC, Yu W, Wu XB, Ando T, Li Y, Iqbal J, Eldeiry L, Rajendren G, Blair HC, et al. Cell. 2003;115:151–162. doi: 10.1016/s0092-8674(03)00771-2. [DOI] [PubMed] [Google Scholar]
- 27.Sun L, Blair HC, Davies TF, Abe E, Zaidi M. Ann NY Acad Sci. 2006;1068:304–318. doi: 10.1196/annals.1346.033. [DOI] [PubMed] [Google Scholar]
- 28.Hayer S, Steiner G, Gortz B, Reiter E, Tohidast-Akrad M, Amling M, Hoffmann O, Redlich K, Zwerina J, Skriner K, et al. J Exp Med. 2005;201:903–914. doi: 10.1084/jem.20040852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kipp DE, Grey CE, McElvain ME, Kimmel DB, Robinson RG, Lukert BP. J Nutr. 1996;126:2044–2049. doi: 10.1093/jn/126.8.2044. [DOI] [PubMed] [Google Scholar]
- 30.Pasco JA, Henry MJ, Wilkinson LK, Nicholson GC, Schneider HG, Kotowicz MA. J Women's Health (Larchmt) 2006;15:295–300. doi: 10.1089/jwh.2006.15.295. [DOI] [PubMed] [Google Scholar]
- 31.Kaptoge S, Welch A, McTaggart A, Mulligan A, Dalzell N, Day NE, Bingham S, Khaw KT, Reeve J. Osteoporos Int. 2003;14:418–428. doi: 10.1007/s00198-003-1391-6. [DOI] [PubMed] [Google Scholar]
- 32.Mohan S, Kapoor A, Singgih A, Zhang Z, Taylor T, Yu H, Chadwick RB, Chung YS, Donahue LR, Rosen C, et al. J Bone Miner Res. 2005;20:1597–1610. doi: 10.1359/JBMR.050406. [DOI] [PubMed] [Google Scholar]
- 33.Morton DJ, Barrett-Connor EL, Schneider DL. J Bone Miner Res. 2001;16:135–140. doi: 10.1359/jbmr.2001.16.1.135. [DOI] [PubMed] [Google Scholar]
- 34.Li P, Schwarz EM, O'Keefe RJ, Ma L, Boyce BF, Xing L. J Bone Miner Res. 2004;19:207–213. doi: 10.1359/JBMR.0301233. [DOI] [PubMed] [Google Scholar]
- 35.Schett G, Redlich K, Hayer S, Zwerina J, Bolon B, Dunstan C, Gortz B, Schulz A, Bergmeister H, Kollias G, et al. Arthritis Rheum. 2003;48:2042–2051. doi: 10.1002/art.11150. [DOI] [PubMed] [Google Scholar]
- 36.Redlich K, Gortz B, Hayer S, Zwerina J, Doerr N, Kostenuik P, Bergmeister H, Kollias G, Steiner G, Smolen JS, Schett G. Am J Pathol. 2004;164:543–555. doi: 10.1016/S0002-9440(10)63144-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.