Abstract
Despite initial virus control by CD8+ cytotoxic T lymphocytes (CTLs), noncytopathic or variably cytopathic viruses (e.g., hepatitis B and C viruses, HIV) are able to establish persistent infections. The role of neutralizing antibodies (nAbs) in controlling disease progression is unclear. Therefore, the phenomenon of viral evasion from the nAb response and its implications for virus persistence remain controversial. Here we demonstrate nAb-mediated viral clearance in CTL-deficient mice infected with the prototypic noncytopathic lymphocytic choriomeningitis virus (strain WE). During prolonged CTL absence, neutralization-resistant virus mutants were selected in individual mice within 70–90 days. In naïve animals infected with these virus variants only low nAb responses were induced, resulting in an increased tendency of virus to persist.
Many viruses that are minimally or noncytopathic in vivo tend to establish chronic infections in their hosts. These comprise hepatitis B or C viruses in humans and lymphocytic choriomeningitis virus (LCMV) in mice and may include HIV and simian immunodeficiency virus. The infections initially are controlled by a strong specific CD8+ cytotoxic T lymphocyte (CTL) response (1), either by direct lysis of infected cells (2) or through production of noncytolytic mediators (3). The cellular immune system alone, however, fails to control these infections in the longer term (4–6).
Neutralizing antibody (nAb) responses to noncytopathic viruses usually are generated late in the course of infection, generally after the initial control of viremia (7–9). Nevertheless, several observations indicate that nAb may contribute to long-term control. First, passive immunization studies in animal models show partial protection (10, 11). Second, nAb titers correlate with lack of disease progression in long-term survivors during HIV infection (12). Third, virus reappears in mice lacking nAb responses (B cell-deficient or CD4+ T cell-deficient mice) 70–150 days after efficient initial viral control during LCMV infection (5, 6). Finally, variant viruses resistant to neutralization by polyclonal autologous sera have been found during HIV and simian immunodeficiency virus infections (13–15). Although immune evasion through antigenic variation of neutralizing epitopes has been demonstrated in vivo for several viruses exposed to mAbs (16, 17) and is a major force in the evolution of influenza viruses within the human population (18), virus evasion from polyclonal antibody responses in one individual and perhaps focused on several neutralizing epitopes, is more difficult to explain (19).
Although CTLs play an important role in the immune control of murine infection with LCMV (20), kinetics of virus elimination sometimes revealed variably efficient clearance and unexplained viral re-emergence (21–23). We therefore examined the correlation between nAb responses and LCMV elimination or persistence. We found delayed, but efficient, control of LCMV by nAb responses in the absence of CD8+ T cells. Unexpectedly, virus variants emerged that escaped the established polyclonal Ab response. Thus, under conditions where CD8+ T cell responses are, or become, low or absent LCMV may be able to persist within a single host by changing neutralizing surface determinants.
Materials and Methods
Mice.
CD8−/− (24), P−/− (2), IFN-αβR−/− (25), IFN-γR−/− (26), RAG-1−/−, and μMT mice (27) as well as C57BL/6 (B6), BALB/c and wild-type (wt)129 mice were obtained from the Institut für Labortierkunde (University of Zürich, Switzerland). All animals were kept under specific pathogen-free conditions.
Viruses and Neutralizing Activity.
LCMV strain WE originally was obtained from F. Lehmann-Grube, Heinrich Pette Institut, Hamburg, Germany, and propagated on L929 cells. LCMV nAb-escape variants were isolated from spleen homogenates of CD8−/− mice, grown on MC57 cells for 48 h, and subsequently plaque-purified two times in vitro as described (17). LCMV titers and nAb titers in blood, or virus titers of centrifuged tissue homogenates, and of virus stock solutions were determined with an immunological focus assay (28).
In Vivo Depletion of CD8+ T Cells.
Mice were treated with 0.2 ml of a tested rat anti-CD8 mAb (YTS 169.4; ref. 29) i.p. 3 days and 1 day before LCMV infection (8). The degree of depletion was assessed by flow cytometric analysis (FACScan, Becton Dickinson) using FITC-conjugated anti-CD8 mAb (PharMingen) and was always > 95% in blood and spleen.
Molecular Analysis.
Sequence analysis of LCMV-WE Glycoprotein (GP)-1 was performed as described (17).
Results
CTL-Independent Clearance of LCMV by nAbs.
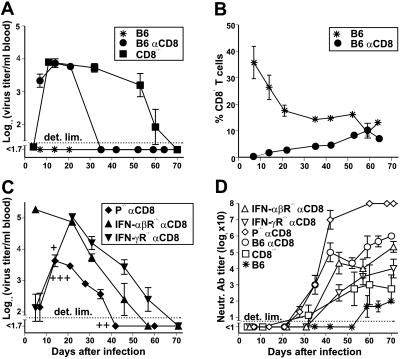
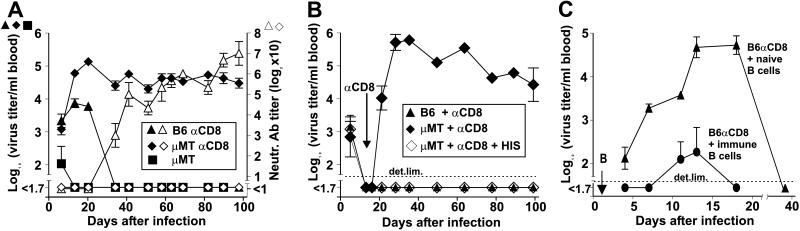
We have observed previously that mice depleted of CD8+ T cells in vivo by treatment with mAb (29) are sometimes capable of clearing LCMV eventually (9). Although the degree of CD8-depletion was > 95% in blood and spleen as assessed by flow cytometric analysis (8), CD8+ T cells reappeared in the blood after 10–15 days, reaching control levels after 60 days (Fig. 1B). To investigate the role of re-emerging CD8+ T cells for virus clearance, we compared CD8-depleted B6 mice and CD8−/− mice (24) for their ability to control a low dose (200 plaque-forming units, pfu) i.v. infection with LCMV-WE. Although immunocompetent B6 mice cleared the virus within 1 week, viremia was controlled between days 30 and 40 in CD8-depleted B6 mice and between days 60 and 70 in CD8−/− mice (Fig. 1A). Direct ex vivo (12–15 h), as well as secondary 51Cr-release assays after in vitro restimulation failed to detect MHC class I restricted cytotoxicity in CD8−/− mice on days 8, 50, and 100 after infection (data not shown, ref. 24). BALB/c mice or wt129 mice depleted of CD8+ T cells controlled viremia similar to B6 mice (data not shown). To evaluate whether cytolytic or noncytolytic mediators released by reappearing CD8+ T cells were responsible for the earlier virus clearance in CD8-depleted mice compared with CD8−/− mice, we additionally examined anti-CD8-treated mice deficient either in perforin or IFN receptors [P−/− mice (2), IFN-αβR−/− mice (25), and IFN-γR−/− mice (26)] infected with 200 pfu of LCMV-WE. Non-CD8-depleted control P−/−, IFN-αβR−/−, and IFN-γR−/− mice died of CD8+ T cell-mediated immunopathology 20–40 days after infection (Fig. 1C, refs. 2 and 30). Under conditions of transient CD8+ T cell deficiency, viremia was controlled in P−/− mice with similar kinetics as in B6 mice, whereas a longer period was needed for clearance in mice deficient in IFN receptors, paralleling the findings in CD8−/− mice (Fig. 1C). Thus, interferons (particularly IFN-γ) seem to be of greater importance than perforin for long-term LCMV control, in agreement with recent studies (3, 6, 30, 31). The surprising finding, however, was that mice permanently lacking either CTLs or different effector mediators were able to eventually control this low-dose LCMV infection, indicating additional antiviral mechanisms. CD8-depleted mice, as well as CD8−/− mice, generated stronger and earlier nAb responses in comparison to control animals (Fig. 1D), correlating with absence of CD8+ T cell-mediated immunosuppression and increased virus titers (8, 9). Together with earlier findings (5, 6) showing LCMV re-emergence in B cell-deficient mice (μMT mice, ref. 27), the observed correlation between low nAb titers and delayed virus control in the different CD8-depleted mice studied (Fig. 1 A, C, and D), suggested that the nAb response might be involved in LCMV clearance in the absence of CD8+ T cells. To test this hypothesis, μMT mice were in vivo depleted of CD8+ T cells and infected with 200 pfu of LCMV-WE i.v. In contrast to untreated μMT mice, these mice were not able to clear the virus for up to 100 days (Fig. 2A). μMT mice may represent a complex phenotype (32) and may have some defects in T help. It has been hypothesized that specific B cells might be involved in priming of T help (27, 32). This hypothesis requires further evaluation, but seems an unlikely explanation because specific B cell frequencies are too low (33). In addition, immunity to LCMV-Armstrong may differ from that to LCMV-WE (32). In a subsequent experiment, LCMV-infected μMT mice were depleted of CD8+ T cells once viremia was controlled by administration of anti-CD8 mAbs on days 15 and 17 after infection (Fig. 2B). Viremia was detectable again 2 days after anti-CD8 treatment and virus persisted at high levels, in contrast to control CD8-depleted B6 mice, which controlled viremia during the whole observation period of 100 days. Interestingly, administration of polyclonal anti-LCMV-WE serum (200 μl; pooled from immune B6 mice) between the two CD8-depleting mAb injections (day 16) and weekly thereafter, prevented re-emergence of the virus in μMT mice (Fig. 2B). To further confirm the role of nAbs in controlling viremia under the conditions tested here, we adoptively transferred i.v. 107 LCMV-immune, magnetic cell sorting-purified B cells (pooled from spleens of CD8-depleted donor B6 mice, 30 days after infection with 200 pfu LCMV, of > 90% purity as assessed by flow cytometry) into CD8-depleted B6 recipient mice, 1 day after infection with 200 pfu of LCMV i.v. Mice that received B cells from uninfected donor animals were able to clear the virus from blood after about 40 days. In comparison, recipients of LCMV-immune B cells exhibited drastically reduced virus titers in the blood and controlled viremia more rapidly by day 18 (Fig. 2C).
Figure 1.
Control of viremia in CD8-depleted mice (αCD8) and CD8−/− mice infected with a low dose of LCMV-WE. CD8−/− mice and in vivo CD8-depleted B6, P−/−, IFN-αβR−/−, and IFN-γR−/− mice were infected i.v. with 200 pfu of LCMV-WE. Nondepleted mice were used as controls. Sequential blood samples were analyzed for virus titers (A and C) for assessment of CD8 depletion by flow-cytometric analysis (B) and for neutralizing activity against LCMV-WE (D). Nondepleted P−/− (+), IFN-αβR−/− (++), and IFN-γR−/− mice (+++) died by days 20–40 as a consequence of CD8+ T cell-mediated immunopathology. Only virus titers before death are indicated for these mice. Data shown are the mean of three mice per group ± SEM in one of two similar experiments.
Figure 2.
Influence of nAb-producing B cells on LCMV viremia in CD8-depleted (αCD8) mice. All mice were infected i.v. with 200 pfu LCMV-WE. (A) Virus titers (filled symbols) and nAb titers (open symbols) in CD8-depleted B6 and μMT mice and nondepleted control μMT mice. (B) B6 mice and μMT mice first were infected (day 0) and CD8-depleted after initial viral clearance (arrow; administration of anti-CD8 mAbs on days 15 and 17 after infection). In a subgroup of μMT mice (◊), 200 μl LCMV-WE hyperimmune serum (HIS) was additionally injected i.v. on day 16 and weekly thereafter. Viral titers were measured in sequential blood samples. (C) Magnetic cell sorting-purified B cells, pooled either from spleens of uninfected B6 mice (▴) or spleens of LCMV-WE-immune B6 mice (CD8-depleted on days −3 and −1, immunized on day 0, B cells taken 30 days after infection; ●) were adoptively transferred i.v. into recipient CD8-depleted mice on day 1 (arrow) after infection. Virus titers in the blood were sequentially determined. Data shown are the mean of three mice per group ± SEM in one of two similar experiments.
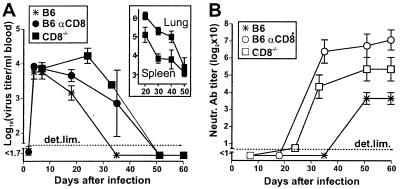
We then reasoned that the known (8) enhancement of the nAb response by high-dose LCMV-WE infection possibly would lead to earlier virus control during long-lasting CD8+ T cell deficiency when compared with 200 pfu infections (Fig. 1A). Therefore, CD8−/− mice were infected with 2 × 106 pfu of LCMV-WE. Viremia in these mice indeed was controlled earlier and with similar kinetics as in CD8-depleted B6 mice (Fig. 3A). Clearance correlated again with an enhanced nAb response (Fig. 3B). Virus was controlled not only in the blood, but also in solid organs (spleen, lung), where virus titers decreased by 100- to 1,000-fold within 50 days in CD8−/− mice (Fig. 3A Inset). Virus titers in organs (spleen, liver, lung, kidney) were below detection level by day 60 in CD8-depleted mice (data not shown).
Figure 3.
Early control of viremia in CD8−/− mice infected with a high dose (2 × 106 pfu) of LCMV-WE. Virus titers (A) and neutralizing activity against LCMV-WE (B) were determined in sequential blood samples. Organs from CD8−/− mice were harvested 20–50 days after infection for quantitation of LCMV titers in spleen and lung (A Inset). Data shown are the mean of six mice per group ± SEM in two independent experiments.
Virus Escape from the Polyclonal nAb Response During CD8+ T Cell Deficiency.
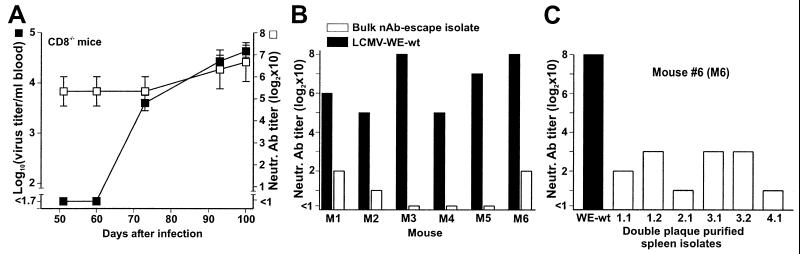
Surprisingly, a longer observation period of blood virus titers in CD8−/− mice infected with 2 × 106 pfu of LCMV-WE revealed a new wave of viremia in all of the mice (6/6) 2–3 weeks after viral clearance from blood or about 70 days after infection (Fig. 4A). Renewed viremia was similarly observed between days 90 and 100 in the following CD8-depleted mice: 2/6 P−/− mice, 2/6 IFN-αβR−/− mice, 2/6 IFN-γR−/− mice, and 3/3 thymectomized B6 mice infected with 200 pfu of LCMV-WE (data not shown). The remaining mice still controlling viremia at this time point had low virus titers in several organs (Table 1). This finding indicated again an important contribution of nAb for virus control in solid organs, probably by preventing or reducing continuous hematogenic virus spread.
Figure 4.
Virus re-emerging in CD8−/− mice escaped the autologous nAb response. (A) Blood from the same CD8−/− mice as in Fig. 3 was tested at later time points for virus titers (■) and neutralizing activity against the immunizing LCMV-WE (□). Data shown are the mean of six mice per group ± SEM in two independent experiments. (B and C) Sera obtained from individual CD8−/− mice (M1-M6) 100 days after infection were tested for neutralizing activity against the immunizing virus (LCMV-WE-wt, solid bars) and against contemporaneous virus isolated from the blood or the spleen of the corresponding mouse M1-M6 (open bars). Variants were used in the neutralization assays either as bulk isolates (B) or after double plaque purification of bulk isolates; shown for mouse #6 (C).
Table 1.
LCMV control in the absence of CTLs or selected antiviral mediators 100 days after infection with 200 pfu
| Genotype | Virus titer, log10
pfu/organ
|
Renewed viremia | |||
|---|---|---|---|---|---|
| Spleen | Liver | Kidney | Lung | ||
| wt* αCD8 | <1.6 | <1.6 | <1.0 | <1.0 | − |
| <1.6 | <1.6 | <1.0 | <1.0 | − | |
| <1.6 | <1.6 | <1.0 | <1.0 | − | |
| P−/− αCD8 | 2.7 | 3.0 | 2.5 | 2.8 | + |
| <1.6 | <1.6 | <1.0 | <1.0 | − | |
| <1.6 | <1.6 | 2.7 | <1.0 | − | |
| IFN-α/βR−/− αCD8 | 5.2 | 5.5 | 6.4 | 6.2 | + |
| <1.6 | <1.6 | <1.0 | <1.0 | − | |
| <1.6 | <1.6 | <1.0 | <1.0 | − | |
| IFN-γR−/− αCD8 | 3.2 | <1.6 | 2.8 | 2.2 | + |
| 2.6 | <1.6 | 4.2 | 3.2 | + | |
| <1.6 | <1.6 | 3.6 | <1.0 | − | |
| CD8−/− | 4.7 | 5.3 | 5.0 | 5.6 | + |
| 4.7 | 6.2 | 6.0 | 4.6 | + | |
| 4.8 | 4.7 | 5.0 | 5.5 | + | |
Mice of the indicated genotype were CD8-depleted (αCD8; except CD8−/− mice) and infected with 200 pfu of LCMV-WE. Organs were harvested 100 days after infection for quantitation of viral titers by plaque assay. LCMV organ titers of individual mice in one of two similar experiments are shown. Virus re-emerged in the mice indicated by +. Renewed viremia before day 100 was observed in 6/6 CD8−/− mice, 2/6 P−/− mice, 2/6 IFN-α/βR−/− and 2/6 IFN-γR−/− mice.
B6 mice and wt 129 mice were used as controls. Infected but not CD8-depleted P−/− and IFN-R−/− mice all died by days 20–40.
The observation of renewed viremia in the presence of high nAb titers (Fig. 4A) suggested that failure of long-lasting virus control might be caused by the emergence of neutralization-resistant virus mutants. We performed neutralization experiments with sequential autologous sera and virus isolates from CD8−/− mice. We found that sera taken after virus reappearance were able to neutralize the originally injected LCMV-WE wt virus, but could not neutralize the bulk virus rescued from the blood or the spleen of the corresponding donor animal 100 days after infection (Fig. 4B). Further analysis of these variants after double-plaque purification on MC57 cells revealed that the majority of the isolated virus clones resisted neutralization by autologous sera (shown for mouse #6 in Fig. 4C). Only four of 26 spleen isolates rescued from CD8−/− mice 100 days after LCMV infection were still sensitive to autologous neutralization. In comparison to LCMV-WE-wt, variants were neutralized to a lesser degree by polyclonal serum pooled from LCMV-WE-immune B6 mice or by several mAbs against the envelope glycoprotein GP-1 (KL25, WEN1, and WEN4; derived from immunizations of mice with LCMV-WE, ref. 34), as demonstrated in Table 2.
Table 2.
Neutralization of nAb-escape LCMV-WE variants by mAbs
| Antibody | Neutralization titer against nAb-escape
variants
|
||||||
|---|---|---|---|---|---|---|---|
| LCMV-WE-wt | WE-M4-1.1 | WE-M4-2.1 | WE-M5-2.1 | WE-M6-1.1 | WE-M6-2.1 | WE-M6-3.1 | |
| KL25 | 320 | 40 | 40 | <10 | 40 | 20 | 80 |
| WEN1 | 640 | 160 | 160 | <10 | 80 | 20 | 80 |
| WEN4 | 1280 | 40 | 80 | <10 | 10 | 20 | 40 |
| HIS | 640 | 40 | 80 | 40 | 40 | 40 | 40 |
Neutralization of nAb escape variants was tested in an infectious focus assay with the LCMV-WE GP1-specific neutralizing mAbs KL25, WEN1, and WEN4 (40 μg/ml) and a polyclonal LCMV-neutralizing mouse hyperimmune serum (HIS), and compared to neutralization of LCMV-WE-wt. Antibodies were ×10 prediluted and titrated in 2-fold dilution steps. LCMV nAb-escape variants were isolated from the spleen of CD8−/− mice (#4–6 in Figs. 4 and 5) 100 days after infection with 2 × 106 pfu LCMV-WE.
The two parts of the single envelope glycoprotein structure of LCMV (GP1 and GP2, amino acids 59–262 and 263–498, respectively) are derived from a precursor polypeptide and encoded on the small (S) segment of the LCMV RNA genome (35), together with the internal nucleoprotein. Competitive binding assays with mAbs have demonstrated the presence of a single, conformational neutralizing epitope on the GP1 of LCMV-WE (36). Sequence analysis of the neutralization-resistant spleen virus isolates from CD8−/− mice revealed various aa mutations in GP1 (Fig. 5). Only 1–3 aa substitutions were found in this region per viral isolate, confirming previous findings of minimal genetic variation of LCMV during chronic infection (37). Noncoding mutations were very rare: only three viral isolates of those characterized in Fig. 5 showed 1–2 silent nucleotide substitutions (not shown), arguing in favor of important selective advantages for the detected aa changes in GP1 in vivo. Importantly, aa substitutions were not present in the GP1 gene of 12 independent viral isolates from persistently infected μMT (three mice) and RAG-1−/− (three mice); this finding correlated with absence of selective pressure exerted by nAb on the virus (Fig. 5). The virus isolates that were still sensitive to autologous neutralization showed no mutation in their GP1 gene (not shown). None of the mutations shown in Fig. 5 led to additional potential sites for N-linked glycosylation (NXS or NXT), a condition associated with a limitation of the humoral response through epitope masking by carbohydrates (38–40).
Figure 5.
LCMV-WE nAb-escape variants contain amino acid-changing point mutations within the sequence coding for the viral envelope GP GP1 (amino acids 59–262). Positions of alterations in deduced amino acid residues are indicated by vertical lines along GP1. Unequivocal amino acid-changes between LCMV-WE-wt and double plaque-purified virus variants are shown. Variants were isolated from spleens of six CD8−/− mice (M1-M6 in Fig. 4) 100 days after infection with 2 × 106 pfu of LCMV-WE and from persistently infected, B cell-deficient μMT and RAG-1−/− mice.
In Vivo Analysis of nAb-Resistant Viruses.
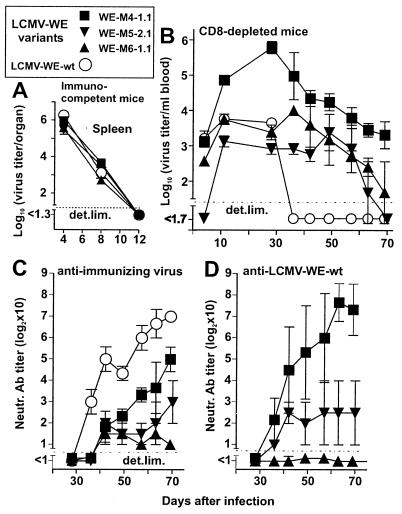
The following experiments were performed to ensure that persistence of spleen isolates was not caused by factors other than resistance to neutralization. Three distinct double plaque-purified nAb escape variants (WE-M4–1.1, WE-M5–2.1, and WE-M6–1.1 in Fig. 5) were chosen for these experiments. Their identity as nAb-escape mutants was confirmed by the demonstration that they were not neutralized by autologous sera from the same time point, although these sera still neutralized LCMV-WE-wt (not shown). In the first experiment, immunocompetent B6 mice were infected with 2 × 104 pfu of these nAb-escape variants to investigate their replication properties. Virus titers in spleens on day 4 after infection were not higher than those of control (LCMV-WE-wt infected) mice (Fig. 6A), and in all instances titers dropped below detection levels by day 12 after infection, not only in the spleen (Fig. 6A) but also in liver and lung. In addition, in vitro infection of MC57 cells with these variants (multiplicity of infection of 0.01) confirmed similar replication capacities (data not shown). Histological analysis of different organs by staining for LCMV with LCMV-immune serum (41) on days 4, 8, and 12 after infection with 2 × 104 pfu of LCMV variants failed to show differences in cell or organ tropism (data not shown). Thus, the variants were not of the LCMV docile or clone 13 type, as they tended not to persist in normal mice (37, 42).
Figure 6.
In vivo control of LCMV-WE nAb-escape variants in naive hosts. B6 mice (A) or CD8-depleted B6 mice (B–D) were infected with 2 × 104 pfu of the indicated nAb escape variants or with LCMV-WE-wt. Nondepleted mice were sacrificed on days 4, 8, and 12, and spleens were harvested for virus quantitation (A). Sequential blood samples from CD8-depleted mice were obtained for quantitation of LCMV titers (B), autologous nAb titers against immunizing virus (C), and reciprocal nAb titers against LCMV-WE-wt (D). Data shown are the mean for three mice per group ± SEM.
Could reappearing LCMV represent CTL-escape mutants (43) rather than nAb-escape mutants? Target MC57 cells infected with the same variants were readily recognized in a 51Cr release assay using splenocytes from mice immunized with LCMV-WE, excluding this possibility (data not shown). In addition, nucleotide sequences showed no mutations in the two immunodominant GP-specific CTL epitopes.
Because the nAb-escape variants were as rapidly controlled as LCMV-WE-wt in immunocompetent mice (probably via efficient virus clearance by CD8+ T cells), we next explored whether they would have a selective advantage in situations where viral control is predominantly mediated by nAb, i.e., during the transient absence of CD8+ T cells. Anti-CD8-treated B6 mice infected i.v. with 2 × 104 pfu of nAb-escape variants (WE-M4–1.1, WE-M5–2.1, and WE-M6–1.1, which have been characterized in Figs. 5 and 6A) showed prolonged viremia beyond day 60, in contrast to LCMV-WE-wt-infected mice, which controlled the virus within 30–40 days (Fig. 6B) and in contrast to CD8+ T cell-competent mice (Fig. 6A and data mentioned above). A more detailed analysis of the nAb response in these mice showed markedly decreased autologous neutralizing activity, compared with LCMV-WE-wt infected mice (Fig. 6C). Despite the slightly higher nAb titers induced against WE-M4–1.1, in comparison to the other variants, virus persisted for longer periods in WE-M4–1.1-infected mice (Fig. 6B). Therefore, additional parameters (e.g., different susceptibilities to IFN) might of course be involved, which also would explain the higher virus titers observed in these mice early after infection (37).
In addition to autologous neutralization assays, sera taken from these CD8-depleted mice infected with variant viruses were tested for their ability to neutralize the ancestor virus LCMV-WE-wt. Although after infection with WE-M5–2.1 and WE-M6–1.1 similar low neutralizing activity was detected against the LCMV-WE-wt virus, the sera of mice infected with WE-M4–1.1 neutralized LCMV-WE-wt much better than the immunizing virus (Fig. 6D), a situation reminiscent of the “original antigenic sin” phenomenon of influenza virus-specific antibodies (44). Notable differences to the influenza situation are: the LCMV mutants arose and persisted in individual mice and the affected animals had never encountered the ancestor virus before.
Taken together, neutralization-resistant viral mutants evolving in one individual show enhanced viral persistence in new CD8-incompetent hosts. This finding correlated with the variants' failure to induce adequate de novo humoral immune responses.
Discussion
This study provides evidence that, together with CTLs, nAbs play a major role in long-term control of low- and high-dose infection with LCMV-WE, a virus that persists at very low levels in immune mice (41). Therefore, general and specific immunopathology and suppression of the nAb response early after infection (8, 9, 45) contribute to virus persistence. If the antibody-mediated pressure on LCMV increases during infection, nAb-escape mutants are selected, particularly if the CTL response is weak or absent.
What is the general importance of nAb-escape mutants in host-parasite relationships? Our findings may help to explain several earlier observations of re-emerging LCMV in presence of ongoing or transferred immunity (21–23). Several organisms (African trypanosomes, Giardia lamblia, Borrelia sp., and Neisseria gonorrhoeae) may switch surface immunodominant protective antigenic determinants by specific strategies (e.g., gene rearrangement, ref. 46) within one single host. Also, the phenomenon described here in individual mice resembles the antigenic drift of influenza viruses that develops at the world population level (18). Escape of virus from a polyclonal antibody response has been suggested for several lentiviruses (13–15). In the absence of direct evidence of a role for nAb in virus control in vivo, it remained, however, unclear whether in these examples the envelope GP variation was driven by the evolution of cell tropism or by antibodies (19).
Here, virus control by nAb was directly associated with emergence of neutralization-resistant variants. The role of immune pressure by nAb for selection of virus variants was not only indicated by the lack of aa substitutions in variants isolated from persistently infected B cell-deficient mice (μMT, RAG-1−/−) and diminished sensitivity of variants to neutralization by polyclonal hyperimmune serum (Table 2), but also by the change of N119 (variants M5–2.1 and M5–2.2 in Fig. 5). Amino acid changes at this position have been selected before in LCMV carrier mice expressing the neutralizing anti-GP1 mAb KL25 as a transgene (17). This finding also correlates with the complete resistance of these variants to neutralization by KL25 (Table 2).
As the three-dimensional structure of LCMV-GP1 is still unknown, it is difficult to assess whether the aa substitutions described in our study are local changes in the neutralizing epitope or mutations at distal sites leading to conformational changes. Most aa changes seem to be clustered in three variable regions (amino acids 119–133, 175–185, and 211–214, Fig. 5), and it would be tempting to speculate that these specific aa sequences make up the neutralizing epitope.
One hypothesis raised to explain the emergence of variants with a neutralization-resistant phenotype within one individual is the presence of a restricted oligoclonal primary antibody response (19). In our study, nAb-escape mutants failed to induce efficient de novo nAb responses, suggesting that variants were less immunogenic or perhaps more immunosuppressive. Quite similar neutralization escape mutants have been described recently in experimental lentiviral infections of macaques (40, 47, 48).
CD8+ T cell deficiency greatly enhanced the appearance of nAb-escape mutants during LCMV infection, paralleling observations in a human/simian immunodeficiency virus model infection (48). Interestingly, β2m−/− mice largely lacking MHC class I and CTL responses in general fail to clear LCMV (11, 49). As β2m−/− mice also have a defect in antibody metabolism (50, 51), this latter phenotype requires further analysis. What is the relevance of our findings for initially immunocompetent animals? Several conditions have been characterized, where CD8+ T cell responses decrease or disappear. First, CTL-escape virus variants in vivo have been demonstrated not only for LCMV (43), but also during HIV and hepatitis B and C viral infections (reviewed in ref. 4). Second, exhaustion or unresponsiveness of CTLs may occur (42, 52). Third, CTL responses may be low initially (53). Thus, even in initially immunocompetent individuals, conditions of low, declining, or absent CTL responses during establishment of persistent viral infections may arise, facilitating virus escape from the nAb response.
In conclusion, the direct correlation between LCMV clearance by nAbs, virus evasion from humoral immunity, and subsequent viral persistence, as documented in this study, strongly suggests that vaccines against viruses that tend to persist should elicit early and strong humoral responses in addition to cellular responses to be effective.
Acknowledgments
We thank A. Trkola, B. Ludewig, and L. Hangartner for helpful discussions, S. Oehen and K. McCoy for critical reading of the manuscript, and A. Althage, D. Zimmermann, and his group for expert technical assistance. This work was supported by Swiss National Foundation Grants 31.50900.97 and 31.50884.97, the Kanton of Zurich, and the Wellcome Trust.
Abbreviations
- CTL
cytotoxic T lymphocyte
- LCMV
lymphocytic choriomeningitis virus
- nAb
neutralizing antibody
- pfu
plaque-forming units
- wt
wild type
- GP
glycoprotein
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.040558797.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.040558797
References
- 1.Schmitz J E, Kuroda M J, Santra S, Sasseville V G, Simon M A, Lifton M A, Racz P, Tenner-Racz K, Dalesandro M, Scallon B J, et al. Science. 1999;283:857–860. doi: 10.1126/science.283.5403.857. [DOI] [PubMed] [Google Scholar]
- 2.Kagi D, Ledermann B, Bürki K, Seiler P, Odermatt B, Olsen K J, Podack E R, Zinkernagel R M, Hengartner H. Nature (London) 1994;369:31–37. doi: 10.1038/369031a0. [DOI] [PubMed] [Google Scholar]
- 3.Guidotti L G, Borrow P, Brown A, McClary H, Koch R, Chisari F V. J Exp Med. 1999;189:1555–1564. doi: 10.1084/jem.189.10.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McMichael A J, Phillips R E. Annu Rev Immunol. 1997;15:271–296. doi: 10.1146/annurev.immunol.15.1.271. [DOI] [PubMed] [Google Scholar]
- 5.Thomsen A R, Johansen J, Marker O, Christensen J P. J Immunol. 1996;157:3074–3080. [PubMed] [Google Scholar]
- 6.Planz O, Ehl S, Furrer E, Horvath E, Brundler M A, Hengartner H, Zinkernagel R M. Proc Natl Acad Sci USA. 1997;94:6874–6879. doi: 10.1073/pnas.94.13.6874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moore J P, Cao Y, Ho D D, Koup R A. J Virol. 1994;68:5142–5155. doi: 10.1128/jvi.68.8.5142-5155.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Battegay M, Moskophidis D, Waldner H, Brundler M A, Fung-Leung W-P, Mak T W, Hengartner H, Zinkernagel R M. J Immunol. 1993;151:5408–5415. [PubMed] [Google Scholar]
- 9.Planz O, Seiler P, Hengartner H, Zinkernagel R M. Nature (London) 1996;382:726–729. doi: 10.1038/382726a0. [DOI] [PubMed] [Google Scholar]
- 10.Shibata R, Igarashi T, Haigwood N, Buckler-White A, Ogert R, Ross W, Willey R, Cho M W, Martin M A. Nat Med. 1999;5:204–210. doi: 10.1038/5568. [DOI] [PubMed] [Google Scholar]
- 11.Baldridge J R, McGraw T S, Paoletti A, Buchmeier M J. J Virol. 1997;71:755–758. doi: 10.1128/jvi.71.1.755-758.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cao Y, Qin L, Zhang L, Safrit J, Ho D D. N Engl J Med. 1995;332:201–208. doi: 10.1056/NEJM199501263320401. [DOI] [PubMed] [Google Scholar]
- 13.Arendrup M, Nielsen C, Hansen J E, Pedersen C, Mathiesen L, Nielsen J O. J Acquired Immune Defic Syndr. 1992;5:303–307. [PubMed] [Google Scholar]
- 14.Bradney A P, Scheer S, Crawford J M, Buchbinder S P, Montefiori D C. J Infect Dis. 1999;179:1264–1267. doi: 10.1086/314711. [DOI] [PubMed] [Google Scholar]
- 15.Burns D P, Collignon C, Desrosiers R C. J Virol. 1993;67:4104–4113. doi: 10.1128/jvi.67.7.4104-4113.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Poignard P, Sabbe R, Picchio G R, Wang M, Gulizia R J, Katinger H, Parren P W, Mosier D E, Burton D R. Immunity. 1999;10:431–438. doi: 10.1016/s1074-7613(00)80043-6. [DOI] [PubMed] [Google Scholar]
- 17.Seiler P, Senn B M, Brundler M A, Zinkernagel R M, Hengartner H, Kalinke U. J Immunol. 1999;162:4536–4541. [PubMed] [Google Scholar]
- 18.Webster R G, Laver W G, Air G M, Schild G C. Nature (London) 1982;296:115–121. doi: 10.1038/296115a0. [DOI] [PubMed] [Google Scholar]
- 19.Parren P W H I, Moore J P, Burton D R, Sattentau Q J. AIDS. 1999;13:S137–S162. [PubMed] [Google Scholar]
- 20.Buchmeier M J, Welsh R M, Dutko F J, Oldstone M B A. Adv Immunol. 1980;30:275–331. doi: 10.1016/s0065-2776(08)60197-2. [DOI] [PubMed] [Google Scholar]
- 21.Larsen J H, Volkert M. Acta Pathol Microbiol Scand. 1967;70:95–106. [PubMed] [Google Scholar]
- 22.Oldstone M B A. Curr Top Microbiol Immunol. 1987;134:211–229. doi: 10.1007/978-3-642-71726-0_9. [DOI] [PubMed] [Google Scholar]
- 23.Ahmed R, Jamieson B D, Porter D D. J Virol. 1987;61:3920–3929. doi: 10.1128/jvi.61.12.3920-3929.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fung-Leung W-P, Kündig T M, Zinkernagel R M, Mak T W. J Exp Med. 1991;174:1425–1429. doi: 10.1084/jem.174.6.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Müller U, Steinhoff U, Reis L F, Hemmi S, Pavlovic J, Zinkernagel R M, Aguet M. Science. 1994;264:1918–1921. doi: 10.1126/science.8009221. [DOI] [PubMed] [Google Scholar]
- 26.Huang S, Hendriks W, Althage A, Hemmi S, Bluethmann H, Kamijo R, Vilcek J, Zinkernagel R M, Aguet M. Science. 1993;259:1742–1745. doi: 10.1126/science.8456301. [DOI] [PubMed] [Google Scholar]
- 27.Kitamura D, Roes J, Kuhn R, Rajewsky K. Nature (London) 1991;350:423–426. doi: 10.1038/350423a0. [DOI] [PubMed] [Google Scholar]
- 28.Battegay M, Cooper S, Althage A, Baenziger J, Hengartner H, Zinkernagel R M. J Virol Methods. 1991;33:191–198. doi: 10.1016/0166-0934(91)90018-u. [DOI] [PubMed] [Google Scholar]
- 29.Cobbold S P, Jayasuriya A, Nash A, Prospero T D, Waldmann H. Nature (London) 1984;312:548–551. doi: 10.1038/312548a0. [DOI] [PubMed] [Google Scholar]
- 30.Nansen A, Jensen T, Christensen J P, Andreasen S O, Ropke C, Marker O, Thomsen A R. J Immunol. 1999;136:6114–6122. [PubMed] [Google Scholar]
- 31.Tishon A, Lewicki H, Rall G, von Herrath M, Oldstone M B. Virology. 1995;212:244–250. doi: 10.1006/viro.1995.1477. [DOI] [PubMed] [Google Scholar]
- 32.Homann D, Tishon A, Berger D P, Weigle W O, von Herrath M G, Oldstone M B. J Virol. 1998;72:9208–9216. doi: 10.1128/jvi.72.11.9208-9216.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Williams S G, Oxenius A, Hengartner H, Benoist C, Mathis D. Eur J Immunol. 1998;28:3763–3772. doi: 10.1002/(SICI)1521-4141(199811)28:11<3763::AID-IMMU3763>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 34.Seiler P, Kalinke U, Rulicke T, Bucher E M, Bose C, Zinkernagel R M, Hengartner H. J Virol. 1998;72:2253–2258. doi: 10.1128/jvi.72.3.2253-2258.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Romanowski V, Matsuura Y, Bishop D H. Virus Res. 1985;3:101–114. doi: 10.1016/0168-1702(85)90001-2. [DOI] [PubMed] [Google Scholar]
- 36.Parekh B S, Buchmeier M J. Virology. 1986;153:168–178. doi: 10.1016/0042-6822(86)90020-6. [DOI] [PubMed] [Google Scholar]
- 37.Matloubian M, Somasundaram T, Kolhekar S R, Selvakumar R, Ahmed R. J Exp Med. 1990;172:1043–1048. doi: 10.1084/jem.172.4.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wright K E, Salvato M S, Buchmeier M J. Virology. 1989;171:417–426. doi: 10.1016/0042-6822(89)90610-7. [DOI] [PubMed] [Google Scholar]
- 39.Reitter J N, Means R E, Desrosiers R C. Nat Med. 1998;4:679–684. doi: 10.1038/nm0698-679. [DOI] [PubMed] [Google Scholar]
- 40.Kimata J T, Kuller L, Anderson D B, Dailey P, Overbaugh J. Nat Med. 1999;5:535–541. doi: 10.1038/8414. [DOI] [PubMed] [Google Scholar]
- 41.Ciurea A, Klenerman P, Hunziker L, Horvath E, Odermatt B, Ochsenbein A F, Hengartner H, Zinkernagel R M. Proc Natl Acad Sci USA. 1999;96:11964–11969. doi: 10.1073/pnas.96.21.11964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moskophidis D, Lechner F, Pircher H, Zinkernagel R M. Nature (London) 1993;362:758–761. doi: 10.1038/362758a0. [DOI] [PubMed] [Google Scholar]
- 43.Pircher H, Moskophidis D, Rohrer U, Bürki K, Hengartner H, Zinkernagel R M. Nature (London) 1990;346:629–633. doi: 10.1038/346629a0. [DOI] [PubMed] [Google Scholar]
- 44.Fazekas de St. Groth S, Webster R G. J Exp Med. 1966;140:355–360. doi: 10.1084/jem.124.3.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Odermatt B F, Eppler M, Leist T P, Hengartner H, Zinkernagel R M. Proc Natl Acad Sci USA. 1991;88:8252–8256. doi: 10.1073/pnas.88.18.8252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Borst P. Immunol Today. 1991;12:A29–A33. doi: 10.1016/S0167-5699(05)80009-X. [DOI] [PubMed] [Google Scholar]
- 47.Karlsson G B, Halloran M, Schenten D, Lee J, Racz P, Tenner-Racz K, Manola J, Gelman R, Etemad-Moghadam B, Desjardins E, et al. J Exp Med. 1998;188:1159–1171. doi: 10.1084/jem.188.6.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Igarashi T, Endo Y, Englund G, Sadjadpour R, Matano T, Buckler C, Buckler-White A, Plishka R, Theodore T, Shibata R, et al. Proc Natl Acad Sci USA. 1999;96:14049–14054. doi: 10.1073/pnas.96.24.14049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lehmann-Grube F, Lohler J, Utermöhlen O, Gegin C. J Virol. 1993;67:332–339. doi: 10.1128/jvi.67.1.332-339.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ghetie V, Hubbard J G, Kim J K, Tsen M F, Lee Y, Ward E S. Eur J Immunol. 1996;26:690–696. doi: 10.1002/eji.1830260327. [DOI] [PubMed] [Google Scholar]
- 51.Junghans R P, Anderson C L. Proc Natl Acad Sci USA. 1996;93:5512–5516. doi: 10.1073/pnas.93.11.5512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zajac A J, Blattman J N, Murali-Krishna K, Sourdive D J, Suresh M, Altman J D, Ahmed R. J Exp Med. 1998;188:2205–2213. doi: 10.1084/jem.188.12.2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Moskophidis D, Battegay M, van den Broek M F, Laine E, Hoffmann Rohrer U, Zinkernagel R M. J Gen Virol. 1995;76:381–391. doi: 10.1099/0022-1317-76-2-381. [DOI] [PubMed] [Google Scholar]