Abstract
Cytokines and reactive oxygen intermediates (ROI) are frequent companions at sites of acute inflammation. We have shown previously that in human monocytes, bacterial lipopolysaccharide, IL-1, and tumor necrosis factor-α induce a rapid down-regulation of the monocyte chemotactic protein-1 receptor CCR2 (CC chemokine receptor-2). These stimuli also induce production of ROI. In this paper, we investigate the influence of antioxidants and/or ROI on chemokine-receptor expression. In human monocytes, the antioxidant pyrrolidine dithiocarbamate (PDTC) rapidly inhibited CCR2 (95–100% of inhibition) and CCR5 (77–100% of inhibition) mRNA expression by strongly decreasing transcript stability. CCR2 half-life was decreased from 1.5 h to 45 min; CCR5 half-life was decreased from 2 h to 70 min. This inhibitory activity also included CXCR4 (CXC chemokine receptor-4) but not CXCR2 receptor and, although to a lesser extent, was shared by the antioxidants N-acetyl-l-cysteine and 2-mercaptoethanol. In contrast, the ROI-generating system xanthine/xanthine oxidase increased CCR5 and CXCR4 mRNA expression and counteracted the inhibitory effect of PDTC. Accordingly, H2O2 and the glutathione-depleting drug buthionine sulfoximine increased to different extents CCR2, CCR5, and CXCR4 mRNA expression. The PDTC-mediated inhibition of CCR5 and CXCR4 mRNA expression was associated with decreased chemotactic responsiveness (>90% inhibition) and with a marked inhibition of surface-receptor expression. In contrast, xanthine/xanthine oxidase opposed the bacterial lipopolysaccharide- and tumor necrosis factor-α-mediated inhibition of CCR5 and CXCR4 mRNA expression and increased both the CCR5 surface expression and the cell migration (3-fold) in response to macrophage inflammatory protein-1β. These results suggest that the redox status of cells is a crucial determinant in the regulation of the chemokine system.
Inflammation is associated with the generation of reactive oxygen intermediates (ROI), including superoxide anion (O2−), hydrogen peroxide (H2O2), and the hydroxyl radical (OH⋅) (1). ROI, in addition to being efficient antimicrobial effector molecules, are also key mediators of inflammation (1–3) and have profound effects on gene transcription (4). Consequently, several studies have shown a protective effect of antioxidants in animal models of inflammatory diseases. In particular, pyrrolidine dithiocarbamate (PDTC) blocks NF-κB activation, which is involved in the activation of a number of immediate-early genes during inflammation and in the NF-κB-dependent replication of human immunodeficiency virus type 1 (HIV-1) (5, 6), and similar results were reported with N-acetyl-l-cysteine (NAC) (7, 8).
It now clearly is established that chemokine receptors are required for primate lentiviruses such as HIV-1, HIV-2, and simian immunodeficiency virus to fuse with and infect target cells, either in combination with CD4 or independently (9). In particular, CC chemokine receptor-5 (CCR5) is a major determinant of the interaction of macrophage-tropic HIV-1 with mononuclear phagocytes (10–14), whereas T cell-tropic HIV-1 isolates target T lymphocytes through the CXCR4 receptor (15). Chemokines, as well as classical chemotactic agonists such as formylated peptides (of which fMet-Leu-Phe is the prototype) and C5a, bind to and activate a family of rhodopsin-like, GTP-binding protein-coupled seven-transmembrane domain receptors (16–18). Nine receptors for C-C chemokines (now named CCR1 through CCR9) and five receptors for CXC chemokines (CXCR1 to CXCR5) have been identified and cloned (17–21). Recent results (22–24) indicate that, in addition to agonist production, regulation of chemokine-receptor expression is likely a crucial point in the regulation of the chemokine system. In particular, certain primary proinflammatory signals [e.g., bacterial lipopolysaccharide (LPS)] rapidly inhibit chemokine-receptor expression by targeting transcript stability (22). The aim of this paper is to investigate the possibility that ROI might regulate the levels of chemokine receptors. For this purpose, we investigated the effect of antioxidants, including PDTC, NAC, and 2-mercaptoethanol, and of ROI generated in vitro on chemokine-receptor expression. Finally, to investigate the role of endogenous glutathione (GSH), we used the inhibitor of GSH synthesis buthionine sulfoximine (BSO). Our results indicate that ROI up-regulate certain chemokine receptors and that antioxidants antagonize this effect.
Materials and Methods
Cells.
Human monocytes were separated from peripheral blood of human healthy donors by Percoll gradient centrifugation (22). Monocytes (≥98% pure as assessed by morphology) were resuspended at 107 monocytes per ml in RPMI medium 1640 supplemented with 10% FBS, 2 mM glutamine, and antibiotics. All reagents contain <0.125 unit/ml of endotoxin as checked by Limulus amebocyte lysate assay (BioWhittaker). In all the conditions used in the test cells, viability, as determined by trypan blue exclusion, was >95%.
Cytokines and Reagents.
LPS (E. coli O55:B5) was purchased from Difco. PDTC, NAC, BSO, xanthine (X), and H2O2 were purchased from Sigma; 2-mercaptoethanol was purchased from Merck; xanthine oxidase (XO) was purchased from Roche Molecular Biochemicals. Human monocyte-chemotactic protein (MCP)-1 was determined by a specific monoclonal sandwich ELISA, as described elsewhere (25). Determination of human macrophage inflammatory protein (MIP)-1β was carried out by using ELISA kits purchased from Amersham Pharmacia.
Migration Assay.
Cell migration was evaluated by using a chemotaxis microchamber technique as previously described (22, 26). Chemoattractant solution or control RPMI medium 1640 with 1% FCS (27 μl) was added to the lower wells of a chemotaxis chamber (Neuroprobe, Plesanton, CA). A polycarbonate filter (5-μm pore size; Neuroprobe) was layered on the wells and covered with a silicon gasket and with the top plate. Cell suspension (50 μl; 1.5 × 106 monocytes per ml in peripheral blood mononuclear cells) was seeded in the upper chamber. The chamber was incubated at 37°C in air with 5% CO2 for 90 min. At the end of the incubation, filters were removed and stained with Diff-Quick (Baxter Scientific Products, Rome), and the cells in five high-power oil-immersion fields were counted.
Northern Blot Analysis.
Total RNA was isolated by the guanidium isothiocyanate method as previously described (27). Total RNA (15 μg) from each sample was electrophoresed under denaturing conditions, blotted onto Nytran membranes (Schleicher & Schuell), and cross-linked by UV irradiation. cDNAs were labeled by random priming and [α-32P]dCTP. CCR2B cDNA was obtained by PCR amplification of the reported sequence (22). CCR5 cDNAs were obtained as previously described (19). The CXCR2 cDNA clone (28) was kindly donated by Ji Ming Wang (National Cancer Institute, Frederick, MD). CXCR4 was kindly provided by Timothy N. C. Wells (Geneva Biomedical Research Institute, Glaxo Wellcome Research and Development, Geneva). Densitometric analysis was performed with a Scanning Densitometer GS300 (Hoefer Scientific Instruments, San Francisco). Relative densitometric values were defined as ratio to control (=1).
Fluorescence-Activated Cell Sorter Analysis.
Cell staining was performed by using human monoclonal antibodies anti-CCR5 (clone 2D7; PharMingen) and anti-CXCR4 (clone 12G5; PharMingen) and irrelevant control mouse IgG2aκ (UPC10; Sigma), followed by FITC-conjugated, affinity-purified, isotype-specific goat anti-mouse antibody (Southern Biotechnology Associates). For CCR2 phenotype analysis, indirect immunofluorescence was performed with the human anti-CCR2 mAb (clone 48607.211; R & D Systems), irrelevant control mouse IgG2b (clone 20116.11; R & D Systems), and phycoerythrin-streptavidin (PharMingen). Analysis was performed by using a FACStar (Becton Dickinson).
Results
Effects of Antioxidants (PDTC and NAC) on Chemokine-Receptor Expression in Human Monocytes.
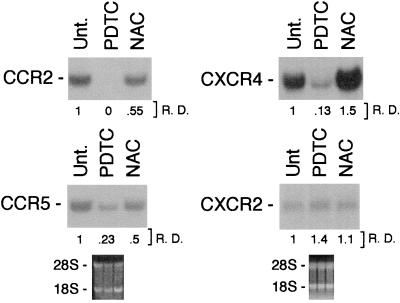
In the experiment shown in Fig. 1, freshly isolated human monocytes were cultured for 4 h with the antioxidants PDTC and NAC. Monocytes expressed high levels of CCR2 and CCR5 transcripts, whereas CCR3 and CCR4 were poorly detectable under these conditions (data not shown), in agreement with previous reports (22–24). PDTC (50 μM) markedly lowered the expression of CCR2 and CCR5. The mRNA levels of these receptors were decreased only slightly by NAC (10 mM). The mRNA expression of two members of the CXCR chemokine-receptor family (CXCR2 and CXCR4) was also analyzed. PDTC markedly decreased the mRNA expression of CXCR4 but did not affect CXCR2.
Figure 1.
Effect of PDTC and NAC on CCR2, CCR5, CXCR4, and CXCR2 mRNA expression. Total RNA was purified from fresh human monocytes incubated for 4 h as indicated. Results are representative of four different experiments. PDTC, 50 μM; NAC, 10 mM; Unt., untreated; R. D., relative densitometry.
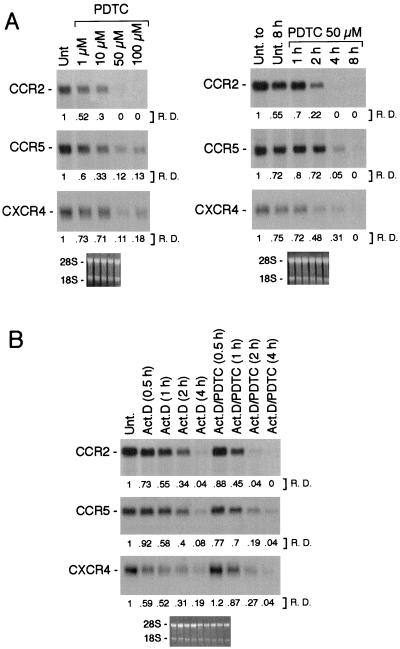
Because of the crucial role played by CCR2, CCR5, and CXCR4 in the recruitment of leukocytes and in their infection by HIV-1 (22), we focused our study on these three receptors. Fig. 2A shows the dose–response and kinetic analysis of the PDTC-mediated inhibition of CCR2, CCR5, and CXCR4 mRNA expression. The estimated ED50 of PDTC was ≈1 μM, and half-maximal effect was reached with an optimal dose in 1.5 h for CCR2, 2 h for CCR5, and 1 h for CXCR4. We also estimated the effects of PDTC on the CCR2, CCR5, and CXCR4 mRNA half-life. For this purpose, actinomycin D (ActD; 1 μg/ml) was added to fresh human monocytes in the presence or absence of PDTC (50 μM), and total RNA was extracted at different times as indicated (Fig. 2B). As shown in Fig. 2B, the estimated half-life of the transcripts was about 1.5 h for CCR2, 2 h for CCR5, and 1 h for CXCR4. Although cotreatment with ActD and PDTC considerably decreased transcript stability of CCR2 and CCR5, resulting in a half-life of ≈45 min for CCR2 and ≈70 min for CCR5, CXCR4 was affected only slightly. These data indicate that the inhibitory action of PDTC on CCR2 and CCR5 gene expression is, at least in part, posttranscriptional.
Figure 2.
(A) Dose–response (Left) and time course (Right) of the effect of PDTC on CCR2, CCR5, and CXCR4 mRNA expression. For the dose–response analysis, total RNA was purified from monocytes incubated for 4 h with PDTC at the indicated doses. In kinetic analysis, the total RNA was purified from fresh human monocytes incubated with 50 μM PDTC for the indicated times. Results in A are representative of three separate experiments. (B) Effect of PDTC on the CCR2, CCR5, and CXCR4 mRNA half-lives. ActD (1 μg/ml) was added to fresh human monocytes in the presence or absence of PDTC (50 μM), and total RNA was extracted at different times as indicated. Results in B are representative of two different experiments.
Effect of Oxidants on CCR2, CCR5, and CXCR4 Expression.
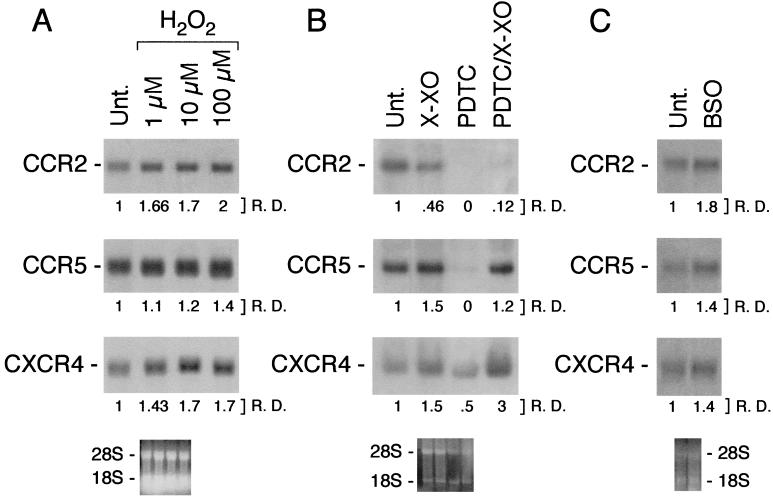
To evaluate the effect of ROI on CCR2, CCR5, and CXCR4 mRNA expression, monocytes were incubated with increasing concentrations of H2O2 (Fig. 3A). As shown, H2O2 increased in a dose–response manner the mRNA expression of CCR2, CCR5, and CXCR4. We also studied the effect of the production of the superoxide-generating system, X/XO, by using the same experimental model. In Fig. 3B, monocytes were cultured 4 h with X/XO, PDTC, or a combination of PDTC plus X/XO as indicated. It can be seen that X/XO markedly increased CCR5 and CXCR4 mRNA expression and completely prevented their PDTC-mediated inhibition. In contrast, X/XO had a minor effect on the level of CCR2 mRNA expression. Similarly, BSO (3 mM), an effective inhibitor of γ-glutamylcysteine synthetase (the enzyme that catalyzes the first step of biosynthesis of GSH) (29), increased to different extents CCR2, CCR5, and CXCR4 mRNA expression (Fig. 3C).
Figure 3.
(A) Effect of H2O2 on CCR2, CCR5, and CXCR4 mRNA expression. Monocytes were incubated for 4 h with H2O2 at the indicated doses. (B) Effect of X/XO on the inhibitory action of PDTC on CCR2, CCR5, and CXCR4 mRNA expression. X was used at 0.5 mM; XO was used at 10 milliunits/ml. Total RNA was purified from fresh human monocytes incubated for 4 h as indicated. (C) Effect of BSO on CCR2, CCR5, and CXCR4 mRNA expression. Cells were incubated with 3 mM BSO for 18 h. Results are representative of three different experiments.
PDTC Down-Regulates the Chemotactic Response to MCP-1, MIP-1β, and Stromal Cell-Derived Factor (SDF)-1 in Human Monocytes.
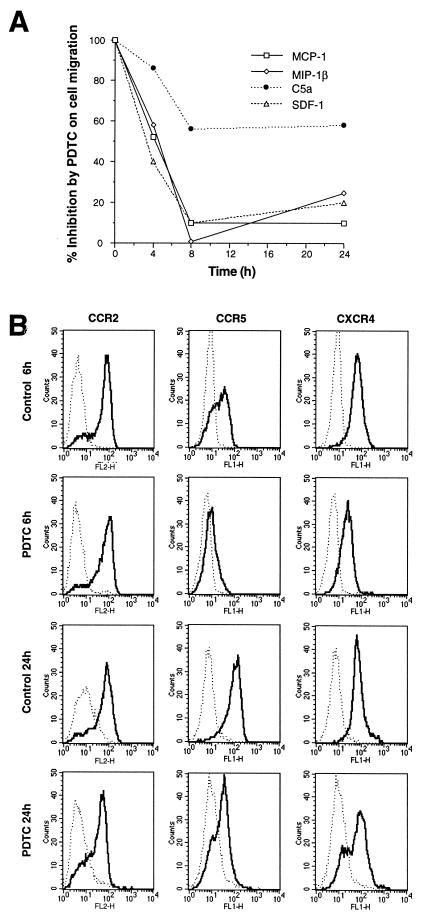
MCP-1, MIP-1β, and SDF-1 are high-affinity ligands for the CCR2, CCR5, and CXCR4 receptors, respectively (14, 30). Thus, decreased expression of these chemokine receptor transcripts by PDTC was expected to result in a diminished chemotactic responsiveness to MCP-1, MIP-1β, and SDF-1. As shown in Fig. 4A, 8-h treatment with PDTC basically abolished the monocyte migration in response to MCP-1, MIP-1β, and SDF-1. Thus, in human monocytes, antioxidant-mediated inhibition of CCR2, CCR5, and CXCR4 expression results in the suppression of chemotactic response toward their ligands. In agreement with this finding, a significant time-dependent inhibition of the binding of 125I-labeled ligands was observed after PDTC treatment (data not shown). We analyzed the CCR2, CCR5, and CXCR4 surface-receptor expression by cytofluorimetry. As shown in Fig. 4B, PDTC treatment (50 μM) resulted in a marked down-regulation of CCR5 and CXCR4 already after 6 h, which also lasted after 24 h. Unexpectedly, CCR2 surface expression was not affected by PDTC treatment.
Figure 4.
(A) Inhibition of monocyte migration by PDTC. Fresh human monocytes were incubated with 50 μM PDTC and assayed for chemotactic response after different periods as indicated. Results in A are representative of five different experiments. (B) Effect of PDTC on CCR2, CCR5, and CXCR4 surface expression. Surface expression was determined by flow cytometry, using human anti-CCR2, anti-CCR5, or anti-CXCR4 antibodies. Dotted line, irrelevant mAbs; continuous line, cells stained with anti-CCR2, anti-CCR5, or anti-CXCR4 as indicated. Results in B are representative of four different experiments. FL1-H, fluorescence one height; FL2-H, fluorescence two height.
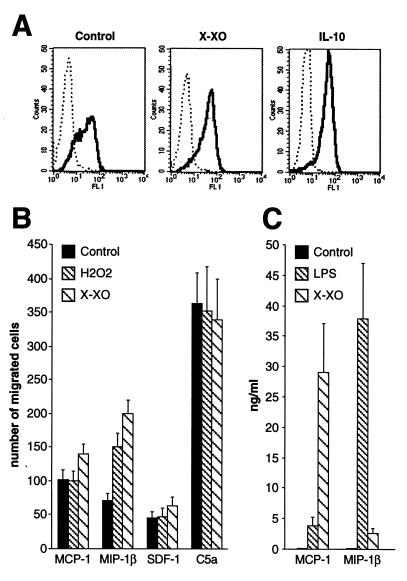
X/XO Up-Regulates CCR5 Surface Expression and Migration in Response to MIP-1β.
To evaluate the functional effect of ROI on chemokine systems, monocytes were incubated for different periods with X/XO, and the surface expression of CCR2, CCR5, and CXCR4 was determined by cytofluorimetry. As shown in Fig. 5A, X/XO treatment resulted in a significant increase of the CCR5 surface expression. In contrast, no appreciable variations were observed for CCR2 and CXCR4 (data not shown). The observed X/XO-dependent increase of CCR5 surface expression was paralleled by a rise in the number of monocytes that migrated in response to MIP-1β. Indeed, as shown in Fig. 5B, X/XO-activated monocytes increased their chemotactic activity in response to MIP-1β, and similar results were observed in H2O2-treated monocytes. In addition, the chemotactic response of monocytes slightly increased in response to SDF-1, but not to MCP-1. The effect of ROI on chemokine production was then considered (Fig. 5C). X/XO-treated monocytes secreted extremely high levels of MCP-1 and significant amounts of MIP-1β.
Figure 5.
(A) Effect of ROI on CCR5 surface expression. Fresh human monocytes were incubated with the ROI-generating system X/XO (X, 50 μM; XO, 1 milliunit/ml) for 18 h. Surface expression was determined by flow cytometry, using human anti-CCR5 antibody. Dotted line, irrelevant mAbs; continuous line, cells stained with anti-CCR5 as indicated. Results in A are representative of four different experiments. (B) Fresh human monocytes were incubated with X/XO or H2O2 and assayed as indicated for chemotactic response after 18 h. Results in B are representative of five different experiments. (C) Effect of ROI on MCP-1 and MIP-1β secretion by monocytes. Cells were activated with X/XO for 18 h, and supernatants were assayed by ELISA for MCP-1 and MIP-1β proteins. Results in C are representative of three separate experiments.
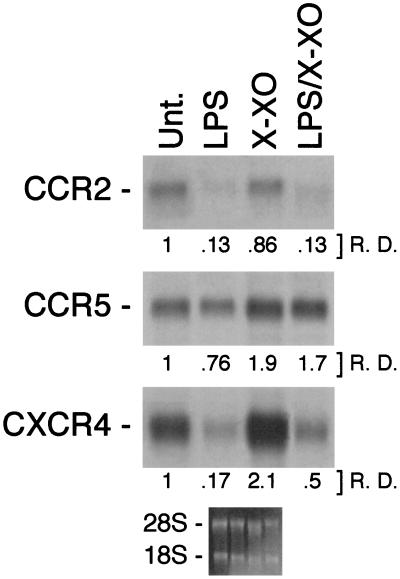
X/XO Reverts the Inhibitory Action of LPS on CCR5 and CXCR4 mRNA Expression.
We previously have reported that LPS and certain proinflammatory cytokines (e.g., IL-1 and tumor necrosis factor-α) inhibit CCRs mRNA expression in human monocytes (22). Among these, CCR2 was inhibited strongly, whereas CCR5 and CCR1 were inhibited only partially. To investigate whether oxidative signals may counteract the inhibitory action of LPS, human monocytes were incubated with LPS, either alone or in combination with X/XO. CCR2, CCR5, and CXCR4 mRNA levels were estimated by Northern blot analysis (Fig. 6). As shown, whereas LPS inhibited to a different extent the mRNA expression of these chemokine receptors, X/XO treatment significantly enhanced the constitutive expression of CCR5 and CXCR4. It is noteworthy that in LPS-treated monocytes, X/XO completely prevented the inhibition of CCR5 and partially prevented the inhibition of CXCR4 mRNA expression, although it had no effect on the inhibition of CCR2. Similarly, X/XO prevented the tumor necrosis factor-α-mediated inhibition of CCR5 and CXCR4 mRNA expression (data not shown).
Figure 6.
X/XO prevents the inhibitory action of LPS (10 ng/ml) on CCR5 and CXCR4. Fresh human monocytes were incubated for 4 h as indicated, and total RNA was extracted after 4 h and analyzed by Northern blot for CCR2, CCR5, and CXCR4 mRNA levels. Results are representative of four different experiments.
Discussion
The results presented in this paper show that in human monocytes, antioxidants (particularly PDTC) decrease expression of CCR2, CCR5, and CXCR4, three chemokine receptors that bind MCP-1 and MIP-1α, MIP1-β, and RANTES and SDF-1, respectively. NAC and 2-mercaptoethanol were less potent than PDTC but still decreased CCR2 and CCR5 in the same experimental system. Thus, we tried further to characterize the effect of PDTC on chemokine receptors and chemokine responses.
We present evidence that PDTC acts, at least in part, at a posttranscriptional level by affecting transcript stability. In particular, CCR2 mRNA half-life, estimated as 1.5 h, was reduced at ≈45 min when cells where cotreated with ActD and PDTC, and CCR5 half-life was reduced from 2 h to ≈70 min under the same conditions. CXCR4 mRNA stability was affected only slightly. Inhibition of CCR2, CCR5, and CXCR4 receptors was functionally relevant because PDTC-treated monocytes showed a reduced capability to respond chemotactically to MCP-1, MIP-1β, and SDF-1. The decrease in CCR2, CCR5, and CXCR4 mRNA expression was accompanied by a decrease both in surface expression and in the chemotactic response to their ligands. The fact that antioxidants decrease constitutive CCR2, CCR5, and CXCR4 expression suggests that transcriptional and posttranscriptional events controlling their gene expression are somewhat regulated by a redox-sensitive mechanism. On the contrary, addition of H2O2 or of a superoxide-generating system (X/XO) augmented mRNA expression of CCR2, CCR5, and CXCR4. In addition, the effect of BSO suggests that not only exogenously administered antioxidants but also endogenous GSH might control the expression of certain chemokine receptors. Interestingly enough, the addition of NAC, although slightly inhibiting CCR expression in the absence of BSO, did not reverse the up-regulating effect of BSO (data not shown). This result could be explained by the fact that NAC is a precursor of GSH synthesis, and GSH repletion by NAC cannot occur in the presence of BSO, which is an inhibitor of GSH synthetase (29). Thus, overproduction of ROI, as observed during the oxidative burst of phagocytes in response to infectious agents, could increase the chemokine responsiveness of phagocytes.
In view of our previous reports that LPS down-regulates CCRs and the present observation that ROI oppose the down-regulatory effects of LPS and tumor necrosis factor-α (data not shown), one could speculate that at inflammatory sites, after a rapid and drastic down-regulation of chemokine receptors, LPS- and cytokine-mediated activation of ROI production by phagocytes (1–3) provides an efficient autocrine signal to restore the chemokine-receptor expression in monocytes. Though differences likely may exist between the molecular mechanisms responsible for the LPS-mediated or PDTC-mediated down-regulation of CCR2, CCR5, and CXCR4 expression in monocytes, oxidation favors chemokine-receptor expression and may counteract these mechanisms of inhibition of their expression. Consistent with this hypothesis, we have observed that although incubation of monocytes with LPS or PDTC inhibits CCR2, CCR5, and CXCR4 mRNA expression, cotreatment with X/XO efficiently prevented CCR5 and CXCR4 down-regulation, but not CCR2 down-regulation. Thus, ROI may display a certain selectivity of their action on chemokine-receptor expression, which functionally was proven in our experiments by the specific increase of both the CCR5 surface expression and the chemotactic response to MIP-1β. Our data should also be considered in view of the fact that antioxidants such as PDTC and NAC inhibit both the activation of NF-κB and the expression of NF-κB-inducible chemokine genes, such as MCP-1 and IL-8 (30–33). In agreement with these data, we observed increased production of MCP-1 and MIP-1β in monocytes activated with X/XO. Though ROI-induced NF-κB activity may play a role in the induction of chemokine-receptor expression, this occurrence seems partially unlikely in this scenario, because potent activators of NF-κB, such as LPS, IL-1, and tumor necrosis factor-α, down-regulate these genes. However, the study of transcriptional and posttranscriptional events mediated by these signals on chemokine-receptor genes may better define this aspect. Thus, ROI accumulation may up-regulate certain chemokine/chemokine-receptor systems by promoting their expression. This finding may be particularly important in ischemic diseases where an inflammatory infiltrate is a component of the pathogenesis and ROI are the trigger for the induction of inflammatory cytokines and chemokines. Thus, prolonged and high expression of chemokine receptors and agonists, such as those likely promoted by ROI in inflammatory sites, may represent a detrimental factor for the control and extinction of the inflammatory response, which could favor inflammatory disorders. Our data also suggest that activation of the chemokine system during oxidation may have a potential impact in HIV infection (34). Indeed, the chemokine receptors CCR5 and CXCR4 are the main coreceptors for macrophage- and T cell-tropic HIV-1 strains, respectively. Our observations indicate that in addition to the observed increase of the NF-κB-dependent HIV-1 long-terminal repeat activity, cell oxidation may facilitate HIV infection by increasing chemokine-receptor expression. Thus, the combination of these events drastically may favor HIV-1 infection and replication, particularly considering the lower GSH levels observed in HIV patients (5, 6, 35).
In conclusion, our data suggest that ROI have an important role in maintaining high expression of certain chemokine receptors, and they provide a mechanism that supports the concept that targeting ROI with antioxidants might be useful in the therapy of infective and inflammatory diseases.
Acknowledgments
This work was supported by the Associazione Italiana Ricerca sul Cancro in Italy; by the special project AIDS from the Istituto Superiore Sanitá; by the Target Project on “Biotechnology” at the Consiglio Nazionale delle Ricerche in Rome (99.00119.PF49); and by the Ministero Università Ricerca Scientifica e Tecnologica in Italy.
Abbreviations
- ROI
reactive oxygen intermediates
- HIV-n
HIV type n
- CCR
CC chemokine receptor
- PDTC
pyrrolidine dithiocarbamate
- NAC
N-acetyl-l-cysteine
- GSH
glutathione
- MIP
macrophage inflammatory protein
- LPS
bacterial lipopolysaccharide
- X
xanthine
- XO
xanthine oxidase
- MCP
monocyte-chemotactic protein
- ActD
actinomycin D
- BSO
buthionine sulfoximine
- SDF
stromal cell-derived factor
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Winrow V R, Winyard P G, Morris C J, Blake D R. Br Med Bull. 1993;49:506–522. doi: 10.1093/oxfordjournals.bmb.a072627. [DOI] [PubMed] [Google Scholar]
- 2.Janssen Y M W, Van Houten B, Borm P J A, Mossman B T. Lab Invest. 1993;69:261–274. [PubMed] [Google Scholar]
- 3.Weiss S J. N Engl J Med. 1989;320:365–376. doi: 10.1056/NEJM198902093200606. [DOI] [PubMed] [Google Scholar]
- 4.Sun Y, Oberley L W. Free Radical Biol Med. 1996;21:335–348. doi: 10.1016/0891-5849(96)00109-8. [DOI] [PubMed] [Google Scholar]
- 5.Schreck R, Rieber P, Baeuerle P A. EMBO J. 1991;10:2247–2258. doi: 10.1002/j.1460-2075.1991.tb07761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schreck R, Meyer B, Mannel D N, Droge W, Baeuerle P A. J Exp Med. 1992;175:1181–1194. doi: 10.1084/jem.175.5.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Staal F J T, Roederer M, Herzenberg L A, Herzenberg L A. Proc Natl Acad Sci USA. 1990;87:9943–9947. doi: 10.1073/pnas.87.24.9943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roederer M, Staal F J T, Raju P A, Ela S W, Herzenberg L A, Herzenberg L A. Proc Natl Acad Sci USA. 1990;87:4884–4888. doi: 10.1073/pnas.87.12.4884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chan D C, Kim P S. Cell. 1998;93:681–684. doi: 10.1016/s0092-8674(00)81430-0. [DOI] [PubMed] [Google Scholar]
- 10.Deng H K, Liu R, Ellmeier W, Choe S, Unutmaz S, Burkhart M, Di Marzio M, Marmon S, Sutton R E, Hill C M, et al. Nature (London) 1996;381:661–666. doi: 10.1038/381661a0. [DOI] [PubMed] [Google Scholar]
- 11.Dragic T, Litwin V, Allaway G P, Martin S R, Huang Y X, Nagashima K A, Cayanan C, Maddon P J, Koup R A, Moore J P, Paxton W A. Nature (London) 1996;381:667–673. doi: 10.1038/381667a0. [DOI] [PubMed] [Google Scholar]
- 12.Choe H, Farzan M, Sun Y, Sullivan N, Rollins B, Ponath P D, Wu L J, Mackay C R, Larosa G, Newman W, et al. Cell. 1996;85:1135–1148. doi: 10.1016/s0092-8674(00)81313-6. [DOI] [PubMed] [Google Scholar]
- 13.Doranz B J, Rucker J, Yi Y J, Smyth R J, Samson M, Peiper S C, Parmentier M, Collman R G, Doms R W. Cell. 1996;85:1149–1158. doi: 10.1016/s0092-8674(00)81314-8. [DOI] [PubMed] [Google Scholar]
- 14.Alkhatib G, Combadiere C, Broder C C, Feng Y, Kennedy P E, Murphy P M, Berger E A. Science. 1996;272:1955–1958. doi: 10.1126/science.272.5270.1955. [DOI] [PubMed] [Google Scholar]
- 15.Feng Y, Broder C C, Kennedy P E, Berger E A. Science. 1996;272:872–877. [Google Scholar]
- 16.Horuk R. Immunol Today. 1994;15:169–174. doi: 10.1016/0167-5699(94)90314-X. [DOI] [PubMed] [Google Scholar]
- 17.Murphy P M. Annu Rev Immunol. 1994;12:593–633. doi: 10.1146/annurev.iy.12.040194.003113. [DOI] [PubMed] [Google Scholar]
- 18.Sozzani S, Locati M, Allavena P, Van Damme J, Mantovani A. Int J Clin Lab Res. 1996;26:69–82. doi: 10.1007/BF02592349. [DOI] [PubMed] [Google Scholar]
- 19.Baggiolini M, Dewald B, Moser B. Annu Rev Immunol. 1997;15:675–705. doi: 10.1146/annurev.immunol.15.1.675. [DOI] [PubMed] [Google Scholar]
- 20.Rollins B J. Blood. 1997;90:909–928. [PubMed] [Google Scholar]
- 21.Luster A D. N Engl J Med. 1998;338:436–445. doi: 10.1056/NEJM199802123380706. [DOI] [PubMed] [Google Scholar]
- 22.Sica A, Saccani A, Borsatti A, Power C A, Wells T N C, Luini W, Polentarutti N, Sozzani S, Mantovani A. J Exp Med. 1997;185:969–974. doi: 10.1084/jem.185.5.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sozzani S, Iannolo G, Luini W, Borsatti A, Polentarutti N, Sica A, Mackay C, Wells T N C, Ghezzi S, Vincenzi E, et al. J Exp Med. 1998;187:439–444. doi: 10.1084/jem.187.3.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Penton-Rol G, Polentarutti N, Luini W, Borsatti A, Mancinelli R, Sica A, Sozzani S, Mantovani A. J Immunol. 1998;160:3869–3874. [PubMed] [Google Scholar]
- 25.Peri G, Milanese C, Matteucci C, Ruco L, Zhou D, Sozzani S, Coletta I, Mantovani A. J Immunol Methods. 1994;174:249–257. doi: 10.1016/0022-1759(94)90029-9. [DOI] [PubMed] [Google Scholar]
- 26.Falk W, Goodwin R H, Jr, Leonard E J. J Immunol Methods. 1980;33:239–247. doi: 10.1016/0022-1759(80)90211-2. [DOI] [PubMed] [Google Scholar]
- 27.Sica A, Wang J M, Colotta F, Dejana E, Mantovani A, Oppenheim J J, Larsen C G, Zachariae C O, Matsushima K. J Immunol. 1990;144:3034–3038. [PubMed] [Google Scholar]
- 28.Lloyd A R, Biragyn A, Johnston J A, Taub D D, Xu L L, Michiel D, Sprenger H, Oppenheim J J, Kelvin D J. J Biol Chem. 1995;270:28188–28192. doi: 10.1074/jbc.270.47.28188. [DOI] [PubMed] [Google Scholar]
- 29.Meister A, Anderson M E. Annu Rev Biochem. 1983;52:711–760. doi: 10.1146/annurev.bi.52.070183.003431. [DOI] [PubMed] [Google Scholar]
- 30.Charo I F, Myers S J, Herman A, Franci C, Connolly A J, Coughlin S R. Proc Natl Acad Sci USA. 1994;91:2752–2756. doi: 10.1073/pnas.91.7.2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stein D, Baldwin A. Mol Cell Biol. 1993;13:7191–7198. doi: 10.1128/mcb.13.11.7191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yasumoto K, Okamoto S-I, Mukaida N, Murakami S, Mai M, Matsushima K. J Biol Chem. 1992;267:22506–22511. [PubMed] [Google Scholar]
- 33.Freeter R R, Alberta J A, Hwang G Y, Wrentmore A L, Stiles C D. J Biol Chem. 1996;271:17417–17424. doi: 10.1074/jbc.271.29.17417. [DOI] [PubMed] [Google Scholar]
- 34.Greenspan H C, Aruoma O I. Immunol Today. 1994;15:209–213. doi: 10.1016/0167-5699(94)90245-3. [DOI] [PubMed] [Google Scholar]
- 35.Kotler D P. Am J Clin Nutr. 1998;67:7–9. doi: 10.1093/ajcn/67.1.7. [DOI] [PubMed] [Google Scholar]