Abstract
The pancreatic β cell can respond in the long term to hyperglycemia both with an increased capacity for insulin production and, in susceptible individuals, with apoptosis. When glucose-induced apoptosis offsets the increasing β cell capacity, type 2 diabetes results. Here, we tested the idea that the pathway of glucose metabolism that leads to the modification of intracellular proteins with the O-linked monosaccharide N-acetylglucosamine (O-GlcNAc) is involved in the glucose-induced apoptosis. This idea is based on two recent observations. First, the β cell expresses much more O-GlcNAc transferase than any other known cell, and second, that the β cell-specific toxin, streptozotocin (STZ), itself a GlcNAc analog, specifically blocks the enzyme that cleaves O-GlcNAc from intracellular proteins. As a consequence, we now show that hyperglycemia leads to the rapid and reversible accumulation of O-GlcNAc specifically in β cells in vivo. Animals pretreated with STZ also accumulate O-GlcNAc in their β cells when hyperglycemic, but this change is sustained upon re-establishment of euglycemia. In concert with the idea that STZ toxicity results from the sustained accumulation of O-GlcNAc after a hyperglycemic episode, we established a low-dose STZ protocol in which the β cells' toxicity of STZ was manifest only after glucose or glucosamine administration. Transgenic mice with impaired β cell glucosamine synthesis treated with this protocol are resistant to the diabetogenic effect of STZ plus glucose yet succumb to STZ plus glucosamine. This study provides a causal link between apoptosis in β cells and glucose metabolism through glucosamine to O-GlcNAc, implicating this pathway of glucose metabolism with β cell glucose toxicity.
The pancreatic β cell is the primary regulator of glucose flux into stored energy in vertebrates. To accomplish this function, β cells must sense the plasma glucose concentration and secrete the appropriate amount of insulin to direct glucose uptake and storage of its chemical energy in the fat, muscle, and liver. Considerable progress has been made in the understanding of glucose sensing by the β cell. Glucose enters the β cell through the GLUT2 glucose transporter (1), and it is phosphorylated by glucokinase, which has a Km for glucose that allows substantial glucose phosphorylation to proceed only when plasma glucose concentrations exceed 5 mM (2). Once glucose is phosphorylated, it can enter a variety of metabolic pathways, including glycolysis. An increased ATP/ADP from glucose metabolism generally is believed to regulate the immediate release of insulin (3, 4). The normal β cell is also capable of adapting its capacity for insulin release depending on long-term nutritional status. For example, exposure to a higher than normal carbohydrate load can condition the β cell to secrete even more insulin after exposure to the same load several hours later (5). Finally, the β cell is capable of even more long-term adaptation by increasing the β cell number through hyperplasia (6, 7). However, this hyperplasia can be offset by concomitant apoptosis (8, 9). It is the failure of these long-term feedback adaptations in genetically susceptible individuals with concurrent insulin resistance that appears to result in the complete phenotype of type 2 diabetes (6).
In addition to being a substrate for energy generation, glucose is used in macromolecular synthesis. Of interest with regard to the β cell is the role of glucose in the synthesis of glycoproteins and in particular those intracellular proteins modified by O-linkage of the monosaccharide, N-acetylglucosamine (O-GlcNAc) to serine or threonine residues in the protein backbone. There is evidence that this protein modification may be specifically important in the β cell in that the enzyme, O-GlcNAc transferase (OGT), is very highly enriched in these cells relative to other known cell types (10, 11). Furthermore, the β cell-specific toxin and GlcNAc analog (12), streptozotocin (STZ), blocks the removal of this O-GlcNAc modification from proteins in vitro through the inhibition of the O-GlcNAc-selective N-acetyl-β-d-glucosaminidase (O-GlcNAcase) (10, 11). This activity of STZ, the β cell-specific toxicity of STZ, and the great abundance of OGT in the β cell together suggest that the utilization of glucose for intracellular protein modification by O-GlcNAc may be of vital importance for β cell adaptation to glucose loads.
The role of O-GlcNAc protein modification is not fully understood. This modification has been detected in many transcription factors (13) and nuclear pore proteins (14, 15). There is now evidence that modification of transcription factors by O-GlcNAc may alter the stability of some of these proteins (16, 17), control the protein interactions involved in transcriptional activation (18), or compete with the phosphorylation of serine residues that control transcription factor function (19). Thus, it is reasonable to suggest that the O-GlcNAc modification of transcription factors may play a role in the control of transcription. Furthermore, there is recent evidence that certain cytoplasmic proteins involved in insulin signaling also may be modified by O-GlcNAc (20), raising the possibility that this protein modification plays some role in signaling from cell surface receptors. However, for there to be any role for O-GlcNAc in the β cell in its response to glucose would require measurable alterations in the abundance of O-GlcNAc on intracellular proteins in response to changes in the extracellular glucose concentration. In this paper, we show that the abundance of O-GlcNAc in β cells markedly increases in response to hyperglycemia in animals and that this increase is rapidly reversible. Furthermore, we establish in vivo that STZ blocks the removal of this modification in β cells. Using a transgenic mouse model in which glucose metabolism to glucosamine is impaired, we show that this action of STZ on O-GlcNAc turnover is necessary for the apoptotic death of the β cell. These findings establish a direct connection between the pathway of glucose metabolism to O-GlcNAc and β cell toxicity.
Materials and Methods
Glucose and Glucosamine Infusion and STZ Treatment in Rats.
Male Sprague–Dawley rats (Charles River Breeding Laboratories, body weight 150–175 g) were fasted overnight. The rats were infused in the right femoral vein with either dextrose (initial rate, 0.125 mg⋅g−1⋅min−1, slowed to 0.042 mg⋅g−1⋅min−1 after 30 min) or glucosamine (0.025 mg⋅g−1⋅min−1) by a electronically controlled syringe pump (KD Scientific, New Hope, PA), whereas control rats were infused with PBS solution (pH 7.4). Blood glucose was determined before and during the infusion at 15-min intervals on blood derived from a tail bleed by using an Advantage blood glucose monitor (Boehringer Mannheim). Human insulin (Eli Lilly) was administrated i.v. The STZ (200 mg⋅kg−1 freshly dissolved in 100 mM citrate buffer, pH 4.2) was injected i.v. 15 min before the glucose infusion. At the various time points and blood glucose levels, the pancreas was removed and formaldehyde-fixed immediately. Animal treatments were performed in an approved manner in a certified animal care facility.
Tissue Immunohistochemistry.
Formaldehyde-fixed and paraffin-embedded pancreas sections were immunostained as described (10) by using RL2 ascites (1/20) or anti-insulin (1/50). To facilitate immunostaining, the sections were pretreated with 2 M HCl for 20 min at 37°C and exposed to 0.01% trypsin at 37°C for 5 min.
Treatment of SJLXB6 Mice with STZ in Combination with Glucose or Glucosamine.
A total of 74 overnight fasted male SJLXB6 mice (body weight 19.6–27.4 g) were divided into three groups. The mice in the first group (n = 26) were injected i.p. with STZ (50 mg⋅kg−1) and PBS (pH 7.4, 30 min after the treatment of STZ). The mice in the second group (n = 23) were injected i.p. with STZ (50 mg⋅kg−1) followed in 30 min by glucose (8.76–10.0 mg⋅g−1). The mice in the third group (n = 25) were injected i.p. with STZ (50 mg/kg) followed in 30 min by glucosamine (4.98 mg⋅g−1). Additionally, 50 mice were divided into five groups and injected i.p. with STZ at doses of 55, 60, 65, 75, and 80 mg⋅kg−1. The blood glucose was monitored frequently before sacrifice 26 hr later.
In Situ Hybridization.
The paraffin and frozen sections of mouse pancreas were prepared and hybridized as described (21). The antisense insulin 35S-cRNA riboprobe was synthesized from the 350-bp rat insulin I cDNA in pBlueScript KS (Stratagene), using T3 polymerase. The sense 35S-cRNA riboprobe, to detect the transgenic antisense mouse glutamine:fructose-6-phosphate amidotransferase (GFAT) mRNA, was synthesized from the 2.1-kb mouse GFAT cDNA in pT7T3 (Amersham Pharmacia), using T7 RNA polymerase. After hybridization, the slides were exposed to x-ray film for 3–10 days. The slides also were dipped in photographic emulsion, exposed, developed, and counterstained with hematoxylin and eosin, then subjected to microscopic examination.
Terminal Deoxynucleotide Transferase-Mediated dUTP Nicked-End Labeling (TUNEL) Assay.
The TUNEL assay was performed as described (22) in paraffin-embedded sections of the pancreases by using an in situ cell death detection kit (Boehringer Mannheim). The endogenous perioxidase activity was blocked by immersing the sections in 0.3% H2O2 in methanol for 30 min before cell permeablization. Nonspecific binding of the peroxidase-coupled antifluorescein antibody was blocked with PBS containing 3% BSA for 20 min. Positive cells were visualized by using peroxidase substrate enhancer and 3,3′-diaminobenzidine tetrahydrochloride substrate (Boehringer Mannheim), and sections were counterstained with hematoxylin. For a pancreas to be scored as apoptotic, all islets had to display TUNEL positivity.
Generation of Transgenic Mice with β Cell-Specific Expression of Mouse GFAT Antisense Gene.
The 2.2-kb mouse GFAT cDNA (23), consisting of 150 bp 5′ untranslated region and complete coding sequence, was inserted in the antisense direction between the rat insulin II promoter (RIP) (24) and the simian virus 40 (SV40) small T-antigen intron and polyadenylation sequences. The entire 4.4-kb fragment, containing the RIP-mGFAT (antisense)-SV40 construct, was excised from the cloning vector, purified, and microinjected into fertilized eggs from SJLXB6 mice. Of the 29 living births, seven founder mice were identified by PCR analysis of tail tip DNA using oligonucleotide primers hybridizing to the RIP and mGFAT sequence. The resulting 1.28-kb PCR product spans the RIP-mGFAT junction. Gene dose was determined by slot-blot analysis, and the transgenic mouse line, termed 3–4, with the highest gene dose was used for the majority of studies. For consistency, 3- to 4-month-old male mice from this line were used for the subsequent studies, although similar results were obtained with the other lines, females, and animals over 5 months old. In situ hybridization with a sense-oriented mouse GFAT [35S]cRNA probe was performed to determine the β cell-specific expression of the antisense transgene in islets. Immunohistochemical staining with RL2 mAbs was performed as before in the transgenic mice and their wild-type littermates 1.5 hr after i.p. injection with STZ (50 mg⋅kg−1) and glucose (10.0 mg⋅g−1, 30 min after the treatment of STZ).
Treatment of RIP-mGFAT (AS) Mice with Multiple Low Doses of STZ.
Multiple low doses of STZ treatment (5 consecutive days, 40 mg⋅kg−1) was performed as described (25) in 14 transgenic mice and 16 littermates (from three different lines). The diabetes was assessed by blood glucose measurements every 3 days and histological analysis 14 and 28 days after the last injection of STZ.
Treatment of RIP-mGFAT (AS) Mice with STZ in Combination with Glucose or Glucosamine.
The trangenic mice and their littermates were injected with either STZ (50 mg⋅kg−1) and glucose (8.76–10 mg⋅g−1, n = 41, 21 transgenic mice and 20 wild-type littermates), or STZ (50 mg⋅kg−1) and glucosamine (4.98 mg⋅g−1, n = 35, 19 transgenic mice and 16 wild-type littermates). The blood glucose was monitored frequently, and the mice were sacrificed for histological analysis, immunohistochemical staining with anti-insulin polyclonal antibody, in situ hybridization for insulin transcripts, and TUNEL assay 26 hr later.
Statistical Analysis.
All values are expressed as mean ± SE. Statistical analysis is carried out with unpaired t test and χ2 test. Differences are considered statistically significantly at P < 0.05 or P < 0.01.
Results
O-GlcNAc Level in β Cells Is Rapidly Responsive to the Blood Glucose in Vivo.
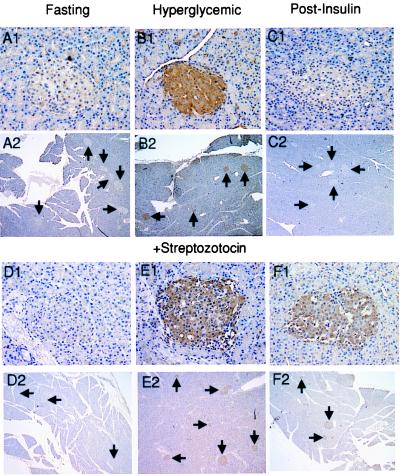
For the glucose concentration to be transduced into a change in the O-GlcNAc content in β cells requires that glucose first be converted to glucosamine, and secondly, that the increased availability of UDP-GlcNAc as a substrate would drive increased protein modification by OGT. The rate-limiting step in glucosamine synthesis is the enzyme GFAT. Provision of substrate for GFAT requires a glucose concentration sufficient for phosphorylation by the β cell glucokinase. Furthermore, GFAT is known to be regulated by feedback inhibition by UDP-GlcNAc (26). While in other cell types, we have shown that altered glucose concentrations can be reflected by changes in the O-GlcNAc modification of intracellular proteins (23, 27), the pancreatic β cell has not been shown to respond in this manner. To determine the effect of changing plasma glucose concentrations on β cell O-GlcNAc content in vivo, we established hyperglycemia in Sprague–Dawley rats by i.v. glucose infusion and assessed β cell O-GlcNAc by immunohistochemical staining with the RL2 mAb. The binding of the antibody to this epitope strictly depends on the presence of the O-GlcNAc moiety (14, 16, 23, 27) and can be blocked by preadsorption of the antibody with GlcNAc (ref. 27; data not shown) or STZ (10) but not other sugars. Elevation of the plasma glucose concentration to approximately 20 mM resulted in a marked and β cell-specific increase in the level of intracellular O-GlcNAc modification within 30 min of the development of hyperglycemia (Fig. 1B1). The effect was readily seen in all islets (Fig. 1B2) at glucose concentrations above 15 mM, although a systematic dose-response relationship was not established. No change in O-GlcNAc content was detected in the exocrine pancreas. After establishment of hyperglycemia (28 mM) by the same protocol, the blood glucose was returned to the fasting level with an infusion of human insulin. The pancreases were examined at a time point defined as 15 min after the blood glucose concentration had decreased to 4.0 ± 0.2 mM (mean ± SE). This point occurred about 1 hr after the blood glucose had decreased through 10 mM. At this time, the O-GlcNAc modification level had returned to the level seen in control fasted rats (Fig. 1 C1 and C2). These observations indicate that the level of O-GlcNAc in β cells is responsive in both directions to the extracellular glucose concentration. This specific O-GlcNAc response to hyperglycemia in the β cells likely results from both the gating effect of the glucokinase, which restricts substrate for glucosamine synthesis under fasting conditions, and the high abundance of the transferase in these cells.
Figure 1.
The effect of hyperglycemia and STZ on O-GlcNAc accumulation in pancreatic islets in vivo. Fasted rats were infused with glucose either without STZ or 15 min after the STZ-pretreatment, and the pancreases were examined for the distribution of RL2-detectable glycoprotein. (A and D) Overnight fasted. (B and E) 45 min after initiation of glucose infusion (30 min with [glucose]>20 mM. (C and F) The glucose infusion was terminated after 45 min and replaced with an insulin infusion that was continued for a further 135 min. The blood glucose was <10 mM for about 1 hr when the pancreases were examined. (D–F) STZ pretreatment. (Magnification: frames 1, ×200; frames 2, ×25.).
STZ Causes O-GlcNAc Accumulation by Blocking Its Removal from the Proteins.
Although we have proposed that STZ is specifically toxic to the β cell because it can block the O-GlcNAcase in vitro (10, 11) and OGT is selectively abundant in β cells (10, 11), other mechanisms for this specific toxicity also have been proposed. STZ has been shown to be a DNA alkylating agent (28, 29), and NO donor (30) and its specificity to the β cell was suggested to result from the preferential ability of the GLUT2 to transport this agent into β cells (31). To determine whether STZ behaves as an inhibitor of the β cell O-GlcNAcase in intact animals, we pretreated the Sprague–Dawley rats with STZ before the initiation of the glucose infusion. The islets were examined again by immunohistochemical staining with the RL2 antibody. In the absence of hyperglycemia, STZ did not alter the RL2 reactivity of β cells (Fig. 1 D1 and D2), even though the RL2 antibody appears to recognize STZ (10). This observation is in agreement with our earlier determination that STZ itself is not metabolized as a substrate of OGT in cultured cells (10). As expected, when hyperglycemia was achieved after the STZ dose, the specific increase in β cell O-GlcNAc was observed again (Fig. 1 E1 and E2). In contrast, when normoglycemia (3.9 ± 0.2 mM) was re-established by insulin infusion as described above, the O-GlcNAc content in the STZ-treated animals failed to decrease in the islets in response to the falling blood sugar, indicating that the removal of the O-GlcNAc modification was blocked by STZ (Fig. 1 F1 and F2). Thus, STZ inhibits the β cells O-GlcNAcase in vivo.
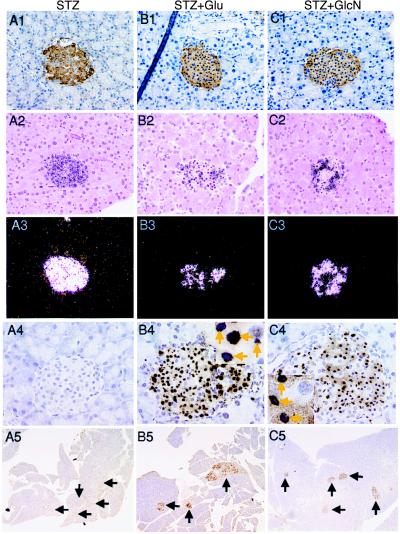
Glucose and Glucosamine Potentiate STZ Toxicity and Cause β Cell Apoptosis.
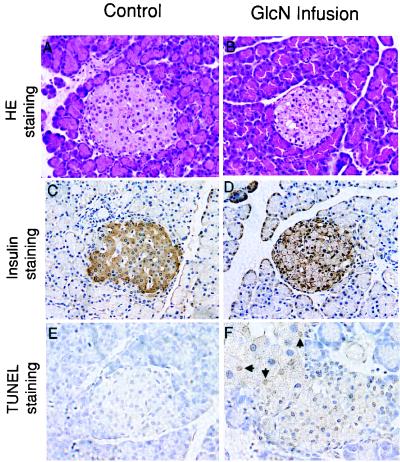
To further correlate the accumulation of O-GlcNAc in β cells with STZ toxicity, we tested the diabetogenic effect of a single dose of STZ in SJLXB6 mice. This strain of mice exhibits reduced sensitivity to this drug relative to the rat (32). Although a single dose lower than 75 mg⋅kg−1 of STZ was insufficient to cause β cell death, treatment with either glucose or glucosamine after an injection of 50 mg⋅kg−1 of STZ resulted in extensive β cell death within 26 hr in 78.3% and 76.0% of treated mice, respectively (Table 1). This cell death was evident on histological sections by the appearance of the pyknotic nuclei in every islet of affected animals and was accompanied by a diminution of insulin mRNA as determined by in situ hybridization (Fig. 2). To determine whether the β cell death was apoptotic in nature, sections from the same pancreases were stained by using the TUNEL assay. Normal islets and the islets in the unaffected animals treated with the threshold dose of STZ alone (50 mg⋅kg−1) showed no evidence of nicked DNA whereas the islets of the affected animals that had been treated with STZ followed by glucose or glucosamine showed numerous apoptotic cells in every islet examined (Fig. 2, rows 4 and 5). It has been previously observed that glucose potentiates the toxic effect of STZ on β cells (33); however, the mechanism has not been understood. This potentiation of STZ toxicity with both glucose and glucosamine is compatible with the notion that the sustained accumulation of O-GlcNAc caused by the combination of STZ and glucose administration is behind this toxicity. Furthermore, a high concentration of glucose recently has been shown to induce apoptosis in cultured islets (34) and hyperglycemia induces β cell apoptosis in certain rodent strains (9). However, we were unable to detect significant islet pathology or apoptosis after an 8-hr i.v. glucose infusion in Sprague–Dawley rats. In contrast, an 8-hr i.v. infusion of glucosamine in these rats resulted in islet pathology. Islets from the glucosamine-infused rats exhibited nuclear pyknosis (Fig. 3B) with several cells being modestly positive for DNA nicking by the TUNEL assay (Fig. 3F). There were fewer apoptotic cells and the staining was less intense after the 8 hr of glucosamine exposure than that seen 26 hr after STZ plus glucose or glucosamine. The glucosamine infusion, at a dose that would allow phosphorylation by the β cell glucokinase, could not be sustained much beyond 8 hr because of general toxicity. We also observed depletion of immunoreactive insulin in the β cells in the glucosamine-treated rats (Fig. 3D). No significant pathological changes were found in the exocrine pancreas.
Table 1.
The effect of glucose and glucosamine on the β cell toxicity of STZ in RIP-GFAT (AS) transgenic and wild-type SJLXB6 mice
| Mice | Treatment | # Mice Examined | # Mice
|
χ2 test | |
|---|---|---|---|---|---|
| Apoptotic* (%) | Normal | ||||
| Wild type | |||||
| (1) | STZ (50 mg⋅kg−1) | 26 | 0 (0) | 26 | |
| (2) | STZ (55 mg⋅kg−1) | 10 | 0 (0) | 10 | |
| (3) | STZ (60 mg⋅kg−1) | 10 | 0 (0) | 10 | |
| (4) | STZ (65 mg⋅kg−1) | 10 | 0 (0) | 10 | |
| (5) | STZ (70 mg⋅kg−1) | 10 | 2 (20) | 8 | P = 0.020 vs. (1) |
| (6) | STZ (75 mg⋅kg−1) | 10 | 8 (80) | 2 | P < 0.0001 vs. (1) |
| (7) | STZ (80 mg⋅kg−1) | 10 | 8 (80) | 2 | P < 0.0001 vs. (1) |
| (8) | STZ (50 mg⋅kg−1) + Glc | 23 | 18 (78.3) | 5 | P < 0.0001 vs. (1) |
| (9) | STZ (50 mg⋅kg−1) + GlcN | 25 | 19 (76.0) | 6 | P < 0.0001 vs. (1) |
| Transgenic mice | |||||
| (10) | STZ (50 mg⋅kg−1) + Glc | 21 | 6 (28.6) | 15 | P = 0.0010 vs. (8) |
| (11) | STZ (50 mg⋅kg−1) + GlcN | 19 | 15 (78.9) | 4 | P = 0.0015 vs. (10) |
| Wild-type littermates | |||||
| (12) | STZ (50 mg⋅kg−1) + Glc | 20 | 16 (80.0) | 4 | P = 0.0009 vs. (10) |
| (13) | STZ (50 mg⋅kg−1) + GlcN | 16 | 13 (81.3) | 3 | P = 0.8653 vs. (11) |
The experimental methods are described in Materials and Methods. Statistical analysis was carried out with χ2 test in microexcel statistics software. Difference were considered statistically significant at P < 0.01.
*Affected animals exhibited β cell apoptosis by TUNEL assay in every islet.
Figure 2.
The effect of glucose and glucosamine on the cytotoxicity of STZ to β cells. (A1–A5) Single-dose STZ (50 mg⋅kg−1). (B1–B5) Single-dose STZ (50 mg⋅kg−1) followed by glucose. (C1–C5) Single-dose STZ (50 mg⋅kg−1) followed by glucosamine. (Row 1) The pancreas was stained by immunohistochemistry with a polyclonal insulin antibody (magnification: ×400). (Row 2) Bright-field view of the in situ hybridization with a 35S-UTP-labeled insulin antisense riboprobe (magnification: ×200). Six animals from each treatment group were examined. (Row 3) Dark-field view of the same sections shown in Row 2. The hybridization signal appears as dark grains in the bright-field and white grains in the dark-field views. (Row 4) In situ TUNEL assay for DNA nicking (magnification: ×300). (Insets, B4 and C4) Apoptotic cell nuclei are indicated by arrows (magnification: ×500). (Row 5) In situ TUNEL assay for DNA nicking (magnification: ×50). Arrows indicate location of all islet of Langerhans in this section.
Figure 3.
The effect of an 8-hr i.v. glucosamine infusion on the pancreas of the Sprague–Dawley rats. Treatment and analysis were as indicated on the figure. Apoptotic nuclei are indicated by arrows. (Magnifications: A–D, ×200; E and F, ×500.)
Trangenic Mice with Impaired Glucosamine Synthesis Are Resistant to the Diabetogenic Effect of STZ.
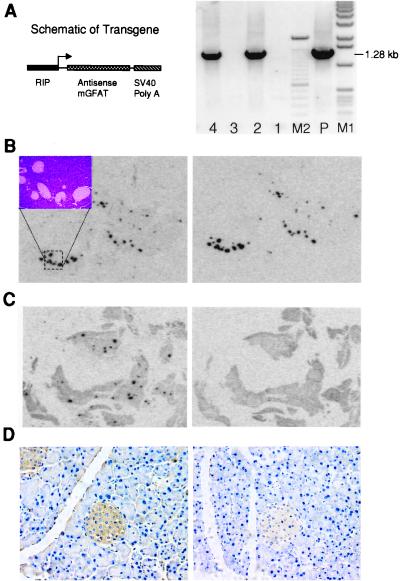
To establish a causal link between β cell glucosamine metabolism and STZ toxicity, we developed a transgenic mouse line in which we expressed in β cells an antisense GFAT construct that we had shown can block the glucose-stimulated increase in O-GlcNAc modification in cultured mouse cells (23). Integration of the transgene was detected by PCR in DNA extracted from the mouse tails (Fig. 4) and by Southern blot analysis (not shown). In situ hybridization confirmed the β cell-specific expression of the antisense transgene in all islets (Fig. 4B). The intensity of the antisense GFAT hybridization signal was comparable to the insulin signal, although the molar specific activity of the GFAT probe was 7-fold higher than that of the insulin probe. In normal mice, the endogenous sense GFAT mRNA was not detectable in the islets (Fig. 4C) exposed to film for the same time, indicating that the antisense transgene was expressed at a markedly higher level than is the endogenous GFAT mRNA. As shown previously in cultured cells (23), this antisense construct was able to blunt the STZ and glucose-induced O-GlcNAc accumulation in the β cells of the transgenic mice as compared with the nontransgenic litermates (Fig. 4D).
Figure 4.
Characterization of the antisense-GFAT transgenic mice. (A) (Left) The schematic diagram of the transgene. (Right) The PCR analysis of DNA derived from the tails of F1 mice (lanes 2 and 4 are gene positive). M1: 1-kb markers; M2: 100-bp markers; P: 0.5 pg plasmid DNA template. The expected amplified target sequence is about 1.28 kb. (B) In situ hybridization of a transgenic mouse pancreas. The adjacent tissue slices, mounted on microscope slides, were exposed to x-ray film for 3 days. (Left) The probe was antisense insulin. (Inset) A photomicrograph of the hematoxylin/eosin-stained islets corresponding to the hybridization signal. (Right) The probe was sense GFAT indicating the location and intensity of expression of the antisense transgene (magnification: ×3). (C) In situ hybridization of a wild-type mouse pancreas. The adjacent tissue slices, mounted on microscope slides, were exposed to x-ray film for 3 days. (Left) Probing for insulin mRNA. (Right) Probing for the endogenous GFAT mRNA (magnification: ×3). (D) The effect of the antisense GFAT transgene on the β cell O-GlcNAc accumulation in hyperglycemic mice. The mice were injected with STZ followed by glucose, and the pancreas was examined by immunohistochemical staining with RL2 antibodies. (Left) The pancreas from a wild-type littermate mouse after receiving treatment with STZ and glucose (blood glucose = 14 mM) (magnification: ×400). (Right) The pancreas from a RIP-mGFAT(AS) transgenic mouse after receiving treatment with STZ and glucose (blood glucose = 19 mM).
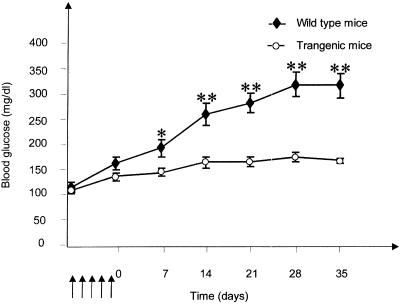
A multiple low-dose STZ protocol has been developed to induce diabetes in mice. Application of this protocol (25) to the mice expressing the antisense GFAT transgene failed to induce diabetes whereas their wild-type littermates developed hyperglycemia and islet destruction within 21 days after the same treatment (Fig. 5). To prove that it is impaired glucosamine synthesis in the antisense GFAT transgenics that confers this STZ resistance, we used our observation that both glucose and glucosamine potentiate the diabetogenic effect of a single-threshold dose of STZ (Fig. 2). Concurrent treatment with glucose and STZ (50 mg/kg) failed to cause β cell apoptosis in 71.4% of the transgenic mice, but caused extensive β cell apoptosis in 80.0% of their wild-type littermates. In contrast, when the mice were administered concurrent treatment with glucosamine and STZ, extensive β cell apoptosis was observed in 78.9% of the transgenic mice and 81.3% of their wild-type littermates (Table 1). This observation strongly supports the idea that glucose must be metabolized by GFAT to glucosamine to potentiate the toxicity of STZ. The glucosamine administered with the STZ bypassed the block in glucose metabolism induced by the antisense GFAT transgene, thereby implicating the transgene in the STZ resistance. This effect of the antisense transgene was observed in three independently developed transgenic lines, ruling out an integration site-specific mechanism for the STZ resistance.
Figure 5.
Blood glucose response after treatment with multiple low doses of STZ. The arrows indicate the timing of the STZ injections (40 mg⋅kg−1, 5 consecutive days). Blood glucose was determined in samples from a tail bleed at the indicated times. Results are given as the mean ± SE for 14 transgenic and 16 wild-type littermate mice. *, P < 0.05; **, P < 0.01 (unpaired t test).
Discussion
The β cell as a glucose sensor has adapted to respond both instantaneously and more chronically to changes in the nutritional load. The long-term adaptation that switches the β cell from the famine mode with limited insulin-secretory capacity to the surplus mode with increased capacity is poorly understood. In insulin-resistant animal models (6) or after partial pancreatectomy (7), the rate of β cell proliferation increases. However, in those animal models susceptible to the development of type 2 diabetes, this proliferative response ultimately is offset by an increase in apoptosis (8, 9) that results in a failure of the net β cell mass to increase sufficiently to fulfill the insulin demand. Both the proliferative (7) and apoptotic response (8, 9) appear to be driven by hyperglycemia. Indeed, the apoptotic response may be part of the glucose toxicity that results in reduced insulin secretion after prolonged hyperglycemia (35–37).
We have linked the metabolism of glucose to O-GlcNAc with β cell apoptosis by using the recently discovered activity of STZ as an inhibitor of O-GlcNAcase (10, 11). Although we show that the normal β cell has a rapid on- and off-rate of O-GlcNAc, STZ arrests this cycle, resulting in the stable and β cell-specific accumulation of the modification in vivo. The enhancement of the apoptotic response to STZ by glucose is blunted in transgenic animals with impaired glucosamine synthesis from glucose, indicating that it is this pathway of glucose metabolism that is required for the β cell-specific toxic and apoptotic effect of STZ.
No in vivo role for the O-GlcNAc modification of proteins previously has been ascertained. Our results linking the O-GlcNAc modification of proteins to β cell apoptosis will open up the search for the protein targets of OGT that lead to the initiation of this process. Candidate targets may arise from the idea that the O-GlcNAc response in β cells to hyperglycemia likely serves some physiological role besides causing apoptosis. OGT contains nuclear localization signals and is enriched in the nucleus (38) and there is evidence that the O-GlcNAc modification may play an important role in the control of gene transcription. Many transcription factors are modified with O-GlcNAc (13). Sp1, an O-GlcNAc modified transcription factor (39), has been found to be regulated in a complex manner by this modification. First, the stability of Sp1 has been shown to be regulated by the proteasome. Under condition of O-GlcNAc depletion, Sp1 is rapidly degraded whereas glucosamine treatment of cells results in the stabilization of Sp1 through the inhibition of proteasomal degradation in vivo (16) and in vitro (17). These changes in the abundance of Sp1 or perhaps other transcription factors may alter the transcription of a wide variety of genes (23, 27, 40, 41), including those TATA-less genes that encode the housekeeping proteins (42). Thus, the O-GlcNAc response to glucose could up-regulate the expression of many β cell genes leading to cellular hypertrophy. However, it also remains possible that this inhibition of proteasomal degradation of Sp1 may extend to other proteins known to be degraded by the proteasome and involved in apoptosis (43). Another effect of O-GlcNAc on Sp1 is in the control of the Sp1 protein interactions involved in transcriptional activation (18). This observation raises the possibility that sustained O-GlcNAc modification of a transcription activation domain of Sp1 could preclude the protein interactions required for transcriptional activation and lead to impaired gene transcription. This concept also may apply to intramolecular domain interactions. Indeed, there is some evidence that the basic C-terminal domain of p53 interacts with and represses the DNA-binding domain of this transcription factor (44). Modification of the C-terminal domain with O-GlcNAc may abrogate this intramolecular interaction and activate p53 as a DNA-binding protein, leading to the transcription of genes that are involved in cell cycle arrest and apoptosis (45).
Recently, poly(ADP ribose) polymerase (PARP)-deficient mice were found to be protected from STZ-induced β cell death (46–48). PARP is stimulated by DNA breaks as part of the repair response (e.g., ref. 49), suggesting that the activation of this enzyme is a downstream event in the apoptotic cascade. Our observation that mice expressing antisense GFAT in β cells did not develop DNA breaks in response to STZ suggests that this construct blocked apoptosis at an earlier point than did the PARP deficiency. Nevertheless, it remains possible that the O-GlcNAc and PARP pathways interact. For example, p53 can be modified both by O-GlcNAc and poly(ADP ribosyl)ation (49), and these modifications could interact to complete the apoptotic process.
Our studies strongly support the notion that STZ-induced β cell apoptosis results from blockade of O-GlcNAcase activity and the subsequent accumulation of O-GlcNAc on certain proteins in β cells. Although glucose itself can cause β cell apoptosis in certain rodent models (8, 9), the accumulation of O-GlcNAc in β cells in response to short-term hyperglycemia alone, at least in this strain of rat, was not sufficient to induce a detectable level of apoptosis, whereas pushing this pathway with an 8-hr glucosamine infusion did result in apoptosis, albeit, less extensive than seen 26 hr after STZ. The difference in behavior of islets treated with glucose from those treated with glucose in combination with STZ may relate to the cyclical nature of the O-GlcNAc modification. That is, the on-rate for the modification normally may be matched by the off-rate even though the steady-state level of modification may be high during hyperglycemia. We infer that interruption of this cycle with STZ results in the prolonged accumulation of O-GlcNAc on the critical substrates that become involved in the apoptotic response. Although delineation of the mechanism of O-GlcNAc-induced apoptosis awaits identification of these substrates, our antisense GFAT transgenic model provides strong evidence that the pathway of glucose metabolism that culminates in the modification of proteins by O-GlcNAc is the pathway that is involved in glucose toxicity. This pathway is then a worthy target for intervention in the glucose toxicity that leads to β cell failure and the pathogenesis of type 2 diabetes.
Acknowledgments
We thank Dr. Robert Konrad for thoughtful discussions and collaborations on isolated pancreatic islets. We thank Drs. D. Hanahan for the rat insulin promoter, L. Gerace for the RL2 antibody, and C. Pinkert for help with the transgenic mice. These studies were supported by National Institutes of Health Grant DK55262.
Abbreviations
- O-GlcNAc
O-linked N-acetylglucosamine
- STZ
streptozotocin
- OGT
O-GlcNAc transferase
- O-GlcNAcase
O-GlcNAc-selective N-acetyl-β-d-glucosaminidase
- GFAT
glutamine:fructose-6-phosphate amidotransferase
- TUNEL
terminal deoxynucleotide transferase-mediated dUTP nicked-end labeling
- RIP
rat insulin II promoter
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Thorens B, Sarkar H K, Kaback H R, Lodish H F. Cell. 1988;55:281–290. doi: 10.1016/0092-8674(88)90051-7. [DOI] [PubMed] [Google Scholar]
- 2.Matschinsky F M. Diabetes. 1996;45:223–241. doi: 10.2337/diab.45.2.223. [DOI] [PubMed] [Google Scholar]
- 3.Inagaki N, Gonoi T, Clement J P, 4th, Namba N, Inazawa J, Gonzalez G, Aguilar-Bryan L, Seino S, Bryan J. Science. 1995;270:1166–1170. doi: 10.1126/science.270.5239.1166. [DOI] [PubMed] [Google Scholar]
- 4.Aguilar-Bryan L, Nichols C G, Wechsler S W, Clement J P, 4th, Boyd A E, 3rd, Gonzalez G, Herrera-Sosa H, Nguy K, Bryan J, Nelson D A. Science. 1995;268:423–426. doi: 10.1126/science.7716547. [DOI] [PubMed] [Google Scholar]
- 5.Efendic S, Cerasi E, Luft R, Gladnikoff G. Diabetes. 1976;25:949–954. doi: 10.2337/diab.25.10.949. [DOI] [PubMed] [Google Scholar]
- 6.Polonsky K S, Sturis J, Bell G I. N Engl J Med. 1996;334:777–783. doi: 10.1056/NEJM199603213341207. [DOI] [PubMed] [Google Scholar]
- 7.Brockenbrough J S, Weir G C, Bonner-Weir S. Diabetes. 1988;37:232–236. doi: 10.2337/diab.37.2.232. [DOI] [PubMed] [Google Scholar]
- 8.Pick A, Clark J, Kubstrup C, Levisetti M, Pugh W, Bonner-Weir S, Polonsky K S. Diabetes. 1998;47:358–364. doi: 10.2337/diabetes.47.3.358. [DOI] [PubMed] [Google Scholar]
- 9.Donath M Y, Gross D J, Cerasi E, Kaiser N. Diabetes. 1999;48:738–744. doi: 10.2337/diabetes.48.4.738. [DOI] [PubMed] [Google Scholar]
- 10.Roos M D, Xie W, Su K, Clark J A, Yang X, Chin E, Paterson A J, Kudlow J E. Proc Assoc Am Physicians. 1998;110:422–432. [PubMed] [Google Scholar]
- 11.Hanover J A, Lai Z, Lee G, Lubas W A, Sato S M. Arch Biochem Biophys. 1999;362:38–45. doi: 10.1006/abbi.1998.1016. [DOI] [PubMed] [Google Scholar]
- 12.Herr R R, Jahnke J K, Argoudelis A D. J Am Chem Soc. 1967;89:4808–4809. doi: 10.1021/ja00994a053. [DOI] [PubMed] [Google Scholar]
- 13.Hart G W. Annu Rev Biochem. 1997;66:315–335. doi: 10.1146/annurev.biochem.66.1.315. [DOI] [PubMed] [Google Scholar]
- 14.Snow C M, Senior A, Gerace L. J Cell Biol. 1987;104:1143–1156. doi: 10.1083/jcb.104.5.1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lubas W A, Smith M, Starr C M, Hanover J A. Biochemistry. 1995;34:1686–1694. doi: 10.1021/bi00005a025. [DOI] [PubMed] [Google Scholar]
- 16.Han I-O, Kudlow J E. Mol Cell Biol. 1997;17:2550–2558. doi: 10.1128/mcb.17.5.2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Su K, Roos M D, Yang X, Han I, Paterson A J, Kudlow J E. J Biol Chem. 1999;274:15194–15202. doi: 10.1074/jbc.274.21.15194. [DOI] [PubMed] [Google Scholar]
- 18.Roos M D, Su K, Baker J R, Kudlow J E. Mol Cell Biol. 1997;17:6472–6480. doi: 10.1128/mcb.17.11.6472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hart G W, Greis K D, Dong L Y, Blomberg M A, Chou T Y, Jiang M S, Roquemore E P, Snow D M, Kreppel L K, Cole R N, et al. Adv Exp Med Biol. 1995;376:115–123. [PubMed] [Google Scholar]
- 20.Patti M E, Virkamaki A, Landaker E J, Kahn C R, Yki-Jarvinen H. Diabetes. 1999;48:1562–1571. doi: 10.2337/diabetes.48.8.1562. [DOI] [PubMed] [Google Scholar]
- 21.Chin E, Michels K, Bondy C A. J Clin Endocrinol Metab. 1994;78:156–164. doi: 10.1210/jcem.78.1.7507121. [DOI] [PubMed] [Google Scholar]
- 22.Stagg A R, Fleming J C, Baker M A, Sakamoto M, Cohen N, Neufeld E J. J Clin Invest. 1999;103:723–729. doi: 10.1172/JCI3895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sayeski P P, Kudlow J E. J Biol Chem. 1996;271:15237–15243. doi: 10.1074/jbc.271.25.15237. [DOI] [PubMed] [Google Scholar]
- 24.Hanahan D. Nature (London) 1985;315:115–122. doi: 10.1038/315115a0. [DOI] [PubMed] [Google Scholar]
- 25.Like A A, Rossini A A. Science. 1976;193:415–417. doi: 10.1126/science.180605. [DOI] [PubMed] [Google Scholar]
- 26.McKnight G L, Mudri S L, Mathewes S L, Traxinger R R, Marshall S, Sheppard P O, O'Hara P J. J Biol Chem. 1992;267:25208–25212. [PubMed] [Google Scholar]
- 27.Roos M D, Han I-O, Paterson A J, Kudlow J E. Am J Physiol. 1996;270:C803–C811. doi: 10.1152/ajpcell.1996.270.3.C803. [DOI] [PubMed] [Google Scholar]
- 28.Kroncke K D, Fehsel K, Sommer A, Rodriguez M L, Kolb-Bachofen V. Biol Chem Hoppe-Seyler. 1995;376:179–185. doi: 10.1515/bchm3.1995.376.3.179. [DOI] [PubMed] [Google Scholar]
- 29.Eizirik D L, Sandler S, Ahnstrom G, Welsh M. Biochem Pharmacol. 1991;42:2275–2282. doi: 10.1016/0006-2952(91)90230-3. [DOI] [PubMed] [Google Scholar]
- 30.Turk J, Corbett J A, Ramanadham S, Bohrer A, McDaniel M L. Biochem Biophys Res Commun. 1993;197:1458–1464. doi: 10.1006/bbrc.1993.2641. [DOI] [PubMed] [Google Scholar]
- 31.Schnedl W J, Ferber S, Johnson J H, Newgard C B. Diabetes. 1994;43:1326–1333. doi: 10.2337/diab.43.11.1326. [DOI] [PubMed] [Google Scholar]
- 32.Rossini A A, Appel M C, Williams R M, Like A A. Diabetes. 1977;26:916–920. doi: 10.2337/diab.26.10.916. [DOI] [PubMed] [Google Scholar]
- 33.Brosky G, Logothetopoulos J. Diabetes. 1969;18:606–611. doi: 10.2337/diab.18.9.606. [DOI] [PubMed] [Google Scholar]
- 34.Efanova I B, Zaitsev S V, Zhivotovsky B, Kohler M, Efendic S, Orrenius S, Berggren P O. J Biol Chem. 1998;273:33501–33507. doi: 10.1074/jbc.273.50.33501. [DOI] [PubMed] [Google Scholar]
- 35.Unger R H, Grundy S. Diabetologia. 1985;28:119–121. doi: 10.1007/BF00273856. [DOI] [PubMed] [Google Scholar]
- 36.Leahy J L, Cooper H E, Deal D A, Weir G C. J Clin Invest. 1986;77:908–915. doi: 10.1172/JCI112389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rossetti L, Giacarri A, DeFronzo R A. Diabetes Care. 1990;13:610–630. doi: 10.2337/diacare.13.6.610. [DOI] [PubMed] [Google Scholar]
- 38.Lubas W A, Frank D W, Krause M, Hanover J A. J Biol Chem. 1997;272:9316–9324. doi: 10.1074/jbc.272.14.9316. [DOI] [PubMed] [Google Scholar]
- 39.Jackson S P, Tjian R. Cell. 1988;55:125–133. doi: 10.1016/0092-8674(88)90015-3. [DOI] [PubMed] [Google Scholar]
- 40.Wang J, Liu R, Hawkins M, Barzilai N, Rossetti L. Nature (London) 1998;393:684–688. doi: 10.1038/31474. [DOI] [PubMed] [Google Scholar]
- 41.Kolm-Litty V, Sauer U, Nerlich A, Lehmann R, Schleicher E D. J Clin Invest. 1998;101:160–169. doi: 10.1172/JCI119875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pugh B F, Tjian R. Genes Dev. 1991;5:1935–1945. doi: 10.1101/gad.5.11.1935. [DOI] [PubMed] [Google Scholar]
- 43.Lopes U G, Erhardt P, Yao R, Cooper G M. J Biol Chem. 1997;272:12893–12896. doi: 10.1074/jbc.272.20.12893. [DOI] [PubMed] [Google Scholar]
- 44.Hupp T R, Meek D W, Midgley C A, Lane D P. Cell. 1992;71:875–886. doi: 10.1016/0092-8674(92)90562-q. [DOI] [PubMed] [Google Scholar]
- 45.Shaw P, Freeman J, Bovey R, Iggo R. Oncogene. 1996;12:921–930. [PubMed] [Google Scholar]
- 46.Burkart V, Wang Z Q, Radons J, Heller BBB, Herceq Z, Stingl L, Wagner E F, Kolb H. Nat Med. 1999;5:314–319. doi: 10.1038/6535. [DOI] [PubMed] [Google Scholar]
- 47.Masutani M, Suzuki H, Kamada N, Watanabe M, Ueda O, Nozaki T, Jishage K, Watanabe T, Sugimoto T, Hakagama H, et al. Proc Natl Acad Sci USA. 1999;96:2301–2304. doi: 10.1073/pnas.96.5.2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pieper A A, Brat D J, Krug D K, Watkins C C, Gupta A, Blackshaw S, Verma A, Wang Z Q, Snyder S H. Proc Natl Acad Sci USA. 1999;96:3059–3064. doi: 10.1073/pnas.96.6.3059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Simbulan-Rosenthal C M, Rosenthal D S, Luo R, Smulson M E. Cancer Res. 1999;59:2190–2194. [PubMed] [Google Scholar]