Abstract
It was believed that tumors originated from the transformation of their tissue-specific stem cells. However, bone marrow-derived cells (BMDCs), which possess an unexpected degree of plasticity and often reside in other tissues, might also represent a potential source of malignancy. To study whether BMDCs play a role in the source of other tumors, BMDCs from mice were treated with 3-methycholanthrene until malignant transformation was achieved. Here we show that transformed BMDCs could form many tumor types, including epithelial tumors, neural tumors, muscular tumors, tumors of fibroblasts, blood vessel endothelial tumors, and tumors of poor differentiation in vivo. Moreover, a single transformed BMDC has the ability to self-renew, differentiate spontaneously into various types of tumor cells in vitro, express markers associated with multipotency, and form teratoma in vivo. These data suggest that multipotent cancer stem cells seemed to originate from transformed BMDCs. Conclusively, these findings reveal that BMDCs might be a source of many tumor types, even teratoma. In addition, multipotent cancer stem cells might originate from malignant transformed BMDCs.
Keywords: Bone marrow-derived cells, source of tumor, multiple tumor types, cancer stem cells, multipotency
Introduction
It was believed that tumors originated from the transformation of their tissue-specific stem cells. The discovery of multipotent progenitor cells with the capacity for self-renewal (stem cells) outside the hematopoietic system raises the possibility that cancer stem cells could arise from other tissue stem cells and initiate other cancer types, including solid cancers [1]. Experimental evidence regarding tissue-specific stem cells contributing to tumors other than the tissue of origin did not exist until Houghton et al. [2] found that gastric cancers could originate from bone marrow-derived sources and indicated that mesenchymal stem cell (MSC) was the most likely candidate. However, it remained unclear whether cancer types, except for gastric cancer, could arise from other tissue stem cells. Recent studies [3,4] have indicated that MSCs in the bone marrow can be transformed into cancer cells. In addition, bone marrow-derived cells (BMDCs), which possess an unexpected degree of plasticity and often reside in other tissues [5–7], might also represent a potential source of malignancy. Therefore, we hypothesized that BMDCs were likely to be a source of many tumor types.
To study this hypothesis, we treated BMDCs with 3-methycholanthrene (MCA), a potent carcinogen, to derive transformed BMDCs. Then we studied the characteristics of tumorigenesis, and the morphology and differentiation of transformed BMDCs. We found that transformed BMDCs could give rise to different tumor types in vitro and in vivo. Our findings further support the idea that tumors can originate from transformed BMDCs.
Materials and Methods
Isolation and Culture of BMDCs from the Bone Marrow of Mice
Bone marrow was isolated from the leg bones of 3- to 4-week-old mice (male Kuming mouse). BMDCs were isolated by culturing whole marrow in knockout Dulbecco's modified Eagle's medium (DMEM; Invitrogen, Carlsbad, CA) containing 10% fetal bovine serum (FBS; PAA Laboratory, Linz, Austria) and 1% L-glutamine for 2 days, discarding nonadherent cells and retaining adherent cells as BMDCs [7]. BMDCs were selected by their plastic adherence and rapid proliferation. When cultures reached confluence, the medium was changed to DMEM (Invitrogen), with low glucose containing 10% FBS.
Induction of BMDCs Treated with MCA
Primary BMDCs were cultured for 6 to 8 days in DMEM containing 10% FBS and 1% L-glutamine. Secondary passages were randomly divided into two groups. One group of secondary BMDCs was treated with 1 µg/ml MCA (Sigma, St. Louis, MO) in culture medium (DMEM containing 20% FBS and 1% L-glutamine) for a week. The medium was changed twice weekly. MCA was dissolved in dimethyl sulfoxide (DMSO; Sigma) to yield a stock solution of 200 µg/ml, which was stored in the dark at 4°C [8]. It was added to the medium so that the final concentration of DMSO was 0.5%. The other group was treated with 0.5% DMSO as control.
Test of Malignant Transformation
Transformed cells were tested for malignancy by the examination of piled-up colonies on an inverted microscope stained with Giemsa. The piled-up colonies of transformed cells heavily were stained with Giemsa, whereas control cells remained as monolayers and were lightly stained. About 4000 cells were added to a semisolid medium containing 2.7% methyl cellulose (Sigma), DMEM, and 10% FBS. After 8 weeks, the colonies with 16 cells or more were counted.
One hundred of thousands of transformed cells were injected subcutaneously into 4-week-old immune-deficient mice (female nude mice). Some mice were injected with control BMDCs pretreated with DMSO only. Most tumors appeared in the mice within 8 weeks. Two of these primary tumors were excised from the mice and injected into secondary mice.
Tissue Collection and Analysis
Mice were killed at 6 to 8 weeks. The tumors were removed and fixed in 10% neutral-buffered formalin for 24 hours, then embedded in paraffin wax for analysis. Tumors were sectioned at 4 µm thickness and were stained using routine hematoxylin and eosin (H&E).
Fluorescence In Situ Hybridization (FISH)
To identify donor cells in a recipient mouse, in situ hybridization was performed on a tissue section for the detection of the Y chromosome, following the manufacturer's protocol. In brief, the tissue section was treated with alcohol at 65°C for 1 hour and digested with 200 µg/ml proteinase K. The section was quenched with protease and dehydrated through graded alcohols before air drying. The section was denatured by incubating in a denaturation solution at 85°C for 5 minutes, dehydrated with graded alcohols, and dried at room temperature. A fluorescein isothiocyanate (FITC)-labeled Y-chromosome paint (star-FISH; Cambio, Cambridge, UK) was used in the supplier's hybridization mix. A probe mixture was incubated at 65°C for 10 minutes and held for 30 minutes at 37°C before being added to the section, and then incubated overnight at 37°C. The section was washed with 2x standard saline citrate (SSC) containing 50% formamide then washed with 1x SSC. Sections were counterstained with 4,6-diamidino-2-phenylindole (DAPI; Invitrogen) before viewing. The hepatic tissue of a female mouse was used as a negative control.
Histology and Immunohistochemistry (IHC)
IHC was performed in combination with various immunostains to identify tumor types. Immunocytochemistry was carried out using standard protocol. Primary antibodies were diluted as follows: vimentin (Vim, 7 µg/ml; Chemicon, Temecula, CA), desmin (Des, 1:150; Chemicon), α-smooth muscle actin (α-SMA, 5 µg/ml; Chemicon), pan-cytokeratin (pan-CK, 1:400; Sigma), epithelial membrane antigen (EMA, clone MH1, 1:200; Lab Vision, Fremont, CA), S-100 (1:120; Chemicon), microtubule-associated protein-2a + b (Map2a + b, 1:100; Chemicon), neurofilament (NF), glial fibrillary acidic protein (GFAP, 1:100; Zymed, San Francisco, CA), von Willebrand factor (vWF, 1:400; Abcam, Cambridge, UK), and neuron-specific enolase (NSE, 1:200; Dako). Immunodetection was performed using SP kit (Zymed). The counterstain of preference for nuclear details was hematoxylin.
Isolation of a Single Cell
Transformed BMDCs were serially diluted into an expansion medium in 96-well plates so that only a single cell was expanded.
Indirect Cytoimmunofluorescence
Transformed BMDCs from the colony of single cells were plated at 5 x 104 cells/well and grown on coverslips (placed at the bottom of the well) overnight, fixed with acetone, and incubated with various primary antibodies for 1 hour at room temperature. Primary antibodies included the following: Des (1:150; Chemicon), α-SMA (5 µg/ml; Chemicon), vWF (1:400; Abcam), pan-CK (1:500; Sigma), GFAP (1:100; Zymed), NF (1:100; Sigma), Vim (7 µg/ml; Chemicon), and Map2a + b (1:100; Chemicon). Secondary goat anti-mouse or rabbit antibodies conjugated to Cy5 (1:400; Jackson, West Grove, PA), FITC (1:300; Chemicon), or Cy3 (1:400; Jackson) fluorescent dyes were used for detection. Cell nuclei were stained with DAPI.
RNA Isolation and Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
Total RNA was extracted from cells using TRIzol (Invitrogen). Before the synthesis of first-strand cDNA, total RNA was treated with RNase-free DNase I (Invitrogen) to eliminate any residual genomic DNA. The mixture of total RNA was reverse-transcribed using cDNA synthesis kit (Invitrogen). The cDNA was amplified with forward (cag aag agg atc acc ttg gg) and reverse (gtg agt gat ctg ctg tag gg) primers to detect OCT4; with forward (acc tac agc atg tcc tac tcg) and reverse (ggg cag tgt gcc gtt aat gg) primers to detect SOX2; and with forward (agg gtc tgc tac tga gat gct ctg) and reverse (caa cca ctg gtt ttt ctg cca ccg) primers to detect NANOG, whereas the internal-control detection of β-actin was performed with forward (tcg tcg aca acg gct ccg gca tgt) and reverse (cca gcc agg tcc aga cgc agg at) primers. In the case of OCT4, PCR conditions were as follows: 30 cycles of 94°C for 30 seconds; 60°C for 1 minute; 72°C for 1 minute; final extension at 72°C for 5 minutes. In the case of SOX2, PCR conditions were as follows: 28 cycles of 94°C for 30 seconds; 56°C for 1 minute; 72°C for 1 minute; final extension at 72°C for 5 minutes. In the case of NANOG, PCR conditions were as follows: 28 cycles of 94°C for 28 seconds; 55°C for 1 minute; 72°C for 1 minute; final extension at 72°C for 5 minutes. In the case of β-actin, PCR conditions were as follows: 28 cycles of 94°C for 30 seconds; 62°C for 1 minute; 72°C for 1 minute; final extension at 72°C for 5 minutes. PCR products were electrophoresed on a 1.5% agarose gel and visualized by ethidium bromide staining. Primary BMDCs without treatment were used as negative control.
Results
Phenotypic Characterization of BMDCs
BMDCs were obtained from the bone marrow of 4-week-old male mice and were isolated based on their plastic adherence [7]. Hematopoietic stem cells (HSCs) and other nonadherent cells were removed with changes in the medium. BMDCs displayed a small round, spindle-shaped, or flat polygonal morphology (Figure 1A).
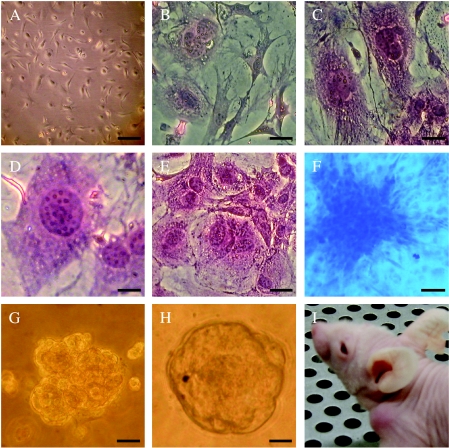
Figure 1.
Detection of malignant transformation. (A) Morphologic characteristics of BMDCs before treatment. (B–I) BMDC treatment with MCA produced characteristics of malignant transformation. (B–E) Morphologic changes of treated BMDCs (stained with H&E). (B) Cellular and nuclear atypia. (C) Nuclear atypia. (D) The number of nucleolus increased. (E) Multinuclear giant cell. (F) Treated BMDCs formed piled-up foci (stained with Giemsa). (G and H) Treated BMDCs formed clones in the semisolid medium containing 2.7% methyl cellulose. (I) Treated BMDCs formed a tumor in the subcutaneous layer. Scale bars: (A) 40 µm; (B, C, E–H) 20 µm; (D) 10 µm.
BMDCs Undergo Malignant Transformation
About 8 weeks after treatment with MCA, the cells demonstrated properties of malignant transformation. The cells showed morphologic changes (Figure 1, B–E), rapid growth, anchorage independence (Figure 1F), loss of contact inhibition (Figure 1, G and H), and loss of polarity. We also analyzed the clonal frequency of transformed BMDCs in the semisolid medium containing 2.7% methyl cellulose and found that about 18 of 4000 total transformed cells gave rise to colonies within 8 weeks. To determine tumorigenic ability, transformed BMDCs were injected subcutaneously into 4-week-old immune-deficient mice (female nude mice) and formed tumors (Figure 1I) in 33 of 33 mice. Every mouse formed tumors in the position of injection of transformed cells. Most tumors appeared in the mice within 6 weeks. Tissue sections showed properties of malignant tumors, such as cellular atypia, tumor giant cells, and pathological mitosis. In some cases, invasive growth into skeletal muscles and fatty tissues was observed. Two of these primary tumors were excised from mice and injected into secondary mice to examine their ability to self-renew. After 2 weeks, two of two injections gave rise to tumors. The secondary tumors resembled the original tumors, whereas the group of injected mice that underwent BMDC treatment with DMSO did not form tumors until their death.
These findings suggested that transformed cells possessed all the characteristics of malignant transformed cells, especially the capacity to self-renew and to form tumors. Therefore, BMDCs transformed into cancer cells. These results were reproducible. We derived transformed BMDCs in three independent experiments.
Transformed BMDCs Produce Different Tumor Types In Vivo
Tumor tissue sections were analyzed by histology and immunochemistry to identify tumor types. Immunochemically, the tumors were tested for different tissue-specific markers, including epithelial, neural, and mesenchymal markers (Figure W1). Immunochemical results showed that these tumors expressed different antigen profiles (Table 1). Tumors expressed markers of mesenchymal tissues (Vim, Des, α-SMA, and vWF), epithelial tissues (pan-CK and EMA), or neural tissues (NF, GFAP, S-100, and Map2a + b). Some tumors contained more than one histologic type of cancer tissue and expressed markers of different tissues simultaneously. There were also tumors that were poorly differentiated and did not express any antigens that had been tested (Table 1). According to histologic characteristics and immunochemical data (Figure 1), these tumors were divided into muscular tumors, blood vessel endothelial tumors, tumors of fibroblasts, epithelial tumors, neural tumors, and tumors of poor differentiation (Figure 2, A–H).
Table 1.
IHC Showing the Expression of Tissue-Specific Markers in Tumors Expressed in Different Antigen Profiles.
| Tumors (n) | Vim | Des | SMA | F VIII | Pan-CK | EMA | S-100 | GFAP | NF | Map2 |
| 8 | + | + | ± | - | + | ± | - | - | - | - |
| 2 | + | - | - | - | + | ± | - | - | - | - |
| 3 | - | - | - | - | + | ± | - | - | - | - |
| 1 | + | + | - | + | - | - | - | - | - | - |
| 3 | + | + | ± | - | ± | - | + | - | + | + |
| 2 | + | - | - | - | - | - | + | + | + | + |
| 5 | + | + | - | - | - | - | - | - | - | - |
| 3 | + | - | - | - | - | - | - | - | - | - |
| 6 | + | - | - | - | - | - | - | - | - | - |
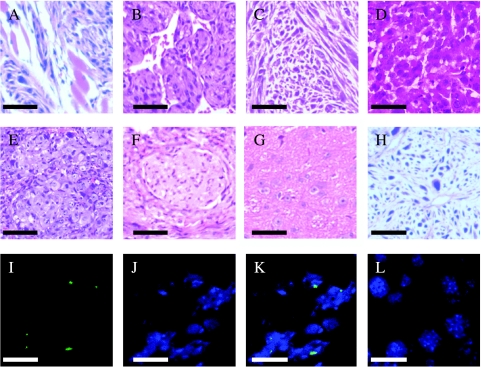
Figure 2.
(A–H) The sections showed different tumor types (stained with H&E). Based on phenotypic characterization and immunochemical analysis, the sections were divided into different tumor tissue types. (A) Leiomyoma. (B) Hemangioendothelioma. (C) Fibrosarcoma. (D) Sarcomatoid carcinoma. (E) Epithelial tumor. (F) Neurilemoma. (G) Mixed tumor of neurocyte and gliacyte. (H) Tumor of poor differentiation. (I–K) FISH-confirmed tumors derived from donor cells (green dot in nuclei). (I) The Y chromosome (FITC-conjugated; green). (J) DAPI staining of cell nuclei (blue). (K) The overlay of the Y chromosome (green dot) within the nuclei (blue). (L) The FISH result for the Y chromosome, using the hepatic tissue of the female mouse that was used as control, was negative. Scale bars: (A–F, H) 100 µm; (G) 25 µm; (I–L) 10 µm.
Tumors were identified as donor-derived by the presence of the Y chromosome in female mice. Tumors from male-to-female transplants showed Y chromosomes (green) within the nuclei (blue) (Figure 2, I–L). The results confirmed that these tumors derived from donor cells of transformed BMDCs.
These results demonstrated that transformed cells produced various tumor tissue types during the course of tumor formation. Consequently, our results suggested that transformed BMDCs could give rise to different tumor types in vivo.
Transformed BMDCs Produce Many Types of Tumor Cells In Vitro
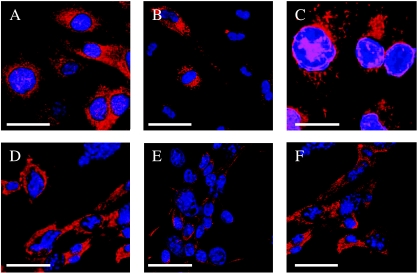
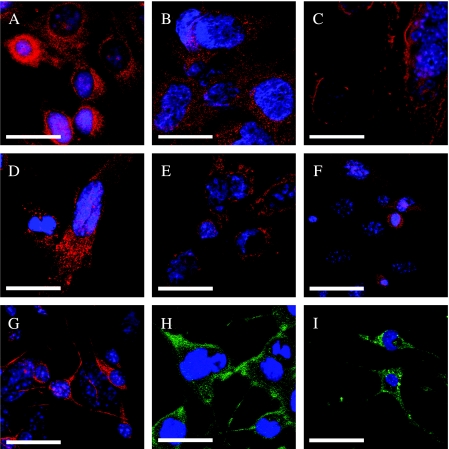
During the routine passage of transformed cells, more than one cell phenotype were frequently observed in culture. We studied the differentiation of transformed BMDCs by testing for tissue-specific markers. The cells stained positively for antigens of pan-CK, vWF, GFAP, Vim, NF, or Des (Figure 3). These data showed that transformed BMDCs differentiated spontaneously into epithelial tumor cells, blood vessel endothelial tumor cells, glial tumor cells, neuronal tumor cells, and muscular tumor cells in vitro. Multiple differentiations of transformed BMDCs were subjected to standard culture conditions (DMEM and 10% FBS). Therefore, transformed BMDCs also possessed the ability to produce various tumor cells in vitro.
Figure 3.
In vitro-transformed cells from BMDCs were tested for the ability to differentiate into multiple lineages at single-cell level. Cultures were examined with indirect immunofluorescence confocal microscopy of differentiated cells decorated with antibodies against pan-CK vWF, Des, NF, GFAP, and Vim. (A) Anti-pan-CK labeled with Cy5. (B) Anti-vW-Cy3. (C) Anti-Des-Cy3. (D) Anti-Vim-Cy5. (E) Anti-GFAP-Cy3. (F) Anti-NF-Cy5. Scale bar: 10 µm.
Transformed BMDCs not only could give rise to various types of tumor cells in vitro but also could undergo in vivo differentiation to produce many tumors similar to those in diseases of mouse tumors. Therefore, the findings demonstrated that various tumor types could originate from BMDCs.
However, what kind of cells in transformed BMDCs is associated with the formation of so many tumor types? Many researches have shown that cancer stem cells, which form only a small proportion of the total tumor cell population, were the only tumor cells with the capacity to keep tumors growing [9–11]. Moreover, the experiments here showed that the clonal frequency of transformed BMDCs in the semisolid medium containing 2.7% methyl cellulose was only 18 of 4000. It means that not all transformed BMDCs had the ability to propagate. Therefore, animal implant and in vitro studies showed that transformed BMDCs could give rise to multiple tumor types, suggesting that there were either multipotent cancer stem cells or mixtures of committed progenitor cells with restricted potential [12,13] in transformed BMDCs. We wanted to know whether a multipotent cancer stem cell presented in transformed cells is responsible for these tumor types. If it were true that there existed multipotent cancer stem cells in transformed BMDCs, some single cells should produce multiple tumor types.
Transformed BMDCs Exhibit Properties of Multiple Cancer Stem Cells in Culture
Transformed BMDCs were serially diluted into an expansion medium in 96-well plates so that only a single cell was expanded. Clonal transformed BMDCs were derived from a single cell.
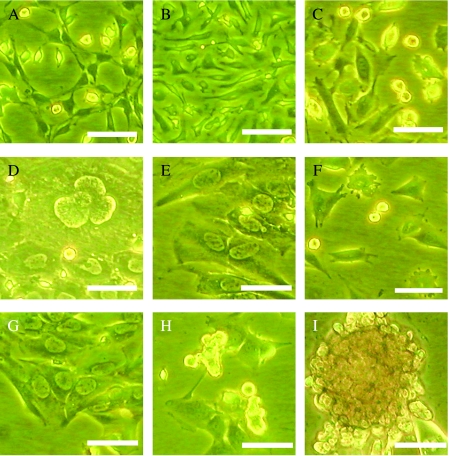
We found that the cells that expanded from a single cell showed various phenotypes (Figure 4), as well as expressed different tissue-specific antigens in vitro (Figure 5). Thus, the results suggested that a single cell could produce various types of tumor cells in vitro. In addition, a single cell has been passaged up to 10 times to date without diminution of its colony-forming ability. To our interest, some cells can grow as multicellular spheroids after they had been cultured for about 2 weeks (Figure 4I). Together, transformed BMDCs at the single-cell level that possess the ability for self-renewal and multiple differentiation indicated that multipotent stem cells seemed to present in transformed BMDCs.
Figure 4.
Phase-contrast micrographs of a single cell's derivatives. The single cell produced various tumor cells in a dish. The cells showed different phenotypes in culture. (A) Neural-like cells. (B) Spindle-shaped cells. (C) Small round cells and spindle-shaped cells. (D) Flat polygonal cells. (E) Flat polygonal cells. (F) Small round cells, spindle-shaped cells, and epithelial-like cells. (G) Flat polygonal cells. (H) Spheroids of small round cells. (I) Multicellular spheroids formed after a 2-week culture. Scale bar: 20 µm.
Figure 5.
Marker expression of the differentiated progeny from single transformed BMDCs. (A) Anti-pan-CK labeled with Cy5. (B) Anti- Vim-Cy5. (C) Anti-Des-Cy3. (D)Anti-SMA-Cy3. (E) Anti-vW-Cy3. (F) Anti-Nestin-Cy3. (G) Anti-GFAP-Cy3. (H) Anti-NF-FITC. (I) Anti-Map2-FITC. Scale bar: 10 µm.
Clonal Transformed BMDCs Express Genes Related to Multipotency In Vitro
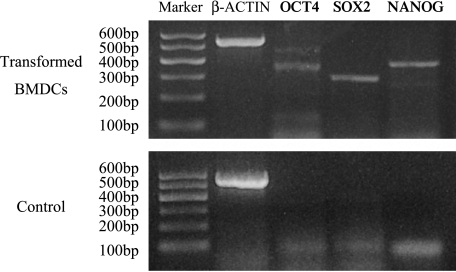
Critical transcription factors, notably OCT4, SOX2, and NANOG, are necessary to maintain self-renewal and pluripotency—two characteristics of embryonic stem (ES) cells [14,15]. Thus, the expressions of OCT4, SOX2, and NANOG are specific markers associated with pluripotency and self-renewal [14,15]. To further analyze the multipotency of clonal transformed BMDCs, we examined their expression of OCT4, SOX2, and NANOG. RT-PCR results showed that clone-transformed BMDCs expressed the genes of OCT4, SOX2, and NANOG (Figure 6). The expression of three markers indicated a possible multipotent nature of transformed BMDCs. This finding further supported that some transformed BMDCs possessed the abilities of self-renewal and pluripotency in vitro.
Figure 6.
RT-PCR analysis of gene expression related to multipotency and self-renewal in clonal transformed BMDCs. β-Actin band, 520 bp; OCT4 band, 324 bp; SOX2 band, 276 bp; and NANOG band, 364 bp. Primary BMDCs were used as controls.
Clonal Transformed BMDCs Form Various Tumors In Vivo
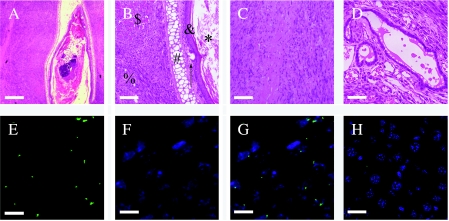
To examine whether clonal transformed BMDCs possessed the ability for self-renewal and pluripotency in vivo, clonal transformed BMDCs were injected subcutaneously into 4-week-old immune-deficient mice (female nude mice) and formed tumors in five of five mice. Every mouse formed tumors in the position of injection of the cells. Another five mice injected primary BMDCs without treatment, and no tumors were found until the mice had died. Histologic examination revealed that one of five tumors contained three germ layers, two contained two germ layers, one contained neural tumor cells, and one had low differentiation (Table 2). The tumor containing three germ layers showed properties typical of teratoma. The teratoma contained the derivatives of all three germ layers, including muscles, cartilages, keratinocytes, squamous epithelium, glandular epithelium, sebaceous glands, tissues of glia, and tissues of neural fibers (Figure 7; and W2). These findings showed that clonal transformed BMDCs possessed the abilities for self-renewal, pluripotency, and tumorigenesis. In vivo studies further confirmed that some cells in transformed BMDCs functioned as multiple cancer stem cells.
Table 2.
Types of Tumor Produced by Clonal Transformed BMDCs In Vivo.
| Tumors (n) | Type of Tumor Tissues | Vim | Des | Pan-CK | EMA | S-100 | GFAP | NF | Map2 |
| 1 | Muscular and epithelial tissues | + | + | + | + | - | - | - | - |
| 1 | Epithelial and neural tissues | + | + | - | - | + | + | + | + |
| 1 | Three germ layers | + | + | + | + | + | + | + | + |
| 1 | Neural tissues | - | - | - | - | + | + | + | + |
| 1 | Low-differentiation tissues | - | - | - | - | - | - | - | - |
Figure 7.
Histology of differentiated elements found in the subcutaneous teratoma after the inoculation of clonal transformed BMDCs. (A) Squamous epithelium, keratinocytes, cartilages, muscles, neural fibers, and glia. (B and C) Magnification of (A). (B) $Muscle; %tissue of glia; #cartilage; &squamous epithelium; ↑ sebaceous gland; *keratinocytes. (C) Tissue of neural fibers. (D) Glandular epithelium. (E–H) FISH confirms the teratoma derived from donor cells (green dot in nuclei). (E) The Y chromosome (FITC-conjugated; green). (F) DAPI staining of cell nuclei (blue). (G) The overlay of the Y chromosome (green dot) within the nuclei (blue). (H) The FISH result for the Y chromosome, using the hepatic tissue of the female mouse that was used as control, was negative. Scale bars: (A) 250 µm; (B–D) 100 µm; (E–G) 10 µm; (H) 20 µm.
The three aspects of experiments demonstrated fully that transformed BMDCs contained multiple cancer stem cells, which answers why transformed BMDCs gave rise to different tumor types. Therefore, the presence of multiple cancer stem cells further supported the hypothesis that BMDCs might be a source of various tumor types.
Discussion
In the present study, malignant transformed BMDCs were induced by MCA. Our experiments indicated that transformed BMDCs could produce various types of tumors in vivo (e.g., epithelial tumors, muscular tumors, tumors of fibroblasts, neural tumors, blood vessel endothelial tumors, tumors of poor differentiation, mixed tumors, and teratomas). These results demonstrated that many tumor types could originate from BMDCs. Houghton et al. [2] found that gastric cancers can arise from BMDCs. Our experiments were similar to the findings of Houghton et al. and extended the concept that tumors could originate from other tissue cells.
The passage of transformed BMDCs produced various types of tumor cells in vitro and, when inoculated in nude mice, these cells gave rise to different tumor types in vivo. Therefore, it was possible that multipotent stem cells that existed in transformed BMDCs could produce many types of cancer cells. Of course, it was also possible that there were populations that were mixtures of committed stem cells, each with restricted potential in transformed BMDCs. Our experiments revealed that multipotent stem cells seemed to exist in transformed BMDCs because transformed BMDCs at the single-cell level could: 1) self-renew; 2) differentiate into various types of tumors cells in vitro; 3) express markers associated with multipotency; and 4) form teratomas containing three germ layers in vivo. The discovery of multiple cancer stem cells provided an idea that some tumors containing multiple germ layers might relate to multiple cancer stem cells. Although our studies indicated that multipotent cacer stem cells were present in transformed BMDCs, further studies in this field will be needed to know fully the characteristics of multiple cancer stem cells.
Teratomas could be derived from transformed BMDCs, providing evidence of a possible origin of teratomas from BMDCs. However, teratomas were usually believed to originate from ES cells. For the first time, we reported that teratomas also could originate from adult cells or adult stem cells.
The experiments here showed that transformed BMDCs possessed a multipotent nature and an ability for self-renewal. These properties may be inherent in BMDCs, rather than acquired over time, indicating that multipotent cancer stem cells derived seemingly from some undifferentiated cells or multipotent progenitor cells. As we know, adult BMDCs include two populations of bone marrow stem cells: HSCs and MSCs [16]. First, the cell culture condition we used was not compatible with HSCs. Second, cultured BMDCs were identical to MSCs in terms of phenotype [5,7]. In addition, MSCs have been shown to differentiate into many different cell lineages [5–7,17,18]. Finally, recent in vitro studies suggested that adult MSCs might be a target for neoplastic transformation [3,4]. Hence, MSCs in BMDCs were the most likely candidates responsible for cancer stem cells in transformed BMDCs. However, all features of transformed BMDCs did not ascribe to MSCs in BMDCs. The BMDCs seemed to obtain some properties of embryonic cells during the course of transformation and long cultures, which were inherent to some cells in BMDCs, because the primary BMDCs did not show the same nature in the present experiments. Of course, it was also possible that there were more immature stem cells in the bone marrow [19] that might contribute to multiple cancer stem cells.
Cell fusion has recently been suggested as an explanation for stem cell plasticity [20,21]. Our case cannot be explained by the same mechanism. Because transformed BMDCs have never been cocultured with tissue-specific cells or ES cells (which differentiate into cells of three germ layers), the in vitro multiple differentiation potential of transformed BMDCs cannot be attributed to cell fusion. Moreover, transformed BMDCs could form teratomas after subcutaneous transplantation.
In conclusion, these findings suggest that various tissue tumors may originate from BMDCs. Multipotent cancer stem cells were involved in transformed BMDCs, contributing to various tumor types. For the first time, we show that teratomas also can originate from adult cells or adult stem cells. These concepts will help us to better understand the origin of tumors and to develop more effective treatments.
Supplementary Material
Acknowledgements
We thank Xiaofei Jiang, Min Li, Weiwei Liu, and Yong Lin for encouragement and support, and Ming Xu for critical reading and editing of the manuscript. We are grateful to Chengyong Shen and Kejing Zhang for helpful discussions. We also thank Xiaoyan Ding and the personnel of the Laboratory of Cell Biology, Shanghai Institutes for Biochemistry and Cell Biology, for excellent support.
Footnotes
This work was supported by a grant from the Shanghai Medical Key Discipline.
This article refers to supplementary materials, which are designated by “W” (i.e., Figures W1 and W2) and are available online at www.bcdecker.com.
References
- 1.Beachy PA, Karhadkar SS, Berman DM. Tissue repair and stem cell renewal in carcinogenesis. Nature. 2004;432:324–337. doi: 10.1038/nature03100. [DOI] [PubMed] [Google Scholar]
- 2.Houghton J, Stoicov C, Nomura S, Rogers AB, Carlson J, Li H, Cai X, Fox JG, Goldenring JR, Wang TC. Gastric cancer originating from bone marrow-derived cells. Science. 2004;306:1568–1571. doi: 10.1126/science.1099513. [DOI] [PubMed] [Google Scholar]
- 3.Serakinci N, Guldberg P, Burns JS, Abdallah B, Schrodder H, Jensen T, Kassem M. Adult human mesenchymal stem cell as a target for neoplastic transformation. Oncogene. 2004;23:5095–5098. doi: 10.1038/sj.onc.1207651. [DOI] [PubMed] [Google Scholar]
- 4.Rubio D, Garcia-Castro J, Martin MC, de la Fuente R, Cigudosa JC, Lloyd AC, Bernad A. Spontaneous human adult stem cell transformation. Cancer Res. 2005;65:3035–3039. doi: 10.1158/0008-5472.CAN-04-4194. [DOI] [PubMed] [Google Scholar]
- 5.Krause DS, Theise ND, Collector MI, Henegariu O, Hwang S, Gardner R, Neutzel S, Sharkis SJ. Multi-organ, multi-lineage engraftment by a single bone marrow-derived stem cell. Cell. 2001;105:369–377. doi: 10.1016/s0092-8674(01)00328-2. [DOI] [PubMed] [Google Scholar]
- 6.Jiang Y, Jahagirdar BN, Reinhardt RL, Schwartz RE, Keene CD, Ortiz-Gonzalez XR, Reyes M, Lenvik T, Lund T, Blackstad M, et al. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418:41–49. doi: 10.1038/nature00870. [DOI] [PubMed] [Google Scholar]
- 7.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 8.Reznikoff CA, Bertram JS, Brankow DW, Heidelberger C. Quantitative and qualitative studies of chemical transformation of cloned C3H mouse embryo cells sensitive to postconfluence inhibition of cell division. Cancer Res. 1973;33:3239–3249. [PubMed] [Google Scholar]
- 9.Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, Henkelman RM, Cusimano MD, Dirks PB. Identification of human brain tumour initiating cells. Nature. 2004;432:396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- 10.Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 11.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci. 2003;100:3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harris RG, Herzog EL, Bruscia EM, Grove JE, Van Arnam JS, Krause DS. Lack of a fusion requirement for development of bone marrow-derived epithelia. Science. 2004;305:90–93. doi: 10.1126/science.1098925. [DOI] [PubMed] [Google Scholar]
- 13.Minguell JJ, Fierro FA, Epunan MJ, Erices AA, Sierralta WD. Nonstimulated human uncommitted mesenchymal stem cells express cell markers of mesenchymal and neural lineages. Stem Cells Dev. 2005;14:408–414. doi: 10.1089/scd.2005.14.408. [DOI] [PubMed] [Google Scholar]
- 14.Boyer LA, Lee TI, Cole MF, Johnstone SE, Levine SS, Zucker JP, Guenther MG, Kumar RM, Murray HL, Jenner RG, et al. Core transcriptional regulatory circuitry in human embryonic stem cells. Cell. 2005;122:947–956. doi: 10.1016/j.cell.2005.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Orkin SH. Chipping away at the embryonic stem cell network. Cell. 2005;122:828–830. doi: 10.1016/j.cell.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 16.Grove JE, Bruscia E, Krause DS. Plasticity of bone marrow-derived stem cells. Stem Cells. 2004;22:487–500. doi: 10.1634/stemcells.22-4-487. [DOI] [PubMed] [Google Scholar]
- 17.Phinney DG, Kopen G, Isaacson RL, Prockop DJ. Plastic adherent stromal cells from the bone marrow of commonly used strains of inbred mice: variations in yield, growth, and differentiation. J Cell Biochem. 1999;72:570–585. [PubMed] [Google Scholar]
- 18.Woodbury D, Reynolds K, Black IB. Adult bone marrow stromal stem cells express germline, ectodermal, endodermal, and mesodermal genes prior to neurogenesis. J Neurosci Res. 2002;69:908–917. doi: 10.1002/jnr.10365. [DOI] [PubMed] [Google Scholar]
- 19.Kucia M, Reca R, Campbell FR, Zuba-Surma E, Majka M, Ratajczak J, Ratajczak MZ. A population of very small embryonic-like (VSEL) CXCR4(+)SSEA-1(+)Oct-4(+) stem cells identified in adult bone marrow. Leukemia. 2006;20:857–869. doi: 10.1038/sj.leu.2404171. [DOI] [PubMed] [Google Scholar]
- 20.Ying QL, Nichols J, Evans EP, Smith AG. Changing potency by spontaneous fusion. Nature. 2002;416:545–548. doi: 10.1038/nature729. [DOI] [PubMed] [Google Scholar]
- 21.Terada N, Hamazaki T, Oka M, Hoki M, Mastalerz DM, Nakano Y, Meyer EM, Morel L, Petersen BE, Scott EW. Bone marrow cells adopt the phenotype of other cells by spontaneous cell fusion. Nature. 2002;416:542–545. doi: 10.1038/nature730. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.