Abstract
Oceanic archipelagos of volcanic origin have been important in the study of evolution because they provide repeated natural experiments allowing rigorous tests of evolutionary hypotheses. Ongoing volcanism on these islands may, however, affect the evolutionary diversification of species. Analysis of population structure and phylogeographic patterns in island populations can provide insight into evolutionary dynamics on volcanic islands. We analysed genetic and morphological variation in the gecko Tarentola boettgeri on the island of Gran Canaria and compared it with Tarentola delalandii on Tenerife, a neighbouring volcanic island of similar age but distinctly different geological past. Intraspecific divergence of mitochondrial haplotypes indicates long-term persistence of Tarentola on each island, with a phylogeographic signal left by older volcanic events. More recent volcanic eruptions (approximately 0.2 million years ago on Tenerife, approximately 2.2 million years ago on Gran Canaria) have left a signature of population expansion in the population genetic structure, the strength of which depends on the time since the last major volcanic eruption on each island. While these stochastic events have left traces in morphological variation in Tenerife, in Gran Canaria geographical variation was solely associated with environmental variables. This suggests that historically caused patterns in morphology may be overwritten by natural selection within 2 million years.
Keywords: volcanism, phylogeography, geographical variation, natural selection, Canary islands, Tarentola
1. Introduction
Oceanic islands have served for more than a century as model systems for the study of the evolution of animals and plants (Williamson 1981). Importantly, oceanic archipelagos provide repeated natural experiments allowing tests of evolutionary hypotheses (Losos et al. 1998). Many oceanic archipelagos used in evolutionary studies are of volcanic origin (i.e. the Hawaiian, Galapagos, Lesser Antilles and Canary islands). It has been argued that population extinctions, recolonizations and associated bottlenecks, which may be caused by volcanism, affect the evolutionary diversification of species (Carson & Templeton 1984; Carson et al. 1990). Although it is disputed whether these population processes facilitate adaptive shifts (Barton & Charlesworth 1984), it is obvious that they can leave characteristic signatures in the genetic and morphological variation of populations. Fossil records on volcanic islands are usually scarce, thus in most cases no direct evidence of past evolutionary events is available. However, organisms may record past population decline and growth in their population structure and genetic variation (Beheregaray et al. 2003). Natural selection can rapidly alter phenotypic means in small experimental populations (Malhotra & Thorpe 1991; Losos et al. 1997). However, we have little understanding of how long it takes natural selection to alter patterns of geographical variation caused by stochastic events. Comparing geological (i.e. volcanic) records, organismal history as recorded in genetic variation and morphological variation may illuminate the time-scales over which stochastic events and natural selection play a role in shaping geographical variation.
The islands of the Canary Archipelago provide an excellent opportunity for such studies because their geological history has been studied in great detail. The two large islands in the centre of the archipelago, Tenerife and Gran Canaria, are of special interest because of their considerable geological age (more than 11 million years), their immense ecological heterogeneity and great biological diversity. Both islands are of comparable size and have been shaped by volcanic periods. Their volcanic histories, however, differ in several aspects. Tenerife is the result of a central volcano joining two or three ancient precursor islands (Ancochea et al. 1990) and has experienced major eruptions 0.2–2 million years ago (Myr) (Ancochea et al. 1990, 1999; Huertas et al. 2002). In contrast, Gran Canaria was established early as a single shield volcano, which after a long quiescent period experienced two major volcanic cycles (Rothe 1996), the Roque Nublo 3.5–4.3 Myr (Perez-Torrado et al. 1995), the Llanos de la Paz 2.2–2.9 Myr (van den Boogaard et al. 1998) and smaller volcanic eruptions localized mainly in the north east of the island during the last 1.5 million years (My).
Geckos of the genus Tarentola are wide spread throughout the Macaronesian (Canary and Madeiran Archipelago) and Cape Verde Islands. Tarentola species have colonized these islands several times from the African mainland (Nogales et al. 1998; Carranza et al. 2000, 2002). Mitochondrial sequence data suggest that populations of T. delalandii and T. boettgeri have persisted on Tenerife and Gran Canaria, respectively, for several million years (Nogales et al. 1998; Gübitz et al. 2000; Carranza et al. 2000, 2002). This long colonization history and the fact that Tarentola inhabit a variety of habitats makes them excellent systems to study the effects of historic volcanism and natural selection on island populations. On Tenerife, allopatric populations of T. delalandii on two or three precursor islands have been brought into contact by the Teide volcanism and more recent eruptions (about 0.2 Myr) may have caused population contractions into and expansions from these refugia (Gübitz et al. 2000). These historic events and natural selection have shaped the patterns of morphological variation in T. delalandii on Tenerife (Gübitz et al. 2000).
Here, we analyse the causes of phylogeographic patterns and geographical variation in morphology in T. boettgeri on the island of Gran Canaria. We show that recurrent within-island volcanism may be sufficient to cause phylogeographic patterns, with even older events leaving long-lasting signatures. While historic volcanism has left a trace in morphological variation on Tenerife, morphological variation is not associated with phylogeography on Gran Canaria but is correlated with environmental variables, indicating that it may take natural selection less than 2 My to overwrite historically caused patterns.
2. Material and methods
(a) Phylogenetic analysis
In order to reconstruct the phylogeography of T. boettgeri on Gran Canaria, we used mitochondrial cytochrome b sequence data (see Electronic Appendix), which is a particularly suitable marker for phylogeographic studies (Moore 1995; Avise 2000). Tissue samples of T. boettgeri were obtained from tail tip biopsies from 30 localities around Gran Canaria. A blood sample from T. b. bischoffi from Selvagem Grande was donated by U. Joger, Darmstadt, Germany. T. delalandii from Tenerife (Adeje and Teno) and from La Palma were used as closely related outgroups.
Phylogenetic relationships among haplotypes were reconstructed using different methods allowing the consistency of phylogenetic estimation to be evaluated (Avise 1994). Modeltest 3 was used to determine the evolutionary model that best fitted the data. Bayesian analysis was conducted using MrBayes (Huelsenbeck & Ronquist 2001) with the HKY model (Hasegawa et al. 1985) and invariant sites and gamma correction employing partitioning into codons. Maximum parsimony (MP) and maximum likelihood (ML; Felsenstein 1981) was performed with heuristic search methods in PAUP* v. 4.0b10 (Swofford 1998). Further details are available in the Electronic Appendix.
(b) Relative rate and neutrality tests
The two-cluster test (Takezaki et al. 1995) as implemented in the program Phyltest v. 2.0 (Kumar 1996) was used to examine the constancy of a molecular clock among the major lineages. This test allows the inclusion of multiple sequences representing two lineages and calculates the difference between the average branch length of each of the two compared lineages in relation to a given outgroup. Statistical testing for neutrality was conducted using Tajima's D (Tajima 1989) and Fu and Li's test statistics F and D, with and without outgroup (Fu & Li 1993), and the McDonald–Kreitman test (McDonald & Kreitman 1991) as implemented in Dnasp v. 3.0 (Rozas & Rozas 1999). Samples from the closest clade, as shown in figure 1, were used as the outgroup in these tests.
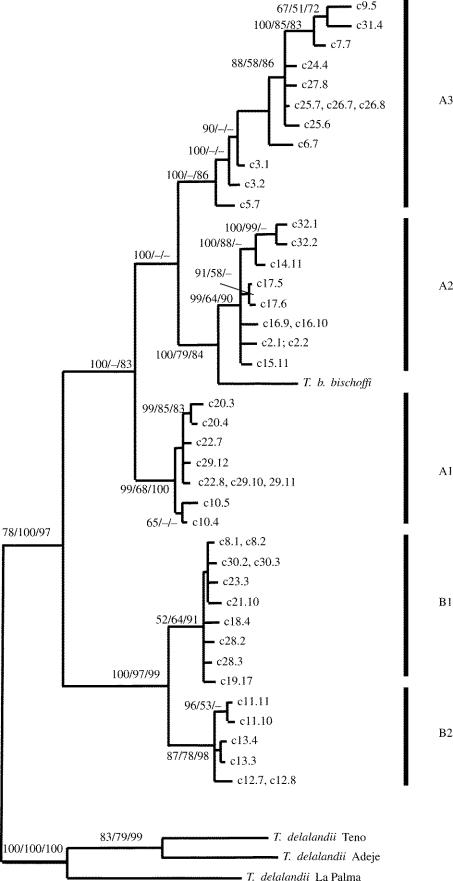
Figure 1.
Bayesian phylogram of T. boettgeri mitochondrial (mt) haplotypes. Samples from Gran Canaria are marked with ‘c’, the following number gives the locality number, the number after the dot gives specimen number. Outgroups are T. delalandii from Teno, Adeje area and La Palma. Major clades are indicated by bars. Numbers on branches (≥65) give Bayesian posterior probabilities and bootstrap values for ML and MP reconstruction, respectively.
(c) Population growth test
Population growth was evaluated using Metropolis–Hastings simulation as implemented in Fluctuate v. 1.4 (Kuhner et al. 1998). This program uses a coalescence approach to calculate ML estimates of the parameters θ (in haploids θ=2Neμ) and the exponential growth (or decline) rate g (32), where μ is the mutation rate per site and g is the exponential growth rate of the population, and the effective population size. The parameter (θ) and the time (t) ago are calculated as θt=θpresent e−gt (where θpresent is the estimate obtained from the present dataset). Positive values of g indicate growth, negative values, decline.
(d) Hypotheses testing by matrix correspondence
Hypotheses relating to multidimensional (i.e. geographical) data may be tested by matrix correspondence tests (MCT) where the association between observed (dependent) and hypothesized patterns, represented by dissimilarity matrices among populations, is measured as a correlation, or absolute standardized regression, and the probability on the null hypothesis of no association is assessed by repeatedly randomizing (10 000 times) the dependent matrix to produce a distribution of association coefficients.
(e) Morphological variation
Morphological variation in 15 body dimensions and 10 scalation characters measured from 471 individuals of T. boettgeri in 30 localities on Gran Canaria was summarized by canonical variate analysis (CVA) in both sexes of T. boettgeri. The Mahalanobis distance was calculated from centroids derived from the CVA and was used in order to characterize the overall differences in each of the character systems between groups (localities). The corresponding matrix was used as the dependent variable in MCT. Phylogeography (average patristic distance between haplotype sequences in each locality), altitude, rainfall, climate type, vegetation type (Garcia Rodriguez et al. 1990) and a northeast–southwest ecotone hypothesis (Brown & Thorpe 1991) were used as independent variables. (More details are available in the Electronic Appendix.)
3. Results
(a) Phylogenetic and phylogeographic analysis
Among 48 sequences of 543 bp length obtained from T. boettgeri specimens from Gran Canaria, 39 different cytochrome b haplotypes were identified (GenBank accession numbers AY841905–AY841944). The average transition/transversion ratio was 3.3. The haplotype diversity (Nei 1987) was 0.990 (±0.007). The nucleotide diversity (π; Nei 1987) was 0.074 and the maximal divergences between haplotypes was 11%. These relatively large values are similar to those found in a few other vertebrate species (Malhotra & Thorpe 2000; Thorpe & Stenson 2003; Takehana et al. 2003; Vences et al. 2004).
The Hasegawa–Kishino–Yano model (Hasegawa et al. 1985) with invariant sites and gamma correction was selected as the best-fitting evolutionary model. The Bayesian tree, the ML and MP tree have basically the same topology and showed T. boettgeri to be monophyletic (in relation to T. delalandii; figure 1). Bayesian posterior probabilities and bootstrap values strongly supported two major clades (A and B) within T. boettgeri (figure 1). Clade A is found in the south and the western half of the island, while clade B is found in the northeast and east of the island (figure 2). Within clade A, at least three subclades can be differentiated (figure 1), which are geographically localized (figure 2). Clade B comprises two subclades (figure 1), which are found in the southeast (B1) and northeast (B2) of the island (figure 2). In general, haplotypes of different clades or subclades do not co-occur (with a single exception, see Electronic Appendix). The sequence of T. boetggeri bischoffi from Selvagem Grande is nested within clade A, suggesting that the Selvagem Islands were colonized from Gran Canaria. T. b. bischoffi is most closely related to haplotypes in clade A2, with which it builds a strongly supported clade (figure 1). The uncorrected pairwise distance (P-distance) between haplotypes in clade A2 and T. b. bischoffi was 6.1%. The average divergence between clade A and B was 10.9%. Average divergence among subclades A1, A2 and A3 range from 6.9 to 8.1% and clade B1 and B2 differed by 4.6%.
Figure 2.
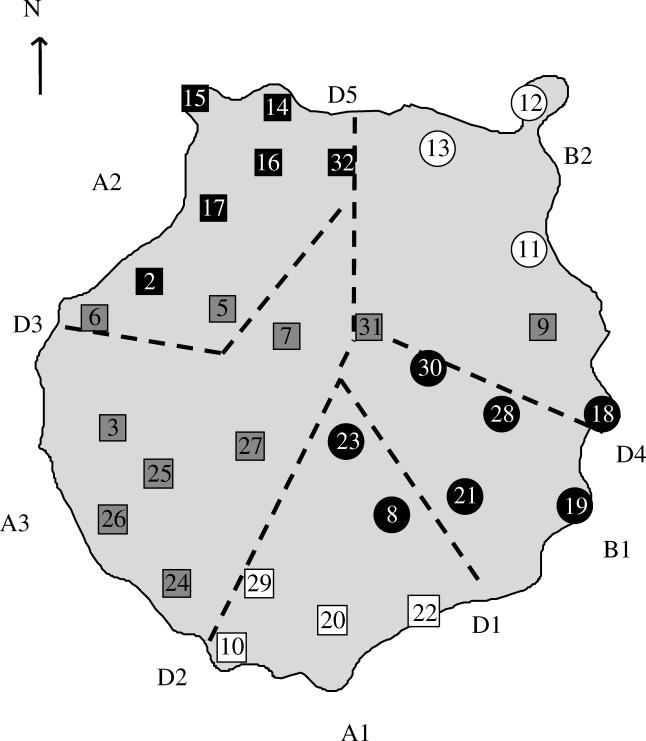
Map showing the distribution of major haplotype clades of Tarentola boettgeri on Gran Canaria. Numbers represent locality numbers, squares indicate clade A and circles indicate clade B. Subclades are indicated by white squares (A1), black squares (A2), grey squares (A3), black circles (B1) and white circles (B2). Dashed lines indicate expected phylogeographic breaks (D1–D5) on the basis of vicariance events resulting from volcanic eruptions.
Branch lengths of clades A2 and A3 appeared to be slightly larger than those of other subclades (figure 1). However, relative rate tests did not reject the null hypothesis of rate constancy in T. boettgeri in relation to T. delalandii or among the subclades of T. boettgeri (results not shown). The neutrality tests performed on the dataset did not show significant deviations from neutral expectations (results not shown). Estimating the population growth rate, g, for each subclade of T. boettgeri on Gran Canaria and T. delalandii on Tenerife suggested positive growth rates on Gran Canaria and even larger growth rates on Tenerife (table 1). Although the approximated 95% confidence limits for the log-likelihood surface of the population growth rate estimates were generally very wide (data not shown), they suggested positive growth values. The Adeje clade samples on Tenerife, which is thought to have expanded most (Gübitz et al. 2000), gave the most narrowly defined estimate for population growth (99% confidence for g>102).
Table 1.
Metropolis–Hastings simulation of the coalescent with changing population size for T. boettgeri clades A1–A3, B1, B2 on Gran Canaria and T. delalandii clades Teno, North (N) Anaga and Adeje on Tenerife. (Estimates for population growth parameter g (±estimated standard deviation) suggest larger growth rates in T. delalandii.)
| clade | A1 | A2 | A3 | B1 | B2 | Teno | N-Anaga | Adeje |
|---|---|---|---|---|---|---|---|---|
| g | 764±146 | 121±38 | 150±23 | 618±81 | 358±119 | 929±431 | 1182±345 | 1660±201 |
Pairwise and partial MCT showed significant association of the observed distribution of haplotypes or the average patristic distance of haplotypes between localities, respectively, with geographical distances and a hypothetical model as predicted from geological data (all p<0.0001, except partial MCT for average patristic distance with geographical distance regressed out p=0.0191).
Analysis of geographical variation in body dimensions (BD) and scalation (SC) in T. boettgeri on Gran Canaria showed an association with environmental variables, but not with patristic distance (table 2). In both sexes, BD was significantly associated with the NE–SW ecotone hypothesis in pairwise MCT. SC was significantly associated with altitude in both sexes and in addition with vegetation in males.
Table 2.
Pairwise matrix correspondence test of Mahalanobis distances (from CVA), representing morphological differences between localities, against independent variables (see text). (Regression coefficient and uncorrected significance level (p in italics) are given. Significant p-values and regression coefficients after row-wise Bonferroni correction are in bold.)
| variable | phylogeny | altitude | rainfall | climate | vegetation | north–south |
|---|---|---|---|---|---|---|
| female body dimensions | 0.04 (0.4422) | 0.17 (0.1505) | 0.19 (0.1032) | 0.08 (0.3877) | 0.13 (0.0796) | 0.12 (0.0022) |
| male body dimensions | 0.05 (0.3501) | 0.15 (0.1586) | 0.26 (0.0346) | 0.13 (0.1735) | 0.12 (0.1118) | 0.19 (<0.0001) |
| female scalation | 0.04 (0.4635) | 0.22 (0.0300) | 0.15 (0.1154) | 0.12 (0.1329) | 0.14 (0.0360) | 0.04 (0.1916) |
| male scalation | 0.03 (0.4933) | 0.28 (0.0155) | 0.14 (0.1389) | 0.21 (0.0188) | 0.18 (0.0138) | 0.03 (0.3691) |
4. Discussion
(a) Volcanism and intra-island phylogeography
Volcanic events can affect island phylogeographies in two ways, either by joining islands, leading to secondary contact between previously allopatric populations (Thorpe et al. 1995; Thorpe & Malhotra 1996; Gübitz et al. 2000) or by fragmenting a population, resulting in temporarily isolated populations (Carson et al. 1990; Desalle & Templeton 1992; Pestano & Brown 1999). The neighbouring islands of Tenerife and Gran Canaria provide examples of these two scenarios, respectively.
On Gran Canaria, periods of volcanic activity (Schmincke 1976, 1998) may have caused a phylogeographic pattern in T. boettgeri. The deep divergences between mt haplotypes in clade A and B of about 11% indicate that Gran Canaria has been colonized by T. boettgeri for a substantial period of time. Molecular clocks are difficult to calibrate (Hillis et al. 1996), especially in the absence of a detailed fossil record. Using vicariance events as calibration points, evolutionary rates of 1.0–1.6% (Gübitz et al. 2000) and 2.4% (Carranza et al. 2000, 2002) sequence divergence per million years are obtained for mt cytochrome b in Tarentola. Given these rates, the two major clades in T. boettgeri on Gran Canaria would have diverged about 11.0–4.6 Myr and hence during a period of volcanic quiescence before the volcanism of the Roque Nublo cycle (Perez-Torrado et al. 1995). Hence the Roque Nublo cycle and the younger Llano de la Paz volcanism, concentrated in the north of the island (van den Boogaard et al. 1998), have probably affected the phylogeography of T. boettgeri on Gran Canaria. The clear phylogeographic patterns, present in the western, southern and southeastern parts of the island, may have resulted from the volcanism of the Roque Nublo cycle (figure 2 and Electronic Appendix fig. S1a). The divergences between clades A and B (table 1) are consistent with this hypothesis as their separation clearly predates the time of the Roque Nublo cycle. Comparison of the distribution of haplotypes belonging to clades A1, A2 and A3 (figure 2) with the pattern of volcanic flows of the Roque Nublo cycle (fig. S1a in Electronic Appendix) suggests that these volcanic flows may have led to their isolation. However, this hypothesis requires assumption of evolutionary rates of 1–1.6% per million years. The volcanic period of the Llano de la Paz formation affected most parts of the northeast of the island (fig. S1b in Electronic Appendix). Owing to strong erosion it is not possible to decide whether the entire northeast was covered by volcanic flows or whether the furthest northeastern part remained unaffected. In the latter case, it would be possible that the ancestor of clade B2 haplotypes became fixed in a northeastern isolate (figure 2 and Electronic Appendix fig. S1b). The area covered by the volcanic flows was apparently recolonized from several source populations (figure 2 and Electronic Appendix fig. S1b).
Stochasticity in the coalescent can create phylogeographic patterns without temporary isolation or bottlenecking (Knowles & Maddison 2002). However, MCT strongly support the hypothesis that the successive volcanism of the Roque Nublo and Llano de la Paz formation has caused the observed phylogeographic pattern in T. boettgeri. Parallelism of phylogeographic patterns in unrelated taxa may provide support for the hypothesis of a common historical cause (Walker & Avise 1998). Phylogenetic studies of the beetle genus Pimelia on Gran Canaria suggest that this genus colonized the island before the Roque Nublo cycle (Contreras-Diaz et al. 2003). Phylogeographic breaks in the distribution of mt haplotype clades in Pimelia sparsa (Contreras-Diaz et al. 2003) coincide with those in T. boettgeri (figure 2) and may have been caused by the Roque Nublo volcanism (D1, D2, D3 in Electronic Appendix fig. S1a) and the Llanos de la Paz formation (D4, D5 in Electronic Appendix fig. S1b). The scincid lizard Chalcides sexlineatus may have colonized Gran Canaria during the Roque Nublo cycle (Pestano & Brown 1999). Delineation of three C. sexlineatus haplotype clades also resembled those in T. boettgeri (D1, D3 and D4 in Electronic Appendix fig. S1a,b). Interestingly no admixture of mt haplotypes has been observed in T. boettgeri, C. sexlineatus or P. sparsa, except in the eastern part of the island, where some admixture/gene flow has been found in all three taxa. However, the phylogeographic patterns resulting from colonization of larger areas by different species may not necessarily coincide, owing to species-specific idiosyncrasies (such as difference in migration rate and colonization capability; Gübitz et al. 2000). While a single mt lineage in C. sexlineatus appears to have colonized the northern part of Gran Canaria, the same area has been colonized by three mt lineages of T. boettgeri (clades A2, A3 and B2), which most probably colonized from different source populations. Similarly, but at a larger scale, some European (plant and animal) taxa, which colonized the post-glacial central and northern Europe, share refugia and colonization routes but differ in their phylogeographic patterns (Hewitt 1996; Taberlet et al. 1998). However, it is surprising that the mitochondrial phylogeography in T. boettgeri should still reflect such relatively old volcanic events (more than 2 Myr) at a relatively small geographical scale. Possible explanations for this apparent low mitochondrial gene flow could be low vagility and extreme philopatry in females.
Consistent with a scenario involving population expansion, coalescent simulations of the mt populations suggest positive population growth rates in T. boettgeri on Gran Canaria (table 1). Notably, estimates obtained for T. delalandii populations on Tenerife indicated even larger population growth rates (table 1). This may be because on Tenerife population expansion probably started only about 0.2 Myr, while on Gran Canaria the volcanic events, which may have been followed by population expansion, are longer ago in the past (approximately 2 Myr) and hence the signature of population growth is weaker.
(b) Changing morphological variation
Geographical variation in morphology can be established by drift during population contractions. However, shifts in morphology caused by drift in small populations will rarely be adaptive but are more likely to be disadvantageous. In addition, bottlenecked populations have reduced standing variation and are unlikely to adapt rapidly to new ecological conditions (Barton & Charlesworth 1984). Hence we would expect that recently expanded populations (after a bottleneck) require some time to adapt to environments into which they have extended. Accordingly, phylogeography in T. delalandii on Tenerife still shows some association with morphological characters (BD and SC but not colour pattern; Gübitz et al. 2000). In contrast, geographical variation in morphology in T. boettgeri on Gran Canaria bears no trace of a historical effect but is rather determined by environmental variables. Given the time-scales since the last major volcanic events on Tenerife and Gran Canaria, we propose that it may take natural selection less than 2 My to overwrite any signature in morphological variation caused by historic events.
Acknowledgments
We thank U. Joger for the T. boetggeri bischoffi blood sample, M. Baez for obtaining permissions and D. Charlesworth, N. Barton and two anonymous referees for comments on the manuscript. T.G. thanks Dan Irwin, Ewald Gübitz and Marion Rozowski for their support during fieldwork.
Footnotes
As this paper exceeds the maximum length normally permitted, the authors have agreed to contribute to production costs.
Supplementary Material
References
- Ancochea E, Fuster J.M, Ibarrola E, Cendrero A, Coello J, Hernan F, Cantagrel J.M, Jamond C. Volcanic evolution of the island of Tenerife (Canary-Islands) in the light of new K–Ar data. J. Volcanol. Geotherm. Res. 1990;44:231–249. [Google Scholar]
- Ancochea E, Huertas M.J, Cantagrel J.M, Coello J, Fuster J.M, Arnaud N, Ibarrola E. Evolution of the Canadas edifice and its implications for the origin of the Canadas Caldera (Tenerife, Canary Islands) J. Volcanol. Geotherm. Res. 1999;88:177–199. [Google Scholar]
- Avise J.C. Natural history and evolution. Chapman & Hall; New York: 1994. Molecular markers. [Google Scholar]
- Avise J.C. The history and formation of species. Havard University Press; Cambridge, MA: 2000. Phylogeography. [Google Scholar]
- Barton N.H, Charlesworth B. Genetic revolutions, founder effects, and speciation. Annu. Rev. Ecol. Syst. 1984;15:133–164. [Google Scholar]
- Beheregaray L.B, Ciofi C, Geist D, Gibbs J.P, Caccone A, Powell J.R. Genes record a prehistoric volcano eruption in the Galapagos. Science. 2003;302:75. doi: 10.1126/science.1087486. [DOI] [PubMed] [Google Scholar]
- Brown R.P, Thorpe R.S. Within-island microgeographic variation in body dimensions and scalation of the skink Chalcides sexlineatus, with testing of causal hypotheses. Biol. J. Linn. Soc. 1991;44:47–64. [Google Scholar]
- Carranza S, Arnold E.N, Mateo J.A, Lopez-Jurado L.F. Long-distance colonization and radiation in gekkonid lizards, Tarentola (Reptilia: Gekkonidae), revealed by mitochondrial DNA sequences. Proc. R. Soc. B. 2000;267:637–649. doi: 10.1098/rspb.2000.1050. 10.1098/rspb.2000.1050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carranza S, Arnold E.N, Mateo J.A, Geniez P. Relationships and evolution of the North African geckos, Geckonia and Tarentola (Reptilia: Gekkonidae), based on mitochondrial and nuclear DNA sequences. Mol. Phylogenet. Evol. 2002;23:244–256. doi: 10.1016/S1055-7903(02)00024-6. [DOI] [PubMed] [Google Scholar]
- Carson H.L, Templeton A.R. Genetic revolutions in relation to speciation phenomena—the founding of new populations. Annu. Rev. Ecol. Syst. 1984;15:97–131. [Google Scholar]
- Carson H.L, Lockwood J.P, Craddock E.M. Extinction and recolonization of local populations on a growing shield volcano. Proc. Natl Acad. Sci. USA. 1990;87:7055–7057. doi: 10.1073/pnas.87.18.7055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras-Diaz H.G, Moya O, Oromi P, Juan C. Phylogeography of the endangered darkling beetle species of Pimelia endemic to Gran Canaria (Canary Islands) Mol. Ecol. 2003;12:2131–2143. doi: 10.1046/j.1365-294x.2003.01884.x. [DOI] [PubMed] [Google Scholar]
- Desalle R, Templeton A.R. The mtDNA genealogy of closely related Drosophila silvestris. J. Hered. 1992;83:211–216. doi: 10.1093/oxfordjournals.jhered.a111195. [DOI] [PubMed] [Google Scholar]
- Felsenstein J. Evolutionary trees from DNA sequences—a maximum likelihood approach. J. Mol. Evol. 1981;17:368–376. doi: 10.1007/BF01734359. [DOI] [PubMed] [Google Scholar]
- Fu Y.X, Li W.H. Statistical tests of neutrality of mutations. Genetics. 1993;133:693–709. doi: 10.1093/genetics/133.3.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia Rodriguez, J.-L., Hernandez, J. H., Cabrera Armas, L. G., Diaz de la Paz, A. & Perez, L. A. 1990 Atlas interinsular de Canarias. Editorial Interinsular Canarias, S.A., Santa Cruz de Tenerife.
- Gübitz T, Thorpe R.S, Malhotra A. Phylogeography and natural selection in the Tenerife gecko Tarentola delalandii: testing historical and adaptive hypotheses. Mol. Ecol. 2000;9:1213–1221. doi: 10.1046/j.1365-294x.2000.00997.x. [DOI] [PubMed] [Google Scholar]
- Hasegawa M, Kishino H, Yano T.A. Dating of the human–ape splitting by a molecular clock of mitochondrial DNA. J. Mol. Evol. 1985;22:160–174. doi: 10.1007/BF02101694. [DOI] [PubMed] [Google Scholar]
- Hewitt G.M. Some genetic consequences of ice ages and their role in divergence and speciation. Biol. J. Linn. Soc. 1996;58:247–276. [Google Scholar]
- Hillis D.M, Moritz C, Mable B.K. 2nd edn. Sinauer Associates; Sunderland, MA: 1996. Molecular systematics. [Google Scholar]
- Huelsenbeck J.P, Ronquist F. MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics. 2001;17:754–755. doi: 10.1093/bioinformatics/17.8.754. [DOI] [PubMed] [Google Scholar]
- Huertas M.J, Arnaud N.O, Ancochea E, Cantagrel J.M, Fuster J.M. Ar-40/Ar-39 stratigraphy of pyroclastic units from the Canadas Volcanic Edifice (Tenerife, Canary Islands) and their bearing on the structural evolution. J. Volcanol. Geotherm. Res. 2002;115:351–365. [Google Scholar]
- Knowles L.L, Maddison W.P. Statistical phylogeography. Mol. Ecol. 2002;11:2623–2635. doi: 10.1046/j.1365-294x.2002.01637.x. [DOI] [PubMed] [Google Scholar]
- Kuhner M.K, Yamato J, Felsenstein J. Maximum likelihood estimation of population growth rates based on the coalescent. Genetics. 1998;149:429–434. doi: 10.1093/genetics/149.1.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S. Institute of Molecular Genetics and Department of Biology, The Pennsylvania State University; PA, USA: 1996. PHYLTEST. [2.0] [Google Scholar]
- Losos J.B, Warheit K.I, Schoener T.W. Adaptive differentiation following experimental island colonization in Anolis lizards. Nature. 1997;387:70–73. [Google Scholar]
- Losos J.B, Jackman T.R, Larson A, de Queiroz K, Rodriguez-Schettino L. Contingency and determinism in replicated adaptive radiations of island lizards. Science. 1998;279:2115–2118. doi: 10.1126/science.279.5359.2115. [DOI] [PubMed] [Google Scholar]
- Malhotra A, Thorpe R.S. Experimental detection of rapid evolutionary response in natural lizard populations. Nature. 1991;353:347–348. [Google Scholar]
- Malhotra A, Thorpe R.S. The dynamics of natural selection and vicariance in the Dominican anole: patterns of within-island molecular and morphological divergence. Evolution. 2000;54:245–258. doi: 10.1111/j.0014-3820.2000.tb00025.x. [DOI] [PubMed] [Google Scholar]
- McDonald J.H, Kreitman M. Adaptive protein evolution at the Adh locus in Drosophila. Nature. 1991;351:652–654. doi: 10.1038/351652a0. [DOI] [PubMed] [Google Scholar]
- Moore W.S. Inferring phylogenies from mt DNA variation—mitochondrial-gene trees versus nuclear-gene trees. Evolution. 1995;49:718–726. doi: 10.1111/j.1558-5646.1995.tb02308.x. [DOI] [PubMed] [Google Scholar]
- Nei M. Columbia University Press; New York: 1987. Molecular evolutionary genetics. [Google Scholar]
- Nogales M, Lopez M, Jimenez Asensio J, Larruga J.M, Hernandez M, Gonzalez P. Evolution and biogeography of the genus Tarentola (Sauria: Gekkonidae) in the Canary Islands, inferred from mitochondrial DNA sequences. J. Evol. Biol. 1998;11:481–494. [Google Scholar]
- Perez-Torrado F.J, Carracedo J.C, Mangas J. Geochronology and stratigraphy of the Rogue Nublo Cycle, Gran-Canaria, Canary-Islands. J. Geol. Soc. 1995;152:807–818. [Google Scholar]
- Pestano J, Brown R.P. Geographical structuring of mitochondrial DNA in Chalcides sexlineatus within the island of Gran Canaria. Proc. R. Soc. B. 1999;266:805–812. 10.1098/rspb.1999.0709 [Google Scholar]
- Rothe P. 2nd edn. Gebr. Borntraeger; Berlin: 1996. Kanarische Inseln. [Google Scholar]
- Rozas J, Rozas R. DnaSP version 3: an integrated program for molecular population genetics. Bioinformatics. 1999;15:174–175. doi: 10.1093/bioinformatics/15.2.174. [DOI] [PubMed] [Google Scholar]
- Schmincke H.-U. The geology of the Canary Islands. In: Kunkel G, editor. Biogeography and ecology of the Canary Islands. Junk; The Hague: 1976. pp. 67–184. [Google Scholar]
- Schmincke H.-U. Zeitliche, strukturelle und vulkanische Entwicklung der Kanarischen Inseln, der Selvagens Inseln und des Madeira-Archipels. In: Bischoff W, editor. Die Reptilien der Kanarischen Inseln, der Selvagens-Inseln und des Madeira-Archipels. Aula-Verlag; Wiesbaden: 1998. pp. 27–69. [Google Scholar]
- Swofford D.L. Sinauer Associates; Sunderland, MA: 1998. PAUP*: phylogenetic analysis using parsimony (* and other methods). [4.0b2] [Google Scholar]
- Taberlet P, Fumagalli L, Wust Saucy A.G, Cosson J.F. Comparative phylogeography and postglacial colonization routes in Europe. Mol. Ecol. 1998;7:453–464. doi: 10.1046/j.1365-294x.1998.00289.x. [DOI] [PubMed] [Google Scholar]
- Tajima F. Statistical-method for testing the neutral mutation hypothesis by DNA polymorphism. Genetics. 1989;123:585–595. doi: 10.1093/genetics/123.3.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takehana Y, Nagai N, Matsuda M, Tsuchiya K, Sakaizumi M. Geographic variation and diversity of the cytochrome b gene in Japanese wild populations of Medaka, Oryzias latipes. Zool. Sci. 2003;20:1279–1291. doi: 10.2108/zsj.20.1279. [DOI] [PubMed] [Google Scholar]
- Takezaki N, Rzhetsky A, Nei M. Phylogenetic test of the molecular clock and linearized trees. Mol. Biol. Evol. 1995;12:823–833. doi: 10.1093/oxfordjournals.molbev.a040259. [DOI] [PubMed] [Google Scholar]
- Thorpe R.S, Malhotra A. Molecular and morphological evolution within small islands. Phil. Trans. R. Soc. B. 1996;351:815–822. [Google Scholar]
- Thorpe R.S, Stenson A.G. Phylogeny, paraphyly and ecological adaptation of the colour and pattern in the Anolis roquet complex on Martinique. Mol. Ecol. 2003;12:117–132. doi: 10.1046/j.1365-294x.2003.01704.x. [DOI] [PubMed] [Google Scholar]
- Thorpe R.S, Malhotra A, Black H, Daltry J.C, Wuster W. Relating geographic pattern to phylogenetic process. Phil. Trans. R. Soc. B. 1995;349:61–68. [Google Scholar]
- van den Boogaard P, Schmicke H.U, Freundt A, Hall C.M, York D. Eruption ages and magma supply rates during the Miocene of Gran Canaria. Single-crystal 40Ar/39Ar laser ages. Naturwissenschaften. 1998;75:616–617. [Google Scholar]
- Vences M, Chiari Y, Raharivololoniaina L, Meyer A. High mitochondrial diversity within and among populations of Malagasy poison frogs. Mol. Phylogenet. Evol. 2004;30:295–307. doi: 10.1016/s1055-7903(03)00217-3. [DOI] [PubMed] [Google Scholar]
- Walker D, Avise J.C. Principles of phylogeography as illustrated by freshwater and terrestrial turtles in the southeastern United States. Annu. Rev. Ecol. Syst. 1998;29:23–58. [Google Scholar]
- Williamson M. Oxford University Press; Oxford: 1981. Island populations. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.