Abstract
Airway hyperresponsiveness and remodeling are defining features of asthma. We hypothesized that impaired superoxide dismutase (SOD) antioxidant defense is a primary event in the pathophysiology of hyperresponsiveness and remodeling that induces apoptosis and shedding of airway epithelial cells. Mechanisms leading to apoptosis were studied in vivo and in vitro. Asthmatic lungs had increased apoptotic epithelial cells compared to controls as determined by terminal dUTP nick-end labeling-positive cells. Apoptosis was confirmed by the finding that caspase-9 and -3 and poly (ADP-ribose) polymerase were cleaved. On the basis that SOD inactivation triggers cell death and low SOD levels occur in asthma, we tested whether SOD inactivation plays a role in airway epithelial cell death. SOD inhibition increased cell death and cleavage/activation of caspases in bronchial epithelial cells in vitro. Furthermore, oxidation and nitration of MnSOD were identified in the asthmatic airway, correlating with physiological parameters of asthma severity. These findings link oxidative and nitrative stress to loss of SOD activity and downstream events that typify asthma, including apoptosis and shedding of the airway epithelium and hyperresponsiveness.
Asthma is commonly diagnosed using physiological measures, but alterations in airway structure are the defining features of asthma. Damage to airway epithelium, eosinophil infiltration, smooth muscle hyperplasia, thickening and aberrant collagen, and protein composition of the basement membrane are well established elements of the asthmatic airway.1,2 The injury to the bronchial epithelium in asthma is marked by loss of columnar epithelial cells. Extensive loss of cells and denuded basement membrane with few basal cells remaining on the airway surface are noted in severe asthma, but shedding of airway epithelium is present even in clinically mild asthma.2,3 Physical loss of epithelial lining cells is considered one proximate cause of the airway hyperresponsiveness to inhaled mediators, and has been speculated to contribute to asthmatic airway remodeling, in particular abnormal collagen synthesis. Evidence from organ culture systems supports the concept of an epithelial-mesenchymal unit in which loss of epithelium leads to mucosal myofibroblast and fibroblast proliferation, and collagen deposition.2,4–6 Thus, if the epithelial injury and loss could be understood and prevented in asthma, the clinical symptoms of airway hyperresponsiveness and long-term progressive sequelae in the airways, which contribute to fixed airflow limitation, might be prevented.
Several reports have proposed that loss of epithelial cells is because of apoptosis based on immunostaining for the proteins that regulate apoptosis, or by detection of DNA strand breaks by immunostaining with the terminal dUTP nick-end labeling (TUNEL) assay.7–11 However, not all reports have confirmed increased TUNEL positivity in airways.9 Furthermore, if airway epithelial cells are undergoing increased cell death, it is unclear whether this is because of an inherent cell defect or a response to the asthmatic airway environment. Although nonspecific events related to increased levels of reactive oxygen and nitrogen species in the asthmatic airway have been postulated to lead to epithelial cell loss, the precise mechanisms of effect are unknown.12–15 In general, the human lung has an effective, well-integrated antioxidant system to combat reactive oxygen species and reactive nitrogen species injury,12 however, the respiratory tract antioxidant capacity is impaired in asthma.16–18 Activity of superoxide dismutase (SOD) is lower in asthmatic lungs as compared to healthy controls, and decreases further during an asthmatic attack.16,17 Inhibition of SOD activity in malignant cells leads to free radical mediated damage to mitochondrial membranes, release of cytochrome c, and entry of cells into apoptosis.19 In this context, we hypothesized that the loss of airway epithelial cells in asthma is because of apoptosis triggered by SOD modification and inactivation, which is specifically related to the inflammatory environment of the asthmatic airway.
To definitively assess apoptosis in asthma, we evaluated expression and activation of caspases, a family of aspartate-directed intracellular proteases required for the terminal stages of apoptosis. Here, we show that caspase-9, an initiator of apoptosis, and caspase-3, an effector of apoptosis, are activated in asthmatic epithelial cells. Validation that loss of SOD in asthmatic airways can activate the apoptotic pathways in epithelial cells is provided by the complementary approaches of both pharmacological inhibition of SOD activity and molecular silencing of MnSOD mRNA. Physiological relevance to asthma is supported by a strong and statistically significant inverse relationship between epithelial cell SOD activity and lung function. Finally, SOD inactivation is linked to oxidative modification of MnSOD in vivo via nitration and hydroxylation, protein modifications promoted, respectively, by NO-derived oxidants (peroxynitrite or peroxidase-catalyzed reactions) and hydroxyl radical-like oxidants such as those generated by redox active transition metal ions during Fenton and Haber-Weiss oxidation chemistry. Taken together, the present studies provide evidence of ongoing profound oxidative and nitrosative stress in asthmatic airways with downstream consequences of SOD inactivation and airway epithelial cell apoptosis, a defining characteristic of airway remodeling.
Materials and Methods
Study Population
To evaluate apoptosis in the respiratory system in vivo, the study population included 9 healthy nonsmoking individuals and 46 asthmatic individuals. Exclusion criteria for the two groups included age younger than 18 years or older than 65 years, pregnancy, human immunodeficiency virus infection, and history of respiratory infection in the previous 6 weeks, prolonged exposure to second hand smoke at home or at work, exposure to dusty environments or known pulmonary disease-producing agents. Asthma was defined based on the National Asthma Education Prevention Program Guidelines, which include: episodic respiratory symptoms, reversible airway obstruction by documentation of variability of FEV1 and/or FVC by 12% and 200 ml either spontaneously or after two puffs of inhaled Albuterol, and/or a positive methacholine challenge.20 None of the patients had a recent asthma exacerbation, hospitalization, or change in medications for 6 weeks before the study. The study was approved by the Institutional Review Boards of the Cleveland Clinic Foundation and the University of Pittsburgh Medical Center, and written informed consent was obtained from all individuals.
Isolation of Bronchial Epithelial Cells
Individuals underwent bronchoscopy to obtain samples of human airway epithelial cells with cytology brushings from second- and third-order bronchi with a 1-mm cytology brush (Microvasive, Watertown, MA) as previously described.17 The brush sample was immediately placed into sterile media, RPMI 1640 (Life Technologies, Inc., Rockville, MD) and an aliquot taken for cytology and cell differential determination.
Cell Culture
BET1A, a human bronchial epithelial cell line, was cultured in serum-free Lechner and LaVeck medium (LHC-8; Biofluids, Inc., Rockville, MD) with additives 0.33 nmol/L retinoic acid, 2.75 mmol/L epinephrine, and the antibiotic combination, 1% penicillin/streptomycin, on plates precoated with coating media containing 29 μg/ml collagen (Vitrogen; Collagen Corp., Palo Alto, CA), 10 μg/ml bovine serum albumin (Biofluids), and 10 μg/ml fibronectin (Calbiochem, La Jolla, CA) for 5 minutes. Human airway epithelial cells obtained by bronchial brushing were cultured in serum-free Lechner and LaVeck media (LHC8) on plates precoated with coating media.21 To evaluate oxidant stress and antioxidants in apoptotic events, BET-1A cells were stimulated at 70% confluence with SOD inhibitor, 2-methoxyestradiol (2-ME) (Sigma, St. Louis, MO), or pyrogallol, a superoxide generating compound (J.T. Baker Inc., Phillipsburg, NJ),22 or hydrogen peroxide (Sigma) in a dose- and time-dependent manner. 293T cells, a clone of 293 (human embryonic kidney fibroblast cells) that expresses the Simian virus 40 large-T antigen, were maintained in Dulbecco’s modified Eagle’s medium (Invitrogen, Carlsbad, CA) with 10% fetal calf serum.
Antioxidant Assays
Bronchial epithelial cells (BET1A) exposed to 5 μmol/L 2-ME for 30 minutes to 24 hours, or serum of asthmatic individuals, were assayed for glutathione (GSH), glutathione peroxidase (GPx), catalase, and SOD activity. SOD activity was determined by the rate of reduction of cytochrome c, with 1 unit (U) of SOD activity defined as the amount of SOD required to inhibit the rate of cytochrome c reduction by 50%.23 The final reaction volume was 3 ml and included 50 mmol/L potassium phosphate buffer, 2 mmol/L cytochrome c, 0.05 mmol/L xanthine, and a 0.1 mmol/L ethylenediaminetetraacetic acid solution. Xanthine oxidase (Sigma) was added at a concentration sufficient to induce a 0.020 per minute change in absorbance at 550 nm. GSH levels were measured as previously described.24
Cell Viability and Apoptosis Detection
Cell viability was assessed by bright-field microscopy using a trypan blue dye (0.4%) exclusion method. The mean survival was determined by examining four different low-power fields. Annexin V binding was used to detect apoptotic cells as previously described.7 Briefly, cells were incubated with 1.0 μg/ml of annexin V-fluorescein isothiocyanate and 2.5 μg/ml of propidium iodide (BD Biosciences, Palo Alto, CA). The stained cells were analyzed with a FACScan (Becton Dickinson, San Jose, CA), using an argon ion laser at 488 nm and emission recorded at 520 nm with band pass and short pass filters. Gating was done on the forward angle and right angle light scatter only to exclude debris and cell clumps. A minimum of 10,000 cells was measured per condition and all values are expressed as relative fluorescence index. The relative fluorescence index was calculated using the ratio of the linearized mean fluorescence of the cell populations, as provided by the CellQuest software (Becton Dickinson). Apoptotic cells are identified as the annexin-positive PI-negative fraction.
Caspase-3-Like Enzyme Activity
Caspase-3-like activity was measured by a spectrophotometric assay (BD PharMingen, San Diego, CA). This assay measures active caspase-3 binding to fluorogenic Ac-DEVD-AMC substrate and its cleavage to release the fluorescent AMC. AMC fluorescence is quantified by UV spectrofluorometry with an excitation wavelength of 380 nm and an emission wavelength range of 420 to 460 nm. The percentage increase in protease activity was determined by comparing the levels of caspase activity in cells recovered from asthmatic versus control patients.
Assay for DNA Nicking
Human bronchial epithelial brushings and bronchial biopsy from controls and asthmatic individuals were evaluated for cell death by the In Situ cell death detection kit AP, (Boehringer Mannheim, Indianapolis, IN). After paraffin removal and rehydration for lung tissue, the TUNEL assay was used. Briefly, cell death was visualized by labeling of DNA strand breaks by terminal deoxynucleotidyl transferase (TdT), which catalyzes polymerization of labeled nucleotides to free 3′-OH DNA ends in a template-independent manner (TUNEL reaction). The detection of the incorporated fluorescein occurs by an anti-fluorescein antibody conjugated with alkaline phosphatase, which is converted by Vector Red alkaline phosphatase substrate K (Vector Laboratories, Burlingame, CA) or by NBT/BCIP (Roche Diagnostics Co., Indianapolis, IN). Bronchial brushings were smeared on to slides. The positive control cells, A549 treated with DNase I (1.5 mg/ml in 50 mmol/L Tris-HCl, pH 7.5, 1 mg/ml bovine serum albumin for 30 minutes), were sedimented on to glass slides using Cytospin Shandon (Milford, MA). Cell samples were air-dried and fixed with a freshly prepared paraformaldehyde solution (4% in phosphate-buffered saline, pH 7.4) for 1 hour at room temperature. The slides were evaluated by light microscopy.
Western Blot Assay
Airway epithelial cells freshly obtained by bronchoscopic brushing from asthmatics and healthy controls, or BET1A cells, were suspended in buffer (3 mmol/L dithiothreitol, 5 μg/ml aprotinin, 1 μg/ml leupeptin and pepstatin A, 0.1 mmol/L phenylmethyl sulfonyl fluoride, 1% Nonidet P-40, and 40 mmol/L Hepes, pH 7.5) and cell lysate prepared by three cycles of freeze/thaw. Total protein was measured by using the Coomassie protein assay (Pierce, Rockford, IL). Whole cell lysate protein was denatured and reduced by treatment with buffer containing 0.05 mol/L Tris (pH 6.8), 1% sodium dodecyl sulfate (SDS), 10% glycerol, 0.00125% bromphenol blue, and 0.5% β-mercaptoethanol for 3 minutes at 95°C. Total protein was separated by electrophoresis on a 10% SDS-polyacrylamide gel, and then electrophoretically transferred onto nitrocellulose (NitroBind EP4HY315F5; Fisher Scientific, Pittsburgh, PA) or polyvinylidene difluoride membrane (Pierce) for 1 hour at 4°C. Membranes were incubated with blocking buffer [5% nonfat dry milk in Tris-buffered saline (20 mmol/L Tris-HCl, pH 7.0, and 137 mmol/L NaCl) with 0.1% Tween] for 1 hour at room temperature to block nonspecific binding and then probed with a primary antibody in blocking buffer overnight at 4°C. After washing, a peroxidase-conjugated secondary antibody was incubated with the membrane for 1 hour at room temperature followed by washes with Tris-buffered saline-0.1% Tween. The detection of signals was performed with an enhanced chemiluminescent system (Amersham Laboratories, Piscataway, NJ). The primary antibodies were anti-mouse monoclonal BAX (Transduction Laboratories, Lexington, KY), poly (ADP-ribose) polymerase (PARP), caspase-8 (BD PharMingen) and β-actin (Sigma), anti-rabbit polyclonal caspase-3 and caspase-9 (BD PharMingen), monoclonal anti-nitrotyrosine antibody (Upstate Biotechnology, Lake Placid, NY), and anti-MnSOD polyclonal antibody (OxisResearch, Portland, OR).
Immunoprecipitation
Airway epithelial cells freshly obtained by bronchoscopic brushing from asthmatics and healthy controls were lysed in ice-cold nonreducing lysis buffer (50 mmol/L Tris-HCl, pH 7.4, 150 mmol/L NaCl, 1 mmol/L ethylenediaminetetraacetic acid, 0.5% Nonidet P-40, 10% glycerol, 1 mmol/L phenylmethyl sulfonyl fluoride, 5 μg/ml leupeptin, 10 μg/ml pepstatin A, 20 μg/ml aprotinin, and 200 μmol/L NaOV). Immunoglobulins were removed by preincubating the cell lysis with protein G-Sepharose (Amersham Laboratories). The supernatant was further incubated with anti-MnSOD antibody (OxisResearch) followed by protein G-Sepharose incubation. The captured beads were washed and boiled in denaturing, nonreducing buffer. The released proteins were analyzed by Western blot as described above. Blots were subsequently evaluated by polyclonal anti-MnSOD antibody to confirm equivalent protein loading.
Two-Dimentional Gel Electrophoresis
BET1A cells and human airway epithelial cells unstimulated or stimulated with 2-ME for 24 hours were harvested with a lysis buffer composed of 7 mol/L urea, 2 mol/L thiourea, 4% CHAPS, 1% dithiothreitol, 0.5% Triton X-100, and 2% IPG ampholytes (pH 3 to 10) at room temperature. Samples were sonicated and clarified by centrifugation. Total protein was measured by using Coomassie protein assay (Pierce). Two-dimensional gel electrophoresis was performed with the isoelectric focusing system (Bio-Rad, Hercules, CA). Eleven-cm linear (pH 3 to 10), immobilized pH gradient strips were used for first dimension. The immobilized pH gradient strips were rehydrated with sample at 50 V for 14 hours and isoelectric focusing performed by a linear increase to 250 V for 20 minutes followed by linear increase to 8000 V throughout 2 hours and 50 minutes and then held at 8000 V until a total of 43 kVh was reached. For the second dimension, the IPG strips were equilibrated for 15 minutes in 50 mmol/L Tris-HCl (pH 8.8), 6 mol/L urea, 30% glycerol, 2% SDS, 1% dithiothreitol, and bromphenol blue, and then 15 minutes in 50 mmol/L Tris-HCl (pH 8.8), 6 mol/L urea, 30% glycerol, 2% SDS, 2% iodoacetamide, and bromphenol blue. The strips were embedded in 1% (wt/vol) agarose on top of 12.5% acrylamide gel containing 4% stacking gel. The second dimension was performed essentially according to Laemmli.25 After completion of the run, the acrylamide gel was soaked in transfer buffer (20 mmol/L Tris-HCl, 96 mmol/L glycine, 20% methanol) and partially transferred to the polyvinylidene difluoride membrane. The gels were stained with colloidal Coomassie blue (Pierce), and Western blot was performed as described for the monoclonal anti-nitrotyrosine antibody. Pure and nitrated MnSOD were used as controls. Briefly, 100 μg of Pure MnSOD (1 mg/ml in 100 mmol/L Tris/HCl, pH 7.5; Sigma) was nitrated at room temperature for 30 minutes by 12 mmol/L tetranitromethane (Sigma). Reaction was stropped by adding gel-loading buffer with β-mercaptoethanol.
Quantification of Purified MnSOD for Protein-Bound Oxidation and Nitration
MnSOD was purified by immunoprecipitation. One hundred μl of protein L gel (Pierce) was added to immunoprecipitated MnSOD to deplete IgG. The purity of MnSOD was demonstrated by SDS-polyacrylamide gel electrophoresis and colloidal blue stain. Purified MnSOD was precipitated by ice-cold acetone, and dried in nitrogen. Protein-bound nitrotyrosine, chlorotyrosine, bromotyrosine, di-tyrosine, o-tyrosine, and m-tyrosine were quantified by stable isotope dilution liquid chromatography-tandem mass spectrometry on a triple quadruple mass spectrometer (API 4000; Applied Biosystems, Foster City, CA) interfaced to an Aria LX Series HPLC multiplexing system (Cohesive Technologies, Franklin, MA) using methods as previously described.26 Briefly, synthetic [13C6]-labeled standards for each analyte were prepared and added to purified MnSOD samples and used as internal standards for quantification of natural abundance analytes. Simultaneously, universally labeled precursor amino acids, [13C9,15N1]tyrosine and [13C9,15N1]phenylalanine (Cambridge Isotopes Inc., Andover, MA), were added. After desalting and delipidation, proteins were hydrolyzed under argon atmosphere in methane sulfonic acid, and then samples passed over mini solid-phase C18 extraction columns (Supelclean LC-C18-SPE minicolumn, 3 ml; Supelco, Inc., Bellefonte, PA) before mass spectrometry analysis. Results were normalized to the content of the precursor amino acid tyrosine (for chlorotyrosine, nitrotyrosine, bromotyrosine, and dityrosine) and phenylalanine (for o-tyrosine and m-tyrosine), which were simultaneously monitored within the same injection using characteristic parent-daughter ion transitions for each isotopomer of each analyte. Intrapreparative formation of [13C9,15N1]-labeled oxidized tyrosine (chlorotyrosine, nitrotyrosine, dityrosine, and bromotyrosine) and phenylalanine (o- and m-tyrosine) species was routinely monitored and negligible (ie, < 5% of the level of the natural abundance analytes observed) under the conditions used.
Transfection of siRNA MnSOD
MnSOD siRNA was synthesized by Ambion (Austin, TX). The sense and anti-sense MnSOD siRNA were 5-GGAACAACAGGCCUUAUUCtt-3 (sense) and 5-GAAUAAGGCCUGUUGUUCCtt-3 (anti-sense). Silencer-negative control no. 1 siRNA (siRNA control) (Ambion) was used as a control. 293T cells, at 60% confluence in 100-cm plates, were transfected in serum-free medium (Dulbecco’s modified Eagle’s medium, Invitrogen) by using lipofectamine reagent (Invitrogen) according to the manufacturer’s instructions. For the silencing experiments, cells were transfected with 10, 50, and 100 μmol/L of MnSOD siRNA or with 50 nmol/L of Silencer Negative Control no. 1 siRNA. Forty-eight hours after transfection, cells were washed, trypsinized, and harvested for evaluation of mRNA, protein expression, and caspase-3 activity.
Northern Analysis of MnSOD Expression
Total RNA from 293T cells was extracted by the GTC [(4 mol/L guanidium thiocyanate, 25 mmol/L sodium citrate pH 7.0), 0.5% sarkosyl, and 0.1 mol/L β-mercaptoethanol]-CsCl gradient method and evaluated by Northern analysis using a 32P-labeled MnSOD (pHMn-SOD4), or as control γ-actin cDNA (pHFγA-1), and then subjected to autoradiography.
Protein Identification
Anti-nitrotyrosine immunopositive spots were matched with the Coomassie stained two-dimensional gel and identified according to Hanna and colleagues.27 The selected protein spots were cored from the gels and placed in a siliconized microcentrifuge tube that had been rinsed with ethanol, water, and ethanol. The gel pieces were washed and destained in 500 μl of 50% methanol/5% acetic acid overnight at room temperature before dehydration in 200 μl of acetonitrile and complete drying in a vacuum centrifuge. The proteins were reduced by addition of 50 μl of 10 mmol/L dithiothreitol and alkylated by addition of 50 μl of 100 mmol/L iodoacetamide. To exchange the buffer, the gel pieces were dehydrated in 200 μl of acetonitrile, hydrated in 200 μl of 100 mmol/L ammonium bicarbonate, and dehydrated again with 200 μl of acetonitrile. The dehydrated gel pieces were then dried completely in a vacuum centrifuge and rehydrated in 50 μl of 20 ng/μl ice-cold, sequencing-grade modified porcine trypsin (Promega, Madison, WI) for 5 minutes on ice. Any excess trypsin solution was removed and the digestion performed overnight at 37°C. The peptides produced in the digest were collected by successive extractions with 50 μl of 50 mmol/L ammonium bicarbonate and 50 μl of 50% acetonitrile/5% formic acid, combining the extracts in a siliconized 0.6-ml microcentrifuge tube that had been previously rinsed with ethanol, water, and ethanol. The total extract was concentrated in a vacuum centrifuge to 20 μl for analysis. The μLC-MS system consisted of a Finnigan LCQ (ThermoQuest) ion-trap mass spectrometer with a Protana nanospray ion source interfaced to a self-packed 8 cm × 75 μm i.d. Phenomenex Jupiter 10 μm C18 reverse-phase capillary column; 0.5 μl (2.5%) vol of peptide extract were injected and the peptides eluted from the column with an acetonitrile/0.1 mol/L acetic acid gradient (2 to 85% acetonitrile in 30 minutes) at a flow rate of 0.25 μl minute−1. The microspray ion source was operated at 2.8 kV. The digest was analyzed using a full data-dependent acquisition routine in which a full-scan mass spectrum (MS) to determine peptide molecular masses was acquired in one scan and product-ion (MS/MS) spectra to determine amino acid sequence were acquired in the four scans before the cycle repeats. This mode of analysis produces ∼500 MS/MS spectra of peptides ranging in abundance throughout several orders of magnitude. Not all MS/MS spectra are derived from peptides. The resulting MS/MS spectra were automatically batch-analyzed for each spot using either Ms-fit (http://prospector.ucsf.edu/htmlucsf3.0/msfit.htm) or Mascot (http://www.matrixscience.com).
Immunohistochemical Analysis of MIB-1
MIB-1, an antibody directed against recombinant parts of the Ki-67 antigen,28 allows reliable determination of proliferating cells.28 Endobronchial biopsies from asthmatic and control individuals were used for immunostaining. Tissues were fixed in 10% buffered formalin, embedded in paraffin, and 5-μm sections were placed on charged slides for immunohistochemistry. Slides were stained with MIB-1 (dilution 1:25; Immunotech, Marseille, France) as previously described.
Statistical Analysis
All data are expressed as the mean and SEM. The comparisons between the three groups were performed using analysis of variance. A value of P < 0.05 was considered significant. Linear regression fit of data were performed using GB-STAT 6.5 ƒ.
Results
Clinical Characteristics
Healthy control and asthmatic individuals were similar in terms of gender and age, but varied as to their race [age (years): control 37 ± 3, asthma 36 ± 2; gender (male/female): control 4/5, asthma 24/22; race (African American/Caucasian) control 4/5, asthma 8/38). Asthmatics had positive methacholine challenge and/or evidence of spontaneous airway reactivity [forced vital capacity (FVC % predicted), asthma, 89 ± 3; forced expiratory volume in 1 second (FEV1 % predicted), 73 ± 3; %FEV1/FVC, 71 ± 3]. Numbers of individuals studied for each experiment are stated in the text.
Increased Apoptosis in Asthmatic Airway Epithelial Cells
Airways were examined for histological changes and apoptosis. Hematoxylin or hematoxylin and eosin (H&E) staining of lung tissue from controls revealed an epithelium consisting of basal, ciliated, and secretory cells (Figure 1A). However, asthmatic epithelium showed marked damage including loss of the bronchial epithelial cells and thickening of the basement membrane, characteristics of remodeling events (Figure 1, C and E). Epithelial cells from asthmatic endobronchial biopsies were strongly TUNEL-positive (Figure 1, D and F). Evaluation of epithelial cells obtained by bronchial brushing further demonstrated apoptosis, by increased TUNEL staining in asthmatic samples (% TUNEL-positive: asthma, 28 ± 3; controls, 0.40 ± 0.16; P < 0.05; Figure 1, G to I). Polarized airway epithelial cells have a relatively low rate of cell proliferation under healthy conditions, with less than 1% cell turnover.28 Along with increased cell death, airway epithelial cell proliferation was increased in asthmatic airways as shown by increased immunopositivity for the proliferation marker MIB-1, detected with an antibody directed against part of the Ki-67 antigen (% MIB-1-positive: asthma, 19.7 ± 2.5; controls, 1.8 ± 0.2; Figure 2).
Figure 1.
Immunohistochemical analysis of apoptosis in airway epithelial cells from control (A, B, G) and asthmatic patients (C–E, F, H). A to H: Increased numbers of TUNEL-positive epithelial cells in endobronchial (D, F) and brush biopsies (H) of the asthmatic airway as compared to healthy controls (B, G). In addition to routine hematoxylin (A, C) and H&E staining (E), sections or cells were subjected to TUNEL assay with no counterstaining (B, D, F), or with eosin counterstaining (G, H). Healthy control bronchial mucosa in endobronchial biopsy (B) or brush biopsy (G) was negative for TUNEL. Architecture of healthy control airway mucosa (A) is contrasted to asthmatic mucosa with thickened basement membrane (C) and marked loss of epithelium in some areas (C–E, F). D, F, and H: Red nuclei indicate TUNEL positivity in asthmatic epithelial cells, whereas only minimal positivity is found in healthy controls (B and G). I: The graph shows the mean ± SE of TUNEL-positive cells in brush biopsies from five healthy controls and four asthmatics. Endobronchial biopsies are representative of seven asthmatic and three control individuals.
Figure 2.
Cell proliferation was detected by anti-human MIB-1. Brown nuclear stain indicates positive MIB-1 staining in the asthmatic epithelial cells (A) and healthy controls (B). C: The graph shows MIB-1-positive cells (mean ± SD) of three healthy controls and four asthmatics. Some fields in asthmatic airways show more than 80% MIB-1-positive cells. Arrows show positive cells.
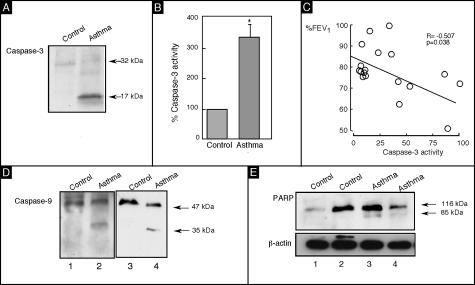
To verify the apoptotic events in the asthmatic airway epithelial cells, we quantitated caspase-3 cleavage and activation. Caspase-3 activity and cleavage (17 kd) was detectable in asthmatic epithelium, with asthma showing the highest activity (Figure 3, A and B). The increase in caspase-3 activity was related to %FEV1 of asthmatic patients (r = −0.507, P = 0.038; Figure 3C). Next we examined activation of the upstream caspase-9, known to be required for caspase-3 activation through the mitochondrial pathway and a key cellular target of caspase-3 and PARP. Evaluation of the key apoptotic targets in asthma revealed that cleavage fragments of caspase-9 (35 kd) and PARP (85 kd) were present in asthmatic epithelial cells (Figure 3; D to E), but not in healthy controls. Taken together, the fact that caspase-3 and -9, and PARP cleavage products are found in asthmatic epithelial cells and that caspase-3 activity is increased and correlated with airflow in asthma, we conclude that apoptosis occurs in a disproportionately higher number of asthmatic airway epithelial cells and is related to the pathophysiology of asthma.
Figure 3.
Apoptosis in asthmatic epithelial cells. Immunoblots of lysates from freshly obtained human airway epithelial cells. A: Asthmatic airway epithelial cells have activation of caspase-3 as shown by the presence of the cleavage product (17 kd). B: Caspase-3 activity assay confirms significant increase in caspase activity in asthmatic airway epithelial cells as compared to controls (P < 0.05). C: Caspase-3 activity measured in asthmatic airway epithelial cells is inversely correlated with %FEV1. Immunoblots of lysates from freshly obtained human airway epithelial cells of asthmatic individuals shows increased cleavage products of caspase-9 (35 kd) (D) and PARP (85 kd) (E), molecular evidence of increased apoptosis in asthmatic airway epithelial cells as compared to healthy control airway epithelial cells.
Effects of Oxidative Stress on Airway Epithelial Cells
We have previously reported that asthmatic airways have diminished SOD activity with increased loss after antigen challenge.12,17 Furthermore, other reports have shown that loss of SOD can initiate apoptosis in some cell types,29 therefore we hypothesized that diminished SOD in airway epithelial cells might be a central event mediating airway epithelial cell apoptosis. To address whether inactivation of SOD and/or increasing reactive oxygen species play a role in airway apoptosis, we treated BET1A cells with pyrogallol, a superoxide-producing agent, 2-ME, a SOD activity inhibitor or hydrogen peroxide, in a dose- and time-dependent manner. 2-ME at 5 μmol/L effectively blocked the activity of SOD by up to 86% at 3 hours (P = 0.004, Figure 4A). However, it did not cause a decrease of SOD protein (data not shown). Quantitative measures of trypan blue showed decrease in cell viability (Figure 4A). The loss of cell viability because of apoptosis in 2-ME-treated cells is validated by two techniques. First, annexin V staining demonstrated an increase in apoptotic cells as early as 17 hours after 2-ME exposure (Figure 4B). Second, caspase-3 activity was significantly increased in a time-dependent matter (Figure 4C). Previous reports have shown that reactive oxygen species lead to apoptosis.29–31 As indicated in Figure 4D, H2O2 and the superoxide-producing compound pyrogallol also result in loss of SOD activity and increased apoptosis. To verify that loss of SOD may initiate apoptosis, we blocked MnSOD RNA using siRNA technique (Figure 5, A and C). Loss of MnSOD leads to caspase-3 activation (Figure 5B). Taken together, these data support the conclusion that loss of SOD, and specifically MnSOD, is one mechanism of apoptosis in epithelial cells. Of note, MnSOD is central for scavenging intramitochondrial reactive oxygen species. Loss of MnSOD has been shown to lead to opening of permeability transition pores in the outer mitochondrial membrane and accelerate the release of cytochrome c, triggering apoptosis through activation of caspases.31 To examine if mitochondria are involved in the apoptotic events in epithelial cells treated with 2-ME, we investigated upstream mediators of caspase-3 activation, ie, BAX, a death-promoting member of the BclII family. The up-regulation of BAX levels after 2-ME treatment suggests that oxidative processes and the mitochondria are involved in the activation of caspase-9 and caspase-3 and entry into apoptosis (Figure 6A). Previous work has shown that oxidative stress decreases intracellular GSH through efflux, and GSH efflux has been identified as a proximal mediator of apoptosis through induction and activation of BAX.32,33 Here, inhibition of SOD activity also caused rapid depletion of intracellular GSH, consistent with increased intracellular oxidant stress (Figure 6B). Thus, our results also link BAX activation to oxidative stress and decreased intracellular GSH in vitro. Furthermore BET1A cells with SOD inhibition by 2-ME had an increase in tyrosine-nitrated proteins, a marker of peroxynitrite (Figure 6C). Human airway epithelial cells exposed to 2-ME showed a similar pattern of nitration (data not shown). Lysates from 2-ME-treated BET1A cells were evaluated by two-dimensional gels and corresponding immunopositive proteins were excised from the parent acrylamide gel, digested in-gel with trypsin, and tryptic peptides were analyzed with mass spectroscopy. Database searching with the peptide masses identified several proteins (Table 1). Collectively, these data support the notion that apoptosis of airway epithelial cells occurs in response to inhibition of SOD and an increase of reactive oxygen and nitration species. Subsequent decrease in intracellular GSH, which occurs in response to oxidative and nitrative stress, may trigger BAX induction and activation, followed by procaspase-9 and -3 activation.
Figure 4.
Loss of SOD leads to activation of caspase-3. A: Inhibition of SOD activity by 5 μmol/L 2-ME leads to apoptotic death of BET1A cells. Graph shows loss of SOD activity (•) and increased apoptotic cells (○) in BET1A cells after treatment with 2-ME. B: Histograms of the fluorescence of BET1A cells stained with annexin V-fluorescein isothiocyanate to demonstrate apoptosis at various times after exposure to 2-ME. Number of cells in each channel are shown plotted against a logarithmic abscissa. C: Inhibition of SOD activity by 5 μmol/L 2-ME in BET1A cells increases caspase-3 activity. D: Exposure of BET1A cells to pyrogallol and H2O2 leads to a decrease in SOD activity and an increase in caspase-3 activity. All data are representative of at least three experiments.
Figure 5.
Silencing of MnSOD. A and C: Northern analysis and immunoblot analysis of 293T cells at 48 hours after transfection with MnSOD siRNA. γ- and β-actin were used as a control for sample loading. B: MnSOD siRNA (48 hours) increases caspase-3 activity (analysis of variance, P = 0.038). As negative control cells were transfected with 50 nmol/L siRNA control. All data are representative of three experiments.
Figure 6.
A: Inhibition of SOD in BET1A cells leads to time-dependent induction of BAX levels. β-Actin was used as a control for equal loading of protein. B: Decreased levels of intracellular GSH at various times after inhibition of SOD confirms loss of reducing potential (C) whereas increased tyrosine nitration of proteins in BET1A cells after SOD inhibition indirectly indicates oxidative and nitrative stress. All data are representative of three experiments.
Table 1.
Identification of Nitrated Proteins in Airway Epithelial Cells Exposed to the SOD Inhibitor 2-Methoxyoestradiol
| Protein | pI | Molecular weight (kd) | Accession no. |
|---|---|---|---|
| Fascin | 6.8 | 55 | 2498357 |
| Dihydrolipoamide | 7.6 | 55 | 66123 |
| Alpha enolase | 6.9 | 47 | 119339 |
| Voltage-dependent anion channel 2 | 6.8 | 30 | 31890058 |
| Ran full-length protein chain c | 9.4 | 21 | 5107637 |
| Citrate synthase | 8.4 | 51 | 14,603,295 |
| Actin | 5.8 | 40 | 16359158 |
| Phosphoglycerate kinase | 8.3 | 44 | 2144428 |
| Fructose-biphosphate aldolase | 8.3 | 39 | 68183 |
| 1-Lactate dehydrogenase | 8.4 | 36 | 65,922 |
| Glyceraldehyde-3-phosphate dehydrogenase | 8.5 | 36 | 625203 |
| Voltage-dependent anion selective channel | 8.6 | 30 | 130683 |
| Histone h3/b | 11.2 | 15 | 18202621 |
| Histone h2b | 10.3 | 13 | 7381193 |
| Peptidylprolyl isomerase | 7.6 | 18 | 118102 |
Nitrated proteins found on two-dimensional gel shown in Figure 6C were identified by peptide mass mapping using product-ion (MS/MS) spectra.
Nitration and Oxidation of MnSOD in Asthmatic Airway Epithelial Cells
Previous studies indicate that MnSOD is susceptible to oxidative and nitrative modifications, which lead to inactivation.30,34–36 To investigate whether or not MnSOD protein is modified in asthmatic airways, epithelial cells were recovered during bronchoscopy, the cells lysed, and then MnSOD was immunoprecipitated, followed by Western blot analyses using anti-nitrotyrosine antibody. Nitrated MnSOD was identified in the freshly obtained asthmatic airway epithelial cells (Figure 7A). To investigate the degree of nitration and oxidation, MnSOD was purified by immunoprecipitation and molecular markers of multiple distinct oxidative pathways were quantified by stable isotope dilution tandem mass spectrometry (Table 2). Interestingly, the oxidation of phenylalanine to m-Tyr and o-Tyr such as via exposure to hydroxyl radical-like oxidants, chlorination of tyrosine (a specific molecular marker for myeloperoxidase-catalyzed halogenation), and oxidative cross-linking of tyrosine as monitored by dityrosine (a product of tyrosyl radical) were the dominant modifications noted. This pattern of oxidative modification is consistent with MnSOD exposure to both Fenton/Haber-Weiss reaction mechanisms, ie, redox active transition metal ion-catalyzed oxidation and myeloperoxidase-catalyzed oxidation, even in airways of mild asthmatics. Consistent with our immunodetection studies, nitration of tyrosine was also present in MnSOD recovered from asthmatic airway epithelial cells, indicating exposure to nitrating oxidants such as peroxynitrite/peroxycarboxynitrite or peroxidase-mediated reactive nitrogen species.14,37 Although the oxidative modifications monitored only represent a subfraction of total oxidative insults experienced by epithelial cell MnSOD within asthmatic airways, the quantification of this diverse array of distinct oxidative modifications provides insight into the potential degree of SOD functional impairment from oxidative processes. Given that there are 10 tyrosine residues per monomer and 4 monomers form an active MnSOD tetramer, if modification of only 1 tyrosine per MnSOD tetramer is sufficient to affect activity, then the observed cumulative modification burden of 1.13 to 1.73 mmol/mol tyrosine in isolated MnSOD predicts up to a 6% loss of MnSOD activity in these mild asthmatics. It is interesting to speculate that loss of activity may be greater in asthma exacerbation and in severe asthma conditions in which generation of reactive oxygen and nitrogen species is greatly increased.14,15,38
Figure 7.
Nitration of SOD in asthmatic airway epithelial cells. A: Lysates from asthmatic or control airway epithelial cells were immunoprecipitated using anti-MnSOD Ab, run on a 4 to 20% gradient gel, and immunoblotted with anti-nitrotyrosine antibody (top panel, lanes 1 and 2). The lower band confirms equal amount of MnSOD after immunoprecipitation. Pure MnSOD and MnSOD nitrated in vitro served as controls (lanes 3 and 4). Experiments were done in triplicate. B: Protein-bound nitrotyrosine of MnSOD purified from asthmatic airway epithelium was quantified by stable isotope dilution LC-MS interfaced to an HPLX system.
Table 2.
Oxidative Modifications in MnSOD from Asthmatic Airway Epithelial Cells
| NO2Y/Y | BrY/Y | ClY/Y | mY/Phe | oY/Phe | DiY/Y | Total |
|---|---|---|---|---|---|---|
| 0.10–0.13 | 0.02–0.04 | 0.11–0.47 | 0.03–0.24 | 0.16–0.55 | 0.31–0.71 | 1.13–1.73 |
Data presented represent ranges in values observed in MnSOD isolated from epithelial cell brushings from mild asthmatic subjects. Results are normalized to the content of the precursor amino acid (mmol oxidation product/mol precursor tyrosine or phenylalanine) that is monitored within the same injection. All data are representative of four asthmatic individuals. Y, tyrosine; NO2Y, nitrotyrosine; ClY, chlorotyrosine; BrY, bromotyrosine; DiY, dityrosine; oY, o-tyrosine; mY, m-tyrosine; Phe, phenylalanine.
Relation of SOD to Clinical Features of Asthma
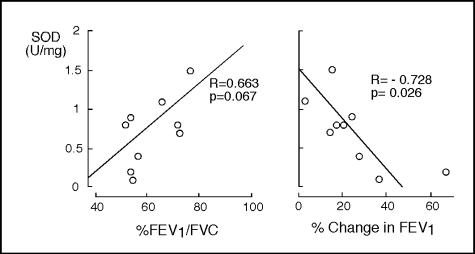
Loss of airway epithelial cells has been postulated to be a contributing mechanism to the airway hyperresponsiveness of asthma.18 On the basis that reduction of SOD activity is directly linked to apoptotic death of bronchial epithelial cells, we hypothesized that diminished SOD activity might be related to physiological parameters of asthma in vivo. To test this, we evaluated airway activity of antioxidant enzymes in nine asthmatics in relation to airflow and responsiveness to inhaled bronchodilator. SOD activity in airway epithelial cells of asthmatics correlated with %FEV1/FVC, and demonstrated significant inverse correlation with airway reactivity as determined by percent change in FEV1 after bronchodilator (Figure 8), although FEV1 itself did not correlate with SOD activity. Interestingly, SOD activity was the only antioxidant enzyme that correlated with pulmonary function of asthmatic individuals (Table 3).
Figure 8.
SOD loss is related to airflow limitation in asthma. Airway epithelial cell SOD activity is inversely correlated to airway response to albuterol (% change in FEV1) and correlate with %FEV1/FVC.
Table 3.
Correlation of Lung Functions with Asthmatic Airway Epithelial Cell Antioxidant Enzymes
| Lung functions | SOD | GPx | Catalase |
|---|---|---|---|
| % FEV1/FVC | r=0.663 | r=0.088 | r=−0.400 |
| P=0.067 | P=0.821 | P=0.286 | |
| % change in FEV1 | r=−0.728 | r=0.326 | r=0.383 |
| P=0.026 | P=0.391 | P=0.309 |
FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity.
Discussion
Recent progress has revealed asthma as a chronic inflammatory disease.13–15,39–41 Current understanding suggests that inflammation leads to remodeling events in the airway, which are often progressive and contributory to severe morbidity and refractoriness to treatment. However the specific mechanisms by which inflammation leads to asthmatic airway remodeling are unclear.1–3,42 Here, we reveal apoptosis as a mechanism for airway epithelial cell loss, a hallmark of remodeling in asthma, and identify loss of catalytically active SOD as an initiating event for entry into programmed cell death. Apoptosis in asthmatic airway epithelial cells was confirmed from three lines of evidence. First, immunostaining of endobronchial or brush biopsies reveals a striking increase in TUNEL-positive bronchial epithelial cells in asthma as compared to healthy nonsmoking controls. Previous studies have suggested epithelial cell apoptosis in the airways through observation of TUNEL-positive cells8 and caspase-3 and PARP immunostaining in biopsies of asthmatic individuals.10 However, Druilhe and colleagues9 noted a failure to detect differences in the number of TUNEL-positive bronchial epithelial cells between control and asthmatic airway endobronchial biopsies, perhaps because of detachment and loss of many of the apoptotic epithelial cells into the lumen during the process of biopsy. Others have indicated that the loss of epithelium in asthmatic biopsies may be an artifact of sampling.5 In this study, cells obtained by gentle brushing of the airway from asthmatic and healthy controls show an increase in TUNEL-positive cells in asthmatic biopsies, whereas only rare cells are TUNEL-positive in healthy control brushings. The marked increase of proliferative cells in asthma as determined by increased MIB-1, together with a previous report of increased expression of epidermal growth factor receptor,6 provides conclusive evidence for apoptosis, as enhanced proliferation of cells is a repair mechanism that occurs in association with accelerated apoptosis and loss of cells.2 Ongoing repair also substantiates that epithelial damage and shedding is in progress in vivo. Finally, the terminal stages of apoptosis require the activation of caspases by proteolytic cleavage, which then proteolytically cleave cellular proteins, including PARP. Hence, the detection of the cleaved form of caspase-3 and the increased activity is undeniable evidence of ongoing apoptosis in asthmatic airway epithelial cells.
SOD enzymes exist as three isoforms, the cellular form, Cu/ZnSOD, the extracellular form, and the mitochondrial form, MnSOD. SODs function as a first line of defense against oxidant stress, and are essential for aerobic life, eg, deletion mutations of the MnSOD gene in mice result in death.43,44 In further support of a role for SOD in cell survival, squamous cell carcinoma cells transfected with anti-sense MnSOD show an increase in levels of proapoptotic proteins such as BAX, which increase susceptibility to anti-cancer drug therapy.45 Here, we show that loss of SOD activity, specific inhibition of MnSOD, and/or increased production of superoxide leads to increased levels of BAX, cleavage and activation of caspase-3, and changes in the redox state of the cells. Previously, we have shown that airway epithelial cells exposed to oxidative stress rapidly shunt out glutathione, resulting in increased extracellular, but transient depletion of intracellular glutathione.22 Interestingly, efflux of glutathione reproducibly activates BAX and cytochrome c release in epithelial cells in vitro and is one established mechanism for induction of apoptosis.32,33,46 In support of increased transcellular glutathione fluctuation in the upstream events leading to epithelial cell apoptosis in asthma, glutathione levels are higher than normal in the asthmatic airway lining fluid, indicating increased efflux from epithelial cells in vivo.42,47–49
An overwhelming number of studies support oxidative and nitrative processes in the pathogenesis of asthma.38,50–52 For example, higher than normal levels of oxidants, nitric oxide, and reactive nitrogen species are present in the airways of asthmatics, and more severe obstruction in asthma is correlated with increased spontaneous and stimulus-induced generation of oxidants by inflammatory cells in the airway lumen.38,50–52 Nitrotyrosine is a valid and semiquantitative marker for the presence of reactive nitrogen species, specifically peroxynitrite or peroxidase-catalyzed oxidative and nitrative events.13,26,53 Here, evidence for reactive oxygen and nitrogen species involvement in airway epithelial cell apoptosis in asthma include the finding of increased nitrotyrosine in epithelial cells after inhibition of SOD in vitro, and in airway epithelial cells in vivo in asthma in other studies.13,22,54 Other reports have shown that MnSOD is a target for tyrosine nitration and oxidation,30,55 which leads to loss of enzyme function, subsequent oxidative damage of mitochondrial proteins, and ultimately cell death.30,56 Here, we provide quantitative data on MnSOD oxidation and nitration in human asthmatic lungs. Between 5% and 7% of MnSOD recovered from asthmatic airway epithelial cells possess at least one oxidative modification, with the majority of modifications related to Fenton-Haber Weiss reaction chemistry and/or peroxidase-catalyzed oxidation. Although not evaluated in this study, Alvarez and colleagues36 recently showed that peroxynitrite causes oxidative modifications of the CuZnSOD and loss of activity through formation of histidinyl radicals. Hence, oxidative inactivation of CuZn SOD may also contribute to the loss of total SOD activity noted in asthma.17,18 The loss of SOD activity likely reflects the increased oxidative and nitrative stress in the asthmatic airway, and may serve as a marker of asthma severity. In support of SOD as a surrogate marker of oxidant stress and asthma severity, Smith and colleagues18 showed a correlation between the degree of airway reactivity to methacholine and SOD activity levels. Here, reactivity measured as the change in FEV1 after bronchodilator confirms the association of airway hyperreactivity to SOD activity. Together, these findings support a link between SOD activity and physiological parameters of asthma severity. Murine models of asthma also provide evidence of a link between antioxidants and airway hyperresponsiveness. For example, transgenic mice that overexpress SOD have decreased allergen-induced physiological changes in the airway in comparison to controls.57 In other acute and chronic inflammation models, SOD mimics reduced PARP immunofluorescence, providing further evidence of a role for SOD in inhibition of apoptosis and inflammation.58,59 Similarly, treatment with SOD mimics reduces the magnitude of ovalbumin-induced airway hyperresponsiveness to methacholine in murine models of asthma.60 Based on this study and others, we propose that loss of SOD activity in asthma occurs, in part, as a consequence of MnSOD protein modifications in the oxidative and nitric oxide-rich environment of the asthmatic airway, and that SOD inactivation and oxidant stress trigger apoptosis and loss of airway epithelial cells, which contributes significantly to airway remodeling and hyperreactivity of asthma.
Acknowledgments
We thank J. Lang for artwork, C. Harris for BET1A cells, H. Markgraaff and D. Schmitt for technical assistance, and the Cleveland Clinic Lerner Research Institute Proteomics Core and M. Kinter for assistance with mass spectroscopy analyses.
Footnotes
Address reprint requests to Serpil C. Erzurum, M.D., Cleveland Clinic Foundation, 9500 Euclid Ave., A90, Cleveland, OH 44195. E-mail: erzurus@ccf.org.
Supported by NIH Grants AI 70640, HL 04265 N01 RR018390, HL076491 and HL 61878.
References
- Bousquet J, Jeffery PK, Busse WW, Johnson M, Vignola AM. Asthma. From bronchoconstriction to airways inflammation and remodeling. Am J Respir Crit Care Med. 2000;161:1720–1745. doi: 10.1164/ajrccm.161.5.9903102. [DOI] [PubMed] [Google Scholar]
- Davies DE, Wicks J, Powell RM, Puddicombe SM, Holgate ST. Airway remodeling in asthma: new insights. J Allergy Clin Immunol. 2003;111:215–226. doi: 10.1067/mai.2003.128. [DOI] [PubMed] [Google Scholar]
- Busse WW, Banks-Schlegel S, Wenzel SE. Pathophysiology of severe asthma. J Allergy Clin Immunol. 2000;106:1033–1042. doi: 10.1067/mai.2000.111307. [DOI] [PubMed] [Google Scholar]
- Hocking DC. Fibronectin matrix deposition and cell contractility: implications for airway remodeling in asthma. Chest. 2002;122:275S–278S. doi: 10.1378/chest.122.6_suppl.275s. [DOI] [PubMed] [Google Scholar]
- Ordonez C, Ferrando R, Hyde DM, Wong HH, Fahy JV. Epithelial desquamation in asthma: artifact or pathology? Am J Respir Crit Care Med. 2000;162:2324–2329. doi: 10.1164/ajrccm.162.6.2001041. [DOI] [PubMed] [Google Scholar]
- Puddicombe SM, Polosa R, Richter A, Krishna MT, Howarth PH, Holgate ST, Davies DE. Involvement of the epidermal growth factor receptor in epithelial repair in asthma. FASEB J. 2000;14:1362–1374. doi: 10.1096/fj.14.10.1362. [DOI] [PubMed] [Google Scholar]
- Trautmann A, Akdis M, Schmid-Grendelmeier P, Disch R, Brocker EB, Blaser K, Akdis CA. Targeting keratinocyte apoptosis in the treatment of atopic dermatitis and allergic contact dermatitis. J Allergy Clin Immunol. 2001;108:839–846. doi: 10.1067/mai.2001.118796. [DOI] [PubMed] [Google Scholar]
- Trautmann A, Schmid-Grendelmeier P, Kruger K, Crameri R, Akdis M, Akkaya A, Brocker EB, Blaser K, Akdis CA. T cells and eosinophils cooperate in the induction of bronchial epithelial cell apoptosis in asthma. J Allergy Clin Immunol. 2002;109:329–337. doi: 10.1067/mai.2002.121460. [DOI] [PubMed] [Google Scholar]
- Druilhe A, Wallaert B, Tsicopoulos A, Lapa e, Silva JR, Tillie-Leblond I, Tonnel AB, Pretolani M. Apoptosis, proliferation, and expression of Bcl-2, Fas, and Fas ligand in bronchial biopsies from asthmatics. Am J Respir Cell Mol Biol. 1998;19:747–757. doi: 10.1165/ajrcmb.19.5.3166. [DOI] [PubMed] [Google Scholar]
- Bucchieri F, Puddicombe SM, Lordan JL, Richter A, Buchanan D, Wilson SJ, Ward J, Zummo G, Howarth PH, Djukanovic R, Holgate ST, Davies DE. Asthmatic bronchial epithelium is more susceptible to oxidant-induced apoptosis. Am J Respir Cell Mol Biol. 2002;27:179–185. doi: 10.1165/ajrcmb.27.2.4699. [DOI] [PubMed] [Google Scholar]
- O’Sullivan MP, Tyner JW, Holtzman MJ. Apoptosis in the airways: another balancing act in the epithelial program. Am J Respir Cell Mol Biol. 2003;29:3–7. doi: 10.1165/rcmb.F273. [DOI] [PubMed] [Google Scholar]
- Comhair SA, Erzurum SC. Antioxidant responses to oxidant-mediated lung diseases. Am J Physiol. 2002;283:L246–L255. doi: 10.1152/ajplung.00491.2001. [DOI] [PubMed] [Google Scholar]
- Dweik RA, Comhair SA, Gaston B, Thunnissen FB, Farver C, Thomassen MJ, Kavuru M, Hammel J, Abu-Soud HM, Erzurum SC. NO chemical events in the human airway during the immediate and late antigen-induced asthmatic response. Proc Natl Acad Sci USA. 2001;98:2622–2627. doi: 10.1073/pnas.051629498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacPherson JC, Comhair SA, Erzurum SC, Klein DF, Lipscomb MF, Kavuru MS, Samoszuk MK, Hazen SL. Eosinophils are a major source of nitric oxide-derived oxidants in severe asthma: characterization of pathways available to eosinophils for generating reactive nitrogen species. J Immunol. 2001;166:5763–5772. doi: 10.4049/jimmunol.166.9.5763. [DOI] [PubMed] [Google Scholar]
- Wu W, Samoszuk MK, Comhair SA, Thomassen MJ, Farver CF, Dweik RA, Kavuru MS, Erzurum SC, Hazen SL. Eosinophils generate brominating oxidants in allergen-induced asthma. J Clin Invest. 2000;105:1455–1463. doi: 10.1172/JCI9702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comhair SA, Bhathena PR, Dweik RA, Kavuru M, Erzurum SC. Rapid loss of superoxide dismutase activity during antigen-induced asthmatic response. Lancet. 2000;355:624. doi: 10.1016/S0140-6736(99)04736-4. [DOI] [PubMed] [Google Scholar]
- De Raeve HR, Thunnissen FB, Kaneko FT, Guo FH, Lewis M, Kavuru MS, Secic M, Thomassen MJ, Erzurum SC. Decreased Cu, Zn-SOD activity in asthmatic airway epithelium: correction by inhaled corticosteroid in vivo. Am J Physiol. 1997;272:L148–L154. doi: 10.1152/ajplung.1997.272.1.L148. [DOI] [PubMed] [Google Scholar]
- Smith LJ, Shamsuddin M, Sporn PH, Denenberg M, Anderson J. Reduced superoxide dismutase in lung cells of patients with asthma. Free Radic Biol Med. 1997;22:1301–1307. doi: 10.1016/s0891-5849(96)00550-3. [DOI] [PubMed] [Google Scholar]
- Huang P, Feng L, Oldham EA, Keating MJ, Plunkett W. Superoxide dismutase as a target for the selective killing of cancer cells. Nature. 2000;407:390–395. doi: 10.1038/35030140. [DOI] [PubMed] [Google Scholar]
- Bethesda: National Institutes of Health,; Guidelines for the Diagnosis and the Management of Asthma, Expert Panel Report II. 1997:pp 97–4051. [Google Scholar]
- Reddel RR, Ke Y, Gerwin BI, McMenamin MG, Lechner JF, Su RT, Brash DE, Park JB, Rhim JS, Harris CC. Transformation of human bronchial epithelial cells by infection with SV40 or adenovirus-12 SV40 hybrid virus, or transfection via strontium phosphate coprecipitation with a plasmid containing SV40 early region genes. Cancer Res. 1988;48:1904–1909. [PubMed] [Google Scholar]
- Comhair SA, Bhathena PR, Farver C, Thunnissen FB, Erzurum SC. Extracellular glutathione peroxidase induction in asthmatic lungs: evidence for redox regulation of expression in human airway epithelial cells. FASEB J. 2001;15:70–78. doi: 10.1096/fj.00-0085com. [DOI] [PubMed] [Google Scholar]
- Nebot C, Moutet M, Huet P, Xu JZ, Yadan JC, Chaudiere J. Spectrophotometric assay of superoxide dismutase activity based on the activated autoxidation of a tetracyclic catechol. Anal Biochem. 1993;214:442–451. doi: 10.1006/abio.1993.1521. [DOI] [PubMed] [Google Scholar]
- Comhair SA, Lewis MJ, Bhathena PR, Hammel JP, Erzurum SC. Increased glutathione and glutathione peroxidase in lungs of individuals with chronic beryllium disease. Am J Respir Crit Care Med. 1999;159:1824–1829. doi: 10.1164/ajrccm.159.6.9810044. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Zheng L, Nukuna B, Brennan ML, Sun M, Goormastic M, Settle M, Schmitt D, Fu X, Thomson L, Fox PL, Ischiropoulos H, Smith JD, Kinter M, Hazen SL. Apolipoprotein A-I is a selective target for myeloperoxidase-catalyzed oxidation and functional impairment in subjects with cardiovascular disease. J Clin Invest. 2004;114:529–541. doi: 10.1172/JCI21109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna SL, Sherman NE, Kinter MT, Goldberg JB. Comparison of proteins expressed by Pseudomonas aeruginosa strains representing initial and chronic isolates from a cystic fibrosis patient: an analysis by 2-D gel electrophoresis and capillary column liquid chromatography-tandem mass spectrometry. Microbiology. 2000;146:2495–2508. doi: 10.1099/00221287-146-10-2495. [DOI] [PubMed] [Google Scholar]
- Boers JE, Ambergen AW, Thunnissen FB. Number and proliferation of basal and parabasal cells in normal human airway epithelium. Am J Respir Crit Care Med. 1998;157:2000–2006. doi: 10.1164/ajrccm.157.6.9707011. [DOI] [PubMed] [Google Scholar]
- Siwik DA, Tzortzis JD, Pimental DR, Chang DL, Pagano PJ, Singh K, Sawyer DB, Colucci WS. Inhibition of copper-zinc superoxide dismutase induces cell growth, hypertrophic phenotype, and apoptosis in neonatal rat cardiac myocytes in vitro. Circ Res. 1999;85:147–153. doi: 10.1161/01.res.85.2.147. [DOI] [PubMed] [Google Scholar]
- Macmillan-Crow LA, Cruthirds DL. Invited review: manganese superoxide dismutase in disease. Free Radic Res. 2001;34:325–336. doi: 10.1080/10715760100300281. [DOI] [PubMed] [Google Scholar]
- Fujimura M, Morita-Fujimura Y, Kawase M, Copin JC, Calagui B, Epstein CJ, Chan PH. Manganese superoxide dismutase mediates the early release of mitochondrial cytochrome C and subsequent DNA fragmentation after permanent focal cerebral ischemia in mice. J Neurosci. 1999;19:3414–3422. doi: 10.1523/JNEUROSCI.19-09-03414.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghibelli L, Fanelli C, Rotilio G, Lafavia E, Coppola S, Colussi C, Civitareale P, Ciriolo MR. Rescue of cells from apoptosis by inhibition of active GSH extrusion. FASEB J. 1998;12:479–486. doi: 10.1096/fasebj.12.6.479. [DOI] [PubMed] [Google Scholar]
- Ghibelli L, Coppola S, Fanelli C, Rotilio G, Civitareale P, Scovassi AI, Ciriolo MR. Glutathione depletion causes cytochrome c release even in the absence of cell commitment to apoptosis. FASEB J. 1999;13:2031–2036. doi: 10.1096/fasebj.13.14.2031. [DOI] [PubMed] [Google Scholar]
- MacMillan-Crow LA, Cruthirds DL, Ahki KM, Sanders PW, Thompson JA. Mitochondrial tyrosine nitration precedes chronic allograft nephropathy. Free Radic Biol Med. 2001;31:1603–1608. doi: 10.1016/s0891-5849(01)00750-x. [DOI] [PubMed] [Google Scholar]
- Guo W, Adachi T, Matsui R, Xu S, Jiang B, Zou MH, Kirber M, Lieberthal W, Cohen RA. Quantitative assessment of tyrosine nitration of manganese superoxide dismutase in angiotensin II-infused rat kidney. Am J Physiol. 2003;285:H1396–H1403. doi: 10.1152/ajpheart.00096.2003. [DOI] [PubMed] [Google Scholar]
- Alvarez B, Demicheli V, Duran R, Trujillo M, Cervenansky C, Freeman BA, Radi R. Inactivation of human Cu, Zn superoxide dismutase by peroxynitrite and formation of histidinyl radical. Free Radic Biol Med. 2004;37:813–822. doi: 10.1016/j.freeradbiomed.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Guo FH, Uetani K, Haque SJ, Williams BR, Dweik RA, Thunnissen FB, Calhoun W, Erzurum SC. Interferon gamma and interleukin 4 stimulate prolonged expression of inducible nitric oxide synthase in human airway epithelium through synthesis of soluble mediators. J Clin Invest. 1997;100:829–838. doi: 10.1172/JCI119598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun WJ, Reed HE, Moest DR, Stevens CA. Enhanced superoxide production by alveolar macrophages and air-space cells, airway inflammation, and alveolar macrophage density changes after segmental antigen bronchoprovocation in allergic subjects. Am Rev Respir Dis. 1992;145:317–325. doi: 10.1164/ajrccm/145.2_Pt_1.317. [DOI] [PubMed] [Google Scholar]
- Barnes PJ. Chronic obstructive pulmonary disease. N Engl J Med. 2000;343:269–280. doi: 10.1056/NEJM200007273430407. [DOI] [PubMed] [Google Scholar]
- Drazen JM, Israel E, O’Byrne PM. Treatment of asthma with drugs modifying the leukotriene pathway. N Engl J Med. 1999;340:197–206. doi: 10.1056/NEJM199901213400306. [DOI] [PubMed] [Google Scholar]
- Kaminsky DA, Mitchell J, Carroll N, James A, Soultanakis R, Janssen Y. Nitrotyrosine formation in the airways and lung parenchyma of patients with asthma. J Allergy Clin Immunol. 1999;104:747–754. doi: 10.1016/s0091-6749(99)70283-6. [DOI] [PubMed] [Google Scholar]
- Kelly EA, Busse WW, Jarjour NN. Increased matrix metalloproteinase-9 in the airway after allergen challenge. Am J Respir Crit Care Med. 2000;162:1157–1161. doi: 10.1164/ajrccm.162.3.9908016. [DOI] [PubMed] [Google Scholar]
- Lebovitz RM, Zhang H, Vogel H, Cartwright J, Jr, Dionne L, Lu N, Huang S, Matzuk MM. Neurodegeneration, myocardial injury, and perinatal death in mitochondrial superoxide dismutase-deficient mice. Proc Natl Acad Sci USA. 1996;93:9782–9787. doi: 10.1073/pnas.93.18.9782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Huang TT, Carlson EJ, Melov S, Ursell PC, Olson JL, Noble LJ, Yoshimura MP, Berger C, Chan PH, Wallace DC, Epstein CJ. Dilated cardiomyopathy and neonatal lethality in mutant mice lacking manganese superoxide dismutase. Nat Genet. 1995;11:376–381. doi: 10.1038/ng1295-376. [DOI] [PubMed] [Google Scholar]
- Ueta E, Yoneda K, Kimura T, Tatemoto Y, Doi S, Yamamoto T, Osaki T. Mn-SOD antisense upregulates in vivo apoptosis of squamous cell carcinoma cells by anticancer drugs and gamma-rays regulating expression of the BCL-2 family proteins, COX-2 and p21. Int J Cancer. 2001;94:545–550. doi: 10.1002/ijc.1513. [DOI] [PubMed] [Google Scholar]
- Jungas T, Motta I, Duffieux F, Fanen P, Stoven V, Ojcius DM. Glutathione levels and BAX activation during apoptosis due to oxidative stress in cells expressing wild-type and mutant cystic fibrosis transmembrane conductance regulator. J Biol Chem. 2002;277:27912–27918. doi: 10.1074/jbc.M110288200. [DOI] [PubMed] [Google Scholar]
- Kelly FJ, Mudway I, Blomberg A, Frew A, Sandstrom T. Altered lung antioxidant status in patients with mild asthma. Lancet. 1999;354:482–483. doi: 10.1016/S0140-6736(99)01812-7. [DOI] [PubMed] [Google Scholar]
- Meerschaert J, Kelly EA, Mosher DF, Busse WW, Jarjour NN. Segmental antigen challenge increases fibronectin in bronchoalveolar lavage fluid. Am J Respir Crit Care Med. 1999;159:619–625. doi: 10.1164/ajrccm.159.2.9806053. [DOI] [PubMed] [Google Scholar]
- Smith LJ, Houston M, Anderson J. Increased levels of glutathione in bronchoalveolar lavage fluid from patients with asthma. Am Rev Respir Dis. 1993;147:1461–1464. doi: 10.1164/ajrccm/147.6_Pt_1.1461. [DOI] [PubMed] [Google Scholar]
- Bast A, Haenen GR, Doelman CJ. Oxidants and antioxidants: state of the art. Am J Med. 1991;91:2S–13S. doi: 10.1016/0002-9343(91)90278-6. [DOI] [PubMed] [Google Scholar]
- Postma DS, Renkema TE, Noordhoek JA, Faber H, Sluiter HJ, Kauffman H. Association between nonspecific bronchial hyperreactivity and superoxide anion production by polymorphonuclear leukocytes in chronic air-flow obstruction. Am Rev Respir Dis. 1988;137:57–61. doi: 10.1164/ajrccm/137.1.57. [DOI] [PubMed] [Google Scholar]
- Sedgwick JB, Calhoun WJ, Vrtis RF, Bates ME, McAllister PK, Busse WW. Comparison of airway and blood eosinophil function after in vivo antigen challenge. J Immunol. 1992;149:3710–3718. [PubMed] [Google Scholar]
- Aulak KS, Miyagi M, Yan L, West KA, Massillon D, Crabb JW, Stuehr DJ. Proteomic method identifies proteins nitrated in vivo during inflammatory challenge. Proc Natl Acad Sci USA. 2001;98:12056–12061. doi: 10.1073/pnas.221269198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleh D, Ernst P, Lim S, Barnes PJ, Giaid A. Increased formation of the potent oxidant peroxynitrite in the airways of asthmatic patients is associated with induction of nitric oxide synthase: effect of inhaled glucocorticoid. FASEB J. 1998;12:929–937. [PubMed] [Google Scholar]
- MacMillan-Crow LA, Crow JP, Kerby JD, Beckman JS, Thompson JA. Nitration and inactivation of manganese superoxide dismutase in chronic rejection of human renal allografts. Proc Natl Acad Sci USA. 1996;93:11853–11858. doi: 10.1073/pnas.93.21.11853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacMillan-Crow LA, Crow JP, Thompson JA. Peroxynitrite-mediated inactivation of manganese superoxide dismutase involves nitration and oxidation of critical tyrosine residues. Biochemistry. 1998;37:1613–1622. doi: 10.1021/bi971894b. [DOI] [PubMed] [Google Scholar]
- Larsen GL, White CW, Takeda K, Loader JE, Nguyen DD, Joetham A, Groner Y, Gelfand EW. Mice that overexpress Cu/Zn superoxide dismutase are resistant to allergen-induced changes in airway control. Am J Physiol. 2000;279:L350–L359. doi: 10.1152/ajplung.2000.279.2.L350. [DOI] [PubMed] [Google Scholar]
- Salvemini D, Mazzon E, Dugo L, Riley DP, Serraino I, Caputi AP, Cuzzocrea S. Pharmacological manipulation of the inflammatory cascade by the superoxide dismutase mimetic, M40403. Br J Pharmacol. 2001;132:815–827. doi: 10.1038/sj.bjp.0703841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvemini D, Mazzon E, Dugo L, Serraino I, De Sarro A, Caputi AP, Cuzzocrea S. Amelioration of joint disease in a rat model of collagen-induced arthritis by M40403, a superoxide dismutase mimetic. Arthritis Rheum. 2001;44:2909–2921. doi: 10.1002/1529-0131(200112)44:12<2909::aid-art479>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Chang LY, Crapo JD. Inhibition of airway inflammation and hyperreactivity by an antioxidant mimetic. Free Radic Biol Med. 2002;33:379–386. doi: 10.1016/s0891-5849(02)00919-x. [DOI] [PubMed] [Google Scholar]