Abstract
Interleukin (IL)-6 is a pleiotropic cytokine that has been shown to inhibit the growth of early stage and to promote the proliferation of advanced stage melanoma cells in vitro. In patients with metastasizing melanomas, highly increased IL-6 blood levels correlate with a poor response to chemotherapy and a worse overall prognosis, suggesting that IL-6 promotes melanoma progression in vivo. Here, we analyzed the role of IL-6 in melanoma development and progression in a transgenic mouse model. We bred IL-6-deficient mice with MT-ret transgenic animals predisposed for melanomas. While MT-ret transgenic animals develop severe melanosis of the skin and subcutis and subsequent melanomas at an incidence of 80% during their first year of life, MT-ret mice devoid of IL-6 developed preneoplastic melanosis and consecutive melanomas significantly less frequently (47%; P < 0.05). Moreover, the tumors were significantly smaller in the groups of MT-ret mice lacking one (P < 0.05) or both (P < 0.01) copies of the IL-6 gene. Immunoblot analysis revealed that ret transgene expression was not reduced in the skin of mice lacking IL-6, indicating that the observed decrease of melanoma incidence and of tumor sizes was not because of a down-regulation of transgene expression. Taken together, these results indicate that IL-6 enhances both the development of melanoma precursor lesions and the subsequent growth of the resulting tumors in the MT-ret model of melanoma development.
Interleukin (IL)-6 is a pleiotropic cytokine that induces the acute phase response, stimulates B- and T-lymphocytes, and regulates the growth, differentiation, and death of several cell populations including neurons and melanocytes.1–3 IL-6 promotes the development and progression of plasmacytomas4 and gliomas4,5 in vivo. It is also a growth-promoting factor for human basal cell carcinoma, Kaposi’s sarcoma, and prostate cancer cells in vitro.3,6
The IL-6 receptor system consists of IL-6 receptor α (IL-6Rα), the primary IL-6 receptor, and the ubiquitous gp130 signal-transducing receptor subunit. Binding of IL-6 to its receptor complex leads to the activation of janus kinases (Jaks) and subsequent phosphorylation, dimerization, and nuclear translocation of signal transducer and activator of transcription 3 (STAT3).2,7 STAT3 promotes tumor progression by regulating the expression of genes involved in growth control such as c-myc, anti-apoptosis [Bcl-x(L) and Mcl-1] and angiogenesis (vascular endothelial growth factor).8–11 In a recent study, STAT3 was found to be constitutively activated in some, but not all human melanoma cell lines and tumor specimens analyzed.12 However, blocking experiments revealed that STAT3 activation in these cell lines was apparently mainly caused by Src tyrosine kinase activity and not by Jak activation.12
In vitro growth of early stage melanoma cells has been shown to be inhibited by IL-6 in several studies.13–16 In the A375 cell line, this inhibition is mediated by IL-6-induced STAT activation.17,18 Cells derived from advanced melanomas at metastatic stages often lose this anti-proliferative response to IL-6.16,19,20 In fact, anti-sense oligonucleotides blocking IL-6 gene expression inhibited the growth of these cell lines, suggesting that IL-6 promotes advanced stage melanoma cell growth by an autocrine mechanism.19 In support of this hypothesis, melanoma cells, and especially cells from advanced tumors, have been shown to express both IL-6 and IL-6 receptor α.19,21–26 Moreover, IL-6 is probably produced in significant amounts as a paracrine stimulator by stromal cells and by cells in the invaded tissue in the vicinity of melanomas including cells of reactive inflammatory infiltrates. In addition, ultraviolet light alters the expression of many genes in keratinocytes with a consequent release of cytokines such as IL-1α, IL-6, and IL-12 in vitro, 27 suggesting that exposure to sunlight might increase IL-6 levels in skin.
Supporting the hypothesis that IL-6 promotes late-stage melanoma progression, elevated IL-6 serum levels have been found in patients with metastatic melanoma; IL-6 elevation was associated with larger tumor burden, resistance to chemo- and immunotherapy, and shorter median survival rate.28–30 Taken together, IL-6 effects on melanomas appear to be variable, depending on the tumor cell lines studied and on the stage of the disease.
To elucidate the functional role of IL-6 in the pathogenesis of melanomas in vivo, we made use of a mouse model that reflects the full range of progressive conversions of nonneoplastic melanocytes to melanoma cells. MT-ret transgenic mice express the human cellular Ret oncogene under the control of a mouse metallothionein-I (MT) promotor-enhancer.31 Metallothionein is expressed in mouse and human skin.32–34 In MT-ret transgenic animals, the c-Ret protein is expressed in the skin, mainly in the inner and outer root sheaths of hair follicles.35 Highest expression levels are found soon after birth.35 Homozygous MT-ret mice die in utero.36 Heterozygous MT-ret (MT-ret+/−) transgenic 304BL/6 mice are viable and develop severe systemic melanosis of the skin and the subcutis; melanocytic skin tumors develop from these precursor lesions and progress to malignant melanomas.36 The development of these malignant melanomas, most of which are phenotypically related to small cell melanomas in humans, resembles malignant transformation of human giant congenital melanocytic nevus and neurocutaneous melanosis.
To analyze the contribution of IL-6 to the development and progression of melanomas in MT-ret mice, we crossed these mice with IL-6-deficient animals.37 We report here that ablation of IL-6 leads to a reduction in tumor incidence and tumor size in this model of spontaneous melanoma.
Materials and Methods
Metallothionein/Ret Transgenic and IL-6-Deficient Mice
MT-ret transgenic mice36 were crossed with IL-6-deficient mice.37 All mice had been backcrossed for at least 10 generations to the C57BL/6 background. To produce MT-ret+/−/IL-6+/− and MT-ret+/−/IL-6−/− mice, MT-ret+/− mice were crossed with IL-6−/− mice and heterozygous MT-ret+/−/IL-6+/− offspring was crossed again to yield the MT-ret+/−/IL-6−/− genotype. Genotyping was performed by polymerase chain reaction of DNA isolated from tail biopsies (MT-ret+/− sense, 5′TCC CTT TTT GAT CAT ATC TAC ACC A3′ and MT-ret+/− anti-sense, 5′AAT CCA TGT GGA AGG GAG GGC TCG A3′, IL-6−/− 5′TTC CATC CAG TTG CCT TCT TGG3′, and 5′TTC TCA TTT CCA CGA TTT CCC AG′3, or neo 5′-CCG GAG AAC CTG CGT GCA ATC C3′). Details of the genotyping procedure are available on request. Tail DNA of all mice was stored at 4°C for regenotyping. The mice were monitored daily. Five weeks after the first detection of a visible or palpable tumor, the animals were killed using CO2. The carcasses were dissected and inspected carefully. The number of tumor nodules was counted. The diameters of all tumors in whole mount sections of the tumor-infiltrated areas were measured using a microscopic scale bar. Animals that did not develop a tumor were killed at 65 weeks of age. Tumor incidence and acceleration was monitored in Kaplan-Meier curves. These results were analyzed statistically using Wilcoxon’s signed-rank test. The extent of tumor necrosis in each group was determined by counting the number of tumor nodules containing necrotic areas. These data were evaluated statistically using the χ2 test. All experiments were performed according to the laws of the State of Bern governing the welfare of animals.
Sample Preparation
Normal skin biopsies, preneoplastic and neoplastic skin lesions, metastases, and all major organs were fixed in 4% buffered paraformaldehyde overnight at room temperature and embedded in paraffin. Paraffin sections were stained with hematoxylin and eosin (H&E) and analyzed histologically to assess tumor morphology, spread, vascularization, and interactions with adjacent tissues. In addition, unfixed material was snap-frozen in liquid nitrogen and stored at −80°C for cryostat sectioning and for molecular biological and enzymatic studies.
Immunohistochemistry and Lectin Staining
Immunostainings were performed using a polyclonal antiserum against S100 protein (DAKO, Glostrup, Denmark) and polyclonal antisera against STAT3 (Santa Cruz Biotechnology, Santa Cruz, CA) as well as activated, tyrosine 705 phosphorylated pY-STAT3 (Cell Signaling Technology, Beverly, MA). Consecutive 3- to 5-μm paraffin sections and cryostat sections were subjected to immunostaining using the alkaline phosphatase technique. Tissue sections were pretreated by boiling either in a pressure cooker for 5 minutes in citrate buffer at pH 6.0 (S100, STAT3) or in a microwave oven for 3 × 5 minutes in 5% urea buffer (pY-STAT3) and then washed for 5 minutes in Tris-buffered saline. Sections were then incubated for 30 minutes at 37°C with 10% normal goat serum before overnight incubation at 4°C with the primary antibody diluted 1:300 in 10% normal goat serum. Bound antibody was visualized using biotinylated goat anti-rabbit IgG or goat anti-mouse IgG (DAKO), alkaline phosphatase-conjugated streptavidin (DAKO), and new fuchsin-naphtol, followed by counterstaining with Mayer’s hematoxylin. To ensure the specificity of the primary antibodies, consecutive tissue sections were incubated either in the absence of primary antibody or with IgG of a nonimmunized rabbit. In these control sections no immunostaining was detected (not shown). Positive controls for the STAT3 and pY-STAT3 immunostaining included paraffin sections of 1) sections of brains of transgenic mice overexpressing the v-src oncogene in astrocytes under the control of the GFAP promoter, thereby constitutively activating STAT3;5 2) human glioblastomas, which overexpress STAT3 and accumulate activated pY-STAT3;5,38 and 3) mouse dorsal root ganglion neurons, which constitutively express IL-6 and IL-6Rα.39 In addition, staining with Bandeiraea simplicifolia lectin (Sigma, St. Louis, MO) was used to label lymphocytes as described.40 Lymph nodes of MT-ret mice served as positive controls. Paraffin sections from seven tumor-bearing mice of each group were stained using the lectin. The total number of tumor nodules as well as the number of nodules containing labeled cells was counted.
Generation of an MT-Ret Melanoma Cell Line and Cell Culture Conditions
A cell line (Mel25) was generated from a primary neck melanoma of a 29-week-old MT-ret+/− mouse. Tumor cells were dissociated by sieving through cell strainers (Falcon Becton Dickinson, Basel, Switzerland) and cultured in Dulbecco’s modified Eagle’s medium supplemented with 6% bovine calf serum and 6% horse serum. The cultures were treated with 100 μg/ml of cis-hydroxyproline (Sigma) as described41 until complete removal of fibroblasts. The cell line has now been cultured for more than 40 passages and is morphologically stable. It is immunoreactive for S100 protein, a melanoma cell marker (Figure 1h), and shows overexpression of Ret (Figure 3a). To determine whether Mel 25 cells express IL-6 and IL-6 receptor, reverse transcriptase-polymerase chain reaction was performed using the following primer sequences: mu IL-6 for 5′AGT TGC CTT CTT GGG ACT GA3′ and mu IL-6 reverse 5′CAG AAT TGC CAT TGC ACA AC3′. Mu IL-6R for 5′AAG CTT GGT TCC GAT TTC CT3′ and mu IL-6R reverse 5′TTC GCC TGA AGT CCT GAG AT3′. The β-actin gene served as a control: mu β-actin for 5′GCT ACA GCT TCA CCA CCA CA3′ and mu β-actin reverse 5′AAG GAA GGC TGG AAA AGA GC3′.
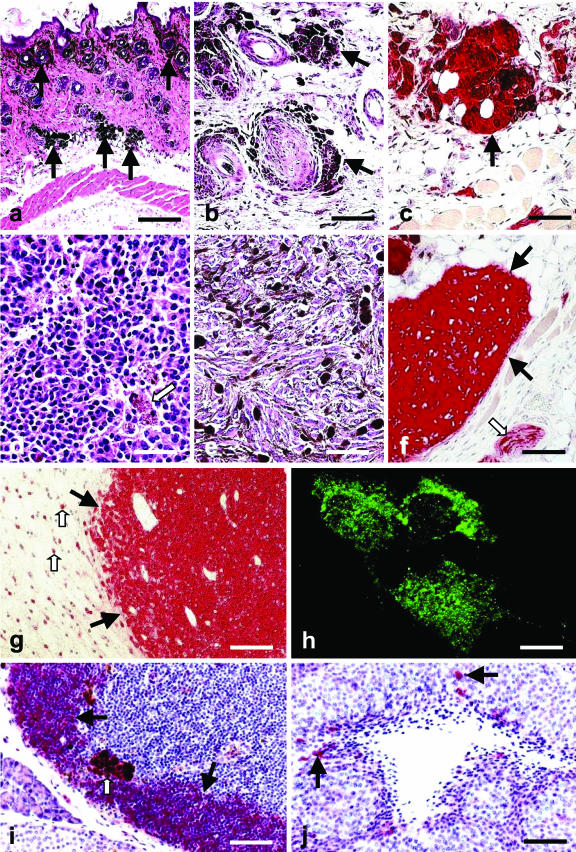
Figure 1.
a: Skin and subcutis of an adult MT-ret+/−/IL-6−/− mouse with several nests of pigmented preneoplastic cells (arrows). Paraffin section, H&E. b: Nodules of preneoplastic pigmented cells (arrows) in the subcutis of an adult MT-ret+/− mouse. Paraffin section, H&E. c: The preneoplastic cells are S100-immunoreactive (arrow). Paraffin section, hematoxylin counterstain. d: Slightly pigmented (arrow) skin melanoma in an adult MT-ret+/− mouse consisting of relatively isomorphic round cells. Still, this tumor proliferated rapidly and metastasized to several distant sites. Paraffin section, H&E. e: Skin melanoma in an adult MT-ret+/− mouse composed of highly polymorphic pigmented cells. Paraffin section, H&E. f: MT-ret+/− melanomas (filled arrows) are S100-immunoreactive (red). An adjacent subcutaneous nerve fascicle containing S100-immunoreactive Schwann cells is marked by an open arrow. Paraffin section, hematoxylin counterstain. g: S100-immunoreactive (red) cerebral melanoma metastasis (filled arrows) in an adult MT-ret+/− mouse. Adjacent reactive astrocytes are also S100-immunoreactive (open arrows). Paraffin section, hematoxylin counterstain. h: S100 immunofluorescence of Mel25 cells in culture. i: Prominent B. simplicifolia labeling of lymphocytes (filled arrows) in a lymph node of a MT-ret+/− mouse. Melanophages (open arrow) are also labeled. Paraffin section, hematoxylin counterstain. H&E. j: Few cells in the vicinity of a necrosis in a of a MT-ret+/− mouse melanoma are B. simplicifolia-positive (arrows). These cells can be classified morphologically as monocytes/macrophages because of their irregular shape and cytoplasmic extensions. Paraffin section, hematoxylin counterstain. H&E. Scale bars: 400 μm (a); 100 μm (b, c, i, j); 80 μm (d, e); 130 μm (f); 150 μm (g); 6 μm (h).
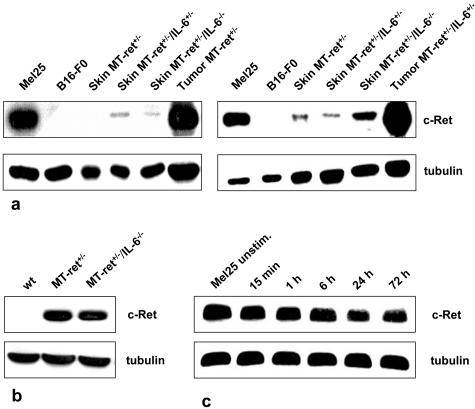
Figure 3.
a: Analysis of ret expression in the skin of adult MT-ret+/− mice by two independent immunoblots. Ret protein levels are rather low in the skin of MT-ret+/− mice, and there is no detectable decrease in ret expression in mice lacking one (IL-6+/−) or both (IL-6−/−) copies of the IL-6 gene. Melanomas of an MT-ret+/− and an MT-ret+/−/IL-6+/− mouse express larger amounts of ret protein, as does the Mel25 cell line. As expected, the B16-F0 cell line does not show any ret expression. b: Immunoblot analysis of MT-ret+/− melanomas. Tumors derived from IL-6+/+ and IL-6−/− mice show similar ret protein expression. Wt: skin from wild-type BL/6 mouse. c: No increase in ret protein expression in immunoblots of Mel25 cells after stimulation with H-IL-6 for 15 minutes to 72 hours.
To assess the response of Mel25 cells to exogenous IL-6 and IL-6Rα, melanoma cells were starved for 2 hours in serum-free medium and then cultured in Dulbecco’s modified Eagle’s medium for 15 and 30 minutes in the presence or absence of 10 to 100 ng/ml of recombinant IL-6 (R&D Systems, Abingdon, UK) or incubated for 0 minutes, 15 minutes, 1 hour, 6 hours, 24 hours, and 72 hours with 100 ng/ml of hyper-IL-6 (H-IL-6), a fusion protein of sIL-6R and IL-6, linked by a flexible peptide chain42 (generous gift by Prof. S. Rose-John, University of Kiel, Kiel, Germany). After incubation with H-IL-6, the survival and proliferation of Mel25 cells was analyzed using the MTT assay as described.43 The melanoma cell lines A375 (human) and B16-F0 (mouse, both obtained from American Type Culture Collection, Rockville, MD) served as controls. The pharmacological blockers, LY294002 and PD90059, were purchased from Sigma and Calbiochem (San Diego, CA), respectively. Using the MTT assay and these blockers, we determined survival-promoting effects of the PI3K/Akt and of the MAPK pathways, respectively, in Mel25 cells under full serum conditions.
Cell Lysis and Western Blot Analysis
Mouse tissues were snap-frozen in liquid nitrogen immediately on removal and weighed. Tissues were homogenized in ice-cold buffer (10% w/v) containing 0.32 mol/L sucrose, 0.5% Nonidet P-40, 0.5% sodium deoxycholate plus NaVO4, and Complete Protease Inhibitor Cocktail (Roche Diagnostics, Rotkreuz, Switzerland). Cultured melanoma cells were lysed on the plate with RIPA lysis buffer, Complete Protease Inhibitor Cocktail, and NaVO4. The lysates were cleared by centrifugation at 14,000 × g.
Protein concentration was determined by use of the BCA kit (Pierce, Rockford, IL) using bovine serum albumin as a standard. Equal amounts of protein on each lane were separated on 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis gels. The samples were then transferred (wet) on nitrocellulose paper. After blocking with 5% skim milk, blots were incubated with polyclonal antisera against Ret and STAT3 (both Santa Cruz Biotechnology), tyrosine-705 phosphorylated pY-STAT3 (Cell Signaling Technology), polyclonal anti-phospho-Akt (phospho-Ser 473) (Cell Signaling Technology); polyclonal anti-Akt 1:1000 (Cell Signaling Technology); monoclonal anti-phospho-p44/42 MAPK (Cell Signaling Technology) at 1:2000; monoclonal anti-MAPK at 1:5000 (BD Transduction Laboratories, Franklin Lakes, NJ) in 1% bovine serum albumin in Tris-buffered saline-Tween overnight followed by three 1-hour washes in Tris-buffered saline-Tween, and incubated with horseradish peroxidase-conjugated goat anti-rabbit antibodies (Pierce). After washing three times for 1 hour in Tris-buffered saline-Tween, blots were incubated for 1 minute with the ECL reagent (Pierce). Chemiluminescence was detected exposing the membranes to Kodak X-Omat films (Eastman-Kodak, Rochester, NY) for periods of 30 seconds to 12 hours.
Results
Decreased Incidence of Melanotic Precursor Lesions in MT-Ret+/−/IL-6−/− Mice
To assess the functional role of IL-6 in the pathogenesis of spontaneously arising malignant melanoma we compared the incidence of the melanoma precursor lesions, melanosis, and of malignant melanomas in MT-ret+/−, MT-ret+/−/IL-6+/− (heterozygous IL-6 knockout), and MT-ret+/−/IL-6−/− (homozygous IL-6 knockout) mouse strains. Melanosis of the skin, the subcutis, and the adjacent skeletal muscles was detected in 36 of 45 (80%) MT-ret+/− mice, and in 13 of 19 (68%) MT-ret+/−/IL-6+/− animals. The difference in the incidence of melanosis between the MT-ret+/− and the MT-ret+/−/IL-6+/− mice was not statistically significant (P > 0.1). However, only 7 of 15 (47%) MT-ret+/− mice that did not express any IL-6 (MT-ret+/−/IL-6−/− mice) developed melanosis. The difference in the incidences of melanosis between the MT-ret+/− group and the MT-ret+/−/IL-6−/− group was statistically significant (P < 0.02), indicating that the absence of IL-6 affects the development of the preneoplastic skin lesions. In those animals that developed melanosis, the extent of involvement of the skin and of adjacent structures showed no apparent difference whether the animals expressed IL-6 or not. The melanotic cells were S100-immunoreactive and often accumulated in the vicinity of hair follicles (Figure 1; a to c). The genotype of the MT-ret+/− mice of all three groups that did not develop melanosis was determined a second time using new tail biopsies as well as the DNA from the first biopsies that had been stored at 4°C. The initial genotyping of all nonmelanotic MT-ret+/−, MT-ret+/−/IL-6+/− and MT-ret+/−/IL-6−/− mice was confirmed by this analysis.
Decreased Melanoma Incidence in MT-Ret+/−/IL-6−/− Mice
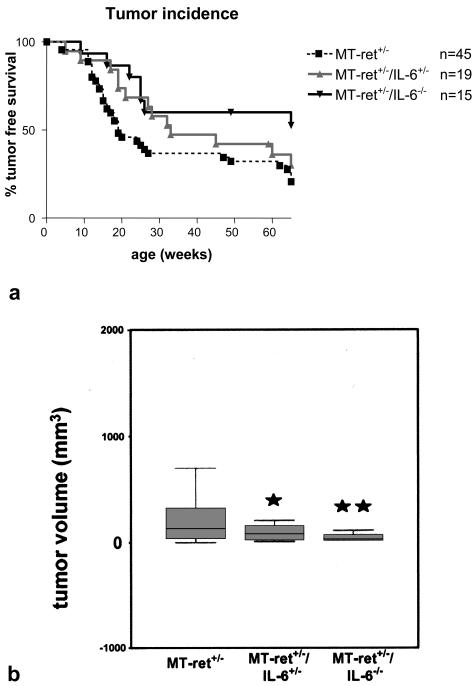
All animals that had developed melanosis also developed visible skin tumors during the observation period of 65 weeks, regardless of their IL-6 gene status. On the other hand, the nonmelanotic animals never showed any tumors. Thus, melanomas were significantly less frequent in MT-ret+/−/IL-6−/− mice compared to MT-ret+/− mice (P < 0.02; Figure 2a). The average age of development of a visible or palpable tumor was not different in the three groups.
Figure 2.
a: Kaplan-Meier curves depicting melanoma incidence in MT-ret+/− mice expressing IL-6 (MT-ret+/−), in MT-ret+/− mice heterozygous for IL-6 (MT-ret+/−/IL-6+/−), and in MT-ret+/− mice lacking the IL-6 gene (MT-ret+/−/IL-6−/−). b: Box plot summarizing the results of the tumor size measurements. Tumor sizes are significantly smaller in the IL-6+/− and IL-6−/− groups compared to the IL-6+/+ group of MT-ret+/− mice (P < 0.05 and P < 0.01, respectively).
In several lines of mice transgenic for oncogenes, the growth and development of tumors is dependent on continuous transgene expression.44 Thus, a direct effect of the lack of IL-6 on MT-ret transgene expression had to be excluded as a possible cause of the decreased incidence of melanosis and melanomas in the IL-6−/− mice. Therefore, we determined c-Ret protein levels in skin biopsies of mice of all experimental groups by immunoblotting. Ret protein levels were not reduced in the skin of MT-ret+/− mice lacking IL-6 (n = 3) compared to mice expressing one (n = 3) or both (n = 3) alleles of the IL-6 gene (Figure 3a), indicating that transgene expression is not decreased in mice lacking IL-6. In line with this observation, MT-ret-melanomas derived from IL-6+/+ and IL-6−/− mice showed similar Ret protein expression levels (Figure 3, a and b), and Mel25 cell stimulation with hIL-6 did not cause increased Ret expression (Figure 3c).
Smaller Primary Melanomas in MT-Ret+/−/IL-6−/− and MT-Ret+/−/IL-6+/− Mice Compared to MT-Ret+/− Animals
In all three groups, most primary tumors were found in the faces and the necks of the animals; another frequent primary location was the hip and upper hind limb area. Interestingly, when measuring the sizes of the nodules, we found that tumors were significantly smaller in the MT-ret+/− animals lacking one (P < 0.05) or both (P < 0.01) functional copies of the IL-6 gene (Figure 2b) compared to MT-ret+/− mice harboring two functional copies of the gene.
In all animals, these tumors infiltrated adjacent soft tissue and skeletal muscle and metastasized in transit and along lymphatic pathways as well as to distant sites such as lungs, mediastinum, heart, abdomen, and brain. The primary melanomas and metastases of all groups had a nodular architecture and consisted of round to spindle-shaped cells that showed a variable degree of pigmentation (Figure 1, d and e). Both the primary tumors and the metastases were S-100-immunoreactive (Figure 1, f and g). The number of metastases and the organs involved were similar in the MT-ret+/−, MT-ret+/−/IL-6+/−, and MT-ret+/−/IL-6−/− mice (not shown). There was no apparent reactive inflammation within the tumors or in their vicinity in any group. B. simplicifolia lectin staining revealed only very minor, if any, accumulations of lymphocytes and of activated monocytes/macrophages in tumors of each group; stained cells were almost exclusively found in the vicinity of the rare necroses (Figure 1, i and j).
Necrosis and Inflammation in MT-Ret Melanomas
The frequencies of tumor nodules containing necrotic areas were low in each group: 21 of 913 (2.4%) in the MT-ret+/− group, 2 of 194 (1.0%) in the MT-ret+/−/IL-6+/− group, and 1 tumor nodule of 141 (0.7%) in the MT-ret+/−/IL-6−/− group contained necrotic areas. Using the χ2 test, we found no significant difference in the incidence of necrosis in the three experimental groups. Using the lectin B. simplicifolia, we analyzed the amount of lymphocytic infiltration within and adjacent to MT-ret melanomas. This lectin is known to be a marker for T lymphocytes40 and strongly stained lymphocytes and, to a lesser extent, monocytes in mice of all groups. B. simplicifolia-labeled lymphocytes were almost completely absent from the MT-ret melanomas of all three experimental groups, and only very few monocytic cells located in the vicinity of the small necroses in some tumors were stained (Figure 1, i and j).
STAT3 in MT-Ret Mouse Skin and MT-Ret Melanomas
Using the MTT assay, we evaluated the response of Mel25 cells to recombinant human IL-6 at concentrations of 10 to 100 ng/ml in serum-free medium and in medium containing various concentrations of serum. Under these conditions, there was no detectable increase or decrease in proliferation and survival of this MT-ret melanoma cell line. However, we think that these in vitro results are difficult to apply to the situation in vivo. First, the cell line could be shown to express both IL-6 and IL-6 receptor α itself, suggesting that an autocrine mechanism of IL-6 stimulation might be active. Second, the highly proliferative Mel25 cell line is hard to compare to the tumor development from benign precursor lesions that took place in vivo. Still, our results demonstrate that, even though lack of IL-6 leads to fewer and smaller melanomas in MT-ret mice, IL-6 does not (any more) influence the survival and proliferation of end-stage Mel25 MT-ret melanoma cells.
STAT3 in MT-Ret Mouse Skin and MT-Ret Melanomas
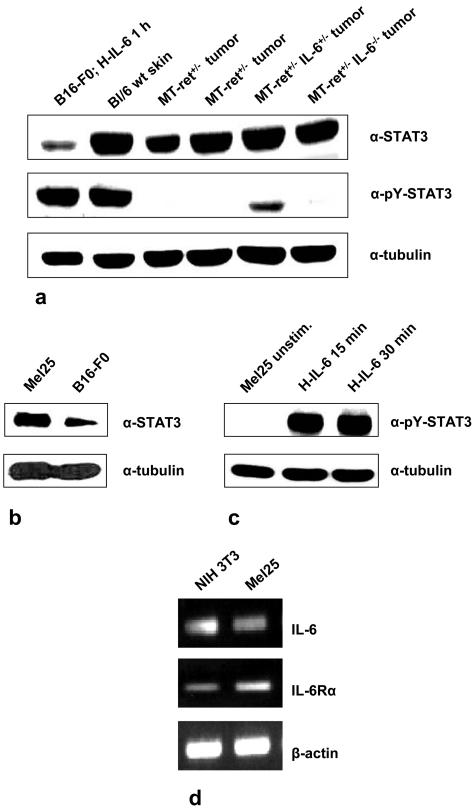
The STAT proteins reside in a latent form in the cytoplasm and are phosphorylated after activation of Jaks by the IL-6 receptor complex.2,7 Because STAT3 is a major, albeit not the only IL-6 signal transducer,2,7 we examined its expression and activation in MT-ret+/− mouse skin and MT-ret+/− melanomas by immunoblotting and immunohistochemistry. In our immunohistochemical analysis of paraffin-embedded MT-ret melanomas of all groups (MT-ret+/−, n = 13; MT-ret+/−/IL-6+/−, n = 1; MT-ret+/−/IL-6−/−, n = 6), we observed expression of latent STAT3 in tumor cells as well as in cells of the adjacent skin, but only minor labeling for activated, tyrosine-705 phosphorylated STAT3 (pY-STAT3) was detected in tumor cells (not shown). Accordingly, no alteration of the levels of STAT3 activation was detected in the skin of MT-ret+/− mice compared to MT-ret+/−/IL-6+/− and MT-ret+/−/IL-6−/− animals by immunoblotting (not shown). Also in accordance with the immunohistochemical results, the signals for latent STAT3 were prominent in immunoblots of MT-ret+/− melanomas of all groups, whereas tyrosine-705-phosphorylated, activated STAT3 was found only in minor amounts, if any (Figure 4a).
Figure 4.
a: Immunoblot analysis demonstrating that STAT3 protein expression in melanomas is not altered in tumors derived from MT-ret+/−/IL-6+/− and MT-ret+/−/IL-6−/− compared to MT-ret+/−/IL-6+/+ mice and does not differ from STAT3 protein expression in wild-type mouse skin. Activation levels of STAT3 measured by tyrosine-705-phosphorylated STAT3 immunoblotting are actually low to undetectable in MT-ret melanomas of all groups. The pY-STAT3 band detected in lane 5 might have been caused by a contamination of the tumor tissue by adjacent nonneoplastic skin tissue. B16-F0 melanoma cells stimulated for 1 hour with H-IL-6 (100 ng/ml) as well as wild-type mouse skin served as positive controls. b, c: Immunoblot analysis of STAT3 and pY-STAT3 in Mel25 cells. Unstimulated Mel25 cells also showed considerable constitutive expression of STAT3, but only minor to nondetectable expression of tyrosine-705-phosphorylated STAT3. Stimulation with H-IL-6 (100 ng/ml), however, leads to rapid activation of STAT3 demonstrated by the increase in tyrosine-705-phosphorylated STAT3 within 15 minutes. d: Reverse transcriptase-polymerase chain reaction demonstrating that Mel25 cells express IL-6 and IL-6 receptor α.
PI3K and MAPK Activation in MT-Ret Melanomas
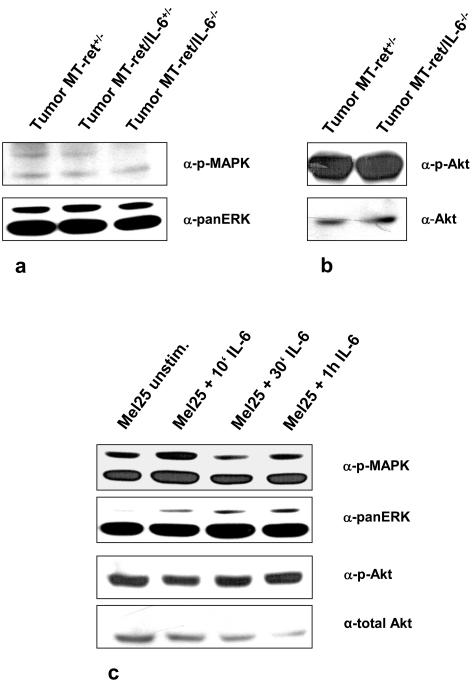
Because the STAT3 pathway was not activated in MT-ret melanomas and Mel25 cells, we investigated whether the remaining two well-characterized signaling pathways activated by IL-6, the PI3K/Akt and MAPK pathways, are involved. By immunoblotting, we found high levels of pMAPK in Mel25 cells. However, even though MAPK was found to be constitutively activated in all tumors investigated (Figure 5a), confirming earlier results,36 these levels were rather low in all groups regardless of the IL-6 genotype. In contrast, the PI3K/Akt pathway was constitutively activated in MT-ret melanomas regardless of their IL-6 genotype. The MT-ret melanoma cell line Mel25 was also found to constitutively express activated, phosphorylated Akt (Figure 5). Adding IL-6 to these cells, which express both IL-6 and IL-6 receptor themselves, did not increase the activation neither of the MAPK nor the PI3K/Akt pathway (Figure 5).
Figure 5.
a, b: Immunoblot analysis showing constitutive activation of the Akt and MAPK pathways in tumors of MT-ret+/−, MT-ret+/−/IL-6+/−, and in MT-ret+/−/IL-6−/− mice. MAPK activation is detectable, but rather low in tumors from all lines; prominent activation of pAkt is found independent of IL-6 expression. c: Stimulation of Mel25 cells with IL-6 (100 ng/ml) for 10 minutes to 1 hour does not alter MAPK and Akt levels.
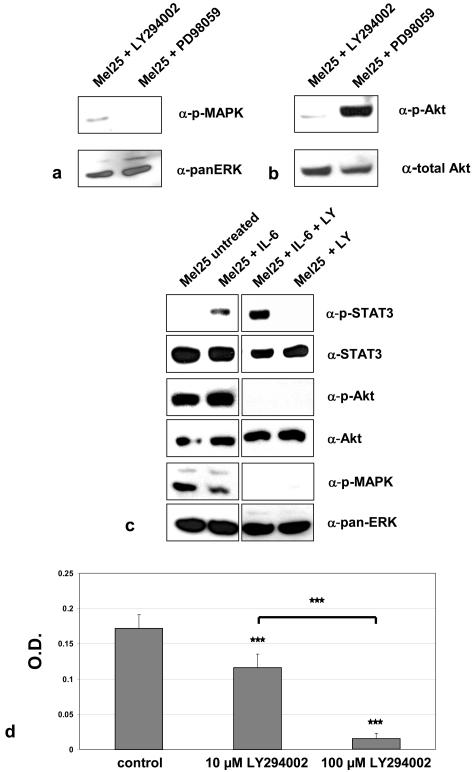
To further scrutinize the canonical pathways that mediate IL-6 effects (STAT3, PI3K, and MAPK) in MT-ret tumors, we analyzed the effects of selective blockers of the MAPK (PD98059) and PI3K (LY294002) pathways on Mel25 cells. We found that the constitutive activation of the PI3K pathway is associated with reduced STAT3 signaling in Mel25 cells (Figure 6c), confirming and extending earlier findings by others in two human melanoma cell lines.45 We also found that inhibition of PI3K activation decreased pMAPK levels in Mel25 cells (Figure 6c). Such cross-talk between the MAPK and PI3K signaling pathways has already been described.46 Interestingly, the PI3K inhibitor LY294002 markedly increased the amount of STAT3 activation achieved by stimulating cells with IL-6 (Figure 6c), suggesting that activation of the PI3K/Akt pathway in Mel25 cells leads to an inhibition of STAT3 activation. Another interesting point was that addition of LY294002 at concentrations of 10 and 100 μmol/L markedly decreased the survival of Mel25 cells under full serum conditions (Figure 6d). In the context of our finding that both Mel25 cells and MT-ret melanomas show high levels of constitutively activated Akt (see above) this result suggests that Mel25 cells and possibly also MT-ret tumors are dependent on the constitutive activation of the PI3K pathway.
Figure 6.
a: The MAPK pathway inhibitor PD98059 blocks MAPK activation in Mel25 cells, as does LY294002, which is commonly used as a PI3K inhibitor. b: LY294002 inhibits Akt activation in Mel25 cells, whereas PD98059 does not affect this pathway. c: LY294002 enhances IL-6-induced STAT3 activation, but blocks Akt and MAPK activation in Mel25 cells. d: LY294002 at concentrations of 10 and 100 μmol/L inhibits survival of Mel25 cells under full serum conditions.
Discussion
Using the MT-ret mouse model for studying in vivo melanoma development, we have demonstrated that lack of IL-6 leads to a significant decrease in the incidence of tumors in MT-ret+/−/IL-6−/− mice because of a reduced occurrence of the preneoplastic precursor lesions. In addition, in those MT-ret+/−/IL-6+/− and MT-ret+/−/IL-6−/− mice that developed melanomas, the skin tumors were significantly smaller compared to MT-ret+/− mice harboring two intact copies of the IL-6 gene. Previous in vitro studies have demonstrated that IL-6 can function as a growth inhibitory factor for early stage13–16 and as a growth-promoting agent for later stage melanoma cells.16,19,20 Our results show that, at least for the MT-ret+/− mouse model of spontaneously developing melanomas, the tumor-promoting effect of IL-6 appears to be decisive, because reduction of IL-6 levels in MT-ret+/− mice leads to a decrease both in melanoma incidence and in the size of primary melanoma nodules.
STAT3 is the major IL-6 signal transducer. Constitutive activation of STAT3 induces neoplastic transformation, establishing STAT3 as an oncogene.47 Thus, we analyzed STAT3 expression and activation in our experimental model. Immunoblot analysis revealed that activation of STAT3 was unaltered in the skin of MT-ret mice lacking IL-6. Moreover, even though ample STAT3 protein was detected in MT-ret melanomas, there was only minor, if any, STAT3 activation in these tumors, and the levels of activated, tyrosine-phosphorylated STAT3 were not decreased in tumors of mice lacking IL-6 compared to the MT-ret+/− animals expressing IL-6. These results suggest that STAT3 is not involved in the development and malignant progression of the MT-ret+/− skin lesions. They are in line with previous reports that showed that activated STAT3 does not induce features of increased malignancy in certain melanoma cell lines. For example, STAT3 activation actually led to growth inhibition of A37517 and WM35 melanoma cells.18
Two other canonical IL-6-signaling pathways, PI3K/Akt and MAPK,3,7,48–51 have been characterized thus far. Among these, the PI3K/Akt pathway appeared to be more relevant for our model, because Akt was highly activated in both MT-ret melanomas and Mel25 cells. Even though pMAPK was also found at high levels in Mel25 cells, levels of activated MAPK were rather low in MT-ret tumors. Interestingly, the PI3K/Akt pathway inhibitor LY294002 both increased STAT3 activation levels in Mel25 cells in response to IL-6 and caused the death of these cells, suggesting that this pathway, and potentially its activation by IL-6, is important for the maintenance of MT-ret melanomas.
IL-6 may enhance tumor growth by promoting tumor angiogenesis.52–54 In this context, it is interesting to note that, in the present study, there was no increase in the amount of tumor necrosis in the MT-ret+/− mice lacking IL-6. This observation suggests that blood supply to these tumors was not critically impaired because of the lack of IL-6. It was also hypothesized that IL-6 can affect the immune system’s ability to recognize and destroy tumor cells.55 Indeed, depending on the cellular context and the signaling pathways, activated IL-6 can have pro- or anti-inflammatory effects.2 In one intriguing example of IL-6 function in an experimental melanoma model, transfection of B78-H1 melanoma cells with IL-6 and IL-6Rα greatly reduced tumor formation in mice injected with these cells.56 This effect was probably because of an enhancement of the immune response against the tumor cells, because IL-6 and IL-6Rα transfected B78-H1 cells actually showed increased proliferation in vitro compared to untransfected cells.56 In the present study, lack of the IL-6 gene was associated with reduced incidence and size of melanomas suggesting that this immunostimulatory activity did not play a role in our model of spontaneous melanoma development. In fact, there was no major inflammatory infiltrate within or adjacent to tumors of any group, indicating that the cellular immune response against these melanomas was negligible.
Studies analyzing effects of IL-6 on invasive and metastatic capabilities of melanoma cell lines have yielded conflicting results. In one such study, treatment of endothelial cells with IL-6 resulted in a remarkable increase in the invasion of B16-F10 mouse melanoma cells into vascular endothelial monolayers; this effect was abolished by the addition of anti-IL-6 antibodies.57 The same B16-F10 melanoma cell line, cultured in the presence of IL-6, showed a clear increase in its metastatic spread to liver and lungs after transplantation.58 However, the tumor formation and metastatic spread of B78 melanoma cells in vivo was inhibited by transfection with IL-6 and sIL-6Rα.59 Similarly, metastatic growth of B16-F10.9 cells in mice was reduced by IL-6, presumably by an indirect immune-mediated mechanism.60 In our model, the incidence and pattern of metastasis was similar whether the MT-ret+/− mice harbored a functional IL-6 gene or not.
Compared to animal models using transplanted tumor cell lines, the MT-ret+/− mice offered major advantages. In contrast to models based on transplantation, influences of IL-6 during the entire period of tumor development including the preneoplastic precursor lesions could be monitored. In addition, injected tumor cell lines are allo- or even xenotransplanted; possible interference by immunological phenomena because of the rejection of allo- or xenotransplanted cells was excluded in our model. Finally, melanoma cells passaged in vitro undergo an evolution that differs from tumor cells maintained in vivo, because they are primarily selected based on their ability to grow well in culture.
In our model, we had to consider whether IL-6 would directly interfere with the metallothionein promoter of the ret transgene. In fact, an up-regulation of the metallothionein-I gene in brain, heart, and liver by IL-6 has been reported previously.61,62 However, three lines of evidence suggest that the impaired tumor growth in the MT-ret+/−/IL-6−/− mice cannot be explained by a decrease of MT-ret transgene expression because of lack of IL-6. First, Ret protein expression levels measured by immunoblotting were not decreased in the skin of MT-ret+/−/IL-6−/− compared to MT-ret+/− mice. Second, Ret levels were also neither decreased in MT-ret+/− tumors lacking the IL-6 gene nor increased in Mel25 cells stimulated with H-IL-6, suggesting that IL-6 does not alter Ret expression by MT-ret+/− tumor cells (Figure 3). Third, levels of MT expression in the skin have already been shown to be similar in IL-6−/− compared to IL-6+/+ mice both before and 48 hours after UVB irradiation, even though the increase of IL-6 expression during the first 2 days after irradiation was delayed in IL-6−/− mice.63 Thus, it is unlikely that direct influences of endogenous IL-6 on the MT promoter of the MT-ret transgene are important determinants of the neoplastic phenotype in our model.
In conclusion, our results indicate that lack of IL-6 leads to a decrease of both melanoma incidence and size in MT-ret+/− mice, suggesting that, at least in this experimental mouse model, IL-6 promotes development and progression of spontaneous melanoma. Because alterations in STAT3 signaling do not seem to be responsible for these effects of IL-6 ablation, future studies will analyze the contribution of alternative IL-6 signal transduction pathways in the development and progression of MT-ret+/− melanomas. Candidates include the Notch pathway that has been shown to be activated by IL-6-type cytokines64 and has recently been implicated in melanoma development from melanocytic precursors.65
Acknowledgments
We thank K. Boschung, B. Rohrbach, M. Economou, and A. Kappeler for technical support; M. Suter, H. Hirsiger, A. Krüttgen, and S. Saxena for helpful discussions; S. Rose-John, University of Kiel, for providing Hyper-IL-6; and to M. Kopf, ETH Zürich, for providing IL-6−/− mice.
Footnotes
Address reprint requests to J. Weis, Institute of Neuropathology, RWTH University Hospital, Pauwelsstrasse 30, D-52074 Aachen, Germany. E-mail: jweis@ukaachen.de.
Supported by the Dr. med. h.c. E. Braun Foundation, Basel, Switzerland.
References
- Marz P, Otten U, Rose-John S. Neuronal activities of IL-6-type cytokines often depend on soluble cytokine receptors. Eur J Neurosci. 1999;11:2995–3004. doi: 10.1046/j.1460-9568.1999.00755.x. [DOI] [PubMed] [Google Scholar]
- Kamimura D, Ishihara K, Hirano T. IL-6 signal transduction and its physiological roles: the signal orchestration model. Rev Physiol Biochem Pharmacol. 2003;149:1–38. doi: 10.1007/s10254-003-0012-2. [DOI] [PubMed] [Google Scholar]
- Kallen KJ. The role of transsignalling via the agonistic soluble IL-6 receptor in human diseases. Biochim Biophys Acta. 2002;1592:323–343. doi: 10.1016/s0167-4889(02)00325-7. [DOI] [PubMed] [Google Scholar]
- Kovalchuk AL, Kim JS, Park SS, Coleman AE, Ward JM, Morse HC, III, Kishimoto T, Potter M, Janz S. IL-6 transgenic mouse model for extraosseous plasmacytoma. Proc Natl Acad Sci USA. 2002;99:1509–1514. doi: 10.1073/pnas.022643999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissenberger J, Loeffler S, Kappeler A, Kopf M, Lukes A, Afanasieva TA, Aguzzi A, Weis J. IL-6 is required for glioma development in a mouse model. Oncogene. 2004;23:3308–3316. doi: 10.1038/sj.onc.1207455. [DOI] [PubMed] [Google Scholar]
- Steiner H, Godoy-Tundidor S, Rogatsch H, Berger AP, Fuchs D, Comuzzi B, Bartsch G, Hobisch A, Culig Z. Accelerated in vivo growth of prostate tumors that up-regulate interleukin-6 is associated with reduced retinoblastoma protein expression and activation of the mitogen-activated protein kinase pathway. Am J Pathol. 2003;162:655–663. doi: 10.1016/S0002-9440(10)63859-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrich PC, Behrmann I, Haan S, Hermanns HM, Muller-Newen G, Schaper F. Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochem J. 2003;374:1–20. doi: 10.1042/BJ20030407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catlett-Falcone R, Landowski TH, Oshiro MM, Turkson J, Levitzki A, Savino R, Ciliberto G, Moscinski L, Fernandez-Luna JL, Nunez G, Dalton WS, Jove R. Constitutive activation of Stat3 signaling confers resistance to apoptosis in human U266 myeloma cells. Immunity. 1999;10:105–115. doi: 10.1016/s1074-7613(00)80011-4. [DOI] [PubMed] [Google Scholar]
- Bromberg J. Signal transducers and activators of transcription as regulators of growth, apoptosis and breast development. Breast Cancer Res. 2000;2:86–90. doi: 10.1186/bcr38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu G, Wright KL, Huang M, Song L, Haura E, Turkson J, Zhang S, Wang T, Sinibaldi D, Coppola D, Heller R, Ellis LM, Karras J, Bromberg J, Pardoll D, Jove R, Yu H. Constitutive Stat3 activity up-regulates VEGF expression and tumor angiogenesis. Oncogene. 2002;21:2000–2008. doi: 10.1038/sj.onc.1205260. [DOI] [PubMed] [Google Scholar]
- Kiuchi N, Nakajima K, Ichiba M, Fukada T, Narimatsu M, Mizuno K, Hibi M, Hirano T. STAT3 is required for the gp130-mediated full activation of the c-myc gene. J Exp Med. 1999;189:63–73. doi: 10.1084/jem.189.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu G, Bowman T, Huang M, Shivers S, Reintgen D, Daud A, Chang A, Kraker A, Jove R, Yu H. Roles of activated Src and Stat3 signaling in melanoma tumor cell growth. Oncogene. 2002;21:7001–7010. doi: 10.1038/sj.onc.1205859. [DOI] [PubMed] [Google Scholar]
- Swope VB, Abdel-Malek Z, Kassem LM, Nordlund JJ. Interleukins 1 alpha and 6 and tumor necrosis factor-alpha are paracrine inhibitors of human melanocyte proliferation and melanogenesis. J Invest Dermatol. 1991;96:180–185. doi: 10.1111/1523-1747.ep12460991. [DOI] [PubMed] [Google Scholar]
- Florenes VA, Lu C, Bhattacharya N, Rak J, Sheehan C, Slingerland JM, Kerbel RS. Interleukin-6 dependent induction of the cyclin dependent kinase inhibitor p21WAF1/CIP1 is lost during progression of human malignant melanoma. Oncogene. 1999;18:1023–1032. doi: 10.1038/sj.onc.1202382. [DOI] [PubMed] [Google Scholar]
- Fontaine V, Mahieu M, Content J. Interferon-gamma and interleukin-6 inhibit proliferation in human melanoma cells by different signalling pathways. Melanoma Res. 1998;8:24–30. doi: 10.1097/00008390-199802000-00005. [DOI] [PubMed] [Google Scholar]
- Lu C, Vickers MF, Kerbel RS. Interleukin 6: a fibroblast-derived growth inhibitor of human melanoma cells from early but not advanced stages of tumor progression. Proc Natl Acad Sci USA. 1992;89:9215–9219. doi: 10.1073/pnas.89.19.9215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kortylewski M, Heinrich PC, Mackiewicz A, Schniertshauer U, Klingmuller U, Nakajima K, Hirano T, Horn F, Behrmann I. Interleukin-6 and oncostatin M-induced growth inhibition of human A375 melanoma cells is STAT-dependent and involves upregulation of the cyclin-dependent kinase inhibitor p27/Kip1. Oncogene. 1999;18:3742–3753. doi: 10.1038/sj.onc.1202708. [DOI] [PubMed] [Google Scholar]
- Bohm M, Schulte U, Funk JO, Raghunath M, Behrmann I, Kortylewski M, Heinrich PC, Kues T, Luger TA, Schwarz T. Interleukin-6-resistant melanoma cells exhibit reduced activation of STAT3 and lack of inhibition of cyclin E-associated kinase activity. J Invest Dermatol. 2001;117:132–140. doi: 10.1046/j.0022-202x.2001.01372.x. [DOI] [PubMed] [Google Scholar]
- Lu C, Kerbel RS. Interleukin-6 undergoes transition from paracrine growth inhibitor to autocrine stimulator during human melanoma progression [published erratum appears in J Cell Biol 1993 Apr; 121(2): following 477]. J Cell Biol. 1993;120:1281–1288. doi: 10.1083/jcb.120.5.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvani A, Ferrari G, Paonessa G, Toniatti C, Parmiani G, Colombo MP. Down-regulation of interleukin 6 receptor alpha chain in interleukin 6 transduced melanoma cells causes selective resistance to interleukin 6 but not to oncostatin M. Cancer Res. 1995;55:2200–2205. [PubMed] [Google Scholar]
- Lee JD, Sievers TM, Skotzko M, Chandler CF, Morton DL, McBride WH, Economou JS. Interleukin-6 production by human melanoma cell lines. Lymphokine Cytokine Res. 1992;11:161–166. [PubMed] [Google Scholar]
- Francis GM, Krohn EG, Woods KV, Buzaid AC, Grimm EA. Interleukin-6 production and secretion in human melanoma cell lines: regulation by interleukin-1. Melanoma Res. 1996;6:191–201. doi: 10.1097/00008390-199606000-00002. [DOI] [PubMed] [Google Scholar]
- Candi E, Knight RA, Spinedi A, Guerrieri P, Melino G. A possible growth factor role of IL-6 in neuroectodermal tumours. J Neurooncol. 1997;31:115–122. doi: 10.1023/a:1005706019048. [DOI] [PubMed] [Google Scholar]
- Mattei S, Colombo MP, Melani C, Silvani A, Parmiani G, Herlyn M. Expression of cytokine/growth factors and their receptors in human melanoma and melanocytes. Int J Cancer. 1994;56:853–857. doi: 10.1002/ijc.2910560617. [DOI] [PubMed] [Google Scholar]
- Moretti S, Pinzi C, Spallanzani A, Berti E, Chiarugi A, Mazzoli S, Fabiani M, Vallecchi C, Herlyn M. Immunohistochemical evidence of cytokine networks during progression of human melanocytic lesions. Int J Cancer. 1999;84:160–168. doi: 10.1002/(sici)1097-0215(19990420)84:2<160::aid-ijc12>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Ciotti P, Rainero ML, Nicolo G, Spina B, Garre C, Casabona F, Santi PL, Bianchi-Scarra G. Cytokine expression in human primary and metastatic melanoma cells: analysis in fresh bioptic specimens. Melanoma Res. 1995;5:41–47. doi: 10.1097/00008390-199502000-00005. [DOI] [PubMed] [Google Scholar]
- Eberlein-Konig B, Jager C, Przybilla B. Ultraviolet B radiation-induced production of interleukin 1alpha and interleukin 6 in a human squamous carcinoma cell line is wavelength-dependent and can be inhibited by pharmacological agents. Br J Dermatol. 1998;139:415–421. doi: 10.1046/j.1365-2133.1998.02404.x. [DOI] [PubMed] [Google Scholar]
- Mouawad R, Khayat D, Merle S, Antoine EC, Gil-Delgado M, Soubrane C. Is there any relationship between interleukin-6/interleukin-6 receptor modulation and endogenous interleukin-6 release in metastatic malignant melanoma patients treated by biochemotherapy? Melanoma Res. 1999;9:181–188. doi: 10.1097/00008390-199904000-00011. [DOI] [PubMed] [Google Scholar]
- Mouawad R, Benhammouda A, Rixe O, Antoine EC, Borel C, Weil M, Khayat D, Soubrane C. Endogenous interleukin 6 levels in patients with metastatic malignant melanoma: correlation with tumor burden. Clin Cancer Res. 1996;2:1405–1409. [PubMed] [Google Scholar]
- Tartour E, Dorval T, Mosseri V, Deneux L, Mathiot C, Brailly H, Montero F, Joyeux I, Pouillart P, Fridman WH. Serum interleukin 6 and C-reactive protein levels correlate with resistance to IL-2 therapy and poor survival in melanoma patients. Br J Cancer. 1994;69:911–913. doi: 10.1038/bjc.1994.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwamoto T, Takahashi M, Ito M, Hamatani K, Ohbayashi M, Wajjwalku W, Isobe K, Nakashima I. Aberrant melanogenesis and melanocytic tumour development in transgenic mice that carry a metallothionein/ret fusion gene. EMBO J. 1991;10:3167–3175. doi: 10.1002/j.1460-2075.1991.tb04878.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Oord JJ, De Ley M. Distribution of metallothionein in normal and pathological human skin. Arch Dermatol Res. 1994;286:62–68. doi: 10.1007/BF00375845. [DOI] [PubMed] [Google Scholar]
- Karasawa M, Nishimura N, Nishimura H, Tohyama C, Hashiba H, Kuroki T. Localization of metallothionein in hair follicles of normal skin and the basal cell layer of hyperplastic epidermis: possible association with cell proliferation. J Invest Dermatol. 1991;97:97–100. doi: 10.1111/1523-1747.ep12478393. [DOI] [PubMed] [Google Scholar]
- Miles AT, Hawksworth GM, Beattie JH, Rodilla V. Induction, regulation, degradation, and biological significance of mammalian metallothioneins. Crit Rev Biochem Mol Biol. 2000;35:35–70. doi: 10.1080/10409230091169168. [DOI] [PubMed] [Google Scholar]
- Kato M, Takeda K, Kawamoto Y, Tsuzuki T, Dai Y, Nakayama S, Toriyama K, Tamada Y, Takahashi M, Nakashima I. RET tyrosine kinase enhances hair growth in association with promotion of melanogenesis. Oncogene. 2001;20:7536–7541. doi: 10.1038/sj.onc.1204918. [DOI] [PubMed] [Google Scholar]
- Kato M, Takahashi M, Akhand AA, Liu W, Dai Y, Shimizu S, Iwamoto T, Suzuki H, Nakashima I. Transgenic mouse model for skin malignant melanoma. Oncogene. 1998;17:1885–1888. doi: 10.1038/sj.onc.1202077. [DOI] [PubMed] [Google Scholar]
- Kopf M, Baumann H, Freer G, Freudenberg M, Lamers M, Kishimoto T, Zinkernagel R, Bluethmann H, Kohler G. Impaired immune and acute-phase responses in interleukin-6-deficient mice. Nature. 1994;368:339–342. doi: 10.1038/368339a0. [DOI] [PubMed] [Google Scholar]
- Rahaman SO, Harbor PC, Chernova O, Barnett GH, Vogelbaum MA, Haque SJ. Inhibition of constitutively active Stat3 suppresses proliferation and induces apoptosis in glioblastoma multiforme cells. Oncogene. 2002;21:8404–8413. doi: 10.1038/sj.onc.1206047. [DOI] [PubMed] [Google Scholar]
- Thier M, Marz P, Otten U, Weis J, Rose-John S. Interleukin-6 (IL-6) and its soluble receptor support survival of sensory neurons. J Neurosci Res. 1999;55:411–422. doi: 10.1002/(SICI)1097-4547(19990215)55:4<411::AID-JNR2>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Sowalsky RA, Fox BS. Pattern of lectin binding to murine T lymphocytes. Immunology. 1992;75:92–98. [PMC free article] [PubMed] [Google Scholar]
- Kao WW, Prockop DJ. Proline analogue removes fibroblasts from cultured mixed cell populations. Nature. 1977;266:63–64. doi: 10.1038/266063a0. [DOI] [PubMed] [Google Scholar]
- Rakemann T, Niehof M, Kubicka S, Fischer M, Manns MP, Rose-John S, Trautwein C. The designer cytokine hyper-interleukin-6 is a potent activator of STAT3-dependent gene transcription in vivo and in vitro. J Biol Chem. 1999;274:1257–1266. doi: 10.1074/jbc.274.3.1257. [DOI] [PubMed] [Google Scholar]
- Thier M, Simon R, Kruttgen A, Rose John S, Heinrich PC, Schroder JM, Weis J. Site-directed mutagenesis of human CNTF: functional analysis of recombinant variants. J Neurosci Res. 1995;40:826–835. doi: 10.1002/jnr.490400614. [DOI] [PubMed] [Google Scholar]
- Weinstein IB. Cancer. Addiction to oncogenes—the Achilles heal of cancer. Science. 2002;297:63–64. doi: 10.1126/science.1073096. [DOI] [PubMed] [Google Scholar]
- Krasilnikov M, Ivanov VN, Dong J, Ronai Z. ERK and PI3K negatively regulate STAT-transcriptional activities in human melanoma cells: implications towards sensitization to apoptosis. Oncogene. 2003;22:4092–4101. doi: 10.1038/sj.onc.1206598. [DOI] [PubMed] [Google Scholar]
- Gonzalez I, Tripathi G, Carter EJ, Cobb LJ, Salih DA, Lovett FA, Holding C, Pell JM. Akt2, a novel functional link between p38 mitogen-activated protein kinase and phosphatidylinositol 3-kinase pathways in myogenesis. Mol Cell Biol. 2004;24:3607–3622. doi: 10.1128/MCB.24.9.3607-3622.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromberg JF, Wrzeszczynska MH, Devgan G, Zhao Y, Pestell RG, Albanese C, Darnell JE., Jr Stat3 as an oncogene. Cell. 1999;98:295–303. doi: 10.1016/s0092-8674(00)81959-5. [DOI] [PubMed] [Google Scholar]
- Birkenkamp KU, Esselink MT, Kruijer W, Vellenga E. An inhibitor of PI3-K differentially affects proliferation and IL-6 protein secretion in normal and leukemic myeloid cells depending on the stage of differentiation. Exp Hematol. 2000;28:1239–1249. doi: 10.1016/s0301-472x(00)00529-4. [DOI] [PubMed] [Google Scholar]
- Kuo ML, Chuang SE, Lin MT, Yang SY. The involvement of PI 3-K/Akt-dependent up-regulation of Mcl-1 in the prevention of apoptosis of Hep3B cells by interleukin-6. Oncogene. 2001;20:677–685. doi: 10.1038/sj.onc.1204140. [DOI] [PubMed] [Google Scholar]
- Hideshima T, Nakamura N, Chauhan D, Anderson KC. Biologic sequelae of interleukin-6 induced PI3-K/Akt signaling in multiple myeloma. Oncogene. 2001;20:5991–6000. doi: 10.1038/sj.onc.1204833. [DOI] [PubMed] [Google Scholar]
- Leu CM, Wong FH, Chang C, Huang SF, Hu CP. Interleukin-6 acts as an antiapoptotic factor in human esophageal carcinoma cells through the activation of both STAT3 and mitogen-activated protein kinase pathways. Oncogene. 2003;22:7809–7818. doi: 10.1038/sj.onc.1207084. [DOI] [PubMed] [Google Scholar]
- Wei LH, Kuo ML, Chen CA, Chou CH, Lai KB, Lee CN, Hsieh CY. Interleukin-6 promotes cervical tumor growth by VEGF-dependent angiogenesis via a STAT3 pathway. Oncogene. 2003;22:1517–1527. doi: 10.1038/sj.onc.1206226. [DOI] [PubMed] [Google Scholar]
- Masui T, Hosotani R, Doi R, Miyamoto Y, Tsuji S, Nakajima S, Kobayashi H, Koizumi M, Toyoda E, Tulachan SS, Imamura M. Expression of IL-6 receptor in pancreatic cancer: involvement in VEGF induction. Anticancer Res. 2002;22:4093–4100. [PubMed] [Google Scholar]
- Loeffler S, Fayard B, Weis J, Weissenberger J: IL-6 induces transcriptional activation of vascular endothelial growth factor in astrocytes in vivo and regulates VEGF promoter activity in glioblastoma cells via direct interaction between STAT3 and SP1. Int J Cancer (in press) [DOI] [PubMed] [Google Scholar]
- Okuno Y, Takahashi T, Suzuki A, Fukumoto M, Nakamura K, Fukui H, Koishihara Y, Ohsugi Y, Imura H. Acquisition of growth autonomy and tumorigenicity by an interleukin 6-dependent human myeloma cell line transfected with interleukin 6 cDNA. Exp Hematol. 1992;20:395–400. [PubMed] [Google Scholar]
- Mackiewicz A, Gorny A, Laciak M, Malicki J, Murawa P, Nowak J, Wiznerowicz M, Hawley RG, Heinrich PC, Rose John S. Gene therapy of human melanoma. Immunization of patients with autologous tumor cells admixed with allogeneic melanoma cells secreting interleukin 6 and soluble interleukin 6 receptor. Hum Gene Ther. 1995;6:805–811. doi: 10.1089/hum.1995.6.6-805. [DOI] [PubMed] [Google Scholar]
- Kitamura Y, Morita I, Nihei Z, Mishima Y, Murota S. Effect of IL-6 on tumor cell invasion of vascular endothelial monolayers. Surg Today. 1997;27:534–541. doi: 10.1007/BF02385807. [DOI] [PubMed] [Google Scholar]
- Garcia de G, Boyano D, Smith-Zubiaga I, Alvarez A, Canton I, Canavate L. Involvement of interleukin-6 in the biology and metastatic activity of B16F10 melanoma cells. Eur Cytokine Netw. 1998;9:187–192. [PubMed] [Google Scholar]
- Mackiewicz A, Wiznerowicz M, Roeb E, Karczewska A, Nowak J, Heinrich PC, Rose John S. Soluble interleukin 6 receptor is biologically active in vivo. Cytokine. 1995;7:142–149. doi: 10.1006/cyto.1995.1019. [DOI] [PubMed] [Google Scholar]
- Katz A, Shulman LM, Porgador A, Revel M, Feldman M, Eisenbach L. Abrogation of B16 melanoma metastases by long-term low-dose interleukin-6 therapy. J Immunother. 1993;13:98–109. doi: 10.1097/00002371-199302000-00004. [DOI] [PubMed] [Google Scholar]
- Molinero A, Penkowa M, Hernandez J, Camats J, Giralt M, Lago N, Carrasco J, Campbell IL, Hidalgo J. Metallothionein-I overexpression decreases brain pathology in transgenic mice with astrocyte-targeted expression of interleukin-6. J Neuropathol Exp Neurol. 2003;62:315–328. doi: 10.1093/jnen/62.3.315. [DOI] [PubMed] [Google Scholar]
- Sato M, Yamaki J, Hamaya M, Hojo H. Synergistic induction of metallothionein synthesis by interleukin-6, dexamethasone and zinc in the rat. Int J Immunopharmacol. 1996;18:167–172. doi: 10.1016/0192-0561(95)00118-2. [DOI] [PubMed] [Google Scholar]
- Nishimura N, Reeve VE, Nishimura H, Satoh M, Tohyama C. Cutaneous metallothionein induction by ultraviolet B irradiation in interleukin-6 null mice. J Invest Dermatol. 2000;114:343–348. doi: 10.1046/j.1523-1747.2000.00862.x. [DOI] [PubMed] [Google Scholar]
- Chojnacki A, Shimazaki T, Gregg C, Weinmaster G, Weiss S. Glycoprotein 130 signaling regulates Notch1 expression and activation in the self-renewal of mammalian forebrain neural stem cells. J Neurosci. 2003;23:1730–1741. doi: 10.1523/JNEUROSCI.23-05-01730.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoek K, Rimm DL, Williams KR, Zhao H, Ariyan S, Lin A, Kluger HM, Berger AJ, Cheng E, Trombetta ES, Wu T, Niinobe M, Yoshikawa K, Hannigan GE, Halaban R. Expression profiling reveals novel pathways in the transformation of melanocytes to melanomas. Cancer Res. 2004;64:5270–5282. doi: 10.1158/0008-5472.CAN-04-0731. [DOI] [PubMed] [Google Scholar]