Abstract
The pulmonary vascular endothelial paracellular pathway and zonula adherens (ZA) integrity are regulated, in part, through protein tyrosine phosphorylation. ZA-associated protein tyrosine phosphatase (PTP)s are thought to counterregulate tyrosine phosphorylation events within the ZA multiprotein complex. One such receptor PTP, PTPμ, is highly expressed in lung tissue and is almost exclusively restricted to the endothelium. We therefore studied whether PTPμ, in pulmonary vascular endothelia, associates with and/or regulates both the tyrosine phosphorylation state of vascular endothelial (VE)-cadherin and the paracellular pathway. PTPμ was expressed in postconfluent human pulmonary artery and lung microvascular endothelial cells (ECs) where it was almost exclusively restricted to EC-EC boundaries. In human lung microvascular ECs, knockdown of PTPμ through RNA interference dramatically impaired barrier function. In immortalized human microvascular ECs, overexpression of wild-type PTPμ enhanced barrier function. PTPμ-VE-cadherin interactions were demonstrated through reciprocal co-immunoprecipitation assays and co-localization with double-label fluorescence microscopy. When glutathione S-transferase-PTPμ was incubated with purified recombinant VE-cadherin, and when glutathione S-transferase-VE-cadherin was incubated with purified recombinant PTPμ, PTPμ directly bound to VE-cadherin. Overexpression of wild-type PTPμ decreased tyrosine phosphorylation of VE-cadherin. Therefore, PTPμ is expressed in human pulmonary vascular endothelia where it directly binds to VE-cadherin and regulates both the tyrosine phosphorylation state of VE-cadherin and barrier integrity.
The pulmonary vascular endothelium presents a selective barrier that actively regulates paracellular movement of circulating macromolecules and cells into extravascular tissues and compartments.1,2 We3,4 and others5–8 have demonstrated that the endothelial paracellular pathway through which macromolecules flux is regulated, in part, through protein tyrosine phosphorylation. Established mediators of vascular permeability, including endotoxin and counteradhesive proteins, increase tyrosine phosphorylation of endothelial cell (EC) proteins almost exclusively restricted to the intercellular boundaries whereas prior protein tyrosine kinase (PTK) inhibition protects against opening of the paracellular pathway in response to these same agonists.3,4 Several substrates for agonist-induced tyrosine phosphorylation have been identified as components of the EC-EC adherens junction or zonula adherens (ZA),4,6 an intercellular junctional complex that modulates homophilic cell-cell adhesion.1,9–11
Vascular endothelial (VE)-cadherin, a membrane-spanning glycoprotein with an ectodomain that dictates homophilic adhesive specificity and a cytoplasmic domain that is indirectly tethered to the actin cytoskeleton, is central to ZA organization in ECs.9,10 Although multiple cadherins can be co-expressed and differentially distributed in ECs,12 VE-cadherin appears to be unique to ECs and is localized to their intercellular junctions.13 Further, anti-VE-cadherin antibodies increase transendothelial flux of macromolecules and established mediators of EC injury alter VE-cadherin distribution. At least three cytoplasmic proteins, collectively termed catenins, form multiprotein complexes that participate in anchoring the cytoplasmic domain of cadherins to actin microfilaments.14–16 This ZA/peripheral actin band forms a continuous belt around the apical portion of the cell where it is strategically localized to modulate EC-EC interactions and the paracellular pathway.1,11
The state of ZA protein tyrosine phosphorylation is central to the regulation of the ZA-actin cytoskeletal linkage and homophilic cell-cell adhesion17–19 and, as we3,4 and others5,6 have shown, to the maintenance of endothelial barrier function. More specifically, the tyrosine phosphorylation state of VE-cadherin appears to influence the endothelial paracellular pathway.6,20 Whether tyrosine phosphorylation of its cytoplasmic domain directly regulates homophilic interactions between its ectodomains is unclear. Protein tyrosine phosphatases (PTPs) are thought to play a crucial role in regulating the state of ZA protein tyrosine phosphorylation and assembly and endothelial barrier function. Increased expression of a number of PTPs parallels increases in cell density.21–24 In contact-inhibited confluent human umbilical vein ECs (HUVECs), membrane-associated PTP activity is increased ∼10-fold compared to subconfluent ECs.22,24 In postconfluent pulmonary vascular ECs, we have found that for some mediators, rigorous PTP inhibition is required for a consistent, reproducible, agonist-induced phosphotyrosine signal.4 Esser and colleagues6 reported similar findings in vascular endothelial growth factor-stimulated HUVECs. Further, co-administration of PTP inhibitors with some of these same agonists, at concentrations that alone do not alter barrier function, enhances mediator-induced increments in transendothelial albumin flux.3,4 Several such agonists have been shown to alter PTP expression or activity25–27 and/or PTP-substrate interactions.7 Finally, PTP inhibition itself induces dose- and time-dependent increments in protein tyrosine phosphorylation, intercellular gap formation, and loss of barrier function both in vitro5,28 and in vivo.8 The phosphotyrosine-containing proteins are immunolocalized predominantly to the intercellular boundaries and several have been identified as ZA proteins.5,28 These combined data suggest that pulmonary vascular ECs express PTPs that associate with and dephosphorylate ZA and possibly other intercellular junctional proteins. In fact, a growing number of ZA-associated PTPs have been demonstrated in various epithelia and other tissues.29–36 The receptor PTPs, PTPμ,23,37,38 PTPK,32 PTPλ,33 VE-PTP, also known as PTPβ,35 density-enhanced phosphatase (DEP)-1,36,39 and a member of the leukocyte common antigen-related protein (LAR)-PTP family,34 each have been shown to bind to and/or dephosphorylate ZA proteins. In HUVECs, the SH2 domain-containing nonreceptor PTP, SHP-2, binds to β-catenin and restrains phosphorylation of β-, γ-, and p120 catenins.7 In chick retinal tissue, another nonreceptor PTP, PTP1B, associates with N-cadherin and dephosphorylates β-catenin.19 It appears that one or more PTPs, possibly in concert, regulate tyrosine phosphorylation events within the ZA multiprotein complex of various cells.
The in vivo tissue expression of these ZA-associated PTPs are distinct; some are ubiquitous whereas others are restricted.40,41 One such PTP, PTPμ, is highly expressed in lung,37,41 is almost exclusively restricted to the vascular endothelium,41,42 and is co-localized with flk-1, a murine EC-specific receptor for vascular endothelial growth factor, with high expression in the pulmonary vasculature. In other in vivo studies of adult rat and porcine tissues, PTPμ can be immunolocalized almost exclusively to vascular EC-EC junctions where it co-localizes with cadherin-catenin complexes.42 These combined studies suggest that PTPμ may be relevant to the regulation of the tyrosine phosphorylation-responsive pulmonary vascular endothelial paracellular pathway.
PTPμ is a multidomain and apparently multifunctional protein that may influence the endothelial paracellular pathway through one or more mechanisms. The extracellular domain of this 195-kd protein contains in tandem an N-terminal MAM (Meprins A and B, Xenopus A5 glycoprotein, PTPmu) domain, an immunoglobulin (Ig)-like repeat, and four fibronectin III-like repeats.40 The ectodomain requires its Ig-like repeat to participate in homophilic adhesion with an identical molecule on neighboring cells.40,43 The intracellular segment contains two catalytic domains, the first or NH2-terminal of which is active.40 A single (C→S) amino acid mutation within the first catalytic domain renders PTPs catalytically inactive and such mutants can act as dominant-negative molecules.44 Double (Y→F, D→A) amino acid mutations within the same catalytic domain result in a substrate-trapping, catalytically impaired mutation that might retain low residual PTP activity.45,46 Such mutations impair catalysis without affecting affinity for substrates, thereby stabilizing PTPμ-substrate interactions and potentially interfering with downstream signaling events. In PTPμ, the ∼70-amino acid juxtamembranous segment between the membrane-spanning domain and first catalytic site shares ∼20% amino acid identity with the conserved, cytoplasmic portion of cadherin.37,38,40 This segment interacts with both phosphatase domains and may regulate catalytic activity.47 In the extracts of rat lung and various cell lines, PTPμ interacts with the classical cadherins, E-cadherin, N-cadherin, and cadherin-430 and possibly one or more of the catenins.31 Among the classical cadherins, the VE-cadherin cytoplasmic domain is the least conserved.48 Whether PTPμ can similarly bind to the vascular EC-specific cadherin, VE-cadherin, is unknown. In the current studies, we determined whether PTPμ catalytic activity regulates the human microvascular endothelial paracellular pathway. Further, we tested whether PTPμ expressed in pulmonary vascular endothelia directly/indirectly associates with and/or regulates the tyrosine phosphorylation state of VE-cadherin.
Materials and Methods
EC Culture
Human pulmonary artery and lung microvascular ECs (Clonetics Corp., San Diego, CA) were cultured in EC growth medium (EBM-2, Clonetics) containing 5% fetal bovine serum (Hyclone Laboratories, Logan, UT), human recombinant epidermal growth factor, human recombinant insulin-like growth-factor-1, human basic fibroblast growth factor, vascular endothelial growth factor, hydrocortisone, ascorbic acid, gentamicin, and amphotericin B. Only ECs at passages 2 to 7 were studied. HMEC-1 cells, a simian virus (SV) 40 T antigen transformed human dermal microvascular EC line (CDC, Atlanta, GA), were also cultured with EBM-2 in the presence of 10% fetal bovine serum.
Reverse Transcriptase (RT)-Polymerase Chain Reaction (PCR) for PTPμ
Poly(A)+ RNA was isolated from confluent human pulmonary artery ECs, human lung microvascular ECs, and HMEC-1 cells. Complementary DNA was generated from RNA using oligo(dT) primers and AMV reverse transcriptase. This cDNA was used as a template for amplification with DyNAzyme EXT DNA polymerase and primers A (5′-CGGTGCVATGGACATCCTGCC-3′) and B (5′-CTTGTACTGATCCAGGAGGTC-3′) that corresponded to the cytoplasmic domain of PTPμ (3652 bp to 4297 bp). The PCR was performed in a Perkin-Elmer GeneAmp PCR system 2400 (Applied Biosystems, Foster City, CA). After an initial 2 minutes of denaturation at 94°C, 30 cycles compromising 30 seconds at 94°C (denaturation), 1 minute at 60°C (annealing), 1 minute at 72°C (extension) was completed followed by a 7-minute final extension at 72°C. The purified PCR products were subcloned into a TA vector and the subcloned constructs were then expressed in XL-1 subcloning cells, isolated, purified, and sequenced to confirm the presence of PTPμ.
Immunoblotting for PTPμ and ZA Proteins
Postconfluent ECs were lysed with ice-cold lysis buffer as previously described in detail.3,4 The lysates were centrifuged, assayed for protein concentration, resolved by electrophoresis on a 6% sodium dodecyl sulfate (SDS)-polyacrylamide gel (Novex, San Diego, CA) and transferred onto polyvinylidene difluoride (PVDF) membranes (Millipore, Bedford, MA). The blots were probed with a murine monoclonal antibody raised against the intracellular juxtramembranous segment of PTPμ (SK 7)49 followed by horseradish peroxidase (HRP)-conjugated rabbit anti-mouse IgG (Transduction Laboratories, Lexington, KY), and developed with enhanced chemiluminescence (ECL) (Amersham, Arlington Heights, IL). To confirm equivalent protein loading, blots were stripped with 100 mmol/L 2-mercaptoethanol, 2% SDS, 62.5 mmol/L Tris-HCl, pH 6.7, and reprobed with 0.5 mg/ml of murine anti-physarum β-tubulin IgG2b (Boehringer-Mannheim, Indianapolis, IN) followed by HRP-conjugated anti-mouse IgG (Transduction Laboratories)3,4 and developed with ECL. In selected experiments with HMEC-1 cells, the EC lysates were processed for immunoblotting with murine monoclonal antibodies raised against the ZA proteins, VE-cadherin (cadherin-5, Transduction Laboratories), and α-, β-, γ-, and p120-catenins (Transduction Laboratories). The cadherin-5 anti-VE-cadherin antibody was raised against a peptide corresponding to a portion of the ectodomain (amino acids 26 to 194).
Immunolocalization of PTPμ and Its Co-Localization with VE-Cadherin by Epifluorescence Microscopy
For localization of PTPμ, ECs were cultured to postconfluence, washed [phosphate-buffered saline (PBS) without Ca2+/Mg2+] three times, fixed (3% paraformaldehyde at 37°C for 5 minutes), permeabilized (0.5% Triton X-100 for 5 minutes), blocked [2% bovine serum albumin (BSA)/5% horse serum (Life Technologies, Inc., Grand Island, NY) for 0.5 hours], and incubated for 1 hour with murine anti-PTPμ antibodies followed by fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse IgG (Molecular Probes Inc., Eugene, OR) as described3 with minor modifications. For co-localization of PTPμ with VE-cadherin, monolayers were probed with monoclonal SK7 anti-PTPμ antibody and goat anti-human VE-cadherin IgG (Santa Cruz Biotechnology, Santa Cruz, CA) at 1:50 dilution in 0.1% BSA in PBS followed by Cy-3-labeled goat anti-mouse IgG and FITC-conjugated donkey anti-goat IgG (3 μg/ml) (Molecular Probes Inc.). After multiple washes, the monolayers were processed for standard epifluorescence microscopy.
PTPμ Knockdown by RNA Interference
Small interfering RNA (siRNA) duplex products were designed and prepared (Dharmacon, Lafayette, CO) to target PTPμ. These duplex siRNAs included four distinct sequences GGGCAGAACUGGCCAUUAG (425 to 443) GGAAGAACGUCCUCGAAGA (1878 to 1896), CGACGAGGCUUUCUCAUUC (2406 to 2424), and GCAAUUAUAUCGAUGGUUA (2867 to 2885). An irrelevant duplex siRNA that does not correspond to any known sequence in the human genome was similarly prepared as a control. The four PTPμ siRNAs mixed together in equivalent concentrations and the control both were preincubated with TransMessenger transfection reagent (Qiagen, Valencia, CA) according to the manufacturer’s protocol and the transfection complexes presented to human lung microvascular ECs cultured to ∼80% confluence for 3 hours in the absence of serum. At increasing times after transfection, ECs were lysed and processed for immunoblotting with SK-15 anti-PTPμ antibody (Oncogene, Boston, MA). To confirm equivalent protein loading, blots were stripped and reprobed with anti-β-tubulin antibodies and developed with ECL as described above. Autoradiographs were scanned by laser densitometry (Molecular Dynamics, Sunnyvale, CA) and the PTPμ-immunoreactive bands analyzed.
Overexpression of Wild-Type (WT) PTPμ and Catalytically Impaired PTPμ in HMEC-1 Cells
A retrovirus-mediated gene transfer system (pRevTRE2; Clontech, Palo Alto, CA) was applied to stably infect HMEC-1 cells. In selected experiments, the system was tetracycline-responsive. pRP265/PTPμ38 was used as a template for overlapping PCR to generate the mutation of Asp1063 (G!T) to Ala (GCT). The resulting plasmid, pRP265/μDA, was used as the template for inverse PCR to generate the mutation of Tyr929(T!C) to Phe(TTC) to give pRP265/μY9292F,D1063A. A NgoM IV/XbaI fragment (nucleotides 2596 to 4356, PTPμ from pRP265/μYFDA was ligated to NgoM IV/XbaI digested pMT2/μ37 to produce pMT2/μYFDA. WT and YFDA PTPμ mutant constructs each were subcloned into the NotI site of pRevTRE2 (Clontech) and diagnostic digestion with XbaI was performed to confirm correct orientation. A hemagglutinin (HA)-tagged C→S mutant (amino acid 1095) was generated from the WT PTPμ retrovirus, pRev-TRE2-PTPμ, using a Quikchange site-directed mutagenesis kit (Stratagene, La Jolla, CA). Final sequence was confirmed by automated dideoxy DNA sequencing. The pRev-TRE2 plasmid encoding either WT PTPμ, the catalytically impaired YFDA PTPμ mutant, or the catalytically inactive C→S PTPμ mutant, each was introduced into PT67 cells (Clontech), and selected with hygromycin B 400 mg/ml (Hoffmann-La Roche Inc., Nutley, NJ). Similarly, a pRev-Tet-Off plasmid (Clontech) was transfected into PT67 cells and was selected with 800 mg/ml of G418 (Invitrogen, Carlsbad, CA). The packaged retroviral particles containing either WT PTPμ or the YFDA mutant were presented to subconfluent HMEC-1 cells, which were then selected with 200 mg/ml of hygromycin B. For experiments with the PTPμ C→S mutant, HMEC-1 cells were simultaneously co-infected with pRev-TRE-2 C→S mutant and pRev-Tet-Off. These cells were selected with both 200 mg/ml of hygromycin B and 400 mg/ml of G418 and the stably infected selectants cultured in the presence or absence of 1 μg/ml of doxycycline. Total (ectopic and endogenous) PTPμ protein expression was monitored with quantitative PTPμ immunoblotting with anti-PTPμ SK7 antibody whereas ectopic PTPμ expression was determined by immunoblotting with anti-HA antibody.
Assay for Endothelial Barrier Development
Transendothelial 14C-bovine serum albumin (BSA) flux was used as a measure of endothelial paracellular permeability as previously described.3,4 Human lung microvascular ECs were seeded at 2.5 × 105 cells in 0.5 ml of media per assay chamber and cultured to confluence. After establishment of the baseline barrier function of each monolayer, ECs were transfected with either PTPμ or control siRNAs or incubated with either the transfection reagent or media alone. After 3 hours, the monolayers were washed and transendothelial 14C-BSA flux assayed every 24 hours. In other experiments, HMEC-1 cells stably infected with pRev-TRE2 encoding for either WT PTPμ, the catalytically impaired YFDA PTPμ mutant, the catalytically inactive C→S PTPμ mutant, or the empty vector alone, each were seeded at an equivalent density of 20,000 ECs in 0.5 ml of media onto gelatin-impregnated polycarbonate filters (13-mm diameter, 0.4-μm pore size) (Nucleopore Inc.) mounted in polystyrene chemotactic chambers (ADAPS) inserted into the wells of 24-well plates. The cells were cultured for 48 hours, after which an equivalent amount of tracer molecule, 14C-BSA (specific activity, 89 μCi/mg protein; Sigma Chemical Co., St. Louis, MO), was applied to each upper compartment for 1 hour at 37°C, after which the lower compartment was counted for 14C activity. The cells stably co-infected with both pRev-TRE2 encoding for the C→S PTPμ mutant and pRev-Tet-Off were cultured in the presence and absence of 1 μg/ml of doxycycline. Transendothelial 14C-BSA flux was expressed as pmol/h.
Co-Immunoprecipitation Assays
Postconfluent human lung microvascular ECs were thoroughly rinsed with ice-cold HEPES buffer and solubilized with a low-stringency lysis buffer containing 20 mmol/L Tris, pH 7.5, 2 mmol/L CaC2, 1% Triton X-100, 5 mg/ml leupeptin, 5 mg/ml aprotinin, 1 mmol/L benzamidine, 200 μmol/L PAO, 1 mmol/L vanadate, and 0.1 mmol/L molybdate as described.29,50 The EC lysates were precleared by incubation for 1 hour at 4°C with protein G cross-linked to agarose (Sigma), preloaded with a species- and isotype-matched irrelevant antibody (AFAP IgG1, Transduction Laboratories). The lysates were then incubated overnight at 4°C with anti-PTPμ (SK7) antibody (7.5 μg of antibody/500 μg of lysate)49 or an equivalent concentration of the irrelevant antibody control. The resultant immune complexes were immobilized by incubation with protein G cross-linked to agarose for 2 hours at 4°C, centrifuged, washed five times, boiled for 7 minutes in sample buffer, and again centrifuged. The supernatants were processed for immunoblotting as described above. The blots were probed with specific murine monoclonal antibodies raised against VE-cadherin (cadherin-5, Transduction Laboratories; 30Q8A and 30Q6F, ICOS Corp., Bothell, WA). Each of the three anti-VE-cadherin antibodies (cadherin-5, 30Q8A, and 30Q6F) were raised against peptides corresponding to portions of the NH2-terminal ectodomain. In other experiments, VE-cadherin immunoprecipitates were similarly processed and probed with anti-PTPμ antibodies. The anti-VE-cadherin immunoprecipitating antibody used was 30Q8A (ICOS Corp.). The blots were subsequently incubated with HRP-conjugated anti-mouse IgG (Transduction Laboratories) and developed with ECL. To control for loading and transfer of immunoprecipitates, blots were stripped and reprobed with the immunoprecipitating antibody followed by HRP-conjugated, species-appropriate secondary IgG and were developed with ECL.
Glutathione S-Transferase (GST)-PTPμ- and VE-Cadherin-Binding Assays
EC lysates were incubated for 3 hours at 4°C with GST fusion proteins of the COOH-terminal cytoplasmic domain of either VE-cadherin (amino acids 629 to 793)28 or PTPμ (amino acids 774 to 1452)49 coupled to glutathione-Sepharose 4B beads (Pharmacia, Piscataway, NJ). The PTPμ-and VE-cadherin-binding proteins bound to the beads were extensively washed, boiled in sample buffer, resolved by SDS-polyacrylamide gel electrophoresis (PAGE), and transferred to PVDF membrane. The GST-PTPμ-binding proteins were probed with anti-VE-cadherin (30Q8A, ICOS Corp.) antibodies and the GST-VE-cadherin-binding proteins were probed with anti-PTPμ antibodies. Simultaneous GST bead controls were performed. In selected experiments, the GST-fusion proteins each containing either a thrombin or factor Xa recognition site, were subjected to protease cleavage with either thrombin (1 U/μl PBS) or factor Xa (1 U/μl in 50 mmol/L Tris-HC1, 150 mmol/L NaCl, 1 mmol/L CaCl2, pH 7.5) cleavage buffers in a GST trap column (Amersham Pharmacia, Piscataway, NJ) to remove the GST tag. After cleavage, proteins were eluted off the column, collected, resolved by SDS-PAGE, and predicted gel mobility confirmed with protein staining. Purified recombinant VE-cadherin was incubated for 3 hours at 4°C with either GST-PTPμ or GST-β-catenin each coupled to beads or with beads alone whereas purified recombinant PTPμ was incubated with either GST-VE-cadherin or GST-β-catenin each coupled to beads or with beads alone. The PTPμ-binding and β-catenin-binding proteins were processed for immunoblotting with affinity-purified, goat polyclonal anti-VE-cadherin antibodies raised against a peptide corresponding to the COOH-terminal cytoplasmic domain (Santa Cruz Biotechnology Inc.) and VE-cadherin-binding and β-catenin-binding proteins were processed for immunoblotting with anti-PTPμ antibodies. Purified recombinant proteins were used as simultaneous positive controls.
Effect of Overexpression of WT PTPμ on Tyrosine Phosphorylation State of VE-Cadherin
HMEC-1 cells were stably infected with either WT PTPμ, or the empty pRev-TRE2 vector, and cultured in the absence of doxycycline. Cells were lysed, and the lysates immunoprecipitated with either anti-VE-cadherin (30Q8A, ICOS) or anti-β-catenin (Transduction Laboratories) antibodies as described in the co-immunoprecipitation assays above. The VE-cadherin and β-catenin immunoprecipitates were resolved by SDS-PAGE, transferred to PVDF membrane, and the blots probed with antiphosphotyrosine antibody (PY-plus; Zymed, South San Francisco, CA) as previously described in detail.3,4 To control for loading and transfer of immunoprecipitates, blots were stripped and reprobed with the immunoprecipitating antibody followed by HRP-conjugated species appropriate secondary IgG. The blots were developed with ECL and the bands of interest normalized to the appropriate loading control.
Statistical Methods
Analysis of variance was used to compare the mean responses among experimental and control groups for all experiments. The Dunnett and Scheffé F-tests were used to determine between which group’s significant differences existed. A P value of <0.05 was considered significant.
Results
PTPμ Expression and Immunolocalization in Pulmonary Vascular Endothelia
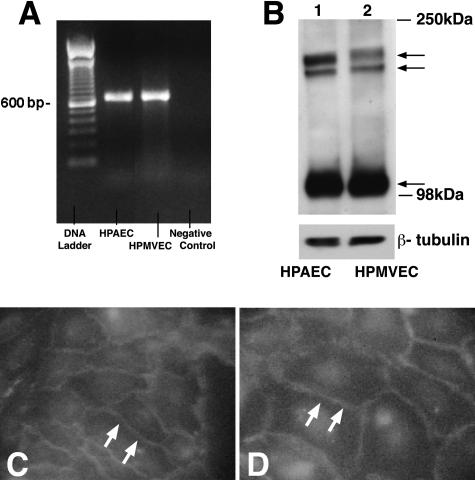
Human pulmonary artery and lung microvascular ECs were cultured to confluence under identical conditions. Using RT-PCR, PTPμ mRNA was detected in both endothelia (Figure 1A). EC lysates were immunoblotted with antibodies raised against PTPμ (Figure 1B). To ensure equal protein loading, blots were stripped and reprobed for β-tubulin. In both endothelia, PTPμ-immunoreactive bands that migrated with apparent Mr of 200,000 and 100,000 were revealed. On a 6% gel, the 200-kd band resolved into a doublet with apparent Mr of ∼210,000 and 195,000. PTPμ protein expression in the two endothelia was comparable. Therefore, PTPμ is expressed at both the mRNA and protein levels in both pulmonary artery and lung microvascular ECs where full-length PTPμ is proteolytically processed into ∼100-kd cleavage products as has been described in both epithelia23,42 and HUVECs.24
Figure 1.
PTPμ expression and immunolocalization in pulmonary vascular endothelia. A: RT-PCR was used to detect PTPμ mRNA in postconfluent human pulmonary artery ECs (HPAEC)s and lung microvascular ECs (HPMVECs). The control DNA ladder is shown on the left and the negative control is on the right. B: PTPμ immunoblotting was used to screen for PTPμ protein in pulmonary vascular ECs. Lysates from postconfluent ECs were resolved by SDS-PAGE and were transferred to PVDF membranes. The blots were probed with murine monoclonal anti-PTPμ antibodies (SK7) followed by HRP-conjugated anti-mouse IgG and developed with ECL. To confirm equivalent protein loading, each blot was stripped and reprobed with anti-β-tubulin antibody. Mr of protein standards in kd are shown on the right. Arrows on right indicate PTPμ-immunoreactive bands at ∼200 kd and ∼100 kd. Each blot is representative of two experiments. Human pulmonary artery (C) and lung microvascular (D) ECs, each were cultured to postconfluence, fixed, permeabilized, blocked, incubated with murine anti-PTPμ SK7 antibodies followed by FITC-conjugated anti-mouse IgG, and analyzed by epifluorescence microscopy. Arrows indicate PTPμ signal at intercellular boundaries. Original magnifications, ×750.
To determine the subcellular localization of PTPμ in pulmonary vascular ECs, postconfluent pulmonary artery and lung microvascular ECs were probed with murine anti-PTPμ (SK7) antibodies followed by FITC-conjugated goat anti-mouse IgG and analyzed by epifluorescence microscopy (Figure 1, C and D). Both endothelia displayed a fluorescence signal that was almost exclusively restricted to the intercellular boundaries, and to a much lesser degree, to perinuclear regions. These results indicate that in postconfluent pulmonary vascular ECs, PTPμ is enriched to EC-EC junctions.
PTPμ Regulates Endothelial Barrier Function
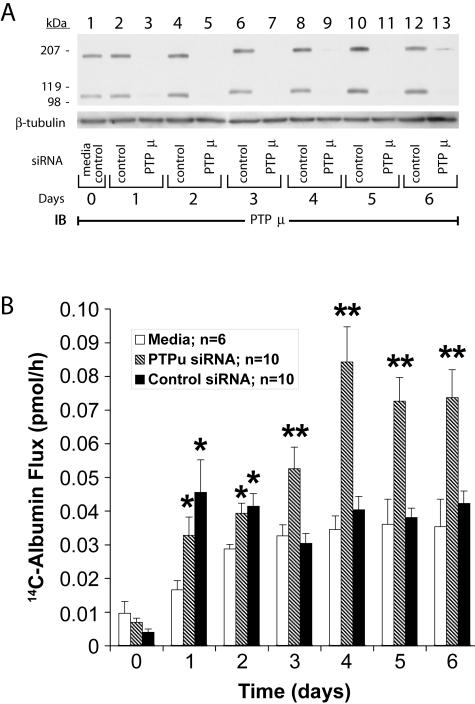
To determine whether PTPμ might influence EC-EC association and monolayer barrier function, postconfluent human lung microvascular EC monolayers were transfected with either PTPμ-targeting or control siRNA (Figure 2A). PTPμ protein was knocked down >95% compared to the simultaneous controls from days 1 to 5. PTPμ protein abundance in ECs transfected with control siRNA was no different from that seen in the media control (Figure 2A, lane 1 versus lanes 2, 4, 6, 8, 10, and 12). 14C-BSA flux across monolayers transfected with PTPμ siRNA was dramatically increased on days 3, 4, 5, and 6 compared to monolayers transfected with the control siRNA (P < 0.02) (Figure 2B). Throughout this same time period, mean 14C-BSA flux across monolayers transfected with control siRNA was not different from flux across the simultaneous media controls. On days 1 and 2, 14C-BSA flux across monolayers transfected with either control or PTPμ siRNA was increased compared to the simultaneous media controls but was not significantly different from each other or from flux across monolayers incubated with the transfection reagent alone (data not shown). These data indicate that the early barrier dysfunction could be ascribed to the transfection reagent whereas the loss of barrier function on day ≥3 was because of selective knockdown of PTPμ.
Figure 2.
Effect of knockdown of PTPμ through siRNA transfection on endothelial barrier function. A: Postconfluent human lung microvascular ECs were transfected with PTPμ-targeting (lanes 3, 5, 7, 9, 11, and 13) or control (lanes 2, 4, 6, 8, 10, and 12) siRNA and after increasing times (days 1 to 6), were lysed and the lysates resolved by SDS-PAGE, transferred to PVDF membrane, and immunoblotted for PTPμ. To confirm equivalent loading, each blot was stripped and reprobed with anti-β-tubulin antibody. B: Human lung microvascular ECs were cultured to confluence in barrier function assay chambers when baseline barrier function was established (day 0). The postconfluent ECs were cultured in media alone or were transfected with PTPμ-targeting or control siRNAs after which transendothelial 14C-BSA flux was assayed every 24 hours. Vertical bars represent mean (±SE) 14C-BSA flux in pmol/hour across media control EC monolayers or monolayers transfected with either PTPμ or control siRNA. *, Significantly increased compared to the simultaneous media control monolayers at P < 0.02. **, Significantly increased compared to the simultaneous siRNA control at P < 0.006.
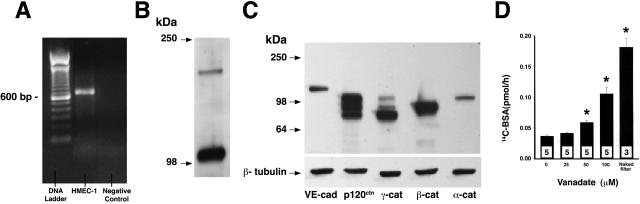
In selected experiments, HMEC-1 cells, immortalized human dermal microvascular ECs, were used to manipulate PTPμ expression and catalytic activity. To help define this experimental system, we determined whether HMEC-1 cells express PTPμ and ZA proteins and whether their paracellular pathway was responsive to PTP inhibition.28 Using RT-PCR, PTPμ mRNA was confirmed in HMEC-1 cells (Figure 3A). Lysates from postconfluent HMEC-1 cells were processed for immunoblotting for PTPμ (Figure 3B), as well as VE-cadherin, and α-, β-, γ-, and p120-catenins (Figure 3C). PTPμ-immunoreactive bands that migrated with apparent Mr of 200,000 and 100,000 were revealed. Of note, the ∼200-kd band did not migrate as a doublet as seen in the two primary cultured pulmonary vascular endothelia (Figure 1B). VE-cadherin and its associated catenins each were expressed with the anticipated gel mobilities (Figure 3C). PTP inhibition with vanadate resulted in concentration-dependent loss of barrier function in postconfluent HMEC-1 monolayers (Figure 3D). Therefore, HMEC-1 cells express PTPμ and all five ZA proteins for which we screened and contains a paracellular pathway that responds to PTP inhibition.
Figure 3.
PTPμ and ZA protein expression and barrier responsiveness to PTP inhibition in HMEC-1 cells. A: RT-PCR was used to detect PTPμ mRNA in HMEC-1 cells. The control DNA ladder is shown on the left and the negative control is on the right. B and C: Lysates of postconfluent HMEC-1 cells were resolved by SDS-PAGE, transferred to PVDF membrane, and immunoblotted for PTPμ (B), VE-cadherin, and α-, β-, γ-, and p120 catenins (C). C: The blot was stripped and reprobed for β-tubulin. Molecular weight markers in kd are indicated. D: Vertical bars represent mean (±SE) transendothelial 14C-BSA flux in pmol/hour immediately after 6 hours of exposure to increasing concentrations of vanadate or media alone. n indicates the number of monolayers studied. Mean (±SE) 14C-BSA flux across naked filters without EC monolayers is shown. *, Significantly increased compared to the simultaneous media control monolayers at P < 0.05.
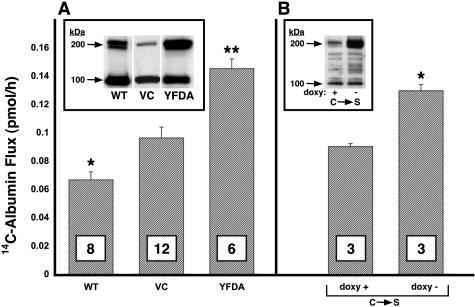
HMEC-1 cells overexpressing WT PTPμ, the catalytically impaired PTPμ YFDA mutant, or the empty vector were selected with hygromycin B. HMEC-1 cell lysates from pooled selectants were resolved by SDS-PAGE, transferred to PVDF membrane, and the blots probed with anti-PTPμ SK7 antibody. Full-length (∼200 kd) PTPμ expression in HMEC-1 cells stably infected with either WT PTPμ or the YFDA mutant was increased ∼7- to 20-fold relative to cells infected with the empty vector (Figure 4A, inset). When ECs overexpressing either WT PTPμ or the YFDA mutant were seeded into the wells of 12-well plates (5 × 104 ECs/well), cultured for 72 hours, and stained with Trypan blue, mean (±SE) percent viability (97.24 ± 0.36, n = 3, and 94.45 ± 0.45, n = 3, respectively) was comparable to the vector control (96.53 ± 0.67%, n = 3) or parental HMEC-1 cells (96.51 ± 0.78%, n = 3).
Figure 4.
Effect of overexpression of WT and catalytically impaired PTPμ mutants on endothelial barrier function. A: HMEC-1 cells stably infected with pRev-TRE-2 encoding for WT PTPμ, the catalytically impaired YFDA mutant, or the empty vector control (VC), each were seeded at equivalent densities in assay chambers and transendothelial 14C-BSA flux assayed at 48 hours. *, Significantly decreased compared to the vector control at P < 0.05. **, Significantly increased compared to the vector control at P < 0.05. Inset: PTPμ immunoblot of lysates of pooled selectants of HMEC-1 cells stably infected to overexpress WT PTPμ, the vector control, or the YFDA mutant. B: HMEC-1 cells stably co-infected with pRev-TRE-2 encoding for the catalytically inactive C→S PTPμ mutant and pRev-Tet-Off were cultured for 48 hours in assay chambers in the presence and absence of doxycycline after which transendothelial 14C-BSA flux was assayed. *, Significantly increased compared to the doxy + control at P < 0.002. Inset: HA immunoblot of lysates of pooled selectants of HMEC-1 cells stably co-infected to overexpress the HA-tagged C→S PTPμ mutant after withdrawal of doxycycline. Arrows on left indicate 200-kd full-length and 100-kd proteolytically cleaved PTPμ protein. In both A and B, each vertical bar represents mean (±SE) 14C-BSA flux in pmol/hour across EC monolayers overexpressing WT PTPμ, the vector control, the catalytically impaired YFDA mutant, or the tetracycline-responsive, catalytically inactive C→S mutant. n is indicated in each bar.
HMEC-1 cells overexpressing WT PTPμ or the empty vector alone were cultured in barrier function assay chambers for 48 hours after which transendothelial 14C-BSA flux was assayed (Figure 4A). The vector control cells achieved tight barrier function. The pooled and selected HMEC-1 cells overexpressing WT PTPμ formed monolayers that permitted even less transendothelial albumin flux than did the vector control (P < 0.05). In contrast, comparable overexpression of the catalytically impaired YFDA PTPμ mutant failed to enhance barrier function (Figure 4A). In other experiments, HMEC-1 cells stably co-infected with pRev-TRE2 encoding for the catalytically inactive C→S PTPμ mutant and pRev-Tet-Off were cultured in barrier function assay chambers in the presence and absence of 1 μg/ml of doxycycline for 48 hours after which transendothelial 14C-BSA flux was assayed (Figure 4B). After removal of doxycycline, both ectopic expression of the C→S PTPμ mutant and 14C-BSA flux were increased (P < 0.002) compared to expression and flux in the presence of doxycycline. Although increased expression of WT PTPμ decreased albumin flux, increased expression of either of two catalytically impaired PTPμ mutants, each with only one (C→S) or two (YFDA) amino acid substitutions, failed to do so (Figure 4, A and B). These combined data indicate that full catalytic activity is required for PTPμ to enhance barrier function. That overexpression of either catalytically impaired mutant failed to enhance barrier function indicates that overexpression of intact PTPμ ectodomain, together with its regulatory noncatalytic domains, was insufficient to reproduce the WT PTPμ effect. Overexpression of either of the two catalytically impaired PTPμ mutants not only failed to display the barrier-enhancing effect of WT PTPμ, each profoundly impaired barrier function (Figure 4, A and B).
PTPμ Interacts with VE-Cadherin
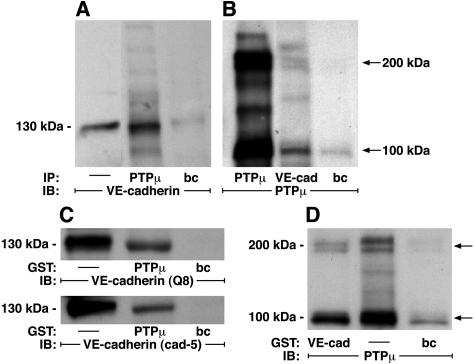
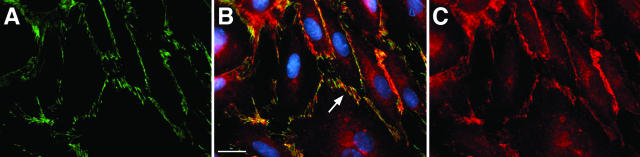
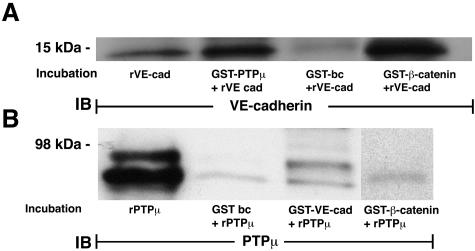
Since PTPμ could be localized to the intercellular boundaries of postconfluent ECs, we asked whether it associated with the membrane-spanning, EC-restricted cadherin, VE-cadherin. Immunoprecipitation of PTPμ with SK7 in lung microvascular ECs lysates, co-immunoprecipitated VE-cadherin (Figure 5A) compared to the simultaneous bead controls preloaded with a species- and isotype-matched irrelevant antibody. In reciprocal manner, immunoprecipitation of VE-cadherin co-immunoprecipitated both full-length PTPμ and the ∼100-kd PTPμ fragments (Figure 5B). In other experiments, lysates of postconfluent human lung microvascular ECs were incubated with GST fusion proteins of either the intracellular segment of PTPμ (Figure 5C), or the cytoplasmic domain of human VE-cadherin (Figure 5D), each coupled to glutathione Sepharose beads, or incubated with a GST bead control. The PTPμ-binding proteins were processed for immunoblotting with either of two distinct anti-VE-cadherin antibodies (Figure 5C). The GST-PTPμ-bound VE-cadherin was detected by either of the two anti-VE-cadherin antibodies whereas none was detected in the GST bead control. When the VE-cadherin-binding proteins were probed for PTPμ, GST-VE-cadherin bound PTPμ compared to the GST bead control (Figure 5D). Each of the two PTPμ-immunoreactive bands in the doublet bound to GST-VE-cadherin. These data indicate that PTPμ directly or indirectly interacts with VE-cadherin in vitro. To determine whether PTPμ co-localizes with VE-cadherin in an intact EC system, immunofluorescence microscopy was applied using simultaneous dual-antibody labeling (Figure 6). As anticipated, PTPμ (Figure 6C) and VE-cadherin (Figure 6A) each localized to intercellular boundaries. Merging the images obtained from identical fields with antibodies against each of these proteins revealed a high degree of co-localization of PTPμ and VE-cadherin in intercellular junctions (Figure 6B, regions of co-localization appear yellow). To determine whether the PTPμ-VE-cadherin interaction might be direct, the in vitro binding assays were performed with purified recombinant proteins in the absence of EC lysates (Figure 7). Purified recombinant VE-cadherin (amino acids 629 to 723) cleaved from GST was incubated with GST-PTPμ coupled to beads or with beads alone (Figure 7A). In reciprocal pull-down experiments, purified recombinant PTPμ (amino acids 774 to 1452) cleaved from GST was incubated with GST-VE-cadherin coupled to beads or beads alone (Figure 7B). The purified recombinant VE-cadherin bound to GST-PTPμ as well as the GST-β-catenin-positive control but not to the bead control (Figure 7A) and the purified recombinant PTPμ bound to the GST-VE-cadherin but not to either GST-β-catenin or the bead control (Figure 7B). These combined data indicate that PTPμ directly and specifically associates with VE-cadherin.
Figure 5.
PTPμ-VE-cadherin interactions. A and B: PTPμ and VE-cadherin co-immunoprecipitation assays. Lysates of postconfluent lung microvascular ECs were immunoprecipitated with either anti-PTPμ (SK7) or anti-VE-cadherin (30Q8A, ICOS) antibodies. The immunoprecipitates were resolved by SDS-PAGE, transferred onto PVDF membrane, and the blots incubated with either anti-VE-cadherin (A) or anti-PTPμ (B) antibodies followed by HRP-conjugated anti-murine antibody, and developed with ECL. Arrows on right indicate 200-kd and 100-kd PTPμ-immunoreactive bands. In A, the positive control is a total cell lysate indicated by —. C and D:GST-PTPμ- and GST-VE-cadherin-binding assays. Lysates from postconfluent lung microvascular ECs were incubated for 3 hours at 4°C with GST fusion proteins of the cytoplasmic domains of either PTPμ (C) or VE-cadherin (D) coupled to beads or incubated with beads alone. The PTPμ- and VE-cadherin-binding proteins were washed, boiled in sample buffer, resolved by SDS-PAGE, and transferred to PVDF membrane. The blots of PTPμ-binding and VE-cadherin-binding proteins were probed with two distinct anti-VE-cadherin antibodies (30Q8A, ICOS, and cadherin-5; Transduction Laboratories) and an anti-PTPμ antibody, respectively, followed by HRP-conjugated anti-murine antibody and developed with ECL. Whole EC lysates without previous incubation with GST-PTPμ or GST-VE-cadherin were immunoblotted as positive controls that are indicated by —. The molecular weights in kd are indicated on the left. Arrows on right indicate 200-kd and 100-kd PTPμ-immunoreactive bands. IP, immunoprecipitate; IB, immunoblot; bc, bead control. Each blot is representative of three experiments.
Figure 6.
Co-localization of PTPμ and VE-cadherin. Postconfluent human lung microvascular ECs were cultured on fibronectin (25 mg/ml)-coated glass coverslips for 48 hours, after which they were fixed and permeabilized with 3% paraformaldehyde and 0.5% Triton X-100, and stained with goat polyclonal anti-VE-cadherin (Santa Cruz) and monoclonal SK7 anti-PTPμ antibodies, followed by fluorescein- and Cy-3-labeled secondary antibodies, respectively. Nuclei were visualized with bis-benzimide. A: VE-cadherin. B: Merged image of VE-cadherin and PTPμ with Hoescht reagent. Regions of co-localized VE-cadherin and PTPμ appear yellow and are indicated by the arrows and nuclei appear blue. C: PTPμ. Scale bar, 20 μm. Original magnifications, ×600.
Figure 7.
GST-PTPμ and GST-VE-cadherin-binding assays with purified recombinant binding partners. The GST-PTPμ- and GST-VE-cadherin-binding assays were repeated as described in Figure 5, C and D, but in these experiments the GST fusion proteins coupled to beads were incubated with recombinant binding partners that were proteolytically cleaved from GST and purified in a GST trap column. GST-PTPμ and GST-β-catenin were incubated with purified VE-cadherin cleaved from GST (A) and GST-VE-cadherin and GST-β-catenin were incubated with purified PTPμ cleaved from GST (B). In each case, simultaneous empty bead controls were performed. The PTPμ-binding, VE-cadherin-binding, and β-catenin-binding proteins were processed for immunoblotting with goat anti-human VE-cadherin (A) (Santa Cruz) and anti-PTPμ (SK7) (B) antibodies, respectively. Purified recombinant proteins were used for the positive controls. The molecular weights in kd are indicated on the left. IB, immunoblot; bc, bead control. Each blot is representative of three experiments.
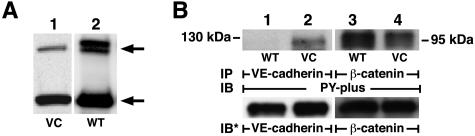
Overexpression of WT PTPμ in HMEC-1 Cells Modulates the Tyrosine Phosphorylation of VE-Cadherin
To determine whether VE-cadherin might be an in vivo substrate for PTPμ, WT PTPμ was overexpressed (Figure 8A) and changes in VE-cadherin and β-catenin tyrosine phosphorylation sought (Figure 8B). Lysates of HMEC-1 cells overexpressing WT PTPμ or the empty vector alone were immunoprecipitated with antibodies raised against VE-cadherin or β-catenin and the immunoprecipitates processed for phosphotyrosine immunoblotting (Figure 8B). In ECs overexpressing WT PTPμ, tyrosine phosphorylation of VE-cadherin (Figure 8B, lanes 1 and 2) was decreased compared to the vector controls, suggesting that VE-cadherin is a potential in vivo substrate for PTPμ catalytic activity. In contrast, overexpression of WT PTPμ did not decrease tyrosine phosphorylation of β-catenin (Figure 8B, lanes 3 and 4). Therefore, overexpression of PTPμ selectively dephosphorylated VE-cadherin without modifying another closely associated ZA component, β-catenin. It is conceivable that other as yet unidentified PTPμ substrates are also involved. Phosphotyrosine immunoblotting of total cell lysates of ECs, in which either WT PTPμ was overexpressed ∼10-fold or >90% PTPμ was knocked down with PTPμ siRNA, failed to demonstrate any consistent differences in phosphotyrosine signal compared to their respective controls (data not presented).
Figure 8.
Overexpression of WT PTPμ modulates tyrosine phosphorylation states of VE-cadherin. A: HMEC-1 cells were stably infected with packaged retroviral particles containing pRev-TRE2 encoding for WT PTPμ or the empty vector control (VC), and were selected with hygromycin B. HMEC-1 cell lysates from pooled selectants were resolved by SDS-PAGE, transferred to PVDF membrane, and the blots probed with anti-PTPμ SK7 antibody followed by HRP-conjugated anti-murine antibody and developed with ECL. The arrows on the right indicate the ∼200-kd full-length PTPμ and the ∼100-kd cleavage products. B: Lysates of HMEC-1 cells stably infected with pRev-TRE2 encoding for WT PTPμ or the empty vector (VC), each were immunoprecipitated with antibodies raised against VE-cadherin (30Q8A, ICOS) and β-catenin. In B, the VE-cadherin and β-catenin immunoprecipitates were resolved by SDS-PAGE, transferred to PVDF membrane, and the blots incubated with HRP-conjugated antiphosphotyrosine PY-plus antibody and developed with ECL. For normalization of the phosphotyrosine signal to the immunoprecipitated protein, blots were stripped and reprobed with the immunoprecipitating antibodies. IP, immunoprecipitate; IB, immunoblot; IB*, immunoblot after stripping; VC, vector control. Molecular weights in kd are indicated on each side. Each blot is representative of more than two experiments.
Discussion
In this report, we have demonstrated that PTPμ is expressed at the mRNA and protein levels in both human pulmonary artery and lung microvascular endothelia where it is enriched to EC-EC junctions. In both endothelia, PTPμ-immunoreactive bands that migrated with apparent Mr of 200,000 and 100,000 were revealed and protein expression in the two endothelia were comparable. Overexpression of WT PTPμ enhanced endothelial barrier function whereas comparable overexpression of either of two catalytically impaired PTPμ mutants not only failed to tighten the barrier but profoundly diminished it. These results indicate that an intact catalytic domain is required for the barrier-enhancing activity of WT PTPμ. Whereas overexpression of WT PTPμ enhanced barrier function, knockdown of PTPμ through siRNA opened the paracellular pathway. In postconfluent human lung microvascular ECs, the cytoplasmic domain of PTPμ directly associated with the intracellular segment of VE-cadherin. Overexpression of WT PTPμ in HMEC-1 cells demonstrated VE-cadherin as a potential in vivo PTPμ substrate. These combined data suggest that PTPμ is expressed in pulmonary vascular endothelia where it directly associates with and regulates the tyrosine phosphorylation state of VE-cadherin as well as EC-EC barrier integrity.
A tyrosine phosphorylation-responsive endothelial paracellular pathway, in which agonists that increase paracellular permeability also increase tyrosine phosphorylation of one or more ZA proteins, has been described.3–6 PTPs are expressed in various epithelia where they associate with and/or dephosphorylate ZA proteins.29–34 However, less is known about ZA-associated PTPs in endothelia.7,35,36,42 We asked whether PTPμ may provide such counterregulation within the human pulmonary vascular endothelium. Recently, PTPμ protein was found to be expressed in vascular ECs in adult rats and swine although PTPμ immunostaining was more prominent in arteries, arterioles, and the vasa vasorum than in veins and capillaries.42 In the current report, we found that in human postconfluent pulmonary artery and lung microvascular ECs, PTPμ protein expression in the two lung endothelia in vitro was comparable (Figure 1, A and B). PTPμ expression in EC monolayers reportedly increases with confluence.24 It is conceivable that in cultured, contact-inhibited ECs, PTPμ expression approaches a maximal level whereas in vivo, other environmental stimuli are operative.41 In both pulmonary vascular endothelia, full-length PTPμ resolved into a doublet (Figure 1B). Although in most reports full-length PTPμ appears as a single band, the PTPμ doublet has been described in COS cells transiently transfected with full-length PTPμ,24 Sf9 insect cells infected with recombinant baculovirus expressing full-length PTPμ,38 and in disassociated cells in chick retinal explants.51 Whether the ∼200-kd doublet in our EC system represents alternatively spliced variants, multiple phosphorylation states, proteolytic processing, and/or other posttranslational modifications is unclear. Such alternative splicing events have been described in the closely related receptor PTP, PTPρ.52
To evaluate whether PTPμ may regulate endothelial barrier function, PTPμ protein expression in ECs was genetically manipulated. In human lung microvascular ECs, PTPμ depletion through RNA interference disrupted barrier function (Figure 2) indicating that PTPμ, directly or indirectly, is absolutely required for barrier maintenance. In HMEC-1 cells, overexpression of WT PTPμ enhanced barrier function (Figure 4A). In contrast, comparable overexpression of either of two catalytically impaired PTPμ mutants, each with only one or two amino acid substitutions, displayed no barrier-enhancing activity (Figure 4A). These findings suggest that the ability of WT PTPμ to promote barrier function requires an intact catalytic domain and may be mediated through tyrosine dephosphorylation of VE-cadherin. Overexpression of the C→S PTPμ mutant that contains an intact PTPμ ectodomain but no catalytic activity did not diminish transendothelial albumin flux (Figure 4, A and B). This indicates that increased homophilically interacting ectodomain alone does not enhance paracellular pathway function. Similarly, overexpression of the putative regulatory, noncatalytic domains of PTPμ was insufficient to enhance barrier function. That increased availability of these potential, protein-binding domains does not tighten barrier function suggests that their increased association with potential binding partners does not explain the barrier-enhancing activity. Not only did each of the two catalytically impaired PTPμ mutants fail to simulate the WT PTPμ barrier-enhancing effect, they dramatically compromised barrier function (Figure 4, A and B). Overexpression of either of these two catalytically impaired, substrate-trapping mutants may sequester binding partners competitively displacing WT PTPμ thereby preventing downstream signaling events.
Since PTPμ could be localized to the intercellular boundaries of postconfluent lung microvascular ECs (Figures 1C and 6C), we asked whether it associated with the established EC-EC adherens junctional protein VE-cadherin. In the extracts of rat lung and various cell lines, PTPμ interacts with the classical cadherins, E-cadherin, N-cadherin, and cadherin-4.30,53 Although multiple cadherins can be co-expressed and differentially distributed in ECs,12 VE-cadherin is unique to ECs and is localized to their intercellular junctions where they regulate paracellular pathway function.13 Among the classical cadherins, the cytoplasmic domain of VE-cadherin is the least conserved.48 It shares only ∼36% amino acid identity with E-cadherin. It was therefore unclear whether PTPμ also binds VE-cadherin. In recent double-label co-localization fluorescent microscopy studies in HUVECs, PTPμ co-localized with VE-cadherin.42 We now have demonstrated in a cell-free system, that purified recombinant cytoplasmic domain of PTPμ binds to immobilized GST-VE-cadherin (Figure 7B) and purified recombinant cytoplasmic domain of VE-cadherin binds to immobilized GST-PTPμ (Figure 7A). These data clearly indicate that the cytoplasmic domain of PTPμ directly associates with the intracellular segment of VE-cadherin. The COOH-terminal 38-amino acid portion of E-cadherin is required for its association with PTPμ.29,30 The same sequence in human VE-cadherin shares ∼60% identity and ∼80% similarity with E-cadherin (BLAST, www.ncbi.nih.gov). It may be through this PTPμ-VE-cadherin interaction that PTPμ localizes to the EC-EC adherens junction.
To determine whether VE-cadherin could be an in vivo PTPμ substrate, we manipulated PTPμ catalytic activity in HMEC-1 cells. Overexpression of full-length WT PTPμ, containing the two catalytic domains and potential protein-binding domains, consistently resulted in tyrosine dephosphorylation VE-cadherin (Figure 8B). If the intracellular pool of a PTPμ-binding partner is finite or rate limiting, overexpression of WT PTPμ might indiscriminately dephosphorylate substrates that are in close proximity. In the current studies, a wide range of levels of WT PTPμ overexpression consistently demonstrated VE-cadherin as a substrate for tyrosine dephosphorylation (data not shown) whereas the VE-cadherin-binding partner, β-catenin, remained unchanged (Figure 8B). It is possible that other as yet unidentified PTPμ substrates are also involved. It is also possible that PTPμ regulates the paracellular pathway independent of its catalytic activity. Of note, introduction of PTPμ in prostate carcinoma cells that lack PTPμ restores E-cadherin-dependent adhesion but does so independent of PTPμ catalytic activity.54
VE-cadherin appears to be a potential in vivo substrate for PTPμ (Figure 8B). Although VE-cadherin has been implicated in multiple aspects of EC behavior,55 it is unclear whether any of these VE-cadherin functions or VE-cadherin-protein interactions are tyrosine phosphorylation-dependent. In one study, vascular endothelial growth factor-induced tyrosine phosphorylation of VE-cadherin in HUVECs was temporally coincident with increases in both paracellular permeability to FITC-dextran and EC migration.6 In another study, tumor necrosis factor-α similarly increased both tyrosine phosphorylation of VE-cadherin and movement of a permeability tracer across EC monolayers.20 These studies imply that increased VE-cadherin tyrosine phosphorylation is associated with opening of the endothelial paracellular pathway. Whether these and other tumor necrosis factor-α- and vascular endothelial growth factor-induced EC responses, including angiogenesis, can be causally related to VE-cadherin tyrosine phosphorylation, and whether PTPμ might counterregulate one or more of these VE-cadherin functions is unknown. In the current studies, overexpression of WT PTPμ decreased tyrosine phosphorylation of VE-cadherin (Figure 8B, lanes 1 and 3) and enhanced barrier function (Figure 4). These combined data indicate that PTPμ regulates both the tyrosine phosphorylation state of VE-cadherin and paracellular pathway function. Whether a causal relationship between these two EC responses exists and the mechanism(s) through which the intracellular modification might be coupled to functional changes outside the cell remain unknown. Interestingly, there is evidence that PTPμ regulates N-cadherin function.44 Down-regulation of PTPμ catalytic activity through either anti-sense or overexpression of a catalytically inactive PTPμ mutant decreased neurite outgrowth on an N-cadherin substrate, suggesting that PTPμ regulates N-cadherin-mediated homophilic adhesion.
The regulation of EC-EC interactions and the paracellular pathway through protein tyrosine phosphorylation is not well understood. Our findings indicate that in pulmonary vascular ECs, PTPμ directly associates with the cytoplasmic domain of VE-cadherin. That PTPμ can directly interact with the cadherin-catenin complex suggests its importance to ZA organization and function. Here, PTPμ dephosphorylates VE-cadherin and possibly other substrates. Such modification of VE-cadherin may, through inside-to-outside signaling, regulate VE-cadherin ectodomain homophilic adhesion. Under physiological conditions, PTPμ, and possibly other PTPs, appears to maintain basal endothelial barrier function through the restraint of tyrosine phosphorylation within the ZA multiprotein complex. Loss of this counterregulatory dephosphorylation may be operative during pathological conditions associated with opening of the pulmonary microvascular endothelial paracellular pathway (eg, acute respiratory distress syndrome) and dysregulated angiogenesis (eg, diabetic retinopathy). The mechanism(s) through which PTPμ regulates EC-EC engagement/disengagement may have implications for tissue morphogenesis and remodeling, and angiogenesis within the context of wound healing and tumor cell survival.
Acknowledgments
We thank Ms. Shirley A. Taylor for excellent secretarial support and Mr. Seth Crawford for photography and graphics.
Footnotes
Address reprint requests to Simeon E. Goldblum, M.D., Mucosal Biology Research Center, University of Maryland School of Medicine, 22 Penn St., HSF II, Room 351, Baltimore, MD 21201. E-mail: sgoldblu@mbrc.umaryland.edu.
Supported by the National Institutes of Health (grants HL63217, HL70155, and HL58064 to S.E.G. and GM55989 to N.K.T.) and the American Heart Association (grant 0151465U to S.E.G.).
This article is featured in a commentary by A. Verin (Am J Pathol 2005, 166:955–957), published in this issue.
References
- Dudek SM, Garcia JGN. Cytoskeletal regulation of pulmonary vascular permeability. J Appl Physiol. 2001;91:1487–1500. doi: 10.1152/jappl.2001.91.4.1487. [DOI] [PubMed] [Google Scholar]
- Cines DB, Pollak ES, Buck CA, Loscalzo J, Zimmerman GA, McEver RP, Pober JS, Wick TM, Konkle BA, Schwartz BS, Barnathan ES, McCrae KR, Hug BA, Schmidt AM, Stern DM. Endothelial cells in physiology and in the pathophysiology of vascular disorders. Blood. 1998;91:3527–3561. [PubMed] [Google Scholar]
- Bannerman DD, Goldblum SE. Endotoxin induces endothelial barrier dysfunction through protein tyrosine phosphorylation. Am J Physiol. 1997;273:L217–L226. doi: 10.1152/ajplung.1997.273.1.L217. [DOI] [PubMed] [Google Scholar]
- Goldblum SE, Young BA, Wang P, Murphy-Ullrich JE. Thrombospondin-1 induces tyrosine phosphorylation of adherens junction proteins and regulates an endothelial paracellular pathway. Mol Biol Cell. 1999;10:1537–1551. doi: 10.1091/mbc.10.5.1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staddon JM, Herrenknecht K, Smales C, Rubin LL. Evidence that tyrosine phosphorylation may increase tight junction permeability. J Cell Sci. 1995;108:609–619. doi: 10.1242/jcs.108.2.609. [DOI] [PubMed] [Google Scholar]
- Esser S, Lampugnani MG, Corada M, Dejana E, Risau W. Vascular endothelial growth factor induces VE-cadherin tyrosine phosphorylation in endothelial cells. J Cell Sci. 1998;111:1853–1865. doi: 10.1242/jcs.111.13.1853. [DOI] [PubMed] [Google Scholar]
- Ukropec JA, Hollinger MK, Salva SM, Woolkalis MJ. SHP2 association with VE-cadherin complexes in human endothelial cells is regulated by thrombin. J Biol Chem. 2000;275:5983–5986. doi: 10.1074/jbc.275.8.5983. [DOI] [PubMed] [Google Scholar]
- Adamson RH. Protein tyrosine phosphorylation modulates microvessel permeability in frog mesentery. Microcirculation. 1996;3:245–247. doi: 10.3109/10739689609148297. [DOI] [PubMed] [Google Scholar]
- Dejana E, Corada M, Lampugnani MG. Endothelial cell-to-cell junctions. FASEB J. 1995;9:910–918. [PubMed] [Google Scholar]
- Lampugnani MG, Dejana E. Interendothelial junctions: structure, signalling and functional roles. Curr Opin Cell Biol. 1997;9:674–682. doi: 10.1016/s0955-0674(97)80121-4. [DOI] [PubMed] [Google Scholar]
- Wong MK, Gotlieb AI. Endothelial cell monolayer integrity. I. Characterization of dense peripheral band of microfilaments. Arteriosclerosis. 1986;6:212–219. doi: 10.1161/01.atv.6.2.212. [DOI] [PubMed] [Google Scholar]
- Navarro P, Ruco L, Dejana E. Differential localization of VE- and N-cadherins in human endothelial cells: VE-cadherin competes with N-cadherin for junctional localization. J Cell Biol. 1998;140:1475–1484. doi: 10.1083/jcb.140.6.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corada M, Mariotti M, Thurston G, Smith K, Kunkel R, Brockhaus M, Lampugnani MG, Martin-Padura I, Stoppacciaro A, Ruco L, McDonald DM, Ward PA, Dejana E. Vascular endothelial-cadherin is an important determinant of microvascular integrity in vivo. Proc Natl Acad Sci USA. 1999;96:9815–9820. doi: 10.1073/pnas.96.17.9815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aberle H, Schwartz H, Kemler R. Cadherin-catenin complex: protein interactions and their implications for cadherin function. J Cell Biochem. 1996;61:514–523. doi: 10.1002/(SICI)1097-4644(19960616)61:4%3C514::AID-JCB4%3E3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Herrenknecht K, Ozawa M, Eckerskorn C, Lottspeich F, Lenter M, Kemler R. The uvomorulin-anchorage protein alpha catenin is a vinculin homologue. Proc Natl Acad Sci USA. 1991;88:9156–9160. doi: 10.1073/pnas.88.20.9156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulsken J, Birchmeier W, Behrens J. E-cadherin and APC compete for the interaction with beta-catenin and the cytoskeleton. J Cell Biol. 1994;127:2061–2069. doi: 10.1083/jcb.127.6.2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukita S, Oishi K, Akiyama T, Yamanashi Y, Yamamoto T, Tsukita S. Specific proto-oncogenic tyrosine kinases of src family are enriched in cell-to-cell adherens junctions where the level of tyrosine phosphorylation is elevated. J Cell Biol. 1991;113:867–879. doi: 10.1083/jcb.113.4.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamaguchi M, Matsuyoshi N, Ohnishi Y, Gotoh B, Takeichi M, Nagai Y. p60v-src causes tyrosine phosphorylation and inactivation of the N-cadherin-catenin cell adhesion system. EMBO J. 1993;12:307–314. doi: 10.1002/j.1460-2075.1993.tb05658.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balsamo J, Arregui C, Leung T-C, Lillien J. The nonreceptor protein tyrosine phosphatase (PTPIB) binds to the cytoplasmic domain of N-cadherin and regulates the cadherin-actin linkage. J Cell Biol. 1998;143:523–532. doi: 10.1083/jcb.143.2.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nwariaku FE, Liu Z, Zhu X, Turnage RH, Sarosi GA, Terada LS. Tyrosine phosphorylation of vascular endothelial cadherin and the regulation of microvascular permeability. Surgery. 2002;132:180–185. doi: 10.1067/msy.2002.125305. [DOI] [PubMed] [Google Scholar]
- Ostman A, Yang Q, Tonks NK. Expression of DEP-1, a receptor-like protein-tyrosine-phosphatase, is enhanced with increasing cell density. Proc Natl Acad Sci USA. 1994;91:9680–9684. doi: 10.1073/pnas.91.21.9680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaits F, Li RY, Ragab A, Ragab-Thomas JM, Chap H. Increase in receptor-like protein tyrosine phosphatase activity and expression level on density-dependent growth arrest of endothelial cells. Biochem J. 1995;311:97–103. doi: 10.1042/bj3110097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebbink MF, Zondag GC, Koningstein GM, Feiken E, Wubbolts RW, Moolenaar WH. Cell surface expression of receptor protein tyrosine phosphatase RPTP mu is regulated by cell-cell contact. J Cell Biol. 1995;131:251–260. doi: 10.1083/jcb.131.1.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campan M, Yoshizumi M, Seidah NG, Lee ME, Bianchi C, Haber E. Increased proteolytic processing of protein tyrosine phosphatase mu in confluent vascular endothelial cells: the role of PC5, a member of the subtilisin family. Biochemistry. 1996;35:3797–3802. doi: 10.1021/bi952552d. [DOI] [PubMed] [Google Scholar]
- Gloor SM, Weber A, Adachi N, Frei K. Interleukin-1 modulates protein tyrosine phosphatase activity and permeability of brain endothelial cells. Biochem Biophys Res Commun. 1997;239:804–809. doi: 10.1006/bbrc.1997.7557. [DOI] [PubMed] [Google Scholar]
- Guo DQ, Wu LW, Dunbar JD, Ozes ON, Mayo LD, Kessler KM, Gustin JA, Baerwald MR, Jaffe EA, Warren RS, Donner DB. Tumor necrosis factor employs a protein-tyrosine phosphatase to inhibit activation of KDR and vascular endothelial cell growth factor-induced endothelial cell proliferation. J Biol Chem. 2000;275:11216–11221. doi: 10.1074/jbc.275.15.11216. [DOI] [PubMed] [Google Scholar]
- Ahmad F, Goldstein BJ. Effect of tumor necrosis factor-alpha on the phosphorylation of tyrosine kinase receptors is associated with dynamic alterations in specific protein-tyrosine phosphatases. J Cell Biochem. 1997;64:117–127. [PubMed] [Google Scholar]
- Young BA, Sui X, Kiser TD, Hyun SW, Wang P, Sakarya S, Angelini DJ, Schaphorst KL, Hasday JD, Cross AS, Romer LH, Passaniti A, Goldblum SE. Protein tyrosine phosphatase activity regulates endothelial cell-cell interactions, the paracellular pathway, and capillary tube stability. Am J Physiol. 2003;285:L63–L75. doi: 10.1152/ajplung.00423.2002. [DOI] [PubMed] [Google Scholar]
- Brady-Kalnay SM, Rimm DL, Tonks NK. Receptor protein tyrosine phosphatase PTPmu associates with cadherins and catenins in vivo. J Cell Biol. 1995;130:977–986. doi: 10.1083/jcb.130.4.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady-Kalnay SM, Mourton T, Nixon JP, Pietz GE, Kinch M, Chen H, Brackenbury R, Rimm DL, Del Vecchio RL, Tonks NK. Dynamic interaction of PTPmu with multiple cadherins in vivo. J Cell Biol. 1998;141:287–296. doi: 10.1083/jcb.141.1.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zondag GC, Reynolds AB, Moolenaar WH. Receptor protein-tyrosine phosphatase RPTPmu binds to and dephosphorylates the catenin p120(ctn). J Biol Chem. 2000;275:11264–11269. doi: 10.1074/jbc.275.15.11264. [DOI] [PubMed] [Google Scholar]
- Fuchs M, Muller T, Lerch MM, Ullrich A. Association of human protein-tyrosine phosphatase kappa with members of the armadillo family. J Biol Chem. 1996;271:16712–16719. doi: 10.1074/jbc.271.28.16712. [DOI] [PubMed] [Google Scholar]
- Cheng J, Wu K, Armanini M, O’Rourke N, Dowbenko D, Lasky LA. A novel protein-tyrosine phosphatase related to the homotypically adhering kappa and mu receptors. J Biol Chem. 1997;272:7264–7277. doi: 10.1074/jbc.272.11.7264. [DOI] [PubMed] [Google Scholar]
- Kypta RM, Su H, Reichardt LF. Association between a transmembrane protein tyrosine phosphatase and the cadherin-catenin complex. J Cell Biol. 1996;134:1519–1529. doi: 10.1083/jcb.134.6.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawroth R, Poell G, Ranft A, Kloep S, Samulowitz U, Fachinger G, Golding M, Shima DT, Deutsch U, Vestweber D. VE-PTP and VE-cadherin ectodomains interact to facilitate regulation of phosphorylation and cell contacts. EMBO J. 2002;21:4885–4895. doi: 10.1093/emboj/cdf497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holsinger LJ, Ward K, Duffield B, Zachwieja J, Jallal B. The transmembrane receptor protein tyrosine phosphatase DEP1 interacts with p120(ctn). Oncogene. 2002;21:7067–7076. doi: 10.1038/sj.onc.1205858. [DOI] [PubMed] [Google Scholar]
- Gebbink MF, van Etten I, Hateboer G, Suijkerbuijk R, Beijersbergen RL, Geurts VK, Moolenaar WH. Cloning, expression and chromosomal localization of a new putative receptor-like protein tyrosine phosphatase. FEBS Lett. 1991;290:123–130. doi: 10.1016/0014-5793(91)81241-y. [DOI] [PubMed] [Google Scholar]
- Gebbink MF, Verheijen MH, Zondag GC, van Etten I, Moolenaar WH. Purification and characterization of the cytoplasmic domain of human receptor-like protein tyrosine phosphatase RPTP mu. Biochemistry. 1993;32:13516–13522. doi: 10.1021/bi00212a017. [DOI] [PubMed] [Google Scholar]
- Lampugnani MG, Zanetti A, Corada M, Takahashi T, Balconi G, Breviario F, Orsenigo F, Cattelino A, Kemler R, Daniel TO, Dejana E. Contact inhibition of VEGF-induced proliferation requires vascular endothelial cadherin, β-catenin, and the phosphatase DEP-1/CD148. J Cell Biol. 2003;161:793–804. doi: 10.1083/jcb.200209019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady-Kalnay SM, Tonks NK. Protein tyrosine phosphatases as adhesion receptors. Curr Opin Cell Biol. 1995;7:650–657. doi: 10.1016/0955-0674(95)80106-5. [DOI] [PubMed] [Google Scholar]
- Fuchs M, Wang H, Ciossek T, Chen Z, Ullrich A. Differential expression of MAM-subfamily protein tyrosine phosphatases during mouse development. Mech Dev. 1998;70:91–109. doi: 10.1016/s0925-4773(97)00179-2. [DOI] [PubMed] [Google Scholar]
- Bianchi C, Sellke FW, Del Vecchio RL, Tonks NK, Neel BG. Receptor-type protein-tyrosine phosphatase mu is expressed in specific vascular endothelial beds in vivo. Exp Cell Res. 1999;248:329–338. doi: 10.1006/excr.1999.4428. [DOI] [PubMed] [Google Scholar]
- Brady-Kalnay SM, Tonks NK. Identification of the homophilic binding site of the receptor protein tyrosine phosphatase PTP mu. J Biol Chem. 1994;269:28472–28477. [PubMed] [Google Scholar]
- Burden-Gulley SM, Brady-Kalnay SM. PTPmu regulates N-cadherin-dependent neurite outgrowth. J Cell Biol. 1999;144:1323–1336. doi: 10.1083/jcb.144.6.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flint AJ, Tiganis T, Barford D, Tonks NK. Development of “substrate-trapping” mutants to identify physiological substrates of protein tyrosine phosphatases. Proc Natl Acad Sci USA. 1997;94:1680–1685. doi: 10.1073/pnas.94.5.1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang SH, Liu J, Kobayashi R, Tonks NK. Identification of the cell cycle regulator VCP (p97/CDC48) as a substrate of the band 4.1-related protein-tyrosine phosphatase PTPH1. J Biol Chem. 1999;274:17806–17812. doi: 10.1074/jbc.274.25.17806. [DOI] [PubMed] [Google Scholar]
- Feiken E, van Etten I, Gebbink MF, Moolenaar WH, Zondag GC. Intramolecular interactions between the juxtamembrane domain and phosphatase domains of receptor protein-tyrosine phosphatase RPTPmu. Regulation of catalytic activity. J Biol Chem. 2000;275:15350–15356. doi: 10.1074/jbc.275.20.15350. [DOI] [PubMed] [Google Scholar]
- Suzuki S, Sano K, Tanihara H. Diversity of the cadherin family: evidence for eight new cadherins in nervous tissue. Cell Regul. 1991;2:261–270. doi: 10.1091/mbc.2.4.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady-Kalnay SM, Flint AJ, Tonks NK. Homophilic binding of PTP mu, a receptor-type protein tyrosine phosphatase, can mediate cell-cell aggregation. J Cell Biol. 1993;122:961–972. doi: 10.1083/jcb.122.4.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannerman DD, Sathyamoorthy M, Goldblum SE. Bacterial lipopolysaccharide disrupts endothelial monolayer integrity and survival signaling events through caspase cleavage of adherens junction proteins. J Biol Chem. 1998;273:35371–35380. doi: 10.1074/jbc.273.52.35371. [DOI] [PubMed] [Google Scholar]
- Burden-Gulley SM, Ensslen SE, Brady-Kalnay SM. Protein tyrosine phosphatase-mu differentially regulates neurite outgrowth of nasal and temporal neurons in the retina. J Neurosci. 2002;22:3615–3627. doi: 10.1523/JNEUROSCI.22-09-03615.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besco JA, Frostholm A, Popesco MC, Burghes AHM, Rotter A. Genomic organization and alternative splicing of the human and morse RPTPrho genes. BMC Genomics. 2001;2:1–13. doi: 10.1186/1471-2164-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiscox S, Jiang WG. Association of PTPmu with catenins in cancer cells: a possible role for E-cadherin. Int J Oncol. 1998;13:1077–1080. doi: 10.3892/ijo.13.5.1077. [DOI] [PubMed] [Google Scholar]
- Hellberg CB, Burden-Gulley SM, Pietz GE, Brady-Kalnay SM. Expression of the receptor protein-tyrosine phosphatase, PTPmu, restores E-cadherin-dependent adhesion in human prostate carcinoma cells. J Biol Chem. 2002;277:11165–11173. doi: 10.1074/jbc.M112157200. [DOI] [PubMed] [Google Scholar]
- Dejana E. Endothelial adherens junctions: implications in the control of vascular permeability and angiogenesis. J Clin Invest. 1997;100:S7–S10. [PubMed] [Google Scholar]