Abstract
Alcoholic liver disease is associated with zinc decrease in the liver. Therefore, we examined whether dietary zinc supplementation could provide protection from alcoholic liver injury. Metallothionein-knockout and wild-type 129/Sv mice were pair-fed an ethanol-containing liquid diet for 12 weeks, and the effects of zinc supplementation on ethanol-induced liver injury were analyzed. Zinc supplementation attenuated ethanol-induced hepatic zinc depletion and liver injury as measured by histopathological and ultrastructural changes, serum alanine transferase activity, and hepatic tumor necrosis factor-α in both metallothionein-knockout and wild-type mice, indicating a metallothionein-independent zinc protection. Zinc supplementation inhibited accumulation of reactive oxygen species, as indicated by dihydroethidium fluorescence, and the consequent oxidative damage, as assessed by immunohistochemical detection of 4-hydroxynonenal and nitrotyrosine and quantitative analysis of malondialdehyde and protein carbonyl in the liver. Zinc supplementation suppressed ethanol-elevated cytochrome P450 2E1 activity but increased the activity of alcohol dehydrogenase in the liver, without affecting the rate of blood ethanol elimination. Zinc supplementation also prevented ethanol-induced decreases in glutathione concentration and glutathione peroxidase activity and increased glutathione reductase activity in the liver. In conclusion, zinc supplementation prevents alcoholic liver injury in an metallothionein-independent manner by inhibiting the generation of reactive oxygen species (P450 2E1) and enhancing the activity of antioxidant pathways.
Zinc depletion in the liver has been well documented in alcoholic patients as well as in animal models of ethanol-induced liver disease.1–3 Investigation of zinc metabolism in alcoholics has demonstrated that ethanol consumption leads to increased zinc excretion in urine and decreased zinc absorption from intestine.4,5 Although zinc depletion has been suggested to play an important role in alcoholic live injury,2 the mechanistic insights into the involvement of zinc depletion in the pathogenesis of alcoholic liver disease have not been achieved.
Ethanol is mainly metabolized in the liver through three major pathways with different subcellular locations: alcohol dehydrogenase (ADH) in the cytosol, aldehyde dehydrogenase (ALDH) in the mitochondria, and microsomal ethanol-oxidizing system in the endoplasmic reticulum.6 All of the three pathways result in reactive oxygen species (ROS) generation. However, the microsomal ethanol-oxidizing system, especially the cytochrome P450 2E1 (CYP2E1), has been shown to play a critical role in ethanol-induced oxidative stress. Long-term ethanol exposure significantly increases the CYP2E1 pathway.7–9 A recent study using CYP2E1 transgenic mice has demonstrated that overexpression of CYP2E1 enhances liver damage by chronic ethanol exposure.10 Inhibition of CYP2E1 activity by inhibitors reduced ethanol-induced liver injury.11,12 On the other hand, chronic ethanol exposure has little effect on ADH activities,7 although ADH is a major pathway for ethanol metabolism under normal physiological conditions. Thus, the shift from ADH to CYP2E1 in ethanol metabolism under chronic ethanol exposure may account for the generation of ROS under chronic ethanol exposure.
Many studies have demonstrated that zinc per se functions as an antioxidant.13 Importantly, zinc has catalytic and structural roles in more than 300 metalloenzymes, as well as regulatory roles in diverse cellular processes such as signaling transduction and gene expression. ADH is a zinc metalloenzyme, and removal of zinc from ADH led to a complete loss of its catalytic activity. Thus, the ethanol-induced zinc depletion is most likely linked to the altered ethanol metabolic pathway, such that a shift from ADH to CYP2E1 results in oxidative stress. Zinc also plays an important role in regulation of cellular glutathione (GSH) that is vital to cellular antioxidant defense.14 Therefore, the present study was undertaken to determine whether dietary zinc supplementation can improve hepatic zinc status under chronic ethanol exposure, thereby preventing oxidative liver injury, and the possible mechanisms by which zinc inhibits ethanol-induced oxidative stress, focusing on ethanol metabolic pathway and GSH-related antioxidants. Because zinc is a potent inducer of metallothionein (MT) synthesis and MT plays an important role in zinc homeostasis,15 a MT-knockout (MT-KO) mouse model and the wild-type (WT) 129/Sv controls were used to define whether the action of zinc is MT-dependent or MT-independent.
Materials and Methods
Animals
Homozygous MT-KO mice and WT controls were obtained from Jackson Laboratories (Bar Harbor, ME). The MT-KO mice lacking MT-I and MT-II, the major mouse hepatic MT isoforms, were produced on the 129/Sv genetic background by a gene-targeting technique.16 All of the mice were treated according to the experimental procedures approved by the Institutional Animal Care and Use Committee.
Improved Chronic Ethanol Feeding Protocol
The Lieber-DeCarli ethanol liquid diet has been widely used to establish animal models for alcoholic liver disease. According to our preliminary experiments, a long-term feeding of mice with the Lieber-DeCarli ethanol liquid diet causes decreases in food intake and body weight gain. To eliminate the possible confounding of malnutrition caused by decreased food intake, we established an improved ethanol feeding protocol for mice. In this protocol, a 1-day-stop on the last day of each week was introduced in the ethanol feeding schedule by replacing ethanol diet with control diet. The content of ethanol in the diet (%, w/v) was gradually increased throughout a 12-week feeding period, starting at 3.2, increasing 0.2% every 2 weeks, and reaching 4.2% at the end. Accordingly, the calorie contribution of ethanol started at 23% of total calories, and reached 30% at the end of 12-week feeding.
Animal Treatments
Ten-week-old male MT-KO and WT 129/Sv mice (∼25 g body weight) were used. Animals were pair-fed with the Lieber-DeCarli liquid diet (Bio-Serv, Frenchtown, NJ), containing either ethanol or isocaloric dextrin maltose. Animals were randomly assigned to eight groups using a 2 × 2 × 2 factorial design, MT × ethanol × zinc. Zinc supplementation was conducted by adding zinc sulfate to the liquid diet at 75 mg zinc element/L. Food intake and body weight were recorded daily and weekly, respectively. At the end of the experiment, the mice were anesthetized with sodium pentobarbital (50 mg/kg). Blood was drawn using a heparinized syringe from the dorsal vena cava, and serum was obtained by centrifugation. The liver was perfused with saline and tissue samples were processed for both pathological and biochemical analysis.
Examination of Blood Ethanol Levels
Blood ethanol levels were examined at 9:00 a.m. in the last week. Blood samples were taken from the tail vein and immediately deproteinized with 6.25% trichloroacetic acid solution. Ethanol concentrations were determined using a Sigma Diagnostics Alcohol kit (procedure no. 332-UV; Sigma, St. Louis, MO).
Zinc and MT Assays
Hepatic zinc concentrations were determined by inductively coupled argon plasma emission spectroscopy (model 1140; Jarrel-Ash, Waltham, MA) after lyophilization and digestion of the tissues with nitric acid and hydrogen peroxide.17 MT concentrations in the liver were determined by a cadmium-hemoglobin affinity assay.18
Assessment of Liver Injury
Histopathological and ultrastructural changes in the liver were examined by light and electron microscopy.19 Serum alanine aminotransferase (ALT) activity was colorimetrically measured using a Sigma Diagnostic kit (procedure no. 505, Sigma). Liver tumor necrosis factor (TNF)-α levels were detected by enzyme-linked immunosorbent assay (ELISA) using a murine kit (BioSource Int., Inc., Camarillo, CA).20
Estimation of Oxidative Stress
Ethanol-induced oxidative stress in the liver was measured by microscopic detection of ROS, 4-hydroxynonenal (4-HNE), and nitrotyrosine, and by biochemical measurements of lipid peroxidation and protein oxidation. Dihydroethidium was used for in situ detection of ROS in the liver according to a previous report.21 Nonfluorescent dihydroethidium is oxidized by ROS to yield red fluorescent product that binds to nucleic acids, staining the nucleus a bright fluorescent red. Cryostat sections of liver were cut at 5 μm and incubated with 5 μmol/L dihydroethidium (Molecular Probes, Eugene, OR) at 37°C for 30 minutes. The red fluorescence from dihydroethidium was detected with a Nikon 2000S fluorescence microscope (Nikon, Melville, NY).
Lipid peroxidation product, 4-HNE, and protein oxidation product, nitrotyrosine, were detected by immunohistochemical staining. Liver tissues were fixed with 4% paraformaldehyde, and sections were cut at 5 μm. A rabbit anti-4-HNE antibody (Alpha Diagnostic, San Antonio, TX) and a rabbit anti-nitrotyrosine antibody (Upstate, Waltham, MA) in combination with a DAKO EnVision+ horseradish peroxidase-conjugated goat anti-rabbit IgG (DAKO, Carpinteria, CA) were used for recognizing the oxidative products. The negative controls were conducted by omitting the primary antibody.
The extent of lipid peroxidation was quantitatively determined by measuring the concentration of thiobarbituric acid-reactive product malondialdehyde.19 Protein peroxidation in the liver was quantified by measuring protein carbonyl content using a sensitive ELISA.22 Protein derivatization was first performed by incubating 15-μl samples (4 mg protein/ml) and 45 μl of 10 mmol/L dinitro-phenylhydrazide. The detect system was a rabbit anti-dinitrophenylhydrazide antibody (Molecular Probes) and horseradish peroxidase-conjugated goat anti-rabbit IgG (Sigma).
Measurements of Ethanol Metabolism and Enzymes
The activities of CYP2E1, ADH, and ALDH in the liver and the ethanol metabolic rate were measured. Microsomal CYP2E1 activity was estimated by colorimetrically measuring the hydroxylation of p-nitrophenol to 4-nitrocatechol, a reaction catalyzed specifically by CYP2E1.23 ADH activity was measured by detecting the reduction of NAD+ at 340 nm in a reaction mixture containing 0.5 mol/L Tris-HCl buffer (pH 7.2), 2.8 mmol/L NAD+, and 5 mmol/L ethanol.24 Mitochondrial ALDH activity was measured by detecting the reduction of NAD+ at 340 nm in a reaction mixture containing 50 mmol/L sodium pyrophosphate (pH 8.8), 0.5 mmol/L NAD+, 0.1 mmol/L pyrazol, 5 mmol/L acetaldehyde, and 2 μmol/L rotenone.25 The protein concentrations of CYP2E1 and ADH were quantitatively measured by an ELISA. The detect systems were a rabbit anti-CYP2E1 antibody (Calbiochem, San Diego, CA) or a mouse anti-ADH antibody (Chemicon, Temecula, CA) in combination with horseradish peroxidase-conjugated anti-rabbit IgG or anti-mouse IgM (Sigma).
Ethanol metabolic rate was estimated by measuring blood ethanol elimination during the last week of feeding. Ethanol diets were replaced with control diets 1 day before the measurement. Mice were given an intragastric bolus of ethanol at 3 g/kg. Blood samples were taken from the tail vein every hour for 4 hours, and the blood ethanol levels were measured using a Sigma Diagnostic Alcohol kit (procedure no. 332-UV, Sigma).
Measurements of GSH and GSH-Related Enzymes
For measurement of subcellular GSH levels, cytosolic and mitochondrial fractions were separated from fresh liver tissue. Briefly, the liver was homogenized in 10 mmol/L HEPES buffer, pH 7.4, containing 200 mmol/L mannitol, 50 mmol/L sucrose, 10 mmol/L KCl, and 1 mmol/L ethylenediamine tetraacetic acid using a Dounce glass homogenizer (Kontes, Vineland, NJ). The crude homogenate was centrifuged at 700 × g, and the supernatant was centrifuged further at 15,000 × g to give a cytosolic supernatant and a mitochondrial pellet. The mitochondrial pellet was washed twice and resuspended in the same buffer. Metaphosphoric acid was used for deproteinization at a final concentration of 5%. After centrifuging at 15,000 × g, the supernatants were used as mitochondrial fraction for GSH assay immediately. The GSH concentrations were measured using a Bioxytech GSH-400 assay kit (OXIS, Portland, OR). Glutathione reductase (GR) activity was assayed by measuring the rate of NADPH oxidation at 340 nm in the presence of 2.4 mmol/L GSSG with a glutathione reductase assay kit (Calbiochem). Glutathione peroxidase (GPx) activity was assayed by measuring the rate of NADPH oxidation at 340 nm with a cellular glutathione peroxidase assay kit (Calbiochem) based on the reduction of organic peroxide, tert-butyl hydroperoxide, by GPx in the presence of GSH.
Statistics
All data are expressed as mean ± SE (n = 4 to 6). The data were analyzed by analysis of variance and Newman-Keuls’ multiple comparison test. Differences between groups were considered significant at P < 0.05.
Results
Characterization of Mice under Chronic Ethanol Exposure
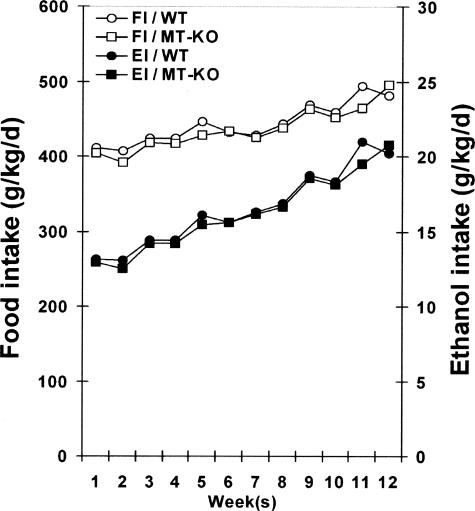
Daily food intake was monitored in the ethanol-exposure WT and MT-KO mice, and pair-fed mice were adjusted for their food supply accordingly. The dietary ethanol levels were increased by 0.2% (w/v) every 2 weeks and the daily food intake showed an increase during the 12-week feeding period with an average of 445 g/kg/day in WT and 434 g/kg/day in MT-KO mice (Figure 1). Accordingly, the calculated daily ethanol intake (g/kg) was gradually increased. The average daily zinc intake was 33 mg/kg. The blood ethanol levels (mg/dl) measured at 9:00 a.m. in the last week were 221 ± 45 in WT mice and 210 ± 30 in MT-KO mice. The liver:body weight ratios at the end of the experiment significantly increased in the ethanol-fed mice (WT, 4.47 ± 0.36; MT-KO, 4.70 ± 0.48) related to the pair-fed groups (WT, 3.16 ± 0.18; MT-KO, 3.22 ± 0.29). No significant differences in all of the above parameters were found between the MT-KO and WT mice. Zinc supplementation to the ethanol-fed mice had no significant effects on all of the parameters measured above.
Figure 1.
Food and ethanol intakes in MT-KO and WT 129/Sv mice chronically fed ethanol for 12 weeks. Food intake was recorded daily. Results are means ± SD (n = 4 to 6). FI, Food intake; EI, ethanol intake.
Zinc Supplementation Attenuated Ethanol-Induced Zinc Decrease and Pathological Changes in the Liver
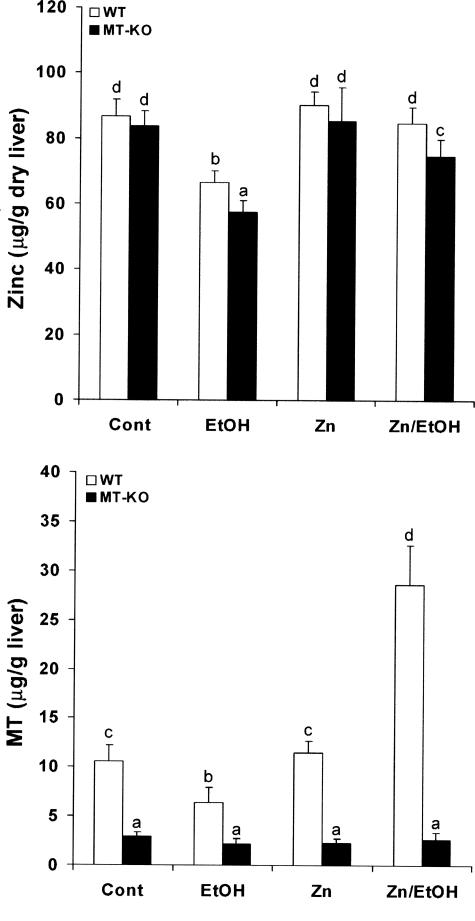
As shown in Figure 2, chronic ethanol exposure caused a significant decrease in hepatic zinc concentrations in both WT and MT-KO mice with a lower value in the latter. Dietary zinc supplementation did not elevate the basal levels, but prevented ethanol-induced decrease, of the hepatic zinc concentrations in both WT and MT-KO mice. Because MT regulates cellular zinc homeostasis, MT concentrations in the liver were measured. In the WT mice, chronic ethanol exposure significantly decreased MT concentrations in the liver. In contrast, the MT concentrations in the liver of mice chronically fed ethanol were significantly increased by zinc supplementation, although zinc did not increase MT concentrations in the liver of pair-feeding WT mice. Only trace amounts of hepatic MT were detected in all groups of MT-KO mice, which represent the assay background.
Figure 2.
Effects of zinc supplementation on hepatic zinc and MT concentrations in MT-KO and WT 129/Sv mice chronically fed ethanol for 12 weeks. Zinc and MT concentrations in the liver were measured by inductively coupled argon plasma emission spectroscopy and by a cadmium-hemoglobin affinity assay, respectively. Results are means ± SD (n = 4 to 6). Significant difference (P < 0.05) is identified by different letters. Cont, Control; EtOH, ethanol.
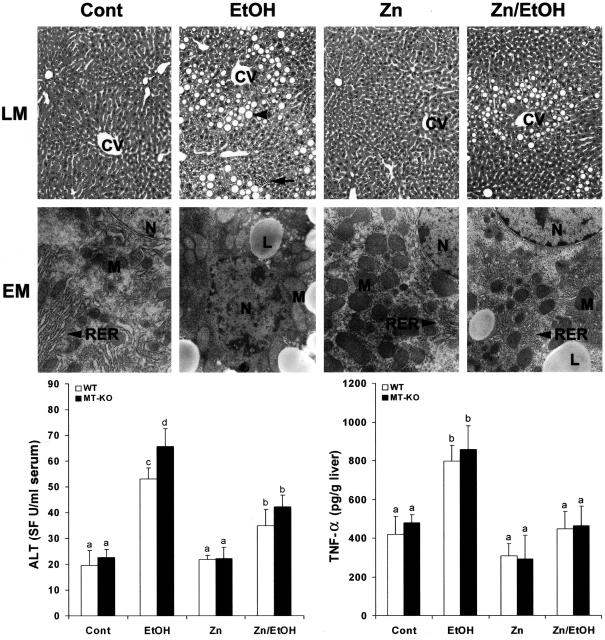
The results presented in Figure 3 show the effects of zinc supplementation on ethanol-induced liver injury. Examination by light microscopy found that zinc attenuated ethanol-induced histopathological changes, including macrovesicular steatosis and focal inflammation in both WT and MT-KO mice. Severe ultrastructural alterations were also observed in the ethanol-exposed mouse liver, including lipid droplet accumulation, enlargement and degeneration of mitochondria, disorganization of rough endoplasmic reticulum, and chromatin condensation. All these ultrastructural abnormalities of hepatocytes under chronic ethanol exposure were primarily inhibited by zinc supplementation in both WT and MT-KO mice (shown here only the data from WT mice). Corresponding to the pathological changes, the plasma ALT levels were significantly elevated in both WT and MT-KO mice with a greater value in the latter. However, zinc supplementation significantly inhibited ethanol-induced ALT elevation equally in the WT and MT-KO mice (Figure 3). Hepatic TNF-α levels were also increased by ethanol exposure, which was abrogated by zinc supplementation in both WT and MT-KO mice (Figure 3).
Figure 3.
Effects of zinc supplementation on liver injury in MT-KO and WT 129/Sv mice chronically fed ethanol for 12 weeks. Light microscopy (LM) shows prominent steatosis (arrowhead) and inflammation (arrow) in the liver of ethanol-fed mice, but these histopathological changes were primarily inhibited by zinc. H&E stain. Electron microscopy (EM) reveals ultrastructural alterations in the hepatocytes, including accumulation of lipid droplets (LD), enlargement and degeneration of mitochondria (M), disorganization of rough endoplasmic reticulum (RER), and condensation of chromatin in the nucleus (N). All these ultrastructural changes were suppressed by zinc. Serum ALT activities were measured using a Sigma Diagnostics kit. Hepatic TNF-α levels were measured using an ELISA kit. Results in serum ALT and hepatic TNF-α levels are means ± SD (n = 4 to 6). Significant difference (P < 0.05) is identified by different letters. CV, Central vein; Cont, control; EtOH, ethanol. Original magnifications: [times130 (LM row); ×9800 (EM row).
Zinc Supplementation Inhibited Ethanol-Induced Oxidative Stress in the Liver
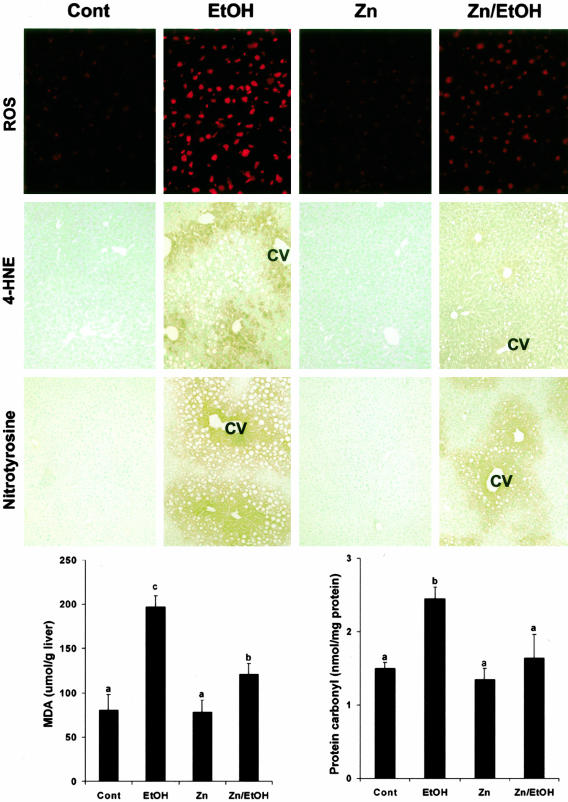
To understand the possible mechanisms by which zinc prevents ethanol-induced liver injury, the effect of zinc supplementation on ethanol-induced oxidative stress in the liver of WT mice was determined as shown in Figure 4. Chronic ethanol exposure enhanced the red fluorescence from dihydroethidium in the liver, but much less in the liver of ethanol-fed mice with zinc supplementation. Immunoperoxidase stain demonstrated that the immunoreactivities to 4-HNE and nitrotyrosine in the liver were increased by chronic ethanol exposure. The positive stain mainly localized around the central vein. Zinc supplementation markedly diminished ethanol-induced 4-HNE and nitrotyrosine stains in the liver. The negative controls showed no background staining on the sections of all treatments. Quantitative measurements of malondialdehyde and protein carbonyl in the liver revealed that zinc significantly diminished ethanol-induced oxidative lipid and protein damages. Although the data presented here were obtained from WT mice, the same results were also obtained from MT-KO mice (data not shown).
Figure 4.
Effect of zinc supplementation on oxidative stress in the liver of WT 129/Sv mice chronically fed ethanol for 12 weeks. ROS generation was detected by dihydroethidium that yields red fluorescence in the nuclei on oxidation by ROS. Red fluorescence from dihydroethidium was strong in the liver of ethanol-fed mice, but much less in mice treated by ethanol plus zinc. Immunohistochemical stains of 4-HNE and nitrotyrosine in the liver show a strong reactivity in ethanol-fed mice, but a weak reactivity in mice treated by ethanol plus zinc. Lipid peroxidation in the liver was quantitatively measured by biochemical assay of malondialdehyde concentration. Protein oxidation in the liver was quantitatively measured by ELISA assay of protein carbonyl concentrations. Results in lipid peroxidation and protein carbonyl are means ± SD (n = 4 to 6). Significant difference (P < 0.05) is identified by different letters. Cont, Control; EtOH, ethanol; CV, central vein. Original magnifications: ×260 (ROS row); ×130 (4-HNE and nitrotyrosine rows).
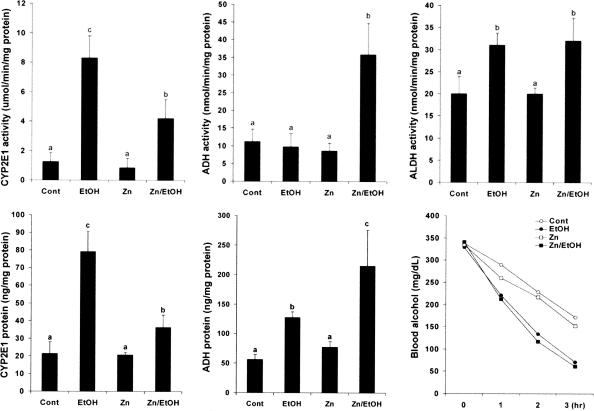
The Effect of Zinc Supplementation on Ethanol Metabolic Pathway
To determine whether zinc inhibits oxidative stress through modulating ethanol metabolic pathways, the effect of zinc supplementation on ethanol metabolism was determined in the WT mice. As shown in Figure 5, chronic ethanol exposure caused a significant increase in the hepatic CYP2E1 activity, which was significantly inhibited by zinc supplementation. In contrast, the ADH activity in the liver was not affected by chronic ethanol exposure, but significantly increased by zinc supplementation. The ALDH activities were significantly increased in mice chronically fed ethanol alone or ethanol plus zinc. Next, possible differences in the protein levels of CYP2E1 and ADH between chronic ethanol-exposed and the zinc-supplemented mice were determined. The protein concentrations of CYP2E1 in the liver were significantly increased by chronic ethanol exposure, which was primarily inhibited by zinc supplementation. In contrast, the protein level of hepatic ADH was significantly higher in mice treated with ethanol plus zinc than ethanol alone, although the latter also induced a significant increase in ADH protein level. To determine whether changes in ethanol metabolic pathway affect ethanol metabolic rate, the blood ethanol elimination was determined after an intragastric bolus of ethanol at 3 g/kg. An increased ethanol clearance was found in ethanol-fed mice, and zinc supplementation did not affect the ethanol-elevated elimination rate.
Figure 5.
Effect of zinc supplementation on alcohol metabolism in WT 129/Sv mice chronically fed ethanol for 12 weeks. CYP2E1 activity in the microsomes was estimated by colorimetrically measuring hydroxylation of p-nitrophenol to 4-nitrocatechol. ADH activity in the cytoplasm was assayed spectrophotometrically using alcohol as substrate by measuring the reduction of NAD+ at 340 nm. ALDH activity in the mitochondria was measured using acetaldehyde as substrate by measuring the reduction of NAD+ at 340 nm. The protein concentrations of CYP2E1 and ADH were quantitatively measured by ELISA. Blood ethanol elimination after an intragastric bolus of ethanol at 3 g/kg was measured to estimate ethanol metabolic rate. Ethanol concentrations were measured using a Sigma Diagnostics Alcohol kit. Results are means ± SD (n = 4 to 6). Significant difference (P < 0.05) is identified by different letters. Cont, Control; EtOH, ethanol.
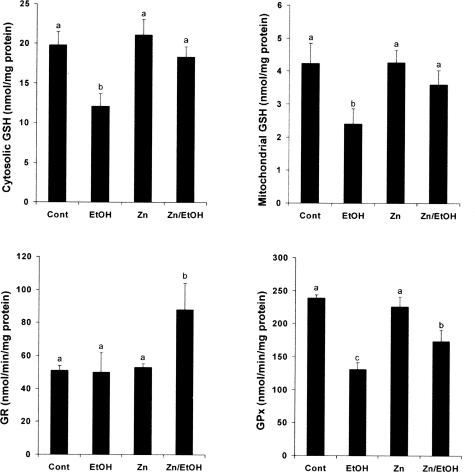
Zinc Supplementation Prevented Ethanol-Induced Decreases in GSH and GSH-Related Enzymes
To determine the effect of zinc on GSH and GSH-related enzymes, hepatic GSH status, and enzymatic activities of GSH reductase (GR) and GSH peroxidase (GPx) in the WT mice were measured (Figure 6). Chronic ethanol exposure induced a significant decrease in GSH concentrations in both cytosol and mitochondria, which was attenuated by zinc supplementation. The hepatic GR activity was not affected by chronic ethanol feeding. However, zinc supplementation significantly increased the GR activity in the mice chronically fed ethanol. The GPx activity in the liver was decreased in the ethanol-fed mice. Zinc supplementation partially inhibited ethanol-induced decrease in GPx activity.
Figure 6.
Effect of zinc supplementation on cytosolic and mitochondrial GSH in the liver of WT 129/Sv mice chronically fed ethanol for 12 weeks. The GSH concentrations in cytosol and mitochondria were measured using a colorimetric Bioxytech GSH-400 assay kit. Activities of GR and GPx were measured using assay kits from Calbiochem. Results are means ± SE (n = 4 to 6). Significant difference (P < 0.05) is identified by different letters. Cont, Control; EtOH, ethanol.
Discussion
The results obtained demonstrated that dietary zinc supplementation prevented ethanol-induced zinc decrease in the liver and inhibited ethanol-induced oxidative liver injury. The suppression of ethanol-induced oxidative injury by zinc supplementation most likely resulted from inhibition of ROS, in particular, superoxide accumulation. The ROS accumulation apparently resulted from the alcohol-enhanced CYP2E1 pathway, which has been shown to be highly responsible for ROS generation.26 Zinc supplementation inhibited the activation of the CYP2E1 pathway and at the same time enhanced the ADH pathway, thus leading to the prevention of the ethanol-induced metabolic shift that is in favor of ROS production. Furthermore, zinc also prevented ethanol-induced decreases in GSH and GPx, but increased GR. These results thus indicate that zinc supplementation results in suppression of alcohol-induced metabolic shift favoring ROS generation and enhanced GSH antioxidant capacity, thus inhibits alcohol-induced oxidative stress leading to prevention of alcoholic liver injury.
The decrease in zinc concentrations in the liver is one of the most consistent observations in alcoholic patients and animal models of alcoholic liver injury.1–3 The consequence of zinc depletion has not been well understood, although it has been suggested that zinc deficiency contributes to the pathogenesis of liver disease.2 MT is a metal-binding protein, and the role of MT in cellular zinc homeostasis has long been recognized.15 Under chronic ethanol exposure, MT concentrations in the liver were found to significantly decrease along with zinc depletion in the liver,27 but dietary zinc supplementation significantly increased MT concentrations in the liver exposed to alcohol.28 Thus, the hepatoprotective effects by zinc supplementation were ascribed to the MT induction in the liver. However, our recent studies have shown that zinc inhibition of acute alcohol liver injury is independent of MT.29–32 By using the MT-KO mouse model in the present study, we demonstrated again that zinc supplementation prevented hepatic zinc decrease and liver injury induced by chronic ethanol exposure in an MT-independent manner. However, the zinc concentrations in the liver were lower in MT-KO mice than WT mice under chronic ethanol exposure, indicating the importance of MT in cellular zinc homeostasis. It is important to note that there were no apparent side effects observed in the mice under long-term zinc supplementation, providing solid evidence that supports the concept that zinc has the advantage of being relatively nontoxic, particularly if taken orally.33
Accumulation of ROS in the liver has been well documented in alcohol-induced liver injury. Dihydroethidium fluorescence microscopy was performed to detect ROS in the liver in the present study. Nonfluorescent dihydroethidium is oxidized to generate red fluorescence product that binds to DNA, leading to a red fluorescent staining on the nuclei. The red fluorescent product was assumed as ethidium.34 However, recent studies with fluorescence spectra, HPLC, and mass spectrometry have shown that superoxide oxidizes dihydroethidium to a red fluorescent product that differs from ethidium by the presence of an additional oxygen atom in its molecular structure, named oxyethidium.35,36 Hydrogen peroxide, hydroxyl radical, and nitric oxide/superoxide-derived oxidants do not react with dihydroethidium to form this characteristic fluorescent product, but these oxidants can oxidize dihydroethidium to form other products.36 Therefore, the red fluorescence from dihydroethidium oxidation may not solely be from its interaction with superoxide. In spite of these limitations, fluorescence microscopy with dihydroethidium is a useful technique for general detection of intracellular ROS. Dihydroethidium fluorescence microscopy in the present study showed that chronic ethanol exposure enhanced the red fluorescence, which is inhibited by zinc supplementation. In an agreement with this result, zinc supplementation attenuated ethanol-induced lipid peroxidation and protein oxidation products. These results suggest that inhibition of ROS accumulation is involved in zinc prevention against ethanol-induced liver injury.
Oxidative stress plays a critical role in the pathogenesis of alcoholic liver injury,6 which is closely associated with ethanol metabolism; in particular, the alteration in ethanol metabolic pathways such as the shift from the ADH to the CYP2E1 pathway, leading to the accumulation of ROS in the liver. The effects of chronic ethanol exposure on hepatic ADH activities have been investigated in both ethanol-fed animals and human alcoholics. Most studies demonstrated that chronic ethanol exposure has little effect on ADH activity,6 although some reports showed decreased or increased ADH activity.37,38 ADH gene expression analysis has demonstrated that chronic ethanol exposure significantly increases the ADH mRNA level in the liver.38 The present study found that the ADH protein level was increased after chronic ethanol exposure, but the enzymatic activity remained unchanged. This could be ascribed to the effect of zinc decrease because ADH activity is zinc-dependent, as shown in a previous study that rats fed a zinc-deficient diet displayed a significant decrease in the hepatic ADH activities.39 Thus, chronic ethanol exposure causes a zinc decrease, which in turn leads to reduced ADH activity. To keep the capacity of ethanol metabolism, an adaptive response for the liver to ethanol exposure is to increase CYP2E1 activity. CYP2E1 has been recognized in most studies as a critical contributor to ethanol-induced ROS generation.6–12 Therefore, the shift of ethanol metabolism from ADH to CYP2E1 pathway is highly responsible for oxidative stress, as shown in the present study. Thus chronic alcohol exposure significantly increased the hepatic malondialdehyde concentrations and nitrotyrosine levels; both were inhibited by zinc supplementation. The inhibitory effect of zinc on nitrotyrosine formation likely reflects the indirect suppression of peroxynitrite, the product of the interaction between nitric oxide and superoxide, which was highly suppressed by zinc supplementation.
The impaired antioxidant defense in the liver has long been recognized in the alcoholic liver disease.40 GSH is the most important molecule involved in cellular antioxidant defense, and selective GSH depletion in mitochondria was repeatedly reported in chronically ethanol-fed animals.40,41 Dietary supplementation with S-adenosyl-l-methionine has been shown to replenish mitochondria GSH pool, thereby attenuating mitochondrial dysfunction and preventing ethanol-induced liver injury.42 A positive correlation also has been found between zinc and GSH. Zinc depletion with zinc chelator induced a dose-dependent decrease in cellular GSH level in cultured keratinocytes, hepatocytes, and hepatic stellate cells.14,43,44 The levels of GSH in blood and liver were also found to correlate well with dietary zinc status.45,46 In the present study, zinc supplementation effectively prevented ethanol-induced GSH decrease in both cytosol and mitochondria. Increase in GR activity by zinc supplementation indicates that zinc protects the GSH pool at least partially through enhancing GSSG reduction to GSH. GPx is one of the primary antioxidant enzymes, and decrease in GPx activity has been repeatedly reported in chronic ethanol exposure.47,48 The present study demonstrated that zinc supplementation prevents ethanol-induced decrease in GPx activity.
TNF-α plays a critical role in the initiation and development of alcoholic liver injury.49 Our recent studies with acute ethanol or lipopolysaccharide hepatotoxicity models demonstrated that zinc suppresses hepatic TNF-α production through protection of intestinal mucosa and inhibition of nuclear factor-κB activation in Kupffer cells.30–32 In the present study, attenuation by zinc supplementation of TNF-α increases in the liver was observed. Because abrogation of TNF-α pathway using TNF-α receptor knockout mouse model has been shown to reduce steatosis, necrosis, and inflammation under chronic ethanol exposure,50 the attenuation of hepatic TNF-α production by zinc supplementation is likely an important action in zinc protection against alcoholic liver injury. However, whether the inhibition of TNF-α production results from the direct action of zinc on the liver such as Kupffer cells or/and indirect action by preserving the intestinal barrier’s integrity leading to inhibition of endotoxemia needs further investigation.
In conclusion, the present study demonstrated that zinc supplementation prevented ethanol-induced liver zinc decrease and alcoholic liver injury. The hepatoprotective effect of zinc on alcoholic liver injury most likely results from inhibition of oxidative stress. Although multiple factors could be involved in the zinc inhibition of oxidative liver injury, the inhibition of CYP2E1 pathway and the enhanced GSH-related antioxidant capacity by zinc would make a significant contribution. These results suggest that zinc may have a therapeutic potential in the prevention and/or treatment of alcoholic liver disease.
Acknowledgments
We thank Xinguo Sun, Aisha Bagshaw, Gwen Dahlen, Kay Keehr, and Laura Idso for technical assistance.
Footnotes
Address reprint requests to Zhanxiang Zhou, Ph.D., University of Louisville School of Medicine, Department of Medicine, 511 South Floyd St., MDR 529, Louisville, KY 40292. E-mail: z0zhou01@louisville.edu.
Supported in part by the National Institutes of Health (grants AA13601 to Z.Z., AA010762 and AA10496 to C.J.M., HL59225 and HL63760 to Y.J.K.), the Kentucky Science and Engineering Foundation (to C.J.M.), and the Veterans Administration (to C.J.M.).
Y.J.K. is a Distinguished University Scholar of the University of Louisville.
References
- Kiilerich S, Dietrichson O, Loud FB, Naestoft J, Christoffersen P, Juhl E, Kjems G, Christiansen C. Zinc depletion in alcoholic liver diseases. Scand J Gastroenterol. 1980;15:363–367. doi: 10.3109/00365528009181484. [DOI] [PubMed] [Google Scholar]
- McClain CJ, Antonow DR, Cohen DA, Shedlofsky S. Zinc metabolism in alcoholic liver disease. Alcohol Clin Exp Res. 1986;10:582–589. doi: 10.1111/j.1530-0277.1986.tb05149.x. [DOI] [PubMed] [Google Scholar]
- Bode JC, Hanisch P, Henning H, Koenig W, Richter FW, Bode C. Hepatic zinc content in patients with various stages of alcoholic liver disease and in patients with chronic active and chronic persistent hepatitis. Hepatology. 1988;8:1605–1609. doi: 10.1002/hep.1840080622. [DOI] [PubMed] [Google Scholar]
- Dinsmore W, Callender ME, McMaster D, Todd SJ, Love AH. Zinc absorption in alcoholics using zinc-65. Digestion. 1985;32:238–242. doi: 10.1159/000199243. [DOI] [PubMed] [Google Scholar]
- Valberg LS, Flanagan PR, Ghent CN, Chamberlain MJ. Zinc absorption and leukocyte zinc in alcoholic and nonalcoholic cirrhosis. Dig Dis Sci. 1985;30:329–333. doi: 10.1007/BF01403841. [DOI] [PubMed] [Google Scholar]
- Lieber CS. Alcohol metabolism, cirrhosis and alcoholism. Clin Chim Acta. 1997;257:59–84. doi: 10.1016/s0009-8981(96)06434-0. [DOI] [PubMed] [Google Scholar]
- Nanji AA, Zhao S, Sadrzadeh SM, Dannenberg AJ, Tahan SR, Waxman DJ. Markedly enhanced cytochrome P450 2E1 induction and lipid peroxidation is associated with severe liver injury in fish oil-ethanol-fed rats. Alcohol Clin Exp Res. 1994;18:1280–1285. doi: 10.1111/j.1530-0277.1994.tb00119.x. [DOI] [PubMed] [Google Scholar]
- Morimoto M, Zern MA, Hagbjork AL, Ingelman-Sundberg M, French SW. Fish oil, alcohol, and liver pathology: role of cytochrome P450 2E1. Proc Soc Exp Biol Med. 1996;207:197–205. doi: 10.3181/00379727-207-43807. [DOI] [PubMed] [Google Scholar]
- Albano E, Clot P, Morimoto M, Tomasi A, Ingelman-Sundberg M, French SW. Role of cytochrome P4502E1-dependent formation of hydroxyethyl free radical in the development of liver damage in rats intragastrically fed with ethanol. Hepatology. 1996;23:155–163. doi: 10.1002/hep.510230121. [DOI] [PubMed] [Google Scholar]
- Morgan K, French SW, Morgan TR. Production of a cytochrome P450 2E1 transgenic mouse and initial evaluation of alcoholic liver damage. Hepatology. 2002;36:122–134. doi: 10.1053/jhep.2002.33720. [DOI] [PubMed] [Google Scholar]
- Morimoto M, Hagbjork AL, Wan YJ, Fu PC, Clot P, Albano E, Ingelman-Sundberg M, French SW. Modulation of experimental alcohol-induced liver disease by cytochrome P450 2E1 inhibitors. Hepatology. 1995;21:1610–1617. [PubMed] [Google Scholar]
- Gouillon Z, Lucas D, Li J, Hagbjork AL, French BA, Fu P, Fang C, Ingelman-Sundberg M, Donohue TM, Jr, French SW. Inhibition of ethanol-induced liver disease in the intragastric feeding rat model by chlormethiazole. Proc Soc Exp Biol Med. 2000;224:302–308. doi: 10.1046/j.1525-1373.2000.22435.x. [DOI] [PubMed] [Google Scholar]
- Powell SR. The antioxidant properties of zinc. J Nutr. 2000;130:1447S–1454S. doi: 10.1093/jn/130.5.1447S. [DOI] [PubMed] [Google Scholar]
- Parat MO, Richard MJ, Beani JC, Favier A. Involvement of zinc in intracellular oxidant/antioxidant balance. Biol Trace Elem Res. 1997;60:187–204. doi: 10.1007/BF02784439. [DOI] [PubMed] [Google Scholar]
- Vallee BL. The function of metallothionein. Neurochem Int. 1995;27:23–33. doi: 10.1016/0197-0186(94)00165-q. [DOI] [PubMed] [Google Scholar]
- Masters BA, Kelly EJ, Quaife CJ, Brinster RL, Palmiter RD. Targeted disruption of metallothionein I and II genes increases sensitivity to cadmium. Proc Natl Acad Sci. 1994;91:584–588. doi: 10.1073/pnas.91.2.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen FH, Zimmerman TJ, Shuler TR. Interactions among nickel, copper, and iron in rats: liver and plasma contents of lipid and trace elements. Biol Trace Elem Res. 1982;4:1225–1243. doi: 10.1007/BF02783253. [DOI] [PubMed] [Google Scholar]
- Eaton DL, Cherian MG. Determination of metallothionein in tissues by cadium-hemoglobin affinity assay. Methods Enzymol. 1991;205:83–88. doi: 10.1016/0076-6879(91)05089-e. [DOI] [PubMed] [Google Scholar]
- Zhou Z, Sun X, Kang YJ. Metallothionein protection against alcoholic liver injury through inhibition of oxidative stress. Exp Biol Med. 2002;227:214–222. doi: 10.1177/153537020222700310. [DOI] [PubMed] [Google Scholar]
- Zhou Z, Wang L, Song Z, Lambert JC, McClain CJ, Kang YJ. A critical involvement of oxidative stress in acute alcohol-induced hepatic TNF-alpha production. Am J Pathol. 2003;163:1137–1146. doi: 10.1016/s0002-9440(10)63473-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter WO, Narayanan PK, Robinson JP. Intracellular hydrogen peroxide and superoxide anion detection in endothelial cells. J Leukoc Biol. 1994;55:253–258. doi: 10.1002/jlb.55.2.253. [DOI] [PubMed] [Google Scholar]
- Buss H, Chan TP, Sluis KB, Domigan NM, Winterbourn CC. Protein carbonyl measurement by a sensitive ELISA method. Free Radic Biol Med. 1997;23:361–366. doi: 10.1016/s0891-5849(97)00104-4. [DOI] [PubMed] [Google Scholar]
- Allis JW, Robison BL. A kinetic assay for p-nitrophenol hydroxlase in rat liver microsomes. Anal Biochem. 1994;219:49–52. doi: 10.1006/abio.1994.1230. [DOI] [PubMed] [Google Scholar]
- Crow KE, Cornell NW, Veech RL. The rate of ethanol metabolism in isolated rat hepatocytes. Alcohol Clin Exp Res. 1977;1:43–50. doi: 10.1111/j.1530-0277.1977.tb05765.x. [DOI] [PubMed] [Google Scholar]
- Tottmar SOC, Pettersson H, Kiessling KH. The subcellular distribution and properties of aldehyde dehydrogenases in rat liver. Biochem J. 1973;135:577–586. doi: 10.1042/bj1350577a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noedmann R, Ribiere C, Rouach H. Implication of free radical mechanisms in ethanol-induced cellular injury. Free Radic Biol Med. 1992;12:219–240. doi: 10.1016/0891-5849(92)90030-k. [DOI] [PubMed] [Google Scholar]
- Wang J, Pierson RN., Jr Distribution of zinc in skeletal muscle and liver tissue in normal and dietary controlled alcoholic rats. J Lab Clin Med. 1975;85:50–58. [PubMed] [Google Scholar]
- Cabre M, Folch J, Gimenez A, Matas C, Pares A, Caballeria AJ, Paternain JL, Rodes J, Joven J, Camps J. Influence of zinc intake on hepatic lipid peroxidation and metallothioneins in alcoholic rats: relationship to collagen synthesis. Int J Vitam Nutr Res. 1995;65:45–50. [PubMed] [Google Scholar]
- Zhou Z, Sun X, Lambert JC, Saari JT, Kang YJ. Metallothionein-independent zinc protection from alcoholic liver injury. Am J Pathol. 2002;160:2267–2274. doi: 10.1016/S0002-9440(10)61174-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert JC, Zhou Z, Wang L, Song Z, McClain CJ, Kang YJ. Prevention of alterations in intestinal permeability is involved in zinc inhibition of acute ethanol-induced liver damage in mice. J Exp Phamacol Ther. 2003;305:880–886. doi: 10.1124/jpet.102.047852. [DOI] [PubMed] [Google Scholar]
- Lambert JC, Zhou Z, Wang L, Song Z, McClain CJ, Kang YJ. Prevention of intestinal structural integrity by zinc is independent of metallothionein in alcohol-intoxicated mice. Am J Pathol. 2004;164:1959–1966. doi: 10.1016/S0002-9440(10)63756-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z, Wang L, Song Z, Saari JT, McClain CJ, Kang YJ. Abrogation of NF-κB activation is involved in zinc inhibition of lipopolysaccharide-induced TNF-α production and liver injury. Am J Pathol. 2004;164:1547–1556. doi: 10.1016/s0002-9440(10)63713-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fosmire GJ. Zinc toxicity. J Clin Nutr. 1990;15:225–227. doi: 10.1093/ajcn/51.2.225. [DOI] [PubMed] [Google Scholar]
- Benov L, Sztejnberg L, Fridovich I. Critical evaluation of the use of hydroethidine as a measure of superoxide anion radical. Free Radic Biol Med. 1998;25:826–831. doi: 10.1016/s0891-5849(98)00163-4. [DOI] [PubMed] [Google Scholar]
- Zhao H, Kalivendi S, Zhang H, Joseph J, Nithipatikom K, Vasquez-Vivar J, Kalyanaraman B. Superoxide reacts with hydroethidine but forms a fluorescent product that is distinctly different from ethidium: potential implications in intracellular fluorescence detection of superoxide. Free Radic Biol Med. 2003;34:1359–1368. doi: 10.1016/s0891-5849(03)00142-4. [DOI] [PubMed] [Google Scholar]
- Fink B, Laude K, McCann L, Doughan A, Harrison DG, Dikalov S. Detection of intracellular superoxide formation in endothelial cells and intact tissues using dihydroethidium and an HPLC-based assay. Am J Physiol. 2004;287:C895–C902. doi: 10.1152/ajpcell.00028.2004. [DOI] [PubMed] [Google Scholar]
- McClearn GE, Bernett EL, Hebert M, Kakehana R, Schlesinger K. Alcohol dehydrogenase activity and previous ethanol consumption in mice. Nature. 1964;203:793–794. doi: 10.1038/203793a0. [DOI] [PubMed] [Google Scholar]
- Badger TM, Hoog JO, Svensson S, McGehee RE, Jr, Fang C, Ronis MJ, Ingelman-Sundberg M. Cyclic expression of class I alcohol dehydrogenase in male rats treated with ethanol. Biochem Biophys Res Commun. 2000;274:684–688. doi: 10.1006/bbrc.2000.3186. [DOI] [PubMed] [Google Scholar]
- Das I, Burch RE, Hahn HK. Effects of zinc deficiency on ethanol metabolism and alcohol and aldehyde dehydrogenase activities. J Lab Clin Med. 1984;104:610–617. [PubMed] [Google Scholar]
- Nordmann R. Alcohol and antioxidant systems. Alcohol Alcohol. 1994;29:513–522. [PubMed] [Google Scholar]
- Hoek JB, Cahill A, Pastorino JG. Alcohol and mitochondria: a dysfunctional relationship. Gastroenterology. 2002;122:2049–2063. doi: 10.1053/gast.2002.33613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieber CS. S-Adenosyl-L-methionine and alcoholic liver disease in animal models: implications for early intervention in human beings. Alcohol. 2002;27:173–177. doi: 10.1016/s0741-8329(02)00230-6. [DOI] [PubMed] [Google Scholar]
- Nakatani T, Tawaramoto M, Opare Kennedy D, Kojima A, Matsui-Yuasa I. Apoptosis induced by chelation of intracellular zinc is associated with depletion of cellular reduced glutathione level in rat hepatocytes. Chem Biol Interact. 2000;125:151–163. doi: 10.1016/s0009-2797(99)00166-0. [DOI] [PubMed] [Google Scholar]
- Kojima-Yuasa A, Ohkita T, Yukami K, Ichikawa H, Takami N, Nakatani T, Opare Kennedy D, Nishiguchi S, Matsui-Yuasa I. Involvement of intracellular glutathione in zinc deficiency-induced activation of hepatic stellate cells. Chem Biol Interact. 2003;146:89–99. doi: 10.1016/s0009-2797(03)00087-5. [DOI] [PubMed] [Google Scholar]
- Shaheen AA, el-Fattah AA. Effect of dietary zinc on lipid peroxidation, glutathione, protein thiols levels and superoxide dismutase activity in rat tissues. Int J Biochem Cell Biol. 1995;27:89–95. doi: 10.1016/1357-2725(94)00053-0. [DOI] [PubMed] [Google Scholar]
- Ozturk A, Baltaci AK, Mogulkoc R, Oztekin E, Sivrikaya A, Kurtoglu E, Kul A. Effects of zinc deficiency and supplementation on malondialdehyde and glutathione levels in blood and tissues of rats performing swimming exercise. Biol Trace Elem Res. 2003;94:157–166. doi: 10.1385/BTER:94:2:157. [DOI] [PubMed] [Google Scholar]
- Rouach H, Fataccioli V, Gentil M, French SW, Morimoto M, Nordmann R. Effect of chronic ethanol feeding on lipid peroxidation and protein oxidation in relation to liver pathology. Hepatology. 1997;25:351–355. doi: 10.1002/hep.510250216. [DOI] [PubMed] [Google Scholar]
- Bailey SM, Patel VB, Young TA, Asayama K, Cunningham CC. Chronic ethanol consumption alters the glutathione/glutathione peroxidase-1 system and protein oxidation status in rat liver. Alcohol Clin Exp Res. 2001;25:726–733. [PubMed] [Google Scholar]
- McClain CJ, Song Z, Barve SS, Hill DB, Deaciuc I. Recent advances in alcoholic liver disease. IV. Dysregulated cytokine metabolism in alcoholic liver disease. Am J Physiol. 2004;287:G497–G502. doi: 10.1152/ajpgi.00171.2004. [DOI] [PubMed] [Google Scholar]
- Yin M, Wheeler MD, Kono H, Bradford BU, Gallucci RM, Luster MI, Thurman RG. Essential role of tumor necrosis factor alpha in alcohol-induced liver injury in mice. Gastroenterology. 1999;117:942–952. doi: 10.1016/s0016-5085(99)70354-9. [DOI] [PubMed] [Google Scholar]