Abstract
Infiltrating macrophages (mφ) can cause injury or facilitate repair, depending on how they are activated by the microenvironment. Studies in vitro have defined the roles of individual cytokines and signaling pathways in activation, but little is known about how macrophages integrate the multiple signals they receive in vivo. We inhibited nuclear factor-κB in bone marrow-derived macrophages (BMDMs) by using a recombinant adenovirus expressing dominant-negative IκB (Ad-IκB). This re-orientated macrophage activation so they became profoundly anti-inflammatory in settings where they would normally be classically activated. In vitro, the lipopolysaccharide-induced nitric oxide, interleukin-12, and tumor necrosis factor-α synthesis was abrogated while interleukin-10 synthesis increased. In vivo, fluorescently labeled BMDMs transduced with Ad-IκB and injected into the renal artery significantly reduced inducible nitric oxide synthase and MHC class II expression when activated naturally in glomeruli of rats with nephrotoxic nephritis. Furthermore, although they only comprised 15% of glomerular macrophages, their presence significantly reduced glomerular infiltration and activation of host macrophages. Injury in nephrotoxic nephritis was also decreased when assessed morphologically and by severity of albuminuria. The results demonstrate the power of Ad-IκB-transduced BMDMs to inhibit injury when activated by acute immune-mediated inflammation within the glomerulus.
Macrophage infiltration is one of the hallmarks of severe or progressive renal disease, and studies over the past decade provide compelling evidence that they cause tissue injury. The severity of injury correlates with the intensity of the macrophage infiltrate in patients with glomerulonephritis and in experimental models.1,2 Immunohistological studies show that macrophages infiltrating the kidneys of patients and rodents with focal necrotizing glomerulonephritis express MHC class II molecules and inducible nitric oxide synthase, as well as other activation markers typical of a pro-inflammatory phenotype. Purified glomerular macrophages from rats with acute nephritis have the characteristics of classically activated (cytotoxic) macrophages.3
Proof of the pathogenetic role of macrophages is provided by macrophage depletion and repletion experiments in rodent models of acute nephritis, which show that depletion abrogates glomerular injury and that this is reversed by adoptive transfer of macrophages. Furthermore, activation of adoptively transferred cells with interferon-γ (IFN-γ) increases their toxicity4 while inhibiting JNK, a key pro-inflammatory signaling pathway greatly decreases it.5 However, the intensity of glomerular macrophage infiltration does not always correlate with the severity of injury,6,7 and there is indirect evidence that they contribute to resolution of glomerular inflammation. The adoptive transfer into the kidney of small numbers of macrophages expressing anti-inflammatory cytokines such as interleukin (IL)-4 and IL-10 provides evidence of the effectiveness of anti-inflammatory macrophages in reducing renal inflammation.8,9 This is consistent with studies of inflammation in lung and skin that clearly demonstrate the importance of macrophages for resolution of injury and for tissue repair.10,11
These results highlight the heterogeneity of inflammatory macrophages, and insights into how this occurs have been gained from in vitro studies analyzing the different activating factors and downstream intracellular signaling pathways. IFN-γ together with pro-inflammatory stimuli such as lipopolysaccharide (LPS) or tumor necrosis factor-α (TNF-α) induce classical macrophage activation characterized by anti-microbial and cytotoxic properties as part of the host responses to infection or autoimmune disease,12 whereas exposure to IL-4 or IL-13 results in alternatively activated macrophages that attenuate inflammation and promote tissue repair.13 Other stimuli result in different, less well-characterized activation states (reviewed in Ref. 14). By contrast, incubation with exogenous IL-10 de-activates macrophages,15 and IL-10 is an important negative feedback regulator in pro-inflammatory macrophages. Accordingly, macrophages activated by ligation of IL-1 and TNF-α superfamily receptors (including Toll-like receptors), oxidative stress, and UV radiation16 rapidly release large amounts of pro-inflammatory mediators, but later, they synthesize large quantities of IL-10.15 The nuclear factor-κB (NF-κB) signaling pathway plays a central role in the pro-inflammatory macrophage responses to LPS, but it is not known how the subsequent IL-10 response is controlled and, specifically, whether NF-κB is involved.
In resting cells, NF-κB is sequestered in the cytoplasm by inhibitor of NF-κB (IκB). Stimulation of signaling pathways leads to activation of IκB kinase complex, which phosphorylates IκB serines at positions 32 and 36. IκB then can be ubiquitinated and degraded by the 26S proteosome, releasing NF-κB, which translocates to the nucleus where it initiates transcription of NF-κB-sensitive genes. The question remains how macrophages with an inhibited NF-κB pathway will respond to the inflammatory environment in vivo and what effect they in turn will have on the progression of injury. Our strategy was to characterize the properties of rat bone marrow-derived macrophages (BMDMs) transduced with a recombinant adenovirus expressing dominant-negative IκB to block NF-κB signaling. The dominant-negative form of IκB has serine 32 and 36 mutated to alanine, so it cannot be phosphorylated and degraded.17 This provides a highly effective biological approach to block NF-κB signaling.
We hypothesized that inhibition of NF-κB by transduction with a constitutively active IκB would inhibit the pro-inflammatory consequences of classically activated macrophages while leaving the delayed IL-10 response intact. If this were the case, then NF-κB-inhibited macrophages entering an acutely inflamed glomerulus or other pro-inflammatory environment would respond by expressing IL-10 without prior release of pro-inflammatory mediators and thus might be profoundly anti-inflammatory. Accordingly, we designed the present experiments, first, to ascertain whether inhibition of NF-κB did abrogate the IL-10 response in vitro, and we then used a novel strategy to determine the effects of infusing small numbers of NF-κB blocked cells on injury in rats with nephrotoxic nephritis. The results demonstrate that NF-κB-blocked macrophages release IL-10 after activation, and that such macrophages do not become classically activated after infusion into the kidneys of rats with nephrotoxic nephritis but instead have a dominant anti-inflammatory effect and attenuate injury.
Materials and Methods
Cells and Reagents
Rat BMDMs were isolated by aspiration of the femur and tibia and suspended in culture medium comprising Dulbecco’s modified Eagle’s medium supplemented with 10% heat inactivated fetal calf serum, 100 U/ml penicillin, and 100 μg/ml streptomycin with the addition of 20% L929 conditioned medium produced using a standard protocol;18 this yields a population of >95% macrophages. For all studies, cells were cultured for 5 days after harvesting, after which time they were transduced with adenovirus. Nephrotoxic serum was produced according to our standard protocol.19
Recombinant Adenoviruses and Transduction
The recombinant adenoviral vectors adenovirus expressing dominant-negative IκBα (Ad-IκBα) and p65RHD were a gift from Christian Jobin (University of North Carolina) and Joseph Anrather (Harvard Medical School, Boston, MA), respectively, and were prepared as described previously.20 Briefly, cDNA coding for dominant-negative IκBα and p65RHD were cloned into the pAC.CMV-pLpASR+vector. The resulting pAC.CMV/IκB plasmid plus the purified fragment of ClaI-digested DNA from E1-deleted adenovirus type 5 and p65RHD/pAC.CMV-pLpASR+ vector with the pJM17 recombination plasmid containing the full-length adenoviral genome with a deletion in the E1 region were coinfected into 293 cells (human embryonic kidney cells). Adenovirus clones obtained by limiting dilution in 293 cells were tested for both Ad-IκBα and p65RHD overexpression using Western blotting. Null adenovirus containing no insert (Ad-null), a gift from Dr. F. Graham (Ontario, Canada), was used as control virus throughout the study. All adenoviruses were produced in 293 cells and subsequently purified on a caesium chloride gradient, dialyzed against 10 mmol/L Tris buffer, pH 7.4, with 10% (v/v) glycerol, and stored at −70°C; viral preparations had low or zero levels of endotoxin. Viral titer was determined by plaque assay on human embryonic kidney 293 cells. Transduction was performed in standard medium with 2% fetal calf serum.21
Electrophoretic Mobility Shift Assay
Rat BMDMs uninfected or infected for 48 hours with 100 pfu/cell of Ad-null, Ad-IκB, or p65RHD were stimulated for 2 hours with 100 ng/ml LPS (Sigma-Aldrich, Dorset, UK) and/or 10 ng/ml IFN-γ (BD Biosciences Pharmingen, Cowley, Oxford, UK) and incubated for 15 minutes with ice-cold buffer containing 10 mmol/L Hepes, 10 mmol/L KCl, 0.1 mmol/L EGTA, 0.1 mmol/L EDTA, and 1 mmol/L dithiothreitol supplemented with a cocktail of protease inhibitors with broad specificity for the inhibition of serine, cysteine, aspartic proteases, and aminopeptidases (Sigma-Aldrich). Cells were lysed by adding 10% Nonidet P-40, supernatant was removed, and the pellet resuspended in ice-cold buffer containing 20 mmol/L Hepes, 0.4 mmol/L NaCl, 1 mmol/L EGTA, 1 mmol/L EDTA, 1 mmol/L dithiothreitol, and 5% glycerol supplemented with protease inhibitors. The mixture was incubated for 60 minutes, and supernatants (nuclear extract) were removed by centrifugation. Equal amounts of nuclear extracts were incubated for 10 minutes at 37°C with [γ-32P]ATP-radiolabeled NF-κB oligonucleotide (5′-AGT TGA GGG GAC TTT CCC AGG C-3′ 3′-TCA ACT CCC CTG AAA GGG TCC G-5′) (Promega, Southampton, United Kingdom), and the DNA protein complexes were separated on a 5% polyacrylamide gel.
Measurement of Cytokines and Nitrite Generation
Normal BMDMs and BMDMs transduced with Ad-null or Ad-IκB for 48 hours were stimulated for 24 hours with 100 ng/ml LPS (Sigma-Aldrich) and/or 10 ng/ml IFN-γ (BD Biosciences Pharmingen) in 1-ml wells. Supernatants were removed, and cells were harvested and counted. The concentration of cytokines in the supernatant was determined by capture enzyme-linked immunosorbent assay using kits specific for TNF-α, IL-10, active TGF-β, (BD Pharmingen) and IL-12 (Biosource International California). Generation of NO by macrophages was assessed by nitrite production assayed using a Greiss reaction22 without reduction of nitrite. To detect cell surface expression of MHC II or CD86, BMDMs were treated with IFN-γ and scraped off wells, and 4 × 105 cells/ml were incubated for 1 hour with 2 μg/ml fluorescein isothiocyanate (FITC)-conjugated monoclonal to either mouse anti-rat MHC class II Ia IgG (Serotec, MCA46FT) or mouse anti rat CD86 (B7-2, 555018; BD Pharmingen) and analyzed by flow cytometry Specific labeling was determined by comparing with nonspecific staining using a FITC-labeled isotype-matched control mAb. Expression of MHC class II or CD86 was determined in terms of specific mean fluorescence relative to isotype control antibody. In each experiment, 10,000 events per sample were collected.
Nephrotoxic Nephritis (NTN) and in Vivo Delivery of Macrophages
Inbred male Sprague-Dawley rats (200–250 g) (Harlan, Bicester, Oxon) were pre-immunized with subcutaneous injection of 1 mg of rabbit IgG (Sigma-Aldrich) in Freund’s complete adjuvant and injected intravenously 1 week later with rabbit nephrotoxic serum. This results in macrophage infiltration, acute glomerular injury, and crescent formation. Normal and transduced macrophages were labeled with a red fluorescent membrane label, PKH-26GL21 and harvested into serum-free media immediately before injection. Labeled macrophages were injected directly into the renal artery 24 hours after induction of NTN by performing a midline laparotomy and isolating the left renal artery. The cells were injected into the artery over 1 to 2 minutes, and renal blood flow re-established in less than 5 minutes. Groups of at least six rats were killed 2, 3, or 6 days after disease induction. The proportion of glomerular macrophages that had been adoptively transferred was measured in two ways. First the proportion of NFκB-blocked macrophages was calculated using immunohistology by relating the number of fluorescent PKH 26GL-labeled (adoptively transferred) macrophages to the total number of ED1-positive glomerular macrophages in 50 consecutive glomeruli. The proportion was also calculated using flow cytometry by determining the proportion of CD11b-positive cells (identified using OX42, Serotec MCA275FT) that also were labeled with PKH 26L.
Albuminuria and Pathology
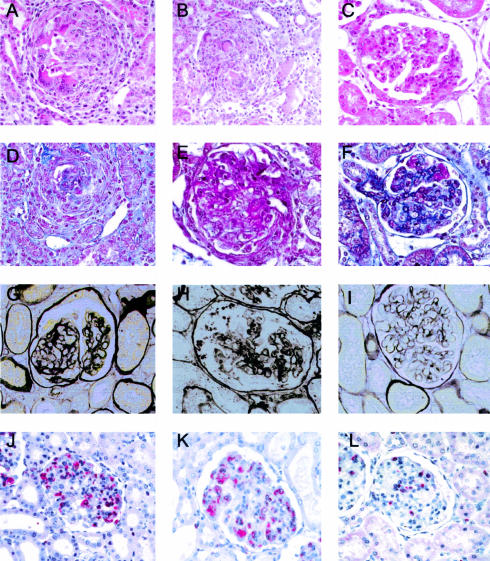
Rats were housed in metabolic cages for 14 hours each day for up to 6 days after disease induction for urine collection. Urine albumin concentration was determined using rocket electrophoresis21 and albuminuria calculated as the total albumin excreted over 24 hours. Sections of renal tissue fixed in methyl Carnoy’s fixative were paraffin embedded, and 3-μm sections were cut before routine staining with hemotoxylin and eosin (H&E), methenamine silver, and Acid Fuchsin Orange G (to highlight necrotic lesions and fibrin deposition) and immunohistochemistry. Tissue sections were examined histologically for severity of glomerular injury by a pathologist blinded to the origin of the sample, using a standard scoring system as follows; necrosis (0, absent; 1, focally present over single glomerular segments; 2, widespread) and sclerosis (0, absent; 1, mild; 2, moderate; 3, severe). Glomerular numbers affected by crescents were counted per 40 glomeruli. Total cellularity was quantified (nuclear counts/glomerular cross-section, mean of 20 glomeruli) regardless of the nature of the cells as infiltrating macrophage numbers were assessed independently. Glomerular macrophage infiltration was assessed in tissue sections that were deparaffinized in xylene and rehydrated in graded alcohol before being incubated with an antibody to CD68 (ED1 Serotec, MCA341R) or MHC class II (OX6, Serotec, MCA46R). The primary antibodies were visualized by alkaline phosphatase-labeled rabbit anti-mouse and mouse APAAP (Dako) and developed using Fast red as a substrate. The number of ED1 and MHC class II-positive cells were counted in 50 glomeruli at ×200 magnification by two independent observers, and the average number per glomerulus was calculated. Mesangium stained weakly for MHC class II in some cases, but strong positive staining was only observed in macrophages as identified by a double stain with ED1.
Isolation of Glomeruli
Kidney was cut into pieces of <1 mm3 in ice-cold Paris buffer (20 mmol/L Tris/HCl, 125 mmol/L NaCl, 10 mmol/L KCl, 10 mmol/L sodium acetate, and 5 mmol/L glucose) and tissue sieved through 250- and 150-μm sieves, and glomeruli were collected on a 63-μm filter. For visualization of the fluorescent macrophages within glomeruli, the isolated glomeruli were labeled with anti-rabbit IgG FITC (Sigma-Aldrich) that binds to the rabbit IgG from the nephrotoxic serum deposited on the glomerular basement membrane, and the number of PKH-26GL-positive, fluorescent cells in 100 glomeruli was counted.
Isolation of Glomerular Cells
Isolated glomeruli were washed in Hanks’ balanced salt solution (HBSS) with calcium and magnesium (Sigma-Aldrich) and then incubated for 10 minutes at 37°C in 4 ml of prewarmed HBSS containing 0.5 mg/ml trypsin, 1 mg/ml collagenase (Type I), and 0.1 mg/ml DNase (Sigma-Aldrich). After washing in HBSS without calcium and magnesium, the partially digested glomeruli were incubated in 2 mmol/L EDTA, disodium salt in the same buffer for 10 minutes at 37°C. For final dissociation, the cell preparation was passed five times through a 23-gauge needle, and cells were washed in HBSS and counted.
Flow Cytometry Analysis
MHC class II-positive macrophages were detected by incubating 8 × 105 isolated glomerular cells/ml for 1 hour with 4 μg/ml of FITC-conjugated monoclonal to anti-rat/mouse I-A IgG (Serotec, MCA46FT). For detection of inducible nitric oxide synthase (iNOS), cells were fixed and permeabilized by Cytofix/cytoperm reagent (BD Pharmingen), blocked with 10% goat serum (Sigma-Aldrich), and incubated for 1 hour with 2 μg/ml anti rat iNOS23 followed by FITC-labeled goat anti-rabbit IgG (Sigma-Aldrich) for 1 hour. Specific labeling was compared with nonspecific staining using a FITC-labeled isotype-matched control antibody. Single-cell preparations of glomeruli were analyzed on a FACSCalibur and using CellQuest software (BD Biosciences). A gate was set to include all PKH-26L-labeled cells, and the FL1 fluorescence from these (ie, those MHC class II or iNOS positive) was displayed as a histogram (Figure 4A). Results were expressed as the percentage of PKH-26L-labeled cells that were MHC class II or iNOS positive relative to isotype control antibody.
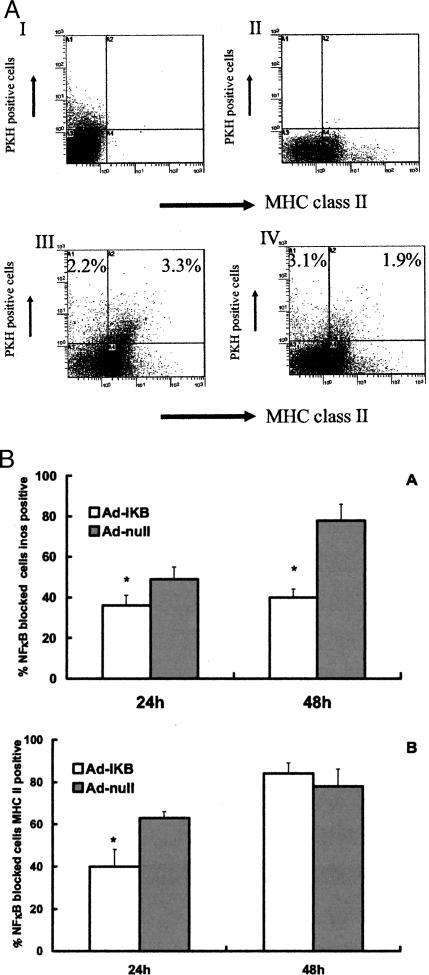
Figure 4.
A: Flow cytometry analysis of PKH 26L-labeled cells after recovery from single-cell preparations of inflamed glomeruli. Controls are indicated by PKH 26GL-positive cells in the presence of MHC class II isotype control (I), and MHC class II-positive cells in a glomerular preparation in the absence of injected PKH 26L-positive cells (II). Bottom histograms indicate the percentage of total glomerular cells that are Ad-null-transduced (III) or Ad-IκB-transduced (IV) injected, PKH 26GL-positive cells that stain (top right quadrant) or do not stain (top left quadrant) for MHC class II. The expression of MHC class II was significantly less in Ad-IκB-transduced macrophages. Dot blots shown are representative for results from at least six rats per group. B: Activation of injected labeled BMDMs after conditioning by inflamed glomeruli. Individual transduced macrophages were recovered from inflamed kidney, and their properties were analyzed by flow cytometry as described in Materials and Methods. Ad-IκB-transduced macrophages exhibited a significant decrease in their expression of iNOS (24 and 48 hours of exposure to an inflamed environment) and MHC class II (after 24 hours) as compared with injected, Ad-null-transduced macrophages. *P < 0.05 compared with Ad-null-transduced cells.
Statistics
Results are presented as mean ± SD, and differences between groups of cells or animals were tested using a two-way analysis of variance followed by a multiple range test (Tukey analysis) or a Mann-Whitney nonparametric ranking test.
Results
Characterization of Macrophages Transduced with Ad-IκB
Our previous work has shown rat BMDMs are transduced with 50 to 150 pfu/cell recombinant adenoviruses with high efficiency and little toxicity.8,9,21 We confirmed this was true for Ad-IκB transduction because of reports that NF-κB inhibition can increase sensitivity to apoptosis.24 Viability was not significantly altered when BMDMs were transduced with Ad-IκB at these concentrations (Table 1). Expression of the dominant-negative IκB protein, assessed by Western blotting, reached a maximum by 48 hours, and the expression was not influenced by LPS or IFN-γ. Consequently, a viral dose of 100 pfu/cell was used in subsequent transductions.
Table 1.
Properties of Ad-IκB-Transduced Macrophages
| Pfu/cell | Percentage of cells transduced | Mean fluorescent intensity | Viability |
|---|---|---|---|
| 50 | 64 ± 4 | 122 | 99 ± 8 |
| 100 | 89 ± 6 | 143 | 100 ± 6 |
| 150 | 86 ± 8 | 137 | 98 ± 11 |
| 200 | 72 ± 5 | 149 | 89 ± 7 |
Transduction efficiency, mean fluorescence intensity, and viability of Ad-IκB-transduced bone marrow-derived macrophages as determined by flow cytometry using an anti-rat IκB antibody and by propidium iodide, respectively. Maximum transduction efficiency with minimum cell death was achieved with 100 pfu/cell.
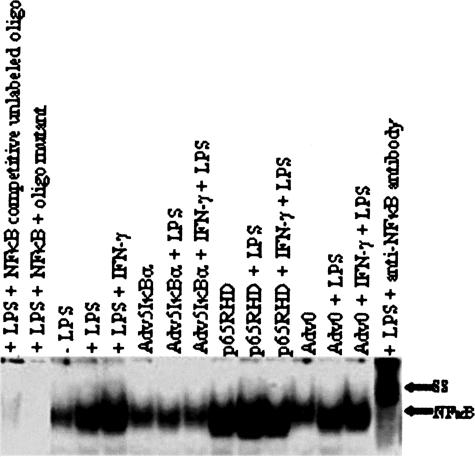
The ability of dominant-negative IκB to suppress NF-κB nuclear translocation was confirmed on electrophoretic mobility shift assay (EMSA). We investigated the presence of Rel/NF-κB in the nuclear extract of either unstimulated or LPS and IFN-γ/LPS-stimulated cells. LPS or IFN-γ/LPS stimulation resulted in an increased Rel/NF-κB translocation to the nucleus (Figure 1). Confirmation that the identified EMSA band was p65 was obtained by transducing macrophages with an adenovirus expressing a dominant-negative form of p65. This gives an intense p65 band that was supershifted by pre-incubation with anti-p65 antibodies. In contrast, transduction of macrophages with Ad-IκB resulted in a marked decrease in nuclear NF-κB that was not increased by either LPS or a combination of IFN-γ/LPS stimulation thus indicating sequestration of RelA/NF-κB in the cytoplasm.
Figure 1.
Ad-IκB suppresses LPS-induced nuclear translocation of NF-κB. BMDMs were transduced with relevant adenoviral vectors and stimulated 48 hours later with either LPS or a combination IFN-γ/LPS. Nuclear extracts were removed and analyzed for the presence of NF-κB using EMSA. Identity of NF-κB was assessed by a supershift (SS) in the NF-κB molecule that resulted from the incubation of cell extract from LPS-stimulated cells with 2 μg/ml of rabbit polyclonal antibody directed against the N-terminal of p65 subunit and by transduction with Ad-p65RHD, which produced a prominent nuclear band.
As expected, NO, TNF-α, and IL-12 responses were profoundly inhibited in Ad-IκB-transduced macrophages (Table 2), demonstrating the effectiveness of blocking pro-inflammatory effects. Also, transduction with either Ad-IκB or Ad-null did not inhibit LPS-induced IL-10 synthesis (Table 2). In fact the response was amplified in Ad-IκB-transduced cells. TGF-β synthesis did not differ in control and Ad-IκB-transduced BMDMs.
Table 2.
Effect of Ad-IκB Transduction on Nonstimulated and LPS-Induced TNF-α, IL-12, Nitrite, IL-10, and Active TGF-β Expression by Rat Bone Marrow-Derived Macrophages
| Nontransduced | Ad-null | LPS
|
||||
|---|---|---|---|---|---|---|
| Ad-IκB | Nontransduced | Ad-null | Ad-IκB | |||
| TNF-α (pg/ml) | 28 ± 2 | 250 ± 31* | 128 ± 11* | 1342 ± 121 | 1721 ± 165 | 572 ± 58† |
| IL-12 (pg/ml) | BLD | BLD | BLD | 172 ± 18 | 198 ± 21 | 100 ± 8† |
| Nitrite (μmol/L) | 12 ± 2 | 39 ± 2* | 27 ± 8* | 166 ± 11 | 176 ± 13 | 62 ± 7† |
| IL-10 (pg/ml) | 88 ± 10 | 64 ± 4 | 130 ± 19* | 1607 ± 156 | 1718 ± 132 | 2058 ± 189† |
| TGF-β (pg/ml) | 3685 ± 333 | 3583 ± 343 | 3201 ± 227 | 3473 ± 510 | 2349 ± 250 | 2577 ± 390 |
Ad-IκB transduction resulted in the inhibition of NO release and TNF-α and IL-12 production by macrophages in response to LPS stimulation while IL-10 concentration was elevated. BLD, below limit of detection. Results are means ± SD (n = 5) and were compared by ANOVA followed by a multiple range test (Tukey analysis).
P < 0.05 compared with nontransduced cells.
P < 0.05 compared with nontransduced and Ad-null transduced cells.
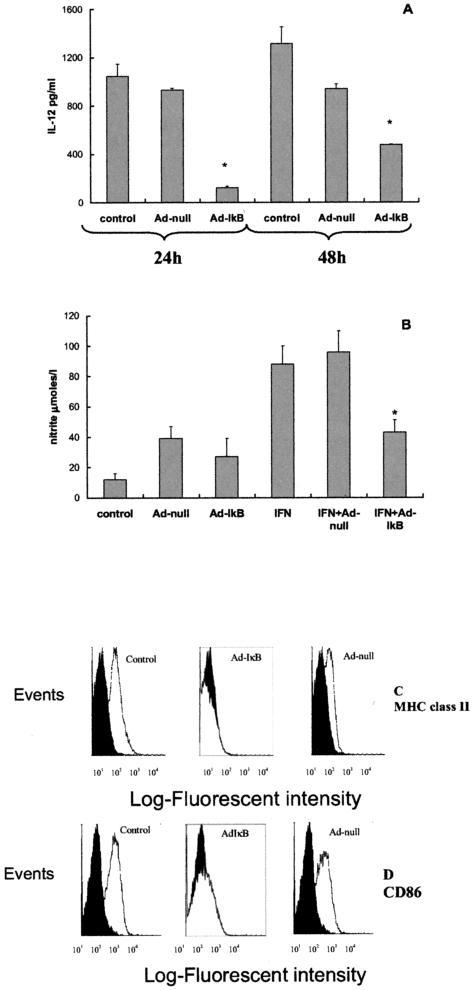
Next, we assessed the ability of Ad-IκB transduction to block classical macrophage activation by the combination of IFN-γ and LPS. The increase in IL-12 was significantly attenuated in Ad-IκB-transduced cells (Figure 2A). Interestingly, the IL-12 response substantially increased by 48 hours, even though levels remained significantly less than those of normal BMDMs and Ad-null-transduced cells. Ad-IκB also blocked the normal IFN-γ-induced increase in NO generation (Figure 2B), MHC class II expression (Figure 2C), and CD-86 expression (Figure 2D) seen in normal and Ad-null-transduced BMDMs. Therefore functioning of the NF-κB pathway was essential for the normal BMDM response to IFN-γ.
Figure 2.
Effects of IFN-γ/LPS and IFN-γ alone on normal and transduced BMDMs. Normal rat BMDMs and BMDMs transduced 48 hours previously with Ad-null or Ad-IκBα were left unstimulated or stimulated for a further 24 hours with IFN-γ or IFN-γ followed 4 hours later by LPS (for determination of IL-12). Supernatant was removed, and IL-12 was determined by enzyme-linked immunosorbent assay (A) and nitrite measured using Greiss reaction (B) (mean ± SD, *P < 0.05). Unstimulated cells have no detectable IL-12 production, and all of the bars in the graph represent cells stimulated with IFN-γ/LPS. Cells were harvested, and cell surface expression of MHC II (C) or CD86 (D) was analyzed by flow cytometry. Solid graphs are cells stained with irrelevant nonspecific FITC-labeled isotype-matched control antibody. Ad-IκB transduction resulted in the inhibition of IL-12 in response to IFN-γ and LPS and inhibition in nitrite and MHC class II and CD86 production by macrophages in response to IFN-γ. Graphs shown are representative for results from four experiments.
Transduction with Ad-IκB radically altered the inflammatory potential of macrophages activated by LPS not only by inhibiting pro-inflammatory mediators but also by enhancing IL-10 expression (Table 2), resulting in a threefold decrease in the overall ratio of TNF-α, IL-12, and nitrite relative to IL-10. Therefore inhibition of NF-κB not only decreases the potential for macrophages to cause injury when exposed to LPS but favors an anti-inflammatory phenotype. To determine whether they behaved in a similar way when activated in the more complex environment in vivo, their properties were examined in a macrophage dependent model of acute immune-mediated inflammation (NTN).
In Vivo Properties of NF-κB-Inhibited Macrophages
Fluorescently labeled BMDMs injected into the renal artery localize poorly to normal glomeruli but efficiently to inflamed glomeruli in rats with NTN8,9,21 with retention times within glomeruli with a t1/2 of approximately 4 days. More than 85% of glomeruli in the injected kidney contained transduced cells (Figure 3) with those glomeruli containing 6.1 ± 2.4 injected BMDMs/glomerulus. Glomerular macrophages in the acute phase of NTN develop a fixed, classically activated phenotype.22,25,26 The effect of transduction with Ad-IκB on this activation was assessed by injecting transduced, fluorescently labeled macrophages into kidney and analyzing single cells purified from the glomeruli by flow cytometry to determine the activation state of only the labeled, injected BMDMs (Figure 4A). After 24 hours of residence in nephritic glomeruli (ie, 48 hours after induction of nephritis), both the number of injected macrophages expressing iNOS (Figure 4B) and the mean fluorescent intensity (MFI) of the positive cells were significantly reduced in Ad-IκB-transduced macrophages (MFI, Ad-IκB 35.4 ± 2.9; Ad-null, 64.7 ± 13.1). The changes were more marked after 48 hours (MFI, Ad-IκB 40.5 ± 5.8; Ad-null, 80.5 ± 9.8). These results are consistent with the decrease in NO production by Ad-IκB-transduced macrophages stimulated with IFN-γ and LPS in vitro. After 24 hours residence, there was a significant increase in the number of Ad-null-transduced BMDMs that expressed MHC class II, and this response was greatly attenuated in Ad-IκB-transduced macrophages (Figure 4, A and B). In contrast to iNOS, the MFI of the positive cells was the same in both groups, suggesting that NF-κB inhibition has a stochastic (ie, all or none) response for MHC class II rather than a modulating one. After 48 hours of residence, there was no significant difference in expression of MHC class II between the groups (Figure 4B).
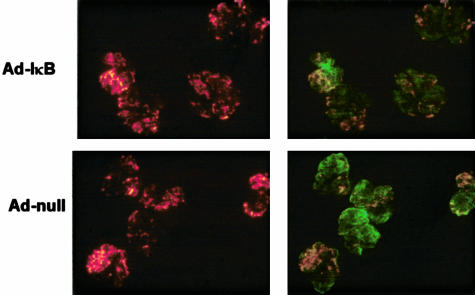
Figure 3.
Glomerular localization of PKH 26L-labeled BMDMs to the glomeruli of rats with NTN. Glomeruli were isolated from kidneys injected 24 hours previously with Ad-IκB-transduced (top) or Ad-null-transduced (bottom) BMDMs. The transduced macrophages were labeled with PKH-26L and fluoresce red (left panels). The glomerular basement membrane has been labeled with an anti-rabbit FITC IgG that binds to the deposited IgG in the glomerulus, showing that large numbers of Ad-IκB- or Ad-null-transduced, fluorescently labeled BMDMs localize efficiently in all of the glomeruli (right panels).
The results demonstrate that Ad-IκB transduction significantly reduced two of the key properties of classically activated macrophages that develop within acutely inflamed glomeruli. This suggests they may have a reduced capacity to induce injury and raises the important question of whether they are actively anti-inflammatory and reduce injury by naturally recruited host macrophages.
Effect of NF-κB-Inhibited Macrophages on Macrophage-Induced Injury in Vivo
Figure 5 shows significant injury in our NTN model with glomerular crescents, fibrosis, and macrophage infiltration 6 days after induction of nephritis. Epithelial cellular crescents with fibrin deposition, some macrophages, and fibrosis but no sclerosis predominate in this model. Injury was reduced in rats treated with Ad-IκB-transduced macrophages (Figure 5, right panel) compared with control nephritic rats (Figure 5, left panel) or rats treated with Ad-null-transduced macrophages (Figure 5, middle panel). There were significantly fewer crescents, significantly less necrosis and sclerosis, and although not statistically significant, less cellularity in glomeruli of rats treated with Ad-IκB, using a standard scoring system for histologically examining the severity of glomerular injury (Table 3).
Figure 5.
Representative histological and immunohistological analysis of glomeruli. A to C: H&E-stained section; D to F: Acid Fuchsin Orange G stain; G to I: silver methenamine stain; J to L: immunostaining of ED-1-positive cells (red cytoplasmic staining). Kidney sections are from control nephritic animals (A, D, G, and J), animals treated with Ad-null-transduced cells (B, E, H, and K), and animals treated with Ad-IκB-transduced cells (C, F, I, and L), showing clear evidence that animals treated with Ad-IkB-transduced cells have reduced glomerular injury. Original magnification, ×400.
Table 3.
Histological Analysis of Kidney Sections at Day 6 from Control Nephritic Rats and Rats Treated with Ad-null or Ad IκB-Transduced Macrophages
| Control | Ad-null | Ad-IκB | |
|---|---|---|---|
| Necrosis | 1.9 ± 0.2 | 2 ± 0.6 | 1.3 ± 0.2* |
| Sclerosis | 0.9 ± 0.3 | 1.5 ± 0.4 | 0.3 ± 0.08* |
| Glomerular cellularity/gcs | 96 ± 10 | 97 ± 8 | 86 ± 6 |
| Crescents/40 glomeruli | 7 ± 2.5 | 7.5 ± 2 | 3 ± 0.6* |
The scoring system for necrosis and sclerosis was translated into numerical values (see Material and Methods) and are averages ± SD calculated from six rats.
P < 0.05 compared with noninjected nephritic rats or rats injected with Ad-null transduced cells (Figure 9).
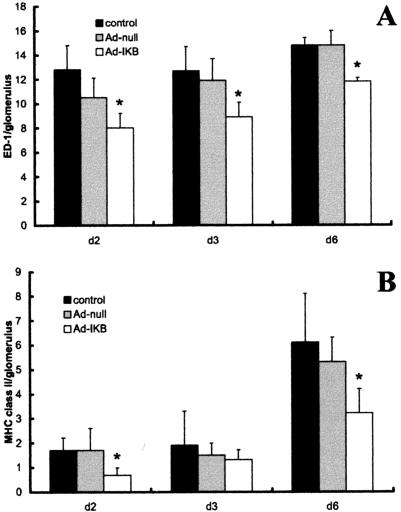
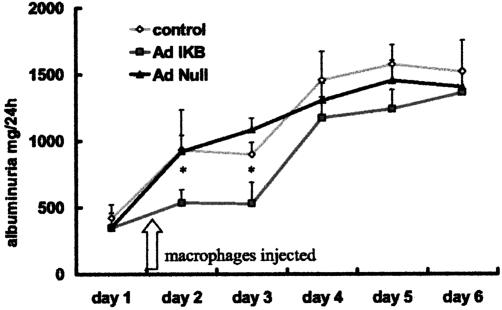
The adoptively transferred Ad-IκB-transduced macrophages comprised only 11 ± 1.5% of the total macrophages infiltrating the glomerulus (in the case of rats injected with Ad-null-transduced cells). Consequently, there were approximately 10-fold more normal host macrophages infiltrating the glomerulus than there were adoptively transferred NF-κB blocked macrophages. Despite this, injection of Ad-IκB-transduced BMDMs significantly reduced the number of host inflammatory macrophages infiltrating glomeruli (Figure 6A). Consequently, the injected BMDMs constituted 15 ± 2% of total macrophages in this group. There was a significant reduction in the number (and proportion) of host macrophages expressing MHC class II in rats treated with Ad-IκB-transduced BMDMs (Figure 6B). At day 3, the decrease in MHC class II was less evident, correlating with results of flow cytometry that did not demonstrate decreased MHC class II at this time point. Ad-IκB-transduced macrophages also significantly attenuated glomerular injury when assessed functionally by the severity of albuminuria, particularly at early time points, (Figure 7) again indicating they had a strong protective effect on disease.
Figure 6.
Effect of NF-κB-inhibited macrophages on macrophage-induced injury in vivo. Comparison of the mean numbers ± SD of ED1 (pan macrophage marker) (A) and MHC class II-positive (B) cells per glomerulus in kidney sections from control nephritic rats (control) and nephritic rats injected with Ad-IκB or Ad-null-transduced macrophages. Injection of NF-κB-inhibited macrophages caused a significant reduction in the number of ED-1 and MHC class II-positive macrophages, indicating NF-κB-inhibited macrophages result in a decrease in inflammation. *P < 0.05 compared with noninjected nephritic rats or rats injected with Ad-null-transduced cells.
Figure 7.
Albuminuria in rats with NTN. Albuminuria was measured as a marker of the severity of disease. Injection of Ad-IκB macrophages into the renal artery resulted in a marked reduction in albuminuria up to day 4 compared with Ad-null-treated animals or control nephritic rats, indicating a reduction in severity of nephritis. *P < 0.05 compared with noninjected nephritic rats or rats injected with Ad-null-transduced cells; n > 6 animals in all groups at each time point.
Discussion
NF-κB is a critical regulator of macrophage pro-inflammatory responses in vitro, and inhibiting it down-regulates these responses. Here, we determined whether blocking NF-κB could also significantly impair the pro-inflammatory potential of macrophages in an in vivo inflamed environment. We demonstrate NF-κB has broader effects on macrophage function as transduction with a dominant-negative IκB not only inhibited release of pro-inflammatory cytokines and nitric oxide on LPS activation but also enhanced IL-10 synthesis. More importantly, NF-κB inhibition prevented classical activation of macrophages infiltrating an inflamed environment in vivo. Instead Ad-IκB-transduced BMDMs are strongly anti-inflammatory, and small numbers of them profoundly attenuated glomerular injury in vivo in nephrotoxic nephritis by reducing both infiltration and activation of host macrophages.
Our demonstration that macrophages expressing dominant-negative IκB have reduced IL-12 and TNF-α synthesis and NO generation when stimulated with LPS is entirely consistent with previous studies.27,28 The combined stimulation of macrophages with LPS and IFN-γ results in enhanced expression of these genes presumptively because their promoter regions contain STAT-binding sites as well as NF-κB sites. IFN-γ itself activates NF-κB via an RNA-dependent protein kinase.29–31 It is not surprising that dominant-negative IκB decreased NO generation by BMDMs activated with IFN-γ alone. More surprisingly, the repressor completely abrogated IFN-γ-induced expression of MHC class II and CD86 by BMDMs. This implies an essential role for NF-κB in at least some STAT 1-dependent IFN-γ responses. The two most likely explanations for this are either that NF-κB facilitates STAT-1 binding to promoter regions of the two genes, or that its inhibition induced the synthesis of molecules that directly inhibit IFN-γ signaling, such as the Suppressors of Cytokine Signaling proteins.32 Regardless of the mechanism(s), the ability of dominant-negative IκB to inhibit classical macrophage activation is important for the in vivo studies because glomerular macrophages in the acute phase of NTN are classically activated.
Many stimuli that increase pro-inflammatory mediators by activating NF-κB also induce the anti-inflammatory cytokine IL-10,15 albeit with a delayed time course. Few studies have examined the influence of NF-κB on this process, and the published results are contradictory. Studies of the NF-κB pathway in genetically deficient mice showed that an IκB kinase 2 deletion caused a significant reduction in LPS-induced bone marrow-derived macrophage IL-10 secretion,33 whereas p50 deficiency, which acts further down the pathway, enhanced it.28 These differences were reflected by results from in vivo studies that demonstrated a reciprocal relation between IL-10 concentrations and the development of atheromatous inflammation. Using a similar strategy to ours, Bondeson et al27 transduced human monocytes with a dominant-negative IκB and found that the transduced cells had no significant decrease in IL-10 expression on exposure to LPS despite a marked attenuation in expression of pro-inflammatory cytokines. A recent study by Wessels et al34 demonstrates a similar principal with BCL-3, an IκB like protein that interacts exclusively with p50 and p65. They demonstrate that LPS-induced BCL-3 functions as an anti-inflammatory regulator in macrophages by attenuating transcription of pro-inflammatory cytokines and activating IL-10 expression. Thus, interruption of the NF-κB pathway either by dominant-negative IκB or p50 deficiency has little effect on cytokine secretion by resting macrophages, but exposure to LPS or classically activating factors results in unrestrained IL-10 synthesis with little or no synthesis of pro-inflammatory cytokines. This potential is clearly demonstrated by the ratios of TNF-α, nitrite, and IL-12 relative to IL-10, which suggests that NF-κB-inhibited macrophages might be anti-inflammatory and immunosuppressive in vivo. However, caution must be exercised in extrapolating in vitro studies to the situation in vivo because of uncertainties about activation by single cytokines rather than the hugely more complex situation in vivo.
Direct studies in vivo are essential, and our use of the rat NTN model provided unique opportunities for analyzing macrophage activation in the much more complex environment. NTN is a reproducible model of immune-mediated renal injury. Macrophage-dependent injury is induced by the host immune response to foreign IgG planted in the kidney after injection of heterologous anti-glomerular basement membrane antibodies.35–39 Fluorescently labeled normal and manipulated macrophages injected into the renal artery localize to nephritic glomeruli, and we have developed methods whereby they can be recovered for analysis after activation in vivo. Infiltrating macrophages are classically activated with increased expression of MHC class II and iNOS.
Inhibiting NF-κB did not affect the ability of cells to localize to inflamed glomeruli, but flow cytometry studies demonstrate they were not activated normally, in that they had less iNOS and MHC class II expression. Interestingly, the administered cells had uniformly lower expression of iNOS after both 24 and 48 hours of residence within the inflamed glomeruli, whereas the MHC class II response only affected a proportion of the cells and was relatively transient, suggesting a stochastic effect. This is reminiscent of the stochastic responses to macrophage activation in vivo that we have previously described within the glomerulus, both in nephrotoxic nephritis3,26 and in the Thy1.1 model of proliferative nephritis;40 and in the eye in uveitis.41 Regardless of the precise type of macrophage activation that occurs, our results show also that IκB-transduced macrophages markedly attenuate the macrophage-dependent glomerular injury in NTN.
The most striking observation is the potency of the anti-inflammatory effect of the Ad-IκB-transduced macrophages. The injected cells comprised only 4 ± 1.2% of total glomerular cells and approximately 15% of the infiltrating macrophages in rats injected with Ad-IκB-transduced cells and 11% in rats injected with Ad-null-transduced cells. This is a critical demonstration of the power of small numbers of these cells to alter the microenvironment within the inflamed glomerulus and thus prevent infiltration and activation of host macrophages. Transduced macrophages remain in the kidney for a short time but have longer term consequences on disease resulting in approximately 20% reduction in naturally infiltrating host macrophages. The increased IL-10 secretion provides a possible but not exclusive mechanism for these effects. We have previously shown that macrophages transduced with an adenovirus expressing IL-10 had similar effects on glomerular injury in the model9 when high local concentrations were achieved. The production of IL-10 from NF-κB modified-cells was significantly increased but unlikely in vivo to reach concentrations produced from macrophages transduced with an IL-10-expressing adenovirus. We are currently investigating this phenomenon as well as the role played by other factors produced by NF-κB-inhibited macrophages in modifying disease.
In summary, we have shown that inhibiting the NF-κB pathway in macrophages by transduction with a dominant-negative IκB has profound effects on their function both in vitro and when they are activated naturally in vivo in NTN. Instead of becoming classically activated and causing injury, they develop profound anti-inflammatory properties, and very small numbers are capable of attenuating glomerular injury by inhibiting influx of host macrophages and preventing their normal activation. Strikingly, these effects operate after the induction of injury. They emphasize the potential of macrophages as a therapeutic target in immune-mediated inflammatory disease.
Footnotes
Address reprint requests to Dr. Heather M. Wilson, Department of Medicine and Therapeutics, University of Aberdeen, Institute of Medical Sciences, Foresterhill, Aberdeen, Scotland, UK, AB25 2ZD. E-mail: h.m.wilson@abdn.ac.uk.
Supported by European Commission (grant QLG1-CT-2002-01215) Biotechnology and Biological Sciences Research Council grant C13027 and NHS Grampian (03/16). D.W. is supported by the National Kidney Research Fund (TF6/2003). D.C.K. is a National Kidney Research Fund Senior Fellow.
A.J.R. and D.C.K. contributed equally to this work.
References
- Nikolic-Paterson DJ, Lan HY, Atkins RC. Macrophages in immune renal injury. Neilson EG, Couser WG, editors. Philadelphia: Lippincott-Raven; Immunologic Renal Diseases. 1997:pp 575–592. [Google Scholar]
- Kluth DC, Erwig LP, Rees AJ. Multiple facets of macrophages in renal injury. Kidney Int. 2004;66:542–557. doi: 10.1111/j.1523-1755.2004.00773.x. [DOI] [PubMed] [Google Scholar]
- Erwig LP, Stewart K, Rees AJ. Macrophages from inflamed but not normal glomeruli are unresponsive to anti-inflammatory cytokines. Am J Pathol. 2000;156:295–301. doi: 10.1016/S0002-9440(10)64730-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikezumi Y, Atkins RC, Nikolic-Paterson DJ. Interferon-gamma augments acute macrophage-mediated renal injury via a glucocorticoid-sensitive mechanism. J Am Soc Nephrol. 2003;14:888–898. doi: 10.1097/01.asn.0000056604.13964.62. [DOI] [PubMed] [Google Scholar]
- Ikezumi Y, Hurst LA, Atkins RC, Nikolic-Paterson DJ. Macrophage-mediated renal injury is dependent on signaling via the JNK pathway. J Am Soc Nephrol. 2004;15:1775–1784. doi: 10.1097/01.asn.0000131272.06958.de. [DOI] [PubMed] [Google Scholar]
- Tam FW, Smith J, Cashman SJ, Wang Y, Thompson EM, Rees AJ. Glomerular expression of interleukin-1 receptor antagonist and interleukin-1 beta genes in antibody-mediated glomerulonephritis. Am J Pathol. 1994;145:126–136. [PMC free article] [PubMed] [Google Scholar]
- Chadban SJ, Tesch GH, Lan HY, Atkins RC, Nikolic-Paterson DJ. Effect of interleukin-10 treatment on crescentic glomerulonephritis in rats. Kidney Int. 1997;51:1809–1817. doi: 10.1038/ki.1997.248. [DOI] [PubMed] [Google Scholar]
- Kluth DC, Ainslie CV, Pearce WP, Clarke D, Anegon I, Rees AJ. Macrophages transfected with adenovirus to express IL-4 reduce inflammation in experimental glomerulonephritis. J Immunol. 2001;166:4728–4736. doi: 10.4049/jimmunol.166.7.4728. [DOI] [PubMed] [Google Scholar]
- Wilson HM, Stewart K, Brown PAJ, Anegon I, Chettibi S, Rees AJ, Kluth DC. Bone marrow derived macrophages (BMDM) genetically modified to produce IL-10 reduce injury in experimental glomerulonephritis. Mol Ther. 2002;6:710–717. doi: 10.1006/mthe.2002.0802. [DOI] [PubMed] [Google Scholar]
- Teder P, Vandivier RW, Jiang D, Liang J, Cohn L, Pure E, Henson PM, Noble PW. Resolution of lung inflammation by CD44. Science. 2002;296:155–158. doi: 10.1126/science.1069659. [DOI] [PubMed] [Google Scholar]
- Nagaoka T, Kaburagi Y, Hamaguchi Y, Hasegawa M, Takehara K, Steeber DA, Tedder TF, Sato S. Delayed wound healing in the absence of intercellular adhesion molecule-1 or L-selectin expression. Am J Pathol. 2000;157:237–247. doi: 10.1016/S0002-9440(10)64534-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flavell RA. The relationship of inflammation and initiation of autoimmune disease: role of TNF super family members. Curr Top Microbiol Immunol. 2002;266:1–9. doi: 10.1007/978-3-662-04700-2_1. [DOI] [PubMed] [Google Scholar]
- Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- Mosser DM. The many faces of macrophage activation. J Leuk Biol. 2003;73:209–212. doi: 10.1189/jlb.0602325. [DOI] [PubMed] [Google Scholar]
- Moore KW, de Waal Malefyt R, Coffman RL, O’Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- Li Q, Verma IM. NF-kappaB regulation in the immune system. Nat Rev Immunol. 2002;2:725–734. doi: 10.1038/nri910. [DOI] [PubMed] [Google Scholar]
- Foxwell B, Browne K, Bondeson J, Clarke C, de Martin R, Brennan F, Feldmann M. Efficient adenoviral infection with IkappaB alpha reveals that macrophage tumor necrosis factor alpha production in rheumatoid arthritis is NF-kappaB dependent. Proc Natl Acad Sci USA. 1998;95:8211–8215. doi: 10.1073/pnas.95.14.8211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lake FR, Noble PW, Henson PM, Riches DW: Functional switching of macrophage responses to tumor necrosis factor-alpha (TNF alpha) by interferons: implications for the pleiotropic activities of TNF alpha. J Clin Invest 199, 93:1661–1669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhan AK, Schneeberger EE, Collins AB, McCluskey RT. Evidence for a pathogenic role of a cell-mediated immune mechanism in experimental glomerulonephritis. J Exp Med. 1978;148:246–260. doi: 10.1084/jem.148.1.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jobin C, Morteau O, Balfour-Sartor R. Specific NF-kappaB blockade selectively inhibits tumour necrosis factor-alpha-induced COX-2 but not constitutive COX-1 gene expression in HT-29 cells. Immunology. 1998;95:537–543. doi: 10.1046/j.1365-2567.1998.00646.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kluth DC, Erwig LP, Rees AJ. Gene transfer into inflamed glomeruli using macrophages transfected with adenovirus. Gene Ther. 2000;7:263–270. doi: 10.1038/sj.gt.3301060. [DOI] [PubMed] [Google Scholar]
- Erwig LP, Kluth DC, Walsh GM, Rees AJ. Initial cytokine exposure determines macrophage function and renders them unresponsive to other cytokines. J Immunol. 1998;161:1983–1988. [PubMed] [Google Scholar]
- Helfrich MH, Evans DE, Grabowski PS, Pollock JS, Ohshima H, Ralston SH. Expression of nitric oxide synthase isoforms in bone and bone cell cultures. J Bone Miner Res. 1997;12:1108. doi: 10.1359/jbmr.1997.12.7.1108. [DOI] [PubMed] [Google Scholar]
- Wang CY, Mayo MW, Baldwin AS. TNF-and cancer therapy-induced apoptosis: potentiation by inhibition of NF-kappaB. Science. 1996;274:784–787. doi: 10.1126/science.274.5288.784. [DOI] [PubMed] [Google Scholar]
- Lianos EA, Orphanos V, Cattell V, Cook T, Anagnou N. Glomerular expression and cell origin of transforming growth factor-beta 1 in anti-glomerular basement membrane disease. Am J Med Sci. 1994;307:1–5. doi: 10.1097/00000441-199401000-00001. [DOI] [PubMed] [Google Scholar]
- Wilson HM, Minto AW, Brown PAJ, Stewart KN, Rees AJ. Transforming growth factor-beta isoforms and glomerular injury in nephrotoxic nephritis. Kidney Int. 2000;57:2434–2444. doi: 10.1046/j.1523-1755.2000.00102.x. [DOI] [PubMed] [Google Scholar]
- Bondeson J, Browne K, Brennan F, Foxwell B, Feldman M. Selective regulation of cytokine induction by adenoviral gene transfer of IκBα into human macrophages: lipopolysaccharide-induced, but not Zymosan-induced, pro-inflammatory cytokines are inhibited, but IL-10 is nuclear factor κB independent. J Immunol. 1999;162:2939–2945. [PubMed] [Google Scholar]
- Kanters E, Gijbels MJ, van der Made I, Vergouwe MN, Heeringa P, Kraal G, Hofker MH, de Winther MP. Hematopoietic NF-kappaB1 deficiency results in small atherosclerotic lesions with an inflammatory phenotype. Blood. 2004;103:934–940. doi: 10.1182/blood-2003-05-1450. [DOI] [PubMed] [Google Scholar]
- Deb A, Haque SJ, Mogensen T, Silverman RH, Williams BR. RNA-dependent protein kinase PKR is required for activation of NF-kappa B by IFN-gamma in a STAT1-independent pathway. J Immunol. 2001;166:6170–6180. doi: 10.4049/jimmunol.166.10.6170. [DOI] [PubMed] [Google Scholar]
- Ramana CV, Grammatikakis N, Chernov M, Nguyen H, Goh KC, Williams BR, Stark GR. Regulation of c-myc expression by IFN-gamma through Stat1-dependent and -independent pathways. EMBO J. 2000;19:263–272. doi: 10.1093/emboj/19.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Yang YL, Flati V, Der S, Kadereit S, Deb A, Haque J, Reis L, Weissmann C, Williams BR. Deficient cytokine signaling in mouse embryo fibroblasts with a targeted deletion in the PKR gene: role of IRF-1 and NF-kappaB. EMBO J. 1997;16:406–416. doi: 10.1093/emboj/16.2.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander WS. Suppressors of cytokine signalling (SOCS) in the immune system. Nat Rev Immunol. 2002;2:410–416. doi: 10.1038/nri818. [DOI] [PubMed] [Google Scholar]
- Kanters E, Pasparakis M, Gijbels MJ, Vergouwe MN, Partouns-Hendriks I, Fijneman RJ, Clausen BE, Forster I, Kockx MM, Rajewsky K, Kraal G, Hofker MH, de Winther MP. Inhibition of NF-kappaB activation in macrophages increases atherosclerosis in LDL receptor-deficient mice. J Clin Invest. 2003;112:1176–1178. doi: 10.1172/JCI18580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessells J, Baer M, Young HA, Claudio E, Brown K, Siebenlist U, Johnson PF. BCL-3 and NF-{kappa}B p50 attenuate lipopolysaccharide-induced inflammatory responses in macrophages. J Biol Chem. 2004;279:49995–50003. doi: 10.1074/jbc.M404246200. [DOI] [PubMed] [Google Scholar]
- Ikezumi Y, Hurst LA, Masaki T, Atkins RC, Nikolic-Paterson DJ. Adoptive transfer studies demonstrate that macrophages can induce proteinuria and mesangial cell proliferation. Kidney Int. 2003;63:83–95. doi: 10.1046/j.1523-1755.2003.00717.x. [DOI] [PubMed] [Google Scholar]
- Chow F, Ozols E, Nikolic-Paterson DJ, Atkins RC, Tesch GH. Macrophages in mouse type 2 diabetic nephropathy: correlation with diabetic state and progressive renal injury. Kidney Int. 2004;65:116–128. doi: 10.1111/j.1523-1755.2004.00367.x. [DOI] [PubMed] [Google Scholar]
- Timoshanko JR, Holdsworth SR, Kitching AR, Tipping PG. IFN-gamma production by intrinsic renal cells and bone marrow-derived cells is required for full expression of crescentic glomerulonephritis in mice. J Immunol. 2002;168:4135–4141. doi: 10.4049/jimmunol.168.8.4135. [DOI] [PubMed] [Google Scholar]
- Timoshanko JR, Sedgwick JD, Holdsworth SR, Tipping PG. Intrinsic renal cells are the major source of tumor necrosis factor contributing to renal injury in murine crescentic glomerulonephritis. J Am Soc Nephrol. 2003;14:1785–1793. doi: 10.1097/01.asn.0000073902.38428.33. [DOI] [PubMed] [Google Scholar]
- Kitching AR, Tipping PG, Holdsworth SR. IL-12 directs severe renal injury, crescent formation and Th1 responses in murine glomerulonephritis. Eur J Immunol. 1999;29:1–10. doi: 10.1002/(SICI)1521-4141(199901)29:01<1::AID-IMMU1>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Minto AW, Erwig LP, Rees AJ. Heterogeneity of macrophage activation in anti-Thy-1.1 nephritis. Am J Pathol. 2003;163:2033–2041. doi: 10.1016/S0002-9440(10)63561-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson MJ, Erwig LP, Liversidge J, Forrester JV, Rees AJ, Dick AD. Retinal microenvironment controls resident and infiltrating macrophage function during uveoretinitis. Invest Ophthalmol Vis Sci. 2002;43:2250–2257. [PubMed] [Google Scholar]