Abstract
Estradiol prevents fatty streak formation in chow-fed atherosclerosis-prone apolipoprotein E (ApoE)-deficient mice. We previously reported that fatty streak development of immunodeficient ApoE−/−/recombination activating gene 2 (RAG-2−/−) double-deficient mice was insensitive to estradiol. In the present work, we demonstrate that the reconstitution of ApoE−/−/RAG-2−/− with bone marrow from immunocompetent ApoE−/−/RAG-2+/+ mice restores the protective effect of estradiol on fatty streak constitution. We extended this demonstration to the model of low-density lipoprotein receptor-deficient mice, establishing the obligatory role of mature lymphocytes in this process. We then investigated whether the protective effect of estradiol was mediated by a specific lymphocyte subpopulation by studying the hormonal effect on fatty streak constitution in recently developed models of ApoE−/− mice deficient in selective T-lymphocyte subsets (either TCRαβ+, CD4+, CD8+, or TCRγδ+ lymphocytes) or B lymphocytes. In all these specifically immunodeficient mice, estradiol administration to ovariectomized mice conferred protection as in immunocompetent ApoE−/− mice, clearly demonstrating that no single lymphocyte subpopulation was specifically required for this effect. These results point to additional lymphocyte-dependent mechanisms such as modulating the interactions among lymphocytes and between lymphocytes and endothelial and/or antigen-presenting cells.
Fuller understanding of the mechanism of atherosclerosis prevention by estrogens is urgently needed.1 Two controlled prospective and randomized studies did not demonstrate a beneficial effect of hormone replacement therapy whether in secondary2 or in primary prevention.3 In contrast to these clinical data, estrogen hormones have been shown to decrease macrophage-derived foam-cell infiltration in different animal species including atherosclerosis-prone apolipoprotein E-deficient (ApoE−/−) mice4,5 although the mechanisms of this effect have remained obscure.
Recent cumulative evidence have suggested that both innate and adaptive immune responses modulate the rate of lesion progression.6–8 Indeed, several studies have confirmed the importance of T lymphocytes present in early lesions of atherosclerosis.9–12 Furthermore, previous observations have demonstrated the particular role for specific T-lymphocyte subsets. For example, Zhou and colleagues13 showed that CD4+ T cells aggravate the atherosclerotic process.
In this context, we previously reported that ApoE−/− mice with homozygous disruption at the recombination activating gene 2 (RAG-2−/−) loci presented a reduced level of atherosclerotic lesions that were insensitive to estradiol (E2).14 In the present studies, we first demonstrated that the reconstitution of ApoE−/−/RAG-2−/− with bone marrow from immunocompetent ApoE−/−/RAG-2+/+ mice restores the protective effect of E2 on fatty streak constitution and extended this demonstration to the model of low-density lipoprotein receptor (LDLr)-deficient mice. We then hypothesized that E2 could target a specific lymphocyte subset to exert its protective effect on fatty streak constitution. To solve this question, we compared the effect of E2 in immunocompetent ApoE−/− mice and in models of ApoE−/− mice deficient in specific lymphocyte subsets developed in our laboratory. We observed that no T- or B-lymphocyte subpopulation specifically mediated the protective effect of E2, pointing to additional lymphocyte-dependent mechanisms.
Materials and Methods
Animals
The specific pathogen-free conditions of animal care and regular chow diet feeding as well as the production of ApoE- and RAG-2-deficient mice (ApoE−/−/RAG-2−/−) have been described previously.14,15 The ApoE−/−/RAG-2−/− mice had been backcrossed into a C57BL/6 background for six generations.
Low-density lipoprotein receptor-deficient (LDLr−/−) mice were purchased from Charles River (L’arbresle, France). RAG-2-deficient (RAG-2−/−) mice were purchased from CDTA (Orléans, France). Both strains had been backcrossed into a C57BL/6 background for more than 10 generations. Female LDLr−/− mice were crossed with male RAG-2−/− mice in our animal facility to obtain LDLr and RAG-2 double-deficient mice (LDLr−/−/RAG-2−/−). RAG-2 and LDLr gene disruptions were assessed by polymerase chain reaction genotyping as previously described.16,17 The production of the double-deficient models is reported elsewhere.12 Briefly, TCRβ-deficient (TCRβ−/−), CD4-deficient (CD4−/−), CD8deficient (CD8−/−), TCRδ-deficient (TCRδ−/−) male mice were crossed with female ApoE−/− mice. B-lymphocyte-deficient mice were obtained similarly by crossing μmt-deficient18 B−/−) male mice with female ApoE−/− mice. Heterozygous ApoE−/−/TCRβ+/−, ApoE−/−/CD4+/−, ApoE−/−/CD8+/−, ApoE−/−/TCRδ+/−, ApoE−/−/B+/− populations were generated and used as the parental genotypes. The offspring of these heterozygous strains, TCRβ+/+, CD4+/+, CD8+/+, TCRδ+/+, B+/+ and TCRβ−/−, CD4−/−, CD8−/−, TCRδ−/−, B−/− served as the subjects of our studies. Confirmation of gene disruption was screened by polymerase chain reaction genotyping and phenotyping of blood lymphocytes or splenocytes by flow cytometry.12 All strains had been backcrossed into a C57BL/6 background for more than 10 generations.
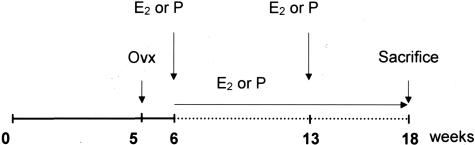
Only female animals were used in the present studies. As shown in Figure 1, mice were ovariectomized at 5 weeks of age and, 1 week later, were administered with either 60-day time-release placebo or 0.1 mg of estradiol-17β pellets (Innovative Research of America, Sarasota, FL) implanted subcutaneously into the back of the animals, using a sterile trochar and forceps. New pellets were reimplanted 7 weeks later. The dose of 0.1 mg of E2, releasing 80 μg/kg/day, had previously been defined as adequate for a maximal effect on fatty streak constitution in female mice.15 ApoE−/− mice were maintained under chow diet throughout the experiments, whereas LDLr−/− mice were switched to a high-fat diet (15% fat, 1.25% cholesterol, no cholate, TD96335; Harlan Teklad, WI) at 5 weeks of age. After E2 or placebo treatment for 12 weeks, all mice were sacrificed with an overdose of ketalar after a 16-hour fast. Blood was collected by orbital punction for serum lipid analysis.15 Uterus was weighted to assess the efficacy of E2 treatment. All experimental procedures were performed in accordance with the recommendations of the European Accreditation of Laboratory Animal Care Institute.
Figure 1.
Protocol to study fatty streak formation in immunocompetent or immunodeficient ApoE-deficient mice. Ovx, ovariectomy; E2, estradiol-17β pellet; Pl, placebo pellet.
Bone Marrow Transplantation
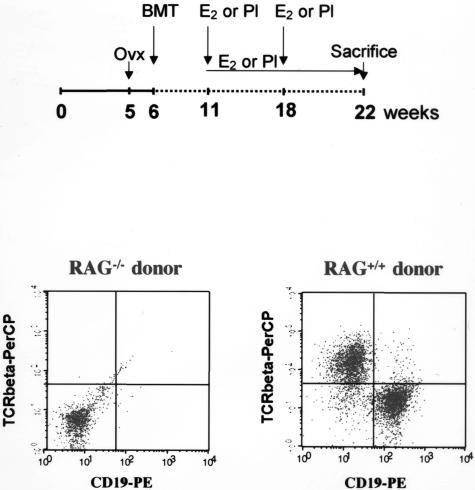
As shown in Figure 2, ApoE−/−/RAG-2−/− and LDLr−/−/RAG-2−/− mice were ovariectomized at 5 weeks of age and received a sublethal dose of whole-body irradiation (400 rads) 1 week later. The day after irradiation, donor ApoE−/− or ApoE−/−/RAG-2−/−, C57BL/6 or RAG-2−/− mice were killed, and their femurs and tibias removed aseptically. Marrow cavities were flushed, and single-cell suspensions were prepared. The irradiated recipients received 15 × 106 bone marrow cells in 0.2 ml of phosphate-buffered saline by tail vein injection. One week before and 4 weeks after the bone marrow transplantation, Bactrim (sulfamethoxazole 200 mg/ml, trimethoprim 48 mg/ml) was added to drinking water. After 5 additional weeks, all transplanted mice were implanted subcutaneously with placebo or E2 pellets and LDLr−/−/RAG-2−/− mice were switched to the high-fat diet to induce atherosclerotic lesion formation. Mice were sacrificed 11 weeks later (at 22 weeks of age). Blood and tissues were collected as described above.
Figure 2.
Protocol of bone marrow transplantation (BMT) and flow cytometry analysis of spleen lymphocyte repopulation of ApoE−/−/RAG-2−/− mice transplanted using bone marrow from ApoE−/−/RAG-2−/− or ApoE−/−/RAG-2+/+ donor mice. Splenocytes were co-labeled with anti-TCRβ-PerCP/anti-CD19-PE-conjugated antibodies.
Tissue Preparation and Lesion Analysis
The circulatory system was perfused with 0.9% NaCl by cardiac intraventricular canalization. The heart and ascending aorta were removed and kept frozen. Surface lesion area was measured by computer-assisted image quantification in the aortic root, by a trained observer blinded to the genotype and treatment of the mice, as previously described15 but using a Leica image analyzer. The rest of the entire aortic tree was removed and cleaned of adventitia, split longitudinally to the iliac bifurcation, and pinned flat on a dissection pan for analysis by en face preparation. Images were captured using a Sony-3CCD video camera and fraction covered by lesions evaluated as a percentage of the total aortic area.
Immunohistochemistry
Cryostat sections from the proximal aorta were fixed in acetone, air-dried, and reacted with a primary rat monoclonal anti-mouse macrophage (clone MOMA-2 from Serotec, Oxford, UK) used at a 1:50 dilution or a primary goat polyclonal anti-CD3 (clone M-20 from Santa Cruz Biotechnology, Santa Cruz, CA) used at a 1:100 dilution. Then, sections were incubated with corresponding preadsorbed secondary biotinylated antibodies (Vector Laboratories, Burlingame, CA): binding of rat monoclonal anti-macrophage was revealed using biotinylated rabbit anti-rat IgG and binding of goat polyclonal anti-CD3 was revealed using biotinylated horse anti-goat IgG. The binding of the biotinylated antibodies was visualized with an avidin DH-biotinylated peroxidase complex (Vectastain ABC kit, Vector Laboratories) and AEC peroxidase substrate kit (Vector Laboratories). Countercoloration was performed using Mayer’s hemalun. Macrophage and T-cell quantification was determined by scoring samples from at least four sections per animal. A minimum of three animals was analyzed per group. Two investigators who were blinded to the sample identity performed analysis.
Analysis of Plasma Lipids and Lipoproteins
Serum cholesterol concentrations were determined by an enzymatic assay adapted to microtiter plates using commercially available reagents (Roche Molecular Biochemicals, Germany). Lipoprotein cholesterol profiles were obtained by Fast Protein liquid chromatography as previously described.19
Statistical Analysis
The results are expressed as means ± SEM. For each parameter (body weight, total cholesterol, lesion area), the effects of genotype were studied by comparing each immunodeficient group with its corresponding immunocompetent group of mice. The effect of E2 treatment was studied comparing placebo- and E2-treated mice in selective immunodeficient or in immunocompetent mice. A one-factor analysis of variance was used (Bonferroni/Dunn’s test); P < 0.05 was considered as significant. Statistical analyses were performed using the Statview statistical software (Abacus Concepts, Inc., Berkeley, CA). When appropriate, an unpaired t-test was also performed.
Results
Immunocompetent Bone Marrow Transplantation Restored Both the Level of Lesions and E2 Sensitivity in ApoE−/−/ RAG-2−/− and LDLr−/−/RAG-2−/− Mice
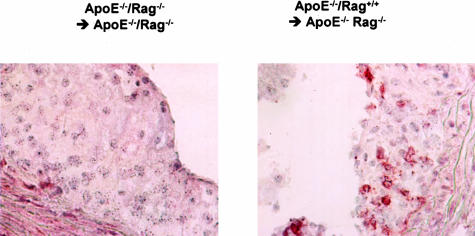
To explore the role of lymphocytes in fatty streak constitution and E2 prevention, ApoE−/−/RAG-2−/− ovariectomized female mice received bone marrow transplantation from ApoE−/−/RAG-2−/− (ApoE−/−/RAG-2−/− → ApoE−/−/RAG-2−/−) or from ApoE−/−/RAG-2+/+ (ApoE−/−/RAG-2+/+ → ApoE−/−/RAG-2−/−) mice (Figure 2), and then were treated with placebo or E2 pellets. Sixteen weeks after bone marrow transplantation, ApoE−/−/RAG-2+/+ → ApoE−/−/RAG-2−/− placebo-treated mice presented a significantly higher level of fatty streaks when compared with ApoE−/−/RAG-2−/− → ApoE−/−/RAG-2−/− placebo-treated mice (Table 1). Immunohistochemical analysis showed the presence of CD3-reactive cells in lesions obtained from ApoE−/−/RAG-2+/+ → ApoE−/−/RAG-2−/− mice but not in lesions obtained from ApoE−/−/RAG-2−/− → ApoE−/−/RAG-2−/− mice, irrespective of placebo or E2 treatment (Figure 3 and data not shown). Importantly, although E2 was still ineffective in ApoE−/−/RAG-2−/− → ApoE−/−/RAG-2−/− mice, the protective effect of the hormone was restored in ApoE−/−/RAG-2+/+ → ApoE−/−/RAG-2−/− mice (Table 1).
Table 1.
Body Weight, Total Cholesterol, and Aortic Root Lesion Area in Placebo (Pl)- or Estradiol-17ß (E2)-Treated Ovariectomized ApoE−/−/RAG−/− Female Mice after Bone Marrow Transplantation from ApoE−/−/RAG−/− or ApoE−/−/RAG+/+ Donors
| Donor genotype | Treatment | Body weight (g) | Total cholesterol (g/L) | Lesion area (μm2) |
|---|---|---|---|---|
| ApoE−/−/RAG+/+ | Pl | 25.3 ± 0.7 | 5.9 ± 0.4 | 66662 ± 5838 |
| E2 | 23.5 ± 0.6 | 4.8 ± 0.4 | 34919 ± 6532* | |
| ApoE−/−/RAG−/− | Pl | 26.8 ± 1.7 | 5.4 ± 0.4 | 33451 ± 5816† |
| E2 | 24.3 ± 0.3 | 4.4 ± 0.7 | 41844 ± 5294 |
Results are means ± SEM (n = 7).
P < 0.05 versus the corresponding Pl-treated mice.
P < 0.05 versus ApoE−/−/RAG+/+-transplanted Pl-treated mice.
Figure 3.
Immunohistochemical analysis of representative lesions from individual ApoE−/−/RAG-2−/− transplanted using bone marrow from ApoE−/−/RAG-2+/+ or ApoE−/−/RAG-2−/− donor mice using anti-CD3 antibodies.
Because ApoE-deficiency could be involved in these observations and because the RAG-2-deficient mice used were not fully backcrossed into the C57/BL6 background, similar experiments were performed in the LDLr-deficient mice. We first confirmed that E2 significantly decreased body weight (26.1 ± 0.6 g versus 23.6 ± 0.5 g, P < 0.05), serum cholesterol (11.1 ± 0.4 g/L versus 7.9 ± 0.7 g/L, P < 0.01), and fatty streak deposit (119,400 ± 7400 μm2/section versus 41,400 ± 5400 μm2/section for placebo- and E2-treated mice, respectively; n = 9, P < 0.01) in immunocompetent LDLr−/− mice on a 12-week high-fat diet in agreement with a previous report.20 The effect on fatty streak was abolished in LDLr−/−/RAG-2−/− mice (42,000 ± 13,100 μm2/section versus 40,300 ± 11,300 μm2/section, respectively; n = 8) whereas the effect on body weight (24.4 ± 1.3 g/L versus 22.9 ± 0.9 g/L, P < 0.05) and serum cholesterol (9.9 ± 0.4 g/L versus 7.4 ± 0.7 g/L, P < 0.01) persisted. Bone marrow graft experiments were also performed in this last model of ovariectomized female LDLr−/−/RAG-2−/− mice. As shown in Table 2, Placebo-treated LDLr−/−/RAG-2−/− mice that had received C57BL/6 bone marrow, presented a significantly higher level of fatty streaks when compared with those that had received RAG-2−/− bone marrow. Again, although E2 remained ineffective in RAG-2−/− → LDLr−/−/RAG-2−/− mice, the protective effect of the hormone was restored in C57BL/6 → LDLr−/−/RAG-2−/− mice (Table 2).
Table 2.
Body Weight, Total Cholesterol, and Aortic Root Lesion Area in Placebo (Pl)- or Estradiol-17ß (E2)-Treated Ovariectomized LDLr−/−/RAG−/− Female Mice after Bone Marrow Transplantation from RAG−/− or C57BL/6 Mice
| Donor genotype | Treatment | Body weight (g) | Total cholesterol (g/L) | Lesion area (μm2/section) |
|---|---|---|---|---|
| C57BL/6 | Pl | 28.1 ± 1.2 | 9.3 ± 0.8 | 84480 ± 9185 |
| E2 | 24.9 ± 0.7* | 6.4 ± 0.7* | 35333 ± 8317* | |
| RAG−/− | Pl | 27.1 ± 0.6 | 11.1 ± 0.6 | 35900 ± 5600† |
| E2 | 24.0 ± 0.5* | 9.9 ± 0.5 | 42800 ± 6600 |
The animals had been on HFD for 12 weeks. Results are means ± SEM (n ≥ 7).
P < 0.05 versus C57BL/6-transplanted Pl-treated mice.
P < 0.05 versus the corresponding Pl-treated mice.
Effect of E2 Treatment on Body Weight and Serum Lipids in Immunocompetent and Selectively Immunodeficient ApoE−/− Mice
We then asked whether the protective effect of E2 could be mediated by a specific T-lymphocyte subset or B lymphocytes, considering the hormonal effect in selectively immunodeficient ApoE−/− female mice. The statistical analysis presented in Table 3 refers to comparisons of each group of immunodeficient mice with its corresponding immunocompetent group. Data from a group of 10 ApoE−/− female mice are given for comparison (Table 3, line 1).
Table 3.
Body Weight, Total Cholesterol, and Lesion Area of Ovariectomized Placebo (Pl)- or Estradiol-17ß (E2)-Treated Immunocompetent ApoE−/− Control and Immunodeficient ApoE−/−/TCRß−/−, CD4−/−, CD8−/−, TCRδ−/− or B−/− Female Mice
| Genotype | Body weight (g)
|
Total cholesterol (g/L)
|
||
|---|---|---|---|---|
| NC | Pl | E2 | NC | |
| ApoE−/− | 22.0 ± 0.5 | 28.7 ± 1.5 | 22.9 ± 0.5* | 3.4 ± 0.1 |
| ApoE−/−TCRß−/− | 21.0 ± 0.5 | 20.9 ± 0.6† | 21.5 ± 0.5 | 3.0 ± 0.2 |
| ApoE−/−CD4−/− | 19.5 ± 0.4 | 21.9 ± 1.2† | 21.9 ± 0.6 | 3.0 ± 0.2 |
| ApoE−/−CD8−/− | 21.5 ± 0.5 | 23.5 ± 0.8† | 22.9 ± 0.5 | 3.1 ± 0.2 |
| ApoE−/−TCRδ−/− | 20.1 ± 0.4 | 27.6 ±1.4 | 21.9 ± 0.1* | 2.9 ± 0.1 |
| ApoE−/−B−/− | — | 26.8 ± 0.9 | 24.3 ± 0.4* | — |
Data of intact corresponding mice (NC) have been published previously12 and are indicated in italics for comparison. Results are means ± SEM (n ≥ 8).
P < 0.05 versus corresponding C (placebo-treated) mice.
P < 0.05 versus corresponding immunocompetent mice.
Table 3.
Continued
| Total cholesterol (g/L)
|
Lesion area (μm2/section)
|
|||
|---|---|---|---|---|
| Pl | E2 | NC | Pl | E2 |
| 5.6 ± 0.3 | 3.1 ± 0.2* | 73,214 ± 2963 | 113,465 ± 5288 | 36,299 ± 1979* |
| 4.4 ± 0.2† | 3.1 ± 0.2* | 37,048 ± 4749 | 65,053 ± 7753† | 37,104 ± 4418* |
| 5.8 ± 0.5 | 2.7 ± 0.2* | 77,745 ± 12,629 | 114,835 ± 21,656 | 42,541 ± 5431* |
| 5.6 ± 0.4 | 3.2 ± 0.1* | 76,909 ± 4722 | 110,537 ± 16,142 | 47,782 ± 11,285* |
| 5.6 ± 0.3 | 2.8 ± 0.2* | 57,589 ± 3737 | 101,557 ± 8125 | 27,730 ± 3637* |
| 4.6 ± 0.2† | 2.6 ± 0.3* | — | 93,432 ± 11,183 | 38,348 ± 5752* |
Uterine weight was <20 mg in ovariectomized mice and increased to 172 ± 13 mg on average with E2 treatment, showing that the level of E2 stimulation was similar in all genotypes. Body weight decreased, reflecting mainly adipose tissue reduction, in immunocompetent ApoE−/− control and in immunodeficient ApoE−/−/TCRδ−/− and ApoE−/−/B−/− mice under E2 treatment. In the immunodeficient ApoE−/−/TCRβ−/−, ApoE−/−/CD4−/−, and ApoE−/−/CD8−/− mice, body weight was lower in placebo-treated mice when compared to their immunocompetent littermates and was not influenced by E2, suggesting a role for TCRαβ+ T lymphocytes in weight regulation. Total serum cholesterol was lower in ovariectomized ApoE−/−/TCRβ−/− and ApoE−/−/B−/− when compared with their respective immunocompetent littermates and decreased under E2 treatment in all strains. Fast performance liquid chromatography showed that the E2-induced decrease concerned the very low-density lipoprotein, intermediary/low-density lipoprotein, and high-density lipoprotein fractions (see Supplemental Figure A at http://ajp.amjpathol.org) in agreement with our previous report.15
Effect of E2 Treatment on Lesion Area in Immunocompetent and Selectively Immunodeficient ApoE−/− Mice
At the level of the aortic root, the lesion area of ovariectomized immunodeficient mice given placebo did not differ significantly from the corresponding immunocompetent mice except for the ApoE−/−/TCRβ−/− mice, which presented a decreased level of lesions (Table 3). E2 treatment induced a significant decrease of fatty streak development in all groups of mice, including the ApoE−/−/TCRβ−/− strain. To further analyze the influence of serum cholesterol on the lesion formation, we sought to analyze subgroups of mice with comparable serum cholesterol levels. Such subgroups could be selected among the whole series of immunocompetent mice that serve as control for the immunodeficient groups (ie, a total of 50 Pl-treated and 50 E2-treated mice) with cholesterolemia arbitrarily encompassed between 4 and 6 g/L. In these subgroups of ovariectomized placebo (n = 19)- and E2 (n = 12)-treated mice, with similar serum cholesterol (5.0 ± 0.1 g/L and 4.9 ± 0.1 g/L, respectively; P = 0.67), lesion area still dramatically differed (109,824 ± 4304 μm2/section and 35,722 ± 4206 μm2/section, P < 0.001), strongly suggesting that the E2-induced decrease of serum cholesterol is not the main factor preventing fatty streak formation.
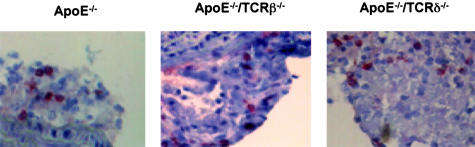
Histo- as well as immunohistochemical analysis showed that, under E2 treatment, residual lesions were essentially fatty streaks containing lipid-laden macrophages, with few characteristics of advanced lesions such as fibrous caps and were substantially less complex than lesions in ovariectomized ApoE−/− control mice (not shown). Remarkably, T lymphocytes were still detectable at a comparable density (2 ± 1%) in these residual lesions (Figure 4). Similar observations were made in all of the series of specifically immunodeficient mice including the ApoE−/−/TCRβ−/− (Figure 4).
Figure 4.
Anti-CD3 immunolabeling of representative lesions from ovariectomized ApoE−/−, ApoE−/−/TCRβ−/−, and ApoE−/−/TCRδ−/− mice after 3 months of treatment with E2 pellets.
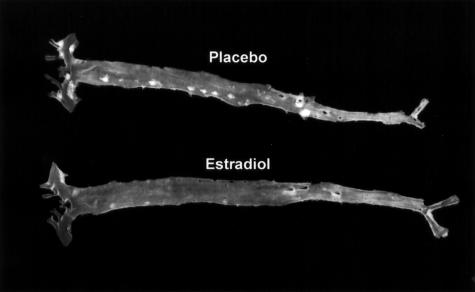
In the rest of the aorta, lesions were identifiable by en face analysis at predilection sites including the aortic arch and the orifices of the brachiocephalic, left subclavian, common carotid, and intercostal arteries. However, the level was low (<3.0% of the total aortic area) except in the ApoE−/−/CD4−/− group of mice (13.5 ± 3.0%, n = 3). In this last group, lesions were observed at the predilection sites and also at the orifice of the large abdominal arteries, in particular the celiac trunk and renal arteries. E2 induced a spectacular (more than fivefold) protective effect at these different sites, especially in the ApoE−/−/CD4−/− group (<3.0%, n = 3; Figure 5).
Figure 5.
Representative en face aorta preparations from placebo- and E2-treated ovariectomized ApoE−/−/CD4−/− mice.
Discussion
The present results definitely demonstrate that, in the C57BL/6 mouse strain, mature lymphocytes are required for the preventive effect of E2 on the atheromatous process irrespective of the model of genetically-induced hypercholesterolemia, namely ApoE−/− and LDLr−/− mice. Indeed, after bone marrow transplantation from immunocompetent donors into immunodeficient mice, lymphocytes were recovered in the lesions and a significant increase in the level of these lesions could be demonstrated. Most importantly, E2 activity was restored after bone marrow transplantation from immunocompetent donors, while E2 was still inactive after bone marrow transplantation from immunodeficient donors to the immunodeficient recipients.
Like our data obtained in intact nonovariectomized mice,12 the measurement of lesion area in placebo-treated ovariectomized mice show a similar level of lesions in immunocompetent and immunodeficient mice, except in the ApoE−/−/TCRβ−/− mice, supporting the deleterious role of αβ T lymphocytes in the atheromatous process. Noteworthy, considering our previous12 and present data, a protective effect of endogenous ovarian estrogens could be demonstrated in all strains because ovariectomized mice developed a higher level of lesions than intact mice. This observation is in accordance with a previous report.21 Moreover, E2 treatment, administered at a dose previously defined as adequate for a maximal effect,15 induced a significant decrease in lesion size in all groups of mice (Table 3). Except in ApoE−/−/TCRβ−/− mice, the residual lesion level was lower than that measured in intact female mice.12 In addition, en face analysis showed that the effect of E2 was not restricted to the aortic sinus. Interestingly, E2 exerted a stronger preventive effect of lesion development in the thoracic and abdominal sites than at the level of the aortic sinus, particularly in ApoE−/−/CD4−/− mice (Figure 5). Although the selective immune deficiency may generate compensatory expansion of other lymphocyte subsets, such as ApoE−/−/CD4−/− mice presenting with a greater number of CD8+ and double-negative CD4-CD8 cells than ApoE−/− mice,12,22 we demonstrate here that E2 was active in all strains, suggesting that no single T-lymphocyte subpopulation directly mediated the protective effect. This included the populations of regulatory T cells able to control the expansion and differentiation of activated T cells23,24 and the TCRγδ+ T cells. E2 has been recently claimed to induce one of these regulatory T-lymphocyte subpopulations25,26 suggesting that it could play a key role in the suppression of harmful immune responses. Our data do not support such a hypothesis in the atherosclerotic process. Finally, the protective effect was also maintained in B-lymphocyte-deficient mice. This excluded a protective role mediated by immunoglobulins that are known to increase under E2 stimulation27 and have been suggested to prevent atherosclerosis.27–30
Interestingly, E2 administration significantly decreased serum cholesterol levels in nearly all conditions analyzed in the present work. However, although serum cholesterol level remains a key determinant of atherosclerosis, several lines of evidence support the fact that the protective effect of E2 occurs mainly at the level of the arterial wall. First, although E2 decreased serum cholesterol levels in immunodeficient LDLr−/−/RAG-2−/− mice (the present work) as well as ApoE−/−/RAG-2−/− mice14 to a similar extent than in immunocompetent mice, it was completely inactive on lesion area. Second, although the maximal decrease of serum cholesterol was obtained with endogenous E2 (Table 3), the maximal decrease in lesion area required higher E2 doses, in line with previous reports.15,21 Third, in subgroups of ovariectomized placebo- or E2-treated ApoE−/− mice arbitrarily selected for similar serum cholesterol levels, fatty streak area was threefold lower in the latter group. Indeed, using cholesterol-clamped rabbits, Holm and colleagues31 had previously demonstrated a plasma lipid-independent anti-atherogenic effect of estrogen, in line with Adams and co-workers,32 who suggested, as early as 1990, a similar conclusion in surgically postmenopausal monkeys.
Altogether, these series of observation points to one (or more) additional lymphocyte-dependent mechanism(s) involved in the protective effect of E2. E2 is a negative regulator of lymphopoiesis, that selectively depletes functional precursors of B and T cells.33 It also inactivates the intrathymic T-cell differentiation pathway and induces thymocyte apoptosis.34 Indeed, we observed a remarkable 80% thymic atrophy (85.2 ± 7.5 mg versus 14.1 ± 1.8 mg) and 50% decrease of circulating lymphocytes in our E2-treated ApoE−/− mice (6804 ± 568 per μl versus 3520 ± 215 per μl; P < 0.001). However, in agreement with Hodgin and colleagues,35 T lymphocytes were still detectable in the residual lesions (Figure 3), showing that, despite their decrease in blood, lymphocytes could still reach and infiltrate the remaining lesions.
The protective effect could also be mediated through the modulation of the interactions between lymphocytes and other cell populations, such as endothelial and/or antigen-presenting cells, leading to a local control of the intimal immune process. First, Shi and colleagues36,37 recently provided strong evidence for the crucial role of endothelial cells rather than hematopoietic cells as determinants of atherosclerosis susceptibility in C57BL/6 mice. Second, decreased proinflammatory38–40 or increased anti-inflammatory cytokine41,42 production resulting from the local interaction between lymphocytes and antigen-presenting cells could explain the protective effect of E2. Indeed, it has recently been reported that estrogens repress Th1 activity and T-cell production of the key inflammatory cytokine tumor necrosis factor-α in bone43 but we reported the opposite effect in antigen-specific CD4+ or NKT cell response.44,45 Further work will be necessary to precisely define the mechanisms of these interactions.
In conclusion, we have demonstrated that lymphocytes are instrumental in the protective effect of E2 but that no single lymphocyte subpopulation is specifically required for this effect. These data point to additional lymphocyte-dependent mechanisms such as modulating the interactions among lymphocytes and between lymphocytes and endothelial and/or antigen-presenting cells.
Supplementary Material
Acknowledgments
We thank Mrs. M.J. Fouque, P. Guillou, and M. Larribe for technical and secretarial assistance.
Footnotes
Address reprint requests to F. Bayard, INSERM U589, IFR31, Institut L. Bugnard, BP 84225, 31432 Toulouse Cédex 4, France. E-mail: bayard@toulouse.inserm.fr.
Supported in part by INSERM, the Ministère de la Recherche et de la Technologie (Université Paul Sabatier), Action Concertée Incitative 2001 and 2003, Association pour la Recherche contre le Cancer, MSD, Theramex Laboratories, European Vascular Genomics Network (grant no.503254), Fondation de France, the Conseil Régional Midi-Pyrénées, and the French Society of Atherosclerosis (to R.E.).
Supplemental material for this article appears on http://ajp.amjpathol.org.
Present address of J.J.: Department of Pharmacology, Jagiellonian University School of Medicine, Grzegorzecka 16, PL 31-531 Krakow, Poland.
References
- Waters DD, Gordon D, Rossouw JE, Cannon RO, III, Collins P, Herrington DM, Hsia J, Langer R, Mosca L, Ouyang P, Sopko G, Stefanick ML. Women’s ischemic syndrome evaluation: current status and future research directions: report of the National Heart, Lung and Blood Institute Workshop: October 2–4, 2002: section 4: lessons from hormone replacement trials. Circulation. 2004;109:e53–e55. doi: 10.1161/01.CIR.0000116209.25597.AE. [DOI] [PubMed] [Google Scholar]
- Hulley S, Grady D, Bush T, Furberg C, Herrington D, Riggs B, Vittinghoff E. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. Heart and Estrogen/Progestin Replacement Study (HERS) Research Group. JAMA. 1998;280:605–613. doi: 10.1001/jama.280.7.605. [DOI] [PubMed] [Google Scholar]
- Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC, Kotchen JM, Ockene J. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- Arnal JF, Gourdy P, Elhage R, Garmy-Susini B, Delmas E, Brouchet L, Castano C, Barreira Y, Couloumiers JC, Prats H, Prats AC, Bayard F. Estrogens and atherosclerosis. Eur J Endocrinol. 2004;150:113–117. doi: 10.1530/eje.0.1500113. [DOI] [PubMed] [Google Scholar]
- Hodgin JB, Maeda N. Minireview: estrogen and mouse models of atherosclerosis. Endocrinology. 2002;143:4495–4501. doi: 10.1210/en.2002-220844. [DOI] [PubMed] [Google Scholar]
- Ross R. Atherosclerosis—an inflammatory disease. N Engl J Med. 1999;340:115–125. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- Hansson GK, Libby P, Schonbeck U, Yan ZQ. Innate and adaptive immunity in the pathogenesis of atherosclerosis. Circ Res. 2002;91:281–291. doi: 10.1161/01.res.0000029784.15893.10. [DOI] [PubMed] [Google Scholar]
- Binder CJ, Chang MK, Shaw PX, Miller YI, Hartvigsen K, Dewan A, Witztum JL. Innate and acquired immunity in atherogenesis. Nat Med. 2002;8:1218–1226. doi: 10.1038/nm1102-1218. [DOI] [PubMed] [Google Scholar]
- Roselaar SE, Kakkanathu PX, Daugherty A. Lymphocyte populations in atherosclerotic lesions of apoE −/− and LDL receptor−/− mice. Decreasing density with disease progression. Arterioscler Thromb Vasc Biol. 1996;16:1013–1018. doi: 10.1161/01.atv.16.8.1013. [DOI] [PubMed] [Google Scholar]
- Song L, Leung C, Schindler C. Lymphocytes are important in early atherosclerosis. J Clin Invest. 2001;108:251–259. doi: 10.1172/JCI11380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reardon CA, Blachowicz L, White T, Cabana V, Wang Y, Lukens J, Bluestone J, Getz GS. Effect of immune deficiency on lipoproteins and atherosclerosis in male apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2001;21:1011–1016. doi: 10.1161/01.atv.21.6.1011. [DOI] [PubMed] [Google Scholar]
- Elhage R, Gourdy P, Brouchet L, Jawien J, Fouque M-J, Fiévet C, Huc X, Barreira Y, Couloumiers J-C, Arnal J-F, Bayard F. Deleting TCRaβ+ or CD4+ T lymphocytes leads to opposite effects on site-specific atherosclerosis in female apolipoprotein E-deficient mice. Am J Pathol. 2004;165:2013–2019. doi: 10.1016/s0002-9440(10)63252-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Nicoletti A, Elhage R, Hansson GK. Transfer of CD4(+) T cells aggravates atherosclerosis in immunodeficient apolipoprotein E knockout mice. Circulation. 2000;102:2919–2922. doi: 10.1161/01.cir.102.24.2919. [DOI] [PubMed] [Google Scholar]
- Elhage R, Clamens S, Reardon-Alulis C, Getz GS, Fievet C, Maret A, Arnal JF, Bayard F. Loss of atheroprotective effect of estradiol in immunodeficient mice. Endocrinology. 2000;141:462–465. doi: 10.1210/endo.141.1.7377. [DOI] [PubMed] [Google Scholar]
- Elhage R, Arnal JF, Pierragi M-T, Duverger N, Fiévet C, Faye JC, Bayard F. Estradiol-17β prevents fatty streak formation in apolipoprotein E-deficient mice. Arterioscl Thromb Vasc Biol. 1997;17:2679–2684. doi: 10.1161/01.atv.17.11.2679. [DOI] [PubMed] [Google Scholar]
- Ishibashi S, Goldstein JL, Brown MS, Herz J, Burns DK. Massive xanthomatosis and atherosclerosis in cholesterol-fed low density lipoprotein receptor-negative mice. J Clin Invest. 1994;93:1885–1893. doi: 10.1172/JCI117179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinkai Y, Rathbun G, Lam KP, Oltz EM, Stewart V, Mendelsohn M, Charron J, Datta M, Young F, Stall AM, Alt FW. RAG-2-deficient mice lack mature lymphocytes owing to inability to initiate V(D)J rearrangement. Cell. 1992;68:855–867. doi: 10.1016/0092-8674(92)90029-c. [DOI] [PubMed] [Google Scholar]
- Kitamura D, Roes J, Kuhn R, Rajewsky K. A B cell-deficient mouse by targeted disruption of the membrane exon of the immunoglobulin mu chain gene. Nature. 1991;350:423–426. doi: 10.1038/350423a0. [DOI] [PubMed] [Google Scholar]
- Duez H, Chao YS, Hernandez M, Torpier G, Poulain P, Mundt S, Mallat Z, Teissier E, Burton CA, Tedgui A, Fruchart JC, Fievet C, Wright SD, Staels B. Reduction of atherosclerosis by the peroxisome proliferator-activated receptor alpha agonist fenofibrate in mice. J Biol Chem. 2002;277:48051–48057. doi: 10.1074/jbc.M206966200. [DOI] [PubMed] [Google Scholar]
- Marsh MM, Walker VR, Curtiss LK, Banka CL. Protection against atherosclerosis by estrogen is independent of plasma cholesterol levels in LDL receptor-deficient mice. J Lipid Res. 1999;40:893–900. [PubMed] [Google Scholar]
- Bourassa P-A, Milos PM, Gaynor BJ, Breslow JL, Aiello RJ. Estrogen reduces atherosclerotic lesion development in apolipoprotein E-deficient mice. Proc Natl Acad Sci USA. 1996;93:10022–10027. doi: 10.1073/pnas.93.19.10022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shedlock DJ, Whitmire JK, Tan J, MacDonald AS, Ahmed R, Shen H. Role of CD4 T cell help and costimulation in CD8 T cell responses during Listeria monocytogenes infection. J Immunol. 2003;170:2053–2063. doi: 10.4049/jimmunol.170.4.2053. [DOI] [PubMed] [Google Scholar]
- Shevach EM, McHugh RS, Piccirillo CA, Thornton AM. Control of T-cell activation by CD4+ CD25+ suppressor T cells. Immunol Rev. 2001;182:58–67. doi: 10.1034/j.1600-065x.2001.1820104.x. [DOI] [PubMed] [Google Scholar]
- Roncarolo MG, Bacchetta R, Bordignon C, Narula S, Levings MK. Type 1 T regulatory cells. Immunol Rev. 2001;182:68–79. doi: 10.1034/j.1600-065x.2001.1820105.x. [DOI] [PubMed] [Google Scholar]
- Matejuk A, Bakke AC, Hopke C, Dwyer J, Vandenbark AA, Offner H. Estrogen treatment induces a novel population of regulatory cells, which suppresses experimental autoimmune encephalomyelitis. J Neurosci Res. 2004;77:119–126. doi: 10.1002/jnr.20145. [DOI] [PubMed] [Google Scholar]
- Polanczyk MJ, Carson BD, Subramanian S, Afentoulis M, Vandenbark AA, Ziegler SF, Offner H. Cutting edge: estrogen drives expansion of the CD4+CD25+ regulatory T cell compartment. J Immunol. 2004;173:2227–2230. doi: 10.4049/jimmunol.173.4.2227. [DOI] [PubMed] [Google Scholar]
- Caligiuri G, Nicoletti A, Poirier B, Hansson GK. Protective immunity against atherosclerosis carried by B cells of hypercholesterolemic mice. J Clin Invest. 2002;109:745–753. doi: 10.1172/JCI07272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verthelyi DI, Ahmed SA. Estrogen increases the number of plasma cells and enhances their autoantibody production in nonautoimmune C57BL/6 mice. Cell Immunol. 1998;189:125–134. doi: 10.1006/cimm.1998.1372. [DOI] [PubMed] [Google Scholar]
- Horkko S, Bird DA, Miller E, Itabe H, Leitinger N, Subbanagounder G, Berliner JA, Friedman P, Dennis EA, Curtiss LK, Palinski W, Witztum JL. Monoclonal autoantibodies specific for oxidized phospholipids or oxidized phospholipid-protein adducts inhibit macrophage uptake of oxidized low-density lipoproteins. J Clin Invest. 1999;103:117–128. doi: 10.1172/JCI4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Major AS, Fazio S, Linton MF. B-lymphocyte deficiency increases atherosclerosis in LDL receptor-null mice. Arterioscler Thromb Vasc Biol. 2002;22:1892–1898. doi: 10.1161/01.atv.0000039169.47943.ee. [DOI] [PubMed] [Google Scholar]
- Holm P, Korsgaard N, Shalmi M, Andersen HL, Hougaard P, Skouby SO, Stender S. Significant reduction of the antiatherogenic effect of estrogen by long-term inhibition of nitric oxide synthesis in cholesterol-clamped rabbits. J Clin Invest. 1997;100:821–828. doi: 10.1172/JCI119597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams MR, Kaplan JR, Manuck SB, Koritnik DR, Parks JS, Wolfe MS, Clarkson TB. Inhibition of coronary artery atherosclerosis by 17-beta estradiol in ovariectomized monkeys. Lack of an effect of added progesterone. Arteriosclerosis. 1990;10:1051–1057. doi: 10.1161/01.atv.10.6.1051. [DOI] [PubMed] [Google Scholar]
- Medina KL, Garrett KP, Thompson LF, Rossi MI, Payne KJ, Kincade PW. Identification of very early lymphoid precursors in bone marrow and their regulation by estrogen. Nat Immunol. 2001;2:718–724. doi: 10.1038/90659. [DOI] [PubMed] [Google Scholar]
- Okasha SA, Ryu S, Do Y, McKallip RJ, Nagarkatti M, Nagarkatti PS. Evidence for estradiol-induced apoptosis and dysregulated T cell maturation in the thymus. Toxicology. 2001;163:49–62. doi: 10.1016/s0300-483x(01)00374-2. [DOI] [PubMed] [Google Scholar]
- Hodgin JB, Krege JH, Reddick RL, Korach KS, Smithies O, Maeda N. Estrogen receptor alpha is a major mediator of 17beta-estradiol’s atheroprotective effects on lesion size in ApoE−/− mice. J Clin Invest. 2001;107:333–340. doi: 10.1172/JCI11320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi W, Haberland ME, Jien ML, Shih DM, Lusis AJ. Endothelial responses to oxidized lipoproteins determine genetic susceptibility to atherosclerosis in mice. Circulation. 2000;102:75–81. doi: 10.1161/01.cir.102.1.75. [DOI] [PubMed] [Google Scholar]
- Shi W, Wang NJ, Shih DM, Sun VZ, Wang X, Lusis AJ. Determinants of atherosclerosis susceptibility in the C3H and C57BL/6 mouse model: evidence for involvement of endothelial cells but not blood cells or cholesterol metabolism. Circ Res. 2000;86:1078–1084. doi: 10.1161/01.res.86.10.1078. [DOI] [PubMed] [Google Scholar]
- Gupta S, Pablo AM, Jiang X, Wang N, Tall AR, Schindler C. IFN-gamma potentiates atherosclerosis in ApoE knock-out mice. J Clin Invest. 1997;99:2752–2761. doi: 10.1172/JCI119465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee TS, Yen HC, Pan CC, Chau LY. The role of interleukin 12 in the development of atherosclerosis in ApoE-deficient mice. Arterioscler Thromb Vasc Biol. 1999;19:734–742. doi: 10.1161/01.atv.19.3.734. [DOI] [PubMed] [Google Scholar]
- Elhage R, Jawien J, Rudling M, Ljunggren HG, Takeda K, Akira S, Bayard F, Hansson GK. Reduced atherosclerosis in interleukin-18 deficient apolipoprotein E-knockout mice. Cardiovasc Res. 2003;59:234–240. doi: 10.1016/s0008-6363(03)00343-2. [DOI] [PubMed] [Google Scholar]
- Mallat Z, Besnard S, Duriez M, Deleuze V, Emmanuel F, Bureau MF, Soubrier F, Esposito B, Duez H, Fievet C, Staels B, Duverger N, Scherman D, Tedgui A. Protective role of interleukin-10 in atherosclerosis. Circ Res. 1999;85:e17–e24. doi: 10.1161/01.res.85.8.e17. [DOI] [PubMed] [Google Scholar]
- Robertson AKL, Rudling M, Zhou X, Gorelik L, Flavell RA, Hansson GK. Disruption of TGF-(beta) signaling in T cells accelerates atherosclerosis. J Clin Invest. 2003;112:1342–1350. doi: 10.1172/JCI18607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cenci S, Toraldo G, Weitzmann MN, Roggia C, Gao Y, Qian WP, Sierra O, Pacifici R. Estrogen deficiency induces bone loss by increasing T cell proliferation and lifespan through IFN-gamma-induced class II transactivator. Proc Natl Acad Sci USA. 2003;100:10405–10410. doi: 10.1073/pnas.1533207100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maret A, Coudert JD, Garidou L, Foucras G, Gourdy P, Krust A, Dupont S, Chambon P, Druet P, Bayard F, Guery JC. Estradiol enhances primary antigen-specific CD4 T cell responses and Th1 development in vivo. Essential role of estrogen receptor alpha expression in hematopoietic cells. Eur J Immunol. 2003;33:512–521. doi: 10.1002/immu.200310027. [DOI] [PubMed] [Google Scholar]
- Gourdy P, Araujo LM, Zhu R, Garmy-Susini B, Diem S, Laurell H, Leite-De-Moraes M, Dy M, Arnal JF, Bayard F, Herbelin A. Relevance of sexual dimorphism to regulatory T cells: estradiol promotes IFN-γ production by invariant natural killer T cells. Blood. 2005;105:2415–2420. doi: 10.1182/blood-2004-07-2819. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.