Abstract
How human cytomegalovirus (CMV) reaches the fetus across the placenta is unknown. The major viral cause of congenital disease, CMV infects the uterine-placental interface with varied outcomes depending on the strength of maternal humoral immunity and gestational age. Covering the surface of villi that float in blood, syncytiotrophoblasts express the neonatal Fc receptor (FcRn) that transports IgG for passive immunity. Immunohistochemical analysis of early-gestation biopsy specimens showed an unusual pattern of CMV replication proteins in underlying villus cytotrophoblasts, whereas syncytiotrophoblasts were spared. Found in placentas with low to moderate CMV-neutralizing antibody titers, this pattern suggested virion transcytosis across the surface. In contrast, syncytiotrophoblasts from placentas with high neutralizing titers contained viral DNA and caveolin-1-positive vesicles in which IgG and CMV glycoprotein B co-localized. In villus explants, IgG-virion transcytosis and macrophage uptake were blocked with trypsin-treatment and soluble protein A. Quantitative analysis in polarized epithelial cells showed that FcRn-mediated transcytosis was blocked by the Fc fragment of IgG, but not F(ab′)2. Our results suggest that CMV virions could disseminate to the placenta by co-opting the receptor-mediated transport pathway for IgG. These findings could explain the efficacy of hyperimmune IgG for treatment of primary CMV infection during gestation and support vaccination.
Although the human placenta functions as a barrier to microorganisms, certain viruses that disseminate in blood, such as human cytomegalovirus (CMV), can be transmitted from the maternal to the fetal compartment. CMV is a ubiquitous virus that infects much of the adult population, causing asymptomatic infections in healthy persons. After a viremic period in primary infection, latency is established in granulocyte-macrophage progenitor cells.1 Development of neutralizing antibodies correlates with clearance of circulating viral DNA and proteins and reduces the chance of fetal infection.2,3 CMV is the leading cause of congenital infection and brain disease in children, with an incidence in the United States of ∼1% of live births.4,5 In 40% of pregnancies complicated by primary CMV infection, virus is transmitted to the fetus. In contrast, reactivation of infection in the mother leads to fetal infection in only 2% of cases. Symptomatic infants die in the neonatal period (12%), and most survivors have permanent, debilitating sequelae, including mental retardation, vision loss, and sensorineural deafness.6 Birth defects from congenital CMV infection depend on maternal neutralizing antibody titers, gestational age,7,8 and the time between primary infection and conception.9 Fetal damage is more severe when infection occurs during the first half of gestation, but the risk of virus transmission is present throughout pregnancy.8 Detection of antibodies with low avidity (ie, poor neutralizing activity) to CMV glycoprotein B (gB), the major neutralizing antigen on virions,10 predicts congenital infection, but the means by which virus is transmitted to the fetus is unknown.
The human placenta has a specialized architecture composed of villi that attach the fetus to the uterus (anchoring villi) and villi that float in maternal blood (floating villi).11,12 The mechanics of supplying maternal blood to the embryo is accomplished by cytotrophoblasts, which are specialized epithelial cells of the placenta. In a stepwise process, these cells leave the basement membrane and differentiate along two independent pathways, depending on their location, to initiate blood flow to the placenta. In the first pathway, cytotrophoblasts fuse into a multinucleate syncytial covering attached at one end to the tree-like fetal portion of the placenta. The syncytiotrophoblast, specialized for exchange of nutrients and waste between maternal and fetal compartments, expresses the neonatal Fc receptor (FcRn), which binds maternal IgG and transcytoses it for passive immunity.13,14 The rest of the villus floats in a stream of maternal blood, which optimizes exchange of substances between the mother and the fetus across the placenta. In the second pathway that gives rise to anchoring villi, cytotrophoblasts aggregate into columns of nonpolarized mononuclear cells that attach to and penetrate the uterine wall. The ends of the columns terminate within the superficial endometrium and give rise to invasive cytotrophoblasts. A subset of these cells, either individually or in clusters, commingle with resident decidual and immune cells. During endovascular invasion, masses of cytotrophoblasts open the termini of uterine arteries and migrate into the vessels, thereby diverting blood flow to the placenta. Together, the two components of cytotrophoblast invasion anchor the placenta to the uterus and permit a steady increase in the supply of maternal blood that is delivered to the developing fetus.
In human pregnancies, patterns of CMV proteins in biopsy specimens from early gestation show that uterine infection spreads to floating and anchoring villi via different routes.15 In the maternal compartment, CMV replicates in the uterine vasculature, glandular epithelium, and stromal fibroblasts in the decidualized endometrium.16 In the placental compartment, the extent of infection is inversely proportional to the level of maternal neutralizing IgG and co-infections.16,17 We also observed an enigmatic pattern of viral infected cell proteins in clusters of underlying cytotrophoblast progenitor cells, whereas the syncytiotrophoblast was spared in placentas with low to moderate CMV-neutralizing antibody titers.16,18 This pattern suggested virions were transported across the surface and infected villus cytotrophoblasts below. Inexplicably, placentas from mothers with strong humoral immunity to CMV (high-avidity IgG) were not infected, but syncytiotrophoblasts contained nucleocapsids.16 Co-localization of IgG and gB suggested retention of immune complexes in vesicular compartments proximal to the microvillus surface in contact with blood. Here we report CMV virions co-opt FcRn-mediated transcytosis and are transported across syncytiotrophoblasts in immune complexes that infect underlying cytotrophoblasts and are captured by macrophages in the villus core.
Materials and Methods
Chorionic Villi Isolation, Explant Cultures, and Tissue Biopsies
First- and second-trimester placentas were obtained from women with normal pregnancies before elective termination for nonmedical reasons. Approval for this project was obtained from the Institutional Review Board of the University of California, San Francisco. Biopsy specimens were analyzed from 45 first-trimester placentas, chorionic villi were dissected, and explants (10 to 20 tree-like villi) were established.18 Dissected villi (5 to 10 mg) were cultured on 12-mm Millicell-CM inserts with 0.4-μm pores (Millipore Inc., Bedford, MA) coated with 100 μl of Matrigel (BD Discovery Lab Inc, Bedford, MA) in 24-well culture dishes.
Cells and Virus
T-84 cells (human colon carcinoma) were a gift from K. Barrett, University of California, San Diego. Cells (2 × 105 cells/ml) were seeded onto semipermeable polycarbonate Transwell filters (6.5 or 12 mm, 3-μm pore size) (Corning Inc., Corning, NY) coated with 10 μg/cm2 of mouse laminin (Sigma-Aldrich Co., St. Louis, MO) and cultured until polarized. Genetic content of laboratory strain AD16919 and clinical strain Toledo20 was published. Construction and properties of the CMV strain Toledo expressing the enhanced green fluorescent protein (EGFP) gene, Toledo β2.7 EGFP, have been reported.21 Briefly, the EGFP gene insertion in plasmid pRC2.7 EGFP was located between the transcription start site and the first open reading frame in the Toledo TRL4 RNA transcript. Filtered and immune-precipitated EGFP-Toledo virions were visualized by immunofluorescence, indicating EGFP-labeled TRL4 transcripts could be packaged in virions.22,23 Villus explants were infected with CMV strains AD169, Toledo, and Toledo-EGFP (5 to 10 × 105 PFU) in serum-free media. After 2 hours of incubation, explants were washed with phosphate-buffered saline and fresh culture medium was added.
Serological Reagents
Murine monoclonal antibodies reacted with CMV immediate-early (IE1&2) proteins (CH160) and gB (CH28).24,25 Guinea pig antiserum to gB was a gift from Chiron Corp. (Emeryville, CA). Rat anti-human cytokeratin antibody (7D3),26 mouse anti-human placental lactogen antibodies, and sheep antiserum to human transferrin receptor were obtained from Serotec Ltd. (Raleigh, NC). Rabbit antiserum to Rab5 was from Quality Controlled Biochemicals (Hopkinton, MA); to Rab11, from Zymed (South San Francisco, CA); and to caveolin-1 from Transduction Laboratories, Lexington, KY. Antibodies to EGFR and isotype controls were purchased from BD Biosciences (San Diego, CA). Rabbit antiserum to FcRn was a generous gift from Neil Simister (Brandeis University, Waltham, MA).27 Goat anti-human IgG and fluorescein isothiocyanate (FITC)-conjugated Affini Pure F(ab′)2 fragment were obtained from Jackson ImmunoResearch, West Grove, PA. Secondary antibodies were goat anti-mouse IgG labeled with FITC or horseradish peroxidase, goat anti-rat IgG labeled with tetramethyl rhodamine isothiocyanate (TRITC), goat anti-guinea pig IgG labeled with TRITC, goat anti-rabbit IgG labeled with FITC, and goat anti-human IgG labeled with FITC, TRITC, or horseradish peroxidase. Nuclei were counterstained with TO-PRO-3 iodide (Molecular Probes, Eugene, OR).
Immunofluorescence and Immunoblotting Assays
The villus explants and placental biopsy specimens were processed for immunohistochemistry as described.18 Briefly, placental tissues were fixed in 3% paraformaldehyde, infiltrated with 5 to 15% sucrose followed by embedding in optimal-cutting temperature compound (OCT), and frozen in liquid nitrogen. Polarized T-84 cells were fixed with 3% paraformaldehyde in phosphate-buffered saline (PBS) (15 minutes) and permeabilized with 0.1% Triton X-100 in PBS (5 minutes). For double labeling, cells were simultaneously incubated with primary antibodies from different species and secondary antibodies labeled with FITC or TRITC. For surface protein staining the primary antibodies were added to live cells and incubated on ice for 30 minutes. Then cells were washed with ice cold PBS and fixed with 3% paraformaldehyde in PBS (5 minutes). Tissue sections and cells were analyzed with a MRC1024 confocal OptiPhot II Nikon microscope using Comos software (Bio-Rad, Hercules, CA). Data analysis was performed using NIH Image and Adobe Photoshop software. For immunoblot assays, lysates of CMV-infected fibroblasts were separated using denaturing 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and reacted with purified maternal IgG (see below). Bands were visualized using enhanced chemiluminescence (GE HealthCare Biosciences Corp., Piscataway, NJ). Internalized and transcytosed IgG-virion complexes were precipitated from cell lysates and output media, respectively, using protein A beads (Sigma-Aldrich Co., St. Louis, MO). The complexes were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and electrotransferred to nitrocellulose. Blots were probed with mouse monoclonal antibody (mAb) CH28 to CMV gB followed by secondary specific antibodies conjugated with horseradish peroxidase. Donkey anti-human horseradish peroxidase-conjugated IgG was used to detect IgG.
Fluorescence in Situ Hybridization (FISH)
Sections (5 to 7 μm) were cut from frozen blocks of tissue fixed as described above on a Hacker-Slee cryostat and collected on silane-coated glass slides. DNA-DNA hybridization was performed using the DNA Detector FISH kit (KPL, Inc., Gaithersburg, MD). YOYO-1-iodide (Molecular Probes) was used for nuclear counterstaining. CMV probe, EcoRI-A, -B, -C, -D, and -E fragments of AD169 (a gift from Deborah Spector, University of California, San Diego, La Jolla, CA28 ) was labeled with biotin-16-dUTP using a nick-translation kit (Roche Diagnostic Inc., Indianapolis, IN).29 Briefly, slides were heated for 3 hours at 65°C and digested with 0.2% pepsin (Sigma-Aldrich Co.) in 0.2 mol/L HCl for 5 minutes at 37°C. Target DNA was denatured in 70% formamide in 2× standard saline citrate for 2 minutes at 70°C before hybridization overnight at 37°C.
Neutralization Assays
Maternal IgG was purified from conditioned medium from villus explants (35 to 70 μg/g tissue) using the ImmunoPure IgG purification kit (Pierce, Rockford, IL). Subclasses were determined using the human IgG subclass profile ELISA kit (Zymed). Virus was incubated with 100 μg/ml of purified IgG, cells were infected, and those expressing CMV IE1&2 were quantified in a rapid infectivity assay.30 Titers were calculated as percent neutralization compared with positive and negative controls.16
Blocking Transcytosis in Villus Explants
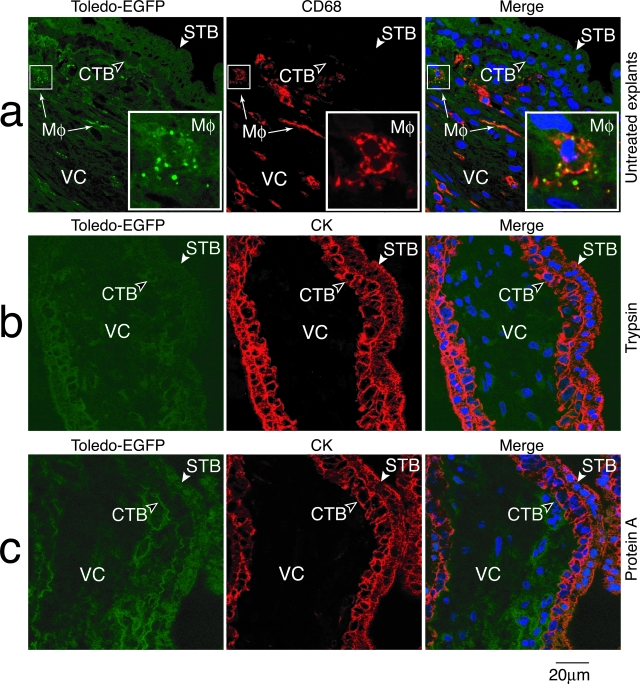
Experiments to block transcytosis by treating villus explants with trypsin and protein A were performed 12 hours after culture. First, villus explants were washed three times with ice-cold serum-free medium containing 20 mmol/L HEPES and incubated with trypsin (1 mg/ml, Sigma-Aldrich Co.) on ice for 30 minutes. Then trypsin was immediately neutralized with 0.5 mg/ml of trypsin inhibitor (Sigma-Aldrich Co.). The explants were washed with ice-cold serum-free medium and Toledo-EGFP (5 × 105 PFU) virions alone or complexed with placenta-associated IgG were added. After 30 minutes adsorption on ice, the explants were washed and incubated in medium containing 10% fetal bovine serum for 60 minutes at 37°C. Untreated control explants were incubated with serum-free medium and subjected to the same handling. In the second set of experiments, villus explants were washed with serum-free medium and incubated for 60 minutes with soluble protein A (10 μg/ml) at 37°C. The medium was aspirated, a fresh mixture containing Toledo-EGFP (5 × 105 PFU) and protein A was added and explants incubated for 60 minutes at 37°C. For untreated controls, explants were incubated with the same titer of Toledo-EGFP.
Transcytosis Assays and Blocking in Polarized Epithelial Cells
Transcellular resistance of T-84 intestinal epithelial cells grown on Transwell filters was monitored using a Millicell-ERS voltohmmeter and reached 1000 to 1200 Ω/cm2 at 10 to 12 days. Polarity was evaluated as described31 by adding horseradish peroxidase-conjugated goat anti-mouse IgG (Fab′)2 (Jackson ImmunoResearch) to the upper filter compartment, and the medium from the lower compartment was assayed photometrically for horseradish peroxidase with o-phenylenediamine dihydrochloride as the substrate. Cells were incubated for 2 hours in serum-free medium buffered to pH 7.4 with 20 mmol/L HEPES (Sigma-Aldrich Co.), washed with ice-cold Hanks’ balanced salt solution (HBSS+) at pH 7.4 with 10 mmol/L HEPES, and cooled for 30 minutes. Before applying IgG-virion complexes, cells were washed with cold 1% bovine serum albumin in HBSS+, pH 6.0 (HBSS+ buffered with 10 mmol/L MES), on the apical (input) surface and with cold 1% bovine serum albumin in HBSS+, pH 7.4 (HBSS+ buffered with 10 mmol/L HEPES), on the opposite (output) surface. IgG-virion complexes were formed by mixing CMV Toledo-EGFP virions, strain AD169 or Toledo (5 × 105 PFU) with human IgG (100 μg/ml) at 37°C for 1 hour. In some experiments, soluble protein A (10 μg/ml) was added to the complexes before applying to cells. IgG-virion complexes in cold 1% bovine serum albumin in HBSS+, pH 6.0, were added to the input surface on ice for 30 minutes for FcRn binding and then incubated at 37°C for 60 to 120 minutes during transcytosis. In some experiments, human IgG Fc fragments or chicken IgY Fc fragments (Jackson ImmunoResearch) in cold 1% bovine serum albumin in HBSS+, pH 6.0, were added to the input surface (100 μg/ml) for 30 minutes. After transcytosis, the media from input and output chambers were collected for polymerase chain reaction (PCR), infectivity, immune precipitation, and immunoblot assays.
Quantitative PCR
QIAamp DNA mini kit (Qiagen Inc., Valencia, CA) was used to extract DNA from media. Products were analyzed using real-time quantitative PCR and TaqMan probes. Primers and probes were designed for the UL83 gene of CMV using Primer Express (Applied Biosystems, Foster City, CA). Forward primer (TGGA-CCTGCGTACCAACATAGA), reverse primer (GCGGAGATTTGTTCTCCTGAAA), probe (CCGGCCCTC-GGTTCTCTGCTG). Primers were manufactured by Qiagen, and FAM/TAMRA-labeled probes by Biosearch Technologies (Novato, CA). Real-time PCR was done in duplicate using the Applied Biosystems 9700HT.
Results
CMV Infects Villus Cytotrophoblasts Underlying Syncytiotrophoblasts
Using immunohistochemistry, we studied the pattern of CMV proteins in chorionic villi of placentas infected in utero. The cytotrophoblasts stained for cytokeratin and the CMV proteins IE1&2, indicating viral infection. Representative placentas showed a remarkable staining pattern (Figure 1a). The syncytiotrophoblast covering the surface was spared whereas underlying villus cytotrophoblasts were susceptible, as indicated by expression of CMV infected-cell proteins IE1&2 in the nuclei. The staining pattern in eight villus explants infected in vitro was remarkably similar to that of natural infection (Figure 1b). Again, we observed CMV IE1&2 protein-positive nuclear staining of isolated clusters of cytotrophoblasts. These patterns suggested that some internalized virions are transported across the syncytiotrophoblast and infect cytotrophoblasts—the earliest steps in transmission.
Figure 1.
CMV infects underlying villus cytotrophoblasts in chorionic floating villi and villus explants. Immunohistological sections of chorionic villi infected with CMV in utero (a) and a villus explant infected in vitro (b). Tissue sections were co-stained for CMV immediate-early proteins (IE) 1 and 2 and cytokeratin, a marker of cytotrophoblasts. STB, syncytiotrophoblast; CTB, villus cytotrophoblasts; VC, villus core.
Vesicles Containing IgG and CMV gB Present Under Microvilli
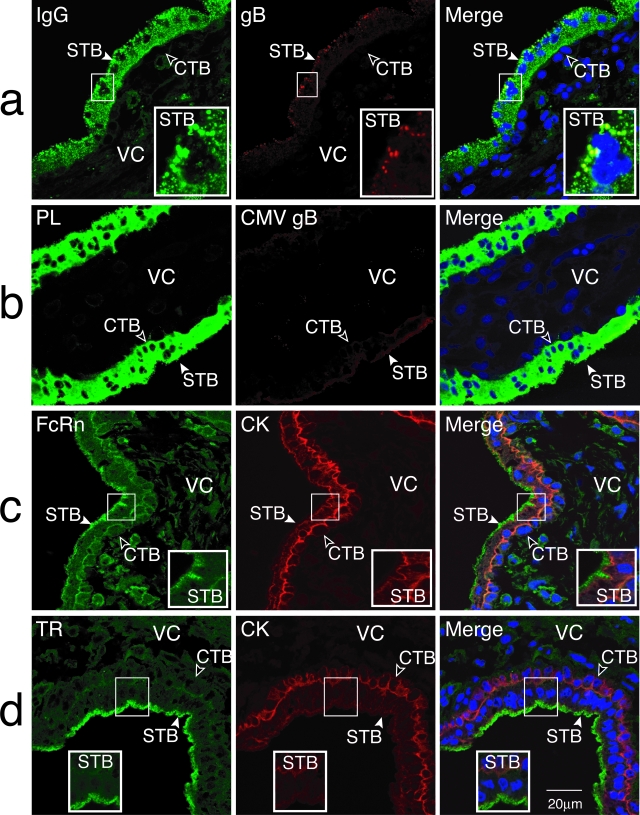
While examining 23 paired decidual and placental biopsy specimens from healthy early-gestation pregnancies, using confocal immunohistochemistry, we often observed a vesicular staining pattern for virion gB in the syncytiotrophoblast that correlated with viral replication in the decidua.16 Frequently, maternal IgG and CMV gB co-localized in large vesicles near the microvillus surface without viral replication (Figure 2a) in donors with moderate to high neutralizing antibody activity. The vesicular pattern, co-stained IgG and gB, contrasted with the homogeneous cytoplasmic staining of placental lactogen, a syncytium-specific protein (Figure 2b). When sections were stained with rabbit antisera to FcRn and a rat monoclonal antibody to cytokeratin, FcRn localized in apical microvilli of the syncytiotrophoblast that covers villus cytotrophoblasts immunostained with cytokeratin, an epithelial cell marker (Figure 2c). Similar staining was seen in 12 placentas. Controls included staining of adjacent sections in parallel with rabbit preimmune serum (data not shown). Localization of the transferrin receptor, a membrane transport protein, was comparable to that of FcRn on the apical surface (Figure 2d).
Figure 2.
Maternal IgG in the syncytiotrophoblast (STB) from early-gestation placentas co-localizes with CMV glycoprotein B (gB) in vesicles without viral replication. Placental biopsy specimens shown are from immune donors with high neutralizing antibody titers. Tissue sections were co-stained for human IgG and gB (a), placental lactogen (PL) and CMV gB (b), FcRn and cytokeratin (CK) (c), and transferrin receptor (TR) and CK (d). Insets show the staining pattern in the syncytiotrophoblast surface that contacts maternal blood. Nuclei counterstained with TO-PRO-3 iodide. CTB, cytotrophoblasts; VC, villus core.
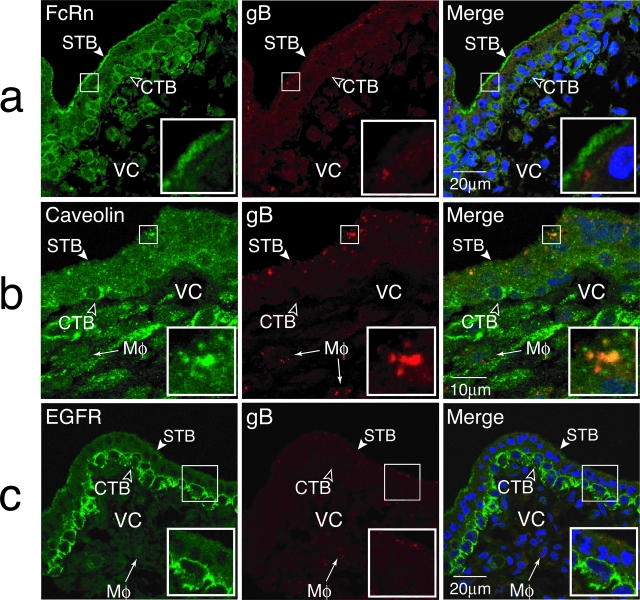
When chorionic villi were co-stained for FcRn and CMV gB, the virion protein was detected in vesicles beneath FcRn-containing microvilli that sometimes co-stained (Figure 3a). To identify vesicles containing IgG-CMV gB complexes, we immunostained sections with antibodies to proteins associated with vesicular trafficking and clathrin- and caveolin-mediated endocytosis. Small gB-containing vesicles weakly co-stained with the GTPases Rab5 and Rab11, which regulate trafficking of endosomes and recycling vesicles, suggesting transient associations (not shown). In contrast, caveolin-1 showed strong co-localization with CMV virion gB-containing vesicular compartments (0.5 to 2 μm in size) located throughout the apical compartment of the syncytiotrophoblast (Figure 3b). In addition, caveolin-1 staining was detected in selected cytotrophoblasts and cells in the villus core. Immunostaining for EGFR, one receptor for CMV virions,32 showed dim staining along the microvillus surface of syncytiotrophoblasts and strong staining in underlying cytotrophoblasts (Figure 3c). This pattern was observed in six placental biopsy specimens. Weak co-staining of EGFR with small gB-containing vesicles was found at the base of these microvilli. Together the results showed that 1) early-gestation placentas express FcRn on microvilli, 2) IgG and CMV gB complexes accumulate in caveolin-1-containing vesicles in syncytiotrophoblasts, and 3) EGFR is expressed in syncytiotrophoblasts and villus cytotrophoblasts. Hence, various cell surface proteins associated with virion entry, endocytosis, and transport of IgG-virion complexes are present at the placenta surface.
Figure 3.
CMV virion gB co-localizes with selected proteins in the syncytiotrophoblast of chorionic villi naturally infected in utero. Tissue sections were co-stained with antibodies to gB and FcRn (a), caveolin-1 (b), and EGF receptor (c). STB, syncytiotrophoblast; CTB, cytotrophoblasts; VC, villus core; Mφ, macrophage.
Floating Chorionic Villi Contain CMV DNA
We previously reported that syncytiotrophoblasts in placentas from seropositive donors contain nucleocapsids proximal to the microvillus surface in the absence of viral replication.16 To ascertain whether the presence of CMV gB correlated with virion-associated DNA, we used FISH to detect viral nucleic acid in biopsy specimens from chorionic villi. Discrete hybridization signals for CMV DNA (red) were found proximal to microvilli and concentrated at the basal membrane of syncytiotrophoblasts (Figure 4, a and b). Some cytotrophoblasts were positive (Figure 4b). Analysis of x-z sections of villi confirmed that signals for viral DNA were cytoplasmic, distinct from nuclei (green) in syncytiotrophoblasts (Figure 4c). In addition, some villus core macrophages near cytotrophoblasts contained punctate signals for CMV DNA (Figure 4, a and b). Parallel sections co-stained with antibodies to CD68 and gB confirmed that selected macrophages contained CMV DNA (data not shown). Similar results were obtained by FISH analysis of six placental biopsy specimens. These findings confirmed the presence of CMV virion DNA in chorionic villi from immune donors without productive infection.
Figure 4.
Chorionic floating villi contain CMV virion DNA without infection. Placental biopsy specimens shown are from immune donors with high neutralizing antibody titers. CMV DNA (red) detected by FISH in syncytiotrophoblast (a and b, inset) and villus core macrophages (a, inset). Nuclei counterstained with YO-YO-1 iodide (green). STB, syncytiotrophoblast; CTB, cytotrophoblasts; VC, villus core, Mφ, macrophages. Sections x-z (a, b) and x-y (c) indicated.
Virion Uptake and Infection in Villus Explants Depend on Neutralizing Activity of Placenta-Derived Maternal IgG
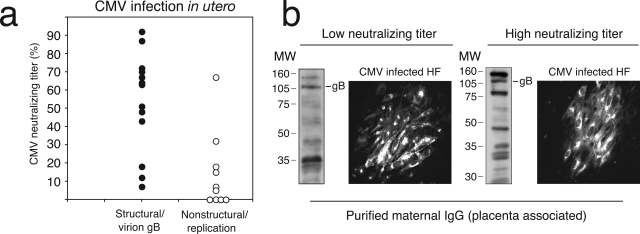
The ease with which CMV could infect cytotrophoblasts purified from early-gestation placentas contrasted sharply with the infrequent infection of chorionic villus explants in vitro (only 7 of 42). To determine whether endogenous maternal antibodies mediated the patterns of infection and protection observed, we evaluated the neutralizing activity of placenta-associated IgG purified from conditioned medium. The samples clustered into groups based on neutralizing activity and the patterns of virion gB and replication proteins in chorionic villi. Chorionic villi from 8 of 13 samples with high titers (>50%) contained CMV virion gB in a vesicular pattern in syncytiotrophoblasts, whereas 8 of 10 samples with low titers (<20%) contained viral replication proteins in cytotrophoblasts (Figure 5a). Although the immunofluorescence reactions of purified IgG could not be distinguished with CMV-infected human fibroblasts, immunoblots showed that the protein profiles recognized by IgG with high and low neutralizing titers differed (Figure 5b). In particular, high neutralizing antibodies reacted strongly with gB and viral replication proteins.
Figure 5.
Neutralizing titers in seropositive women that are associated with patterns of CMV proteins in placentas infected in utero and fibroblasts infected in vitro. a: Neutralizing titer (%) relative to the patterns of CMV structural protein (gB) (filled circles) and nonstructural viral replication proteins (open circles). b: Placenta-associated maternal IgG with high and low neutralizing titers in immunoblot and immunofluorescence reactions with CMV-infected fibroblasts.
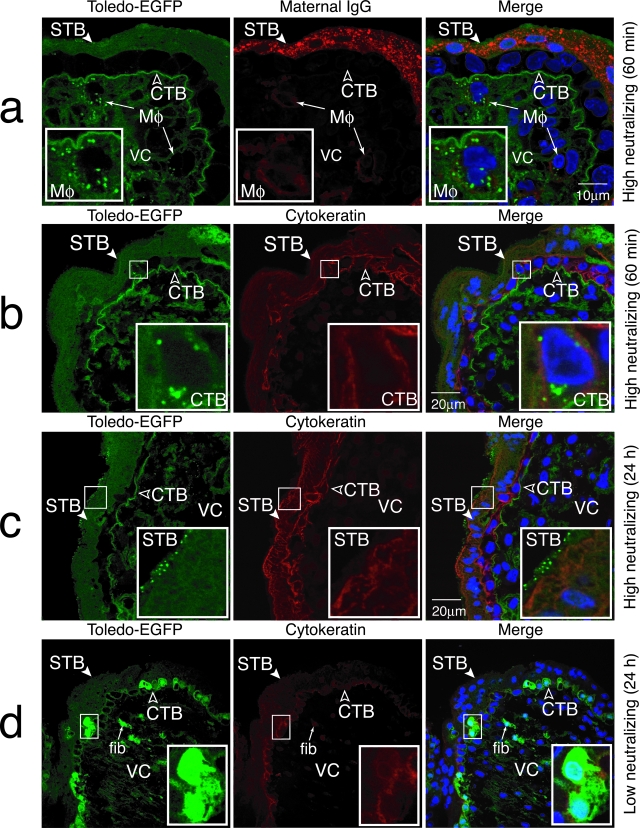
Explants were infected in vitro and then examined for uptake of Toledo-EGFP-labeled virions or productive replication using confocal microscopy. When maternal IgG had high neutralizing activity, CMV failed to replicate, macrophages contained vesicles with Toledo-EGFP-labeled virions by 60 minutes (Figure 6a, inset) and small positive vesicles were detected in microvilli. This pattern suggested that virions rapidly transcytosed across syncytiotrophoblasts were captured by villus core macrophages. The same pattern was seen in explants from donors with a range of neutralizing activity (10 of 11 placentas). Sometimes, isolated cytotrophoblasts contained vesicles with EGFP staining in the cytoplasm, suggesting rapid transcytosis and internalization could occur in 60 minutes without infection (Figure 6b, inset). At 24 hours, villus explants from highly immune donors showed Toledo-EGFP virions accumulated without replication in apical microvilli (Figure 6c, inset). In contrast, an unusual pattern of infection was observed in explants from seropositive women with low or undetectable neutralizing antibodies (Figure 6d, inset). Viral replication in cytotrophoblast progenitors, indicated by strong EGFP expression in infected cells, occurred beneath an intact uninfected syncytiotrophoblast. Stromal fibroblasts in the villus core were also involved (white arrows). This replication pattern was seen in 11 placentas infected in utero, and certain villus explants infected in vitro. The results showed that endogenous maternal IgG protects or promotes cytotrophoblast infection, depending on the strength of maternal immunity.
Figure 6.
Uptake of CMV Toledo-EGFP virions in villus explants and virus replication in underlying villus cytotrophoblasts dependent on neutralizing activity of endogenous maternal IgG. a and b: Placentas containing IgG with high neutralizing activity infected with Toledo-EGFP: virion uptake by villus core macrophages (Hofbauer cells) (a) and villus cytotrophoblasts (b) (60 minutes). c: Toledo-EGFP virions accumulate in microvilli of the syncytiotrophoblast (24 hours). d: Replication of Toledo-EGFP in cytotrophoblasts in villus explants from a donor seropositive for CMV without detectable neutralizing antibodies (24 hours). STB, syncytiotrophoblast; CTB, cytotrophoblasts; VC, villus core; Mφ, macrophages. fib, stromal fibroblasts.
Quantification of placenta-associated neutralizing antibodies showed that samples from seropositive donors had high to low or undetectable levels.16,17 We isolated predominantly IgG1, less IgG3, and trace amounts of IgG2 from conditioned media of villus explants. Virion uptake in syncytiotrophoblasts and capture by villus core macrophages (Figure 6, a–c) occurred predominantly in those placentas with high to moderate neutralizing levels (85 to 35%), whereas infection often took place in conjunction with seropositive samples with very low (<20%) or undetectable neutralizing activity (Figure 6d).
Blocking FcRn-Dependent Transcytosis of IgG-Virion Complexes in Villus Explants
Release of CMV-specific IgG from villus explants and uptake of EGFP-Toledo virions by villus core macrophages, or infection of underlying cytotrophoblasts, suggested FcRn-mediated transcytosis of immune complexes across syncytiotrophoblasts. It was recently reported that FcRn mediates IgG transcytosis across the intestinal epithelial barrier to the mucosal surface, then recycles immune complexes with cognate antigen back across for processing by dendritic cells.33 To determine whether FcRn was involved in uptake of IgG-virion complexes, we modulated transcytosis in villus explants with high neutralizing titers (Figure 7). The explants were briefly treated with ice-cold trypsin to remove all protein receptors on the cell surface. In contrast to untreated explants that contained transcytosed Toledo-EGFP virions captured by villus core macrophages (Figure 7a), trypsin treatment of explants precluded virion uptake (Figure 7b). Anti-cytokeratin staining confirmed the syncytiotrophoblasts were intact. Neither Toledo-EGFP virions alone nor IgG-virion complexes were transcytosed after trypsin treatment. When soluble protein A was added to the villus explants, we failed to detect uptake of EGFP-Toledo by villus core macrophages suggesting the immune complexes failed to bind FcRn and were not transported across the villus surface (Figure 7c). The results were repeated in explants from four different placentas. These findings strongly support a central role for FcRn-mediated transcytosis of IgG-CMV virion complexes across syncytiotrophoblasts to underlying cells, cytotrophoblasts, and villus core macrophages.
Figure 7.
Blocking of FcRn-mediated transcytosis of IgG-virions complexes in villus explants from immune donors. a: Transcytosis of Toledo-EGFP virions (green) and uptake by villus core macrophages stained with CD68 (red) in control untreated explants. Explants briefly treated with trypsin (b), and explants infected in the presence of soluble protein A (c) did not transcytose IgG-virions across syncytiotrophoblasts to villus core macrophages. STB, syncytiotrophoblast; CTB, cytotrophoblasts; VC, villus core; Mφ, macrophages.
Transcytosis of IgG-Virion Complexes in Polarized Cells Expressing FcRn
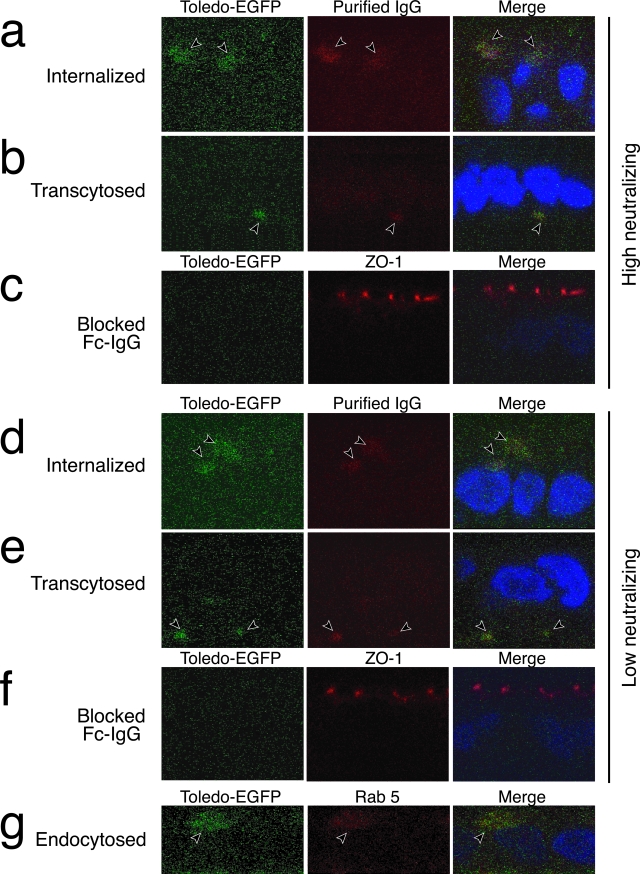
To quantify transcytosis of immune complexes under controlled conditions, we used human intestinal epithelial T-84 cells polarized on permeable filter supports to model transport. T-84 cells endogenously express FcRn, which transcytoses IgG from apical to basolateral cell membranes, releasing it at neutral pH.34,35 The role of human FcRn in trafficking complexes of IgG-Toledo-EGFP virions was evaluated using purified placenta-associated IgG. Virions, mixed with purified IgG of high or low neutralizing activity, were added to the apical filter compartment at low pH. The bound immune complexes were internalized and rapidly transcytosed (60 minutes), as indicated by IgG-virion co-staining in a vesicular pattern below the level of ZO-1 in tight junctions (apical) and below nuclei (basolateral) (Figure 8, a and b). Likewise, immune complexes with low neutralizing activity were internalized and transcytosed (Figure 8, d and e). Virions alone neither infected T-84 cells nor were internalized (data not shown). When cells were pretreated with the Fc fragment of human IgG, internalization and transcytosis were blocked (Figure 8, c and f) showing that FcRn saturation with specific ligand precludes the binding of immune complexes. Next, we immunostained cells with antibodies to proteins associated with early and late endosomes and found that Rab5 co-localized with internalized immune complexes (Figure 8g). These results showed that IgG-virion complexes entered the transcytotic pathway that was FcRn-dependent in polarized T-84 cells.
Figure 8.
FcRn-dependent transcytosis of maternal IgG-virion complexes in polarized T-84 epithelial cells. Immune complexes of Toledo-EGFP virions with purified placenta-associated maternal IgG were applied in the apical compartment, internalized, and transcytosed to the basolateral domain. IgG-virion complexes had high neutralizing activity (a–c) or low neutralizing activity (d–f). Complexes were added to the apical compartment in low pH media and localized at 60 minutes (x-z sections). Toledo-EGFP virions (green) and IgG (red) were internalized into apical endosomes and transcytosed to the basolateral domain below nuclei (blue). Cells pretreated with Fc fragments of human IgG blocked internalization and transcytosis of immune complexes (c, f). g: Co-localization of Toledo-EGFP and Rab5 in apical endosomes. Experiments were repeated four times with similar results.
Human FcRn-Mediated Transcytosis of IgG-Virion Complexes and Infectivity
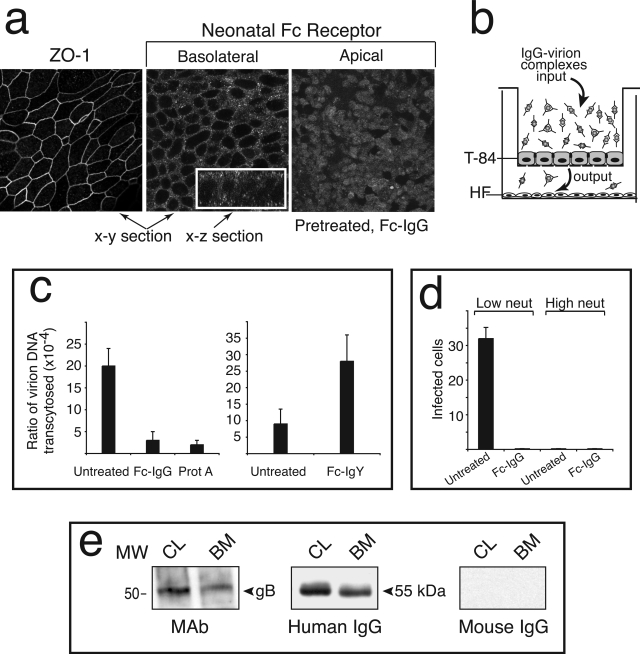
We verified the localization of FcRn in polarized T-84 cells by immunostaining with specific antibodies. Unlike ZO-1, which formed a ring-like pattern, the vesicular staining for FcRn was distributed throughout apical and basolateral membranes (Figure 9a). Addition of Fc-IgG in low-pH medium synchronized receptor transport to the apical membrane in response to ligand (Figure 9a). In experiments to evaluate transcytosis of IgG-virion complexes, we compared the ratio of output to input DNA by quantitative PCR, as described in Materials and Methods. Maternal IgG was isolated from placentas, and immune complexes were formed and added to the upper compartment of polarized cells on filter supports (Figure 9b). Medium from apical and basolateral compartments was removed after 2.5 hours, and the amount of viral DNA was measured. For high-neutralizing IgG-virion complexes, a fraction of input complexes were transcytosed to the basolateral compartment (Figure 9c). A dramatic reduction in transcytosis occurred when cells were pretreated with Fc fragment of IgG and when IgG-virion complexes were pretreated with protein A. In contrast, cells pretreated with the Fc fragment of chicken IgY, which does not bind FcRn, did not reduce transcytosis (Figure 9c). When low-neutralizing IgG-virion complexes were added to T-84 cells, fewer virions were transcytosed, and pretreatment with the Fc fragment blocked transport (data not shown). These results were representative of experiments repeated five times in triplicate. Next, we quantified the infectivity of transcytosed complexes in human foreskin fibroblasts plated in the lower filter chamber or in separate cultures. Only transcytosed IgG-virion complexes with low neutralizing titer were infectious in six experiments (Figure 9d). Pretreatment with the Fc fragment of IgG precluded transcytosis of infectious complexes. Typical immunoblot analysis of immune complexes immunoprecipitated from T-84 cell lysates and the basal medium showed that the complexes were internalized, transcytosed, and released from the basal membrane in three experiments (Figure 9e). Together, the results confirmed the receptor-mediated transcytosis of IgG-virion complexes in polarized epithelial cells expressing human FcRn and the dependence of infectivity on maternal antibodies. IgG with low neutralizing titers isolated from placentas infected in utero promoted focal infection of chorionic villus explants, whereas antibodies from highly immune women was protective.
Figure 9.
Transcytosis of IgG-CMV(AD169) virion complexes in polarized epithelial cells expressing FcRn in vitro. a: Immunostaining of cell-cell tight junctions containing ZO-1 protein and FcRn in apical and basolateral membranes of T-84 cells untreated or pretreated with Fc fragment of human IgG. b: Model for IgG-virion transcytosis in polarized T-84 cells. c: Quantitative analysis of CMV virion DNA in immune complexes transcytosed from the apical to the basal compartment (2.5 hours). Viral DNA was quantified using PCR as described in Materials and Methods. Complexes of purified maternal IgG with high neutralizing activity and AD169 virions were applied to the apical compartment (input). T-84 cells were untreated or pretreated with Fc-IgG or Fc-IgY, and IgG-virion complexes were treated with Staphylococcus protein A (30 minutes on ice). d: Infectivity of transcytosed IgG-virion complexes with low or high neutralizing activity quantified in human foreskin fibroblasts. Experiments were reproduced three times with three replicas each. e: Immunoblot analysis of internalized (CL) and transcytosed (BM) complexes of IgG and virions immunoprecipitated with protein A from cell lysates (CL) and the basal medium (BM).
Discussion
We confirm the early steps of CMV infection in utero and demonstrate for the first time using an in vitro model of chorionic villi and polarized epithelial cells endogenously expressing FcRn that 1) maternal IgG modulates CMV infection in floating chorionic villi, 2) FcRn transcytoses IgG-virion complexes that retain infectivity with low neutralizing antibodies, 3) villus core macrophages capture transcytosed IgG-virions, 4) complexes of IgG and gB accumulate in caveolae, and 5) virion DNA is present in syncytiotrophoblasts as well as some cytotrophoblasts and macrophages without replication. Our original conclusions based on immunohistochemical analysis of naturally infected placentas were strengthened by experimental procedures. We followed virion transport with Toledo-EGFP, detected CMV DNA using FISH, blocked FcRn-mediated transcytosis in villus explants and polarized T-84 cells, and quantified transcytosis in vitro. Receptor-mediated transport and caveolar endocytosis explain the infection patterns in villus cytotrophoblasts and CMV virion gB accumulation in vesicular compartments in utero.16,18 The rapid kinetics of receptor-mediated transport of IgG-virions was similar in syncytiotrophoblasts and polarized T-84 cells: transcytosed immune complexes were detected in cytotrophoblasts, villus core macrophages, or the basal medium by 60 minutes (compare Figures 6a and 7a with Figure 8, a, b and d, e).
FcRn-mediated transcytosis of immune complexes, CMV DNA, and nucleocapsids in the cytoplasm and IgG-gB complexes in caveolae imply different pathways of virion entry in floating villi. Some immune complexes bound to FcRn via IgG could enter the transcytotic pathway crossing syncytiotrophoblasts without de-envelopment. Possibly, IgG virion complexes bind EGFR32 thereby initiating receptor clustering in caveolae.36 Interestingly, CMV gB contains caveolin-1 binding sites (E. Maidji, S. McDonagh and L. Pereira, unpublished) that might favor caveosome formation and accumulation of IgG-gB complexes in caveolar endosomes at neutral pH.37 Storage of these complexes explains the large vesicular compartments in syncytiotrophoblasts containing gB without viral replication (Figure 3b). Why nucleocapsids fail to cause productive infection is puzzling, likely replication could be arrested by the presence of interferons within syncytiotrophoblasts.38,39
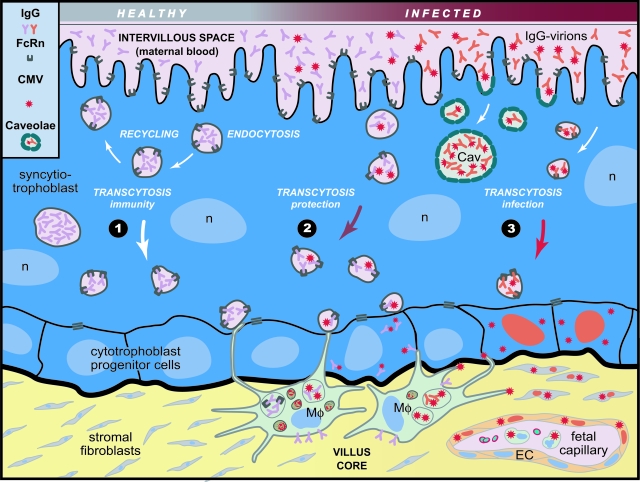
The current studies support previous results16–18 showing the hematogenous route of CMV transmission in the placenta, explain focal infection in floating villi, and accumulation of IgG-gB complexes in syncytiotrophoblasts without productive infection (Figure 10). In endosomes, FcRn could bind IgG from maternal blood at low pH (Figure 10, pathway 1). IgG can be recycled back to the surface or transcytosed to the basal membrane and captured by villus core macrophages expressing other Fc receptors.40 Directly beneath cytotrophoblasts, villus core macrophages project extensions across cell junctions clearing virions and immune complexes.33,41 When intrauterine CMV infection occurs in immune women, neutralized IgG-virion complexes bound to FcRn, are rapidly transcytosed to the basal membrane and phagocytosed by macrophages (Figure 10, pathway 2). In addition, immune complexes endocytosed in small caveolar vesicles at neutral pH might release nucleocapsids into the cytoplasm, accumulate IgG-gB complexes and form caveosomes. In women with recent CMV infections, trans-cytosed IgG-virions may be captured by macrophages or bind to receptors infecting cytotrophoblasts (Figure 10, pathway 3). Focal CMV infection could spread by cell-cell transmission to stromal fibroblasts, villus capillaries, and leukocytes in the fetal compartment, found in infected term placentas.42
Figure 10.
Illustration of hematogenous CMV transmission in chorionic floating villi. Pathway 1: Uninfected syncytiotrophoblasts; IgG pinocytosed from maternal blood binds FcRn in endosomes and is recycled or transcytosed across the syncytium to the basal membrane. Pathway 2: Suppressed infection of IgG-virion complexes with high neutralizing activity that bind FcRn in endocytic vesicles at low pH, are transcytosed across the syncytiotrophoblast and released. IgG-virion envelope gB complexes stored in caveosomes (Cav) at neutral pH. Neutralized IgG-virion complexes transcytosed across syncytiotrophoblasts are captured by villus core macrophages (Mφ) and internalized by cytotrophoblasts. Pathway 3: Focal infection of cytotrophoblasts by IgG-virion complexes with low neutralizing activity. Infection could spread to stromal fibroblasts, blood vessels in the villus core, and leukocytes in the fetal bloodstream.16,42 Symbols for IgG, FcRn, virions (left margin). Red nuclei, infected cells.
Additional evidence supports FcRn transport of IgG and immune complexes. Early studies in mice showed that the transfer of immune complexes, not free antigen, across the neonatal intestine to blood was mediated by a receptor that transports IgG.43 First identified in the murine intestine, FcRn, an MHC class I-related protein associated with β2-microglobulin,44 carries IgG from milk across the intestinal epithelium.45 FcRn protects bound IgG and transports it intact across cells, extending IgG survival time compared with other immunoglobulins.44,46 Consequently, mice lacking β2-microglobulin and those with a defective FcRn gene have IgG with an abnormally short half-life in serum.46,47 Murine FcRn binds both rodent and human IgG1, whereas human FcRn fails to react across species and explains why murine IgG is rapidly cleared from human circulation.14,48 Our results extend this observation to the placenta and show that murine antibodies to CMV gB and Toledo-EGFP virion complexes are not internalized or transcytosed in syncytiotrophoblasts, nor phagocytosed by villus core macrophages (data not shown). Additionally, murine CMV does not cross the rodent placenta, which lacks FcRn,49 and is separated from maternal blood by three cell layers.50 These studies provide indirect support for receptor-mediated transcytosis of CMV-IgG complexes by FcRn in the human placenta.
Our results clarify the association between low-neutralizing maternal antibodies to CMV and congenital infection.10 Paradoxically, IgG1, bound by human FcRn with high affinity,14 the major subclass elicited during infection, recognizes CMV gB that binds cell surface receptors.32,51–53 Consequently, IgG1 with high neutralizing titer functions as an effective barrier suppressing placental infection in immune mothers. Infection could spread from cytotrophoblasts to stromal fibroblasts in the villus core. Macrophages confer additional protection capturing trans-cytosed immune complexes. In addition to the placenta, other tissues—intestine,34 kidney,54 lung,55 and breast56 —are sites of CMV infection,5 suggesting FcRn-mediated transport of IgG-virion complexes in specialized cells.
Perhaps FcRn mediates the vertical transmission of other viruses associated with congenital infection by transcytosing immune complexes from maternal blood. Congenital rubella, greatly reduced by vaccination, involves low-avidity antibodies of the IgG1 subclass generated during primary infection during gestation.57–59 Prenatal transmission of hepatitis B virus, reduced by vaccination of carrier mothers and newborns,60 can occur in areas with endemic chronic hepatitis.61 In certain cases, fetal infection is associated with transplacental transmission of HBe antigen as specific IgG-complexes, in contrast to free HBe antigen at high levels in the blood of asymptomatic carriers and actively infected mothers.62,63 Whether HBe antigen-positive babies become carriers depends on antigen persistence and the level of hepatitis B virus DNA in maternal blood. Some cases of human immunodeficiency virus infection in newborns are associated with transmission of minor antigenic variants that escape neutralization.64,65 Placentas from women infected with multiple human immunodeficiency virus strains contain a few minor variants66,67 suggesting that FcRn could trans-cytose low-avidity IgG1-virion complexes.
As a transporter of maternal IgG, FcRn plays a central role in protecting the fetus by passive immunization. Treatment of pregnant women with hyperimmune antiviral antibodies can suppress infection in the mother and limit fetal disease for congenital varicella-zoster virus68 and CMV.69 In a recent clinical trial, women with primary CMV infection, low-avidity IgG, and congenital infection with intrauterine growth restriction in the fetus were treated with hyperimmune IgG at mid-gestation and monthly until term.70 At delivery, all infants were seropositive but only 3% were symptomatic in the treatment group as compared with 50% in the comparison group, a significantly reduced risk of congenital disease. These results suggest hyperimmune IgG could suppress CMV replication at the maternal-fetal interface and improve fetal outcome. Our proposed model offers testable hypotheses for further evaluation and supports development of novel strategies to increase the barrier function of the placenta, passive immunization with CMV-specific hyperimmune IgG for primary infection during gestation, and vaccination for protective immunity.
Acknowledgments
We thank the members of the Pereira and Fisher laboratories and Drs. Susan Fisher and Sharof Tugizov for thoughtful discussions; Gregory Duke for Toledo-EGFP; Neil Simister for antibodies to FcRn; Zoya Kharitonov, Hsin-Ti Chang, Mirhan Kapidzic, and Jennifer Weston for technical assistance; Doug Beckner and Keith Jones for preparing the illustrations; and Mary McKenney for editing the manuscript.
Footnotes
Address reprint requests to Lenore Pereira, Department of Cell and Tissue Biology, School of Dentistry, University of California San Francisco, 513 Parnassus, San Francisco, San Francisco, CA 94143-0512. E-mail: pereira@itsa.ucsf.edu.
Supported by the National Institutes of Health (grants AI46657, AI53782, and EY13683 to L.P.), the Thrasher Research Fund (grant 02821-7 to L.P.), and University of California San Francisco Academic Senate (to E.M.).
References
- Kondo K, Kaneshima H, Mocarski ES. Human cytomegalovirus latent infection of granulocyte-macrophage progenitors. Proc Natl Acad Sci USA. 1994;91:11879–11883. doi: 10.1073/pnas.91.25.11879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revello MG, Zavattoni M, Sarasini A, Percivalle E, Simoncini L, Gerna G. Human cytomegalovirus in blood of immunocompetent persons during primary infection: prognostic implications for pregnancy. J Infect Dis. 1998;177:1170–1175. doi: 10.1086/515277. [DOI] [PubMed] [Google Scholar]
- Lazzarotto T, Varani S, Spezzacatena P, Pradelli P, Potena L, Lombardi A, Ghisetti V, Gabrielli L, Abate DA, Magelli C, Landini MP. Delayed acquisition of high-avidity anti-cytomegalovirus antibody is correlated with prolonged antigenemia in solid organ transplant recipients. J Infect Dis. 1998;178:1145–1149. doi: 10.1086/515671. [DOI] [PubMed] [Google Scholar]
- Britt WJ. Congenital Cytomegalovirus Infection. Hitchcock PJ, MacKay HT, Wasserheit JN, editors. Washington DC: ASM Press,; 1999:pp 269–281. [Google Scholar]
- Pass BF. Cytomegalovirus. Knipe DM, Howley PM, editors. New York: Lippincott-Raven,; 2001:pp 2675–2705. [Google Scholar]
- Demmler GJ. Congenital cytomegalovirus infection and disease. Adv Pediatr Infect Dis. 1996;11:135–162. [PubMed] [Google Scholar]
- Fowler KB, Stagno S, Pass RF, Britt WJ, Boll TJ, Alford CA. The outcome of congenital cytomegalovirus infection in relation to maternal antibody status. N Engl J Med. 1992;326:663–667. doi: 10.1056/NEJM199203053261003. [DOI] [PubMed] [Google Scholar]
- Stagno S, Pass RF, Cloud G, Britt WJ, Henderson RE, Walton PD, Veren DA, Page F, Alford CA. Primary cytomegalovirus infection in pregnancy. Incidence, transmission to fetus, and clinical outcome. JAMA. 1986;256:1904–1908. [PubMed] [Google Scholar]
- Revello MG, Zavattoni M, Furione M, Lilleri D, Gorini G, Gerna G. Diagnosis and outcome of preconceptional and periconceptional primary human cytomegalovirus infections. J Infect Dis. 2002;186:553–557. doi: 10.1086/341831. [DOI] [PubMed] [Google Scholar]
- Boppana SB, Britt WJ. Antiviral antibody responses and intrauterine transmission after primary maternal cytomegalovirus infection. J Infect Dis. 1995;171:1115–1121. doi: 10.1093/infdis/171.5.1115. [DOI] [PubMed] [Google Scholar]
- Benirschke K, Kaufmann P. Pathology of the Human Placenta. New York: Springer,; 2000 [Google Scholar]
- Zhou Y, Fisher SJ, Janatpour M, Genbacev O, Dejana E, Wheelock M, Damsky CH. Human cytotrophoblasts adopt a vascular phenotype as they differentiate. A strategy for successful endovascular invasion? J Clin Invest. 1997;99:2139–2151. doi: 10.1172/JCI119387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simister NE, Story CM, Chen HL, Hunt JS. An IgG-transporting Fc receptor expressed in the syncytiotrophoblast of human placenta. Eur J Immunol. 1996;26:1527–1531. doi: 10.1002/eji.1830260718. [DOI] [PubMed] [Google Scholar]
- Firan M, Bawdon R, Radu C, Ober RJ, Eaken D, Antohe F, Ghetie V, Ward ES. The MHC class I-related receptor, FcRn, plays an essential role in the maternofetal transfer of gamma-globulin in humans. Int Immunol. 2001;13:993–1002. doi: 10.1093/intimm/13.8.993. [DOI] [PubMed] [Google Scholar]
- Pereira L, Maidji E, McDonagh S, Tabata T. Insights into viral transmission at the uterine-placental interface. Trends Microbiol. 2005;13:164–174. doi: 10.1016/j.tim.2005.02.009. [DOI] [PubMed] [Google Scholar]
- Pereira L, Maidji E, McDonagh S, Genbacev O, Fisher S. Human cytomegalovirus transmission from the uterus to the placenta correlates with the presence of pathogenic bacteria and maternal immunity. J Virol. 2003;77:13301–13314. doi: 10.1128/JVI.77.24.13301-13314.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonagh S, Maidji E, Ma W, Chang HT, Fisher S, Pereira L. Viral and bacterial pathogens at the maternal-fetal interface. J Infect Dis. 2004;190:826–834. doi: 10.1086/422330. [DOI] [PubMed] [Google Scholar]
- Fisher S, Genbacev O, Maidji E, Pereira L. Human cytomegalovirus infection of placental cytotrophoblasts in vitro and in utero: implications for transmission and pathogenesis. J Virol. 2000;74:6808–6820. doi: 10.1128/jvi.74.15.6808-6820.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chee MS, Bankier AT, Beck S, Bohni R, Brown CM, Cerny R, Horsnell T, Hutchison CA, Kouzarides T, Martignetti JA, Preddie E, Satchwell SC, Tomlinson P, Weston KM, Barrell BG. Analysis of the protein-coding content of the sequence of human cytomegalovirus strain AD169. Curr Top Microbiol Immunol. 1990;154:125–170. doi: 10.1007/978-3-642-74980-3_6. [DOI] [PubMed] [Google Scholar]
- Cha TA, Tom E, Kemble GW, Duke GM, Mocarski ES, Spaete RR. Human cytomegalovirus clinical isolates carry at least 19 genes not found in laboratory strains. J Virol. 1996;70:78–83. doi: 10.1128/jvi.70.1.78-83.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Mo C, Kemble G, Duke G. Development of an efficient fluorescence-based microneutralization assay using recombinant human cytomegalovirus strains expressing green fluorescent protein. J Virol Methods. 2004;120:207–215. doi: 10.1016/j.jviromet.2004.05.010. [DOI] [PubMed] [Google Scholar]
- Bresnahan WA, Shenk T. A subset of viral transcripts packaged within human cytomegalovirus particles. Science. 2000;288:2373–2376. doi: 10.1126/science.288.5475.2373. [DOI] [PubMed] [Google Scholar]
- Sarcinella E, Brown M, Tellier R, Petric M, Mazzulli T. Detection of RNA in purified cytomegalovirus virions. Virus Res. 2004;104:129–137. doi: 10.1016/j.virusres.2004.03.008. [DOI] [PubMed] [Google Scholar]
- Dondero DV, Pereira L. Monoclonal Antibody Production. Emmons R, Schmidt N, editors. Washington DC: American Public Health Association,; 1990:pp 101–124. [Google Scholar]
- Qadri I, Navarro D, Paz P, Pereira L. Assembly of conformation-dependent neutralizing domains on human cytomegalovirus glyco-protein B. J Gen Virol. 1992;73:2913–2921. doi: 10.1099/0022-1317-73-11-2913. [DOI] [PubMed] [Google Scholar]
- Damsky CH, Fitzgerald ML, Fisher SJ. Distribution patterns of extracellular matrix components and adhesion receptors are intricately modulated during first trimester cytotrophoblast differentiation along the invasive pathway, in vivo. J Clin Invest. 1992;89:210–222. doi: 10.1172/JCI115565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy KM, Yoong Y, Simister NE. Bidirectional transcytosis of IgG by the rat neonatal Fc receptor expressed in a rat kidney cell line: a system to study protein transport across epithelia. J Cell Sci. 2000;113:1277–1285. doi: 10.1242/jcs.113.7.1277. [DOI] [PubMed] [Google Scholar]
- Spector DH, Hock L, Tamashiro JC. Cleavage maps for human cytomegalovirus DNA strain AD169 for restriction endonucleases EcoRI, BglII, and HindIII. J Virol. 1982;42:558–582. doi: 10.1128/jvi.42.2.558-582.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T, Suzuki S, Radsak K, Hirai K. The UL112/113 gene products of human cytomegalovirus which colocalize with viral DNA in infected cell nuclei are related to efficient viral DNA replication. Virus Res. 1998;56:107–114. doi: 10.1016/s0168-1702(98)00032-x. [DOI] [PubMed] [Google Scholar]
- Navarro D, Paz P, Tugizov S, Topp K, La Vail J, Pereira L. Glycoprotein B of human cytomegalovirus promotes virion penetration into cells, transmission of infection from cell to cell, and fusion of infected cells. Virology. 1993;197:143–158. doi: 10.1006/viro.1993.1575. [DOI] [PubMed] [Google Scholar]
- Gulino D, Delachanal E, Concord E, Genoux Y, Morand B, Valiron MO, Sulpice E, Scaife R, Alemany M, Vernet T. Alteration of endothelial cell monolayer integrity triggers resynthesis of vascular endothelium cadherin. J Biol Chem. 1998;273:29786–29793. doi: 10.1074/jbc.273.45.29786. [DOI] [PubMed] [Google Scholar]
- Wang X, Huong SM, Chiu ML, Raab-Traub N, Huang ES. Epidermal growth factor receptor is a cellular receptor for human cytomegalovirus. Nature. 2003;424:456–461. doi: 10.1038/nature01818. [DOI] [PubMed] [Google Scholar]
- Yoshida M, Claypool SM, Wagner JS, Mizoguchi E, Mizoguchi A, Roopenian DC, Lencer WI, Blumberg RS. Human neonatal Fc receptor mediates transport of IgG into luminal secretions for delivery of antigens to mucosal dendritic cells. Immunity. 2004;20:769–783. doi: 10.1016/j.immuni.2004.05.007. [DOI] [PubMed] [Google Scholar]
- Israel EJ, Taylor S, Wu Z, Mizoguchi E, Blumberg RS, Bhan A, Simister NE. Expression of the neonatal Fc receptor, FcRn, on human intestinal epithelial cells. Immunology. 1997;92:69–74. doi: 10.1046/j.1365-2567.1997.00326.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson BL, Badizadegan K, Wu Z, Ahouse JC, Zhu X, Simister NE, Blumberg RS, Lencer WI. Bidirectional FcRn-dependent IgG transport in a polarized human intestinal epithelial cell line. J Clin Invest. 1999;104:903–911. doi: 10.1172/JCI6968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pol A, Lu A, Pons M, Peiro S, Enrich C. Epidermal growth factor-mediated caveolin recruitment to early endosomes and MAPK activation. Role of cholesterol and actin cytoskeleton. J Biol Chem. 2000;275:30566–30572. doi: 10.1074/jbc.M001131200. [DOI] [PubMed] [Google Scholar]
- Pelkmans L, Burli T, Zerial M, Helenius A. Caveolin-stabilized membrane domains as multifunctional transport and sorting devices in endocytic membrane traffic. Cell. 2004;118:767–780. doi: 10.1016/j.cell.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Bulmer JN, Morrison L, Johnson PM, Meager A. Immunohistochemical localization of interferons in human placental tissues in normal, ectopic, and molar pregnancy. Am J Reprod Immunol. 1990;22:109–116. doi: 10.1111/j.1600-0897.1990.tb00652.x. [DOI] [PubMed] [Google Scholar]
- Sainz B, Jr, Lamarca HL, Garry RF, Morris CA. Synergistic inhibition of human cytomegalovirus replication by interferon-alpha/beta and interferon-gamma. Virol J. 2005;2:14. doi: 10.1186/1743-422X-2-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simister NE. Placental transport of immunoglobulin G. Vaccine. 2003;21:3365–3369. doi: 10.1016/s0264-410x(03)00334-7. [DOI] [PubMed] [Google Scholar]
- Rescigno M, Urbano M, Valzasina B, Francolini M, Rotta G, Bonasio R, Granucci F, Kraehenbuhl JP, Ricciardi-Castagnoli P. Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nat Immunol. 2001;2:361–367. doi: 10.1038/86373. [DOI] [PubMed] [Google Scholar]
- McDonagh S, Maidji E, Chang H-T, Pereira L. Patterns of human cytomegalovirus infection in term placentas: a preliminary analysis. J Clin Virol. 2006;35:210–215. doi: 10.1016/j.jcv.2005.08.011. [DOI] [PubMed] [Google Scholar]
- Abrahamson DR, Powers A, Rodewald R. Intestinal absorption of immune complexes by neonatal rats: a route of antigen transfer from mother to young. Science. 1979;206:567–569. doi: 10.1126/science.493961. [DOI] [PubMed] [Google Scholar]
- Simister NE, Mostov KE. An Fc receptor structurally related to MHC class I antigens. Nature. 1989;337:184–187. doi: 10.1038/337184a0. [DOI] [PubMed] [Google Scholar]
- Israel EJ, Patel VK, Taylor SF, Marshak-Rothstein A, Simister NE. Requirement for a beta 2-microglobulin-associated Fc receptor for acquisition of maternal IgG by fetal and neonatal mice. J Immunol. 1995;154:6246–6251. [PubMed] [Google Scholar]
- Ghetie V, Hubbard JG, Kim JK, Tsen MF, Lee Y, Ward ES. Abnormally short serum half-lives of IgG in beta 2-microglobulin-deficient mice. Eur J Immunol. 1996;26:690–696. doi: 10.1002/eji.1830260327. [DOI] [PubMed] [Google Scholar]
- Roopenian DC, Christianson GJ, Sproule TJ, Brown AC, Akilesh S, Jung N, Petkova S, Avanessian L, Choi EY, Shaffer DJ, Eden PA, Anderson CL. The MHC class I-like IgG receptor controls perinatal IgG transport, IgG homeostasis, and fate of IgG-Fc-coupled drugs. J Immunol. 2003;170:3528–3533. doi: 10.4049/jimmunol.170.7.3528. [DOI] [PubMed] [Google Scholar]
- Ober RJ, Radu CG, Ghetie V, Ward ES. Differences in promiscuity for antibody-FcRn interactions across species: implications for therapeutic antibodies. Int Immunol. 2001;13:1551–1559. doi: 10.1093/intimm/13.12.1551. [DOI] [PubMed] [Google Scholar]
- Ahouse JJ, Hagerman CL, Mittal P, Gilbert DJ, Copeland NG, Jenkins NA, Simister NE. Mouse MHC class I-like Fc receptor encoded outside the MHC. J Immunol. 1993;151:6076–6088. [PubMed] [Google Scholar]
- Adamson SL, Lu Y, Whiteley KJ, Holmyard D, Hemberger M, Pfarrer C, Cross JC. Interactions between trophoblast cells and the maternal and fetal circulation in the mouse placenta. Dev Biol. 2002;250:358–373. doi: 10.1016/s0012-1606(02)90773-6. [DOI] [PubMed] [Google Scholar]
- Urban M, Winkler T, Landini MP, Britt W, Mach M. Epitope-specific distribution of IgG subclasses against antigenic domains on glyco-proteins of human cytomegalovirus. J Infect Dis. 1994;169:83–90. doi: 10.1093/infdis/169.1.83. [DOI] [PubMed] [Google Scholar]
- Feire AL, Koss H, Compton T. Cellular integrins function as entry receptors for human cytomegalovirus via a highly conserved disintegrin-like domain. Proc Natl Acad Sci USA. 2004;101:15470–15475. doi: 10.1073/pnas.0406821101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Huang DY, Huong SM, Huang ES. Integrin alphavbeta3 is a coreceptor for human cytomegalovirus. Nat Med. 2005;11:515–521. doi: 10.1038/nm1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haymann JP, Levraud JP, Bouet S, Kappes V, Hagege J, Nguyen G, Xu Y, Rondeau E, Sraer JD. Characterization and localization of the neonatal Fc receptor in adult human kidney. J Am Soc Nephrol. 2000;11:632–639. doi: 10.1681/ASN.V114632. [DOI] [PubMed] [Google Scholar]
- Spiekermann GM, Finn PW, Ward ES, Dumont J, Dickinson BL, Blumberg RS, Lencer WI. Receptor-mediated immunoglobulin G transport across mucosal barriers in adult life: functional expression of FcRn in the mammalian lung. J Exp Med. 2002;196:303–310. doi: 10.1084/jem.20020400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cianga P, Cianga C, Cozma L, Ward ES, Carasevici E. The MHC class I related Fc receptor, FcRn, is expressed in the epithelial cells of the human mammary gland. Hum Immunol. 2003;64:1152–1159. doi: 10.1016/j.humimm.2003.08.025. [DOI] [PubMed] [Google Scholar]
- Fitzgerald MG, Pullen GR, Hosking CS. Low affinity antibody to rubella antigen in patients after rubella infection in utero. Pediatrics. 1988;81:812–814. [PubMed] [Google Scholar]
- Thomas HI, Morgan-Capner P. Specific IgG subclass antibody in rubella virus infections. Epidemiol Infect. 1988;100:443–454. doi: 10.1017/s0950268800067182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katow S. Rubella virus genome diagnosis during pregnancy and mechanism of congenital rubella. Intervirology. 1998;41:163–169. doi: 10.1159/000024931. [DOI] [PubMed] [Google Scholar]
- Marion SA, Tomm Pastore M, Pi DW, Mathias RG. Long-term follow-up of hepatitis B vaccine in infants of carrier mothers. Am J Epidemiol. 1994;140:734–746. doi: 10.1093/oxfordjournals.aje.a117321. [DOI] [PubMed] [Google Scholar]
- Xu DZ, Yan YP, Choi BC, Xu JQ, Men K, Zhang JX, Liu ZH, Wang FS. Risk factors and mechanism of transplacental transmission of hepatitis B virus: a case-control study. J Med Virol. 2002;67:20–26. doi: 10.1002/jmv.2187. [DOI] [PubMed] [Google Scholar]
- Arakawa K, Tsuda F, Takahashi K, Ise I, Naito S, Kosugi E, Miyakawa Y, Mayumi M. Maternofetal transmission of IgG-bound hepatitis B e antigen. Pediatr Res. 1982;16:247–250. doi: 10.1203/00006450-198203000-00017. [DOI] [PubMed] [Google Scholar]
- Wang Z, Zhang J, Yang H, Li X, Wen S, Guo Y, Sun J, Hou J. Quantitative analysis of HBV DNA level and HBeAg titer in hepatitis B surface antigen positive mothers and their babies: HBeAg passage through the placenta and the rate of decay in babies. J Med Virol. 2003;71:360–366. doi: 10.1002/jmv.10493. [DOI] [PubMed] [Google Scholar]
- Devash Y, Calvelli TA, Wood DG, Reagan KJ, Rubinstein A. Vertical transmission of human immunodeficiency virus is correlated with the absence of high-affinity/avidity maternal antibodies to the gp120 principal neutralizing domain. Proc Natl Acad Sci USA. 1990;87:3445–3449. doi: 10.1073/pnas.87.9.3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinstein A, Goldstein H, Calvelli T, Devash Y, Rubinstein R, Soeiro R, Lyman W. Maternofetal transmission of human immunodeficiency virus-1: the role of antibodies to the V3 primary neutralizing domain. Pediatr Res. 1993;33:S76–S79. doi: 10.1203/00006450-199305001-00440. [DOI] [PubMed] [Google Scholar]
- Wolinsky SM, Wike CM, Korber BT, Hutto C, Parks WP, Rosenblum LL, Kunstman KJ, Furtado MR, Munoz JL. Selective transmission of human immunodeficiency virus type-1 variants from mothers to infants. Science. 1992;255:1134–1137. doi: 10.1126/science.1546316. [DOI] [PubMed] [Google Scholar]
- Menu E, Mbopi-Keou FX, Lagaye S, Pissard S, Mauclere P, Scarlatti G, Martin J, Goossens M, Chaouat G, Barre-Sinoussi F, M’Bopi Keou FX. Selection of maternal human immunodeficiency virus type 1 variants in human placenta. European Network for In Utero Transmission of HIV-1. J Infect Dis. 1999;179:44–51. doi: 10.1086/314542. [DOI] [PubMed] [Google Scholar]
- Enders G. Management of varicella-zoster contact and infection in pregnancy using a standardized varicella-zoster ELISA test. Postgrad Med J. 1985;61(Suppl 4):23–30. [PubMed] [Google Scholar]
- Nigro G, La Torre R, Anceschi MM, Mazzocco M, Cosmi EV. Hyperimmunoglobulin therapy for a twin fetus with cytomegalovirus infection and growth restriction. Am J Obstet Gynecol. 1999;180:1222–1226. doi: 10.1016/s0002-9378(99)70620-4. [DOI] [PubMed] [Google Scholar]
- Nigro G, Adler SP, La Torre R, Best AM. Passive immunization during pregnancy for congenital cytomegalovirus infection. N Engl J Med. 2005;353:1350–1362. doi: 10.1056/NEJMoa043337. [DOI] [PubMed] [Google Scholar]