Fungal cells must deal with a wide variety of potentially toxic environmental challenges during the course of their proliferation. Often these environmental changes are designed to target and eliminate the fungus along with other microorganisms from a specific milieu, such as an animal host. An important example of an environmental challenge to which fungi must rise is the high levels of reactive oxygen species (ROS) produced by neutrophil cells during the oxidative burst (32). The oxidative killing of fungal cells by this host defense mechanism represents an important line of elimination of pathogenic microorganisms (17). Not surprisingly, correlations have been made between the function of the oxidative-stress response in certain pathogenic fungi and their ability to proliferate in the host (93).
A critical feature in this response to oxidative stress is the necessity for rapid signaling of the new stressful environment, which in turn leads to a reprogramming of gene expression and expression of gene products required to buffer the otherwise lethal elevation in ROS. Since the onset of oxidative stress can occur quickly, as illustrated by the oxidative burst (6), the response pathways of fungi must be similarly rapid. The cell must be able to detect the altered redox balance, modulate the activity of appropriate transcriptional regulators, and then induce the expression of the relevant target genes. While fungi use multiple different means of controlling the expression of redox active gene products, a common feature is the necessity of a transcriptional response to oxidative challenge. The goal of this review is to summarize the state of knowledge of various signaling pathways utilized by fungal cells to control the expression of genes involved in the oxidative-stress response.
The mechanisms regulating the fungal response to oxidative challenge can be broadly classified into two types: nuclear localization control and activity regulation via protein phosphorylation. Certain pathways, such as the modulation of the stress-responsive Sty1 protein kinase in Schizosaccharomyces pombe, are influenced by both of these different regulatory mechanisms (reviewed in reference 86). Other oxidant-responsive factors, such as Saccharomyces cerevisiae Yap1p or Hsf1p, appear to be regulated at the level of nuclear localization (46) or phosphorylation (52), respectively. Along with these better-studied examples, many other proteins that are intimately involved in oxidative-stress tolerance have regulatory mechanisms that have so far eluded understanding. S. cerevisiae cells lacking the Skn7p transcription factor are extremely sensitive to H2O2, owing in part to a failure to induce transcription of important antioxidant genes like TRX2 and TRR1 (reviewed in reference 36). However, the control of Skn7p by oxidant challenge remains a mystery.
Finally, transcriptional regulatory systems with a primary role in metal homeostasis will not be considered here. Metal homeostasis is crucial for normal cellular redox balance, and the interested reader is encouraged to examine excellent reviews on this subject (5, 18).
NUCLEAR LOCALIZATION
The compartmentalization of genomic DNA inside the nuclear membrane provides an important avenue for regulation of cytoplasmically synthesized transcriptional regulatory proteins. The nuclear membrane barrier allows access of transcription factors to their gene targets to be controlled through regulation of the localization of these key modulatory proteins. These gene regulators, like all other proteins with a nuclear site of action, must cross the nuclear membrane through the nuclear pore (reviewed in reference 27). Control of nuclear localization is an important experimental area under intense investigation and will be briefly summarized here. Recent reviews provide a more comprehensive consideration of advances in the understanding of the molecular mechanisms underlying nuclear localization (40, 43, 89).
Briefly, the key regulator of directional nuclear movement is the small Ras-like GTPase called Ran in mammalian cells, encoded by the GSP1 gene in S. cerevisiae (8). Gsp1p is maintained in a primarily GTP-bound state inside the nucleus through the action of a guanine nucleotide exchange factor called Prp20p (23), while Gsp1p GTPase activity is stimulated by the Rna1p GTPase-activating protein present in the cytoplasm (7). Proteins containing short signal sequences known as nuclear localization signals interact with karyopherins (importins) that in turn can be imported across the nuclear pore into the nuclear interior. Once arriving inside the nucleus, Gsp1p-GTP stimulates the dissociation of the karyopherin-cargo complex (26). Conversely, nuclear export occurs when a protein containing a nuclear export signal (NES) binds to a karyopherin homologue called an exportin (such as Crm1p in S. cerevisiae and S. pombe) along with Gsp1p-GTP (see reference 49 for a recent review of nuclear export). This complex then moves to the cytoplasm, where Rna1p stimulates the GTPase activity of Gsp1p, leading to complex dissociation.
Fungal cells have targeted nuclear localization of several components of their oxidative-stress response machinery for modulation. This allows preexisting pools of proteins to rapidly be recruited into their roles in the stress response. One of the first examples of a transcription factor in which nuclear localization is regulated by oxidative stress is the basic region-leucine zipper (bZip)-containing protein Yap1p from S. cerevisiae.
Yap1p was first identified on the basis of its biochemical similarity with mammalian AP-1 (33). The DNA sequence of YAP1 indicated that the gene product shared sequence similarity that was limited to the region of the bZip domain (58). Use of an artificial reporter gene containing three AP-1 response elements placed upstream of a TRP5-lacZ reporter gene demonstrated that Yap1p was likely to be a positive regulator of gene expression, but little else was known about the in vivo role of this protein (58).
Work from several labs provided evidence that Yap1p was likely to play a role in redox homeostasis. Cells lacking the YAP1 gene were found to be hypersensitive to H2O2 challenge as well as to high oxygen levels, consistent with a requirement for Yap1p to deal with ROS (72). Two other findings supported and extended this idea. First, Yap1p was shown to be required for the oxidative-stress-inducible transcription of the TRX2 gene (45). TRX2 encodes a thioredoxin that is critical for H2O2 tolerance (25, 45). Second, Yap1p was demonstrated to be involved in transcriptional control of the GSH1 gene (91), a locus encoding the rate-limiting enzyme in glutathione biosynthesis (62, 63). Glutathione is the most prevalent thiol-containing compound in the cell and plays a key role in redox balance (30).
While these and other studies clearly implicated Yap1p in the response to oxidative stress, the mechanism underlying activation of Yap1p by oxidants remained unknown. Use of a green fluorescent protein (GFP)-Yap1p fusion protein provided an important advance in the dissection of Yap1p regulation. GFP-Yap1p was found to be primarily cytoplasmic in cells growing under normal conditions but to rapidly relocate to the nucleus after diamide challenge (46). Mutations in a carboxy-terminal cysteine-rich domain (c-CRD) were found to trap the mutant form of Yap1p in the nucleus under both stressed and nonstressed conditions. This c-CRD had previously been noted in the characterization of a Yap1p homologue from S. pombe called Pap1 (85). Strikingly, three cysteine residues were conserved in the c-CRD regions from these related bZip transcription factors, and transfer of the c-CRD from Yap1p to a heterologous transcription factor conferred diamide-inducible nuclear localization on the chimeric protein (46). Together, these observations presented a compelling case that control of Yap1p activity by diamide could be assigned to regulation of nuclear localization of the factor through the c-CRD.
However, the response of Yap1p to H2O2 was much more complex. A comparative analysis of the domains of Yap1p involved in mediating the response to diamide or H2O2 stress indicated that oxidant-specific defects resulted from deletion or substitution mutations in the protein (90). Importantly, deletion mutations that removed a second, amino-terminally located CRD (n-CRD) caused the resulting mutant protein to be unable to complement the H2O2 hypersensitivity of a Δyap1 strain. One of the n-CRD deletion mutants was found to be hyperresistant to diamide yet hypersensitive to H2O2. Similarly, alanine-scanning mutations removing the extreme C-terminal cysteine residue from the c-CRD produced a mutant factor that conferred H2O2 hypersensitivity but diamide hyperresistance on Δyap1 cells. This alanine-scanning mutant protein also led to high-level, constitutive activation of an artificial Yap1p-dependent reporter gene (ARE-TRP5-lacZ). These data strongly argued that the responses of Yap1p to oxidant stresses elicited by diamide and H2O2 were not equivalent. The discordance between ARE-TRP5-lacZ activation and H2O2 phenotype suggested that there are different requirements for Yap1p activation at different target promoters, a prediction confirmed by further work (see below).
The first information on trans-regulators of Yap1p localization came from studies on the function of the exportin Crm1p. Crm1p was first identified in S. pombe as a factor required for normal chromatin structure (1). Mutant forms of Crm1p were noted to confer staurosporine resistance and to overproduce a protein of 25 kDa. Strikingly, overexpression of the pap1+ gene, encoding the S. pombe Yap1p homologue, also produced these same phenotypes (84). Genetic analyses indicated that Crm1p negatively regulated Pap1 function. Later work on Crm1p in S. cerevisiae demonstrated that this exportin was involved in control of Yap1p nuclear localization (47, 95). Use of a temperature-sensitive form of Crm1p led to trapping of Yap1p in the nuclei of cells shifted to the restrictive temperature. In vitro and in vivo experiments argued that Yap1p and Crm1p interacted in an oxidant-sensitive fashion; increasing the level of oxidant decreased the degree of interaction. Substitution mutations in the c-CRD cysteine residues led to constitutive elimination of Yap1p-Crm1p interactions. Study of the nuclear import of Yap1p failed to find an oxidant-regulated step in the entry of Yap1p to the nucleus (35). These observations led to the model that Yap1p localization control was provided by regulation of the nuclear export of this protein. In nonstressed cells, Yap1p enters the nucleus, interacts with Crm1p, and is rapidly returned to the cytoplasm. However, in oxidant-challenged cells, Yap1p-Crm1p interaction is disturbed via modification of the c-CRD, Yap1p accumulates in the nucleus, and target genes containing a Yap1p response element are upregulated. In the case of an oxidant like diamide, most of the information conferring regulation of Yap1p localization is present in the c-CRD region of the factor.
While the behavior of Yap1 in response to diamide fit well with the simple model presented above, regulation of Yap1p by H2O2 was more complex. There are at least two different aspects to the more elaborate control of Yap1p by H2O2. First, the regulation of Yap1p localization by H2O2 requires at least two different regions of the protein. Second, normal H2O2-regulated induction of gene expression by Yap1p requires regions of the protein that are dispensable for high-level expression of genes during diamide challenge.
Deletion mutagenesis of the n-CRD uncovered the requirement for this Yap1p domain to ensure both normal H2O2 resistance and correct localization in response to this oxidant. Removal of the entire n-CRD produced a protein that failed to complement the H2O2 hypersensitivity of a Δyap1 strain but, surprisingly, was hypertolerant to diamide and exhibited elevated expression of the ARE-TRP5-lacZ gene in the presence of this oxidant (90). Replacement of the most N-terminal cysteine residue located in the n-CRD with alanine (C303A) also produced these same phenotypes (13). Even though the c-CRD was wild type in these mutant proteins, normal oxidant phenotypes were not present. Together, these findings were most consistent with the notion that both the n- and c-CRD must be present for normal H2O2 regulation of Yap1p function.
The importance of the n-CRD in trafficking of Yap1p was further demonstrated by the finding that a disulfide bond appears to form, linking the n-CRD with the c-CRD during oxidative-stress conditions (15). This disulfide bridge is generated only during peroxide challenge and cannot be detected when diamide is used to produce oxidative stress. Examination of the oxidation status of the c-CRD has also confirmed that multiple forms of disulfide bridges form within this segment of the protein in response to oxidative stress (44). These observations suggested that Yap1p might directly sense oxidative stress through action of the cysteine residues in this transcription factor. Interestingly, a recent study (16) has argued for the role of a thiol peroxidase, encoded by the GPX3 gene (4, 34), being required for formation of the disulfide bridge between the n- and c-CRD regions. Gpx3p is required for Yap1p activation in the presence of H2O2 but not in the presence of diamide. This is consistent with Gpx3p acting upstream of Yap1p as the actual H2O2 sensor.
While there is no question that nuclear localization is an essential feature of Yap1p regulation, this controlled trafficking event cannot explain all of the phenotypes of Yap1p mutants. Derivatives of Yap1p that are constitutively released from Crm1p-mediated nuclear export accumulate in the nucleus and can activate artificial reporter genes to a high degree, along with conferring diamide hyperresistance (46, 90). However, these same mutants cannot support normal expression of the thioredoxin-encoding TRX2 gene (13). Yap1p-regulated TRX2 induction is required for normal H2O2 tolerance, and the differential ability of Yap1p mutants to activate TRX2 expression versus artificial reporter genes has been argued to indicate that nonidentical protein-protein interactions must be executed by Yap1p at its different target genes (13). Promoter context-specific transcriptional activation reflects the different demands placed on Yap1p at the genes that must be regulated by this protein in order for normal oxidant tolerance to be achieved.
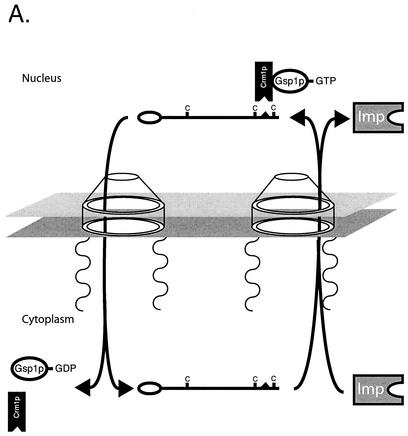
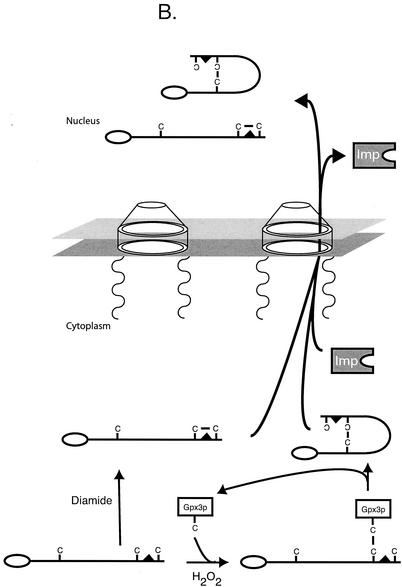
A summary of the oxidant-regulated trafficking of Yap1p is shown in Fig. 1.
FIG. 1.
Oxidant-regulated trafficking of Yap1p. (A) Nonstress conditions. Yap1p associates with importin proteins (Imp) via interaction with an amino-terminally located nuclear localization signal. The importins associated with Yap1p nuclear import are Pse1p and Kap123p (35). This complex is then delivered to the nucleus, and the importin-Yap1p complex dissociates. Under nonstressed conditions, reduced Yap1p can associate with the Crm1p exportin and GTP-loaded Gsp1p, as the NES in Yap1p is accessible. This trimeric complex moves through the nuclear pore and back into the cytoplasm, where it is dissociated. Yap1p can now be bound by importin and recruited back into the nucleus. (B) Oxidative-stress conditions. Yap1p is modified in an oxidant-dependent fashion upon challenge of cells by diamide or H2O2. Diamide-induced oxidative stress may elicit short-range disulfide bond formation that leads to sequestration of the NES. Lack of NES accessibility allows Yap1p to accumulate in the nucleus, since Crm1p interaction is prevented. Similarly, H2O2 also reduces NES accessibility, although via a more complex mechanism. The peroxidase Gpx3p forms a disulfide bond with a C-terminal cysteine residue in Yap1p upon H2O2 challenge (15). Intramolecular disulfide bond rearrangement leads to Gpx3p release and formation of a disulfide bond between the n-CRD and c-CRD of Yap1p, again leading to sequestration of the Yap1p NES and nuclear accumulation of the factor. Nuclear accumulation leads to enhanced target gene expression with associated increases in antioxidant functions that act to return the redox potential of the cell back to a normal range.
OTHER FUNGAL Yap1p HOMOLOGUES
While the oxidant-regulated nuclear localization of Yap1p has been most intensively studied, homologues of this transcription factor from S. pombe and Candida albicans have also been shown to undergo similar regulated recruitment to the nucleus. Pap1 was the first Yap1p homologue identified and is required for resistance of S. pombe to a broad range of different environmental insults along with oxidative-stress tolerance (88). Pap1 also contains both CRD regions, and analysis of the c-CRD has confirmed that this domain is required for oxidant-regulated nuclear localization of Pap1 in S. pombe (87), but this region could not function when expressed in S. cerevisiae (47). Similarly, the Cap1p transcription factor from C. albicans carries out functions equivalent to those of Yap1p (2, 3) and localizes to the nucleus in an oxidant-responsive fashion in this pathogenic yeast (96). While heterologous expression of Cap1p in a Δyap1 S. cerevisiae strain was able to correct some of the oxidant phenotypes of this mutant strain, oxidant-inducible transcription of a Yap1p-regulated reporter gene was not restored (96). Although Pap1, Cap1p, and Yap1p are clearly related at both the levels of sequence and function, the regulation of each of these transcription factors requires features unique to their homologous environments.
Msn2p/Msn4p
One of the first factors shown to be recruited to the nucleus in response to stress was the stress response element binding protein Msn2p (and its homologue Msn4p) (21). Msn2p was found to be required for activation of expression of the cytosolic catalase gene CTT1 in response to oxidative stress (54, 71). While Msn2p has been clearly demonstrated both to be required for CTT1 expression and to be localized to the nucleus in response to other stresses (28), there is little data to link the role of Msn2p to oxidative stress per se. Current models for the regulation of Msn2p/Msn4p argue that these factors are likely to respond to a broad set of stresses and serve to inhibit growth in order to allow cells to adjust to the imposition of stress (see reference 20 for a review). Detailed studies on Msn2p nuclear localization have shown that reductions in cyclic AMP levels can elicit nuclear accumulation, although a second regulatory input appears to inhibit the export of this protein upon stress imposition (29). Thus, Msn2p (and Msn4p) is not directly regulated by redox challenge but rather is regulated by the more global effects caused by stress. It is interesting that, at least in the case of C. albicans, this Msn2p/Msn4p general stress circuitry has been reported not to exist (19).
MAPK CASCADES
One of the most widespread and best studied signaling system involves a series of sequentially acting protein kinases referred to as a mitogen-activated protein kinase (MAPK) cascade. This kinase signaling module consists of an upstream MAPK kinase kinase (MAPKKK) that is activated to phosphorylate its target, a MAPK kinase (MAPKK). MAPKKK phosphorylation of MAPKK then allows this kinase to phosphorylate the ultimate target kinase target in the pathway, the MAPK, leading to activation of this key signaling molecule. Phosphorylated and active MAPK can then regulate its downstream effector molecules, leading to the appropriate regulatory effects.
MAPK pathways have been implicated in oxidative-stress tolerance in animal cells (48) and certain fungal species. The best studied example of a fungal MAPK cascade influencing oxidant resistance is the Sty1/Spc1/Phh1 pathway in S. pombe (37, 55, 76). Sty1 was found to be required for viability under a variety of stress conditions, including osmotic, oxidative, and heat challenges (14, 55, 76). Sty1 activation requires phosphorylation via the MAPKK Wis1. Wis1 in turn is activated by two different MAPKKK proteins, Wis4/Wak1/Wik1 (69, 74, 78) and Win1 (70, 75). Full activation of both Sty1 and Sty1-responsive gene expression requires the presence of both Wis4 (69, 74, 78) and Win1 (70, 75), although Wis4 may play a slightly greater role in the response to oxidant challenge (67). This MAPK pathway is shown schematically in Fig. 2.
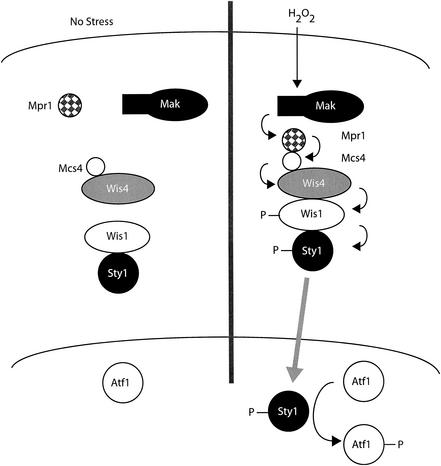
FIG. 2.
Oxidant signaling through the Sty1 pathway. In the absence of oxidative stress, Sty1 (MAPK) is in a low-activity state and localizes to the cytoplasm as a complex with Wis1, the MAPKK. The MAPKKK Wis4 exists as a complex with the Mcs4 protein in the absence of stress. Upon oxidant challenge, the Mak histidine kinases exhibit alterations in their activity, possibly due to modulation of their associated PAS domains. It is presently unknown whether the degree of kinase activity increases or decreases upon oxidant exposure. Mpr1 is then also modified in a yet-unknown fashion to associate with the Mcs4-Wis4 complex. This association likely leads to an increase in Wis4 kinase activity and triggers the sequential phosphorylations of Wis1 and Sty1. Once Sty1 is phosphorylated, it dissociates from Wis1 and moves to the nucleus, where it binds and phosphorylates Atf1. Atf1 can now activate gene expression to restore normal redox potential.
Along with the MAPK module defined by Sty1, upstream regulators of this pathway have been identified. These upstream regulators have been shown to participate in a two-component regulatory pathway. A two-component pathway is so named because, in its simplest form, it contains two conserved components: a histidine kinase protein and a response regulator protein (80). The histidine kinase autophosphorylates on a histidine residue, forming a high-energy phosphohistidine intermediate. This activated phosphate group is then transferred to an aspartate on a receiver domain of the response regulator protein, thereby changing its functional properties.
This two-component scheme has been demonstrated to provide the upstream input that activates Sty1 in response to oxidative stress. Three different histidine kinases have been detected in S. pombe, all of which appear to have some role in redox regulation (12). These three kinases have been termed Mcs4-associated kinases, or Mak, since they appear to act upstream from the response regulator Mcs4 (74, 78). The Mcs4 protein has been demonstrated to bind directly to the Wis4 MAPKKK in a stress-independent fashion (12). Importantly, a protein designated Mpr1 associates with Mcs4 in an oxidant-dependent manner (59). Mpr1 is a homologue of an S. cerevisiae protein called Ypd1p (66) that acts as a phosphorelay protein to transfer a phosphate group from a phosphoaspartate residue on S. cerevisiae Sln1p (the sole histidine kinase in this yeast [94]) to the response regulator Ssk1p (53). When phosphorylated, Ssk1p cannot positively regulate its downstream targets, the MAPKKKKs Ssk2p and Ssk22p (65). The S. cerevisiae Sln1p histidine kinase is regulated by hyperosmolarity but not by oxidative stress, while the Mak histidine kinases in S. pombe are controlled by oxidative challenge but not by osmostress (12). Strikingly, the Mak kinases appear to be cytoplasmic, while Sln1p is localized to the plasma membrane (64).
The precise mechanism of signal transmission from the activated Mak kinases to activation of the Sty1 MAPK cascade is still under investigation, although it is clear that localization of Sty1 to the nucleus is a key feature of the stress response. Sty1 is phosphorylated on two positions by the MAPKK Wis1, with both of these phosphorylation events being required to allow Sty1 to move to the nucleus upon stress challenge (24). Once in the nucleus, Sty1 is retained there by interaction with one of its downstream targets, the transcription factor Atf1 (77, 81). Atf1 is most closely related to the mammalian transcription factor ATF-2, a known substrate for mammalian MAPKs (31). Atf1 is known to be phosphorylated by Sty1, and this phosphorylation is required for activation of Atf1 by oxidant challenge (77, 81).
The central role played by the Sty1 MAPK pathway in the response of S. pombe to oxidant challenge is striking in comparison to MAPK participation in oxidative-stress resistance in S. cerevisiae. S. cerevisiae Hog1p is the MAPK sharing the most sequence similarity with Sty1. Hog1p is a crucial participant in osmotic stress (as is Sty1 in S. pombe) but does not appear to play a significant role in the oxidative-stress response (reviewed in reference 86). Hog1p accumulates in the nucleus upon osmotic challenge but not during H2O2 treatment (22). Although S. cerevisiae MAPKs do not play a major role in oxidant tolerance, this system of protein kinases has been recently found to be required for normal oxidative-stress resistance in the fungus Aspergillus nidulans (38). Deletion of the SakA MAPK from A. nidulans produces a mutant strain that is extremely sensitive to oxidative stress.
OTHER MECHANISMS
Although regulated nuclear localization and function of MAPK cascades have been well described to regulate the oxidative-stress response, other regulatory mechanisms exist. Two related transcriptional regulatory proteins in S. cerevisiae, Hsf1p and Skn7p, clearly illustrate the presence of additional and important regulatory inputs mediating the response to oxidative challenge in this yeast.
The heat shock transcription factor (Hsf1p) from S. cerevisiae shares many features with Hsfs from other organisms (see reference 92 for a discussion). Genes regulated by Hsf1p can be highly inducible by heat shock in addition to other stresses (reviewed in reference 20). These genes are routinely observed to contain multiple copies of the typical binding site for Hsf proteins referred to as heat shock elements, corresponding to repeats of the DNA element AGAAN (92). While historically Hsf1p regulation was evaluated on the basis of heat inducibility, oxidants have been recognized as potent activators of Hsf1p-dependent transcription (52, 68). Stimulation of expression of the copper metallothionein-encoding gene, CUP1, by Hsf1p is required for normal tolerance to heavy metals and menadione (52, 73, 79). CUP1 transcription is also activated via heat shock in an Hsf1p-dependent fashion (73, 79).
Although little is known about the details underlying oxidant activation of Hsf1p function, differential phosphorylation has been implicated in control of the activity of this transcription factor (82). Phosphopeptide mapping experiments indicated that the spectrum of phosphorylation sites used in Hsf1p varied depending on the nature of the stress (52). The precise role of these phosphorylation events, along with the exact mechanism of oxidative stress in activation of Hsf1p, is still under investigation.
Along with the potential modulation of Hsf1p during oxidant challenge by phosphorylation, interaction with a homologous transcription factor called Skn7p is involved in the response to oxidative stress. Skn7p was first cloned as a locus participating in cell wall biosynthesis (11). Analysis of the sequence of Skn7p demonstrated that this protein was a response regulator homologue and a likely participant in a two-component signal transduction pathway (10). Skn7p was later shown to be a downstream target of the Sln1p histidine kinase (39, 50) and to possess a DNA-binding domain with strong sequence similarity to that of Hsf1p (11).
Skn7p function was linked to oxidative-stress tolerance by a genetic screen searching for genes required for normal H2O2 resistance (41). Loss of the POS9 gene produced a cell that was extremely sensitive to H2O2. Cloning of POS9 indicated that this gene was allelic with SKN7 (42). Intriguingly, even though Skn7p contains an aspartate residue that is phosphorylated via Sln1p, this phosphorylation is not required for normal oxidative-stress resistance (57). Other work has shown that some but not all Skn7p functions require the presence of this aspartate (10, 57). Skn7p function was also linked to Yap1p. As mentioned above, mutants lacking YAP1 are highly sensitive to the presence of H2O2 (45, 72). Genetic analysis of the phenotypes of Δyap1 and Δskn7 strains suggested that these two factors function within the same oxidant tolerance pathway (42). More-detailed studies of the ability of Yap1p and Skn7p to regulate gene expression have shed light on the possible overlap of these transcriptional regulators. The thioredoxin-encoding TRX2 gene is induced by H2O2 treatment (45) but only if both Yap1p and Skn7p are present in the cell (56). Loss of either factor blocks H2O2-mediated activation, and even hyperactive forms of Yap1p cannot bypass the requirement for Skn7p (13).
While a large body of data has linked Skn7p to the normal response to oxidative stress, the mode of redox control of Skn7p remains a mystery. Use of a Skn7p-GFP fusion protein indicated that the subcellular distribution of this protein was not altered by changes in the redox environment (68). However, study of the interactions between Hsf1p and Skn7p has provided a potential clarifying link between these two proteins. Biochemical experiments demonstrated that Hsf1p and Skn7p physically interact, while genetic analyses found that a Δskn7 allele elicited a further increase in the oxidant sensitivity of a strain lacking normal Hsf1p function (68). Loss of Skn7p blocked the oxidant inducibility of several heat shock protein-encoding genes but did not eliminate the elevation in gene expression seen for these loci upon heat shock.
Together, these observations suggest that protein-protein interactions between Hsf1p and Skn7p lead to the formation of a heteromeric transcriptional regulatory protein that may explain the induction of heat shock protein-encoding genes by oxidative stress. Interestingly, Skn7p has also been described to form heteromers with the cell cycle transcription factor Mbp1p (9). Changes in the DNA binding specificity of Skn7p as a result of association with Mbp1p or Hsf1p might explain the difficulty in assigning a consensus binding site for Skn7p at relevant target promoters, which seem to vary depending on the gene studied (51, 56).
A protein homologous to Skn7p has been found in S. pombe and designated Prr1 (60). Deletion of prr1+ produced an oxidant-hypersensitive phenotype in S. pombe that is very similar to that seen in S. cerevisiae. Intriguingly, Δprr1 cells are also sterile, a feature distinct from that seen for a Δskn7 strain of S. cerevisiae (61). An Skn7p gene homologue (CaSKN7) (Candida albicans genome sequence assembly 19 accession number Orf19.971) has also been found in the genomic sequence of C. albicans, although the functional analysis of this gene has not been reported.
SUMMARY
While dealing with oxidative stress is a common necessity for fungi, different organisms rely on different mechanisms to detoxify ROS and ensure their survival. S. pombe cells have an elaborate signaling network that activates a downstream transcription factor in response to oxidative stress. The finding of histidine kinases that may serve as the sensors for ROS indicates that important information will soon be forthcoming to explain how this signaling network detects changes in the oxidative environment of a cell. S. cerevisiae cells do not express the analogous histidine kinases seen in S. pombe, consistent with different requirements for oxidant sensing in these two yeasts. An intriguing feature of the S. pombe histidine kinases is the presence of PAS domains in these proteins (12). PAS domains have been demonstrated to serve as redox sensors in several different systems (reviewed in reference 83). Unlike S. cerevisiae Sln1p, the S. pombe Mak kinases do not appear to have transmembrane domains and may function as cytoplasmic sensors of redox status.
S. cerevisiae cells appear to rely on direct oxidant sensing by transcriptional regulators such as Yap1p. In opposition to the more selective appearance of the histidine kinase sensor pathway, all fungal species examined appear to express a transcription factor similar to S. cerevisiae Yap1p. Additionally, Hsf1p and Skn7p gene homologues can be found in S. pombe and C. albicans genomic sequences and are likely present in all fungi. The ubiquitous presence of these transcriptional regulators emphasizes the nonnegotiable status of these proteins as key components in the response to redox challenge.
These transcription factors also provide insight into the complex nature of the response to various oxidants in the cell. The most information about the oxidant-specific defects that appear in the presence of a compromised form of one of these gene regulators comes from studies with S. cerevisiae (summarized in Table 1). Loss of the YAP1 gene causes cells to acquire an extremely oxidative-stress-sensitive phenotype for many but not all oxidants. Peroxide and diamide sensitivity increases greatly in cells lacking Yap1p, but resistance to menadione, a free radical generator, does not appear to increase in Δyap1 strains (72). Removal of the SKN7 gene dramatically enhances peroxide sensitivity but appears to increase tolerance to diamide challenge (56). Finally, functionally compromised forms of Hsf1p fail to support wild-type peroxide or menadione resistance. These findings illustrate the complicated and interacting nature of the antioxidant genes regulated by these transcription factors. A better understanding of this web of target genes and the effects of their gene products on redox balance will be a major experimental step towards understanding how a eukaryotic cell survives oxidative stress.
TABLE 1.
Oxidant-specific regulatory roles of S. cerevisiae transcription factorsa
| Factor | Effect on resistance to:
|
||
|---|---|---|---|
| Peroxide | Diamide | Free radical generator | |
| Yap1p | Positive | Positive | None |
| Skn7p | Positive | Negative | Positive |
| Hsf1p | Positive | Unknown | Positive |
The oxidant-selective effects of several transcription factors from S. cerevisiae are summarized. Positive indicates that the presence of the factor increases resistance to a given oxidant, while negative indicates a decrease in resistance. Yap1p has not been found to significantly influence tolerance to free radical generators, while the role of Hsf1p in diamide tolerance has not yet been reported.
Acknowledgments
I thank Paul Herman for a critical reading of the manuscript.
Work in my laboratory on oxidative stress is supported by NIH grant GM57007.
REFERENCES
- 1.Adachi, Y., and M. Yanagida. 1989. Higher order chromosome structure is affected by cold-sensitive mutations in a Schizosaccharomyces pombe gene crm1+ which encodes a 115-kDa protein preferentially localized in the nucleus and its periphery. J. Cell Biol. 108:1195-1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alarco, A. M., I. Balan, D. Talibi, N. Mainville, and M. Raymond. 1997. AP-1-mediated multidrug resistance in Saccharomyces cerevisiae requires FLR1 encoding a transporter of the major facilitator superfamily. J. Biol. Chem. 272:19304-19313. [DOI] [PubMed] [Google Scholar]
- 3.Alarco, A. M., and M. Raymond. 1999. The bZip transcription factor Cap1p is involved in multidrug resistance and oxidative stress response in Candida albicans. J. Bacteriol. 181:700-708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Avery, A. M., and S. V. Avery. 2001. Saccharomyces cerevisiae expresses three phospholipid hydroperoxide glutathione peroxidases. J. Biol. Chem. 276:33730-33735. [DOI] [PubMed] [Google Scholar]
- 5.Avery, S. V. 2001. Metal toxicity in yeasts and the role of oxidative stress. Adv. Appl. Microbiol. 49:111-142. [DOI] [PubMed] [Google Scholar]
- 6.Babior, B. M. 2000. Phagocytes and oxidative stress. Am. J. Med. 109:33-44. [DOI] [PubMed] [Google Scholar]
- 7.Becker, J., F. Melchior, V. Gerke, F. R. Bischoff, H. Ponstingl, and A. Wittinghofer. 1995. RNA1 encodes a GTPase-activating protein specific for Gsp1p, the Ran/TC4 homologue of Saccharomyces cerevisiae. J. Biol. Chem. 270:11860-11865. [DOI] [PubMed] [Google Scholar]
- 8.Belhumeur, P., A. Lee, R. Tam, T. DiPaolo, N. Fortin, and M. W. Clark. 1993. GSP1 and GSP2, genetic suppressors of the prp20-1 mutant in Saccharomyces cerevisiae: GTP-binding proteins involved in the maintenance of nuclear organization. Mol. Cell. Biol. 13:2152-2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boquin, N., A. L. Johnson, B. A. Morgan, and L. H. Johnston. 1999. Association of the cell cycle transcription factor Mbp1 with the Skn7 response regulator in budding yeast. Mol. Biol. Cell 10:3389-3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brown, J. L., H. Bussey, and R. C. Steward. 1994. Yeast Skn7p functions in a eukaryotic two-component regulatory pathway. EMBO J. 13:5186-5194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown, J. L., S. North, and H. Bussey. 1993. SKN7, a yeast multicopy suppressor of a mutation affecting cell wall beta-glucan assembly, encodes a product with domains homologous to prokaryotic two-component regulators and to heat shock transcription factors. J. Bacteriol. 175:6908-6915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Buck, V., J. Quinn, T. Soto Pino, H. Martin, J. Saldanha, K. Makino, B. A. Morgan, and J. B. A. Millar. 2001. Peroxide sensors for the fission yeast stress-activated mitogen-activated protein kinase pathway. Mol. Biol. Cell 12:407-419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coleman, S. T., E. A. Epping, S. M. Steggerda, and W. S. Moye-Rowley. 1999. Yap1p activates gene transcription in an oxidant-specific fashion. Mol. Cell. Biol. 19:8302-8313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Degols, G., K. Shiozaki, and P. Russell. 1996. Activation and regulation of the Spc1 stress-activated protein kinase in Schizosaccharomyces pombe. Mol. Cell. Biol. 16:2870-2877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Delaunay, A., A. D. Isnard, and M. B. Toledano. 2000. H2O2 sensing through oxidation of the Yap1 transcription factor. EMBO J. 19:5157-5166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Delaunay, A., D. Pflieger, M. B. Barrault, J. Vinh, and M. B. Toledano. 2002. A thiol peroxidase is an H2O2 receptor and redox-transducer in gene activation. Cell 111:471-481. [DOI] [PubMed] [Google Scholar]
- 17.Domer, J. E., and R. I. Lehrer. 1993. Introduction to Candida: systemic candidiasis, p. 49-116. In J. W. Murphy, H. Friedman, and M. Bendinelli (ed.), Fungal infections and immune responses. Plenum Press, New York, N.Y.
- 18.Eide, D. J. 2000. Metal ion transport in eukaryotic microorganisms: insights from Saccharomyces cerevisiae. Adv. Microb. Physiol. 43:1-38. [DOI] [PubMed] [Google Scholar]
- 19.Enjalbert, B., A. Nantel, and M. Whiteway. 2003. Stress-induced gene expression in Candida albicans: absence of a general stress response. Mol. Biol. Cell 14:1460-1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Estruch, F. 2000. Stress-controlled transcription factors, stress-induced genes and stress tolerance in budding yeast. FEMS Microbiol. Rev. 24:469-486. [DOI] [PubMed] [Google Scholar]
- 21.Estruch, F., and M. Carlson. 1993. Two homologous zinc finger genes identified by multicopy suppression in a SNF1 protein kinase mutant of Saccharomyces cerevisiae. Mol. Cell. Biol. 13:3872-3881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ferrigno, P., F. Posas, D. Koepp, H. Saito, and P. A. Silver. 1998. Regulated nucleocytoplasmic exchange of HOG1 MAPK requires the importinβ homologs NMD5 and XPO1. EMBO J. 17:5606-5614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fleischmann, M., M. W. Clark, W. Forrester, M. Wickens, T. Nishimoto, and M. Aebi. 1991. Analysis of yeast prp20 mutations and functional complementation by the human homologue RCC1, a protein involved in the control of chromosome condensation. Mol. Gen. Genet. 227:417-423. [DOI] [PubMed] [Google Scholar]
- 24.Gaits, F., G. Degols, K. Shiozaki, and P. Russell. 1998. Phosphorylation and association with transcription factor Atf1 regulate localization of Spc1/Sty1 stress-activated kinase in fission yeast. Genes Dev. 12:1464-1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garrido, E. O., and C. M. Grant. 2002. Role of thioredoxins in the response of Saccharomyces cerevisiae to oxidative stress induced by hydroperoxides. Mol. Microbiol. 43:143-147. [DOI] [PubMed] [Google Scholar]
- 26.Gilchrist, D., B. Mykytka, and M. Rexach. 2002. Accelerating the rate of disassembly of karyopherin-cargo complexes. J. Biol. Chem. 277:18161-18172. [DOI] [PubMed] [Google Scholar]
- 27.Görlich, D., and U. Kutay. 1999. Transport between the cell nucleus and the cytoplasm. Annu. Rev. Cell Dev. Biol. 15:607-660. [DOI] [PubMed] [Google Scholar]
- 28.Gorner, W., E. Durchschlag, M. T. Martinez-Pastor, F. Estruch, G. Ammerer, B. Hamilton, H. Ruis, and C. Schuller. 1998. Nuclear localization of the C2H2 zinc finger protein Msn2p is regulated by stress and protein kinase A activity. Genes Dev. 12:586-597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gorner, W., E. Durchschlag, J. Wolf, E. L. Brown, G. Ammerer, H. Ruis, and C. Schuller. 2002. Acute glucose starvation activates the nuclear localization signal of a stress-specific yeast transcription factor. EMBO J. 21:135-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grant, C. M., F. H. MacIver, and I. W. Dawes. 1996. Glutathione is an essential metabolite required for resistance to oxidative stress in the yeast Saccharomyces cerevisiae. Curr. Genet. 29:511-515. [DOI] [PubMed] [Google Scholar]
- 31.Hai, T., and M. G. Hartman. 2001. The molecular biology and nomenclature of the activating transcription factor/cAMP responsive element binding family of transcription factors: activating transcription factor proteins and homeostasis. Gene 25:1-11. [DOI] [PubMed] [Google Scholar]
- 32.Hampton, M. B., A. J. Kettle, and C. C. Winterbourn. 1998. Inside the neutrophil phagosome: oxidants, myeloperoxidase and bacterial killing. Blood 92:3007-3017. [PubMed] [Google Scholar]
- 33.Harshman, K. D., W. S. Moye-Rowley, and C. S. Parker. 1988. Transcriptional activation by the SV40 AP-1 recognition element in yeast is mediated by a factor similar to AP-1 that is distinct from GCN4. Cell 53:321-330. [DOI] [PubMed] [Google Scholar]
- 34.Inoue, Y., T. Matsuda, K. Sugiyama, S. Iszawa, and A. Kimura. 1999. Genetic analysis of glutathione peroxidase in oxidative stress response of Saccharomyces cerevisiae. J. Biol. Chem. 274:27002-27009. [DOI] [PubMed] [Google Scholar]
- 35.Isoyama, T., A. Murayama, A. Nomoto, and S. Kuge. 2001. Nuclear import of yeast AP-1-like transcription factor Yap1p is mediated by transport receptor Pse1p and this import step is not affected by oxidative stress. J. Biol. Chem. 276:21863-21869. [DOI] [PubMed] [Google Scholar]
- 36.Jamieson, D. J. 1998. Oxidative stress responses of the yeast Saccharomyces cerevisiae. Yeast 14:1511-1527. [DOI] [PubMed] [Google Scholar]
- 37.Kato, T., K. Okazaki, H. Murakami, P. Stettler, P. Fantes, and H. Okayama. 1996. Stress signal mediated by a Hog1-like MAP kinase controls sexual differentiation in fission yeast. FEBS Lett. 378:207-212. [DOI] [PubMed] [Google Scholar]
- 38.Kawasaki, L., O. Sanchez, K. Shiozaki, and J. Aguirre. 2002. SakA MAP kinase is involved in stress signal transduction, sexual development and spore viability in Aspergillus nidulans. Mol. Microbiol. 45:1153-1163. [DOI] [PubMed] [Google Scholar]
- 39.Ketela, T., J. L. Brown, R. C. Stewart, and H. Bussey. 1998. Yeast Skn7p activity is modulated by the Sln1p-Ypd1p osmosensor and contributes to regulation of the HOG pathway. Mol. Gen. Genet. 259:372-378. [DOI] [PubMed] [Google Scholar]
- 40.Komeili, A., and E. K. O'Shea. 2001. New perspectives on nuclear transport. Annu. Rev. Genet. 35:341-364. [DOI] [PubMed] [Google Scholar]
- 41.Krems, B., C. Charizanis and K.-D. Entian. 1995. Mutants of Saccharomyces cerevisiae sensitive to oxidative and osmotic stress. Curr. Genet. 27:427-434. [DOI] [PubMed] [Google Scholar]
- 42.Krems, B., C. Charizanis and K.-D. Entian. 1996. The response regulator-like protein Pos9/Skn7 of Saccharomyces cerevisiae is involved in oxidative stress resistance. 29:327-334. [DOI] [PubMed]
- 43.Kuersten, S., M. Ohno, and I. W. Mattaj. 2001. Nucleocytoplasmic transport: Ran, beta and beyond. Trends Cell Biol. 11:497-503. [DOI] [PubMed] [Google Scholar]
- 44.Kuge, S., M. Arita, A. Murayama, K. Maeta, S. Izawa, Y. Inoue, and A. Nomoto. 2001. Regulation of the yeast Yap1p nuclear export signal is mediated by redox signal-induced reversible disulfide bond formation. Mol. Cell. Biol. 21:6139-6150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kuge, S., and N. Jones. 1994. YAP1-dependent activation of TRX2 is essential for the response of S. cerevisiae to oxidative stress by hydroperoxides. EMBO J. 13:655-664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kuge, S., N. Jones, and A. Nomoto. 1997. Regulation of yAP-1 nuclear localization in response to oxidative stress. EMBO J. 16:1710-1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kuge, S., T. Toda, N. Iizuka, and A. Nomoto. 1998. Crm1 (Xpo1) dependent nuclear export of the budding yeast transcription factor yAP-1 is sensitive to oxidative stress. Genes Cells 3:521-532. [DOI] [PubMed] [Google Scholar]
- 48.Kyriakis, J. M., and J. Avruch. 1996. Protein kinase cascades activated by stress and inflammatory cytokines. Bioessays 18:567-577. [DOI] [PubMed] [Google Scholar]
- 49.Lei, E. P., and P. A. Silver. 2002. Protein and RNA export from the nucleus. Dev. Cell 2:261-272. [DOI] [PubMed] [Google Scholar]
- 50.Li, S., A. Ault, C. L. Malone, D. Raitt, S. Dean, L. H. Johnston, R. J. Deschenes, and J. S. Fassler. 1998. The yeast histidine protein kinase, Sln1p, mediates phosphotransfer to two response regulators, Ssk1p and Skn7p. EMBO J. 17:6952-6962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li, S., S. Dean, Z. Li, J. Horecka, R. J. Deschenes, and J. S. Fassler. 2002. The eukaryotic two-component histidine kinase Sln1p regulates OCH1 via the transcription factor, Skn7p. Mol. Biol. Cell 13:412-424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu, X.-D., and D. J. Thiele. 1996. Oxidative stress induces heat shock factor phosphorylation and HSF-dependent activation of yeast metallothionein gene transcription. Genes Dev. 10:592-603. [DOI] [PubMed] [Google Scholar]
- 53.Maeda, T., S. M. Wurgler-Murphy, and H. Saito. 1994. A two-component system that regulates an osmosensing MAP kinase cascade in yeast. Nature 369:242-245. [DOI] [PubMed] [Google Scholar]
- 54.Martinez-Pastor, M. T., G. Marchler, C. Schuller, A. Marchler-Bauer, H. Ruis, and F. Estruch. 1996. The Saccharomyces cerevisiae zinc finger proteins Msn2p and Msn4p are required for transcriptional induction through the stress-response element (STRE). EMBO J. 15:2227-2235. [PMC free article] [PubMed] [Google Scholar]
- 55.Millar, J. B., V. Buck, and M. G. Wilkinson. 1995. Pyp1 and Pyp2 PTPases dephosphorylate an osmosensing MAP kinase controlling cell size at division in fission yeast. Genes Dev. 9:2117-2130. [DOI] [PubMed] [Google Scholar]
- 56.Morgan, B. A., G. R. Banks, W. M. Toone, D. Raitt, S. Kuge, and L. H. Johnston. 1997. The Skn7 response regulator controls gene expression in the oxidative stress response of the budding yeast Saccharomyces cerevisiae. EMBO J. 16:1035-1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Morgan, B. A., N. Bouquin, G. F. Merrill, and L. H. Johnston. 1995. A yeast transcription factor bypassing the requirement for SBF and DSC1/MBF in budding yeast has homology to bacterial signal transduction proteins. EMBO J. 14:5679-5689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Moye-Rowley, W. S., K. D. Harshman, and C. S. Parker. 1989. Yeast YAP1 encodes a novel form of the jun family of transcriptional activator proteins. Genes Dev. 3:283-292. [DOI] [PubMed] [Google Scholar]
- 59.Nguyen, A. N., A. Lee, W. Place, and K. Shiozaki. 2000. Multistep phosphorelay proteins transmit oxidative stress signals to the fission yeast stress-activated protein kinase. Mol. Biol. Cell 11:1169-1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ohmiya, R., C. Kato, H. Yamada, H. Aiba, and T. Mizuno. 1999. A fission yeast gene (prr1+) that encodes a response regulator implicated in oxidative stress response. J. Biochem. (Tokyo) 125:1061-1066. [DOI] [PubMed] [Google Scholar]
- 61.Ohmiya, R., H. Yamada, C. Kato, H. Aiba, and T. Mizuno. 2000. The Prr1 response regulator is essential for transcription of ste11+ and for sexual development in fission yeast. Mol. Gen. Genet. 264:441-451. [DOI] [PubMed] [Google Scholar]
- 62.Ohtake, Y., A. Satou, and S. Yabuuchi. 1990. Isolation and characterization of glutathione biosynthesis-deficient mutants in Saccharomyces cerevisiae. Agric. Biol. Chem. 54:3145-3150. [Google Scholar]
- 63.Ohtake, Y., and S. Yabuuchi. 1991. Molecular cloning of the gamma-glutamylcysteine synthetase gene of Saccharomyces cerevisiae. Yeast 7:953-961. [DOI] [PubMed] [Google Scholar]
- 64.Ostrander, D. B., and J. A. Gorman. 1999. The extracellular domain of the Saccharomyces cerevisiae Sln1p membrane osmolarity sensor is necessary for kinase activity. J. Bacteriol. 181:2527-2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Posas, F., and H. Saito. 1998. Activation of the yeast SSK2 MAP kinase kinase kinase by the SSK1 two-component response regulator. EMBO J. 17:1385-1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Posas, F., S. M. Wurgler-Murphy, T. Maeda, E. A. Witten, T. C. Thai, and H. Saito. 1996. Yeast HOG1 MAP kinase cascade is regulated by a multistep phosphorelay mechanism in the SLN1-YPD1-SSK1 “two component” osmosensor. Cell 86:865-875. [DOI] [PubMed] [Google Scholar]
- 67.Quinn, J., V. J. Findlay, K. Dawson, J. B. A. Millar, N. Jones, B. A. Morgan, and W. M. Toone. 2002. Distinct regulatory proteins control the graded transcriptional response to increasing H2O2 levels in fission yeast Schizosaccharomyces pombe. Mol. Biol. Cell 13:805-816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Raitt, D. C., A. L. Johnson, A. M. Erkine, K. Makino, B. A. Morgan, D. S. Gross, and L. H. Johnston. 2000. The Skn7 response regulator of Saccharomyces cerevisiae interacts with Hsf1 in vivo and is required for the induction of heat shock genes by oxidative stress. Mol. Biol. Cell 11:2335-2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Samejima, I., S. Mackie, and P. A. Fantes. 1997. Multiple modes of activation of the stress-responsive MAP kinase pathway in fission yeast. EMBO J. 16:6162-6170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Samejima, I., S. Mackie, E. Warbrick, R. Weisman, and P. A. Fantes. 1998. The fission yeast mitotic regulator win1+ encodes a MAP kinase kinase kinase that phosphorylates and activates Wis1 MAP kinase kinase in response to high osmolarity. Mol. Biol. Cell 9:2325-2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Schmitt, A. P., and K. McEntee. 1996. Msn2p, a zinc finger DNA-binding protein, is the transcriptional activator of the multistress response in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 93:5777-5782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schnell, N., B. Krems and K.-D. Entian. 1992. The PAR1 (YAP1/SNQ3) gene of Saccharomyces cerevisiae, a c-jun homolog, is involved in oxygen metabolism. Curr. Genet. 21:269-273. [DOI] [PubMed] [Google Scholar]
- 73.Sewell, A. K., F. Yokoya, W. Yu, T. Miyagawa, T. Murayama, and D. R. Winge. 1995. Mutated yeast heat shock transcription factor exhibits elevated basal transcriptional activation and confers metal resistance. J. Biol. Chem. 270:25079-25086. [DOI] [PubMed] [Google Scholar]
- 74.Shieh, J. C., M. G. Wilkinson, V. Buck, B. A. Morgan, K. Makino, and J. B. Millar. 1997. The Mcs4 response regulator coordinately controls the stress-activated Wak1-Wis1-Sty1 MAP kinase pathway and fission yeast cell cycle. Genes Dev. 11:1008-1022. [DOI] [PubMed] [Google Scholar]
- 75.Shieh, J. C., M. G. Wilkinson, and J. B. Millar. 1998. The Win1 mitotic regulator is a component of the fission yeast stress-activated Sty1 MAPK pathway. Mol. Biol. Cell 9:311-322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shiozaki, K., and P. Russell. 1995. Cell cycle control linked to extracellular environment by MAP kinase pathway in fission yeast. Nature 378:739-743. [DOI] [PubMed] [Google Scholar]
- 77.Shiozaki, K., and P. Russell. 1996. Conjugation, meiosis, and the osmotic stress response are regulated by Spc1 through Atf1 transcription factor in fission yeast. Genes Dev 10:2276-2288. [DOI] [PubMed] [Google Scholar]
- 78.Shiozaki, K., M. Shiozaki, and P. Russell. 1997. Mcs4 mitotic catastrophe suppressor regulates the fission yeast cell cycle through the Wik1-Wis1-Spc1 kinase cascade. Mol. Biol. Cell 9:409-419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Silar, P., G. Butler, and D. J. Thiele. 1991. Heat shock transcription factor activates expression of a the yeast metallothionein gene. Mol. Cell. Biol. 11:1232-1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Stock, A. M., V. L. Robinson, and P. N. Goudreau. 2000. Two-component signal transduction. Annu. Rev. Biochem. 69:183-215. [DOI] [PubMed] [Google Scholar]
- 81.Takeda, T., T. Toda, K. Kominami, A. Kohnosu, M. Yanagida, and N. Jones. 1995. Schizosaccharomyces pombe atf1+ encodes a transcription factor required for sexual development and entry into stationary phase. EMBO J. 14:6193-6208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tamai, K. T., X. Liu, P. Silar, T. Sosinowski, and D. J. Thiele. 1994. Heat shock transcription factor activates yeast metallothionein gene expression in response to heat and glucose starvation via distinct signalling pathways. Mol. Cell. Biol. 14:8155-8165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Taylor, B. L., and I. B. Zhulin. 1999. PAS domains: internal sensors of oxygen, redox potential and light. Microbiol. Mol. Biol. Rev. 63:479-506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Toda, T., M. Shimanuki, Y. Saka, H. Yamano, Y. Adachi, M. Shirikawa, Y. Kyogoku, and M. Yanagida. 1992. Fission yeast Pap1-dependent transcription is negatively regulated by an essential nuclear protein, crm1. Mol. Cell. Biol. 12:5474-5484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Toda, T., M. Shimanuki, and M. Yanagida. 1991. Fission yeast genes that confer resistance to staurosporine encode an AP-1-like transcription factor and a protein kinase related to the mammalian ERK1/MAP2 and budding yeast FUS3 and KSS1 kinases. Genes Dev. 5:60-73. [DOI] [PubMed] [Google Scholar]
- 86.Toone, W. M., and N. Jones. 1998. Stress-activated signalling pathways in yeast. Genes Cells 3:485-498. [DOI] [PubMed] [Google Scholar]
- 87.Toone, W. M., S. Kuge, M. Samuels, B. A. Morgan, T. Toda, and N. Jones. 1998. Regulation of the fission yeast transcription factor Pap1 by oxidative stress: requirement for the nuclear export factor Crm1 (exportin) and the stress-activated MAP kinase Sty1/Spc1. Genes Dev. 12:1453-1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Toone, W. M., B. A. Morgan, and N. Jones. 2001. Redox control of AP-1-like factors in yeast and beyond. Oncogene 20:2336-2346. [DOI] [PubMed] [Google Scholar]
- 89.Weis, K. 2002. Nucleocytoplasmic transport: cargo trafficking across the border. Curr. Opin. Cell Biol. 14:328-335. [DOI] [PubMed] [Google Scholar]
- 90.Wemmie, J. A., S. M. Steggerda, and W. S. Moye-Rowley. 1997. The Saccharomyces cerevisiae AP-1 protein discriminates between oxidative stress elicited by the oxidants H2O2 and diamide. J. Biol. Chem. 272:7908-7914. [DOI] [PubMed] [Google Scholar]
- 91.Wu, A., and W. S. Moye-Rowley. 1994. GSH1, which encodes γ-glutamylcysteine synthetase, is a target gene for yAP-1 transcriptional regulation. Mol. Cell. Biol. 14:5832-5839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wu, C. 1995. Heat shock transcription factors: structure and regulation. Annu. Rev. Cell Dev. Biol. 11:441-469. [DOI] [PubMed] [Google Scholar]
- 93.Wysong, D. R., L. Christin, A. M. Sugar, P. W. Robbins, and R. D. Diamond. 1998. Cloning and sequencing of a Candida albicans catalase gene and effects of the disruption of this gene. Infect. Immun. 66:1953-1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yamada-Okabe, T., T. Mio, N. Ono, Y. Kashima, M. Matsui, M. Arisawa, and H. Yamada-Okabe. 1999. Roles of three histidine kinase genes in hyphal development and virulence of the pathogenic fungus Candida albicans. J. Bacteriol. 181:7243-7247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yan, C., L. H. Lee, and L. I. Davis. 1998. Crm1p mediates regulated nuclear export of a yeast AP-1-like transcription factor. EMBO J. 17:7416-7429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhang, X., M. De Micheli, S. T. Coleman, D. Sanglard, and W. S. Moye-Rowley. 2000. Analysis of the oxidative stress regulation of the Candida albicans transcription factor, Cap1p. Mol. Microbiol. 36:618-629. [DOI] [PubMed] [Google Scholar]