Abstract
d-Glucose is the preferred carbon and energy source for most eukaryotic cells. Immediately following its uptake, glucose is rapidly phosphorylated to glucose-6-phosphate (Glc-6-P). The yeast Saccharomyces cerevisiae has three enzymes (Hxk1p, Hxk2p, and Glk1p) that convert glucose to Glc-6-P. In the present study, we found that yeast mutants lacking any two of these enzymes retain the ability to efficiently convert glucose to Glc-6-P and thus maintain a low level of cellular glucose. However, a mutant strain lacking all three glucose-phosphorylating enzymes contained up to 225-fold more intracellular glucose than normal. Drugs that inhibit the synthesis or the trimming of the lipid-linked core oligosaccharide Glu3Man9GlcNac2 effectively reduced the accumulation of glucose. Similarly, mutations that block the addition of glucose residues to the core oligosaccharide moiety, such as alg5Δ or alg6Δ, also diminished glucose accumulation. These results indicate that the intracellular glucose accumulation observed in the glucose phosphorylation mutant results primarily from the trimming of glucose residues from core oligosaccharide chains within the endoplasmic reticulum (ER). Consistent with this conclusion, both [14C]glucose exchange and subcellular fractionation experiments indicate that much of the accumulated glucose is retained within an intracellular compartment, suggesting that the efficient transport of glucose from the ER to the cytosol in yeast may be coupled to its rephosphorylation to Glc-6-P. The high level of cellular glucose was associated with an increased level of protein glycation and the release of glucose into the culture medium via its transit through the secretory pathway. Finally, we also found that the accumulation of glucose may lead to a subtle alteration in ion homeostasis, particularly Ca2+ uptake. This suggests that this mutant strain may serve as a useful model to study the consequences of excessive glucose accumulation and protein glycation.
The yeast Saccharomyces cerevisiae contains three enzymes capable of converting glucose to glucose-6-phosphate (Glc-6-P). Two are hexokinases (encoded by the HXK1 and HXK2 genes), whereas the third is a glucokinase (encoded by the GLK1 gene). The presence of any one of these enzymes is sufficient to ensure normal growth rates in medium containing d-glucose as a carbon and energy source (6, 32). However, a yeast strain lacking all three enzymes is unable to metabolize any hexoses or pentoses except d-galactose. d-Galactose is phosphorylated by a specific galactokinase (encoded by the GAL1 gene), which then leads to its entry into the glycolytic pathway after a multistep metabolic conversion to Glc-6-P (22, 23).
Glucose is rapidly phosphorylated to Glc-6-P during or immediately after its transport into the cell (6). This modification has several consequences. First, the negative charge associated with phosphorylation prevents the escape of the molecule once it enters the cell. Second, conversion to Glc-6-P also allows its direct entry into biochemical pathways such as glycolysis. Finally, this modification prevents the accumulation of a high intracellular concentration of glucose, which can lead to adverse consequences such as the nonenzymatic glycation of proteins. Although glucose phosphorylation occurs upon the transport of this sugar from the extracellular environment, it is also necessary for the reutilization of free glucose produced by metabolic processes within the cell. This includes the reutilization of glucose produced from the breakdown of storage carbohydrates such as glycogen and trehalose and core oligosaccharide trimming in the lumen of the endoplasmic reticulum (ER).
In the present study, we examined how yeast cells cope with the glucose generated by cellular metabolism in a yeast strain that is unable to convert glucose to Glc-6-P. Growth of this mutant strain in media containing galactose or lactate as a primary carbon and energy source led to an extremely high level of intracellular glucose. Inhibition of the synthesis of the lipid-linked core oligosaccharide Glu3Man9GlcNac2 or a block in the trimming of glucose residues from this core oligosaccharide greatly decreased glucose accumulation, indicating that glucose was produced primarily from the trimming of core oligosaccharide chains in the ER. Much of this accumulated glucose remained compartmentalized within the cell, suggesting that the efficient transport of glucose from the ER may be tightly coupled to its phosphorylation in the cytosol. Consistent with this finding, a significant fraction of this excess glucose was released from the cell via vesicular transport through the secretory pathway. Finally, we found that high levels of glucose correlated with increased protein glycation and alterations in ion homeostasis.
MATERIALS AND METHODS
Strains used.
The strains used in the present study include DFY1 (MATa lys1-1 SUC2), DFY568 (MATα lys1-1 SUC2 leu2-1 hxk1::LEU2 hxk2::LEU2), DFY582 (MATα lys1-1 SUC2 leu2-1 hxk1::LEU2 glk1::LEU2), DFY583 (MATα lys1-1 SUC2 leu2-1 hxk2::LEU2 glk1::LEU2), and DFY570 (MATα lys1-1 SUC2 leu2-1 hxk1::LEU2 hxk2::LEU2 glk1-E289Q). These strains were kindly provided by D. G. Fraenkel. Although the GLK1 locus in DFY570 was originally reported to contain a glk1::LEU2 disruption (32), our analysis of the GLK1 locus in this strain indicated that a LEU2 insertion was not present. Instead, we identified a glk1-E289Q mutation (GAA→CAA) in DFY570 that was not present in the parent strain, DFY1. Biochemical assays confirmed that this mutation resulted in a loss of glucokinase activity. An alg5Δ strain (MATa his3Δ leu2Δ met15Δ ura3Δ alg5Δ::KnMX; Research Genetics strain record number 1065) and an alg6Δ strain (MATa his3Δ leu2Δ met15Δ ura3Δ alg6Δ::KnMX; Research Genetics strain record number 1778) were each crossed to DFY570 to construct YDB434 (MATα lys1-1 hxk1::LEU2 hxk2::LEU2 glk1-E289Q met15Δ alg5Δ::KnMX) and YDB502 (MATa his3Δ leu2Δ met15Δ hxk1::LEU2 hxk2::LEU2 glk1-E289Q alg6Δ::KnMX). The presence of the hxk1::LEU2, hxk2::LEU2, alg5Δ::KnMX, and alg6Δ::KnMX disruptions in the indicated strains were confirmed by PCR and Southern blotting, whereas the glk1-E289Q mutation was confirmed by DNA sequence analysis.
Culture conditions.
The media used for yeast growth are as described previously (26). YP-galactose (YPGal) medium was supplemented with 2% d-galactose (d-glucose contamination of <0.01%), whereas YP-lactate (YPLct) medium was supplemented with 3% dl-lactate. In all experiments, cultures were grown for five to seven generations in the indicated culture conditions at 30°C to a maximum cell density of ≤1.0 A600 U/ml unless otherwise noted.
Measurement of glucose phosphorylation activity.
Cells were grown in YPGal medium at 30°C and harvested at a cell density of 1.0 A600 U/ml. The cell pellet was washed once in distilled water, and cells were disrupted by glass bead lysis in 40 mM morpholineethanesulfonic acid (MES)-Tris buffer (pH 6.8) supplemented with 2 mM phenylmethylsulfonyl fluoride. The protein concentration of samples was measured by the Lowry method (20) and diluted to 50 to 100 mg/ml with the above buffer. Samples were supplemented with 20 mM d-glucose, 20 mM ATP, and 5 mM MgCl2 and incubated at 30°C. The amount of glucose remaining at various times was measured by using a glucose-oxidase-peroxidase reaction kit (Sigma). Glucose phosphorylation activity was then calculated from the total amount of glucose consumed during the incubation (per unit of time).
Measurement of cellular glucose levels.
For cellular glucose determination, 100 A600 U of yeast cells were harvested by centrifugation at 4,000 × g for 10 min at room temperature. The supernatant was removed, and the cells were washed once in distilled water. The cells were transferred into microcentrifuge tubes and resuspended in 1 ml of distilled water, and the tightly closed tubes were boiled for 10 min. The samples were then centrifuged at room temperature at 15,000 × g for 10 min. The supernatant was separated from the cellular debris and analyzed for glucose content by using a glucose-oxidase-peroxidase reaction kit (Sigma). To ensure that cellular trehalose or glycogen did not degrade to glucose during the processing and/or measurement of samples as described above, we supplemented some samples with 20 mM trehalose or 2% glycogen. We found no significant increase in the measured glucose levels beyond the contaminating glucose present in the trehalose and glycogen preparations.
For normalization of cellular glucose levels we harvested a parallel sample and collected the cells as described above. The samples were transferred into microcentrifuge tubes of known weights and centrifuged at 15,000 × g for 10 min. The cells were washed once in distilled water and centrifuged again. The residual supernatant was carefully removed, and the wet weight of each sample was determined gravimetrically. The samples were then dried in a Savant SpeedVac system until no further loss of weight occurred, and the dry weight of each sample was then measured. The cellular glucose level in each sample was then normalized to the mass of each sample and is presented as millimoles of glucose/kilogram (dry mass) unless indicated otherwise. This method of data presentation was used because we found that the relative water content of yeast cells differed after growth in medium containing glucose, galactose, and lactate as the primary carbon and energy source. Other variables, such as growth phase and treatment with various drugs, also altered the cell volume. To determine the mechanism of glucose release into the culture medium, 224 μg of brefeldin A (BFA; Sigma B-7651)/ml was used to inhibit vesicular transport through the secretory pathway (9).
Measurement of exchangeable versus nonexchangeable glucose levels.
Strains were grown in YPGal medium and harvested at a cell density of 1 A600 U/ml as described above. The cells were then resuspended in YP medium supplemented with [14C]glucose (final glucose concentration, 1 mM) and incubated at 30°C for 15 min. The cell-associated [14C]glucose and total cellular glucose concentrations were measured before and after washing the cells three times in glucose free, ice-cold YP medium.
Inhibition of asparagine-linked glycosylation and glucose trimming.
The N-acetylglucosamine analog tunicamycin was added directly to the YPGal medium, and cells were grown as described above. We found that 1 μM tunicamycin (Sigma T-7765) caused an ∼3-fold reduction in the growth rate of cells. Castanospermin (Sigma C-3784) and 1-deoxynojirimycin (Sigma D-9305) inhibit the glycosidases responsible for the glucose trimming from the core oligosaccharide. The addition of a 1 mM concentration of each of these drugs to the YPGal medium did not cause a detectable decrease in the growth rate of cells.
Fructosamine measurement.
Cultures were grown to <1.0 A600 U/ml in YPGal medium, and 200 A600 U of cells was collected by centrifugation at 4,000 × g for 5 min. Cells were washed briefly in 0.89% NaCl, resuspended in a 3× volume of 1 M NaOH, and then crushed by mechanical agitation with glass beads. After centrifugation as described above, the supernatant was neutralized with an equal volume of 1 M HCl and processed for fructosamine (17) by using a commercial kit (Roche 07-5606-3) and a Hitachi 704 automated analyzer. The protein levels were determined by using the Bradford method (4).
To determine the fructosamine level in the ER, an ER-enriched fraction was isolated from spheroplasts. Briefly, cells were collected and washed as described above. The washed cells were weighed gravimetrically and resuspended in 0.1 M Tris-Cl (pH 9.4) at a 2:1 buffer/cell ratio. The sample was supplemented with 10 mM dithiothreitol and incubated for 10 min at 30°C. The cells were recovered by centrifugation at 4,000 × g and resuspended in 1.2 M sorbitol. The cells were centrifuged again and resuspended in zymolyase buffer (1.2 M sorbitol, 20 mM KPO4 buffer [pH 7.2]) at a 10:1 buffer/cell ratio, 5 mg of zymolyase/ml was added, and the cell suspension was incubated in a gently shaking water bath for 30 min. The spheroplasts were washed twice with 1.2 M sorbitol, transferred into ice-cold homogenization buffer (0.6 M sorbitol, 20 mM Tris-Cl [pH 7.4], 2 mM phenylmethylsulfonyl fluoride), and homogenized in a Dounce homogenizer (25 strokes). The homogenate was then centrifuged at 4,000 × g, and the pellet was discarded. After the supernatant was centrifuged again, the supernatant was centrifuged at 10,000 × g for 20 min. The ER-enriched pellet was dissolved in 1 M NaOH, neutralized, and processed for fructosamine and protein measurements as described above.
Measurement of steady-state ion levels and 45Ca2+ uptake rates.
Total cellular Ca2+ levels were measured by using an Eppendorf Efox 5053 flame photometer as previously described (30). Briefly, cells were grown to a cell density of <1 A600 U/ml and harvested by centrifugation at room temperature (5 min at 10,000 × g). A single sample contained ca. 100 A600 U of cells. The samples were transferred into microcentrifuge tubes of known weight and centrifuged at room temperature at 15,000 × g for 10 min. The supernatants were carefully removed, and the sample measured gravimetrically on an analytical balance. Each sample was dried in a SpeedVac (Savant) vacuum dessiccator for 3 h, and the dry weight of the samples measured gravimetrically. A total of 0.6 ml of 1 M HCl was added to the dry samples, and the samples were vortexed. The samples were incubated on a rocker table for 24 h and then centrifuged at 15,000 × g for 5 min. The supernatants were removed, and the indicated elemental measurements were then carried out.
The rate of high-affinity Ca2+ uptake in intact yeast strains was measured as described previously (8, 11). Exponential phase cells were harvested, washed, and resuspended in buffer containing 40 mM morpholinepropanesulfonic acid-Tris (pH 6.0) and 20 mM d-galactose. Cells were incubated for 10 min at 30°C. The uptake experiment was started by the addition of 45Ca2+ to a final concentration of 1 mCi/ml. At the indicated time points, 1-ml aliquots of cells were filtered through 0.45-mm (pore-size) Gelman GN-6 Metricel filters prewashed with buffer containing 20 mM MgCl2 and 0.2 mM LaCl3. The filters were then washed three times with the same buffer, and cell-associated 45Ca2+ was determined by liquid scintillation counting. In all experiments, triplicate samples were taken at each time point to ensure reproducibility.
RESULTS
A mutant yeast strain lacking hexokinase and glucokinase activities accumulates high levels of intracellular glucose.
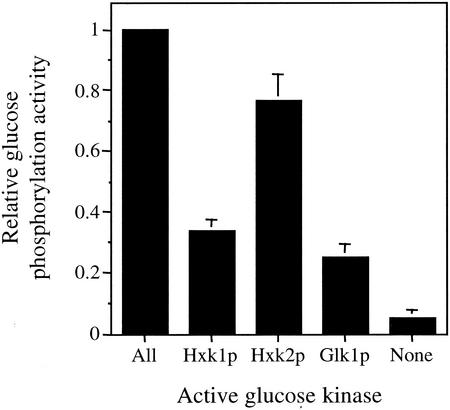
We initially examined the ability of strains expressing only one of the three glucose-phosphorylating enzymes (Hxk1p, Hxk2p, or Glk1p) to phosphorylate glucose when grown in YPGal medium (Fig. 1). We found that the strain expressing only Hxk2p contained ∼77% of normal glucose phosphorylation activity, while the strains containing only Hxk1p or Glk1p contained 34 and 25% of the normal glucose phosphorylation activity, respectively. In the strain lacking the activity of all three enzymes, we measured only very low (∼5%) residual activity. These relative levels are similar to those published previously (32).
FIG. 1.
Relative glucose phosphorylation activity in extracts prepared from strains containing different hexokinase or glucokinase activities. The kinase(s) remaining in each strain is indicated. Glucose phosphorylation was determined in an assay measuring the consumption of glucose as described in Materials and Methods.
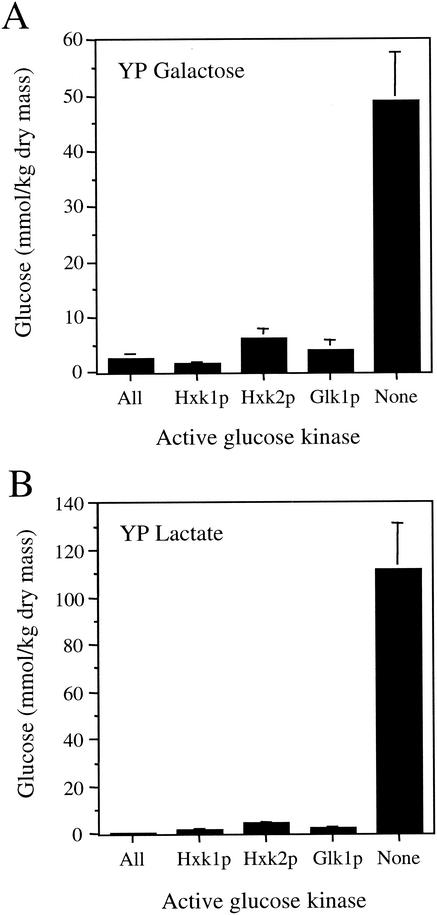
We next assayed the glucose levels present in cell extracts from these strains after an extended growth period (five to seven generations) in YPGal or YPLct medium. In agreement with earlier observations (32), we found that the intracellular glucose levels were relatively low in wild-type and mutant S. cerevisiae strains lacking two of the three glucose-phosphorylating enzymes when grown in medium containing galactose or lactate as a carbon source (Fig. 2). The wild-type strain with all three active kinases contained 0.5 to 2 mmol of glucose/kg (dry mass), depending on the growth conditions. Double mutants that retained only one of three glucose-phosphorylating enzymes generally contained a modestly higher glucose level than the wild-type strain. The strain containing only Hxk1p contained 1.5 to 2 mmol of glucose/kg (dry mass), whereas the strain containing only Hxk2p contained 4 to 6 mmol of glucose/kg (dry mass). The strain that contained only Glk1p activity contained 2 to 4 mmol of glucose/kg (dry mass). This latter finding is consistent with a previous study showing that a strain containing only Glk1p activity accumulates more glucose than normal after glucose is depleted from the growth medium (6).
FIG. 2.
Intracellular glucose levels measured in extracts from strains containing different hexokinase or glucokinase activities. The kinase(s) remaining in each strain is indicated.
In contrast to the small increases in the level of free glucose observed in strains lacking two of the three kinases, we found that a strain lacking all three glucose-phosphorylation enzymes accumulated extremely high levels of glucose. When grown in YPGal medium, this strain contained 49 mmol of glucose/kg (dry mass), a level that was ∼20-fold higher than that of the wild-type strain. When grown in YPLct medium, the glucose level in the mutant strain was 112 mmol of glucose/kg (dry mass) (∼225-fold higher than that of the wild-type strain). These results suggest that glucose produced as a result of some aspect of cellular metabolism leads to the accumulation of high glucose levels as a consequence of the inability of this strain to rephosphorylate free glucose.
The accumulated glucose in the phosphorylation mutant is sequestered in an intracellular compartment.
The Hxk1p and Glk1p of S. cerevisiae are cytosolic enzymes, whereas Hxk2p shuttles between the cytosol and nucleus (24). One purpose of the rapid phosphorylation of transported glucose is to trap it within the cell, since cytosolic glucose is rapidly released from yeast cells in the absence of its conversion to Glc-6-P (3, 6, 29). Based upon this observation, our finding that excess free glucose accumulates to an extremely high level in the glucose phosphorylation mutant suggested that the glucose may be sequestered within an intracellular compartment.
It was previously shown that a yeast mutant that lacked the ability to phosphorylate glucose can still carry out high-affinity glucose transport (29). This allowed us to examine whether the accumulated glucose could readily interchange with radiolabeled glucose transported from the extracellular environment. The mutant strain lacking the ability to phosphorylate glucose was grown in YPGal medium and then shifted to YP medium containing 1 mM [14C]glucose for 15 min. The cell-associated [14C]glucose and total glucose concentrations were then determined before and after the cells were washed three times in glucose-free YP medium. We found that ∼1 mM [14C]glucose was accumulated in cells after the initial incubation, but that [14C]glucose does not readily interchange with accumulated intracellular glucose in the glucose phosphorylation-deficient strain. The mean cellular glucose concentrations for endogenous glucose were 39.5 ± 6.6 and 37.9 ± 3.9 mM before and after washing, respectively. The mean cellular glucose concentrations for [14C]glucose were 1.11 ± 0.09 and 0.018 ± 0.005 mM before and after washing, respectively. Thus, a large majority of this [14C]glucose (∼98%) could be released from the cells by the wash procedure. In contrast, only ∼5% of the total cellular glucose was lost during the washes, indicating that the bulk of the accumulated glucose resides in a nonexchangeable pool that is distinct from the recently transported cytosolic [14C]glucose pool. These results indicate that the accumulated glucose is sequestered in an intracellular compartment in this strain. Consistent with this conclusion, centrifugation of a cell lysate prepared at 10,000 × g led to the recovery of a significant fraction of this glucose in the pellet fraction (data not shown).
Excess glucose accumulation is attributable to core oligosaccharide trimming in the ER.
Only a limited number of catabolic reactions result in the production of free glucose. First, glucose can be produced from the degradation of reserve polysaccharides, such as glycogen and trehalose in S. cerevisiae. Free glucose results from 8 to 10% of glucose units liberated from glycogen by the 1,6-glycosidase, whereas glucose linked by 1,4-glycoside bonds are cleaved and phosphorylated by glycogen phosphorylase (31). Glucose can also be produced by the breakdown of trehalose [α-d-glucopyranosyl-(1-1)-(α-d-glucopyranoside)], which can be cleaved to two glucose molecules directly by neutral trehalase (1, 18). However, these enzymes and their substrates are largely cytosolic. Hence, their contribution to organellar (compartmentalized) glucose accumulation is unlikely.
In contrast to the cytosolic degradation of glycogen or trehalose, the processing of N-linked oligosaccharides within the ER could result in the generation of a compartmentalized pool of glucose. In S. cerevisiae, the biosynthesis of the lipid-linked oligosaccharide precursor, Glc3Man9GlcNAc2, is similar to that seen in higher eukaryotes (12, 14, 33). After the translocation of the core oligosaccharide from dolichol phosphate to a susceptible asparagine on the nascent protein in the lumen of the ER, three glucoses and a mannose are removed by glucosidases (16). Subsequently, the protein containing the trimmed core oligosaccharide is transported to the Golgi apparatus for final oligosaccharide side chain processing.
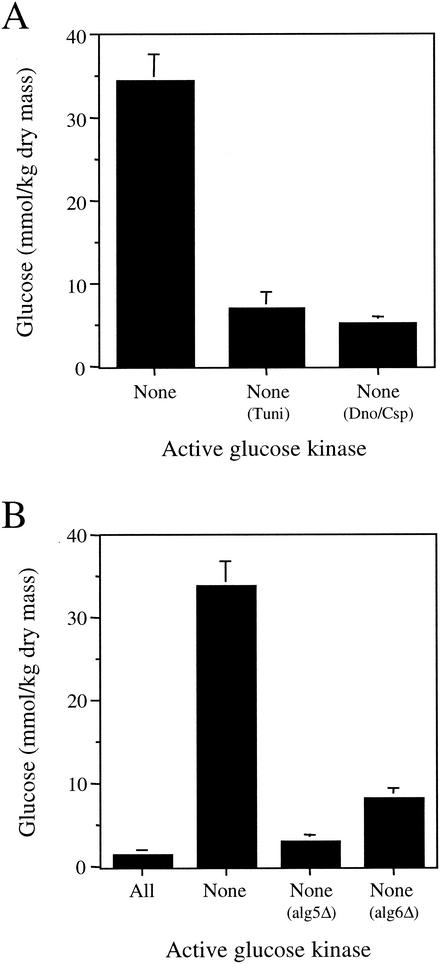
Tunicamycin inhibits the enzyme UDP-N-acetylglucosamine-1-P transferase by competitively blocking the linkage of N-acetylglucosamine to dolichol phosphate (19, 33). We found that the addition of 1 μM tunicamycin to the culture medium of the strain that lacks glucose phosphorylation activity reduced the glucose accumulation observed in the glucose phosphorylation mutant strain by more than fivefold (Fig. 3A). However, the addition of 1 μM tunicamycin also reduced the growth rate of this strain significantly. This raised the possibility that the reduction in glucose accumulation was a secondary consequence of the slower growth rate.
FIG. 3.
Compounds that inhibit N-linked oligosaccharide processing reduce cellular glucose levels in the glucose phosphorylation mutant. (A) Effect of 1 μM tunicamycin (Tuni) or a combination of 1 mM 1-deoxynojirimycin and 1 mM castanospermine (Dno/Csp) on cellular glucose levels in the yeast mutant unable to phosphorylate glucose. (B) Effect of deleting the ALG5 or ALG6 genes on cellular glucose levels in the yeast mutant unable to phosphorylate glucose.
As a separate means of examining the contribution of core oligosaccharide trimming to glucose accumulation in the glucose phosphorylation mutant, we next examined the effect of inhibiting the enzymes responsible for glucose trimming. Castanospermine and 1-deoxynojirimycin inhibit the glucosidases that trim the glucose residues from the core oligosaccharide (28). Treatment of the glucose phosphorylation mutant with a combination of 1-deoxynojirimycin and castanospermine resulted in a sixfold reduction of cellular glucose levels (Fig. 3A). Unlike tunicamycin treatment, the addition of these glucosidase inhibitors did not alter the growth rate of cells. These results provide additional evidence that the trimming of core oligosaccharides within the ER is responsible for the majority of the glucose accumulation observed in this strain.
To confirm this conclusion, we next used a genetic approach to prevent the generation of free glucose via the trimming of asparagine-linked oligosaccharides in the ER. We constructed two new strains that lacked the three enzymes responsible for the phosphorylation of glucose. In addition, these strains also contained a deletion of either the gene encoding UDP-glucose dolichol phosphate glucosyltransferase (alg5Δ) or dolichol-phosphate-mannose-protein mannosyltransferase (alg6Δ). It has previously been shown that both alg5Δ and alg6Δ mutants are unable to attach glucose residues to the core oligosaccharide during its synthesis (13, 15, 27). Consequently, the release of free glucose by trimming of the core oligosaccharide chain should not occur in these strains. We found that the glucose phosphorylation-deficient alg5Δ strain accumulated 11-fold-less glucose, whereas the glucose phosphorylation-deficient alg6Δ strain accumulated 4-fold-less glucose than the parent glucose phosphorylation strain (Fig. 3B). These results confirm that the trimming of glucose from asparagine-linked oligosaccharides is largely responsible for the elevation of cellular glucose in the strain that lacks the capacity to phosphorylate glucose to Glc-6-P.
Glucose is released from the glucose phosphorylation-deficient strain and accumulates in the culture medium via transit through the secretory pathway.
Our results suggest that glucose accumulates as a result of core oligosaccharide trimming in the glucose phosphorylation mutant strain. Since the trimming of these oligosaccharide chains takes place exclusively in the ER, a compartment that comprises only a small fraction of the total volume of a yeast cell, the actual glucose concentration in this organelle must be significantly higher. In addition, we hypothesized that the presence of this large reservoir of glucose in a compartment of the secretory pathway could lead to the passive release of glucose from the cell.
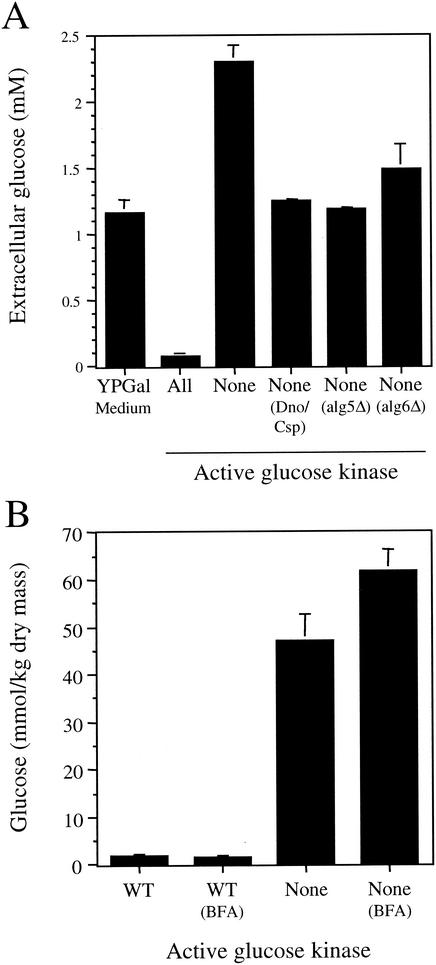
To test this possibility, we assayed for the net appearance of glucose in the culture medium. YPGal medium normally contains a small amount of glucose (∼1.2 mM) due to its presence as a trace contaminant in one or more components of this medium (Fig. 4A). To test for the appearance of glucose in this growth medium, we grew the strains for 36 h, which was a sufficient length of time to metabolize all of the galactose present in the culture medium. We found that the small amount of free glucose initially present in the culture medium was consumed by the wild-type strain (probably before the utilization of galactose was initiated). In contrast, we measured a level of ∼2.3 mM glucose in the YPGal medium used to grow the glucose phosphorylation mutant strain. This represented a net increase of ∼1.1 mM glucose to the glucose concentration of the starting culture medium and an increase of ∼2.2 mM glucose above the concentration measured in the medium in which the wild-type strain was grown. These results indicate that glucose is released from the glucose phosphorylation mutant under these growth conditions. Glucose release into the medium was greatly reduced when the strain was grown either in the presence of 1-deoxymojirimycin and castanospermine or when the ALG5 or ALG6 genes were knocked out. These results demonstrate that a significant amount of the glucose that accumulates from core oligosaccharide trimming can be released into the extracellular environment.
FIG. 4.
The glucose phosphorylation mutant releases glucose into the culture medium via the secretory pathway. (A) Amount of glucose released into the culture medium by the indicated strains grown in YPGal medium or YPGal supplemented with 1 mM 1-deoxynojirimycin and 1 mM castanospermine (Dno/Csp). Cultures were grown for 36 h. (B) Concentrations of glucose accumulated in cells after BFA treatment. Cultures of the wild-type and glucose phosphorylation mutant strains grown in YPGal medium were divided into two aliquots. BFA (224 μg/ml) was added to one aliquot from each strain, and incubation was continued under the same culture conditions for 4.5 h before the cells were harvested and the cellular glucose levels were determined.
Like mammalian cells, the route of glucose release from the glucose phosphorylation mutant could occur through the cytosol or the secretory pathway (10). This led us to test whether glucose release occurs via transit through the secretory pathway in this strain. Since core oligosaccharide trimming and glucose accumulation is a relatively slow process that requires continuing protein synthesis, we felt it was necessary to impose a partial block in vesicular transport over several generations rather than a complete block that would lead to a cessation of protein synthesis. The fungal metabolite BFA acts to disassemble the Golgi complex and collapse it into the ER (5). Yeast strains are relatively impermeant to this compound unless a mutation in sterol synthesis is present (9). However, since we desired only a partial block in Golgi traffic, we examined the effect of BFA on glucose accumulation in our strains in the absence of such a mutation. Cells grown in YPGal medium were incubated with BFA for 4.5 h (∼3 generations), and the amount of cell-associated glucose was determined (Fig. 4B). We found that this BFA treatment had no effect on the low level of cellular glucose present in the wild-type strain. However, we observed a substantial (32%) increase in cell-associated glucose in the glucose phosphorylation mutant. These results indicate that a significant fraction of the glucose lost into the culture medium from this strain is released via transit through the secretory pathway.
Glucose accumulation in the glucose phosphorylation mutant is associated with the nonenzymatic glycation of proteins and alterations in ion homeostasis.
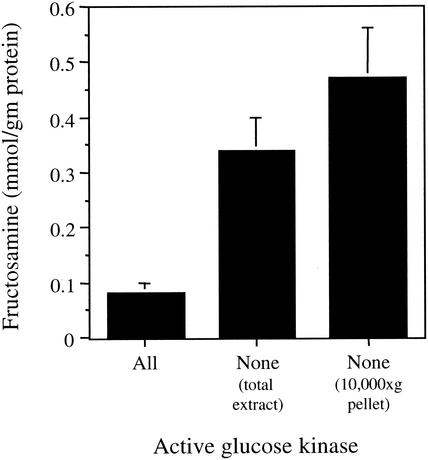
Nonenzymatic biochemical reactions between carbohydrates and proteins can occur at significant levels in biological systems (2). Such glycation reactions represent a central component of the pathology associated with diabetes, particularly in individuals who frequently experience hyperglycemia (25). The measurement of fructosamine is commonly used to monitor plasma protein glycation in diabetic patents. Because we had observed a high concentration of glucose accumulation in the yeast glucose phosphorylation mutant, we next sought to determine whether this also resulted in an increase in protein glycation. The glucose phosphorylation mutant strain was grown in YPGal medium, and the fructosamine concentration was then determined in crude extracts. We found that the fructosamine concentration was ∼4-fold higher in the glucose phosphorylation-deficient mutant than in the wild-type strain (Fig. 5). The fructosamine level was even higher (∼6-fold) than wild type in a 10,000 × g pellet from the glucose phosphorylation mutant. Since the yeast ER is enriched in the 10,000 × g pellet, this result is consistent with the accumulation of glucose in the ER compartment. These results suggest that protein glycation could possibly alter the function of not only resident ER proteins but also any other proteins that transit through the ER on their way to the cell surface.
FIG. 5.
Fructosamine levels measured in cell extracts indicate that nonenzymatic protein glycation is increased in the glucose phosphorylation-deficient strain. Cells were grown in YPGal medium at 30°C.
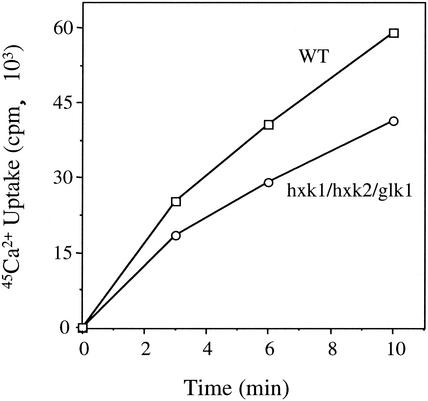
We next sought to determine whether these high levels of cellular glucose had any adverse effects on protein function that could lead to alterations in cellular physiology. Since cellular ion homeostasis is dependent upon an assortment of channels and transporters that transit to their final cellular location (such as the ER, Golgi apparatus, vacuole, and plasma membrane) via the secretory pathway, we first examined the steady-state cellular levels of various cations by flame photometry (Table 1). We found that steady-state ion levels were altered in a subtle manner in the glucose phosphorylation mutant. When the level of divalent cations was examined, we found that the steady-state Ca2+ level was increased 6%, whereas the Mg2+ level was decreased 13%. Similarly, our analysis of monovalent cations indicated that the steady-state Na+ level was increased 15%, whereas the K+ level was decreased 6%. Thus, the ratios of Mg2+/Ca2+ and K+/Na+ were both reduced by 18%. These small, compensatory changes in the relative amounts of both divalent and monovalent cations suggest that increased cellular glucose may correlate with alterations in ion homeostasis in the glucose phosphorylation-deficient strain. Since these changes in the steady-state levels of these ions were relatively small, we next examined the high-affinity 45Ca2+ uptake rate in the glucose phosphorylation-deficient and wild-type strains (Fig. 6). We found that high-affinity Ca2+ uptake was reduced 30 to 40% in the mutant strain in multiple experiments. Taken together, these results demonstrate that ER glucose accumulation leads to significant alterations in cellular ion homeostasis.
TABLE 1.
Cellular ion levels in the glucose phosphorylation-deficient straina
| Active kinase | Mean ion level (mmol/kg [dry mass]) ± SD
|
|||
|---|---|---|---|---|
| Ca2+ | Mg2+ | K+ | Na+ | |
| All | 5.21 ± 0.06 | 58.6 ± 5.2 | 691.2 ± 8.7 | 66.6 ± 0.8 |
| None | 5.53 ± 0.28 | 51.0 ± 1.0 | 650.5 ± 11.9 | 76.7 ± 9.0 |
Ion levels were determined by flame photometry.
FIG. 6.
High-affinity Ca2+ uptake is significantly reduced in the glucose phosphorylation mutant strain. Cells grown in YPGal medium at 30°C were harvested at a cell density of ≤1.0 A600 U/ml, and the rate of Ca2+ uptake was determined as described in Materials and Methods.
DISCUSSION
The results of the present study demonstrate that a yeast strain lacking the three enzymes responsible for the phosphorylation of glucose to Glc-6-P (Hxk1p, Hxk2p, and Glk1p) accumulates a high level of intracellular glucose when grown in medium containing either galactose or lactate as carbon source. The results of our study suggest that this glucose accumulates as a result of the trimming of N-linked core oligosaccharides within the lumen of the ER. We used both [14C]glucose exchange and subcellular fractionation experiments to show that the glucose produced by the trimming of N-linked core oligosaccharides accumulates in an intracellular compartment. Since the enzymes responsible for the rephosphorylation of the glucose generated in this manner are largely cytosolic, the glucose produced by oligosaccharide trimming must normally be transported from the ER to the cytosol prior to its reconversion to Glc-6-P. This predicts the existence of an ER-localized glucose transporter to carry out this important task. In light of this reasoning, our finding that ER glucose accumulation occurs only when the capacity for glucose phosphorylation is completely eliminated suggests that a mechanism exists to couple glucose transport from the ER to its efficient conversion to Glc-6-P in the cytosol. Further studies are required to determine whether a specific glucose transporter resides in the yeast ER membrane where it facilitates the recycling of glucose produced by core oligosaccharide trimming.
Previous studies have demonstrated that some enzymes responsible for glucose phosphorylation can bind to various intracellular membranes. For example, mammalian hexokinase binds to the cytosolic face of both mitochondria and ER membranes (21). Our finding that much of the accumulated glucose is located in the ER suggests that a mechanism coupling glucose transport from the ER to its immediate rephosphorylation to Glc-6-P may have evolved to provide the most efficient reentry of this glucose into cellular metabolic pathways. This model predicts that one or more of the enzymes responsible for the conversion of glucose to Glc-6-P may also associate with the yeast ER membrane. Interestingly, it has been shown that yeast Hxk2p exhibits a dual cytosolic-nuclear localization (24). Since the cortical ER membrane is contiguous with the nuclear membrane, further studies should examine whether this nuclear localization of Hxk2p may also include the associated ER compartment involved in protein translocation and asparagine-linked glycosylation. However, given our finding that the accumulation of ER glucose can be suppressed by the presence of any one of the three enzymes Hxk1p, Hxk2p, or Glk1p, it appears that each of these enzymes can independently trigger glucose transport from the ER and its subsequent phosphorylation to Glc-6-P. This suggests that each of these enzymes may be capable of associating with the ER membrane. Alternatively, the gating of the glucose release channel may be regulated by an indirect mechanism that does not require the direct association of any of these enzymes.
When the glucose phosphorylation mutant strain was grown in YPGal medium, we found that the glucose content was ∼49 mmol/kg (dry mass). Based upon this value and gravimetric determinations of the total wet and dry weights of cells grown under these conditions, we calculate that the total intracellular glucose concentration in the aqueous fraction is remarkably high (∼17 mM). Since the cellular location of core oligosaccharide trimming is the ER, the local concentration of glucose in this compartmentalized pool is likely to be significantly (more than an order of magnitude) higher. Since high concentrations of osmolytes can have a significant effect on protein folding, as well as influence the function of molecular chaperones (7), this high concentration of glucose has the potential to affect protein folding in the ER. Finally, given the volume of cells relative to the total culture volume, we can further calculate that the quantitative release of this glucose would increase the glucose concentration in the culture medium by ∼0.4 mM. When combined with the 1.1 mM glucose that was measured into the culture medium, we conclude that a total of ∼1.5 mM glucose is trapped in this nonmetabolizable state when the capacity to phosphorylate glucose is lost. This amount represents 1.25% of the 120 mM galactose that was originally present in the YPGal culture medium.
Our results also indicate that the high concentration of ER glucose that accumulates in the absence of glucose phosphorylation leads to an elevated level of nonenzymatic protein glycation. This result is strikingly similar to the elevated level of protein glycation observed in diabetic patients. Based upon this observation, we sought to determine whether any adverse consequences that may result from increased protein glycation could be detected. We found that the absolute levels of Ca2+, Na+, Mg2+, and K+ are all slightly altered in the glucose phosphorylation mutant strain. As a result, the ratios of Mg2+/Ca2+ and K+/Na+ were both reduced by 18%. Furthermore, the rate of high-affinity Ca2+ uptake was reduced 30 to 40% in the glucose phosphorylation mutant strain. The compensatory changes in the relative levels of these monovalent and divalent ions and the significantly reduced rate of Ca2+ uptake together suggest that a subset of plasma membrane proteins responsible for ion transport into the cell may be compromised to various extents as a consequence of the excessive level of glucose present in the secretory pathway. Further experiments are required to determine whether these alterations in ion homeostasis are attributable to high glucose levels on protein folding in the ER, to the nonenzymatic glycation of proteins, or to a combination of these mechanisms.
Acknowledgments
We thank Daniel G. Frankel for providing strains and helpful discussions. We also thank Pappné Bácskai Sarolta and Qun Zeng for technical assistance.
This work was supported by Hungarian NSF grants OTKA T-038144 and NKFP grant 1/026/2001 (A.M.) and JDF grant 99502 and AHA (SE affiliate) grant 0255121B (D.M.B.).
REFERENCES
- 1.App, H., and H. Holzer. 1989. Purification and characterization of neutral trehalase from the yeast ABYS1 mutant. J. Biol. Chem. 264:17583-17588. [PubMed] [Google Scholar]
- 2.Baynes, J. W. 2001. The role of AGEs in aging: causation or correlation. Exp. Gerontol. 36:1527-1537. [DOI] [PubMed] [Google Scholar]
- 3.Becker, J. U., and A. Betz. 1972. Membrane transport as controlling pacemaker of glycolysis in Saccharomyces carlsbergensis. Biochim. Biophys. Acta 274:584-597. [DOI] [PubMed] [Google Scholar]
- 4.Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 5.Chardin, P., and F. McCormick. 1999. Brefeldin A: the advantage of being uncompetitive. Cell 97:153-155. [DOI] [PubMed] [Google Scholar]
- 6.Clifton, D., R. B. Walsh, and D. G. Fraenkel. 1993. Functional studies of yeast glucokinase. J. Bacteriol. 175:3289-3294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Diamant, S., N. Eliahu, D. Rosenthal, and P. Goloubinoff. 2001. Chemical chaperones regulate molecular chaperones in vitro and in cells under combined salt and heat stresses. J. Biol. Chem. 276:39586-39591. [DOI] [PubMed] [Google Scholar]
- 8.Fu, L., A. Miseta, D. Hunton, R. B. Marchase, and D. M. Bedwell. 2000. Loss of the major isoform of phosphoglucomutase results in altered calcium homeostasis in Saccharomyces cerevisiae. J. Biol. Chem. 275:5431-5440. [DOI] [PubMed] [Google Scholar]
- 9.Graham, T. R., P. A. Scott, and S. D. Emr. 1993. Brefeldin A reversibly blocks early but not late protein transport steps in the yeast secretory pathway. EMBO J. 12:869-877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guillam, M. T., R. Burcelin, and B. Thorens. 1998. Normal hepatic glucose production in the absence of GLUT2 reveals an alternative pathway for glucose release from hepatocytes. Proc. Natl. Acad. Sci. USA 95:12317-12321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Halachmi, D., and Y. Eilam. 1996. Elevated cytosolic free Ca2+ concentrations and massive Ca2+ accumulation within vacuoles, in yeast mutant lacking PMR1, a homolog of Ca2+-ATPase. FEBS Lett. 392:194-200. [DOI] [PubMed] [Google Scholar]
- 12.Haselbeck, A., and R. Schekman. 1986. Interorganelle transfer and glycosylation of yeast invertase in vitro. Proc. Natl. Acad. Sci. USA 83:2017-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heesen, S., L. Lehle, A. Weissmann, and M. Aebi. 1994. Isolation of the ALG5 locus encoding the UDP-glucose:dolichyl-phosphate glucosyltransferase from Saccharomyces cerevisiae. Eur. J. Biochem. 224:71-79. [DOI] [PubMed] [Google Scholar]
- 14.Herscovics, A., and P. Orlean. 1993. Glycoprotein biosynthesis in yeast. FASEB J. 7:540-550. [DOI] [PubMed] [Google Scholar]
- 15.Huffaker, T. C., and P. W. Robbins. 1983. Yeast mutants deficient in protein glycosylation. Proc. Natl. Acad. Sci. USA 80:7466-7470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jakob, C. A., P. Burda, S. ten Heesen, M. Aebi, and J. Roth. 1998. Genetic tailoring of N-linked oligosaccharides: the role of glucose residues in glycoprotein processing of Saccharomyces cerevisiae in vivo. Glycobiology 8:155-164. [DOI] [PubMed] [Google Scholar]
- 17.Johnson, R. N., P. A. Metcalf, and J. R. Baker. 1983. Fructosamine: a new approach to the estimation of serum glycosylprotein: an index of diabetic control. Clin. Chim Acta 127:87-95. [DOI] [PubMed] [Google Scholar]
- 18.Kopp, M., H. Muller, and H. Holzer. 1993. Molecular analysis of the neutral trehalase gene from Saccharomyces cerevisiae. J. Biol. Chem. 268:4766-4774. [PubMed] [Google Scholar]
- 19.Lennon, K., A. Bird, Y. F. Chen, R. Pretel, and M. A. Kukuruzinska. 1997. The dual role of mRNA half-lives in the expression of the yeast ALG7 gene. Mol. Cell Biochem. 169:95-106. [DOI] [PubMed] [Google Scholar]
- 20.Lowry, O. H., N. J. Rosebrough, A. L. Farr, and R. J. Randall. 1951. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193:265-275. [PubMed] [Google Scholar]
- 21.Magnani, M., G. Serafini, R. Crinelli, A. Antonelli, M. Malatesta, and G. Gazzanelli. 1993. Intracellular distribution of hexokinase in rabbit brain. Mol. Cell Biochem. 122:123-132. [DOI] [PubMed] [Google Scholar]
- 22.Meyer, J., A. Walker-Jonah, and C. P. Hollenberg. 1991. Galactokinase encoded by GAL1 is a bifunctional protein required for induction of the GAL genes in Kluyveromyces lactis and is able to suppress the gal3 phenotype in Saccharomyces cerevisiae. Mol. Cell. Biol. 11:5454-5461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nehlin, J. O., M. Carlberg, and H. Ronne. 1991. Control of yeast GAL genes by MIG1 repressor: a transcriptional cascade in the glucose response. EMBO J. 10:3373-3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Randez-Gil, F., P. Herrero, P. Sanz, J. A. Prieto, and F. Moreno. 1998. Hexokinase PII has a double cytosolic-nuclear localisation in Saccharomyces cerevisiae. FEBS Lett. 425:475-478. [DOI] [PubMed] [Google Scholar]
- 25.Resnick, H. E., and B. V. Howard. 2002. Diabetes and cardiovascular disease. Annu. Rev. Med. 53:245-267. [DOI] [PubMed] [Google Scholar]
- 26.Rose, M. D., F. Winston, and P. Heiter. 1990. Methods in yeast genetics: a laboratory course manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 27.Runge, K. W., T. C. Huffaker, and P. W. Robbins. 1984. Two yeast mutations in glucosylation steps of the asparagine glycosylation pathway. J. Biol. Chem. 259:412-417. [PubMed] [Google Scholar]
- 28.Simons, J. F., M. Ebersold, and A. Helenius. 1998. Cell wall 1,6-β-glucan synthesis in Saccharomyces cerevisiae depends on ER glucosidases I and II, and the molecular chaperone BiP/Kar2p. EMBO J. 17:396-405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smits, H. P., G. J. Smits, P. W. Postma, M. C. Walsh, and K. van Dam. 1996. High-affinity glucose uptake in Saccharomyces cerevisiae is not dependent on the presence of glucose-phosphorylating enzymes. Yeast 12:439-447. [DOI] [PubMed] [Google Scholar]
- 30.Tokes-Fuzesi, M., D. M. Bedwell, I. Repa, K. Sipos, B. Sumegi, A. Rab, and A. Miseta. 2002. Hexose phosphorylation and the putative calcium channel component Mid1p are required for the hexose-induced transient elevation of cytosolic calcium response in Saccharomyces cerevisiae. Mol. Microbiol. 44:1299-1308. [DOI] [PubMed] [Google Scholar]
- 31.Voet, D., and J. G. Voet. 1995. Biochemistry, 2nd ed. John Wiley & Sons, Inc., New York, N.Y.
- 32.Walsh, R. B., D. Clifton, J. Horak, and D. G. Fraenkel. 1991. Saccharomyces cerevisiae null mutants in glucose phosphorylation: metabolism and invertase expression. Genetics 128:521-527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ziegler, F. D., T. R. Gemmill, and R. B. Trimble. 1994. Glycoprotein synthesis in yeast: early events in N-linked oligosaccharide processing in Schizosaccharomyces pombe. J. Biol. Chem. 269:12527-12535. [PubMed] [Google Scholar]