Abstract
Serum albumin is the most abundant protein in the circulatory system. The ability of albumins to undergo a reversible conformational transition, observed with changes in pH, is conserved in distantly related species, suggesting for it a major physiological role possibly related to the transport of small molecules including drugs. We have followed changes of bovine serum albumin (BSA) in volume by densimetry and in adiabatic compressibility during its conformational transition from pH 7–2, using ultrasound measurements. In parallel, circular dichroism was measured. The volume and adiabatic compressibility decrease from pH 4 to 2. The change in ellipticity shows a decrease over the same pH range from 70% to 40% of its α-helix content. Sorbitol, at concentrations from 0 to 2 M, led to the progressive restoration of BSA volume and compressibility values, as well as a substantial recovery of its original α-helix content. This finding implies that the compressibility variation observed reflects the conformational changes during the transition. The mutual interactions of the mechanical properties and structural features of BSA reported here are important in biotechnology for research in material sciences and for the design and the development of new, tailor-made drug carriers.
INTRODUCTION
Serum albumin is the most abundant protein in the circulatory system. At neutral pH, the crystal structure of the 66-kD protein reveals a heart-shaped molecule organized into three similar structural domains, each subdivided into two subdomains. The domains are predominantly α-helical (70%) and include loops and a large number (17) of disulfide bonds. The sequence of bovine serum albumin (BSA) is 76%—identical to that of human serum albumin (HSA)—the main difference being the presence of a second tryptophan residue in position 131 in the bovine protein (1,2). Serum albumin plays a key role in the transport of a large number of metabolites, endogenous ligands, fatty acids, bilirubin, hormones, anesthetics, and commonly used drugs, which may be delivered to the appropriate cellular targets. For example, it has been found that more than 50% of a clinically administered general anesthetic will be bound to HSA (3). A detailed structural basis of the drug-binding specificity of HSA has been derived from crystallographic analyses of 17 different complexes of HSA with a number of drugs and small molecule toxins (4).
The ability of albumins to undergo a major reversible conformational modification, induced by decreasing pH in the 7–2 range, was described several decades ago by Foster (5,6) and has been documented by a wide range of methods (7–10). Since no albumin crystal structure is so far available at low (or high) pH values, the conformational changes induced at these pH values are not known at atomic level resolution. However the conservation of the above transition in a number of animal species suggests for it an important physiological role, probably linked to the ligand/drug release and distribution mechanisms (2), making it an important problem to explore.
The elastic properties of proteins in solution yield information about the amplitude of their structural fluctuations. They have been shown to be particularly sensitive to interactions with solvent and also to the packing of the structure (11–14). Any protein folding-unfolding transition should therefore be reflected by corresponding changes in system volume and compressibility. Recent developments in acoustic techniques have made possible high precision ultrasound velocimetry measurements of small volume samples, leading to estimation of protein compressibility (15). As a consequence, volume and compressibility measurements have been increasingly used by investigators in the last few years for the characterization of protein conformational states (16–23), giving rise to a large body of information, in particular from the Chalikian group in Toronto (24–27).
We have characterized BSA in this work by ultrasound velocity between pH 7 and 2. The variation in partial specific volume and adiabatic compressibility associated with the pH-induced conformational transition have been determined. These have been correlated with the change of the α-helix content using circular dichroism (CD). Furthermore, to investigate the reversal of the conformational transition and its effect on the protein compressibility, we have carried out the refolding of BSA at pH 2 induced by sorbitol. This polyol has been used as a good osmolyte without direct interaction with the peptide backbone (28,29). By progressively increasing the sorbitol concentration from 0 to 3 M at pH 2, we have studied the substantial restoration of the α-helix content of BSA together with the adiabatic compressibility changes induced by the protein refolding. This procedure has enabled us to compare both compressibility and CD variation under similar conditions.
The interpretation of changes in compressibility is not trivial. It involves a number of parameters: mainly the contribution of conformational changes and protein surface hydration and the modification in size of the internal protein cavities. It is therefore difficult to evaluate specific contributions at present and there is a general agreement that adiabatic compressibility of globular proteins cannot be interpreted by correlation with a single variable. Nevertheless our aim is to investigate the possible correlation between compressibility variation and the partial unfolding and refolding of BSA at acid pH, with possible relevance to albumin function.
MATERIALS AND METHODS
Materials
BSA was purchased from Sigma (St. Louis, MO) (A-0281, 99% pure minimum, fatty acid free) and used without further purification. Sorbitol, crystallized 99% pure, was from FLUKA (Buchs, Switzerland). All the other chemicals were analytical grade. Water was of MILLIQ purity.
Sample preparation
BSA was dried under vacuum for at least 24 h and the samples prepared by weighing the dry protein in volumetric flasks (class A ± 0.04 ml) on a model AG 245 Metler Toledo balance (Greitensee, Switzerland) with a precision of ±0.03 mg. The protein concentration of the samples used for measurements was ∼3 mg/ml−1. The solvents used to make up the volume at 20°C, (water or 0.02 M phosphate buffer) were brought to the required pH value with 0.1 M HCl. The pH of the solutions was determined on a PHMB 82 Radiometer (Copenhagen, Denmark) pH meter.
Volumetric measurements
The densities of solvents and protein solutions were determined at 25°C ± 0.01°C, using a vibrating tube Anton Paar DMA 58 digital density meter (Graz, Austria). The precision in density measurements is better than 10−5 g/ml. Each set of determinations was carried out at least 10 times and the averaged value used to calculate the protein apparent specific volume ϕv using the well-known relationship
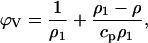 |
(1) |
where (ρ) and (ρ1) are the relative densities of the protein solution and of the reference solvent, respectively, and cp is the protein concentration.
Ultrasound velocity measurements
The method used is based on measured resonance peaks of an ultrasonic resonator (30,31), which offers, in addition to its simplicity, a high precision permitted by recent instrumental and computational advances.
The principle
Ultrasonic resonators consisting of a liquid sample (volume <1 ml) enclosed by two planar piezoelectric transducer plates (32) allow direct and accurate calculation of the ultrasound velocity, u, at a frequency of 5 MHz. The difference between two consecutive resonance peaks f0 and f1 is proportional to the ultrasound velocity variation u1– u0 where u1 and u0 are the ultrasound velocity in the solution and the solvent, respectively:
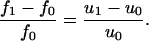 |
(2) |
The relative specific sound velocity increment is
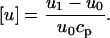 |
(3) |
The system
One of the potential limitations of the custom-built system resides in possible temperature drifts. This difficulty has been circumvented by the use of a set of tandem cells of identical acoustic path (15); experiments are carried out at 25°C, in a temperature-controlled room. The setup allows sequential determination of the ultrasonic frequency difference between a reference and a measuring cell at identical temperature, which achieves a considerable improvement in sensitivity. First, the two cells are filled with the reference liquid and measurements are carried out. In a second step the measuring cell is drained, rinsed, dried, and refilled with the liquid under investigation. Under these experimental conditions the precision obtained in ultrasound velocity variation is on the order of one part in 10−5. This requires perfect parallelism of the two transducers per cell and a quality coefficient of the resonator on the order of one part in 104.
Compressibility calculation
Measurement of the protein solution density (ρ) and that of the sound velocity (u) allows the determination of the solution adiabatic compressibility, β, using Laplace's equation:
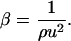 |
(4) |
Equations 1, 3, and 4 were used to calculate the partial specific apparent adiabatic compressibility, ϕk, of BSA using the following relationship:
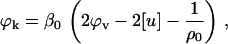 |
(5) |
where β0 is the coefficient of adiabatic compressibility of the solvent (12).
Circular dichroism
CD spectra were recorded using an AVIV model 62A (Aviv Associates, Lakewood, NJ) spectropolarimeter. The temperature of the sample was controlled at 25°C ± 0.1°C. In the far ultraviolet (UV), a 0.1-cm path length cuvette was employed. The results are expressed as mean residue ellipticities, defined as 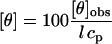 , where [θ]obs is the observed ellipticity in degrees, cp is the protein concentration in residue mol per liter, and l is the length of the cell light path in centimeters. Secondary structure analysis was carried out using the CD Pro suite of programs (33).
, where [θ]obs is the observed ellipticity in degrees, cp is the protein concentration in residue mol per liter, and l is the length of the cell light path in centimeters. Secondary structure analysis was carried out using the CD Pro suite of programs (33).
RESULTS
The BSA acid driven transition
Protonation of carboxylates releases water of electrostriction and generates volume changes, and the raw data are corrected accordingly (17,27,34). These volume changes are subtracted from the total specific volume difference ΔV, leaving the contribution of the conformational change, including all other forms of hydration and packing defects.
Volume
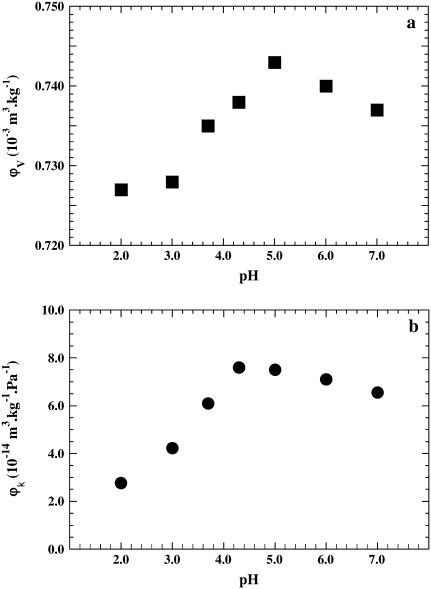
The volume variation curve of BSA as a function of pH is shown in Fig. 1 a. The profile displays two parts: first, an ascending part from pH 7.0 to 5.0, raising the volume from 0.737 to 0.743 × 10−3 m3 kg−1 and then a decrease to pH 3.0, the volume dropping then from 0.743 to 0.728 × 10−3 m3 kg−1. Between pH 3 and 2 no further decrease is observed. For the acid transition, is ΔV = −0.015 × 10−3 m3 kg−1.
FIGURE 1.
(a) Plot of ϕv, the apparent partial specific volume of BSA, as a function of pH. The standard deviation in volume measurements is ±0.003 × 10−3 m3 kg−1. (b) Plot of the partial specific adiabatic compressibility ϕk as a function of pH. The standard deviation in compressibility measurements is ±0.3 × 10−14 m3 kg−1 Pa−1. The contribution for protonation was taken into account (see text).
Adiabatic compressibility
The compressibility curve of BSA as a function of pH shows a profile very similar to that of the volumetric curve, with a shallow ascending part and a steeper descending one (Fig. 1 b). A careful inspection reveals, however, slight differences between the two curves. Between pH 7 and pH 4.3 a progressive increase of ϕk from 6.6 to 7.6 × 10−14 m3 kg−1 Pa−1 is observed: the specific compressibility difference is Δϕk = 1.0 × 10−14 m3 kg−1 Pa−1. The maximum of the compressibility curve is slightly shifted and located at a pH value around 4.3 in contrast to 5.0 in the volumetric curve. The compressibility then decreases continuously from 7.6 × 10−14 m3 kg−1 Pa−1 at pH 4.3 to 2.8 × 10−14 m3 kg−1 Pa−1 at pH 2.0, the difference in compressibility Δϕk being −4.8 × 10−14 m3 kg−1 Pa−1. As reported, the adiabatic compressibility variation during a conformational transition (here a 63% variation) is often larger than that of the protein-specific volume (2% variation) (35).
Circular dichroism
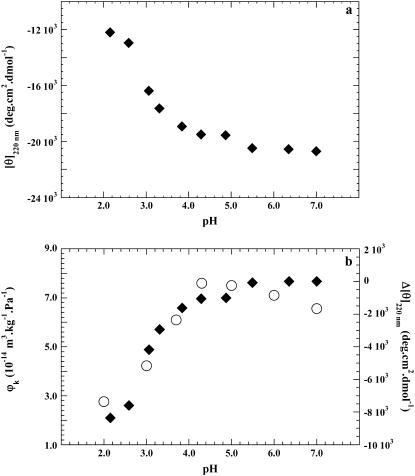
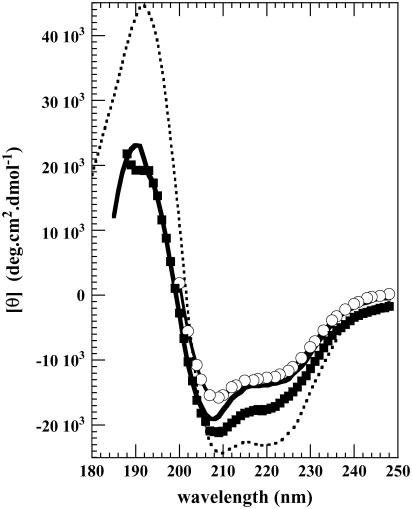
The CD of BSA was monitored in the far UV. Titration of the protein with 0.1 N HCl reveals only marginal changes in the secondary structure of BSA between pH 6.5 and 5 as judged from the ellipticities at 220 nm. Then [θ]220 becomes less negative with decreasing pH from about −22,100 deg cm2 dmol−1 between pH 7 to 5, to −12,700 deg cm2 dmol−1 at pH 2.0 (Fig. 2 a). These values are in good agreement with the literature (9,36). The curves measured by compressibility (on the left) and ellipticity difference Δ[θ]220 (on the right) superimpose well (Fig. 2 b), indicating that the main conformational change associated with changes in hellicity (in the 5–2 pH range) is responsible for the change in compressibility or, in other words, the two methods probe the same phenomenon.
FIGURE 2.
(a) CD of BSA. Plot of ellipticity [θ]220 versus pH. (b) Plot of the ellipticity difference on the right (♦). Δ[θ]220 represents the difference in ellipticity measured at pH 7 and at any pH value below 7. On the left, the partial specific adiabatic compressibility φk of BSA versus pH (○).
The sorbitol-induced transition
Osmolytes have been used by a number of investigators to induce the refolding of proteins and to stabilize them (28,29,37,38). We have used here sorbitol, an electrically neutral, low molecular weight polyol to induce refolding of BSA from its low pH unfolded state. Refolding was monitored by ellipticity, volume, and adiabatic compressibility. As a basis for comparison, we first examined the behavior of the BSA native state at pH 7 as a function of sorbitol concentration in the 0–3 M range.
Volume and compressibility at pH 7
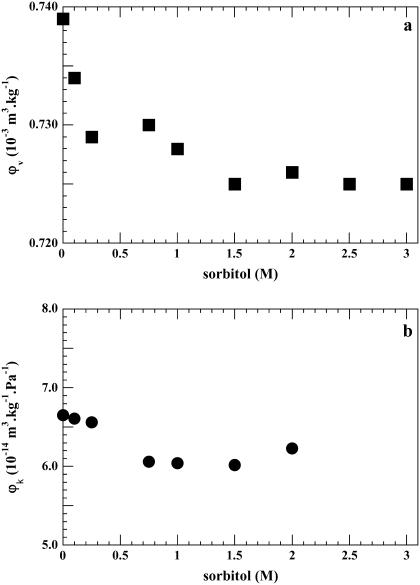
The effect of sorbitol on the specific volume of BSA at neutral pH is shown in Fig. 3 a. The partial specific volume decreases sharply as a function of sorbitol concentration from 0.739 × 10−3 m3 kg−1 in pure water to a value of 0.729 × 10−3 m3 kg−1 in 0.25 M sorbitol. It then decreases slightly to 0.725 × 10−3 m3 kg−1 at 1.5 M, remaining around this value up to 3M of the osmolyte.
FIGURE 3.
BSA at pH 7. (a) Plot of φv, the apparent partial specific volume, as a function of sorbitol molarity (M). (b) Plot of the adiabatic compressibility as a function of sorbitol (M). The standard deviation values are as above.
The partial specific adiabatic compressibility decreases from 6.6 × 10−14 m3 kg−1 Pa−1 in the absence of sorbitol to around 6.0 × 10−14 m3 kg−1 Pa−1 for 1.5 M sorbitol, coming then back to 6.2 × 10−14 m3 kg−1 Pa−1 for 2 M (Fig. 3 b). The observed decrease in volume and compressibility is consistent with the literature (39) and will be discussed below. These changes are not accompanied by changes in the protein conformation as judged from CD measurements (not shown), the overall secondary structure of BSA remaining stable in sorbitol at neutral pH.
Volume and compressibility at pH 2
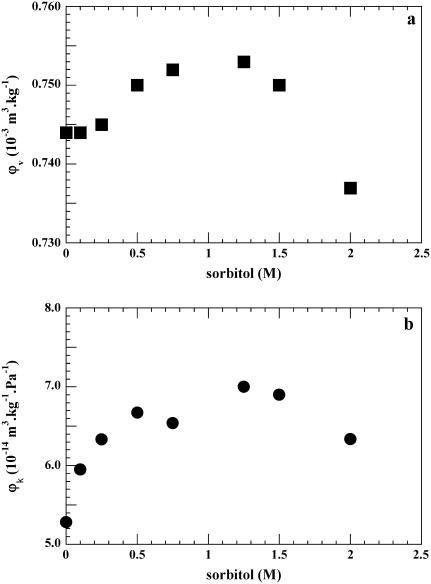
In another set of experiments we titrated the protein from pH 7 to pH 2.0 and kept that pH constant at all sorbitol concentrations. In contrast to the values obtained at pH 7, both volume and compressibility increase at pH 2 as a function of sorbitol (Fig. 4, a and b), both reaching a maximum at a concentration between 1.0 and 1.25 M sorbitol. The partial specific volume of BSA in sorbitol increases progressively from 0.744 × 10−3 m3 kg−1 in the absence of sorbitol at pH 2 to 0.753 × 10−3 m3 kg−1 at 0.5 M sorbitol; it then remains constant within the limits of error at 0.753 × 10−3 m3 kg−1 up to 1.5 M (Fig. 4 a). The volume difference at pH 2 is ΔV = 0.009 × 10−3 m3 kg−1. At higher concentrations the volume decreases to a value close to that of native BSA.
FIGURE 4.
BSA at pH 2. (a) Plot of φv, the apparent partial specific volume, as a function of sorbitol molarity (M). (b) Plot of the adiabatic compressibility φk as a function of sorbitol (M). The standard deviation values are as above. The contribution for protonation was not accounted for (see text).
The compressibility of BSA at pH 2 as a function of sorbitol concentration is shown in Fig. 4 b. It increases from 5.3 × 10−14 m3 kg−1 Pa−1 in the absence of sorbitol at pH 2 to a value of ϕk = 7.0 × 10−14 m3 kg−1 Pa−1 between 1.0 and 1.5 M sorbitol; the difference Δϕk is 1.7 × 10−14 m3 kg−1 Pa−1. At 2 M sorbitol ϕk decreases to a value not significantly different from that of native BSA. In this set of experiments no correction has been made for protonation contribution either volume or compressibility, because in concentrated polyol solutions accurate ionization values for protein residues cannot be estimated (29,40).
Circular dichroism
The far UV CD spectra of BSA measured at pH 2 as a function of sorbitol concentration are shown in Fig. 5. Between 0 and 0.5 M sorbitol, [θ]220 becomes slightly more negative but markedly so at 1 M, changing from −12,700 deg cm2 dmol−1 to −17,600 deg cm2 dmol−1 where it corresponds to 80% of that for native BSA in water at pH 7. No significant change occurs at higher sorbitol concentrations.
FIGURE 5.
CD spectra of BSA in the far UV. At pH 7 (dashed line). At pH 2, in water (○), in 0.5 M sorbitol (solid line), and in 1 M sorbitol (▪).
DISCUSSION
The conformational transition of BSA occurring at acid pH was first reported several decades ago, and the partial unfolding of the protein as a function of pH has been documented (1,2). Thus, the primary focus of this investigation was to explore the extent to which the BSA acid induced conformational transition involves changes in adiabatic compressibility and how the latter phenomenon can be interpreted in terms of the molecular structure. In a previous study (23), we showed that the increase of α-helix content of β-lactoglobulin induced by several alcohols is closely associated with the progressive increase of its compressibility. Whereas in the latter work each point of the transition was measured in a different alcohol-water mixture, in this study the protein remains during the conformational transition in water, so that both structural and volumetric changes arise mainly from the proton binding, which induces partial unfolding (Cl− binding being neglected). We have also used sorbitol to refold BSA at acid pH, knowing that it belongs to a class of neutral additives (osmolytes) that do not interact directly with the protein, although a different interaction of protein surface with water may result (40) from polyol addition, modifying volumetric properties.
Unfolding and compressibility
The secondary structure of BSA displays few changes in a range of pH values between 6.5 and 5, the latter value being close to the isoelectric point of the protein (pI 4.8). Below this value, the ellipticity of the protein measured at 220 nm decreases continuously down to pH 2 (Fig. 1 a), finally losing ∼40% of its initial α-helix. This structural alteration is due to the repulsive forces acting below its isoelectric point within the highly charged protein. The superimposition of the partial unfolding of BSA and the decrease in compressibility (Fig. 2 b) implies that the compressibility variation observed reflects the conformational change during the acid transition, which may also involve some nonhelical parts of the molecule.
An interesting phenomenon, occurring in the range of pH between 7 and 4, concerns the progressive increase in BSA compressibility (Δϕk = 1.0 × 10−14 m3 kg−1 Pa−1) shown in Fig. 2 b, which we found highly reproducible during this investigation (for standard errors see figure legends). In a study of the pH conformational changes of HSA, two different structural events within the 7–4 pH range were observed (9): a loss in protein ellipticity in the near UV CD, and an unexpected sigmoidal rise of the (214) tryptophan fluorescence, reaching a 2.4-fold increase in intensity at pH 4. The same result had been previously reported in the pioneering work of Foster (6). This effect was explained by a change of the tertiary structure of the protein leading to a transition of the fluorophore (located in domain II of HSA) from an aqueous environment to a more hydrophobic one and by the expulsion of water in contact with the aromatic residue. Although the burying of an aromatic residue in a hydrophobic pocket should lead to a small decrease in volume, it can be argued that the parallel expulsion of water from the hydrophobic pocket would increase the final protein volume and compressibility values (because the number of aliphatic residues of the pocket outnumbers the effect of a single aromatic residue), in good agreement with our findings.
As the binding of protons to BSA proceeds with decreasing pH, we observe a progressive decrease of volume and compressibility values, both starting to drop from about pH 5 down to pH 2. The variation of volume and compressibility together originates from at least two phenomena. First, the partial unfolding process of the BSA helices leads to a progressive exposure of the protein surface to the acid aqueous environment, leading to increased hydration. At the same time protein voids and cavities, which in the native protein contribute substantially to the compressibility and to the partial specific volume (17,41,42), are collapsing. Thus both mechanisms contribute to the relative variation of the specific volume and adiabatic compressibility (43–45).
To obtain a more precise picture of the acid transition, the secondary structure of the intact molecule and its three recombinant domains—I, II, and III of HSA—have been investigated by CD (9). It was concluded that the partial loss of periodic structure was due to a structural loosening of the helices located mainly in the C-terminal domain III. In the crystal structure, the two main helices of the C-terminus display few interactions with other parts of the molecule (46). Further, the interactions between albumin domains and subdomains should further weaken below pH 3, leading to a disruption of the intradomain contacts of domain III, within the limits of its disulfide bridges. In fact between pH values of 3 and 2, we observe that the adiabatic compressibility decreases continuously with decreasing pH, indicating again a parallelism between the pH dependence of ellipticity at 220 nm and compressibility changes. If the observed unfolding is restricted mostly to domain III of BSA, it should also be easier to refold it because of the small number of interactions mentioned above.
Compressibility and refolding
The transition examined in this work is reversible (1,2). Here we investigated refolding in a different way using the same experimental techniques but under the influence of osmolytes. Timasheff (40) has shown that such molecules are preferentially excluded from the surface of the protein and do not involve contact with it. Furthermore they raise the Gibbs energy of the protein unfolded state relative to the native one and induce refolding.
Indeed at pH 2, both volume and compressibility of BSA increase as a function of sorbitol concentration (Fig. 4, a and b). Thus whereas the α-helix content of BSA is in the same manner progressively restored and the protein refolded up to 80% of its initial ellipticity value at 220 nm (Fig. 5), compressibility and volume both reach values close to those of the native protein. Moreover the recovery is obtained at a rather low sorbitol concentration (∼1 M), compared to the literature where the sorbitol concentration used by investigators to obtain a comparable refolding reaches 3 M (28,29). This phenomenon could be interpreted by the fact that, as discussed in the preceding paragraph, the conformational disruption induced at pH 2 appears to be restricted mostly to the C-terminal helices of domain III (46).
Bolen and Baskakov (47) explain the effect of osmolytes on proteins by an “osmophobic effect” arising from the unfavorable interaction between an osmolyte and the peptide backbone of the unfolded protein. It shifts the equilibrium toward the native state, as illustrated in BSA (Fig. 5). Thus the decreased exposure of the backbone is the major driving force leading to minimization of the protein surface in contact with the solvent and to refolding.
At neutral pH, a decrease of volume and compressibility of BSA in the presence of glycerol has been reported (39). These results were interpreted as the consequence of water release from the protein interior followed by the collapse of its cavities. This mechanism would entail a decrease in both volume and compressibility of the protein at pH 7, as depicted in Fig. 3, a and b, at low sorbitol concentration. Furthermore at pH 2, once the protein is partially refolded, it behaves toward sorbitol as the native protein at pH 7. This fact could explain the decrease of compressibility at sorbitol concentrations higher than 1 M (Fig. 4, a and b).
Compressibility, structure, and function
Turning now to the BSA domains I, II, and III, how can we connect our compressibility data to the protein function as a drug carrier? It is reported that domain III harbors a number of important binding sites (4) in subdomains A and B. In particular, subdomain IIIA, designated as bonding site 2 and comprising all six helices of the subdomain, is associated with small molecules and drugs such as diazepam and ibuprofen as well as with an anesthetic (diisopropyl phenol), a hormone (thyroxine), and fatty acids. Subdomain IIIB, in addition to fatty acids, also binds thyroxine and oxyphenylbutazone. Furthermore the above authors emphasize the particular adaptability, or flexibility, of drug site 2. From our results it can be inferred that the variation in compressibility reported in this work is related to the conformational flexibility of the domain III helices.
In a recent study on ultrafast hydration dynamics in the unfolding of HSA (48) it was reported that the dynamics of ordered water in the vicinity of domain I, in presence of 6 M guanidine chloride, is nearly the same as that of the native state of the protein domain, in contrast to that of the largely unfolded protein domains II and III. It was concluded that the longer residence time of surface water in domain I must originate in its rigidity and thus lower its conformational flexibility required to modulate ligand binding. It thus seems that there exists a tradeoff between structural stability and function: the greater the stability, the less the function (49). It could be speculated that such a mechanism might explain the adaptability and flexibility of drug site 2 located in domain III, easy to unfold and to refold at a rather low sorbitol concentration (∼1 M), as discussed above. However, great care should be exercised when extrapolating dynamic results.
There is little information about the possible role of the albumin acid transition in drug binding and transport mechanisms. The existence in albumins of a membrane surface-induced conformational change leading to two populations with low and high ligand affinities, respectively, has been reported (2,50). A similar mechanism occurring at a membrane-like interface (51,52) has also been described with a partial loss of HSA α-helix. It could be speculated that given the low pH measured in the vicinity of membrane surfaces of several tissues (2), the transition described in this work might be operative in such an environment.
The data presented here, in addition to representing a step toward elucidation of forces that destabilize/stabilize the native conformation of BSA and govern the control of the fine balance between structure and protein dynamics, could also shed light on relations between albumin and drug binding or/and distribution. The control of protein-drug interactions as well as the optimization of their distribution in relation to variation of structure and compressibility constitute crucial parameters for investigators in pharmacokinetics. It has been pointed out (4) that problems associated with adsorption, distribution, and elimination of drugs add considerably to the complexity and to the cost of developing new ones. They may also be important in protein engineering, for example, the design and development of de novo drug carriers.
Acknowledgments
The authors thank Prof. Roger Pain for advice, suggestions, and critical reading of the manuscript. They are grateful to one of the reviewers for his illuminating remarks.
This work was funded in part by grants from Centre National de la Recherche Scientifique (CNRS) and Agence Nationale pour la Recherche (ANR BLAN-NTO5-3-42548 ACU).
References
- 1.Peters, T., Jr. 1985. Serum albumin. In Advances in Protein Chemistry. C. B. Anfinsen, J. T. Edsall, and F. Richards, editors. Academic Press, New York. 161–245.
- 2.Carter, C. D., and J. X. Ho. 1994. Structure of serum albumin. In Advances in Protein Chemistry. V. N. Schumaker, editor. Academic Press, New York. 153–203. [DOI] [PubMed]
- 3.Bhattacharya, A. A., S. Curry, and N. P. Franks. 2000. Binding of the general anesthetics propofol and halothane to human serum albumin. J. Biol. Chem. 275:38731–38738. [DOI] [PubMed] [Google Scholar]
- 4.Ghuman, J., P. A. Zunszain, I. Petitpas, A. A. Bhattacharya, M. Otagiri, and S. Curry. 2005. Structural basis of the drug-binding specificity of human serum albumin. J. Mol. Biol. 358:38–52. [DOI] [PubMed] [Google Scholar]
- 5.Foster, J. F. 1960. Plasma albumin. In The Plasma Proteins. F. W. Putnam, editor. Academic Press, New York. 179–239.
- 6.Foster, J. F. 1977. Binding properties of albumin. In Albumin Structure, Function and Uses. V. M. Rosenoer, M. Oratz, and M. A. Rothschild, editors. Pergamon Press, Oxford. 53–84.
- 7.Suzukida, M., H. P. Le, F. Shahid, R. A. McPherson, E. R. Birnbaum, and D. W. Darnall. 1983. Resonance energy transfer between cystein-34 and tryptophan-214 in human serum albumin. Distance measurement as a function of pH. Biochemistry. 22:2415–2420. [DOI] [PubMed] [Google Scholar]
- 8.Sadler, P. J., and A. Tucker. 1993. pH induced structural transitions in bovine serum albumin. Eur. J. Biochem. 212:811–817. [DOI] [PubMed] [Google Scholar]
- 9.Dockal, M., D. C. Carter, and F. Rüker. 2000. Conformational transitions of the three recombinant domains of human serum albumin depending on pH. J. Biol. Chem. 275:3042–3050. [DOI] [PubMed] [Google Scholar]
- 10.Gascao Pereira, L. G., O. Théodoly, H. W. Blanch, and C. J. Radke. 2003. Dilatational rheology of BSA conformers at the air/water interface. Langmuir. 19:2349–2356. [Google Scholar]
- 11.Gekko, K., and H. Noguchi. 1979. Compressibility of globular proteins in water at 25°C. J. Phys. Chem. 83:2706–2714. [Google Scholar]
- 12.Sarvazyan, A. P. 1991. Ultrasonic velocimetry of biological compounds. Annu. Rev. Biophys. Biophys. Chem. 20:321–341. [DOI] [PubMed] [Google Scholar]
- 13.Chalikian, T. V., A. P. Sarvazyan, and K. J. Breslauer. 1994. Hydration and partial compressibility of biological compounds. Biophys. Chem. 51:89–109. [DOI] [PubMed] [Google Scholar]
- 14.Chalikian, T. V., M. Totrov, R. Abagyan, and K. Breslauer. 1996. The hydration of globular proteins as derived from volume and compressibility measurements: cross correlating thermodynamic and structural data. J. Mol. Biol. 260:588–603. [DOI] [PubMed] [Google Scholar]
- 15.Sarvazyan, A. P. 1982. Developments of methods of precise ultrasonic measurements in small volumes of liquids. Ultrasonics. 20:151–154. [Google Scholar]
- 16.Gekko, K., and Y. Hasegawa. 1986. Compressibility-structure relationship of globular proteins. Biochemistry. 25:6563–6571. [DOI] [PubMed] [Google Scholar]
- 17.Foygel, K., S. Spector, S. Chatterjee, and P. C. Kahn. 1995. Volume changes of the molten globule transitions of horse heart ferricytochrome c: a thermodynamic cycle. Protein Sci. 4:1426–1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chalikian, T. V., V. S. Gindkin, and K. Breslauer. 1995. Volumetric characterizations of the native, molten globule and unfolded states of cytochrome c at acidic pH. J. Mol. Biol. 250:291–306. [DOI] [PubMed] [Google Scholar]
- 19.Kharakoz, D. P., and V. E. Bychkova. 1997. Molten globule of human lactalbumin: hydration, density, and compressibility of the interior. Biochemistry. 36:1881–1890. [DOI] [PubMed] [Google Scholar]
- 20.Chalikian, T. V., J. Völker, D. Anafi, and K. J. Breslauer. 1997. The native and heat-induced denatured states of alpha-chymotrypsinogen A: a thermodynamic and spectroscopic study. J. Mol. Biol. 274:237–252. [DOI] [PubMed] [Google Scholar]
- 21.Valdez, D., J. Y. Le Huérou, M. Gindre, W. Urbach, and M. Waks. 2001. Hydration and protein folding in water and in reverse micelles: compressibility and volume changes. Biophys. J. 80:2751–2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Taulier, N., and T. V. Chalikian. 2001. Characterization of pH-induced transitions of beta-lactoglobulin: ultrasonic densimetric, and spectroscopic studies. J. Mol. Biol. 314:873–889. [DOI] [PubMed] [Google Scholar]
- 23.Kanjilal, S., N. Taulier, J. Y. Le Huérou, M. Gindre, W. Urbach, and M. Waks. 2003. Ultrasonic studies of alcohol induced transconformation in beta-lactoglobulin. The intermediate state. Biophys. J. 85:3928–3934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taulier, N., and T. V. Chalikian. 2002. Compressibility of protein transitions. Biochim. Biophys. Acta. 1595:48–70. [DOI] [PubMed] [Google Scholar]
- 25.Chalikian, T. V. 2003. Volumetric properties of proteins. Annu. Rev. Biophys. Biomol. Struct. 32:207–235. [DOI] [PubMed] [Google Scholar]
- 26.Chalikian, T. V., and R. Filfil. 2003. How large are the volume changes accompanying protein transitions and binding? Biophys. Chem. 104:489–499. [DOI] [PubMed] [Google Scholar]
- 27.Taulier, N., I. V. Beletskaya, and T. V. Chalikian. 2005. Compressibility changes accompanying conformational transitions of apomyoglobin. Biopolymers. 79:218–229. [DOI] [PubMed] [Google Scholar]
- 28.Xie, G., and S. Timasheff. 1997. Mechanism of the stabilization of ribonuclease A by sorbitol: preferential hydration is greater for the denatured than for the native protein. Protein Sci. 6:211–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kamiyama, T., Y. Sadahide, Y. Nogusa, and K. Gekko. 1999. Polyol-induced molten globule of cytochrome c: an evidence for stabilization by hydrophobic interactions. Biochim. Biophys. Acta. 1434:44–57. [DOI] [PubMed] [Google Scholar]
- 30.Eggers, F., and T. Funck. 1973. Ultrasonic measurements with milliliter liquid samples in the 0.5-100 MHz range. Rev. Sci. Instrum. 44:969–977. [DOI] [PubMed] [Google Scholar]
- 31.Sarvazyan, A. P., and T. V. Chalikian. 1991. Theoretical analysis of an ultrasonic interferometer for precise measurements at high pressures. Ultrasonics. 29:119–124. [DOI] [PubMed] [Google Scholar]
- 32.Eggers, F. 1997. Model calculations for ultrasonic plate-liquid-plate resonators: peak frequency shift by liquid density and velocity variations. Meas. Sci. Technol. 8:643–647. [Google Scholar]
- 33.Sreerama, N., S. Y. Venyaminov, and R. W. Woody. 2001. Analysis of protein CD spectra with a reference protein set based on tertiary structure. Anal. Biochem. 299:271–274. [DOI] [PubMed] [Google Scholar]
- 34.Rasper, J., and W. Kauzmann. 1962. Volume changes in protein reactions. 1 Ionization reactions in proteins. J. Am. Chem. Soc. 84:1771–1777. [Google Scholar]
- 35.Royer, C. A. 2002. Revisiting volume changes in pressure-induced protein unfolding. Biochim. Biophys. Acta. 1595:201–209. [DOI] [PubMed] [Google Scholar]
- 36.Muzammil, S., Y. Kumar, and S. Tayyab. 1999. Molten globule-like state of human serum albumin at low pH. Eur. J. Biochem. 266:20–32. [DOI] [PubMed] [Google Scholar]
- 37.Baskakov, I., and D. W. Bolen. 1998. Forcing thermodynamically unfolded proteins to fold. J. Biol. Chem. 273:4831–4834. [DOI] [PubMed] [Google Scholar]
- 38.Qu, Y., C. L. Bolen, and D. W. Bolen. 1998. Osmolyte driven contraction of a random coil protein. Proc. Natl. Acad. Sci. USA. 95:9268–9273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Priev, A., A. Almagor, S. Yegdar, and B. Gavish. 1996. Glycerol decreases the volume and compressibility of protein interior. Biochemistry. 35:2061–2066. [DOI] [PubMed] [Google Scholar]
- 40.Timasheff, S. N. 2002. Protein hydration, thermodynamic binding, and preferential hydration. Biochemistry. 41:13473–13482. [DOI] [PubMed] [Google Scholar]
- 41.Gekko, K., and Y. Hasegawa. 1989. Effect of temperature on the compressibility of native globular proteins. J. Phys. Chem. 93:426–429. [Google Scholar]
- 42.Frye, K. J., and C. Royer. 1998. Probing the contribution of internal cavities to the volume change of protein unfolding under pressure. Protein Sci. 7:2217–2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ybe, J. A., and P. C. Kahn. 1994. Slow-folding kinetics of ribonuclease A by volume change and circular dichroism: evidence for two independent reactions. Protein Sci. 3:630–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chalikian, T. V., and K. J. Breslauer. 1996. Compressibility as a means to detect and characterize globular proteins. Proc. Natl. Acad. Sci. USA. 93:1012–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tamura, Y., and K. Gekko. 1995. Compactness of thermally and chemically denatured ribonuclease A as revealed by volume and compressibility. Biochemistry. 24:1878–1884. [DOI] [PubMed] [Google Scholar]
- 46.Sugio, S., A. Kashima, S. Moshizuki, M. Noda, and K. Kobayashi. 1999. Crystal structure of human serum albumin at 2.5 Å resolution. Protein Eng. 12:439–446. [DOI] [PubMed] [Google Scholar]
- 47.Bolen, D. W., and I. Baskakov. 2001. The osmophobic effect: natural selection of a thermodynamic force in protein folding. J. Mol. Biol. 310:955–963. [DOI] [PubMed] [Google Scholar]
- 48.Kamal, J. K. A., L. Zhao, and A. H. Zawail. 2004. Ultrafast hydration dynamics in protein unfolding: human serum albumin. Proc. Natl. Acad. Sci. USA. 101:13411–13416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Huber, R., and W. Bennet Jr. 1983. Functional significance of flexibility in proteins. Biopolymers. 22:261–279. [DOI] [PubMed] [Google Scholar]
- 50.Reed, R. G., and C. M. Burrington. 1989. The albumin receptor effect may be due to a surface-induced conformational change in albumin. J. Biol. Chem. 17:9867–9872. [PubMed] [Google Scholar]
- 51.Desfosses, B., N. Cittanova, W. Urbach, and M. Waks. 1991. Ligand binding at membrane mimetic interfaces: human serum albumin in reverse micelles. Eur. J. Biochem. 199:79–87. [DOI] [PubMed] [Google Scholar]
- 52.Desfosses, B., C. Nicot, and M. Waks. 1992. A drug release-facilitating mechanism for human serum albumin in reverse micelles: requirement of a structural switch. Biochemistry Internatl. 26:257–264. [PubMed] [Google Scholar]