Abstract
Mycotoxin contamination associated with head blight of wheat and other grains caused by Fusarium culmorum and F. graminearum is a chronic threat to crop, human, and animal health throughout the world. One of the most important toxins in terms of human exposure is deoxynivalenol (DON) (formerly called vomitoxin), an inhibitor of protein synthesis with a broad spectrum of toxigenicity against animals. Certain Fusarium toxins have additional antimicrobial activity, and the phytotoxin fusaric acid has recently been shown to modulate fungus-bacterium interactions that affect plant health (Duffy and Défago, Phytopathology 87:1250-1257, 1997). The potential impact of DON on Fusarium competition with other microorganisms has not been described previously. Any competitive advantage conferred by DON would complicate efforts to control Fusarium during its saprophytic growth on crop residues that are left after harvest and constitute the primary inoculum reservoir for outbreaks in subsequent plantings. We examined the effect of the DON mycotoxin on ecological interactions between pathogenic Fusarium and Trichoderma atroviride strain P1, a competitor fungus with biocontrol activity against a wide range of plant diseases. Expression of the Trichoderma chitinase genes, ech42 and nag1, which contribute to biocontrol activity, was monitored in vitro and on crop residues of two maize cultivars by using goxA reporter gene fusions. We found that DON-producing F. culmorum and F. graminearum strains repressed expression of nag1-gox. DON-negative wild-type Fusarium strains and a DON-negative mutant with an insertional disruption in the tricothecene biosynthetic gene, tri5, had no effect on antagonist gene expression. The role of DON as the principal repressor above other pathogen factors was confirmed. Exposure of Trichoderma to synthetic DON or to a non-DON-producing Fusarium mutant resulted in the same level of nag1-gox repression as the level observed with DON-producing Fusarium. DON repression was specific for nag1-gox and had no effect, either positive or negative, on expression of another key chitinase gene, ech42. This is the first demonstration that a target pathogen down-regulates genes in a fungal biocontrol agent, and our results provide evidence that mycotoxins have a novel ecological function as factors in Fusarium competitiveness.
Fusarium culmorum (WG Smith) Sacc. and F. graminearum Schwabe [teleomorph, Gibberella zeae (Schwabe) Petch] are causal agents of Fusarium head blight, a disease with a global distribution that greatly reduces the yields of maize, wheat, barley, and other grains (29, 56). Even more devastating losses result from production by these fungi of several mycotoxins. The most important toxin in terms of human exposure is the trichothecene deoxynivalenol (DON), which was first identified in late 1979 in the United States and has since been found worldwide. DON, previously called vomitoxin, is a low-molecular-weight inhibitor of protein synthesis with cell membrane and hemolytic activity. Ingestion of contaminated grain or by-products of exposed animals has severe long-term consequences, including immunosuppression, neurotoxicity, and nutrient uptake alteration (29, 45, 51, 59). In domestic animals, particularly swine, extremely low levels of DON induce protracted feed refusal.
Head blight epidemics resulting in substantial economic losses have been attributed in large part to increasingly widespread implementation of reduced tillage for soil conservation. This technique results in deposition of larger amounts of crop residues on the soil surface, creating an ideal growth environment for Fusarium (61). Much of the disease epidemiology focuses on competitive survival and sporulation of F. culmorum and F. graminearum in the stubble and leaf residues as the major source of inoculum for subsequent crops in a rotation (24). Maize has particular relevance because of the extraordinary amounts of susceptible residues left after harvest and the established link with increased wheat disease when wheat is planted in rotation with maize (4, 17). Recent evidence indicates that trichothecenes have low levels of phytotoxicity and contribute to plant pathogenesis (16, 31). DON production and virulence of wild-type Fusarium strains on wheat are positively correlated (45), and F. graminearum mutants with insertions in the biosynthetic locus tri5 do not produce DON and are significantly less virulent on wheat than the wild-type parental strain in field trials (50). Considering that the greater part of the Fusarium life cycle is saprophytic and depends on retaining occupation of colonized plant debris in competition with numerous competitor microorganisms (6), it is curious that nothing is known about the possible ecological role of Fusarium production of DON or any other mycotoxin during this critical growth stage. Such information should have a critical impact on designing effective control strategies.
Trichoderma spp. are among the principal competitor fungi that aggressively colonize crop residues of maize and wheat throughout the decomposition process (5). This ability, together with the proven history of Trichoderma as a biological control agent for a wide range of aerial and soilborne plant pathogens (10, 32), makes it an excellent candidate for controlling saprophytic growth of Fusarium. Trichoderma biocontrol activity is due in large part to production of cell wall-degrading enzymes, antibiosis, mycoparasitism, and substrate competition (10, 12, 22, 27, 52). Trichoderma atroviride strain P1 (formerly classified as Trichoderma harzianum) (35), which we have used in our work, has previously been shown to have a broad spectrum of biocontrol activity (30). Strain P1 produces an array of fungal cell wall-degrading enzymes that act synergistically to advance mycoparasitism. The most important of these enzymes are the ECH42 endochitinase encoded by ech42 and an N-acetyl-β-d-glucosaminidase encoded by nag1 (42). Disruption of the ech42 gene reduces the biocontrol activity of strain P1 against Botrytis cinerea (60). Both the ech42 and nag1 genes are inducible by fungal cell walls (8, 49), but these genes have distinct regulatory mechanisms (42). The nag1 gene is induced by low-molecular-weight chito-oligosaccharides and its own catabolic products, while ech42 expression is indirectly induced by carbon starvation and other stress conditions (42). Much of the information that is known has resulted from the use of sensitive reporter fusions of the chitinase genes with the Aspergillus niger glucose oxidase-encoding gene, goxA (42). These are the constructs that we used in this study.
Our objective in this study was to evaluate what, if any, influence DON mycotoxin production has on the ecological interaction between pathogenic Fusarium and antagonistic Trichoderma. We found that DON is produced in maize residues colonized by F. culmorum and F. graminearum, and we observed plant host genotype effects. Using three approaches (application of purified toxin, coinoculation with natural producing and nonproducing strains, and coinoculation with insertional mutants lacking DON production), we demonstrated that DON production modulates chitinase gene expression in Trichoderma and is a specific negative signal in pathogen self-defense against antagonism.
MATERIALS AND METHODS
Fungal strains and culture conditions.
For a long time strain P1 was classified as T. harzanium ATCC 74058 based on morphological features, but recent genome sequence analysis has shown that it is more closely aligned with T. atroviride (35). Derivatives of strain P1 carrying gox-reporter gene fusions with the ech42 (strain ech42-gox) and nag1 (strain nag1-gox) chitinase biosynthetic genes have been described previously (42). The strains were stored as spore suspensions in 20% (vol/vol) glycerol at −20°C. Four days before the start of experiments, Trichoderma strains were grown on PDA (4.8 g of potato dextrose broth [Difco, Detroit, Mich.], 12 g of agar [Oxoid, Basingstoke, Hampshire, United Kingdom]; pH 6.5) at 24°C in the dark. F. culmorum strains 9712 and 9713 were isolated from infested maize stubble in Switzerland. F. graminearum field isolate GZ3639 was isolated from scabby wheat in the midwest United States. Strain GZT40 is a derivative of GZ3639 that contains the transformation vector pGZTS4-, which disrupts the tri5 gene and eliminates DON production (50). Fusarium strains were stored on 2% malt extract (Oxoid) agar slants at 3°C. Five days before the start of experiments, Fusarium strains were grown on malt extract agar plates at 24°C in the dark.
Maize cultivars and crop residue preparation.
Maize cultivars Corso (FAL, Zürich, Switzerland) and Magister (Hilleshög-NK, Saint-Sauvin, France) were grown for 4 weeks in the greenhouse (two plants per 5-liter polystyrene pot) in natural soil (Eschikon, Zürich, Switzerland) at 27°C during each 16-h day and at 22°C during each 8-h night. Maize leaves were cut into pieces that were approximately 4 to 5 cm2, surface sterilized for 45 s in a 7% (vol/vol) sodium hypochloride solution (Erne-Chemie, Avenches, Switzerland), and washed twice with sterile water in a sterile inoculation hood. These cut leaf tissues mimicked fresh crop residues. Noninoculated maize tissues were included in experiments as controls for surface disinfection efficacy.
DON production by mycotoxigenic Fusarium in vitro and on maize leaf tissue.
To determine in vitro mycotoxin production, one plug of an actively growing culture of Fusarium strain 9712, 9713, GZ3639, or GZT40 was inoculated onto a 1.5% malt extract agar plate. After 90 h of growth in the dark at 24°C, 10 ml of sterile double-distilled water was added to each plate. After the plates were shaken for 3 h at 100 rpm, the liquid phase containing mycelial fragments was transferred from each plate to a 15-ml polypropylene Falcon centrifuge tube (Greiner Labortechnik, Kremsmünster, Austria) and centrifuged for 5 min at 1,800 × g. The supernatant was used for quantification of DON as described below. Data from three experiments with three replicates each were pooled for the final analysis after exclusion of a trial × treatment interaction (P = 0.439) in a preliminary general linear model (GLM) procedure (Systat, version 9.0; Systat Inc., Evanston, Ill.). Treatment effects were analyzed by using a GLM procedure, and means were compared by using Fisher's protected (P ≤ 0.05) least significant difference (LSD) test.
For determination of DON production in maize leaf tissues, plant material from cultivars Corso and Magister (10 g) was inoculated with two plugs of an actively growing culture of Fusarium strain 9712, 9713, GZ3639, or GZT40. After 14 days of incubation at 24°C in the dark, the plant material was macerated with liquid nitrogen and homogenized with double-distilled water (10 ml/g). DON levels in the supernatant were quantified by a commercial enzyme-linked immunosorbent assay (R-Biopharm, Darmstadt, Germany). This test has negligible cross-reactivity with substances related to DON, such as nivalenol, 15-acetyl-DON, triacetyl-DON, triacetyl-nivalenol, tetra-acetyl-DON, and fusarenon X. The detection limits for DON with the enzyme-linked immunosorbent assay kits were approximately 0.09 μg/cm2 of mycelial growth on agar plates and 0.2 μg/g of leaf tissue. Results from two replicated experiments were analyzed by using a GLM procedure and LSD test (Systat, version 9.0).
Influence of F. culmorum wild-type strains and F. graminearum insertional mutants on Trichoderma chitinase gene expression in vitro.
One plug of an actively growing culture of Fusarium strain 9712 (which produces high levels of DON), 9713 (which does not produce DON), GZ3639 (which produces DON), or GZT40 (a tri5 mutant which does not produce DON) was placed inverted at the side of a 1.5% malt extract agar plate and incubated at 24°C in the dark. Plates were then coinoculated with one plug of an actively growing culture of T. atroviride reporter strain ech42-gox or nag1-gox that was placed inverted at the opposite side of the plate (3.2 cm from the Fusarium plug). After 66 h of growth in the dark at 24°C, 10 ml of phosphate buffer (1.2 g of KH2PO4 per liter, 2.6 g of K2HPO4 per liter; pH 7.1) was spread onto each agar surface, and the plates were placed on an orbital shaker for 30 min at 150 rpm. Each suspension containing mycelial fragments was decanted into a 15-ml Falcon tube and centrifuged for 5 min at 1,800 × g. The supernatant was collected and used for quantification of glucose oxidase activity by the method of Mach et al. (42). No background activity was observed on uninoculated plates or plates inoculated with only Fusarium. Data from three experiments with three replicates each were pooled for the final analysis by a GLM procedure after exclusion of a trial × treatment interaction (P = 0.494), and means were compared by using an LSD test.
Influence of synthetic DON on Trichoderma gene expression in vitro.
A sterile aqueous solution of DON (200 μl of a solution containing 0, 1, 2.5, or 5 μg/g; Sigma-Aldrich Chemie GmbH, Steinheim, Germany) was evenly spread over the surface of a 1.5% malt extract agar plate and allowed to rapidly absorb into the medium. Plates were immediately inoculated by inverting on one side of each plate one plug taken from an actively growing culture of the T. atroviride P1 reporter construct ech42-gox or nag1-gox. After 66 h of growth in the dark at 24°C, 10 ml of phosphate buffer was added to each plate. The plates were shaken for 30 min at 150 rpm, and the liquid containing mycelial fragments was transferred to 15-ml Falcon centrifuge tubes and centrifuged for 5 min at 1,800 × g. The supernatants were used for quantification of glucose oxidase activity (42).
The influence of synthetic DON was further tested in combination with a non-DON-producing Fusarium mutant. Synthetic DON at the concentrations described above was added to plates inoculated on one side with the tri5 insertional DON-negative mutant GZT40. T. atroviride P1 reporter constructs were inoculated 24 h later on the opposite sides of the plates. Glucose oxidase activity was evaluated as described above. Data from three experiments with six replicates each were pooled after exclusion of a trial × treatment interaction (P = 0.405) and were analyzed further by using GLM and LSD tests.
Influence of different F. culmorum and F. graminearum strains on Trichoderma chitinase gene expression on leaf tissues of maize cultivars Corso and Magister.
Five grams of leaf tissue was transferred into a sterile 100-ml flask, and the flask was inoculated with two plugs of an actively growing culture of Fusarium strain 9712, 9713, GZ3639, or GZT40 and two plugs of the T. atroviride P1 derivative nag1-gox. Then the preparations were incubated for 6 days at 24°C in the dark. At the end of the experiment, 20 ml of phosphate buffer was added to each flask. After the flasks were shaken for 10 min at 150 rpm, the liquid phase of each flask was transferred into a 50-ml Falcon tube and centrifuged for 5 min at 1,800 × g. The resultant supernatant was analyzed for glucose oxidase activity. Data from three experiments with eight replicate flasks each were pooled for final analysis after exclusion of a trial × treatment interaction (P = 0.396).
Influence of different media on T. atroviride P1 growth and chitinase gene expression.
One plug from an actively growing culture of a T. atroviride derivative containing either the ech42-gox or the nag1-gox fusion was inoculated onto the following four media: 0.1% malt extract agar, 1.5% malt extract agar, PDA, and SM agar [2.8 g of (NH4)2SO4 per liter, 0.6 g of urea per liter, 4.0 g of KH2PO4 per liter, 0.6 g of CaCl2 · 2H2O per liter, 0.2 g of MgSO4 · 7H2O per liter, 0.01 g of FeSO4 · 7H2O per liter, 0.0028 g of ZnSO4 · 2H2O per liter, 0.0032 g of CoCl2 · 6H2O per liter, 1 g of saccharose per liter, 12 g of agar per liter; pH 5.4]. T. atroviride P1 was grown alone or was coinoculated with the non-DON-producing F. graminearum strain GZT40 at a distance of 3.2 mm. After 66 h of growth in the dark at 24°C, 10 ml of phosphate buffer was added to each plate. After the plates were shaken for 30 min at 150 rpm, the liquid phase in each plate was transferred to a 15-ml Falcon tube and centrifuged for 5 min at 1,800 × g. The supernatant was used for quantification of glucose oxidase activity by the method of Mach et al. (42). Growth was estimated based on mycelial surface measurements taken with a planimeter. Data from three trials with six replicates each were pooled after no trial × treatment interaction was determined in a preliminary GLM analysis (P = 0.176), and treatment means were separated with an LSD test.
RESULTS
DON production.
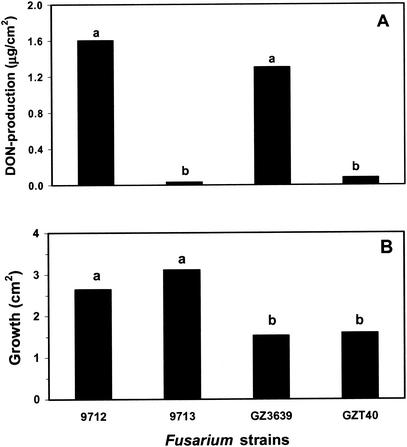
DON production was confirmed for two wild-type strains, F. culmorum 9712 isolated in Switzerland and F. graminearum GZ3639 isolated in the United States, when they were grown on malt extract agar. Wild-type strain 9713 from Switzerland and the tri5 DON biosynthetic gene insertion mutant of F. graminearum GZT40 were found to be negative for DON production (Fig. 1A). The DON production calculated relative to mycelial growth was approximately 1.2 times greater in F. culmorum 9712 than in F. graminearum GZ3639 after 90 h of incubation. Generally, however, the growth of each of the Swiss isolates was similar to the growth of the other Swiss isolates but significantly greater than the growth of the United States isolates (Fig. 1B). The DON production on maize residues differed slightly from the DON production observed in the plate assays. Whereas both 9712 and GZ3639 produced substantial levels of DON on both cultivar Corso and cultivar Magister and the tri5 mutant produced no detectable DON, strain 9713 was found to produce low but detectable levels of the mycotoxin on these substrates. Thus, 9712 and GZ3639 are considered strains that produce high levels of DON, strain 9713 is considered a strain that produces low levels or no DON, and mutant GZ3639 is considered a nonproducer. The maize cultivar had a slight effect on the level of DON produced, and the level produced was generally higher on cultivar Corso (Table 1).
FIG. 1.
Production of DON by (A) and mycelial growth of (B) F. culmorum strains 9712 and 9713 and F. graminearum strains GZ3639 and GZT40 on 1.5% malt extract agar. DON levels are expressed relative to colony surface area as measured with a planimeter. Bars with different letters above them are significantly different according to Fisher's LSD test (P ≤ 0.05).
TABLE 1.
DON production by Fusarium strains on leaf tissue of maize cultivars Corso and Magister
| Strain | DON concn (μg/g)a
|
|
|---|---|---|
| Cultivar Corso | Cultivar Magister | |
| F. culmorum 9712 | 2.51 a | 1.96 a |
| F. culmorum 9713 | 0.38 b | 0.41 b |
| F. graminearum GZ3639 | 2.78 a | 2.11 a |
| F. graminearum GZT40 | ND c | ND c |
DON concentration per gram (fresh weight) of maize leaf tissue determined 14 days after fungal inoculation. The values are means from two experiments with four replicates. Values within a column followed by the same letter are not significantly different according to Fishers protected LSD test (P ≤ 0.05). ND, not detected.
Influence of DON-producing and non-DON-producing Fusarium strains on Trichoderma chitinase gene expression in vitro.
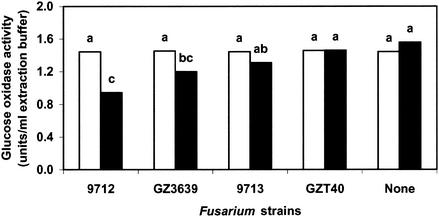
DON-producing strains of Fusarium reduced chitinase gene expression in the T. atroviride nag1-gox reporter strain (Fig. 2). Mycelial contact between the pathogen and the antagonist occurred after approximately 66 h of growth on malt extract agar plates. Repression occurred shortly thereafter (<12 h) when contact was made with either F. culmorum 9712 or F. graminearum GZ3639. The degree of repression varied; the presence of 9712 and GZ3639 resulted in 23 and 39% repression, respectively, compared to the values obtained for controls with just Trichoderma and no Fusarium. No repression was observed when Trichoderma was challenged with either of the Fusarium strains that do not produce DON in vitro (strains 9713 and GZT40) (Fig. 2). In contrast, expression of another chitinase gene, ech42, was not affected either positively or negatively by any of the Fusarium strains, demonstrating that DON does not universally repress all antagonist chitinase genes (Fig. 2).
FIG. 2.
Effects of different F. culmorum and F. graminearum strains on expression of ech42-gox (open bars) and nag1-gox (solid bars) chitinase genes of T. atroviride P1. Malt extract agar plates containing Trichoderma were coinoculated with one of the following Fusarium strains: F. culmorum 9712 (which produces high levels of DON), F. culmorum 9713 (which produces low levels of DON), F. graminearum GZ3639 (which produces high levels of DON), or F. graminearum GZT40 (which does not produce DON). The values are the means of three experiments with three replicates each. Bars with different letters above them are significantly different according to Fisher's LSD test (P ≤ 0.05).
Influence of synthetic DON on Trichoderma chitinase gene expression.
The possible role of DON but not additional pathogen metabolites in chitinase gene repression was confirmed in follow-up experiments performed by using the model described above, except that DON-producing Fusarium was used and the antagonist was challenged with synthetic DON. Exposure of Trichoderma grown alone to increasing concentrations of DON ranging from 0 to 5 μg/g of agar resulted in increasing repression of nag1-gox from 1.3 U with no DON to only 0.8 U with 5 μg of DON per g or an approximately 40% reduction in gene expression (Table 2). Further evidence that there was DON-specific repression of nag1-gox was provided by the fact that addition of synthetic DON to confrontation assay mixtures with the insertion mutant F. graminearum GZT40, which was defective only in the DON biosynthetic gene tri5, restored the repressive nature of this fungus (Table 2). Mutant GZT40 alone did not affect nag1-gox expression (Table 2). Synthetic DON had no effect on expression of the other chitinase reporter gene fusion, ech42-gox, either when the antagonist was grown alone or when it was challenged with the nonproducing pathogen mutant (Table 2). The effects which we observed were limited to gene expression, and in no case did DON affect Trichoderma growth either positively or negatively.
TABLE 2.
Influence of synthetic DON on T. atroviride chitinase gene expression
| DON concn (μg/g)a | Glucose oxidase activity (U/ml)b
|
|||
|---|---|---|---|---|
|
ech42-gox
|
nag1-gox
|
|||
| P1 alone | P1 plus GZT40 | P1 alone | P1 plus GZT40 | |
| 0 | 1.19 ac | 1.22 a | 1.30 a | 1.23 a |
| 1 | 1.20 a | 1.20 a | 1.09 b | 1.04 b |
| 2.5 | 1.20 a | 1.19 a | 0.94 c | 0.87 c |
| 5 | 1.19 a | 1.21 a | 0.79 d | 0.79 c |
DON concentration on malt extract agar plates prior to fungal inoculation.
Expression of chitinase genes in T. atroviride strain P1 inoculated alone or together with F. graminearum GZT40 (a mutant which does not produce DON) was quantified after 66 h of growth by using ech42-gox and nag-gox reporter gene fusions. Glucose oxidase activity is expressed in units per milliliter of extraction buffer.
The values are the means of three experiments with six replicates. Means within a column followed by the same letter are not significantly different according to Fisher's protected LSD test (P ≤ 0.05). No significant differences in expression of either chitinase gene were observed at any DON concentration for treatments with and without Fusarium (within-row comparisons).
Pathogen-mediated repression of antagonist nag1-gox chitinase gene expression in maize leaf tissues.
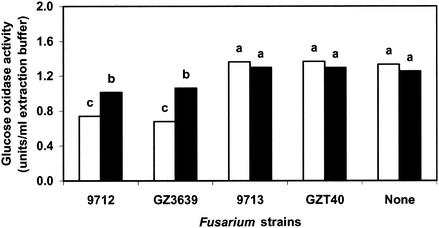
We demonstrated that the activity of DON as a repressor of a Trichoderma biocontrol gene occurred in maize leaf tissues. Both DON-producing wild-type strains, F. culmorum 9712 and F. graminearum GZ3639, significantly repressed the nag1-gox chitinase reporter gene fusion (Fig. 3). The levels of repression were equivalent for the two strains, which reflected the fact that the two strains produced similar amounts of DON on maize leaf tissues (Table 1). This finding supports the prominence of DON as the repressor factor above other pathogen strain differences since strains that belonged to different species but contained the same level of toxin had the same level of repression. F. culmorum 9713, which produced barely detectable levels of DON in crop residues (Table 1), had no significant effect on nag1-gox expression (Fig. 3). Similarly, the insertion mutant F. graminearum GZT40, which produced no DON, had no effect on Trichoderma chitinase gene expression (Fig. 3). Although biocontrol gene repression was observed on both maize cultivars, repression in a confrontation with either 9713 or GZ3639 was greater on cultivar Corso and less severe on cultivar Magister (Fig. 3). DON production by these fungi was not significantly different on the two cultivars.
FIG. 3.
Expression of the nag1-gox gene fusion of T. atroviride P1 grown on leaf and stem pieces from maize cultivars Corso (open bars) and Magister (solid bars) inoculated with one of the following Fusarium strains: F. culmorum 9712 (which produces high levels of DON), F. culmorum 9713 (which produces low levels of DON), F. graminearum GZ3639 (which produces high levels of DON), or F. graminearum GZT40 (which does not produce DON). The values are the means of three experiments with eight replicates each. Bars with different letters above them are significantly different according to Fisher's LSD test (P ≤ 0.05).
Altered chitinase gene expression in response to culture media.
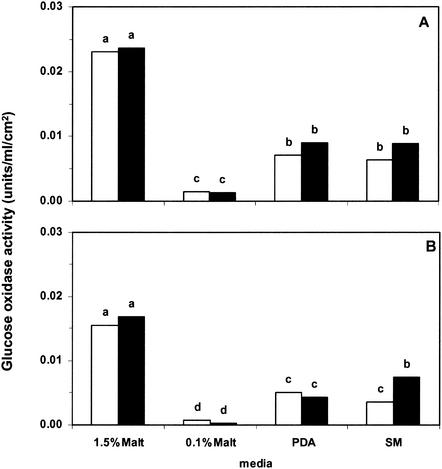
Not surprisingly, the culture medium had a slight effect on the growth of both of the Trichoderma reporter strains (data not shown). Glucose oxidase activity was therefore quantified relative to mycelial surface area. On the four media tested, expression of ech42-gox (Fig. 4A) was only slightly higher than expression of nag1-gox (Fig. 4B). There was substantial variation, however, in the levels of expression between media. Concentrated 1.5% malt extract agar supported the highest levels of expression for both gene fusions, whereas dilute 0.1% malt extract supported the lowest levels, which were just above the detection limit. Low but detectable levels of expression were observed on both PDA and SM minimal salts medium. Expression of both Trichoderma chitinase genes was slightly increased when the non-DON-producing pathogen GZT40 was present, and this effect was most pronounced on SM medium (Fig. 4). This suggests the importance of nutritional variables, other than the previously reported fungal host factors (i.e., chitin), for chitinase gene expression.
FIG. 4.
Expression of the ech42-gox (A) and nag1-gox (B) fusions in T. atroviride P1 after 66 h of growth at 24°C in the dark on four different media. The activity is expressed in units per milliliter of extraction buffer per square centimeter of mycelial surface. Trichoderma was grown alone (open bars) or was coinoculated with the non-DON-producing F. graminearum strain GZT40 (solid bars). The media (1.5 and 0.1% malt extract agar, PDA, and SM medium) are described in Materials and Methods. Each value is the mean of three experiments with six replicate plates. Bars with different letters above them are significantly different according to Fisher's LSD test (P ≤ 0.05).
DISCUSSION
Crop losses in wheat and other grains due to F. culmorum- and F. graminearum-caused disease and mycotoxin contamination cost billions of dollars annually worldwide (7, 55). Although progress has been made in combating head blight, there are still not adequate control measures, and biological control has emerged as a promising strategy (53). To date, biocontrol efforts have been limited to the flower infection stage of wheat head blight. In our work we developed a new biocontrol approach aimed at reducing the primary inoculum in crop residues. Certain antagonists have a unique ability to out-compete pathogens for occupation of crop residues, thus reducing pathogen inoculum buildup in this epidemiologically critical habitat. Trichoderma strains are among the most aggressive competitors of Fusarium in crop residues (37). A key biocontrol mechanism for most if not all Trichoderma strains is mycoparasitism mediated by the production of chitinases and other cell wall-degrading enzymes (10, 39).
We found that the pathogen mycotoxin DON represses expression of one important chitinase gene, nag1, in T. atroviride biocontrol strain P1. This is the first report of negative signaling between a pathogen and a fungal antagonist and one of the few reports of negative signaling described for any microbe-microbe interaction. Expression of this key biocontrol gene was diminished by as much as 50% in maize residues when the antagonist was placed in competition with wild-type DON-producing strains of F. culmorum and F. graminearum. The primary role of DON in this repression was confirmed by adding synthetic mycotoxin to assay mixtures with Fusarium strains that otherwise had no effect on Trichoderma. Synthetic DON induced repression in assays with either a wild-type non-DON-producing isolate of F. culmorum that does not produce DON or a non-DON-producing derivative of a F. graminearum mutant with the tricothecene biosynthesis gene tri5 interrupted that does not produce DON. Further proof was obtained by inducing nag1 repression with synthetic DON added to Trichoderma gnotobiotic cultures. DON was the principal if not sole factor responsible for this repression. This explains why the degree of nag1 repression was equivalent for two very different pathogen isolates, F. culmorum 9712 obtained from maize in Switzerland and F. graminearum obtained from wheat in the United States, as long as they produced equivalent amounts of DON in maize residues. We found that while DON repressed nag1 expression, it did not universally affect chitinase genes in the antagonist. Expression of the endochitinase gene ech42, which has been demonstrated to contribute to disease suppression (60), was neither positively nor negatively affected by DON or contact with Fusarium. However, because various chitinases function synergistically during mycoparasitism (38, 52), repression of one antagonist gene may still provide a level of protection for the pathogen. There is evidence that individual enzyme activity can release signals from the fungal pathogen host that trigger a cascade of other genes involved in mycoparasitic attack (36). Our findings should encourage further investigation of pathogen toxin effects on the entire mycoparasitic process.
Pathogen strains vary widely in the level of DON which they produce in particular environments (2, 28). Plant host genotype is known to affect production during plant colonization (33, 43, 44), and this can be exploited in resistance breeding programs. Host variation is thought to be based on a combination of pathogen growth or infection inhibition and biochemical differences which enable resistant varieties to down-regulate DON biosynthesis (47) or to rapidly degrade DON (46). The focus of previous work has understandably been directed toward reducing grain contamination and thus toward living host tissues. We obtained evidence for the first time that a similar host effect on DON biosynthesis may influence production in crop residues. Higher DON levels were detected on colonized residues of maize cultivar Corso than on colonized residues of maize cultivar Magister. This could affect the quality of dead plant material used for animal feed (i.e., straw, silage) (62). We found that host plant effects on DON production influenced the interaction between toxigenic Fusarium and Trichoderma, and repression was greatest on cultivar Corso. Tissue age could also have an effect on the interaction. The juvenile tissues used in this study to facilitate sterilization were chemically and physically different from the senescent tissues that are colonized by the pathogen in the field after crop harvest. Further study is required to determine if the pathogen signaling identified here has an influence on biocontrol interactions in a field setting.
Pathogen repression of chitinase genes has several important consequences for the application of Trichoderma as a biocontrol agent against Fusarium diseases. We observed that negative pathogen signaling is an additional factor that may contribute to the inconsistent performance often observed with biocontrol. Thus, even though growth of Fusarium did not affect growth of the biocontrol agent, the pathogen was still able to interfere with biocontrol activity by interfering with expression of a chitinase gene involved in mycoparasitism. Identification of factors that interfere with biocontrol activity is important for directing approaches to bolster biocontrol reliability (21). Possible remedies include screening for DON-insensitive Trichoderma strains, combined inoculation of Trichoderma with toxin-degrading microorganisms (3, 23, 34, 57), combined inoculation with microorganisms that block DON biosynthesis in Fusarium (11), and targeting the use of antagonists that rely on other mechanisms of action (e.g., antibiosis) when they are confronted with toxigenic Fusarium. Many filamentous fungi have ABC or MFS transporter proteins for toxin efflux that confer resistance to diverse natural and synthetic antifungal compounds (15). It will be interesting to see which membrane efflux systems are present in Trichoderma and whether they offer certain strains resistance to negative pathogen signals like DON. Trichoderma chitinase genes have been successfully deployed in transgenic crops to improve resistance to fungal diseases (41). In these instances the plants were not tested for resistance to Fusarium. Our findings indicate that in the future during selection of genes and promoters for this purpose workers might also consider potential pathogen self-defense strategies, such as DON-mediated repression.
It remains to be determined if DON-mediated repression is a general phenomenon for Trichoderma or is limited to a small group of isolates. Certain chitinase genes, including those in our study, are widespread among Trichoderma and related mycoparastic fungi (26). However, little is known about the level of genetic conservation of these genes and, more importantly, whether the same chitinase gene may be differentially regulated in different strains. Our recent discovery of a pathogen signal that affects biosynthesis of the Pseudomonas antibiotic 2,4-diacetylphloroglucinol (20, 54) revealed subtle but important differences in how the same biocontrol factor is regulated and should allow more deliberate bacterial strain selection (19). Another open question is whether DON derivatives or other mycotoxins act as additional signals for nag1 and/or whether these compounds affect expression of different biocontrol genes in Trichoderma. Fusarium strains synthesize an extraordinary array of secondary metabolites (1, 25), any one of which may be a candidate for a signal in pathogen-antagonist interactions.
Surprisingly little is known about environmental regulation of chitinase genes. Chitin, which is the most abundant cell wall component in higher fungi, has long been recognized as an inducer in compatible mycoparasitic interactions (38, 58). Additional cell wall components released during the early stages of mycoparasitism appear to be important in further induction of T. atroviridae chitinase genes (36, 63). We describe here induction of both nag1 and ech42 by abiotic factors. Induction did not occur in the absence of favorable medium conditions (e.g., dilute malt extract agar) even with access to a non-DON-producing Fusarium strain, which obviously contained chitin. This raises the possibility that Fusarium strains may possess a mechanism to escape detection by the mycoparasite or to prevent release of autorepressor proteins in Trichoderma (40). Our work supports recent reports (13, 14, 18, 42) that identified the influence of nitrogen and carbon availability on chitinase gene expression. Ammonium starvation and glucose starvation generally stimulate nag1 and ech42 expression. DON biosynthesis in Fusarium is also influenced by environmental regulators (9). Interestingly, the same factors that stimulate the antagonist biocontrol genes, carbon and nitrogen depletion, stimulate DON production by the pathogen (48). Thus, production of this pathogen self-defense compound seems to be coordinated with environmental conditions to diffuse chitinase-mediated antagonism. No cultivar effects on expression of nag1 or ech42 were observed when Trichoderma was inoculated alone, indicating that crop residues have a conducive nitrogen and/or carbon composition. Not only are crop residues an agriculturally important niche for exploring biological control; they may also be a useful model system to study antagonist gene regulation.
Acknowledgments
We gratefully acknowledge M. Lorito for providing the T. atroviride strains, H.-R. Forrer for providing F. culmorum strains, N. Alexander for providing the F. graminearum strains, R. Notz for reading the manuscript, and C. Saez-Wanzenried for technical assistance.
This research was supported by grants to B.D. and G.D. from the Swiss National Foundation for Scientific Research (SPP-Biotechnology project 5002-57815), COST Action 835 (grant BBW/OFES-NB. C 99-0087), and the Swiss National Centre of Competence in Research (NCCR Plant Survival).
REFERENCES
- 1.Bacon, C. W., J. K. Porter, W. P. Norred, and J. F. Leslie. 1996. Production of fusaric acid by Fusarium species. Appl. Environ. Microbiol. 62:4039-4043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bakan, B., L. Pinson, B. Cahagnier, D. Melcion, E. Sémon, and D. Richard-Molard. 2001. Toxigenic potential of Fusarium culmorum strains isolated from French wheat. Food Addit. Contam. 18:998-1003. [DOI] [PubMed] [Google Scholar]
- 3.Bata, A., and R. Laszitity. 1999. Detoxification of mycotoxin-contaminated food and feed by microorganisms. Trends Food Sci. Technol. 10:223-228. [Google Scholar]
- 4.Beck, R., and J. Lepschy. 1997. Aehrenfusariosen: Gefahr aus Maisstoppeln. DLG-Mitteilungen 5:34-38. [Google Scholar]
- 5.Broder, M. W., and G. H. Wagner. 1988. Microbial colonization and decomposition of corn, wheat, and soybean residue. Soil Sci. Soc. Am. J. 52:112-117. [Google Scholar]
- 6.Bruehl, G. W. 1987. Soilborne plant pathogens. MacMillan Publishing Co., New York, N.Y.
- 7.Cardwell, K. F., A. Desjardins, S. H. Henry, G. Munkvold, and J. Robens. August 2001, posting date. Mycotoxins: the cost of achieving food security and food quality. American Phytopathological Society APSNet feature. [Online.] American Phytopathological Society, St. Paul, Minn. http://www.apsnet.org/online/feature/mycotoxin/top.html.
- 8.Carsolio, C., A. Gutierrez, B. Jimenez, M. van Montagu, and A. Herrera-Estrella. 1994. Characterization of ech42, a Trichoderma harzianum endochitinase gene expressed during mycoparasitism. Proc. Natl. Acad. Sci. USA 91:10903-10907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen, L., S. P. McCormick, and T. M. Hohn. 2000. Altered regulation of 15-acetyldeoxynivalenol production in Fusarium graminearum. Appl. Environ. Microbiol. 66:2062-2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chet, I. 1987. Trichoderma: application, mode of action and potential as a biocontrol agent of soilborne plant pathogenic fungi, p. 137-160. In I. Chet (ed.), Innovative approaches to plant disease control. Wiley, New York, N.Y.
- 11.Cooney, J. M., D. R. Lauren, and M. E. di Menna. 2001. Impact of competitive fungi on trichothecene production by Fusarium graminearum. J. Agric. Food Chem. 49:522-526. [DOI] [PubMed] [Google Scholar]
- 12.de la Cruz, J., J. A. Pintor-Toro, T. Benitez, and A. Llobell. 1995. Purification and characterization of an endo-β-1,6-glucanase from Trichoderma harzianum that is related to its mycoparasitism. J. Bacteriol. 177:1864-1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de la Cruz, J., M. Rey, J. M. Loro, A. Hidalgo-Gallego, F. Dominguez, J. A. Pintor-Toro, A. Llobell, and T. Benitez. 1993. Carbon source control on β-glucanases, chitobiase and chitinase from Trichoderma harzianum. Arch. Microbiol. 159:316-322. [Google Scholar]
- 14.de la Mercedes Dana, M. C. Limón, R. Mejías, R. L. Mach, T. Benítez, J. A. Pintor-Toro, and C. Kubicek. 2001. Regulation of chitinase 33 (chit33) gene expression in Trichoderma harzianum. Curr. Genet. 38:335-342. [DOI] [PubMed] [Google Scholar]
- 15.Del Sorbo, G., H.-J. Schoonbeek, and M. A. De Waard. 2000. Fungal transporters involved in efflux of natural toxic compounds and fungicides. Fungal Genet. Biol. 30:1-15. [DOI] [PubMed] [Google Scholar]
- 16.Desjardins, A. E., and T. M. Hohn. 1997. Mycotoxins in plant pathogenesis. Mol. Plant-Microbe Interact. 2:147-152. [Google Scholar]
- 17.Dill-Macky, R., and R. K. Jones. 2000. The effect of previous crop residues and tillage on Fusarium head blight of wheat. Plant Dis. 84:71-76. [DOI] [PubMed] [Google Scholar]
- 18.Donzelli, B. G. G., and G. E. Harman. 2001. Interaction of ammonium, glucose, and chitin regulates the expression of cell wall-degrading enzymes in Trichoderma atroviride strain P1. Appl. Environ. Microbiol. 67:5643-5647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Duffy, B. K., and G. Défago. 1998. A Fusarium pathogenicity factor blocks antibiotic biosynthesis by antagonistic pseudomonads. IOBC wprs Bull. 21:145-148.
- 20.Duffy, B. K., and G. Défago. 1997. Zinc improves biocontrol of Fusarium crown and root rot of tomato by Pseudomonas fluorescens and represses the production of pathogen metabolites inhibitory to bacterial antibiotic biosynthesis. Phytopathology 87:1250-1257. [DOI] [PubMed] [Google Scholar]
- 21.Duffy, B. K., B. H. Ownley, and D. M. Weller. 1997. Soil chemical and physical properties associated with suppression of take-all of wheat by Trichoderma koningii. Phytopathology 87:1118-1124. [DOI] [PubMed] [Google Scholar]
- 22.Elad, Y., I. Chet, and Y. Henis. 1982. Degradation of plant pathogenic fungi by Trichoderma harzianum. Can. J. Microbiol. 28:719-725. [Google Scholar]
- 23.El-Nezami, H. S., A. Chrevatidis, S. Auriola, S. Salminen, and H. Mykkänen. 2002. Removal of common Fusarium toxins in vitro by strains of Lactobacillus and Propionibacterium. Food Addit. Contam. 19:680-686. [DOI] [PubMed] [Google Scholar]
- 24.Fernando, W. G. D., T. C. Paulitz, W. L. Seaman, P. Dutilleul, and J. D. Miller. 1997. Head blight gradients caused by Gibberella zeae from area sources of inoculum in wheat field plots. Phytopathology 87:414-421. [DOI] [PubMed] [Google Scholar]
- 25.Fotso, J., J. F. Leslie, and J. S. Smith. 2002. Production of beauvericin, moniliformin, fusaproliferin, and fumonisins B1, B2, and B3 by fifteen ex-type strains of Fusarium species. Appl. Environ. Microbiol. 68:5195-5197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.García, I., J. M. Lora, J. de la Cruz, T. Benítez, A. Llobell, and J. A. Pintor-Toro. 1994. Cloning and characterization of a chitinase (CHIT42) cDNA from the mycoparasitic fungus Trichoderma harzianum. Curr. Genet. 27:83-89. [DOI] [PubMed] [Google Scholar]
- 27.Ghisalberti, E. L., and C. Y. Rowland. 1993. Antifungal metabolites from Trichoderma harzianum. J. Nat. Prod. 56:1799-1804. [DOI] [PubMed] [Google Scholar]
- 28.Gilbert, J., D. Abramson, B. McCallum, and R. Clear. 2002. Comparison of Canadian Fusarium graminearum isolates for aggressiveness, vegetative compatibility, and production of ergosterol and mycotoxins. Mycopathologia 153:209-215. [DOI] [PubMed] [Google Scholar]
- 29.Gilbert, J., and A. Tekauz. 2000. Recent developments in research on Fusarium head blight of wheat in Canada. Can. J. Plant Pathol. 22:1-8. [Google Scholar]
- 30.Harman, G. E., and T. Björkman. 1998. Potential and existing uses of Trichoderma and Gliocladium for plant disease control and plant growth enhancement, p. 229-265. In G. E. Harman and C. P. Kubicek (ed.), Trichoderma and Gliocladium, vol. 2. Enzymes, biological control and commercial application. Taylor and Francis Ltd., London, United Kingdom.
- 31.Harris, L. J., A. E. Desjardins, R. D. Plattner, P. Nicholson, G. Butler, Y. C. Young, G. Weston, R. H. Proctor, and T. M. Hohn. 1999. Possible role of trichothecene mycotoxins in virulence of Fusarium graminearum on maize. Plant Dis. 83:954-960. [DOI] [PubMed] [Google Scholar]
- 32.Hjeljord, L., and A. Tronsmo. 1998. Trichoderma and Gliocladium in biological control: an overview, p. 129-151. In G. E. Harman and C. P. Kubicek (ed.), Trichoderma and Gliocladium, vol. 2. Enzymes, biological control and commercial application. Taylor and Francis Ltd., London, United Kingdom.
- 33.Jenny, E., A. Hecker, P. Kessler, C. Külling, and H.-R. Forrer. 2000. Getreidefusariosen: Sortenresistenz und Toxingehalte. AGRAR Forschung 7:270-273. [Google Scholar]
- 34.Karlovsky, P. 1999. Biological detoxification of fungal toxins and its use in plant breeding, feed and food production. Nat. Toxins 7:1-23. [DOI] [PubMed] [Google Scholar]
- 35.Kullnig, C. M., T. Krubica, M. Lorito, R. L. Mach, M. Rey, T. Benitez, and C. P. Kubicek. 2001. Confusion abounds over identities of Trichoderma biocontrol isolates. Mycol. Res. 105:769-772. [Google Scholar]
- 36.Kullnig, C. M., R. L. Mach, M. Lorito, and C. P. Kubicek. 2000. Enzyme diffusion from Trichoderma atroviride (= T. harzianum P1) to Rhizoctonia solani is a prerequisite for triggering of Trichoderma ech42 gene expression before mycoparasitic contact. Appl. Environ. Microbiol. 66:2232-2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lipps, P. E., and I. W. Deep. 1991. Influence of tillage and crop rotation on yield, stalk rot, and recovery of Fusarium and Trichoderma spp. from corn. Plant Dis. 75:828-833. [Google Scholar]
- 38.Lorito, M. 1998. Chitinolytic enzymes and their genes, p. 73-99. In G. E. Harman and C. P. Kubicek (ed.), Trichoderma and Gliocladium, vol. 2. Enzymes, biological control and commercial application. Taylor and Francis Ltd., London, United Kingdom.
- 39.Lorito, M., V. Farkas, S. Rebuffat, B. Bodo, and C. P. Kubicek. 1996. Cell wall synthesis is a major target of mycoparasitic antagonism by Trichoderma harzianum. J. Bacteriol. 178:6382-6385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lorito, M., R. L. Mach, P. Sposato, J. Strauss, C. K. Peterbauer, and C. P. Kubicek. 1996. Mycoparasitic interaction relieves binding of the Cre1 carbon catabolite repressor protein to promoter sequences of the ech42 (endochitinase-encoding) gene in Trichoderma harzianum. Proc. Natl. Acad. Sci. USA 93:14868-14872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lorito, M., S. L. Woo, I. G. Fernandez, G. Colucci, G. E. Harman, J. A. Pintor-Toro, E. Filippone, S. Muccifora, C. B. Lawrence, A. Zoina, and F. Scala. 1998. Genes from mycoparasitic fungi as a source for improving plant resistance to fungal pathogens. Proc. Natl. Acad. Sci. USA 95:7860-7865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mach, R. L., C. K. Peterbauer, K. Payer, S. Jaksits, S. L. Woo, S. Zeilinger, C. M. Kullnig, M. Lorito, and C. P. Kubicek. 1999. Expression of two major chitinase genes of Trichoderma atroviride (T. harzianum P1) is triggered by different regulatory signals. Appl. Environ. Microbiol. 65:1858-1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Magg, T., A. E. Melchinger, D. Klein, and M. Bohn. 2002. Relationship between European corn borer resistance and concentration of mycotoxins produced by Fusarium spp. in grains of transgenic Bt maize hybrids, their isogenic counterparts, and commercial varieties. Plant Breed. 121:146-154. [Google Scholar]
- 44.Mesterhazy, A., T. Bartok, C. G. Mirocha, and R. Komoroczy. 1999. Nature of wheat resistance to Fusarium head blight and the role of deoxynivalenol for plant breeding. Plant Breed. 118:97-110. [Google Scholar]
- 45.Miller, J. D., J. W. ApSimon, B. A. Blackwell, R. Greenhalgh, and A. Taylor. 2001. Deoxynivalenol: a 25 year perspective on a trichothecene of agricultural importance, p. 310-320. In B. A. Summerell, J. F. Leslie, D. Backhouse, W. L. Bryden, and L. W. Burgess (ed.), Fusarium: Paul E. Nelson Memorial Symposium. The American Phytopathological Society Press, St. Paul, Minn.
- 46.Miller, J. D., and P. G. Arnison. 1986. Degradation of deoxynivalenol by suspension cultures of the fusarium head blight resistant wheat cultivar Frontana. Can. J. Plant Pathol. 8:147-150. [Google Scholar]
- 47.Miller, J. D., M. Miles, and D. A. Fielder. 1997. Kernel concentrations of 4-acetylbenzoxazolin-2-one and diferuloylputrescine in maize genotypes and Gibberella ear rot. J. Agric. Food Chem. 45:4456-4459. [Google Scholar]
- 48.Miller, J. D., A. Taylor, and R. Greenhalgh. 1983. Production of deoxynivalenol and related compounds in liquid culture by Fusarium graminearum. Can. J. Microbiol. 29:1171-1178. [Google Scholar]
- 49.Peterbauer, C. K., M. Lorito, C. K. Hayes, G. E. Harman, and C. P. Kubicek. 1996. Molecular cloning and expression of the nag1 gene (N-acetyl-β-d-glucosaminidase-encoding gene) from Trichoderma harzianum P1. Curr. Genet. 30:325-331. [DOI] [PubMed] [Google Scholar]
- 50.Proctor, R. H., T. M. Hohn, and S. P. McCormick. 1995. Reduced virulence of Giberella zeae caused by disruption of a trichothecene toxin biosynthetic gene. Mol. Plant-Microbe Interact. 8:593-601. [DOI] [PubMed] [Google Scholar]
- 51.Rotter, B. A., and D. B. Prelusky. 1996. Toxicology of deoxynivalenol (vomitoxin). J. Toxicol. Environ. Health 48:1-34. [DOI] [PubMed] [Google Scholar]
- 52.Schirmbock, M., M. Lorito, Y. L. Wang, C. K. Hayes, I. Arisan-Atac, F. Scala, G. E. Harman, and C. P. Kubicek. 1994. Parallel formation and synergism of hydrolytic enzymes and peptaibol antibiotics, molecular mechanisms involved in the antagonistic action of Trichoderma harzianum against phytopathogenic fungi. Appl. Environ. Microbiol. 60:4364-4370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schisler, D. A., N. I. Khan, and M. J. Boehm. 2002. Biological control of Fusarium head blight of wheat and deoxynivalenol levels in grain via microbial antagonists, p. 53-69. In J. W. DeVries, M. W. Trucksess, and L. S. Jackson (ed.), Mycotoxins and food safety. Kluwer Academic Press/Plenum Publishers, New York, N.Y. [DOI] [PubMed]
- 54.Schnider-Keel, U., A. Seematter, M. Maurhofer, C. Blumer, B. Duffy, C. Gigot-Bonnefoy, C. Reimmann, R. Notz, G. Défago, D. Haas, and C. Keel. 2000. Autoinduction of 2,4-diacetylphloroglucinol biosynthesis in the biocontrol agent Pseudomonas fluorescens CHA0 and repression by the bacterial metabolites salicylate and pyoluteorin. J. Bacteriol. 182:1215-1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stack, R. W. May 1999, posting date. Return of an old problem: Fusarium head blight of small grains. American Phytopathological Society APSNet feature. [Online.] American Phytopathological Society, St. Paul, Minn. http://www.apsnet.org/online/feature/FHB/top.html.
- 56.Tekauz, A., B. McCallum, and J. Gilbert. 2000. Fusarium head blight of barley in western Canada. Can. J. Plant Pathol. 22:9-16. [Google Scholar]
- 57.Toyoda, H., H. Hashimoto, R. Utsumi, H. Kobayashi, and S. Ouchi. 1988. Detoxification of fusaric acid by a fusaric acid-resistant mutant of Pseudomonas solanacearum and its application to biological control of Fusarium wilt of tomato. Phytopathology 78:1307-1311. [Google Scholar]
- 58.Tronsmo, A., and G. E. Harman. 1992. Coproduction of chitinolytic enzymes and biomass for biological control by Trichoderma harzianum on media containing chitin. Biol. Control 2:272-277. [Google Scholar]
- 59.Walker, S. L., S. Leath, W. M. Hagler, and J. P. Murphy. 2001. Variation among isolates of Fusarium graminearum associated with Fusarium head blight in North Carolina. Plant Dis. 85:404-410. [Google Scholar]
- 60.Woo, S. L., B. Donzelli, F. Scala, R. Mach, G. E. Harman, C. P. Kubicek, G. Del Sorbo, and M. Lorito. 1999. Disruption of the ech42 (endochitinase-encoding) gene affects biocontrol activity in Trichoderma harzianum P1. Mol. Plant-Microbe Interact. 12:419-429. [Google Scholar]
- 61.Yi, C. L., H. P. Kaul, E. Kubler, and W. Aufhammer. 2002. Populations of Fusarium graminearum on crop residues as affected by incorporation depth, nitrogen and fungicide application. J. Plant Dis. Plant Prot. 109:252-263. [Google Scholar]
- 62.Yu, W., F.-Y. Yu, D. J. Undersander, and F. S. Chu. 1999. Immunoassays of selected mycotoxins in hay, silage and mixed feed. Food Agric. Immunol. 11:307-319. [Google Scholar]
- 63.Zeillinger, S., C. Galhaup, K. Payer, S. L. Woo, R. L. Mach, C. Fakete, M. Lorito, and C. P. Kubicek. 1999. Chitinase gene expression during mycoparasitic interaction of Trichoderma harzianum with its host. Fungal Genet. Biol. 26:131-140. [DOI] [PubMed] [Google Scholar]