Abstract
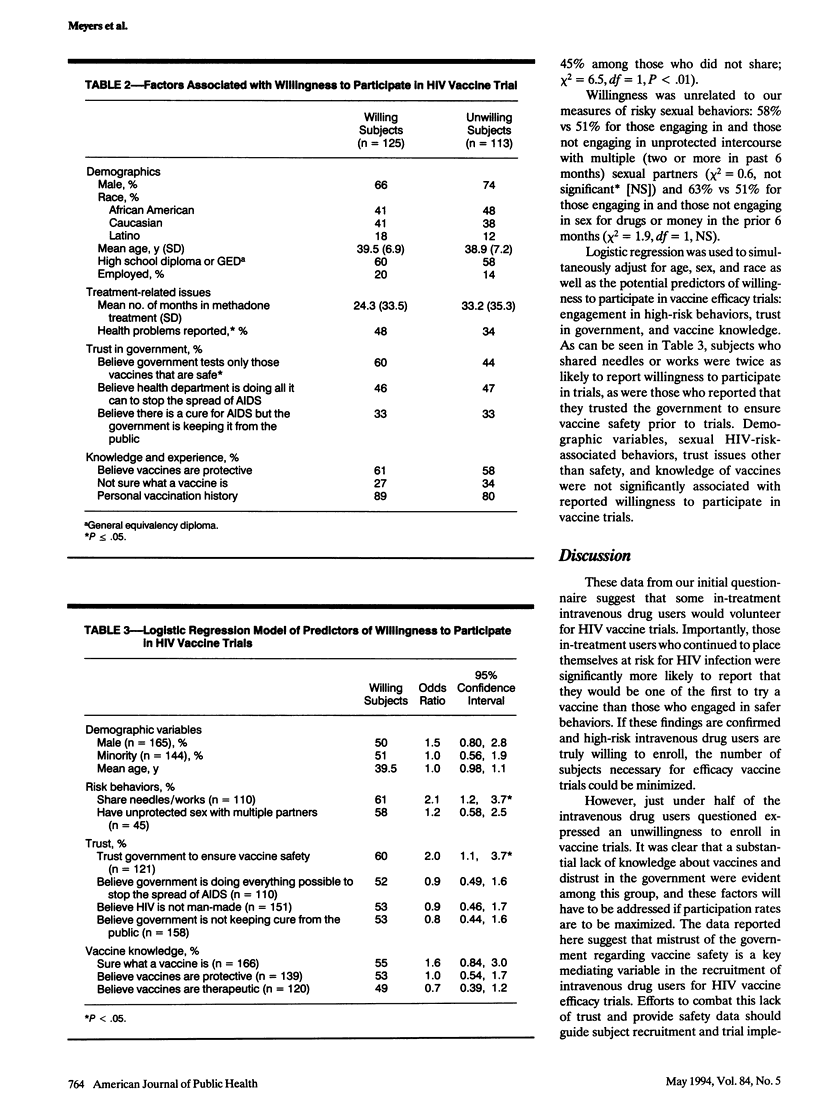
OBJECTIVES. The purpose of this study was to assess the willingness of intravenous drug users to participate in a preventive human immunodeficiency virus (HIV) vaccine efficacy trial. METHODS. Of the 347 intravenous drug users in methadone treatment who were approached for participation, 257 completed a battery of self-administered questionnaires assessing risk behaviors, interest in vaccine trials, and other vaccine-related information. Data from 16 known seropositives and 1 inconsistent responder were dropped from analyses (n = 240). RESULTS. Fifty-two percent of the subjects expressed a willingness to be one of the first individuals to participate in a preventive HIV vaccine efficacy trial. Subjects who had recently shared needles or works and subjects who trusted the government to ensure vaccine safety were both twice as likely to report interest in participation. Twenty-two percent of subjects reported that they would increase needle sharing if vaccinated. Thirty percent did not know what a vaccine was. CONCLUSIONS. These findings suggest that some in-treatment intravenous drug users would volunteer for a preventive HIV vaccine efficacy trial. Education and counseling will be required to ensure that subjects fully understand the trial's purposes, methods, risks and benefits.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Calsyn D. A., Saxon A. J., Wells E. A., Greenberg D. M. Longitudinal sexual behavior changes in injecting drug users. AIDS. 1992 Oct;6(10):1207–1211. doi: 10.1097/00002030-199210000-00023. [DOI] [PubMed] [Google Scholar]
- Coates T. J., Stall R. D., Kegeles S. M., Lo B., Morin S. F., McKusick L. AIDS antibody testing. Will it stop the AIDS epidemic? Will it help people infected with HIV? Am Psychol. 1988 Nov;43(11):859–864. [PubMed] [Google Scholar]
- Enel P., Charrel J., Larher M. P., Reviron D., Manuel C., San Marco J. L. Ethical problems raised by anti-HIV vaccination. Eur J Epidemiol. 1991 Mar;7(2):147–153. doi: 10.1007/BF00237358. [DOI] [PubMed] [Google Scholar]
- Esparza J., Osmanov S., Kallings L. O., Wigzell H. Planning for HIV vaccine trials: the World Health Organization perspective. AIDS. 1991;5 (Suppl 2):S159–S163. [PubMed] [Google Scholar]
- Holmes K. K., Karon J. M., Kreiss J. The increasing frequency of heterosexually acquired AIDS in the United States, 1983-88. Am J Public Health. 1990 Jul;80(7):858–863. doi: 10.2105/ajph.80.7.858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karzon D. T., Bolognesi D. P., Koff W. C. Development of a vaccine for the prevention of AIDS, a critical appraisal. Vaccine. 1992;10(14):1039–1052. doi: 10.1016/0264-410x(92)90114-y. [DOI] [PubMed] [Google Scholar]
- Koff W. C., Fauci A. S. Human trials of AIDS vaccines: current status and future directions. AIDS. 1989;3 (Suppl 1):S125–S129. [PubMed] [Google Scholar]
- Koff W. C., Hoth D. F. Development and testing of AIDS vaccines. Science. 1988 Jul 22;241(4864):426–432. doi: 10.1126/science.3293212. [DOI] [PubMed] [Google Scholar]
- Magura S., Goldsmith D., Casriel C., Goldstein P. J., Lipton D. S. The validity of methadone clients' self-reported drug use. Int J Addict. 1987 Aug;22(8):727–749. doi: 10.3109/10826088709027454. [DOI] [PubMed] [Google Scholar]
- Mariner W. K. Why clinical trials of AIDS vaccines are premature. Am J Public Health. 1989 Jan;79(1):86–91. doi: 10.2105/ajph.79.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCusker J., Stoddard A. M., McDonald M., Zapka J. G., Mayer K. H. Maintenance of behavioral change in a cohort of homosexually active men. AIDS. 1992 Aug;6(8):861–868. doi: 10.1097/00002030-199208000-00015. [DOI] [PubMed] [Google Scholar]
- Metzger D. S., Woody G. E., McLellan A. T., O'Brien C. P., Druley P., Navaline H., DePhilippis D., Stolley P., Abrutyn E. Human immunodeficiency virus seroconversion among intravenous drug users in- and out-of-treatment: an 18-month prospective follow-up. J Acquir Immune Defic Syndr. 1993 Sep;6(9):1049–1056. [PubMed] [Google Scholar]
- Metzger D., Woody G., De Philippis D., McLellan A. T., O'Brien C. P., Platt J. J. Risk factors for needle sharing among methadone-treated patients. Am J Psychiatry. 1991 May;148(5):636–640. doi: 10.1176/ajp.148.5.636. [DOI] [PubMed] [Google Scholar]
- Samuels J. F., Vlahov D., Anthony J. C., Chaisson R. E. Measurement of HIV risk behaviors among intravenous drug users. Br J Addict. 1992 Mar;87(3):417–428. doi: 10.1111/j.1360-0443.1992.tb01942.x. [DOI] [PubMed] [Google Scholar]
- Smith P. G., Hayes R. J., Mulder D. W. Epidemiological and public health considerations in the design of HIV vaccine trials. AIDS. 1991;5 (Suppl 2):S105–S111. doi: 10.1097/00002030-199101001-00015. [DOI] [PubMed] [Google Scholar]
- Zule W. A. Risk and reciprocity: HIV and the injection drug user. J Psychoactive Drugs. 1992 Jul-Sep;24(3):243–249. doi: 10.1080/02791072.1992.10471644. [DOI] [PubMed] [Google Scholar]
- el-Sadr W., Capps L. The challenge of minority recruitment in clinical trials for AIDS. JAMA. 1992 Feb 19;267(7):954–957. [PubMed] [Google Scholar]


