Abstract
Apparently conflicting data regarding the role of SOS-inducible, error-prone DNA polymerase IV (DinB) in spontaneous mutation are resolved by the finding that mutation is reduced by a polar allele with which dinB and neighboring yafN are deleted but not by two nonpolar dinB alleles. We demonstrate the existence of a dinB operon that contains four genes, dinB-yafN-yafO-yafP. The results imply a role for yafN, yafO, and/or yafP in spontaneous mutation.
Understanding of mutation mechanisms has been altered dramatically by the discovery of a superfamily of error-prone DNA polymerases called the polymerase Y (pol Y) superfamily (23), which is evolutionarily conserved throughout all modern domains of life (reviewed in references 9, 21 and 27). Many of these polymerases can insert bases across from DNA lesions, but this translesion synthesis activity is correlated with diminished fidelity on undamaged DNA templates. Escherichia coli contains two DNA polymerases from this family, DNA pol IV and pol V, both of which are upregulated during the SOS DNA damage response. DNA pol IV has a propensity for creating −1 frameshifts at mononucleotide repeats and also G-to-T transversions (12, 28-30). Recently, we found that DNA pol IV (encoded by the dinB gene) is required for “stationary-phase” or “adaptive” mutation in a lac frameshift reversion assay (19). Stationary-phase mutation in the Lac system occurs in slowly growing or nongrowing Lac− cells during starvation on lactose medium, and the mutation mechanism differs from spontaneous mutation during exponential growth in its requirements for different proteins (those required for double-strand break repair) and in producing different kinds of mutations, i.e., mostly −1 frameshifts (reviewed in references 8 and 24). We found that DinB (pol IV) is not required for spontaneous reversion of the same lac allele during exponential growth (growth-dependent mutation) (19). We also detected no effect of a dinB loss-of-function mutation in several other growth-dependent mutation assays (19). This suggests that pol IV does not contribute significantly to spontaneous mutation in rapidly growing cells. However, these observations appear to conflict with the results of others in which a pol IV-deficient mutant displayed a two- to threefold decrease in growth-dependent reversion of the same lac allele that we studied (26). In this study, we examined two possible reasons for this apparent discrepancy and found evidence that the source of the differences in results is the different dinB alleles used in the two studies. We report that dinB is part of a multigene operon and present evidence that one or more of the genes downstream of dinB may affect growth-dependent mutation.
dinB is the first of four open reading frames (ORFs) transcribed in the same orientation (see Fig. 1) (1). The functions of the downstream genes, yafN, yafO, and yafP, are unknown. Transcription of all four ORFs is induced by DNA-damaging agents as part of a LexA-regulated SOS response (5). Although dinB has a LexA-repressed promoter (12), the last three genes have no obvious promoters (5), suggesting that all four may be transcribed as part of an operon from the dinB promoter. There are two major differences between the reported experiments testing the role of DinB (pol IV) in growth-dependent mutation. First, we used a nonpolar dinB substitution mutation that affects the polymerase active site (2, 17, 19, 29, 32), whereas the apparently conflicting results were obtained with a dinB deletion-insertion allele in which part of yafN, as well as dinB, is deleted (12, 26). In addition to truncating yafN, the deletion-insertion could also have polar effects on downstream genes yafO and yafP. Second, we reduced contamination of the growth-dependent Lac+ mutant colony counts (mutants formed prior to plating on lactose medium) with stationary-phase mutant colonies (formed continuously after exposure to lactose medium) by using an internally controlled method to determine the time to count colonies (earlier than when stationary-phase mutant colonies are prevalent), as described in detail previously (11). In the other study, mutant colonies were scored at an arbitrary, and later, 43-h time point (26), which could allow inclusion of Lac+ stationary-phase mutant colonies. Because the latter form dinB+ dependently (19), this could cause an appearance of dinB-dependent, growth-dependent mutation when, in reality, only the stationary-phase mutants contributing to the counts were dinB dependent. We hypothesized (19) that the apparently conflicting results arose from allele differences, specifically, from effects of the downstream genes on growth-dependent mutation in the case of the dinB deletion-insertion allele, or from the difference in the time of scoring of Lac+ mutant colonies for determination of the mutation rates.
FIG. 1.
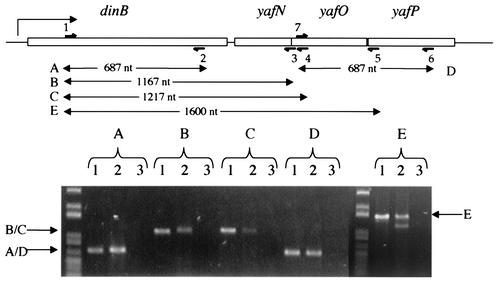
Messages on the same transcript. RT-PCR was performed on RNA extracted from SOS-induced E. coli strain MG1655 (Table 1) with primers (half arrows) complementary to the locations diagrammed at the top. The reactions were run on a 0.75% agarose gel in 1× TAE buffer (25) and stained with ethidium bromide, and a representative photograph is shown. In each set of reactions (A to E) for a given primer pair shown above (A to E), lane 1 shows a PCR performed on genomic DNA to give a size standard. Lane 2 contains the products of an RT-PCR performed on extracted RNA. Lane 3 contains products of a PCR performed on the extracted RNA leaving out the RT to ensure that the products seen are due to amplification of RNA, not contaminating DNA. Primers are complementary to sequences in the locations indicated. Primer 1 is BP381 (5′-ACGCCTACAAAGAAGCCTCA-3′). Primer 2 is BP382 (5′-GATCAGCTTTATTCAGCAGC-3′). Primer 3 is BP90 (5′-AATACCCGCATCCTTATTCCTT-3′). Primer 4 is BP375 (5′-ACGCATCAAGTTCCTCTGCT-3′). Primer 5 is BP 377 (5′-TCGCCAGGCTGATAGTTTCT-3′). Primer 6 is BP380 (5′-TTATGCTTGCGTCCACCGTA-3′). Primer 7 is BP378 (5′-AGCAGAGGAACTTGATGCGT-3′). nt, nucleotides.
We tested these hypotheses by determining growth-dependent rates of mutation to Lac+ in parallel in strains carrying either the nonpolar dinB10 allele we used previously or the Δ(dinB-yafN)::kan allele (referred to in reference 12 as ΔdinB::kan) used by others (26) (Table 1). The strains in which the Lac+ mutation rates were assayed carry two copies of the dinB and yaf genes: one in the chromosome and one in the F′ plasmid carrying the lac frameshift allele (19). To move Δ(dinB-yafN)::kan into both sites, PCR products of the Δ(dinB-yafN)::kan allele were made with primers BP89 5′-GCATTTCTCAAACCCTGAAATC-3′ and BP90 5′-AATACCCGCATCCTTATTCCTT-3′ on template strain AB1157 Δ(dinB-yafN)::kan and were moved via homologous linear replacement (6) into SMR5833, an FC40 derivative carrying recombination-promoting plasmid pKD46 (6). Isolates were screened for positions of insert on the F′ or chromosome as previously (19). One isolate carrying Δ(dinB-yafN)::kan on the chromosome was used as a donor for P1 transduction into FC36 to create SMR6095 [genotype FC36 Δ(dinB-yafN)::kan]. Another isolate, carrying Δ(dinB-yafN)::kan on the F′ plasmid, was mated into SMR4511 to create SMR6094 {genotype MG1655 proAB-81::Tn10 ΔlacX74[F′ Δ(dinB-yafN)::kan proAB+ lacI33ΩlacZ]}. These resulting strains were mated to produce homozygous Δ(dinB-yafN)::kan strain SMR6111 (Table 1). The presence of Δ(dinB-yafN)::kan on both chromosome and F′ was verified by PCR and mating as described previously (19).
TABLE 1.
E. coli K-12 strains used in this study
| Strain(s) | Description | Reference or source |
|---|---|---|
| FC29 | Δ(lac-proAB)XIIIthi ara[F′ Δ(lacI-lacZ)] | 3 |
| FC36 | Δ(lac-proAB)XIIIthi ara Rifr | 3 |
| FC40 | Δ(lac-proAB)XIIIthi ara Rifr[F′ proAB+lacI33ΩlacZ] | 3 |
| AB1157 Δ(dinB-yafN)::kan | 12; via F. Taddei | |
| MG1655 | Wild type | 1 |
| P90C | Δ(lac-proAB)XIII | 4 |
| SMR3804, SMR3805, SMR3807, SMR3855- SMR3859, SMR3861, SMR3863 | 10 independent Lac+ revertants of FC40; dinB+ | 20a |
| SMR4511 | MG1655 ΔlacX74 proAB-81::Tn10 | This workb |
| SMR4562 | Genotype same as that of FC40, independent construction | 18 |
| SMR5816 | P90C[F′ proAB-81::Tn10 lacI33ΩlacZ] | This workc |
| SMR5830 | SMR4562 dinB10[F′ dinB10 proAB+lacI33ΩlacZ]d | 19 |
| SMR5865-SMR5874 | 10 independent Lac+ revertants of SMR5830 | 19a |
| SMR5889 | SMR4562 ΔdinB51[F′ ΔdinB51 proAB+lacI33ΩlacZ]d | This work |
| SMR6111 | SMR4562 Δ(dinB-yafN)::kan[F′ Δ(dinB-yafN)::kan proAB+lacI33ΩlacZ]d | This work |
| SMR6356-SMR6365 | 10 independent Lac+ revertants of SMR6111 | This worka |
| SMR7481-SMR7490 | 10 independent Lac+ revertants of SMR5889 | This worka |
| SMR7491 | SMR4562 Δ(yafN-yafP)602[F′ Δ(yafN-yafP)602 proAB+lacI33ΩlacZ]d | This work |
| SMR7492-SMR7501 | 10 independent Lac+ revertants of SMR7491 | This workb |
Spontaneous Lac+ revertants were isolated, one each from 10 independent cultures.
Construction made by P1 transductions with the proAB-81::Tn10 and ΔlacX74 alleles derived from strains NK5525 (kindly provided by N. Kleckner via the E. coli Genetic Stock Center, Yale University) and CH1504 (16), respectively.
Construction made by P1 transduction of proAB-81::Tn10 derived from strain NK5525 (kindly provided by N. Kleckner) into the F′ derived from FC40 and mating into P90C.
The dinB and yafN-yafP genes are present on both the chromosome and F′, and so homozygous mutant strains were constructed.
The mutation rates were determined by using fluctuation tests as described previously (19), and mutation rates were calculated by using the Lac+ mutant counts taken at two times: First, in the controlled-time method (e.g., see references 11 and 19), known numbers of cells of control strains isogenic to each strain tested [dinB+, dinB10, or Δ(dinB-yafN)::kan, etc.], but carrying a Lac+ reversion mutation, are plated on lactose selective medium in parallel with the fluctuation tests. Colony counts in the fluctuation tests are taken continuously, and the counts corresponding to the time at which 50% of the control colonies have arisen are used for calculation of mutation rates. This method serves the dual functions of reducing contamination with stationary-phase Lac+ mutant colonies (which arise during selection on lactose plates) and also avoiding artificially low observations of mutant colonies in slowly growing strains, which take longer to form colonies under the selective conditions (on lactose plate medium) (11). Second, in the fixed-time method (26), colony counts are taken at 43 h.
For the controlled-time method, 40-culture fluctuation tests were performed with 5-ml cultures that were grown to saturation in M9 medium with 0.1% glycerol, washed three times in M9 wash, and concentrated 10-fold. Between 300 and 350 μl of the cell suspensions was plated with about 2 ×109 FC29 scavenger cells (Table 1), which consume any contaminating nonlactose carbon sources (3), on solid M9 medium with 0.1% lactose, and Lac+ mutant colonies were scored while scoring Lac+ speed-of-growth controls. Mutation rates were calculated by the method of the median (14) using colony counts from the time at which 50% of the Lac+ control colonies were visible and then multiplying by 2 to obtain the numbers of colonies at the time at which 100% of the Lac+ control colonies were visible. The median number of colonies observed ranged between 1 and 15.5 (with a mode of 2) among all of the experiments.
Using the controlled-time method, we found (Table 2 and previously [19]) that the Lac+ mutation rates for the dinB+ and dinB10 strains are indistinguishable from each other at the time when 50% of the control Lac+ colonies have become visible (as shown by overlapping standard errors and failure to reject the null hypothesis of equal rates at P = 0.97 by single-factor analysis of variance [ANOVA] and the Tukey test [31]). However, the mutation rate of the Δ(dinB-yafN)::kan strain is two- to threefold lower than that of the dinB+ control (a statistically significant difference at P = 0.025 by single-factor ANOVA and the Tukey test). Thus, it appears that the difference between the two sets of previous results (19, 26) can be explained by the different dinB alleles used. The Δ(dinB-yafN)::kan allele decreases the level of growth-dependent mutation significantly.
TABLE 2.
Lac+ mutation rate determinations from controlled-time colony counts
| dinB allelea and expt | Mutation rate (no. of mutations/ cell/generation) | Mean mutation rate (±SEM) (no. of mutations/cell/ generation) | P value for difference from dinB+b |
|---|---|---|---|
| dinB+ | |||
| 1 | 11 × 10−10 | 9.4 (±1) × 10−10 | |
| 2 | 8.2 × 10−10 | ||
| 3 | 12 × 10−10 | ||
| 4 | 8.9 × 10−10 | ||
| 5 | 16 × 10−10 | ||
| 6 | 11 × 10−10 | ||
| 7 | 12 × 10−10 | ||
| 8 | 5.8 × 10−10 | ||
| 9 | 5.7 × 10−10 | ||
| 10 | 5.2 × 10−10 | ||
| 11 | 7.6 × 10−10 | ||
| dinB10 | |||
| 1 | 6.6 × 10−10 | 8.2 (±1) × 10−10 | 0.97 |
| 2 | 7.9 × 10−10 | ||
| 3 | 10 × 10−10 | ||
| Δ(dinB-yafN)::kan | |||
| 1 | 3.5 × 10−10 | 5.6 (±0.3) × 10−10 | 0.025 |
| 2 | 5.8 × 10−10 | ||
| 3 | 5.8 × 10−10 | ||
| 4 | 6.8 × 10−10 | ||
| 5 | 6.6 × 10−10 | ||
| 6 | 6.0 × 10−10 | ||
| 7 | 5.5 × 10−10 | ||
| 8 | 4.3 × 10−10 | ||
| 9 | 4.6 × 10−10 | ||
| 10 | 5.4 × 10−10 | ||
| 11 | 7.2 × 10−10 | ||
| ΔdinB51 | |||
| 4 | 9.0 × 10−10 | 10 (±1) × 10−10 | 0.98 |
| 5 | 8.4 × 10−10 | ||
| 6 | 12 × 10−10 | ||
| 7 | 12 × 10−10 | ||
| Δ(yafN-yafP)602 | |||
| 4 | 6.2 × 10−10 | 8.7 (±1) × 10−10 | 0.96 |
| 5 | 6.8 × 10−10 | ||
| 6 | 3.4 × 10−10 | ||
| 7 | 17 × 10−10 | ||
| 8 | 11 × 10−10 | ||
| 9 | 5.6 × 10−10 | ||
| 10 | 9.3 × 10−10 | ||
| 11 | 10 × 10−10 |
The strains assayed were SMR4562, SMR5830, SMR6111, SMR5889, and SMR7491, and the Lac+ timing control strains for them, plated in parallel, were SMR3804, SMR3805, SMR3807, SMR3855 to SMR3859, SMR3861, SMR3863, SMR5865 to SMR5874, SMR6356 to SMR6365, SMR7481 to SMR7490, and SMR7492 to SMR7501 (Table 1).
P values were calculated by using single-factor ANOVA and the Tukey test (31) with SigmaStat 2.03 for Windows by SPSS, Inc., with the mutation rates at three significant figures.
With the fixed-time colony counts, in which the additional Lac+ mutants that appear by 43 h (including stationary-phase mutants) were included in the mutation rate calculations, the calculated mutation rates were slightly higher (Table 3), as expected. Again, we saw that the rates for the dinB+ and Δ(dinB-yafN)::kan strains were different, but with marginal significance (at only P > 0.1 by single-factor ANOVA and the Tukey test), and that dinB10 was not significantly different from din+ (P > 0.5). Because the Δ(dinB-yafN)::kan allele (but not the nonpolar dinB10 allele) decreased mutation significantly even when adaptive mutants were more stringently eliminated from the colony counts (Table 2), we conclude that genuine growth-dependent mutation was decreased by Δ(dinB-yafN)::kan but not by dinB10.
TABLE 3.
Lac+ mutation rate determinations from fixed-time (43-h) colony counts
| dinB allelea and expt | No. of cultures | Mutation rate (no. of mutations/ cell/generation)b | Mean mutation rate (±SEM) (no. of mutations/cell/ generation) |
|---|---|---|---|
| dinB+ | |||
| 1 | 40 | 3.9 × 10−9 | 4.6 (±1) × 10−9 |
| 2 | 30 | 3.3 × 10−9 | |
| 3 | 40 | 6.7 × 10−9 | |
| dinB10 | |||
| 1 | 40 | 1.6 × 10−9 | 2.4 (±0.9) × 10−9 |
| 2 | 28 | 1.3 × 10−9 | |
| 3 | 40 | 4.2 × 10−9 | |
| Δ(dinB-yafN)::kan | |||
| 1 | 40 | 1.2 × 10−9 | 1.8 (±0.7) × 10−9 |
| 2 | 40 | 1.1 × 10−9 | |
| 3 | 40 | 3.1 × 10−9 |
The strains assayed were SMR4562, SMR5830, and SMR6111 (Table 1).
Determinations of mutation rates were performed by fluctuation tests as described in the text for Table 2, except that the colony counts from which mutation rates were calculated were all taken at a fixed time of 43 h of incubation after plating. Mutation rates were calculated by the method of the median (14).
There are at least two possible reasons for the different phenotypes of the different dinB alleles in growth-dependent Lac+ mutation. First, the pol IV protein may play some structural role that is not abolished by the dinB10 substitution mutation, and thus, the null deletion mutant may have a stronger phenotype. That is, dinB10 may be a partial-function allele. Alternatively, loss of function of one or more of the yaf genes may be responsible for the decrease in mutation in Δ(dinB-yafN)::kan cells. To address the possibility that dinB10 is a partial-function allele, we constructed a nonpolar dinB null deletion allele, an in-frame deletion of dinB that does not affect the yafN-yafP genes, ΔdinB51 (Table 1). First, an in-frame dinB deletion-kan insertion, ΔdinB50::kan, was made by PCR using primers dinBwL 5′-GAAATCACTGTATACTTTACCAGTGTTGAGAGGTGAGCAATGCGTAAAATCATTCCGGGGATCCGTCGACC-3′ and dinBwR 5′-TAATAATGCACACCAGAATATACATAATAGTATACATCATAATCCCAGCACTGTAGGCTGGAGCTGCTTC-3′ on template plasmid pKD13 (6) (underlined bases are complementary to the plasmid) and moved via homologous linear replacement (6) into SMR5833 (described above). Isolates were screened for insert positions (19) on the F′ plasmid (SMR5876) or on the chromosome (SMR5875). SMR5878 (genotype FC36 ΔdinB50::kan) was made by transduction of FC36 with SMR5875 as the donor. Next, the kan cassette was removed from SMR5878 as described in reference 6 to create SMR5883 (genotype FC36 ΔdinB51). In this allele, codons 5 to 347 of the 351-codon dinB gene are replaced with FRT (the Flp recombinase target site). SMR5876, carrying ΔdinB50::kan on the F′ plasmid, was mated into SMR4511 to create SMR5877 (genotype MG1655 proAB-81::Tn10 ΔlacX74[F′ ΔdinB50::kan proAB+ lacI33ΩlacZ]), from which the kan cassette was removed to create SMR5882 (genotype MG1655 proAB-81::Tn10 ΔlacX74[F′ ΔdinB51 proAB+ lacI33ΩlacZ]). SMR5882 and SMR5883 were mated to produce homozygous ΔdinB51 mutant strain SMR5889 (Table 1). The presence of ΔdinB51 on both the chromosome and the F′ plasmid was verified as described previously (19).
With the nonpolar deletion ΔdinB51, we found that growth-dependent Lac+ reversion was no different in this strain than in the dinB+ strain (Table 2, P = 0.98) and so conclude that no (nonredundant) function of the DinB protein is needed for growth-dependent mutation. This supports the alternative possibility that one or more of the yafN, yafO, or yafP gene products contribute to growth-dependent mutation and that the depression of mutation in cells carrying the Δ(dinB-yafN)::kan deletion-insertion allele results either from the loss of yafN by deletion or from polar effects on yafO and/or yafP. We tested this hypothesis further by determining whether the yaf genes are cotranscribed with dinB.
To determine whether dinB-yafN-yafO-yafP is a transcriptional unit, we used reverse transcriptase PCR (RT-PCR) to detect transcripts. Because all four ORFs are SOS inducible (5), we induced expression of these genes by treating log-phase cultures of E. coli MG1655 with 20 μg of bleomycin per ml to induce the SOS response (13). Treatment for 20 min at 37°C resulted in a 30-fold decrease in viability. We isolated RNA from the induced cultures with the Qiagen RNeasy kit and used RT-PCR (25) to detect transcripts containing dinB, yafN, yafO, and yafP. Each result was obtained from three independent RNA preparations, and representative data are shown in Fig. 1. For each set of primers (A to E), amplification of chromosomal DNA showed the expected-size product (lane 1), amplification of reverse-transcribed RNA demonstrated the presence of the transcript (lane 2), and a control amplification of RNA without RT showed that the product in lane 2 was not from contaminating DNA (lane 3). Correct identities of the PCR products were verified by restriction mapping (data not shown). Primers within each downstream ORF, yafN, yafO, or yafP, yielded a product with a 5′ primer within the dinB gene (Fig. 1), indicating that a single transcript includes the entire dinB, yafN, and yafO genes and at least part of the yafP gene (Fig. 1, product E). We conclude that these genes constitute an operon. We were able to amplify a product with primers in dinB and the beginning of the yafP ORF, but we were unable to amplify with a primer to the 3′ end of yafP. However, as shown in lanes D of Fig. 1, we were able to detect a transcript including the 5′ end of yafO and the 3′ end of yafP, indicating that there is no significant transcription terminator downstream of the yafP 5′ end, which we detected in transcripts containing dinB. This, coupled with the lack of potential promoter sequences in the yafN-to-yafP region, implies that yafP is also part of the dinB-yafN-yafO operon. The results support the possibility that the Δ(dinB-yafN)::kan allele may confer loss of function not only of the deleted dinB and yafN genes but also of yafO and yafP via polar effects.
A yaf gene(s) may be required for growth-dependent Lac+ reversion either on its own or only when dinB is also absent, as is the case with the Δ(dinB-yafN)::kan allele. We constructed strains with only yafN-yafP deleted (which are dinB+) to test these possibilities. PCR products that create the allele Δ(yafN-yafP)601::kan were made with primers yafNwL 5′-TGTATATTCTGGTGTGCATTATTATGAGGGTATCACTGTATGCATCGAATTATTCCGGGGATCCGTCGACC-3′ andyafPwR 5′-ATACCAGGCGGGCGTTATTTTCATTGCAAGCTGGATTTAATGTTGCGGTTTTGTAGGCTGGAGCTGCTTC-3′ to amplify plasmid pKD13 (6) and moved via homologous linear replacement (6) into SMR5832 [genotype FC36(pKD46)] and SMR6233 [genotype MG1655(pKD46)] to create SMR6352 [genotype FC36 Δ(yafN-yafP)601::kan] and SMR6353 [genotype MG1655 Δ(yafN-yafP)601::kan], respectively. The kan gene was removed from SMR6352 (6) to create SMR7479 [genotype FC36 Δ(yafN-yafP)602]. This deletion replaces codon 5 of the yafN gene through codon 146 of the 150-codon yafP gene with a single FRT site at the deletion junction. Δ(yafN-yafP)601::kan was transduced into SMR5816 from SMR6353, creating SMR7477 {genotype P90C[F′ Δ(yafN-yafP)601::kan proAB+ lacI33ΩlacZ]}. The kan gene was removed (6), creating SMR7478 {genotype P90C [F′ Δ(yafN-yafP)602 proAB+ lacI33ΩlacZ]}. SMR7478 and SMR7479 were mated to produce homozygous Δ(yafN-yafP)602 strain SMR7491 (Table 1).
In the strain with yafN-yafP deleted, mutation rates were too variable for us to distinguish whether or not mutation was decreased. In eight mutation rate determinations (Table 2), the strain carrying Δ(yafN-yafP)602 displayed variable mutation rates that are neither significantly different from those of the isogenic din+ yaf+ strain (P = 0.96) nor significantly different from those of the Δ(dinB-yafN)::kan isogenic strain (P = 0.18). Thus, it remains possible either that a yaf gene(s) affects mutation on its own or that a yaf gene(s) affects mutation only when dinB is also deleted, as is the case for the Δ(dinB-yafN)::kan strain (Table 2). This possibility, i.e., redundant roles of dinB and a yaf gene(s) in growth-dependent mutation, would contrast with the nonredundant role of dinB in adaptive Lac+ reversion (19).
In summary, we have shown that a polar dinB allele causes decreased spontaneous mutation whereas a nonpolar substitution and a nonpolar deletion allele of dinB do not and that dinB is part of an operon of four genes, dinB-yafN-yafO-yafP. All of these genes were shown previously to be LexA controlled and SOS inducible (5). It is likely that the Δ(dinB-yafN)::kan allele disrupts expression of all four genes, and thus, phenotypes ascribed to dinB that were obtained with this allele may result partly or wholly from loss of yafN, yafO, and/or yafP function. This could be important regarding phenotypes previously ascribed to dinB reported for the Δ(dinB-yafN)::kan allele. These include phenotypes in spontaneous mutation (26), translesion synthesis (15, 22), and adaptive mutation (7), although the last has also been demonstrated with the nonpolar dinB10 allele and so its attribution to dinB is not in doubt (19). Here we have shown that one such phenotype, a reported effect of dinB on growth-dependent mutation in a Lac reversion assay (26), is likely to result from loss of function of one or more of the yaf genes.
What are the functions of the other genes in the dinB operon? yafO and yafP have no informative homologs; however, YafN is highly homologous to the protein type (rather than the RNA type) of antitoxins of bacterial toxin-antitoxin systems (10). These toxin-antitoxin systems increase the stability of plasmids within populations by selectively killing cells that lose the plasmid. The role of chromosomal toxin-antitoxin systems is not clear. In these systems, the antitoxin binds the toxin, which would otherwise cause cell death, and inhibits its function (10). The genes encoding toxin-antitoxin pairs are often found in proximity to each other, but neither YafO nor YafP has homology to a known toxin. Thus, YafN may be an SOS-inducible antitoxin that regulates the function of an as yet unidentified toxin. If found, a phenotype of yafN in mutation may result from lack of antitoxin and therefore killing of rare SOS-induced cells that would otherwise experience mutation. Finally, the results suggest that the yafN, yafO, and/or yafP gene products contribute to spontaneous mutation in growing cells. The SOS-regulated nature of their expression makes the mutation phenotype intriguing.
Acknowledgments
The first two authors contributed equally to this work.
We thank Charles Hill and the E. coli Genetic Stock Center for bacterial strains and P. J. Hastings, Megan Hersh, Mary-Jane Lombardo, Joseph Petrosino, Bernard Strauss, and Roger Woodgate for helpful comments on the manuscript.
This work was supported by Department of Defense Breast Cancer Research Program Predoctoral Fellowship DAMD17-99-1-9072 (G.J.M.), an American Society for Microbiology Undergraduate Summer Research Fellowship (P.L.L.), and National Institutes of Health grants T32-GM08231 (D.B.M.) and R01-GM53158.
REFERENCES
- 1.Blattner, F. R., G. Plunkett III, C. A. Bloch, N. T. Perna, V. Burland, M. Riley, J. Collado-Vides, J. D. Glassner, C. K. Rode, G. F. Mayhew, J. Gregor, N. W. Davis, H. A. Kirkpatrick, M. A. Goeden, D. J. Rose, B. Mau, and Y. Shao. 1997. The complete genome sequence of Escherichia coli K-12. Science 277:1453-1474. [DOI] [PubMed] [Google Scholar]
- 2.Boudsocq, F., H. Ling, W. Yang, and R. Woodgate. 2002. Structure-based interpretation of missense mutations in Y-family DNA polymerases and their implications for polymerase function and lesion bypass. DNA Repair 1:343-358. [DOI] [PubMed] [Google Scholar]
- 3.Cairns, J., and P. L. Foster. 1991. Adaptive reversion of a frameshift mutation in Escherichia coli. Genetics 128:695-701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coulondre, C., and J. H. Miller. 1977. Genetic studies of the lac repressor. IV. Mutagenic specificity in the lacI gene of Escherichia coli. J. Mol. Biol. 117:577-606. [DOI] [PubMed] [Google Scholar]
- 5.Courcelle, J., A. Khodursky, B. Peter, P. O. Brown, and P. C. Hanawalt. 2001. Comparative gene expression profiles following UV exposure in wild-type and SOS-deficient Escherichia coli. Genetics 158:41-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Datsenko, K. A., and B. L. Wanner. 2000. One-step gene inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA 97:6640-6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Foster, P. L. 2000. Adaptive mutation in Escherichia coli. Cold Spring Harbor Symp. Quant. Biol. 65:21-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Foster, P. L. 1999. Mechanisms of stationary phase mutation: a decade of adaptive mutation. Annu. Rev. Genet. 33:57-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Friedberg, E. C., R. Wagner, and M. Radman. 2002. Specialized DNA polymerases, cellular survival, and the genesis of mutations. Science 296:1627-1630. [DOI] [PubMed] [Google Scholar]
- 10.Grønlund, H., and K. Gerdes. 1999. Toxin-antitoxin systems homologous with relBE of Escherichia coli plasmid P307 are ubiquitous in prokaryotes. J. Mol. Biol. 285:1401-1415. [DOI] [PubMed] [Google Scholar]
- 11.Harris, R. S., G. Feng, K. J. Ross, R. Sidhu, C. Thulin, S. Longerich, S. K. Szigety, P. J. Hastings, M. E. Winkler, and S. M. Rosenberg. 1999. Mismatch repair is diminished during stationary-phase mutation. Mutat. Res. 437:51-60. [PubMed] [Google Scholar]
- 12.Kim, S.-R., G. Maenhaut-Michel, M. Yamada, Y. Yamamoto, K. Matsui, T. Sofuni, T. Nohmi, and H. Ohmori. 1997. Multiple pathways for SOS-induced mutagenesis in Escherichia coli: an SOS gene product (DinB/P) enhances frameshift mutations in the absence of any exogenous agents that damage DNA. Proc. Natl. Acad. Sci. USA 94:13792-13797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Köppen, A., S. Krobitsch, B. Thoms, and W. Wackernagel. 1995. Interaction with recombination hotspot χ converts the RecBCD enzyme of Escherichia coli into a χ-independent recombinase by inactivation of the RecD subunit. Proc. Natl. Acad. Sci. USA 92:6249-6253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lea, D. E., and C. A. Coulson. 1949. The distribution of the numbers of mutants in bacterial populations. J. Genet. 49:264-285. [DOI] [PubMed] [Google Scholar]
- 15.Lenne-Samuel, N., R. Janel-Bintz, A. Kolbanovskiy, N. E. Geacintov, and R. P. P. Fuchs. 2000. The processing of a benzo(a)pyrene adduct into a frameshift or a base substitution mutation requires a different set of genes in Escherichia coli. Mol. Microbiol. 38:299-307. [DOI] [PubMed] [Google Scholar]
- 16.Lin, R. J., M. Capage, and C. W. Hill. 1984. A repetitive DNA sequence, rhs, responsible for duplications within the Escherichia coli K-12 chromosome. J. Mol. Biol. 177:1-18. [DOI] [PubMed] [Google Scholar]
- 17.Ling, H., F. Boudsocq, R. Woodgate, and W. Yang. 2001. Crystal structure of a Y-family DNA polymerase in action: a mechanism for error-prone and lesion-bypass replication. Cell 107:91-102. [DOI] [PubMed] [Google Scholar]
- 18.McKenzie, G. J., R. S. Harris, P. L. Lee, and S. M. Rosenberg. 2000. The SOS response regulates adaptive mutation. Proc. Natl. Acad. Sci. USA 97:6646-6651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McKenzie, G. J., P. L. Lee, M.-J. Lombardo, P. J. Hastings, and S. M. Rosenberg. 2001. SOS mutator DNA polymerase IV functions in adaptive mutation and not adaptive amplification. Mol. Cell 7:571-579. (Erratum, 7:1119.) [DOI] [PubMed]
- 20.McKenzie, G. J., M.-J. Lombardo, and S. M. Rosenberg. 1998. Recombination-dependent mutation in Escherichia coli occurs in stationary phase. Genetics 149:1163-1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McKenzie, G. J., and S. M. Rosenberg. 2001. Adaptive mutations, mutator DNA polymerases and genetic change strategies of pathogens. Curr. Opin. Microbiol. 4:586-594. [DOI] [PubMed] [Google Scholar]
- 22.Napolitano, R., R. Janel-Bintz, J. Wagner, and R. P. P. Fuchs. 2000. All three SOS-inducible DNA polymerases (pol II, pol IV, and pol V) are involved in induced mutagenesis. EMBO J. 19:6259-6265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ohmori, H., E. C. Friedberg, R. P. P. Fuchs, M. F. Goodman, F. Hanaoka, D. Hinkle, T. A. Kunkel, C. W. Lawrence, Z. Livneh, T. Nohmi, L. Prakash, S. Prakash, T. Todo, G. C. Walker, Z. Wang, and R. Woodgate. 2001. The Y-family of DNA polymerases. Mol. Cell 8:7-8. [DOI] [PubMed] [Google Scholar]
- 24.Rosenberg, S. M. 2001. Evolving responsively: adaptive mutation. Nature Rev. Genet. 2:504-515. [DOI] [PubMed] [Google Scholar]
- 25.Sambrook, J., and D. W. Russell. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 26.Strauss, B. S., R. Roberts, L. Francis, and P. Pouryazdanparast. 2000. Role of the dinB gene product in spontaneous mutation in Escherichia coli with an impaired replicative polymerase. J. Bacteriol. 182:6742-6750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sutton, M. D., and G. C. Walker. 2001. Managing DNA polymerases: coordinating DNA replication, DNA repair, and DNA recombination. Proc. Natl. Acad. Sci. USA 98:8342-8349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tang, M., P. Pham, X. Shen, J. S. Taylor, M. O'Donnell, R. Woodgate, and M. F. Goodman. 2000. Roles of E. coli DNA polymerases IV and V in lesion-targeted and untargeted SOS mutagenesis. Nature 404:1014-1018. [DOI] [PubMed] [Google Scholar]
- 29.Wagner, J., P. Grúz, S. R. Kim, M. Yamada, K. Matsui, R. P. P. Fuchs, and T. Nohmi. 1999. The dinB gene encodes a novel E. coli DNA polymerase, DNA pol IV, involved in mutagenesis. Mol. Cell 4:281-286. [DOI] [PubMed] [Google Scholar]
- 30.Wagner, J., and T. Nohmi. 2000. Escherichia coli DNA polymerase IV mutator activity: genetic requirements and mutational specificity. J. Bacteriol. 182:4587-4595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zar, J. H. 1984. Biostatistical analysis, 2nd ed. Prentice Hall, Englewood Cliffs, N.J.
- 32.Zhou, B. L., J. D. Pata, and T. A. Steitz. 2001. Crystal structure of a DinB lesion bypass DNA polymerase catalytic fragment reveals a classic polymerase catalytic domain. Mol. Cell 8:427-437. [DOI] [PubMed] [Google Scholar]