Abstract
The c-MYC protooncogene is overexpressed in the most malignant primary brain tumor, glioblastoma multiforme (GBM), and has been correlated with the undifferentiated character of several cell types. However, the role of Myc activity in the generation of GBMs is not known. In this report, we show that gene transfer of c-MYC to GFAP-expressing astrocytes in vitro promotes the outgrowth of GFAP-negative, nestin-expressing cells with progenitor-like morphology, growth characteristics and gene-expression pattern. In addition, gene transfer of c-MYC to GFAP-expressing astrocytes in vivo induces GBMs when co-expressed with activated Ras and Akt. Without c-MYC, Ras+Akt induces GBMs from nestin-expressing CNS progenitors but is insufficient in GFAP-expressing differentiated astrocytes. The ability of Myc activity to enhance the oncogenic effects of Ras+Akt appears to be limited to GFAP-expressing astrocytes because nestin-expressing progenitors show no increase in GBM formation with the addition of MYC to Ras+Akt. These studies indicate that one role of MYC activity in the formation of gliomas might be to either promote or reinforce an undifferentiated phenotype required for glioma cells to respond to the oncogenic effects of elevated Ras and Akt activity.
Keywords: Glioblastoma multiforme (GBM), brain tumor, mouse model, oncogene, RCAS/tv-a
INTRODUCTION
Gliomas are classified into four clinical grades (Kleihues and Cavanee, 2000); the most aggressive tumors, grade 4 astrocytomas, are GBMs. In addition to overexpressing c-MYC and n-MYC, other well described alterations in GBMs result in disruption of cell cycle-arrest pathways controlled by INK4AARF (Ichimura et al., 1996) and stimulation of common signal transduction pathways that involve RAS, AKT and other proteins (Feldkamp et al., 1997).
We have modeled the genetic components of gliomagenesis in mice with somatic cell gene transfer using the RCAS/tv-a system (Fisher et al., 1999). This system utilizes avian retroviral replication competent ALV splice acceptor (RCAS) vectors and transgenic mice expressing TVA (the RCAS receptor) from tissue-specific promoters. In these studies we have used two mouse lines: one (Gtv-a) expresses tv-a from the astrocyte-specific promoter of the gene that encodes glial fibrillary acidic protein (GFAP) (Holland and Varmus, 1998); the second (Ntv-a) expresses tv-a from the nestin promoter, which is active in neural and glial progenitors (Holland et al., 1998). GFAP-expressing astrocytes from Gtv-a transgenic mice and nestin-expressing glial progenitors from Ntv-a transgenic mice are susceptible to infection and gene transfer by RCAS vectors both in vitro and in vivo. Mixtures of genes can also be transferred simultaneously to individual cells by infecting with multiple RCAS vectors.
Previously, we demonstrated that combined activation of Ras and Akt can induce GBM formation from nestin-expressing glial progenitors in vivo (RCAS-Ras + RCAS-Akt infection of Ntv-a mice). By contrast, combined activation of Ras and Akt is insufficient to induce GBMs from GFAP-expressing astrocytes (dual infection of Gtv-a mice) (Holland et al., 2000). These data imply that an undifferentiated cell-of-origin might be more sensitive to transformation.
OBJECTIVES
The c-MYC protooncogene is overexpressed in GBMs and is associated with an undifferentiated state (Iovanna et al., 1992; Chin et al., 1995; Hoshimaru et al., 1996; Felsher and Bishop, 1999). We sought to determine if overexpression of MYC sensitizes GFAP-expressing astrocytes to the transforming potential of combined Ras and Akt activation in vivo. We also investigated the effect of MYC expression on the differentiation of astrocytes in culture to determine if enhanced tumorigenic potential results from promoting an undifferentiated phenotype.
METHODS
Constructs
RCAS-Ras carries the gene encoding the G12D point mutant activated K-Ras; the mutant K-Ras cDNA was a gift of gift from Tyler Jacks (MIT) and the RCAS-Ras vector was a gift from Galen Fisher (Varmus lab). RCAS-Akt carries the activated form of Akt, designated Akt-MyrΔ11-60 (Aoki et al., 1998) and was a gift from Peter Vogt (UCSD). RCAS-LacZ encodes beta-galactosidase and was a gift of Yi Li (Varmus lab). RCASMYC includes human c-MYC cDNA and was a gift from Galen Fisher. RCAS-puro carries the gene that encodes puromycin resistance.
Mice
Production of the Gtv-a and Ntv-a mouse lines have been described (Holland et al., 1998; Holland and Varmus, 1998). The genetic backgrounds of the tv-a transgenic mice used were mixes of FVB/N, C57BL6, BALB/C and 129.
Cell culture
DF-1 cells, an immortalized line of chicken cells (Himly et al., 1998; Schaefer-Klein et al., 1998), were a generous gift of Douglas Foster (University of Minnesota). Plasmid forms of RCAS vectors were transfected into DF-1 cells using CaPO4 and allowed to replicate as viral vectors in culture. The supernatants from DF-1 cells infected with and producing either RCAS-MYC or RCAS-LacZ were filtered through a 0.45 μM filter and plated directly onto primary brain cultures from Gtv-a mice. Both DF-1 and cultured cells from Gtv-a mice were maintained in DMEM with 10% fetal calf serum (Gibco BRL).
Infection of transgenic mice
DF-1 cells infected with and producing RCAS vectors were harvested by trypsin digestion and pelleted by centrifugation; the pellets were resuspended in ∼50 μl medium and placed on ice. Using a 10 μl gas-tight Hamilton syringe, a single injection of 1 μl containing 10 000 DF-1 cells was made just anterior to the striatum in the right frontal region of newborn mice, with the tip of the needle just touching the base of the skull.
Western blot analysis, immunohistochemistry and immunocytochemistry
The following antibodies were used: polyclonal rabbit anti-PLP (Oncogene Research); polyclonal rabbit anti-PDGFRa (Upstate); monoclonal anti-human c-MYC and polyclonal anti-HA (Santa Cruz); polyclonal goat anti-vimentin (Chemicon); monoclonal anti-fibronectin and anti-GFAP (Boehringer); and monoclonal anti-nestin (Pharmingen). Antibodies were diluted in antibody dilution buffer (Chem-Mate). Secondary antibodies were obtained from Boehringer for Western analyses and ABC kits from Vector Labs for immunohistochemistry and immunocytochemistry studies.
For Western blot analysis, whole cell lysates were prepared from cultured cells with cold lysis buffer consisting of 100 mM NaCl, 30 mM Tris-HCl pH 7.6, 1% NP-40, 30 mM NaF, 1 mM EDTA, 1 mM Na vanadate, 0.5 mM phenylmethylsulfonyl floride and protease inhibitor cocktail (Boehringer). Samples were incubated on ice for 30 minutes and supernatants recovered after centrifuging at 14 000 rpm at 4°C for 20 minutes. Protein concentrations were determined by the BCA method (Pierce). Proteins were separated by electrophoresis on SDS–PAGE gels under reducing conditions and transferred to nitrocellulose membranes (Osmotics). The membranes were blocked with 5% non-fat dried milk in TBS pH 7.4 and washed in TBS with 0.5% Tween-20.
For immunocytochemistry, cultured cells were fixed in 4% paraformaldehyde in PBS (pH 7.4) at room temperature for 15 minutes, washed three times for 5 minutes each in PBS (pH 7.4) and blocked in 5% horse serum in PBS (pH 7.4) at room temperature for 60 minutes. The cells were then incubated in primary antibody for 60 minutes at room temperature and washed three times (5 minutes each) in PBS (pH 7.4) before staining with secondary antibody and DAB using an ABC kit from Vector Labs.
Brain sectioning and immunohistochemistry
Animals were sacrificed at 12-weeks old or earlier if they developed macrocephaly and lethargy, and the brains fixed in 4% formaldehyde in PBS for 36 hours. The brains were cut into 5 sections, mounted in paraffin and 5 μM sections cut with a Leica microtome. The sections were treated first with antigen unmasking solution (Vector Labs) then stained with hematoxylin and eosin (H&E). Alternatively, the sections were treated with 1% hydrogen peroxide in methanol for 30 minutes to inactivate endogenous peroxidases. The sections were then blocked with 1% goat serum in TBS-T solution for 60 minutes followed by a 60-minute incubation at room temperature after the addition of primary antibodies noted above. The sections were washed extensively with TBS-T and antibody staining visualized with peroxidase–biotin conjugated anti-mouse or anti-rabbit antibody (ABC™ Vector) and counter-stained with hematoxylin.
RESULTS
Initially, we induced c-MYC expression in cultured astrocytes by infecting primary cultures of Gtv-a astrocytes with an RCAS vector carrying the human c-MYC cDNA (RCAS-MYC). Following infection with RCAS-MYC, astrocytes adopted a smaller, more compact morphology compared with astrocytes infected with the control RCAS-LacZ vector that carries the marker gene encoding β-galactosidase (Fig. 1A). Immunocytochemical staining of astrocytes infected with RCAS-MYC demonstrated loss of GFAP expression and increased nestin expression (Fig. 1B). We then performed Western blot analysis of Gtv-a-transgenic brain cultures infected with either RCAS-MYC or RCAS-LacZ and compared the concentrations of proteins such as PLP, fibronectin and vimentin, which are expressed characteristically in glial progenitors but not in astrocytes (Lee et al., 2000). We discovered increased expression of markers for glial progenitors in RCAS-MYC infected cells, which correlated with the level of virally transduced MYC in two independently infected populations of cells (Fig. 1C). Expression of platelet-derived growth factor receptor α (PDGFR-α) was essentially the same in both cell populations, indicating that PDGFR-α expression is relatively unaffected by MYC, unlike other markers of glial progenitors. Last, cells infected with RCAS-MYC had a growth advantage relative to cells infected with RCAS-LacZ (Fig. 1D) and RCAS-MYC-infected cells were immortalized whereas LacZ-infected Gtv-a astrocytes did not exhibit an undifferentiated character and senesced after extended culture (data not shown). Together, these data imply that elevating the activity of MYC in astrocytes in culture results in a rapidly growing population of cells that share many gene-expression characteristics of undifferentiated glia. They also indicate that MYC maintains the undifferentiated phenotype.
Fig. 1.
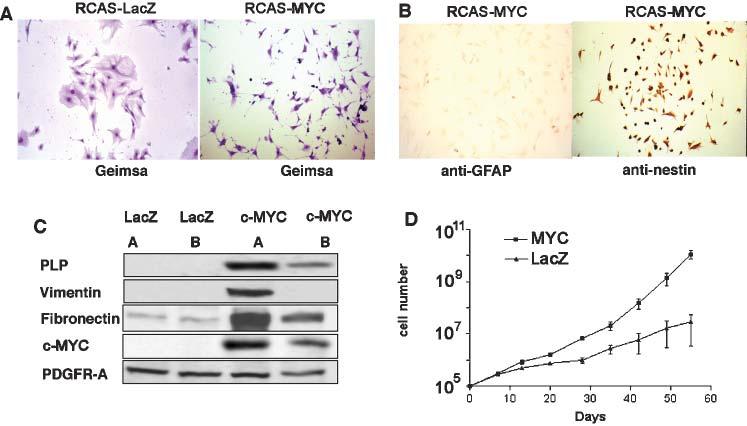
Cell-culture characteristics of astrocytes infected with RCAS-MYC. (A) Morphologic differences between Gtv-a astrocytes infected with RCAS-MYC and RCAS-LacZ are indicated. (B) Immunostaining for either GFAP or nestin in cultured astrocytes from Gtv-a mice infected with RCAS-MYC. (C) Western blot analysis of two, independent, RCAS-MYC-infected Gtv-a astrocyte cultures demonstrating expression of proteins that are not typically expressed in astrocytes but are characteristic of glial progenitors, including PLP, vimentin and fibronectin. (D) Growth rates of RCAS-MYC-infected and RCAS-LacZ (control)-infected Gtv-a transgenic astrocytes in culture. A and B at 100× magnification.
Previously, we demonstrated that combined activation of Ras and Akt induced GBMs only from undifferentiated glial progenitors in Ntv-a mice, and that differentiated astrocytes from Gtv-a mice were insensitive to tumor formation following such stimuli (Holland et al., 2000). The in vitro results indicated that MYC could promote undifferentiated Ntv-a-like characteristics in infected cells from Gtv-a mice. By infecting both mouse strains (Gtv-a and Ntv-a) with Ras+Akt, either with or without MYC, we first confirmed our previous result that MYC is not necessary for tumor formation from nestin-expressing glial progenitors (Ntv-a mice) that are naturally undifferentiated and sensitive to the tumor-inducing capacity of double infection with Ras+Akt. After achieving this goal, we then sought to demonstrate that the addition of MYC is required to promote an undifferentiated phenotype in GFAP-expressing astrocytic cells of origin (Gtv-a mice) sensitizing them to glioma formation by Ras+Akt.
The incidence of tumors in each group is shown in Fig. 2C. To test the hypothesis that MYC induces gliomas when combined with Ras+Akt in Gtv-a but not Ntv-a mice, we created a logistic regression model with MYC, strain (Gtv-a and Ntv-a), and MYC by strain interaction as predictors. Using exact methods, the coefficients for strain and MYC-by-strain interaction, but not MYC-independent-of-strain, were statistically significant (P < 0.001, P < 0.001 and P = 0.5, respectively). This demonstrates that the tumor-promoting effect of MYC, when combined with Ras and Akt, depends on the mouse strain. The upper bound of the 95% confidence interval for MYC was an odds ratio of 1.6, confirming that infection with MYC when combined with Ras+Akt is unlikely to have an important effect independent of mouse strain. Therefore, MYC appears to contribute to oncogenesis from GFAP-expressing astrocytes as the cell of origin but not from nestin-expressing glial progenitors.
Fig. 2.
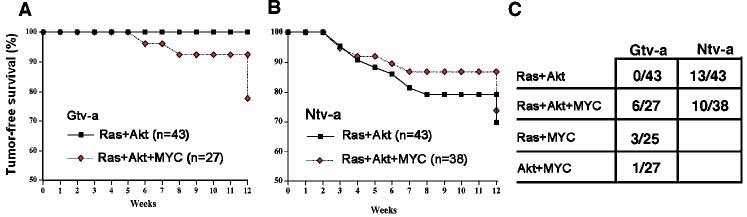
Tumor-free survival and tumor incidence in Gtv-a and Ntv-a-transgenic mice infected with RCAS vectors carrying activated Ras, Akt and human c-MYC. Two cohorts each of Gtv-a (A) and Ntv-a (B) transgenic mice were infected with either RCAS-Ras + RCAS-Akt or RCAS-Ras + RCAS-Akt + RCAS-MYC. Mice were sacrificed either at 12 weeks of age or when they developed signs of intracranial pathology. (C) The incidence of tumors in Ntv-a and Gtv-a mice infected with RCAS vectors. The denominator indicates the number of mice infected at birth and the numerator indicates the number of mice that developed tumors by 12 weeks of age.
The data in vivo is consistent with MYC promoting undifferentiated character in Gtv-a cells, similar to naturally undifferentiated Ntv-a cells that are sensitive to Ras + Akt. In Gtv-a mice, the addition of MYC to Ras+Akt allows tumor formation (6/27; 22%) from more differentiated, GFAP-expressing astrocytic cells of origin, and the largest such tumor is shown in Fig. 3A. By contrast, no tumors (0/43) were observed Gtv-a mice with Ras+Akt in the absence of MYC. This difference was statistically significant (P = 0.002, Fisher's exact test). In Ntv-a mice, where nestin-expressing cells are naturally undifferentiated and sensitive to tumor formation by Ras + Akt, the addition of MYC was unnecessary. There was no significant difference in glioma incidence (P = 0.7 by χ2 test) when comparing double infection (Ras+Akt, 13/43, 30%) to triple infection (MYC + Ras+Akt, 10/38, 26%); moreover, the confidence interval from this statistical analysis further demonstrated that addition of MYC to Ras+Akt had no important effect on tumor growth in Ntv-a mice. The data is consistent with MYC promoting an undifferentiated character of GFAP-expressing astrocytes from Gtv-a mice, which is similar to the naturally (i.e. without MYC) undifferentiated, nestin-expressing tumor cells in Ntv-a mice. These results are further illustrated in the Kaplan-Meier survival curves (Fig. 2A,B).
Fig. 3.
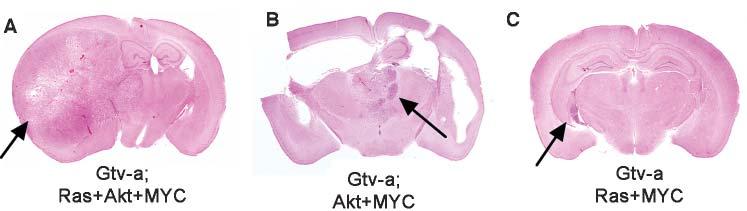
Characteristics of GBMs induced from astrocytes by infection of mice with RCAS-MYC, RCAS-Ras and RCAS-Akt. Whole-mounts illustrating (A) the largest GBM arising in Gtv-a mice after infection with a combination of Ras+Akt+MYC, (B) the only GBM that arose in a Gtv-a mouse after infection with Akt+MYC (with associated hydrocephalus), and (C) the largest GBM arising in Gtv-a mice after infection with Ras+MYC.
To further interrogate the effect of MYC on other oncogenic combinations, we infected Gtv-a mice with double combinations, including MYC+Ras, MYC + Akt and Ras + Akt. As reported previously (Holland et al., 2000), Ras + Akt induced no tumors. MYC+Akt induced one tumor from 27 Gtv-a mice (4%) at 12 weeks (Fig. 3B), which, histologically, resembled the Ras+Akt+MYC gemistocytic astrocytomas. Immunohistochemical analysis of this particular tumor demonstrated expression of exogenous Akt and MYC and endogenous Ras (not shown). Therefore, although MYC+Akt did induce a glioma in one mouse, activation of Ras was achieved spontaneously.
Three out of 25 mice (12%) infected with the double combination of Ras+MYC developed small tumors with the histologic characteristics of lower grade astrocytomas (largest tumor shown in Fig. 3C). These lesions contained abundant cytoplasm when stained with H&E, expressed GFAP as shown by immunohistochemistry, and showed occasional evidence of mitotic activity. Because these lesions exhibited neither pseudopalisading necrosis nor microvascular proliferation, they were classified as anaplastic astrocytoma (grade III astrocytoma) rather than GBM (grade IV astrocytoma). It is possible that these lesions might have acquired additional oncogenic mutations and increased in either size or grade had they been allowed to progress over time. From this data it appears that postnatal gene transfer of Ras + MYC is weakly oncogenic in differentiated astrocytes, but that Ras alone is not under these experimental conditions (Holland et al., 2000).
The gliomas induced by the combination of Ras+Akt+MYC in Gtv-a mice were characterized by large, epithelioid cells that closely resemble human gemistocytic astrocytomas (Bigner et al., 1998). Figure 4 illustrates the microscopic features of these tumors which include a mixture of low and high-grade elements, as seen in human gemistocytic astrocytomas undergoing progression from a low grade tumor to a GBM (Fig. 4A,B). In addition, the mouse tumors contained elements of higher malignancy, comprising an overgrowth of small tumor cells and the classic findings of GBM, including pseudopalisading necrosis and microvascular proliferation.
Fig. 4.
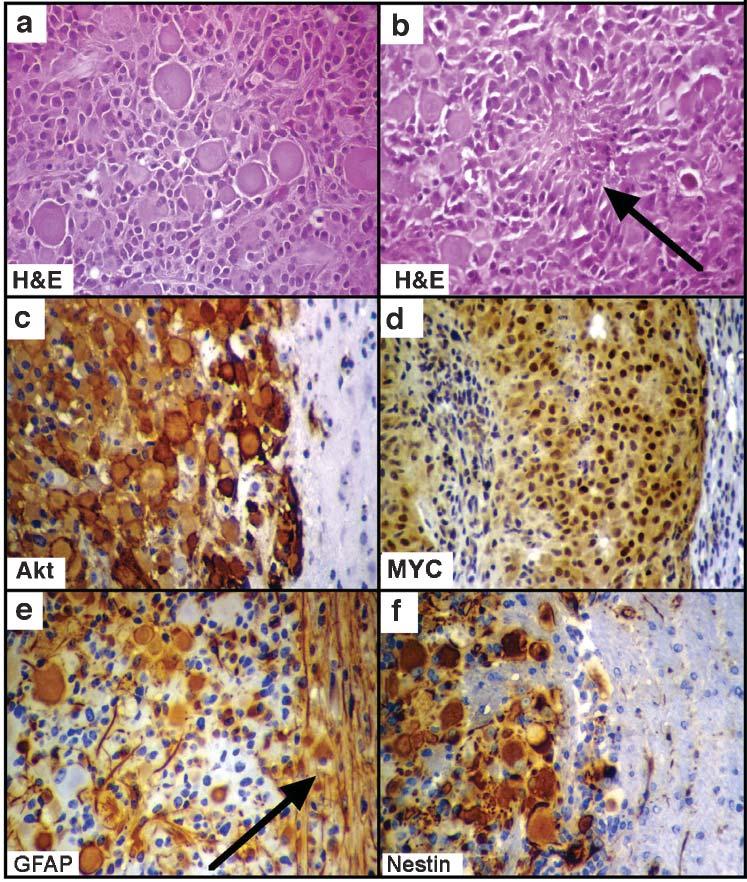
Microscopic characteristics of gliomas arising from astrocytes following infection of Gtv-a mice with RCAS vectors encoding Ras, Akt and MYC. H&E staining illustrates (A) the mixed cell type and (B) pseudopalisading necrosis (arrow). (C–F) Immunohistochemical staining with antibodies to the HA-tag on the virally transduced Akt (to identify the tumor) (C), human c-MYC (D), GFAP (E) and nestin (F). All photographs of immunohistochemical stains include both tumor and adjacent brain tissue for comparison. The progenitor marker nestin is expressed in the tumor arising from GFAP-expressing cells, and GFAP is expressed in both tumor and adjacent reactive brain tissue (arrow in E). All at 400× magnification.
We then sought to determine if the tumors that arose from the GFAP-expressing cells showed evidence of undifferentiated character. These tumors expressed nestin strongly, which is consistent with an undifferentiated phenotype and analogous to the effects of c-MYC that we observed in vitro (Fig. 4C-F). This data indicates that in vivo, c-MYC might convert GFAP-expressing astrocytes in Gtv-a mice to cells that resemble more closely nestin-expressing cells from Ntv-a mice, which are sensitive to the oncogenic effects of Ras+Akt without MYC. The expression of human c-MYC was demonstrated by immunohistochemical staining of glioma sections. Antihuman c-MYC staining in tumors induced in Gtv-a mice following triple infection with Ras+Akt+MYC corresponded to regions of tumor cells that express exogenous Akt, as indicated by staining for the HA epitope tag on the Akt vector.
To characterize further the cells comprising gliomas induced by Ras+Akt+MYC in Gtv-a mice, we performed immunohistochemical staining with antibodies to the astrocyte markers GFAP and S100 protein, and the neuronal markers synaptophysin and NeuN. The tumors expressed variable amounts of GFAP and S100 as occurs in human GBMs (Bigner et al., 1998), which indicates limitation to glial lineage. By contrast, no tumor expressed either of the neuronal markers synaptophysin or NeuN (data not shown), indicating that Gtv-a astrocytes do not dedifferentiate to cells that have features of neurons, such as those found in primitive neuroectodermal tumors.
CONCLUSIONS
MYC either maintains or promotes an undifferentiated phenotype and imparts a growth advantage in GFAP-expressing cultured astrocytes following post-natal somatic cell gene transfer by RCAS in vitro.
MYC+Ras+Akt induces gliomas from GFAP-expressing astrocytes in 22% of mice following post-natal, somatic-cell, gene transfer by RCAS in vivo, whereas Ras+Akt without MYC does not. However, Ras+Akt (without MYC) does induce gliomas from nestin-expressing glial progenitors, and the addition of MYC does not increase frequency of tumor formation.
MYC+Akt induces gliomas from GFAP-expressing astrocytes at low frequency (4%), but there is spontaneous activation of endogenous Ras in the tumor cells.
MYC+Ras is weakly oncogenic (12%) in astrocytes that express GFAP in vivo.
DISCUSSION
The c-MYC protooncogene is overexpressed in human GBMs (Trent et al., 1986; Engelhard et al., 1989) and MYC correlates with the undifferentiated character (Iovanna et al., 1992; Chin et al., 1995; Hoshimaru et al., 1996; Felsher and Bishop, 1999). We demonstrated previously that undifferentiated glial precursors are more sensitive to transformation than differentiated astrocytes (Holland et al., 2000; Dai et al., 2001). Here, we demonstrated that MYC enhances the sensitivity of GFAP-expressing astrocytes to gliomagenesis. By contrast, MYC was neither required nor important in nestin-expressing glial progenitors.
One interpretation of these results is that elevated MYC activity alters the differentiation status of astrocytes by promoting an undifferentiated state that resembles glial progenitors both in vitro and in vivo. The expression of the progenitor marker nestin in gliomas that arise from GFAP-expressing cells (in Gtv-a) mice supports the notion that these oncogenic alterations cause a shift towards an undifferentiated state. A second, complementary interpretation of the data is that the combination of Ras + MYC is weakly oncogenic in astrocytes, and this oncogenic effect is enhanced dramatically by the additional activation of Akt.
From the data presented here, it appears that an undifferentiated phenotype is required for a cell to respond to the oncogenic signals of Ras and Akt. This undifferentiated character might result either from an undifferentiated cell-of-origin (as is the case in Ntv-a mice in our experimental system) or from additional alterations that promote undifferentiated character in cells that were originally differentiated (as is the case in Gtv-a mice with the addition of c-MYC). Additionally, rather than discrete categorization of either differentiated or undifferentiated states, glia might exist in a continuum of differentiation and oncogenic sensitivity, such that less-differentiated cells might be more susceptible to transformation. If so, alterations that either promote or maintain an undifferentiated phenotype, such as those that result in elevated MYC activity, might contribute to gliomagenesis.
There are certain to be many cell types from the Gtv-a and Ntv-a transgenic mice that are infected, and our study does not distinguish between two possibilities: (1) expression of c-MYC promotes an undifferentiated phenotype of astrocytes such that they exhibit features of glial progenitors; and (2) c-MYC expression selects a subset of GFAP-expressing progenitor cells to become maintained in an undifferentiated state. Nonetheless, these data imply that differentiation status and MYC activity might be important, related therapeutic targets for patients with gliomas. Last, although it is not clear from our data whether MYC contributes to tumor initiation or maintenance, or both, therapeutic strategies aimed at either forcing a differentiated gene-expression-pattern on glioma cells or blocking MYC activity might reduce the oncogenic effects of elevated Ras and Akt activities.
ACKNOWLEDGEMENTS
We thank Christina Glaster and Bill Carey for assistance preparing the manuscript. We also thank Joseph C. Celestino and Laura K. Schaefer for technical assistance and Tyler Jacks (MIT) for the mutant K-Ras cDNA, Galen Fisher (Varmus lab) for the RCAS-Ras and RCAS-MYC vectors, Peter Vogt (UCSD) for the RCAS-Akt vector, Yi Li (Varmus lab) for the RCAS-LacZ vector, and Douglas Foster (University of Minnesota) for the DF-1 cells. We also thank Dan Fults (University of Utah) for his input on the project. This work was supported in part by a Basic Research Fellowship from the American Brain Tumor Association and grant number T32 CA009512 from the National Cancer Institute (ABL), and by the Bressler, Seroussi, and Kirby Foundations, and the NIH (ECH).
REFERENCES
- Aoki M, Batista O, Bellacosa A, Tsichlis P, Vogt PK. The Akt kinase: Molecular determinants of oncogenicity. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:14950–14955. doi: 10.1073/pnas.95.25.14950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigner DD, McLendon RE, Bruner JM, Russell DS, Rubinstein LJ. Russell and Rubinstein's Pathology of Tumors of the Nervous System. Hodder Arnold; 1998. [Google Scholar]
- Chin L, Schreiber-Agus N, Pellicer I, Chen K, Lee HW, Dudast M, Cordon-Cardo C, DePinho RA. Contrasting roles for Myc and Mad proteins in cellular growth and differentiation. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:8488–8492. doi: 10.1073/pnas.92.18.8488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai C, Celestino JC, Okada Y, Louis DN, Fuller GN, Holland EC. PDGF autocrine stimulation dedifferentiates cultured astrocytes and induces oligodendrogliomas and oligoastrocytomas from neural progenitors and astrocytes in vivo. Genes and Development. 2001;15:1913–1925. doi: 10.1101/gad.903001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelhard HH, 3rd, Butler A.B.t., Bauer KD. Quantification of the c-myc oncoprotein in human glioblastoma cells and tumor tissue. Journal of Neurosurgery. 1989;71:224–232. doi: 10.3171/jns.1989.71.2.0224. [DOI] [PubMed] [Google Scholar]
- Feldkamp MM, Lau N, Guha A. Signal transduction pathways and their relevance in human astrocytomas. Journal of Neurooncology. 1997;35:223–248. doi: 10.1023/a:1005800114912. [DOI] [PubMed] [Google Scholar]
- Felsher DW, Bishop JM. Reversible tumorigenesis by MYC in hematopoietic lineages. Molecular Cell. 1999;4:199–207. doi: 10.1016/s1097-2765(00)80367-6. [DOI] [PubMed] [Google Scholar]
- Fisher GH, Orsulic S, Holland E, Hively WP, Li Y, Lewis BC, Williams BO, Varmus HE. Development of a flexible and specific gene delivery system for production of murine tumor models. Oncogene. 1999;18:5253–5260. doi: 10.1038/sj.onc.1203087. [DOI] [PubMed] [Google Scholar]
- Himly M, Foster DN, Bottoli I, Iacovoni JS, Vogt PK. The DF-1 chicken fibroblast cell line: transformation induced by diverse oncogenes and cell death resulting from infection by avian leukosis viruses. Virology. 1998;248:295–304. doi: 10.1006/viro.1998.9290. [DOI] [PubMed] [Google Scholar]
- Holland EC, Varmus HE. Basic fibroblast growth factor induces cell migration and proliferation after glia-specific gene transfer in mice. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:1218–1223. doi: 10.1073/pnas.95.3.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland EC, Hively WP, DePinho RA, Varmus HE. A constitutively active epidermal growth factor receptor cooperates with disruption of G1 cell-cycle arrest pathways to induce glioma-like lesions in mice. Genes and Development. 1998;12:3675–3685. doi: 10.1101/gad.12.23.3675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland EC, Celestino J, Dai C, Schaefer L, Sawaya RE, Fuller GN. Combined activation of Ras and Akt in neural progenitors induces glioblastoma formation in mice. Nature Genetics. 2000;25:55–57. doi: 10.1038/75596. [DOI] [PubMed] [Google Scholar]
- Hoshimaru M, Ray J, Sah DW, Gage FH. Differentiation of the immortalized adult neuronal progenitor cell line HC2S2 into neurons by regulatable suppression of the v-myc oncogene. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:1518–1523. doi: 10.1073/pnas.93.4.1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichimura K, Schmidt EE, Goike HM, Collins VP. Human glioblastomas with no alterations of the CDKN2A (p16INK4A, MTS1) and CDK4 genes have frequent mutations of the retinoblastoma gene. Oncogene. 1996;13:1065–1072. [PubMed] [Google Scholar]
- Iovanna JL, Lechene de la Porte P, Dagorn JC. Expression of genes associated with dedifferentiation and cell proliferation during pancreatic regeneration following acute pancreatitis. Pancreas. 1992;7:712–718. doi: 10.1097/00006676-199211000-00013. [DOI] [PubMed] [Google Scholar]
- Kleihues P, Cavanee W. Pathology and Genetics of Tumours of the Nervous System. International Agency for Research on Cancer; 2000. [Google Scholar]
- Lee JC, Mayer-Proschel M, Rao MS. Gliogenesis in the central nervous system. Glia. 2000;30:105–121. doi: 10.1002/(sici)1098-1136(200004)30:2<105::aid-glia1>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Schaefer-Klein J, Givol I, Barsov EV, Whitcomb JM, VanBrocklin M, Foster DN, Federspiel MJ, Hughes SH. The EV-O-derived cell line DF-1 supports the efficient replication of avian leukosis-sarcoma viruses and vectors. Virology. 1998;248:305–311. doi: 10.1006/viro.1998.9291. [DOI] [PubMed] [Google Scholar]
- Trent J, Meltzer P, Rosenblum M, Harsh G, Kinzler K, Mashal R, Feinberg A, Vogelstein B. Evidence for rearrangement, amplification, and expression of c-myc in a human glioblastoma. Proceedings of the National Academy of Sciences of the United States of America. 1986;83:470–473. doi: 10.1073/pnas.83.2.470. [DOI] [PMC free article] [PubMed] [Google Scholar]


