Abstract
Haemoglobin-based oxygen carriers can undergo oxidation of ferrous haemoglobin into a non-functional ferric form with enhanced rates of haem loss. A recently developed human haemoglobin conjugated to maleimide-activated poly(ethylene glycol), termed MP4, has unique physicochemical properties (increased molecular radius, high oxygen affinity and low cooperativity) and lacks the typical hypertensive response observed with most cell-free haemoglobin solutions. The rate of in vitro MP4 autoxidation is higher compared with the rate for unmodified SFHb (stroma-free haemoglobin), both at room temperature (20–22 °C) and at 37 °C (P<0.001). This appears to be attributable to residual catalase activity in SFHb but not MP4. In contrast, MP4 and SFHb showed the same susceptibility to oxidation by reactive oxygen species generated by a xanthine–xanthine oxidase system. Once fully oxidized to methaemoglobin, the rate of in vitro haem loss was five times higher in MP4 compared with SFHb in the fast phase, which we assign to the β subunits, whereas the slow phase (i.e. haem loss from α chains) showed similar rates for the two haemoglobins. Formation of MP4 methaemoglobin in vivo following transfusion in rats and humans was slower than predicted by its first-order in vitro autoxidation rate, and there was no appreciable accumulation of MP4 methaemoglobin in plasma before disappearing from the circulation. These results show that MP4 oxidation and haem loss characteristics observed in vitro provide information regarding the effect of poly(ethylene glycol) conjugation on the stability of the haemoglobin molecule, but do not correspond to the oxidation behaviour of MP4 in vivo.
Keywords: autoxidation, haem dissociation, kinetics, poly-(ethylene glycol) (PEG), PEGylated haemoglobin
Abbreviations: ApoHb, apohaemoglobin; ApoMb, apomyoglobin; DeoxyHb, deoxygenated haemoglobin; Hb, haemoglobin; HBOC, haemoglobin-based oxygen carrier; KCN, potassium ferricyanide; koff, rate of oxygen dissociation; kox, rate of oxidation; LOQ, limit of quantitation; MetHb, methaemoglobin; OxyHb, oxygenated haemoglobin; PEG, poly(ethylene glycol); pO2, partial pressure of oxygen; p50, oxygen partial pressure producing 50% saturation; ROS, reactive oxygen species; SFHb, stroma-free haemoglobin
INTRODUCTION
The clinical use of HBOC [Hb (haemoglobin)-based oxygen carriers] might be hindered by the fast rate of autoxidation of functional ferrous (Fe2+) Hb into ferric (Fe3+) MetHb (methaemo-globin) and, possibly, a ferryl (Fe4+) Hb form. Whereas in the red cell, oxidation is made reversible by an effective redox system catalysed by NADPH-dependent MetHb reductase, extracellular Hb does not benefit from these defence mechanisms. However, it has been shown in vitro that red cells in the presence of ascorbate can reduce extracellular MetHb [1]. MetHb formation in the circulation may be deleterious for at least three reasons: (i) MetHb does not bind oxygen, and thus its formation decreases the oxygen-carrying capacity of blood; (ii) MetHb gives rise to ROS (reactive oxygen species), and accumulating evidence suggests that oxidized haemoproteins may play a role in the development of oxidative damage in vivo [2]; (iii) the chemical bond that stabilizes the haem in native Hb is weakened in MetHb, which therefore releases haem faster, thereby giving rise to a cascade of effects, including induction of haem oxygenase-1 [3,4]. Of interest, it has been observed by us and others that rates of myoglobin or Hb autoxidation correlate positively with p50 oxygen partial pressure producing 50% saturation, i.e. the partial pressure at which Hb is half-saturated with oxygen, a useful index of the Hb–oxygen affinity [5–7].
Presently, a novel Hb product is being developed, MP4, made from human Hb conjugated to maleimide-activated PEG [poly-(ethylene glycol)] [8]. MP4 has an average of six PEG chains (5 kDa each) per Hb tetramer, which create a hydrophilic sphere surrounding the protein surface. The surface modification chemistry is associated with unique physicochemical properties of the product, including increased molecular radius, high oxygen affinity and low cooperativity. Most interestingly, in prospective use as an HBOC, MP4 does not exhibit the typical hypertensive response observed with most cell-free Hb solutions [8].
The present study reports the evaluation of the autoxidation process in MP4 as compared with unmodified SFHb (stroma-free Hb) that is used as the starting material to produce MP4. This evaluation involves various aspects of the chemical, physical and redox properties of these Hb species. Furthermore, in the present study we report that the rate of in vivo oxidation of MP4 in rats and humans is not related to its measured in vitro oxidation behaviour. Taken together, the oxidative behaviour and stability of MP4 observed in vitro does not define its metabolic behaviour in vivo.
MATERIALS AND METHODS
Materials
MP4 and SFHb were prepared as described previously [8]. Another PEG–Hb derivative, P5K2, with two PEGs attached per tetramer, was prepared by the same method described previously for MP4 [8], except that no thiolation was performed prior to the reaction with PEG. In this case, the maleimide–PEG reacts only at the two β93 cysteine residues [9] and was used to differentiate haem binding to the two types of Hb subunits. All reagents and enzymes were from Sigma and were of the highest purity degree available. Maleimide-activated PEG was provided by NOF (Tokyo, Japan).
Preparation of MetHb
OxyHb (oxygenated Hb) was reacted with potassium ferricyanide [1.5 mol/mol of haem for 20 min at room temperature (20–22 °C)], which is a one-electron oxidant that converts the ferrous (Fe2+) haem iron into the ferric (Fe3+) state, forming MetHb [10]. The product of this reaction was purified using 10 kDa and 70 kDa tangential flow filtration (Pall Filtron; Centramate) for SFHb and MP4 respectively. Total conversion into the MetHb form was confirmed by the Evelyn–Malloy assay [11]. To test for the presence of residual KCN (potassium ferricyanide) in the MetHb solution, samples were analysed by size-exclusion chromatography with two Superose-12 columns (AKTA Purifier-10; Amersham Pharmacia) in series against controls of (i) a potassium ferricyanide solution and (ii) unpurified MetHb. Unreacted potassium ferricyanide was not observed in the chromatograms of the purified MetHbs.
Spectrophotometry
Total Hb concentration was calculated on a haem basis by using ϵ523=7.12 mM−1·cm−1 [12]. The levels of MetHb, OxyHb and DeoxyHb (deoxygenated Hb) were measured from the absorbance at λ=560, 576, 630 and 680 nm by the following equations [13]:
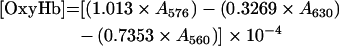 |
(1) |
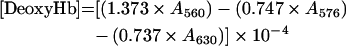 |
(2) |
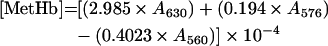 |
(3) |
Spectrophotometric measurements were carried out in a UV-visible diode array spectrophotometer (Agilent HP 8453). In some experiments, the level of MetHb was measured using an IL 682 CO-oximeter (Instrumentation Laboratory).
Hb autoxidation
The autoxidation studies were carried out at the indicated temperatures and at the indicated Hb concentration in PBS with 0.1 mM EDTA (pH 7.3) unless otherwise stated. The solutions were placed in sealed 1 cm path, 3 ml spectrophotometry cells, and spectra were taken at the appropriate times. The change in absorbance at 630 nm was used to fit a single-exponential function to determine the kox (rate of oxidation).
Hb oxidation by xanthine–xanthine oxidase
The susceptibility of Hb to oxidation was measured in the presence of excess ROS generated enzymatically in a system containing xanthine and xanthine oxidase (EC 1.1.3.22), assuming the following model [14]:
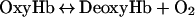 |
(4) |
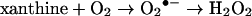 |
(5) |
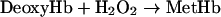 |
(6) |
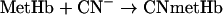 |
(7) |
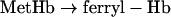 |
(8) |
Reaction 4 depends on the pO2 (partial pressure of oxygen) and the Hb affinity for oxygen. The first step in reaction 5 is catalysed by xanthine oxidase, whereas the next steps are non-enzymatic. Reaction 6 may be considered reversible, but can be made irreversible by reaction 7 that traps Fe3+ by forming a stable adduct. Reaction 8 is an interfering reaction that yields ferryl–Hb, a metastable compound containing Fe4+. The reactions were carried out in quartz cells containing 3 ml of 0.1 mM Hb, 50 mM potassium phosphate, 0.5 mM xanthine and 1 mM EDTA (pH 7.3) at 37 °C. The reaction scheme was started by adding 15 μl of xanthine oxidase at a final concentration of 100 m-units/ml. Spectra were collected at 5 s intervals, and the calculation of the maximal oxidation rate was performed using data collected at λ=576 nm. In some experiments, the medium pO2 was measured by a Clark-type electrode.
Haem loss
When Hb loses haem, it transforms into a species called ApoHb. To measure the rate of this reaction, we measured the absorbance change observed when a haem acceptor, a double-mutant ApoMb (apomyoglobin), binds the haem released from Hb to yield holomyoglobin, a green adduct [15]:
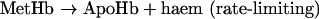 |
(9) |
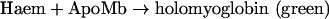 |
(10) |
The scheme may be considered first-order if [ApoMb]/[MetHb]≥10. ApoMb was prepared by an acid/acetone method [16] from a double-mutant myoglobin, H64Y/V68F (a gift from Professor J. S. Olson, Department of Biochemistry and Cell Biology, Rice University, Houston, TX, U.S.A.). The ApoMb precipitate was dissolved and used for the haem dissociation experiments.
The spectrophotometric 1 cm, 3 ml quartz cell was loaded with 0.15 M sodium acetate, 10 mM potassium phosphate, 0.45 M sucrose and 10 μM ApoMb (pH 7.0) at 37 °C. After measuring the baseline spectrum, 1 μM Hb was added and the content quickly mixed by inversion. The fast phase of the reaction was recorded by running one spectrum/min for 30 min, followed by one spectrum every 5 min to record the slow phase. Data taken at λ=410 nm were used for analysis.
In vivo experimental methods
Male Sprague–Dawley rats (mean body weight of 332±4 g) were used in this portion of the study. All animal protocols were approved by Sangart's IACUC, according to the National Institutes of Health guidelines. Prior to the day of the experiment, the rats were habituated to a plastic restraining tube for at least 30 min/session for three sessions. On the day of the experiment, the rats were anaesthetized with isoflurane using an induction chamber (5% in oxygen) and maintained using a nose cone with isoflurane (1.5–2.0% in oxygen). Respiration was spontaneous throughout the surgical procedure. Polyethylene catheters were placed, via bilateral inguinal incisions, in both femoral arteries for withdrawal and sampling of blood. One femoral vein was cannulated for infusion of MP4. Catheters were tunneled subcutaneously to the base of the tail, exited through the skin, and secured in a plastic protector sutured to the skin. Each animal was administered 3 ml of 0.9% NaCl subcutaneously for prophylactic fluid replacement. The inguinal incisions were closed with staples, and the rats were allowed to recover for 30–60 min in their cages.
Following recovery from anaesthesia, rats were placed in a plastic restraining tube and the catheters opened for access. A pre-transfusion baseline blood sample was collected from the arterial catheter into three heparinized capillary tubes. A 50% blood volume exchange transfusion (total blood volume estimated to be 65 ml/kg of body weight) was performed by infusing MP4 into the venous catheter at the rate of 0.5 ml/min with simultaneous withdrawal of arterial blood at the same rate. Upon completion of the transfusion, blood specimens were collected into capillary tubes at 0, 15, 30, 60, 90 and 120 min, and 3, 4, 5, 6, 8, 12, 24, 36 and 48 h post-transfusion. After the 2 h collection time point, animals were returned to their cages with free access to food and water. Each blood specimen was centrifuged and placed on ice immediately following collection to prevent formation of MetHb in the tube. Plasma specimens were collected into a 0.5 ml centrifuge tube and stored on ice immediately following centrifugation and decanting. Plasma specimens were analysed for total Hb and MetHb within 2 h of collection.
Data are expressed as means±S.E.M. ANOVA was used to evaluate differences among groups. If this test resulted in a significant difference, then the Bonferroni multiple comparison test was performed. In the case of two groups, these tests are equivalent to the Student's t test for unpaired data. The significance level was set at P=0.05 (two-tailed).
In vivo clinical studies
Total plasma Hb and MetHb concentrations (g/l) were determined as a function of time in patients from a Phase Ib MP4 dose-escalation clinical trial of orthopaedic surgery conducted in Stockholm, Sweden. The clinical study was performed according to the principles stated in the Declaration of Helsinki and Good Clinical Practices as described by the International Conference on Harmonization. The protocol and consent forms were approved by the Centralized Institutional Review (Ethics) Committee of the Karolinska University Hospital, Stockholm, and the study was approved by the Swedish Medical Products Agency. Written informed consent was given by the subjects. Patients received either 200, 400 or 600 ml of MP4. Blood samples were collected at times defined in the clinical protocol and shipped frozen from participating hospitals to Sangart for testing. Post-thaw plasma total Hb was measured using a Haemocue Plasma/Low Hb photometer and were calculated as the average of duplicate readings. According to the manufacturer's specification, the LOQ (limit of quantitation) of the Haemocue Plasma/Low Hb photometer is 0.3 g/l. The percentage of plasma MetHb was determined using the Evelyn–Malloy assay [11]. The plasma MetHb concentration was calculated from the remaining fraction of plasma Hb (g/l). The LOQ for this assay was determined by plotting the absorbance values at 630 nm for all samples after the Hb had been converted to MetHb by the addition of potassium ferricyanide as a function of Hb concentration, giving a LOQ of 0.029 absorbance units (results not shown). The calculated plasma Hb and MetHb concentrations were normalized to the patients' body weights. Averaged values ± S.E.M. (n=4 in each dosing group) are reported.
RESULTS
In vitro autoxidation rates of MP4 and SFHb
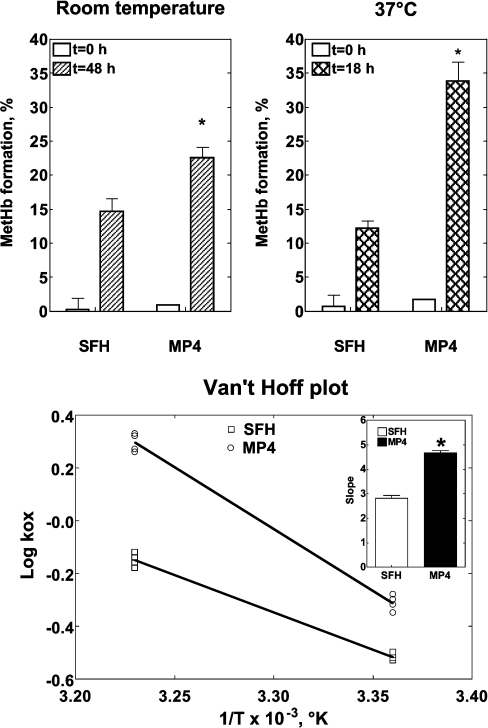
Figure 1 (upper panels) show autoxidation in SFHb and MP4 at room temperature (20–22 °C for 48 h) and at 37 °C (for 18 h). At both temperatures, autoxidation was greater for MP4 than for SFHb (P<0.001). To understand whether temperature has the same effect on SFHb and MP4 oxidation, Figure 1 (lower panel) shows the Van't Hoff isochores (e.g. log kox against 1/°K). If the plots are assumed linear, the steeper slope observed for MP4 indicates stronger temperature dependence for this Hb type.
Figure 1. Autoxidation of SFH and MP4.
Upper panel: autoxidation of SFHb and MP4 at room temperature (20–22 °C; left panel) and 37 °C (right panel). Experiments were performed with four different preparations of each Hb type with a Hb concentration of 0.06 mM in PBS with 0.1 mM EDTA (pH 7.3). P<0.0001 (as determined by ANOVA) for both temperatures. *P<0.001 compared with SFHb (determined by the Bonferroni multiple comparison test). Lower panel: a Van't Hoff plot of the data shown in the upper panel, with the kox reported as a function of 1/°K. The inset shows the slopes of the regression lines separately for SFHb and MP4. *P<0.001 compared with SFHb (Student's t test).
Effect of residual catalase activity in SFHb preparations
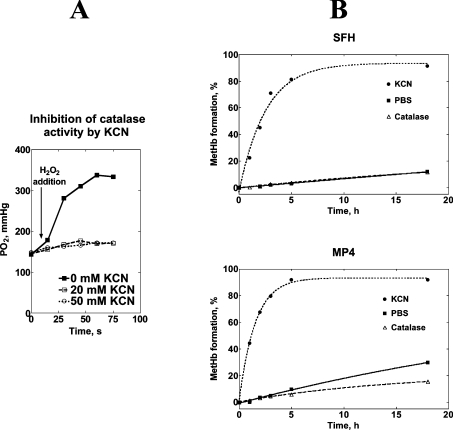
To examine whether the different autoxidation rates for SFHb and MP4 were a consequence of the presence of residual catalase activity in the preparations, we determined the autoxidation rates at 37 °C in the presence of either added catalase or of an inhibitor of catalase activity (KCN). First we assessed at which concentration KCN inhibits catalase activity by measuring the pO2 change upon addition of H2O2 in a sealed cell containing catalase and varying amounts of KCN (Figure 2A). Because no pO2 increase was observed in the presence of 20 mM KCN, we assumed that KCN completely blocked any catalase activity at this concentration, and we determined the Hb autoxidation rate in the presence of either 1000 units/ml catalase or 20 mM KCN (Figure 2B). Although catalase decreased the autoxidation rate for MP4 by half, it had no effect on SFHb, thereby suggesting that SFHb preparations contained some catalase activity that protected Hb from oxidation. Conversely, KCN markedly increased the autoxidation rates for both Hb types, again suggesting that catalase protects against Hb oxidation. Experimental data were fitted to a single-exponential equation in the form:
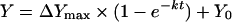 |
(11) |
where Y is the percentage of MetHb, ΔYmax is the total percentage change in MetHb at the end of the reaction, k is the rate constant, t is time and Y0 is the percentage of MetHb at t=0. Table 1 shows that, in the presence of KCN, autoxidation of MP4 was faster than that of SFHb.
Figure 2. Rates of autoxidation of SFH and MP4 in the presence and absence of catalase.
The left panel (A) shows the inhibition of catalase activity by KCN. pO2 was measured in an anaerobic cell containing 2 ml PBS and 0.1 ml catalase (final nominal activity 1000 units/ml). The reaction was started by adding 5 μl of 3% H2O2 (arrow) and pO2 was monitored for an additional 60 s at 37 °C. The reaction was performed at KCN concentrations of 0, 20 and 50 mM. The right panel (B) shows the autoxidation of SFHb and MP4 in the presence of PBS only (■), 1000 units/ml catalase (△) and 50 mM KCN (●). Best fit lines were obtained as explained in the text, and Table 1 reports the values of the rate constants.
Table 1. Results of the best fit of data shown in Figure 2(B) when fitted to eqn (11).
The Table shows the values (mean±S.E.M.) of the rate constant, k (h−1), in the equation above. NS=Not significant.
| PBS (h−1) | PBS+catalase (h−1) | PBS+KCN (h−1) | |
|---|---|---|---|
| SFH | 0.007±0.001 | 0.007±0.004 | 0.410±0.046 |
| MP4 | 0.021±0.001 | 0.010±0.001 | 0.678±0.024 |
| P | <0.0001 | NS | 0.0009 |
Susceptibility to oxidation of MP4 and SFHb in the presence of excess ROS
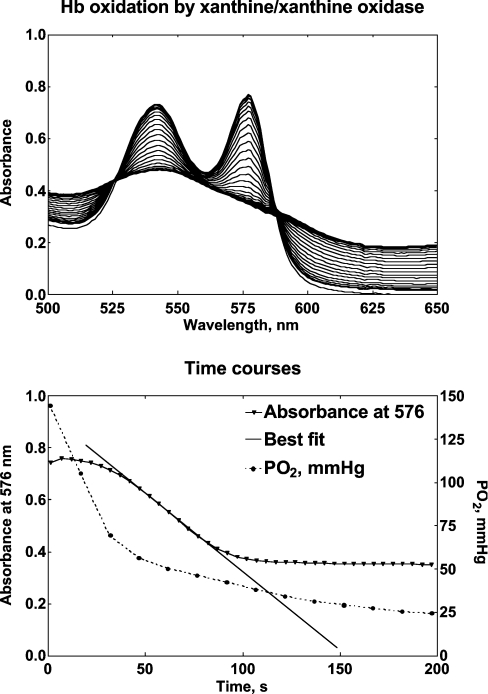
The upper panel in Figure 3 shows the spectra recorded when Hb was exposed to the xanthine/xanthine oxidase system. OxyHb is transformed into a species that resembles MetHb but does not have its characteristic spectrophotometric pattern. This species might be ferryl–Hb (Fe4+), which dismutates more slowly into MetHb. When the absorbance monitored at various wavelengths was plotted against time, a kinetic pattern appears (Figure 3, lower panel), where the disappearance of OxyHb follows a lag-type pattern. At 576 nm, the absorbance change was maximized and allowed for the calculation of the oxidation rate. The pO2 was measured using a Clark electrode in the absence of Hb to assess xanthine oxidase activity under the selected conditions. After a 30 s fast phase, the pO2 fell linearly at a rate of 16 mmHg/min, or 0.036 mM O2/min, in the 30–150 s range. This corresponded to a linear equimolar production of H2O2. The maximal oxidation rates for SFHb and MP4 were 8.6×10−3 and 10.1×10−3 M/min respectively (P=not significant; n=5), indicating the same susceptibility to oxidation by excess ROS.
Figure 3. Hb oxidation by the xanthine/xanthine oxidase system.
The upper panel shows a typical spectral change observed when 0.1 mM Hb in 50 mM potassium phosphate, 0.5 mM xanthine, 50 mM KCN and 1 mM EDTA (pH 7.3) is exposed to 0.5 units of xanthine oxidase at 37 °C. Spectra were obtained every 5 s. The lower panel shows the absorbance changes recorded at λ=576 nm to measure the maximal oxidation rate. The Figure also shows the decay of pO2 (right axis) when the mixture is reacted in the absence of Hb. The maximal oxidation rate of Hb corresponds to the linear portion of the fall in pO2.
Haem loss rates for MP4, SFHb and P5K2
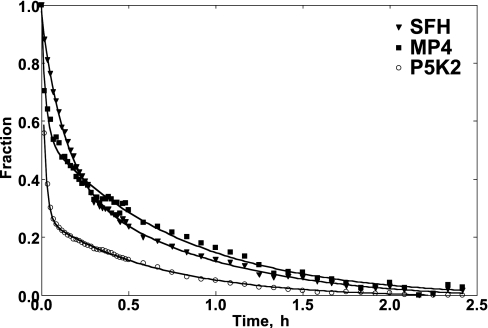
Figure 4 shows the rates of haem loss from the MetHb forms of SFHb, MP4 and P5K2. Averaged data were normalized and fitted to a double-exponential decay of the type:
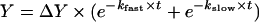 |
(12) |
where kfast and kslow represent the rate constant k (h−1) relative to the fast and slow phases respectively and ΔY is the normalized MetHb concentration at t=0. The values of the rate constants are reported in Table 2. For SFHb and MP4 time courses, the fitted fast and slow rates were constrained to have equal amplitudes to reflect haem loss from β and α chains [15]. The faster haem loss observed for MP4 with respect to SFHb is entirely attributable to higher rate in the fast phase; the slow phases are the same for both Hb types. In additional experiments (results not shown), we ruled out the possibility that the fast phase of the haem loss process might reflect the presence of free haems in the starting Hb solution. To this purpose, we performed the same experiment as described using MetHb solutions aged for 0, 4 and 7 h after their preparation, but the different aging times had no affect on either kfast or kslow. Compared with MP4 or SFHb, P5K2 showed an even higher rate of haem loss in the fast phase and again no change in the slow rate (Table 2). However, unlike the SFHb and MP4 time courses, the P5K2 time course could not be fitted using constrained amplitudes and, in this case, the fitted amplitude for the fast phase comprised approx. 70% of the total time course.
Figure 4. Time course of haem loss from SFHb, MP4 and P5K2 MetHbs.
Averaged data were fitted using a double-exponential decay (see text). The rates are reported in Table 2. n=3 per group.
Table 2. Results of the best fit of data shown in Figure 4 when fitted to eqn (12).
Values are means±S.E.M. For kfast, P<0.0001 (as determined by ANOVA); § significant difference compared with SFH (as determined by the Bonferroni multiple comparison test). For kslow, P=not significant (as determined by ANOVA).
| SFH (h−1) | MP4 (h−1) | P5K2 (h−1) | |
|---|---|---|---|
| kfast | 8.08±0.45 | 40.26±4.27§ | 52.54±2.62§ |
| kslow | 1.22±0.12 | 1.28±0.09 | 1.50±0.03 |
| r2 | 0.9986 | 0.9918 | 0.9996 |
Effect of ascorbate on the in vitro autoxidation of MP4 and SFHb
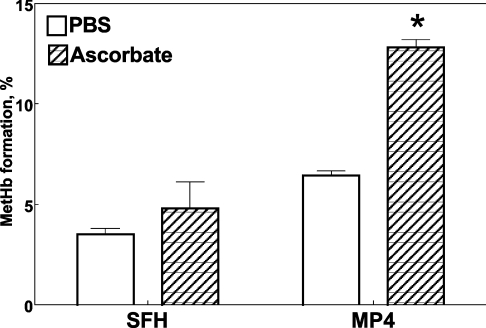
To assess whether the presence of a reducing agent affects the Hb oxidation rate, we incubated either SFHb or MP4 in the presence of 0.1 mM ascorbate (Figure 5). It appears that whereas SFHb autoxidation was not affected by this concentration of ascorbate in PBS, the rate for MP4 oxidation was enhanced approximately 2-fold in the presence of ascorbate (P=0.02).
Figure 5. Effect of 0.1 mM ascorbate on autoxidation of SFHb and MP4 to MetHb in PBS at 37 °C over 3 h.
P<0.001 for both (as determined by ANOVA). *P=0.02 compared with PBS (as determined by Bonferroni's multiple comparison test).
Formation of MP4 MetHb in vivo
Plasma MetHb was measured in vivo in two models: (i) rats following a 50% exchange-transfusion of their blood volume, and (ii) humans in a Phase Ib clinical trial transfused with 200, 400 or 600 ml of MP4 as a blood-volume topload.
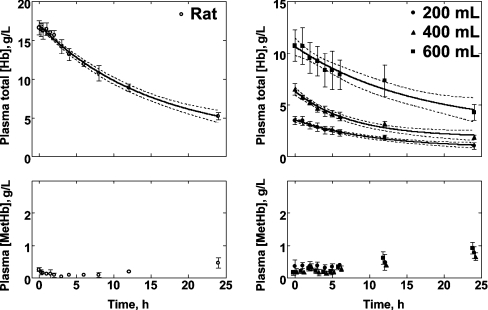
Keipert et al. have shown that unmodified human Hb is cleared in rats with a half-life of approx. 1 h [17]. Therefore we were unable to determine the rate of oxidation of SFHb in rats. By contrast, longer persistence of MP4 in the circulation allowed determination of the formation of MP4 MetHb in vivo (Figure 6, left panel). During the first half-life of plasma MP4 (t1/2=10.6 h) in rats, no change in the concentration of MetHb was measured, and over 24 h the circulating MetHb stayed at ≤10% of the remaining plasma Hb concentration. This is in contrast with the first-order in vitro rate of MP4 autoxidation (Table 1), which if applicable to MP4 oxidation in vivo would have predicted an exponential increase in circulating MetHb to 20–40% of the total plasma Hb concentration over 24 h, depending on catalase activity.
Figure 6. Disappearance of plasma Hb and formation of MetHb.
Left panel: rats exchange-transfused with MP4 (n=4). Right panel: humans transfused with different doses of MP4 (n=4 in each group). Humans received 200, 400 or 600 ml, and data were normalized for body weight. Average and S.E.M. of concentrations (g/l) are shown. The plasma Hb disappearance curves with 95% confidence limits were fitted using a single-exponential decay function. Note different scales.
In humans, there was a dose dependence of plasma Hb concentration. At t=0, i.e. measured at the first blood-collection time point following transfusion, total Hb concentration normalized to body weight (3.5±0.8, 6.5±1.1 and 10.7±3.0 g/l with 200, 400 and 600 ml dosing respectively; Figure 6, right panel). Plasma Hb concentrations decayed mono-exponentially, giving t1/2 values of 12.4, 11.4 and 10.6 h respectively. In contrast with plasma Hb concentration, we could not resolve a dose dependence for plasma MetHb concentration over the t1/2 of MP4; the measured values of plasma MetHb at 12 h were 0.47±0.49, 0.39±0.11 and 0.61±0.39 g/l MetHb for the 200, 400 and 600 ml doses respectively (Figure 6, right panel). At 24 h, the highest dose group had a plasma Hb concentration of 4.3±1.5 g/l with 0.92±0.35 g/l MetHb, showing that, of the remaining circulating plasma Hb, 21% was in the MetHb form. The percentage MetHb increased with decreasing plasma Hb, such that 35% and 83% of the Hb was converted into MetHb in the lower-dose groups, 400 and 200 ml respectively. However, the single-exponential rate for MP4 autoxidation determined in vitro in PBS at 37 °C was 0.021 h−1 (Table 1), predicting approx. 40% MetHb formation at 24 h, regardless of Hb concentration. MetHb formation was higher over time in humans in this experiment compared with the rat experiment, but neither could be predicted by the rate of oxidation measured in vitro.
DISCUSSION
In the present study, we address the ox–redox properties of SFHb and MP4 in vitro. The main results are: (i) Hb autoxidation is faster for MP4 than SFHb; (ii) residual catalase activity in SFHb preparations may in part explain the greater rate of oxidation of MP4; (iii) MP4 and SFHb display the same susceptibility to oxidation in the presence of excess ROS; (iv) MP4 MetHb displays faster haem loss than SFHb; and (v) ascorbate increases autoxidation of MP4 but not that of SFHb. In addition, in rats exchange-transfused or humans transfused with MP4, the formation of MetHb in vivo was not predicted by the rate of MP4 autoxidation measured in vitro.
Faster rates of in vitro Hb autoxidation in MP4 than SFHb can be explained, at least in part, with residual catalase activity in SFHb preparations as opposed to a greater degree of purification and less catalase activity in MP4 preparations. Catalase is a powerful scavenger of H2O2, a ROS which is, at the same time, generated during Hb oxidation and a substrate for the oxidation according to the classical Fenton's scheme. Saturating the antioxidant capacity by excess catalase led to indistinguishable rates of autoxidation for MP4 and SFHb. It should be noted, however, that there might be another factor that eventually comes into play, as the different slopes in the Van't Hoff isochores shown in Figure 1 indicate that temperature is not the single determinant to predict the different oxidation rates in the two Hb types.
In an attempt to better clarify the mechanism of MP4 oxidation, we evaluated the susceptibility of the Hbs to excess oxidizing species. The Fenton chemistry and the reaction:
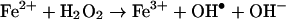 |
(13) |
involve a second-order reaction, whose rate is affected by H2O2 concentration. For this purpose, we built a near-physiological model where H2O2 is generated enzymatically by the xanthine/xanthine oxidase system. The reaction of xanthine oxidase, which catalyses the conversion of hypoxanthine into xanthine and xanthine into urate with production of H2O2, has been described to be a major ROS contributor during reperfusion of ischaemic tissues [18]. Although its relevance in humans has been questioned [19], this reaction is known as a powerful system that produces most of the reperfusion injury in ischaemic tissues. Although MP4 oxidation in the presence of this system was observed to be slightly faster (P=not significant) than SFHb, the small acceleration does not account for the differences observed in the autoxidation experiments. It has been shown that during the reaction of sperm whale myoglobin with H2O2 the initial site of the radical is Tyr103, but then it rapidly migrates to Tyr151 [20]. As the treatment to convert human Hb into MP4 does not involve these residues, it is not surprising that the susceptibility to oxidation by excess ROS appears to remain unaffected in MP4 with respect to SFHb.
We also assessed rates of haem loss from SFHb and MP4 MetHbs. Oxidation of haem iron from Fe2+ to Fe3+ weakens the fifth coordinate bond to proximal histidine residues and increases the probability of haem loss [21]. Therefore haem loss depends on the geometry of the Hb molecule and perhaps the presence of a shield of water surrounding the molecule. In the present study, we demonstrated that haem loss is accelerated in PEG-conjugated Hb compared with SFHb. Analysis of the time courses of haem loss provides a tool to understand the mechanism of haem loss from tetrameric Hb. The fast phase has been associated with haem loss from β chains, whereas the slow phase is relative to the α chains [15]. Since kslow is the same under all the tested conditions, the α chains do not appear to be involved. By contrast, the larger kfast for MP4 than for SFHb suggests that the β chains are less protected in this Hb type. Haem loss from P5K2, a derivative with two PEGs attached to the tetramer bound to Cys93 in the β chains, showed a 6-fold increase in the fast phase associated with β subunits and, again, little change in the rate of haem loss in the slower phase associated with α subunits. If the assignment of the fast and slow phases to β and α chains holds for MP4, then these results suggest that it is modification by PEGylation of β chain Cys93 that destabilizes the haem. Additional PEGylation on the surface of the Hb molecule does not further destabilize the protein, and this destabilization does not appear to be cooperative, i.e. destabilizing the β chain does not affect the α chain. Friedman et al. examined conformational changes of a maleimide PEG-modified Hb with either two or six PEGs attached to the tetramer [9]. They showed similar changes to the Fe-proximal histidine residue stretching frequency compared with unmodified Hb [22], results which appear to be consistent with the decrease in haem stability for MP4 compared with unmodified Hb reported in the present study.
The physiological consequences of MP4 MetHb formation and/or accelerated haem loss were not determined in the present study and may depend on competing oxidative versus cytoprotective mechanisms. Haem can act as a pro-oxidant that reacts with ROS, thereby promoting lipid peroxidation and protein oxidation [23]. Alternatively, free haem may mediate anti-inflammatory defence mechanisms through stimulation of haem oxygenase-1 and endogenous production of bilirubin [3] and carbon monoxide [24]. Haem dissociation can also cause the resulting ApoHb to unfold and become insoluble at physiological pH and temperature. As an example, the ability of red blood cells to prevent Hb oxidation and denaturation is critical to their survival. Precipitated Hb forms Heinz bodies on the inside surface of the red blood cell membrane and oxidative damage to the membrane [25,26]. In sickle cells, the amount of membrane-bound haem is directly related to the degree of haemolysis [27]. Whether the oxidation of cell-free haemoglobin has similar deleterious consequences is unknown.
The results in the present study show a lower plasma concentration of circulating MetHb in rats compared with an earlier observation following administration of a polymerized bovine Hb in sheep [28]. The human data also do not show exponential formation of MetHb that could be predicted by rates of in vitro oxidation, but a greater percentage of MetHb could be seen in humans compared with rats at 24 h. This may be due to endogenous ascorbate production in rats and not humans, leading to higher antioxidant capacity in rats than in humans as discussed in Bompadre et al. [29]. Although a full discovery of mechanisms contributing to the formation and clearance of circulating MetHb in vivo will require further study, data in the present study provide a preliminary understanding of this phenomenon. There might be two, not necessarily exclusive, hypotheses to explain these findings: either the endogenous blood antioxidant capacity inhibits plasma MetHb formation or MetHb is readily cleared from plasma. However, if we assume the following scheme Hb→MetHb→ApoHb and assume that only ApoHb is cleared, then one can consider that the rate of the second reaction (8 or 40 h−1 for SFHb or MP4 respectively) is much faster than that of the first reaction (0.007 or 0.010 h−1 respectively, in the presence of catalase). It should be noted that the experimental conditions selected to determine those rates are similar to the in vivo conditions (pH 7.4 at 37 °C). Even if we consider an increased rate of oxidation of MP4 in the presence of endogenous ascorbate in rat plasma, MetHb formation would still be slower than ApoHb formation following haem loss. Therefore the oxidation of Hb to MetHb is expected to be the rate-limiting step in the in vivo situation. Since we found that clearance of plasma Hb in rats followed a first-order reaction with a rate constant of 0.065 h−1, we conclude that it is unlikely that the observed lack of MetHb build-up in plasma is due to rapid clearance. Rather, the ability of blood to keep cell-free Hb Fe2+ in the reduced state probably depends on the presence of radical-scavenging antioxidants, a heterogeneous group of substances, some synthesized in the body and some derived exclusively from the diet. Serum proteins, for example, can scavenge free radicals and help prevent formation of MetHb if they have available tyrosine or cysteine groups. Uric acid, which is also present in the serum in substantial concentrations, is another major free-radical scavenger. The total antioxidant capacity in normal human blood is roughly in the range 1–2.5 mM [30], which is an order of magnitude higher than any MetHb formed in vivo in these experiments, thereby supporting the view that endogenous defences are able to keep extracellular MP4 in the reduced form.
Ascorbate is a powerful antioxidant because it can donate a hydrogen atom and form a relatively stable ascorbyl free radical. The ascorbyl free radical can be converted back into reduced ascorbate by accepting another hydrogen atom or it can undergo further oxidation to dehydroascorbate. Dehydroascorbate is unstable but is more fat soluble than ascorbate and is taken up 10–20 times more rapidly by erythrocytes, where it will be reduced back to ascorbate by GSH or NADPH from the hexose monophosphate shunt [31]. However, for ascorbate to function as an antioxidant, the ox–redox properties of the pair ascorbate/Hb must be examined. To estimate this effect, we considered the standard redox potential (E′0) for the half-reactions ascorbate→dehydroascorbate (E′0=+0.08 V) and Fe3+→Fe2+ in both SFHb and MP4. There is an inverse correlation between the redox potential of Hb and oxygen affinity: the higher the oxygen affinity, the lower the redox potential [32]. Therefore to estimate E′0 for both Hb types, we used Wyman's linked-function analysis [33] and previously reported data for p50 and nHill values [34] (Table 3):
Table 3. Calculation of E′0 (at 25 °C and pH 7.4) for SFH and MP4 using Wyman's linked-function analysis.
| SFH | MP4 | |
|---|---|---|
| p50 (mmHg) | 5.5 | 3.0 |
| nHill | 3.0 | 1.0 |
| Bohr factor | −0.5 | −0.24 |
| E′0 (V) | +0.15 | +0.04 |
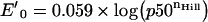 |
(14) |
The value calculated for E′0 for SFHb is consistent with that reported by Bunn (+0.14 V) [35]. With the values reported in Table 3 in mind, it appears that ascorbate reduces SFHb but oxidizes MP4, at least under standard conditions, thereby explaining the data shown in Figure 5. The decrease in the redox potential of haemoproteins has also been correlated with the exposure of the haem to the aqueous solvent [36]. This would potentially make MP4 more susceptible to both autoxidation and haem loss.
The relationship between Hb oxidation rate (kox) and Hb–O2 affinity or p50 might provide a useful tool to assess the validity of the following scheme of Hb autoxidation:
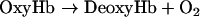 |
(15) |
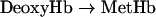 |
(16) |
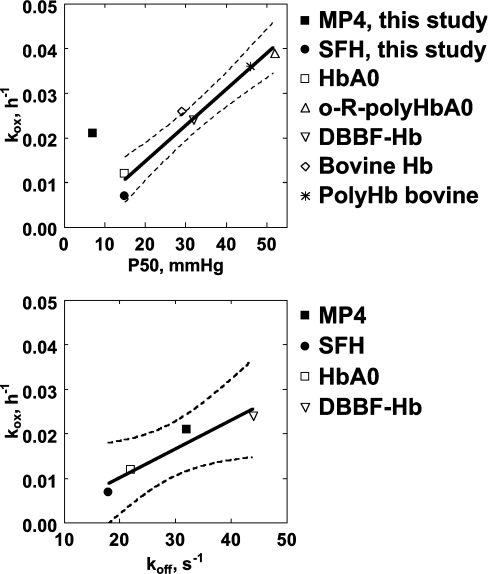
The good correlation between p50 and kox previously reported by Nagababu et al. [7] when using data obtained from HbA (adult human Hb), bovine Hb, DBBF-Hb (diaspirin cross-linked human Hb at αLys99 residues), bovine PolyHb (polymerized Hb) and o-R-polyHbA (o-raffinose polymerized human Hb) suggests that the effect of Hb deoxygenation on kox is greater than any variability in the rates of autoxidation attributed to differences in the initial levels of MetHb [7] and, in our opinion, also on the effects of protein structure on redox properties. Figure 7 (upper panel) shows how data reported in the present study compare with those reported by Nagababu et al. [7]. kox for SFHb is similar to kox for HbA0 but slightly lower. This difference may reflect the catalase activity in SFHb compared with its absence in chromatographically purified HbA0. In contrast, MP4 displays relatively higher kox at even lower p50. When all available data were fitted to a straight line, the result was acceptable (r2=0.75, P=0.01), but a much better regression comes out if the point relative to MP4 is excluded (r2=0.96, P=0.0007). This indicates that MP4 does not comply with the above rule, possibly indicating that the autoxidation process is not coupled to the oxygen affinity. However, it was reported previously that the R-state rate of koff (oxygen dissociation) from OxyHb is 2-fold higher for MP4 compared with SFHb, as opposed to identical rates of oxygen association [34]. Figure 7 (lower panel) shows that the scheme described in equations 15 and 16 might hold true in this specific case, as koff for the dissociation of oxygen from OxyHb correlates with kox with sufficient statistical significance (P=0.04), when using available data from the literature [34,37]. Data shown in Figure 7, however, should be taken with caution as we have shown that traces of catalase activity in the Hb preparations might have profound effects on kox.
Figure 7. Relationship between autoxidation rates and p50 or oxygen dissociation rate constants.
Upper panel: relationship between the apparent kox and Hb–O2 affinity (p50, i.e. the pO2 at which half of Hb is saturated with oxygen) in various Hb types from the present study and from the literature [7]. The line represents the best linear fit when the point referring to MP4 is excluded from the calculation, with the 95% confidence limits, slope=(0.808±0.085)×10−3, intercept=(1.46±2.928)×10−3, P=0.0007. Lower panel: relationship between kox and the rate constant of oxygen dissociation from OxyHb in various Hb types from the literature [34,37]. The line represents the best linear fit when considering all data points, with the 95% confidence limits, slope=(0.648±0.141)×10−3, intercept=(−2.807±4.331)×10−3, P=0.04.
Conclusions
The in vitro oxidation of Hb does not appear to be a valuable predictor of the early phase of oxidation in vivo in either a rat model or in human clinical trials. In vivo, factors others than those normally taken into consideration for in vitro studies are involved. Nevertheless, examining in vitro oxidation rates provides a good opportunity to study several aspects of Hb biochemistry and biophysics. In the present study, we show that PEGylation of the protein to yield a derivative that may be useful as a blood substitute increases the rate of haem loss from MetHb and alters its redox properties. More specifically, the rate of haem loss from the β subunits was five times greater for MP4 than for SFHb, whereas the α chains release the haem at the same rate. By contrast, the apparent increase of the autoxidation rate of the PEGylated derivative seems attributable to a greater degree of purity and lack of catalase activity and not to a greater susceptibility to oxidation. Simple first-order exponential models can be used to evaluate MP4 oxidation and subsequent haem loss in vitro, but systems that control the rate of oxidation and in vivo disappearance of cell-free Hb are more complex. These mechanisms are not yet fully defined but probably depend on redox properties of circulating plasma proteins and red blood cell enzymes.
Acknowledgments
This work was supported by Grant R01 HL 076163 from the NIH (National Institutes of Health, Bethesda, MD, U.S.A.), NHLBI. K.D.V., A.M., E.J., J.L., M.A.Y. and R.M.W. are employees of Sangart, Inc. K.D.V., A.M. E.J., J.L. and M.A.Y. hold stock options in the company. R.M.W. is the President, CEO and Chairman of the Board of Sangart, Inc.
References
- 1.McGown E. L., Lyons M. F., Marini M. A., Zegna A. Reduction of extracellular methemoglobin by erythrocytes. Biochim. Biophys. Acta. 1990;1036:202–206. doi: 10.1016/0304-4165(90)90035-u. [DOI] [PubMed] [Google Scholar]
- 2.Reeder B. J., Svistunenko D. A., Cooper C. E., Wilson M. T. The radical and redox chemistry of myoglobin and hemoglobin. Antioxid. Redox Signal. 2004;6:954–966. doi: 10.1089/ars.2004.6.954. [DOI] [PubMed] [Google Scholar]
- 3.Balla J., Jacob H. S., Balla G., Nath K., Eaton J. W., Vercellotti G. M. Endothelial cell heme uptake from heme proteins: induction of senstitization and desensitization to oxidant damage. Proc. Natl. Acad. Sci. U.S.A. 1993;90:9285–9289. doi: 10.1073/pnas.90.20.9285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Motterlini R., Foresti R., Vandegriff K., Intaglietta M., Winslow R. Oxidative-stress response in vascular endothelial cells exposed to acellular hemoglobin solutions. Am. J. Physiol. 1995;269:H648–H655. doi: 10.1152/ajpheart.1995.269.2.H648. [DOI] [PubMed] [Google Scholar]
- 5.Brantley R. E., Smerdon S. J., Wilkinson A. J., Singleton E. W., Olson J. S. The mechanism of autooxidation of myoglobin. J. Biol. Chem. 1993;268:6995–7010. [PubMed] [Google Scholar]
- 6.Macdonald V., Vandegriff K., Winslow R. Oxidation rates and stability in solution of mono- and bivalently cross-linked human hemoglobin. Artif. Cells Blood Substit. Immobil. Biotechnol. 1991;19:299–520. [Google Scholar]
- 7.Nagababu E., Ramasamy S., Rifkind J. M., Alayash A. I. Site-specific crosslinking of human and bovine hemoglobins differentially alters oxygen binding and redox side reactions producing rhombic heme and heme degradation. Biochemistry. 2002;41:7407–7415. doi: 10.1021/bi0121048. [DOI] [PubMed] [Google Scholar]
- 8.Vandegriff K. D., Malavalli A., Wooldridge J., Lohman J., Winslow R. M. MP4, a new nonvasoactive PEG–Hb conjugate. Transfusion. 2003;43:509–516. doi: 10.1046/j.1537-2995.2003.00341.x. [DOI] [PubMed] [Google Scholar]
- 9.Juszczak L. J., Manjula B., Bonaventura C., Acharya S. A., Friedman J. M. UV resonance Raman study of b93-modified hemoglobin A: chemical modifier-specific effects and added influences of attached poly(ethylene glycol) chains. Biochemistry. 2002;41:376–385. doi: 10.1021/bi011212r. [DOI] [PubMed] [Google Scholar]
- 10.Di Iorio E. E. Preparation of derivatives of ferrous and ferric hemoglobin. Methods Enzymol. 1981;76:57–72. doi: 10.1016/0076-6879(81)76114-7. [DOI] [PubMed] [Google Scholar]
- 11.Evelyn K., Malloy H. Microdetermination of oxyhemoglobin, methemoglobin and sulfhemoglobin in a single sample of blood. J. Biol. Chem. 1938;126:655–662. [Google Scholar]
- 12.Snell S. M., Marini M. A. A convenient spectroscopic method for the estimation of hemoglobin concentrations in cell-free solutions. J. Biochem. Biophys. Methods. 1988;17:25–33. doi: 10.1016/0165-022x(88)90075-9. [DOI] [PubMed] [Google Scholar]
- 13.Benesch R. E., Benesch R., Yung S. Equations for the spectrophotometric analysis of hemoglobin mixtures. Anal. Biochem. 1973;55:245–248. doi: 10.1016/0003-2697(73)90309-6. [DOI] [PubMed] [Google Scholar]
- 14.Samaja M., Motterlini R., Rovida E. Enhanced oxidation of bis(3,5-dibromosalicyl) fumarate α-α cross linked hemoglobin by free radicals generated by xanthine/xanthine oxidase. Artif. Cells Blood Substit. Immobil. Biotech. 1994;22:517–524. doi: 10.3109/10731199409117879. [DOI] [PubMed] [Google Scholar]
- 15.Hargrove M. S., Singleton E. W., Quillin M. L., Ortiz L. A., Phillips G. N., Olson J. S. His64 (E7)-Tyr apomyoglobin as a reagent for measuring rates of hemin dissociation. J. Biol. Chem. 1994;269:4207–4214. doi: 10.2210/pdb1mgn/pdb. [DOI] [PubMed] [Google Scholar]
- 16.Ascoli F., Fanelli M. R., Antonini E. Preparation and properties of apohemoglobin and reconstituted hemoglobins. Methods Enzymol. 1981;76:72–87. doi: 10.1016/0076-6879(81)76115-9. [DOI] [PubMed] [Google Scholar]
- 17.Keipert P. E., Chang T. M. S. Effects of partial and total isovolemic exchange transfusion in fully conscious rats using pyridoxylated polyhemoglobin solution as a colloidal oxygen-delivering blood replacement fluid. Vox Sang. 1987;53:7–14. doi: 10.1111/j.1423-0410.1987.tb04905.x. [DOI] [PubMed] [Google Scholar]
- 18.Granger D., Rutili G., McCord J. Superoxide radicals in feline intestinal ischemia. Gastroenterology. 1981;81:22–29. [PubMed] [Google Scholar]
- 19.Bianciardi P., Scorza R., Ghilardi G., Samaja M. Xanthine oxido-reductase activity in ischemic human and rat intestine. Free Radical Res. 2004;38:919–925. doi: 10.1080/10715760412331273430. [DOI] [PubMed] [Google Scholar]
- 20.Svistunenko D. A., Dunne J., Fryer M., Nicholls P., Reeder B. J., Wilson M. T., Bigotti M. G., Cutruzzola F., Cooper C. E. Comparative study of tyrosine radicals in hemoglobin and myoglobins treated with hydrogen peroxide. Biophys. J. 2002;83:2845–2855. doi: 10.1016/S0006-3495(02)75293-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bunn H. F., Jandl J. H. The renal handling of hemoglobin. J. Biol. Chem. 1968;243:465–475. [PubMed] [Google Scholar]
- 22.Khan I., Dantsker D., Samuni U., Friedman A. J., Bonaventura C., Manjula B., Acharya S. A., Friedman J. M. β93 Modified hemoglobin: kinetic and conformational consequences. Biochemistry. 2001;40:7581–7592. doi: 10.1021/bi010051o. [DOI] [PubMed] [Google Scholar]
- 23.Vincent S. H. Oxidative effects of heme and porphyrins on proteins and lipids. Semin. Hematol. 1989;26:105–113. [PubMed] [Google Scholar]
- 24.Otterbein L. E. Carbon monoxide: innovative anti-inflammatory properties of an age-old gas molecule. Antioxid. Redox Signal. 2002;4:309–319. doi: 10.1089/152308602753666361. [DOI] [PubMed] [Google Scholar]
- 25.Jacob H., Winterhalter K. Unstable hemoglobins: the role of heme loss in Heinz body formation. Proc. Natl. Acad. Sci. U.S.A. 1970;65:697–701. doi: 10.1073/pnas.65.3.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Winterbourn C. C. Oxidative reactions of hemoglobin. Methods Enzymol. 1990;186:265–272. doi: 10.1016/0076-6879(90)86118-f. [DOI] [PubMed] [Google Scholar]
- 27.Kannan R., Labotka R., Low P. S. Isolation and characterization of the hemichrome-stabilized membrane protein aggregates from sickle erythrocytes. J. Biol. Chem. 1988;263:13766–13773. [PubMed] [Google Scholar]
- 28.Lee R., Neya K., Svizzero T. A., VLahakes G. J. Limitations of the efficacy of hemoglobin-based oxygen-carrying solutions. J. Appl. Physiol. 1995;79:236–242. doi: 10.1152/jappl.1995.79.1.236. [DOI] [PubMed] [Google Scholar]
- 29.Bompadre S., Luciana L., Politi A., Battino M. Improved FIA-ABTS method for antioxidant capacity determination in different biological samples. Free Radical Res. 2004;38:831–838. doi: 10.1080/10715760410001715158. [DOI] [PubMed] [Google Scholar]
- 30.Fischer M. A., Gransier T. J., Beckers L. M., Bekers O., Bast A., Haenen G. R. Determination of the antioxidant capacity in blood. Clin. Chem. Lab. Med. 2005;43:735–740. doi: 10.1515/CCLM.2005.125. [DOI] [PubMed] [Google Scholar]
- 31.Hornig D. Distribution of ascorbic acid, metabolites and analogues in man and animals. Ann. N.Y. Acad. Sci. 1975;258:103–118. doi: 10.1111/j.1749-6632.1975.tb29271.x. [DOI] [PubMed] [Google Scholar]
- 32.Edelstein S. J., Gibson Q. H. The effect of functional differences in the α and β chains on the cooperativity of the oxidation-reduction reaction of hemoglobin. J. Biol. Chem. 1975;250:961–965. [PubMed] [Google Scholar]
- 33.Antonini E., Brunori M. New York, U.S.A.: North Holland/American Elsevier; 1971. Hemoglobin and myoglobin in their reactions with ligands; p. 343. [Google Scholar]
- 34.Vandegriff K. D., Bellelli A., Samaja M., Malavalli A., Brunori M., Winslow R. M. Kinetics of NO and O2 binding to a maleimide poly(ethylene glycol)-conjugated human haemoglobin. Biochem. J. 2004;382:183–189. doi: 10.1042/BJ20040156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bunn H. F., Forget B. Philadelphia, U.S.A.: W.B. Saunders; 1998. Hemoglobin: Molecular, Genetic and Clinical Aspects; p. 639. [Google Scholar]
- 36.Stellwagen E. Haem exposure as the determinate of oxidaiton-reduction potential of haem proteins. Nature (London) 1978;275:73–74. doi: 10.1038/275073a0. [DOI] [PubMed] [Google Scholar]
- 37.Vandegriff K. D., Le Tellier Y. C., Winslow R. M., Rohlfs R. J., Olson J. S. Determination of the rate and equilibrium constants for oxygen and carbon monoxide binding to R-state human hemoglobin cross-linked between the α subunits at lysine 99. J. Biol. Chem. 1991;266:17049–17059. [PubMed] [Google Scholar]