Abstract
RecQ DNA helicases function during DNA replication and are essential for the maintenance of genome stability. There is increasing evidence that spontaneous genomic instability occurs primarily during DNA replication, and that proteins involved in the S-phase checkpoint are a principal defence against such instability. Cells that lack functional RecQ helicases exhibit phenotypes consistent with an inability to fully resume replication fork progress after encountering DNA damage or fork arrest. In this review we will concentrate on the various functions of RecQ helicases during S phase in model organisms.
INTRODUCTION
RecQ helicases are a subgroup of DNA helicases that are highly conserved from bacteria to man. The family ofRecQ helicases is named after the recQ gene of Escherichia coli and has the activity of unwinding DNA in the 3′–5′ direction in relation to the DNA strand in which the enzyme is bound. There are at least five homologs in humans, three of which are associated with genetic diseases. The BLM, WRN and RECQL4 genes are mutated in Bloom's syndrome (BS), Werner's syndrome (WS) and Rothmund–Thomson syndrome (RTS), all autosomal recessive disorders. At the cellular level each of these human syndromes exhibit genomic instability that leads ultimately to cancer. However, they also have distinct phenotypes such as infertility and immunological abnormalities for BS, premature aging for WS and skin and skeleton abnormalities for RTS. For a detailed review of RecQ helicases in humans and the disorders associated with their deficiencies we refer readers to other recent review articles (1–3).
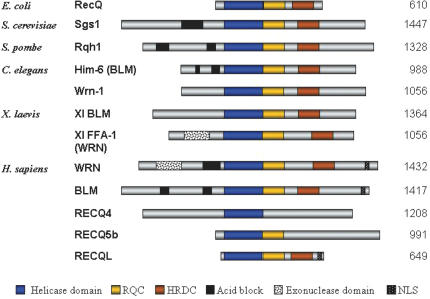
Helicase catalyzed strand separation is generally coupled to ATP hydrolysis, and most helicases contain the conserved Walker A and B ATP-binding motifs. To date the RecQ family from all organisms can be distinguished from other helicases not only by its 400 amino acid helicase domain, but also by the presence of additional conserved regions, the RQC and HRDC domains (Figure 1). The RQC domain is unique to RecQ helicases, while the HRDC domain has also been found in nucleases and is likely involved in binding nucleic acid substrates [for a review see (4)]. The WRN protein and its homolog in Xenopus laevis also contain a conserved 3′–5′ exonuclease domain near the N-terminus (Figure 1). Functional conservation has been demonstrated within the RecQ family by the ectopic expression of either human BLM or WRN protein partially rescuing elevated rates of spontaneous and illegitimate recombination in budding yeast cells lacking Sgs1. However, complementation of both HU sensitivity and reduced lifespan can only be achieved by the BLM protein, not the WRN protein (5,6).
Figure 1.
Members of the RecQ family of DNA helicases from E.coli, S.cerevisiae, S.pombe, C.elegans, X.laevis and H.sapiens. The size of each protein in amino acids is shown on the right and the regions corresponding to the helicase domain, and conserved regions RQC and HRDC are indicated and shown in the key below the figure. The NLS and exonuclease regions unique to the mammalian orthologs are also indicated.
BLM helicase interacts biochemically with DNA topoisomerase III a type IA enzyme that unlinks single-stranded catenanes (7) and the two proteins co-localize in discrete foci in mammalian cells (8). This interaction is conserved in both budding and fission yeast where the N-terminal domain in either Sgs1 or Rqh1 is important for Top3 interaction (9–11). In Saccharomyces cerevisae a TOP3 disruption shows a very pronounced slow growth phenotype, and loss of Sgs1 function suppresses this, giving the helicase its name (slow growth suppressor) (12). The phenotype ismore severe in both Mus musculus and Schizosaccharomyces pombe where the deletion of TOPOIII α or top3+ is lethal, yet viability is restored when coupled with either the deletion of the fission yeast rqh1+ or a mutation of its helicase activity (rqh1K547I) (13,14). The reason why the presence of Sgs1 without Top3 is so detrimental is not clear, but it is possible that Sgs1 dependent structures form and cannot be resolved by any other topoisomerase or cleavage enzyme. The conserved RecQ–Top3 complex is thought to suppress hyper-recombination by resolving strand-exchange structures at the replication fork in a manner that re-establishes functional forks rather than generating a truncated chromosome. This will be examined in more detail below.
Despite the high level of conservation among RecQ helicases, genes encoding these enzymes are generally not essential for cell viability. Biochemical data suggest that RecQ helicase processivity and substrate specificity are very atypical among helicases. RecQ helicases catalyze little or no unwinding of duplexed DNA from blunt ends, from internal nicks, or from partial duplex molecules with single-stranded 3′–5′ tails in vitro (15–19). They do however unwind substrates that have bubbles internally inserted into blunt-ended duplexed DNA, and both BLM and WRN enzymes efficiently unwind synthetic X-junctions that resemble Holliday junction intermediates and G-quadruplex DNA (20–22). Drosophila RECQ5 helicase and budding yeast Sgs1 can disrupt synthetic 3- and 4-way junctions (23,24), substrates resembling both Holliday junction (HJ) recombination intermediates and structures formed at stalled replication forks (25). Indeed, RecQ helicases have been implicated in many cellular capacities where these types of DNA substrates arise including the process of replication, double-strand break (DSB) repair, recombination and telomere maintenance.
In the following review we will focus primarily on RecQ helicases in simple organisms during S phase of the cell cycle where a vast amount of both biochemical and genetic data will be interpreted in the context of DNA replication. For this reason we will not discuss specifically their role in telomere preservation, although it should not be excluded that some of the replication functions are used in the context of telomere replication. The difference is that the outcome of strand exchange can be very different at a telomere than at an internal sequence. In contrast to the human homologs which have been directly implicated in telomere maintenance [see reviews (1–3)], RecQ in budding yeast is likely to have a less important role than its mammalian counterpart, owing to the lack of simple repeat at telomeric ends. Here we will summarize studies from both budding and fission yeast which have been extremely valuable in understanding the roles of RecQ helicases during the process of replication particularly at the molecular level and also studies from Caenorhabditis elegans which have proven to be beneficial for understanding RecQ-deficient phenotypes in multicellular organisms (phenotype summary, Table 1).
Table 1.
Phenotypes associated with mutations in the following RecQ helicases
| Feature | S.cerevisiae | S.pombe | C.elegans | H.sapiens | |
|---|---|---|---|---|---|
| SGS1 | Rqh1 | HIM-6 | BLM | WRN | |
| Replication defects | Sensitive to HU/defects in rDNA | Hypersensitive to HU/defects in rDNA | HU induced and germ line defects | Abnormal replication intermediates, retarded fork progression | Abnormal replication intermediates, sensitive to S-phase specific agents |
| DNA damage response | No UV sensitivity, S-phase checkpoint defect | UV sensitive, defects spindle checkpoint dependent | IR sensitivity, S-phase checkpoint defect | No sensitivity to UV | No sensitivity to UV |
| Aging phenotype | Reduced lifespan | Reduced lifespan | Reduced lifespan/telomere shortening | ||
| Source of genomic instability | Replication fork associated hyper-recombination | Replication fork associated hyper-recombination | Mutator phenotype: random insertions and deletions | Chromosome breakage and rearrangements; sister chromatid exchanges | Variegated translocation mosaicism, deletions |
RECQ HELICASES: CHECKPOINT ACTIVITY AND REPLICATION FORK PRESERVATION
DNA replication does not proceed normally in the absence of a functional RecQ helicase. Human cells lacking functional WRN or BLM proteins accumulate aberrant replication intermediates (26,27). Similar to BLM protein in man, the intracellular levels of Sgs1 in S.cerevisiae are cell-cycle regulated. Sgs1 levels are low in late metaphase and G1-phase cells but the protein is found in bright intra-nuclear foci during S phase, where they co-localize with the origin recognition complex and newly synthesized DNA (28). This is particularly pronounced when cells are synchronized by an arrest and release from mitosis. Chromatin immunoprecipitation experiments have shown that Sgs1 is at replication forks both in the presence and absence of exogenous damage (29), and studies using DNA combing techniques indicate that Sgs1 modulates replication fork progression even without exogenous damage (30). Thus, there is ample evidence indicating that RecQ helicases play a number of roles during replication including the maintenance of replication forks during ‘normal’ S-phase progression.
During replication of the genome the Watson–Crick DNA strands are separated, greatly enhancing the vulnerability of the genome to irreparable loss of genetic information. Cells have evolved a network of response pathways called checkpoints to deal with DNA damage and replication defects, and the intra-S checkpoint reacts to DNA damage that occurs during S phase (31,32). Through a cascade of events this checkpoint blocks late replication origins from firing, promotes the repair of damaged DNA, and helps to reinitiate replication once repair has been achieved (31). RecQ helicases are linked to the S phase checkpoint and in budding yeast cells lacking Sgs1 extend spindles in HU and are partially defective in slowing progression through S-phase in MMS (28), both phenotypes that correlate with a compromised checkpoint response.
Central to the activation of the intra-S-phase checkpoint are ATM-related kinases (Mec1) and the downstream kinase CHK2 (Rad53 in budding yeast). Mec1-dependent phosphorylation of Rad53 occurs in response to strand breaks that arise from replication fork collision with MMS-induced alkylation or in response to fork stalling by high concentrations of HU [reviewed in (33)]. Although Rad53 can be activated through a parallel pathway involving Rad24 and the 9-1-1 complex (Rad17, Ddc1 and Mec3 in budding yeast), in the absence of Rad24, Sgs1 is essential for Rad53 kinase activation in response to stalled forks. Suprisingly, however, this function does not require Sgs1's helicase activity, Rad51 or Top3 (28,34).
This role in checkpoint activation is likely attributed to the physical interaction between Sgs1 and the checkpoint kinase Rad53, which has been mapped to the helicase domain of Sgs1 and the FHA1 domain of Rad53 (34). Consistent with this, in vivo studies have shown that mutation of the Rad53 FHA1 domain compromises its ability to respond to replication fork arrest but is not required for the G2/M Rad9-dependent damage response, which requires the FHA2 domain in Rad53 (35). Additionally, the Claspin protein homolog ScMrc1 was shown to be essential for Rad53 activation, specifically in response to stalled forks (36,37). It has been determined that Sgs1 and Mrc1 are genetically in the same epistasis pathway for Rad53 activation (34), suggesting a model where Sgs1 helps recruit Rad53 through direct interactions with the replication fork (29), facilitating its activation by Mrc1 (34).
RecQ helicases are not only involved in intra-S checkpoint activation per se but likely have a direct function in providing replication fork stability. Replication fork stability is an active process that prevents fork collapse, whereby the replisome and fork are maintained in a competent state to resume replication once the stress is alleviated. Full DNA polymerase α and ɛ association with a stalled fork requires the helicase activity of Sgs1 and its interaction with Top3 (34). Moreover, Mec1 the upstream activator of Rad53 is also necessary for stabilizing DNA polymerases at stalled forks (38). Using a partial loss of function mutant mec1-100 it was demonstrated that Mec1 and Sgs1 contribute to fork stability in an additive fashion, and that the loss of Mec1 is highly synergistic with a disruption in Sgs1 for spontaneous gross chromosomal rearrangements (GCRs) (39). There is a genetic interaction between Sgs1 and Mec1, one which correlates DNA polymerase stability with the suppression of chromosomal breaks. The extensive fork defects observed in cells deficient for both Mec1 and Sgs1 also coincides with the rapid loss of replication protein A (RPA) from HU stalled forks (39). Since RPA promotes the initiation of primer synthesis by DNA polα/primase it is possible that both the Mec1 and the Sgs1 pathways for stabilizing the replisome converge on RPA, which itself is a target of checkpoint kinase modification [reviewed in (40)].
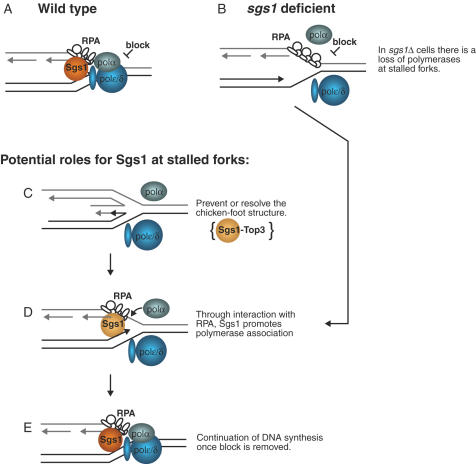
The function of Sgs1 in preserving replisome maintenance could be via more than one mechanism. It has been proposed many times that Sgs1 could reverse or prevent nascent strands from pairing with one another as in the proposed chicken-foot structure which appears to occur at sites of stalled replication (Figure 2C) (25). This is a known in vitro substrate for Sgs1 and if it is processed in vivo the reaction would likely be a rapid because the levels of fork associated RPA do not change dramatically in sgs1 single mutant cells during HU treatment (38). Furthermore, to account for the loss of DNA polymerases at stalled forks in sgs1 cells in has been proposed that Sgs1 could stabilize a particular conformation of RPA, notably the conformation that promotes DNA polα loading (40,41). This function of Sgs1 could be important for maintaining polymerases at forks until the block has been removed, or for reloading the polymerases after the resolution of the chicken-foot structure (Figure 2D).
Figure 2.
During an HU block the Sgs1–Top3 complex functions to maintain DNA polymerase association at replication forks. (A) In wild-type cells the polymerases are maintained at stalled forks. (B) In the absence of Sgs1 helicase activity both DNA polymerase α and ɛ association with stalled forks is defective. Sgs1–Top3 could function to prevent or resolve the ‘chickenfoot’ structure (C) and/or favor DNA polymerase α and ɛ association through interaction with RPA (D), allowing for the resumption of DNA replication after the block is removed (E).
RecQ helicase in fission yeast probably plays a similar role during the process of DNA replication because in the absence of Rqh1 chromosomal rearrangements stemming from blocked replication forks increase dramatically (42). Rqh1 has been proposed to help protect the fork in different ways, either by directly providing stability to the replisome components at blocked forks as shown in S.cerevisae, or by unwinding DNA junctions that might otherwise be cleaved by an endonuclease (9). Cells lacking Rqh1 are also defective in recovery from S-phase arrest when exposed to HU (43), and the interaction between the N-terminus of Rqh1 and Top3 is important for this recovery (9). Interestingly, following HU exposure the survival of rqh1− helicase dead cells was enhanced compared to cells carrying a full disruption of rqh1+, suggesting that some functions are independent of its helicase activity (44). In contrast to sgs1 cells in budding yeast, rqh1− cells are able to fully arrest the cell cycle following HU treatment. Thus, rather than a direct role in the checkpoint response it has been proposed that Rqh1 may be involved in the resumption of growth following genomic insult, perhaps in a pathway that allows the replication fork to bypass DNA damage (45). Uniquely, it was shown that most rqh1− cells enter mitosis with dynamics similar to wild type yet become delayed in anaphase progression, a phenotype dependent on the spindle checkpoint (46).
In C.elegans loss of function mutations in the BLM helicase ortholog HIM-6 result in a partially defective cell-cycle arrest phenotype in response to HU treatment (47). When him-6 worms were grown on plates containing HU they showed an elevated number of mitotic germ cells. This is in contrast to wild type where cells transiently stop dividing in response to HU, but similar to S-phase checkpoint-defective cells which continue to proliferate (48,49). Moreover, in C.elegans him-6 mutants show phenotypic signs of genomic instability including a mutator phenotype, GCRs such as random insertions and deletions, and germ line apoptosis. It has been suggested that genomic instability can adversely affect longevity and defects in the him-6 mutant show a shortened lifespan (50). Interestingly, the genetic interactions for him-6, rad-51 and top-3 differ from those observed in the orthologs in both yeasts. In C.elegans top-3 (RNAi); him-6 mutants display synergistic defects, suggesting that him-6 and top-3 act on partially redundant pathways downstream of rad-51 to prevent the accumulation of recombination intermediates that occur as a consequence of mishaps during replication (47).
The WRN helicase ortholog in C.elegans is WRN-1. It is also implicated in the DNA damage checkpoint and similar to him-6 mutants wrn-1 (RNAi) strains show a shortened lifespan (51). When treating gonads with HU to interrupt DNA synthesis WRN-1 protein was required to activate the S-phase checkpoint in germ cells (51). Furthermore, in the early stages of development S-phase is accelerated in the wrn-1 (RNAi) strain, a phenotype similar to that observed by disrupting the DNA replication checkpoint by chk-1 (RNAi). It has been suggested that WRN-1 works genetically on a pathway with CHK-1 because double RNAi of wrn-1 and chk-1 is very similar to single RNAi of chk-1 (51). Although these studies are indirect in nature they do suggest that a role for RecQ helicases in checkpoint activation is maintained in multicellular organisms.
RECQ: ROLES IN RECOMBINATION AND PROCESSING STALLED FORKS
The DNA replisome frequently encounters DNA lesions, stable secondary structures and DNA-bound protein complexes such as transcription factors, which will stall the replication fork. Stalled replication forks are sites of homologous recombination (HR) (52–54), where this process is required to repair double-strand breaks and restart collapsed forks. Although HR is essential for the preservation of genome integrity there are pathological situations in which excessive HR can destabilize the genome. Therefore, tight and controlled HR at sites of replication is absolutely required. Given its location and substrate preference it is not surprising that there is a direct link between the RecQ helicases and HR resolution at stalled forks. Indeed of more direct character is the observation that Sgs1, such as human BLM protein, shows a physical interaction with the Rad51 recombinase (55). The role of Sgs1 in recombination presumably also involves its binding partner Top3, since top3 homozygous diploids are not capable of going through meiosis unless meiotic recombination is prevented (56). Here we discuss the involvement of the Sgs1–Top3 complex in HR, focusing on the genetic interactions which are relatively well defined in yeast compared to man.
Several synthetic lethal screens have identified genes acting in parallel or epistatic with SGS1 (57,58). The colethality of many of these interactions can be suppressed by eliminating HR, suggesting that a significant fraction of sgs1 phenotypes can be attributed to events downstream of HR.
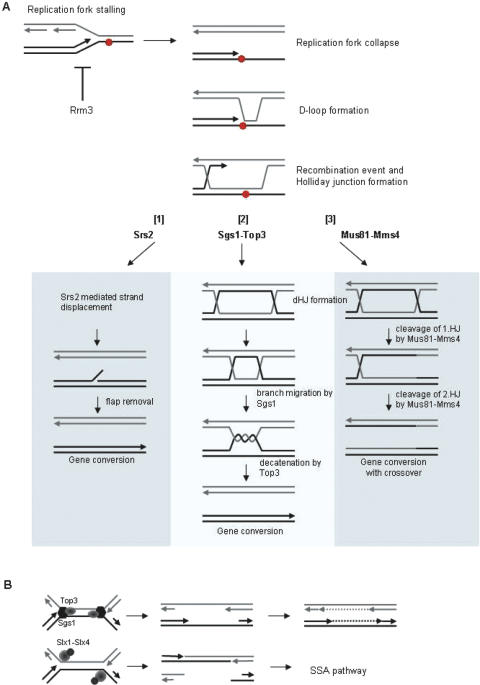
Sgs1 has a Rad51-dependent synthetic lethality with two helicases, the 3′–5′ DNA helicase Srs2 and also with the 5′–3′ DNA helicase Rrm3 (59–61). Similar to sgs1 mutants, srs2 mutants display a hyper-recombination phenotype, which lends support to the notion that SRS2 negatively regulates HR (62). Using yeast two-hybrid and biochemical assays, Srs2 was shown to physically interact with Rad51 like Sgs1 (63). Biochemical analyses and electron microscopy showed that Srs2 efficiently disrupts the presynaptic filament formed by Rad51p, thereby releasing ssDNA which is immediately sequestered by RPA to prevent re-nucleation of Rad51 (63,64). Furthermore, in vivo data revealed that Srs2 suppresses crossover events during DSB repair in mitotic cells (65). From this it was suggested that Srs2 channels recombination intermediates into the synthesis-dependent strand-annealing pathway (SDSA) during DSB repair, thereby reducing unwanted crossover events (Figure 3A, [1]). The Sgs1–Top3 complex also suppresses crossover events during DSB repair (65), however, by a different mechanism than Srs2. Wu and Hickson (66) showed that Sgs1–Top3 can work on preformed double Holliday Junction substrates and resolve these into non-crossover products (Figure 3A, [2]). The sgs1srs2 synthetic phenotype is therefore likely to be a consequence of an accumulation of recombination structures that cannot be resolved in the absence of these helicases or which resolution leads to extensive reciprocal exchange events. This also nicely explains why deletion of not only RAD51, but also RAD52, RAD55 or RAD57 suppresses the sgs1 srs2 phenotype (59).
Figure 3.
Pathways where Sgs1–Top3 function to process collapsed or stalled replication forks. (A) When a replication fork approaches a lesion (red circle) in the DNA a single-stranded gap forms and replication fork collapse may occur. This allows Rad51-dependent D-loop formation to initiate a recombination event via Holliday Junction (HJ) formation. The HJ may be processed in three ways. [1] The invading strand can be disrupted by the Srs2 helicase as part of the SDSA pathway leading to gene conversion without crossover events. [2] The Sgs1–Top3 complex can work in a dissolution pathway, where Sgs1 first promotes branch migration on the formed dHJ substrate. The single-stranded interlinked DNA is subsequent decatenated by Top3 resulting in gene conversion without crossover events. [3] Finally endonucleases such as Mus81–Mms4 may process the dHJ by two subsequent cleavage reactions. This will generate gene conversion products either with, or without an associated crossover event. Note that in the absence of Rrm3 more stalled replication forks will be generated. (B) When replication forks converge and stall at the rDNA, Sgs1–Top3 may allow DNA replication to finish by a DNA polymerase fill in reaction after separating the parental strands of the DNA template. In the absence of Sgs1–Top3, Slx1–Slx4 may cleave at each replication fork thereby creating a double nicked chromatid and a broken sister chromatid. The DSB can be repaired by a RAD52-independent single-strand annealing pathway at the rDNA.
S.pombe cells also display severe growth defects upon deletion of rqh1− and srs2−, however, so far it is unclear whether inactivation of HR suppresses this growth defect since contradictory results have been reported previously (67,68). Similar to Sgs1, Rqh1 in fission yeast likely plays a similar role in the processing of recombination intermediates, although it remains to be verified if the Rqh1–Top3 complex works to resolve and suppress crossover events in the cell. In the absence of Rqh1 there is a remarkable increase in the rate of HR structures and failure to properly segregate their chromosomes following replication arrest (43,69). This defect might be a direct consequence of aberrant recombination arising during DNA replication. In support of this notion, it was shown that overexpression of a E.coli Holliday junction resolvase partially suppressed the UV and HU hypersensitivity as well as aberrant mitosis in rqh1− cells (69).
Rrm3 promotes fork progression past non-nucleosomal protein–DNA complexes, and its absence leads to increased fork stalling and breakage at specific sites located throughout the S.cerevisiae genome (61). It has been suggested that paused or broken forks created in the absence of Rrm3 are processed by Rad51 into intermediates which become toxic for the cell in the absence of a functional Sgs1–Top3 complex, explaining why elimination of HR suppress this genetic interaction. A synthetic lethal interaction between rrm3 and srs2 has also been reported previously (60) which is probably due to the fact that Srs2p limits the number of toxic intermediates by disrupting Rad51 filaments. In the absence of Srs2 more recombination intermediates accumulate and the activity of Sgs1–Top3 is likely no longer sufficient for the cell. This indicates that when cells encounter massive replication stalling and consequently more breakage, both SRS2 and SGS1/TOP3 pathways are needed for survival.
Several slx mutants that require SGS1 for viability have also been identified previously (57). They fall into three phenotypic classes: MMS4 and MUS81, SLX1 and SLX4, and SLX5 and SLX8, and these gene pairs have been suggested to work as heterodimeric complexes. Interestingly, only the synthetic lethal interaction between sgs1 and the mms4–mus81 gene pair is suppressed by eliminating HR (70), indicating that Mus81–Mms4 are part of an alternative pathway to Sgs1 for the processing of recombination intermediates. Mutations in mus81–mms4 confer sensitivity to camptothecin (CPT), a compound known to produce replication-dependent DSBs (71). This sensitivity to CPT is shared by sgs1–top3 mutants and also with the corresponding S.pombe mutants (72). Taken together this suggests that SGS1–TOP3 and MUS81–MMS4 work on parallel pathways in order to process recombination intermediates that form downstream of collapsed replication forks (Figure 3A). Mus81–Mms4 has been characterized as a structure-specific endonuclease with equal affinity for either duplex DNA with a 3′ssDNA branch or completely duplex Y-form (a replication fork) (73). Upon replication fork breakdown strand invasion intermediates will arise and these can be processed by different pathways. If the invading strand switches template, a double HJ (dHJ) structure is generated and it has been suggested that Mus81-Mms4 is able to resolve these by cleavage creating gene conversion products either with or without crossover event (Figure 3A, [3]). In this scenario Sgs1–Top3 works on a parallel pathway, where branch migration and subsequent decatenation leads to dissolution of the dHJ with no reciprocal exchange (Figure 3A, [2]). Homologs of MUS81–MMS4 genes have been identified in other eukaryotes, including humans (74,75), and like the case in S.cerevisiae, double mutants of rqh1− and mus81− are inviable in S.pombe (76).
Similar to Mus81–Mms4, the Slx1–Slx4 complex also acts as a structure-specific endonuclease which is active on branched structures such as simple-Y, 5′-flap and replication fork structures (77). It has been shown that slx4 strains carrying temperature-sensitive alleles of SGS1 encounter problems in the rDNA at the restrictive temperature (78). Pulsed-field gel electrophoresis revealed that chromosome XII was altered and failed to enter the gel after S-phase progression, a defect which reflects incompletely replicated chromosomes (79). However, bulk DNA synthesis was not affected under these conditions, suggesting that the redundant function between Slx1–Slx4 and Sgs1–Top3 is restricted to the nucleolus. At the rDNA locus Sgs1–Top3 could be engaged at the termination of rDNA replication to decatenate stalled forks, and in the absence of Sgs1–Top3, Slx1–Slx4 might cleave these stalled forks (Figure 3B). This model is consistent with the idea that Sgs1–Top3 and Slx1–Slx4 appear to intersect upstream of HR, since the synthetic lethality of sgs1 slx1 and sgs1slx4 is not suppressed by eliminating HR (70). Furthermore, processing of stalled replication forks in the rDNA by Slx1–Slx4 would create a DSB, which in the rDNA can be repaired by RAD52-independent single-strand annealing (80). Further evidence that Sgs1–Top3 plays a role at the rDNA locus comes from observations on high rates of recombination at the rDNA in sgs1 and top3 strains (10,81). Interestingly, throughout much of the genome replication forks move more rapidly in the absence of Sgs1, yet replication is strongly retarded within the rDNA locus, suggestive of more extensive replication fork stalling in this locus that require functional Sgs1–Top3 (30). This stalling is probably associated with the replication fork barriers (RFB), which block replication from moving into the rDNA repeat in a direction opposite that of rDNA transcription when the Fob1 is bound to the RFB sequence (82,83). It has also been suggested that rqh1− phenotypes arise partially from defects in the processing of stalled forks in the rDNA since deletion of reb1+ in S.pombe (similar to FOB1 in S.cerevisae) partially suppresses the rqh1− deficient phenotype, including HU sensitivity (46). Furthermore, acute synthetic lethality of rqh1− and slx1− or slx4− mutations have also been reported, which lends support to the notion that the redundancy between RecQ helicases and Slx1–Slx4 is conserved between the two highly divergent yeast and strengthen the likelihood that this might also be the case in higher eukaryotic organisms (84).
SUMMARY
RecQ helicases are evolutionarily conserved from bacteria to man but the number of RecQ family members present in each organism differs. The individual RecQ helicases in humans appear to be involved exclusively in certain cellular processes based on the different clinical phenotypes associated with the human diseases. Some of the diversity of RecQ functions is certainly related to the complexity of multicellular organisms, however disruptions in these helicases across different species show two consistent features: elevated levels of HR and genomic instability. These specific RecQ-deficient phenotypes suggest that certain roles are evolutionarily conserved and likely carried out by one RecQ helicase in budding yeast and E.coli. We propose that the inherent genomic instability stemming from hyper-recombination during DNA replication is a dominant phenotype in RecQ helicase deficient cells. We have described how RecQ helicases in model organisms can contribute to the maintenance of genomic integrity through more than one cellular mechanism. For example, in budding yeast Sgs1 functions on at least two pathways when replication is blocked, one contributes to the checkpoint response by binding Rad53, and helping mediate its activation. This function does not require its helicase activity, Top3 or Rad51. Sgs1 also contributes to the stabilization of DNA polymerases at stalled forks and resumption of replication. This function does require Top3 interaction and is epistatic to Rad51. These various functions of RecQ helicases protect against replication fork demise by both preventing fork breakdown and restoring productive DNA synthesis after blocks and lesions are encountered and underscore the connection between genomic stability and these processes.
Acknowledgments
J.A.C. thanks the American Cancer Society (PF-01-142-01-CCG) and L.B. acknowledges a grant from the Danish Research Council (21-04-0354) and the Novo Nordisk Foundation. The authors also thank S. Gasser for reading this manuscript and helpful suggestions. Funding to pay the Open Access publication charges for this article was provided by Susan Gasser at the Friedrich Miescher-Institut.
Conflict of interest statement. None declared.
REFERENCES
- 1.Bachrati C.Z., Hickson I.D. RecQ helicases: suppressors of tumorigenesis and premature aging. Biochem. J. 2003;374:577–606. doi: 10.1042/BJ20030491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hickson I.D. RecQ helicases: caretakers of the genome. Nature Rev. Cancer. 2003;3:169–178. doi: 10.1038/nrc1012. [DOI] [PubMed] [Google Scholar]
- 3.Ozgenc A., Loeb L.A. Current advances in unraveling the function of the Werner syndrome protein. Mutat. Res. 2005;577:237–251. doi: 10.1016/j.mrfmmm.2005.03.020. [DOI] [PubMed] [Google Scholar]
- 4.Morozov V., Mushegian A.R., Koonin E.V., Bork P. A putative nucleic acid-binding domain in Bloom's and Werner's syndrome helicases. Trends Biochem. Sci. 1997;22:417–418. doi: 10.1016/s0968-0004(97)01128-6. [DOI] [PubMed] [Google Scholar]
- 5.Heo S.J., Tatebayashi K., Ohsugi I., Shimamoto A., Furuichi Y., Ikeda H. Bloom's syndrome gene suppresses premature ageing caused by Sgs1 deficiency in yeast. Genes Cells. 1999;4:619–625. doi: 10.1046/j.1365-2443.1999.00288.x. [DOI] [PubMed] [Google Scholar]
- 6.Yamagata K., Kato J., Shimamoto A., Goto M., Furuichi Y., Ikeda H. Bloom's and Werner's syndrome genes suppress hyperrecombination in yeast sgs1 mutant: implication for genomic instability in human diseases. Proc. Natl Acad. Sci. USA. 1998;95:8733–8738. doi: 10.1073/pnas.95.15.8733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu L., Davies S.L., North P.S., Goulaouic H., Riou J.F., Turley H., Gatter K.C., Hickson I.D. The Bloom's syndrome gene product interacts with topoisomerase III. J. Biol. Chem. 2000;275:9636–9644. doi: 10.1074/jbc.275.13.9636. [DOI] [PubMed] [Google Scholar]
- 8.Johnson F.B., Lombard D.B., Neff N.F., Mastrangelo M.A., Dewolf W., Ellis N.A., Marciniak R.A., Yin Y., Jaenisch R., Guarente L. Association of the Bloom syndrome protein with topoisomerase IIIalpha in somatic and meiotic cells. Cancer Res. 2000;60:1162–1167. [PubMed] [Google Scholar]
- 9.Ahmad F., Stewart E. The N-terminal region of the Schizosaccharomyces pombe RecQ helicase, Rqh1p, physically interacts with Topoisomerase III and is required for Rqh1p function. Mol. Genet. Genomics. 2005;273:102–114. doi: 10.1007/s00438-005-1111-3. [DOI] [PubMed] [Google Scholar]
- 10.Gangloff S., McDonald J.P., Bendixen C., Arthur L., Rothstein R. The yeast type I topoisomerase Top3 interacts with Sgs1, a DNA helicase homolog: a potential eukaryotic reverse gyrase. Mol. Cell. Biol. 1994;14:8391–8398. doi: 10.1128/mcb.14.12.8391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goodwin A., Wang S.W., Toda T., Norbury C., Hickson I.D. Topoisomerase III is essential for accurate nuclear division in Schizosaccharomyces pombe. Nucleic Acids Res. 1999;27:4050–4058. doi: 10.1093/nar/27.20.4050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wallis J.W., Chrebet G., Brodsky G., Rolfe M., Rothstein R. A hyper-recombination mutation in S.cerevisiae identifies a novel eukaryotic topoisomerase. Cell. 1989;58:409–419. doi: 10.1016/0092-8674(89)90855-6. [DOI] [PubMed] [Google Scholar]
- 13.Li W., Wang J.C. Mammalian DNA topoisomerase IIIα is essential in early embryogenesis. Proc. Natl Acad. Sci. USA. 1998;95:1010–1013. doi: 10.1073/pnas.95.3.1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maftahi M., Han C.S., Langston L.D., Hope J.C., Zigouras N., Freyer G.A. The top3(+) gene is essential in Schizosaccharomyces pombe and the lethality associated with its loss is caused by Rad12 helicase activity. Nucleic Acids Res. 1999;27:4715–4724. doi: 10.1093/nar/27.24.4715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bennett R.J., Sharp J.A., Wang J.C. Purification and characterization of the Sgs1 DNA helicase activity of Saccharomyces cerevisiae. J. Biol. Chem. 1998;273:9644–9650. doi: 10.1074/jbc.273.16.9644. [DOI] [PubMed] [Google Scholar]
- 16.Gray M.D., Shen J.C., Kamath-Loeb A.S., Blank A., Sopher B.L., Martin G.M., Oshima J., Loeb L.A. The Werner syndrome protein is a DNA helicase. Nature Genet. 1997;17:100–103. doi: 10.1038/ng0997-100. [DOI] [PubMed] [Google Scholar]
- 17.Karow J.K., Chakraverty R.K., Hickson I.D. The Bloom's syndrome gene product is a 3′–5′ DNA helicase. J. Biol. Chem. 1997;272:30611–30614. doi: 10.1074/jbc.272.49.30611. [DOI] [PubMed] [Google Scholar]
- 18.Shen J.C., Gray M.D., Oshima J., Loeb L.A. Characterization of Werner syndrome protein DNA helicase activity: directionality, substrate dependence and stimulation by replication protein A. Nucleic Acids Res. 1998;26:2879–2885. doi: 10.1093/nar/26.12.2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Suzuki N., Shimamoto A., Imamura O., Kuromitsu J., Kitao S., Goto M., Furuichi Y. DNA helicase activity in Werner's syndrome gene product synthesized in a baculovirus system. Nucleic Acids Res. 1997;25:2973–2978. doi: 10.1093/nar/25.15.2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Constantinou A., Tarsounas M., Karow J.K., Brosh R.M., Bohr V.A., Hickson I.D., West S.C. Werner's syndrome protein (WRN) migrates Holliday junctions and co-localizes with RPA upon replication arrest. EMBO Rep. 2000;1:80–84. doi: 10.1093/embo-reports/kvd004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karow J.K., Constantinou A., Li J.L., West S.C., Hickson I.D. The Bloom's syndrome gene product promotes branch migration of holliday junctions. Proc. Natl Acad. Sci. USA. 2000;97:6504–6508. doi: 10.1073/pnas.100448097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mohaghegh P., Hickson I.D. DNA helicase deficiencies associated with cancer predisposition and premature ageing disorders. Hum. Mol. Genet. 2001;10:741–746. doi: 10.1093/hmg/10.7.741. [DOI] [PubMed] [Google Scholar]
- 23.Bennett R.J., Keck J.L., Wang J.C. Binding specificity determines polarity of DNA unwinding by the Sgs1 protein of S.cerevisiae. J. Mol. Biol. 1999;289:235–248. doi: 10.1006/jmbi.1999.2739. [DOI] [PubMed] [Google Scholar]
- 24.Ozsoy A.Z., Ragonese H.M., Matson S.W. Analysis of helicase activity and substrate specificity of Drosophila RECQ5. Nucleic Acids Res. 2003;31:1554–1564. doi: 10.1093/nar/gkg243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sogo J.M., Lopes M., Foiani M. Fork reversal and ssDNA accumulation at stalled replication forks owing to checkpoint defects. Science. 2002;297:599–602. doi: 10.1126/science.1074023. [DOI] [PubMed] [Google Scholar]
- 26.Lonn U., Lonn S., Nylen U., Winblad G., German J. An abnormal profile of DNA replication intermediates in Bloom's syndrome. Cancer Res. 1990;50:3141–3145. [PubMed] [Google Scholar]
- 27.Poot M., Hoehn H., Runger T.M., Marin G.M. Impaired S-phase transit of Werner syndrome clls expressed in lymphoblastoid cell lines. Exp. cell Res. 1992;202:267–273. doi: 10.1016/0014-4827(92)90074-i. [DOI] [PubMed] [Google Scholar]
- 28.Frei C., Gasser S.M. The yeast Sgs1p helicase acts upstream of Rad53p in the DNA replication checkpoint and colocalizes with Rad53p in S-phase-specific foci. Genes Dev. 2000;14:81–96. [PMC free article] [PubMed] [Google Scholar]
- 29.Cobb J.A., Bjergbaek L., Shimada K., Frei C., Gasser S.M. DNA polymerase stabilization at stalled replication forks requires Mec1 and the RecQ helicase Sgs1. EMBO J. 2003;22:4325–4336. doi: 10.1093/emboj/cdg391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Versini G., Comet I., Wu M., Hoopes L., Schwob E., Pasero P. The yeast Sgs1 helicase is differentially required for genomic and ribosomal DNA replication. EMBO J. 2003;22:1939–1949. doi: 10.1093/emboj/cdg180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lowndes N.F., Murguia J.R. Sensing and responding to DNA damage. Curr. Opin. Genet. Dev. 2000;10:17–25. doi: 10.1016/s0959-437x(99)00050-7. [DOI] [PubMed] [Google Scholar]
- 32.Paulovich A.G., Toczyski D.P., Hartwell L.H. When checkpoints fail. Cell. 1997;88:315–321. doi: 10.1016/s0092-8674(00)81870-x. [DOI] [PubMed] [Google Scholar]
- 33.Nyberg K.A., Michelson R.J., Putnam C.W., Weinert T.A. Toward maintaining the genome: DNA damage and replication checkpoints. Annu. Rev. Genet. 2002;36:617–656. doi: 10.1146/annurev.genet.36.060402.113540. [DOI] [PubMed] [Google Scholar]
- 34.Bjergbaek L., Cobb J.A., Tsai-Pflugfelder M., Gasser S.M. Mechanistically distinct roles for Sgs1p in checkpoint activation and replication fork maintenance. EMBO J. 2005;24:405–417. doi: 10.1038/sj.emboj.7600511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schwartz M.F., Lee S.J., Duong J.K., Eminaga S., Stern D.F. FHA domain-mediated DNA checkpoint regulation of Rad53. Cell Cycle. 2003;2:384–396. [PubMed] [Google Scholar]
- 36.Alcasabas A.A., Osborn A.J., Bachant J., Hu F., Werler P.J., Bousset K., Furuya K., Diffley J.F., Carr A.M., Elledge S.J. Mrc1 transduces signals of DNA replication stress to activate Rad53. Nature Cell Biol. 2001;3:958–965. doi: 10.1038/ncb1101-958. [DOI] [PubMed] [Google Scholar]
- 37.Kumagai A., Dunphy W.G. Claspin, a novel protein required for the activation of Chk1 during a DNA replication checkpoint response in Xenopus egg extracts. Mol. Cell. 2000;6:839–849. doi: 10.1016/s1097-2765(05)00092-4. [DOI] [PubMed] [Google Scholar]
- 38.Cobb J.A., Schleker T., Rojas V., Bjergbaek L., Tercero J.A., Gasser S.M. Replisome instability, fork collapse, and gross chromosomal rearrangements arise synergistically from Mec1 kinase and RecQ helicase mutations. Genes Dev. 2005;19:3055–3069. doi: 10.1101/gad.361805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Myung K., Kolodner R.D. Suppression of genome instability by redundant S-phase checkpoint pathways in Saccharomyces cerevisiae. Proc. Natl Acad. Sci. USA. 2002;99:4500–4507. doi: 10.1073/pnas.062702199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Binz S.K., Sheehan A.M., Wold M.S. Replication protein A phosphorylation and the cellular response to DNA damage. DNA Repair (Amst.) 2004;3:1015–1024. doi: 10.1016/j.dnarep.2004.03.028. [DOI] [PubMed] [Google Scholar]
- 41.Arunkumar A.I., Klimovich V., Jiang X., Ott R.D., Mizoue L., Fanning E., Chazin W.J. Insights into hRPA32 C-terminal domain—mediated assembly of the simian virus 40 replisome. Nature Struct. Mol. Biol. 2005;12:332–339. doi: 10.1038/nsmbXX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ahn J.S., Osman F., Whitby M.C. Replication fork blockage by RTS1 at an ectopic site promotes recombination in fission yeast. EMBO J. 2005;24:2011–2023. doi: 10.1038/sj.emboj.7600670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stewart E., Chapman C.R., Al-Khodairy F., Carr A.M., Enoch T. rqh1+, a fission yeast gene related to the Bloom's and Werner's syndrome genes, is required for reversible S phase arrest. EMBO J. 1997;16:2682–2692. doi: 10.1093/emboj/16.10.2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Davey S., Han C.S., Ramer S.A., Klassen J.C., Jacobson A., Eisenberger A., Hopkins K.M., Lieberman H.B., Freyer G.A. Fission yeast rad12+ regulates cell cycle checkpoint control and is homologous to the Bloom's syndrome disease gene. Mol. Cell. Biol. 1998;18:2721–2728. doi: 10.1128/mcb.18.5.2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Murray J.M., Lindsay H.D., Munday C.A., Carr A.M. Role of Schizosaccharomyces pombe RecQ homolog, recombination, and checkpoint genes in UV damage tolerance. Mol. Cell. Biol. 1997;17:6868–6875. doi: 10.1128/mcb.17.12.6868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Win T.Z., Mankouri H.W., Hickson I.D., Wang S.W. A role for the fission yeast Rqh1 helicase in chromosome segregation. J. Cell Sci. 2005;118:5777–5784. doi: 10.1242/jcs.02694. [DOI] [PubMed] [Google Scholar]
- 47.Wicky C., Alpi A., Passannante M., Rose A., Gartner A., Muller F. Multiple genetic pathways involving the Caenorhabditis elegans Bloom's syndrome genes him-6, rad-51, and top-3 are needed to maintain genome stability in the germ line. Mol. Cell. Biol. 2004;24:5016–5027. doi: 10.1128/MCB.24.11.5016-5027.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ahmed S., Alpi A., Hengartner M.O., Gartner A. C.elegans. RAD-5/CLK-2 defines a new DNA damage checkpoint protein. Curr. Biol. 2001;11:1934–1944. doi: 10.1016/s0960-9822(01)00604-2. [DOI] [PubMed] [Google Scholar]
- 49.MacQueen A.J., Villeneuve A.M. Nuclear reorganization and homologous chromosome pairing during meiotic prophase require C.elegans chk-2. Genes Dev. 2001;15:1674–1687. doi: 10.1101/gad.902601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Grabowski M.M., Svrzikapa N., Tissenbaum H.A. Bloom syndrome ortholog HIM-6 maintains genomic stability in C.elegans. Mech. Ageing Dev. 2005;126:1314–1321. doi: 10.1016/j.mad.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 51.Lee S.J., Yook J.S., Han S.M., Koo H.S. A Werner syndrome protein homolog affects C.elegans development, growth rate, life span and sensitivity to DNA damage by acting at a DNA damage checkpoint. Development. 2004;131:2565–2575. doi: 10.1242/dev.01136. [DOI] [PubMed] [Google Scholar]
- 52.Lambert S., Watson A., Sheedy D.M., Martin B., Carr A.M. Gross chromosomal rearrangements and elevated recombination at an inducible site-specific replication fork barrier. Cell. 2005;121:689–702. doi: 10.1016/j.cell.2005.03.022. [DOI] [PubMed] [Google Scholar]
- 53.Merrill B.J., Holm C. The RAD52 recombinational repair pathway is essential in pol30 (PCNA) mutants that accumulate small single-stranded DNA fragments during DNA synthesis. Genetics. 1998;148:611–624. doi: 10.1093/genetics/148.2.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zou H., Rothstein R. Holliday junctions accumulate in replication mutants via a RecA homolog-independent mechanism. Cell. 1997;90:87–96. doi: 10.1016/s0092-8674(00)80316-5. [DOI] [PubMed] [Google Scholar]
- 55.Wu L., Davies S.L., Levitt N.C., Hickson I.D. Potential role for the BLM helicase in recombinational repair via a conserved interaction with RAD51. J. Biol. Chem. 2001;276:19375–19381. doi: 10.1074/jbc.M009471200. [DOI] [PubMed] [Google Scholar]
- 56.Gangloff S., de Massy B., Arthur L., Rothstein R., Fabre F. The essential role of yeast topoisomerase III in meiosis depends on recombination. EMBO J. 1999;18:1701–1711. doi: 10.1093/emboj/18.6.1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mullen J.R., Kaliraman V., Ibrahim S.S., Brill S.J. Requirement for three novel protein complexes in the absence of the Sgs1 DNA helicase in Saccharomyces cerevisiae. Genetics. 2001;157:103–118. doi: 10.1093/genetics/157.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tong A.H., Evangelista M., Parsons A.B., Xu H., Bader G.D., Page N., Robinson M., Raghibizadeh S., Hogue C.W., Bussey H., et al. Systematic genetic analysis with ordered arrays of yeast deletion mutants. Science. 2001;294:2364–2368. doi: 10.1126/science.1065810. [DOI] [PubMed] [Google Scholar]
- 59.Gangloff S., Soustelle C., Fabre F. Homologous recombination is responsible for cell death in the absence of the Sgs1 and Srs2 helicases. Nature Genet. 2000;25:192–194. doi: 10.1038/76055. [DOI] [PubMed] [Google Scholar]
- 60.Schmidt K.H., Kolodner R.D. Requirement of Rrm3 helicase for repair of spontaneous DNA lesions in cells lacking Srs2 or Sgs1 helicase. Mol. Cell. Biol. 2004;24:3213–3226. doi: 10.1128/MCB.24.8.3213-3226.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Torres J.Z., Schnakenberg S.L., Zakian V.A. Saccharomyces cerevisiae Rrm3p DNA helicase promotes genome integrity by preventing replication fork stalling: viability of rrm3 cells requires the intra-S-phase checkpoint and fork restart activities. Mol. Cell. Biol. 2004;24:3198–3212. doi: 10.1128/MCB.24.8.3198-3212.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Aguilera A., Klein H.L. Genetic control of intrachromosomal recombination in Saccharomyces cerevisiae. I. Isolation and genetic characterization of hyper-recombination mutations. Genetics. 1988;119:779–790. doi: 10.1093/genetics/119.4.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Krejci L., Van Komen S., Li Y., Villemain J., Reddy M.S., Klein H., Ellenberger T., Sung P. DNA helicase Srs2 disrupts the Rad51 presynaptic filament. Nature. 2003;423:305–309. doi: 10.1038/nature01577. [DOI] [PubMed] [Google Scholar]
- 64.Veaute X., Jeusset J., Soustelle C., Kowalczykowski S.C., Le Cam E., Fabre F. The Srs2 helicase prevents recombination by disrupting Rad51 nucleoprotein filaments. Nature. 2003;423:309–312. doi: 10.1038/nature01585. [DOI] [PubMed] [Google Scholar]
- 65.Ira G., Malkova A., Liberi G., Foiani M., Haber J.E. Srs2 and Sgs1-Top3 suppress crossovers during double-strand break repair in yeast. Cell. 2003;115:401–411. doi: 10.1016/s0092-8674(03)00886-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wu L., Hickson I.D. The Bloom's syndrome helicase suppresses crossing over during homologous recombination. Nature. 2003;426:870–874. doi: 10.1038/nature02253. [DOI] [PubMed] [Google Scholar]
- 67.Maftahi M., Hope J.C., Delgado-Cruzata L., Han C.S., Freyer G.A. The severe slow growth of Deltasrs2 Deltarqh1 in Schizosaccharomyces pombe is suppressed by loss of recombination and checkpoint genes. Nucleic Acids Res. 2002;30:4781–4792. doi: 10.1093/nar/gkf581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang S.W., Goodwin A., Hickson I.D., Norbury C.J. Involvement of Schizosaccharomyces pombe Srs2 in cellular responses to DNA damage. Nucleic Acids Res. 2001;29:2963–2972. doi: 10.1093/nar/29.14.2963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Doe C.L., Dixon J., Osman F., Whitby M.C. Partial suppression of the fission yeast rqh1(−) phenotype by expression of a bacterial Holliday junction resolvase. EMBO J. 2000;19:2751–2762. doi: 10.1093/emboj/19.11.2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fabre F., Chan A., Heyer W.D., Gangloff S. Alternate pathways involving Sgs1/Top3, Mus81/Mms4, and Srs2 prevent formation of toxic recombination intermediates from single-stranded gaps created by DNA replication. Proc. Natl Acad. Sci. USA. 2002;99:16887–16892. doi: 10.1073/pnas.252652399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang C., Roberts T.M., Yang J., Desai R., Brown G.W. Suppression of genomic instability by SLX5 and SLX8 in Saccharomyces cerevisiae. DNA Repair (Amst.) 2006;5:336–346. doi: 10.1016/j.dnarep.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 72.Doe C.L., Ahn J.S., Dixon J., Whitby M.C. Mus81-Eme1 and Rqh1 involvement in processing stalled and collapsed replication forks. J. Biol. Chem. 2002;277:32753–32759. doi: 10.1074/jbc.M202120200. [DOI] [PubMed] [Google Scholar]
- 73.Kaliraman V., Mullen J.R., Fricke W.M., Bastin-Shanower S.A., Brill S.J. Functional overlap between Sgs1-Top3 and the Mms4-Mus81 endonuclease. Genes Dev. 2001;15:2730–2740. doi: 10.1101/gad.932201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Boddy M.N., Gaillard P.H., McDonald W.H., Shanahan P., Yates J.R., III, , Russell P. Mus81-Eme1 are essential components of a Holliday junction resolvase. Cell. 2001;107:537–548. doi: 10.1016/s0092-8674(01)00536-0. [DOI] [PubMed] [Google Scholar]
- 75.Chen X.B., Melchionna R., Denis C.M., Gaillard P.H., Blasina A., Van de Weyer I., Boddy M.N., Russell P., Vialard J., McGowan C.H. Human Mus81-associated endonuclease cleaves Holliday junctions in vitro. Mol. Cell. 2001;8:1117–1127. doi: 10.1016/s1097-2765(01)00375-6. [DOI] [PubMed] [Google Scholar]
- 76.Boddy M.N., Lopez-Girona A., Shanahan P., Interthal H., Heyer W.D., Russell P. Damage tolerance protein Mus81 associates with the FHA1 domain of checkpoint kinase Cds1. Mol. Cell. Biol. 2000;20:8758–8766. doi: 10.1128/mcb.20.23.8758-8766.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fricke W.M., Brill S.J. Slx1-Slx4 is a second structure-specific endonuclease functionally redundant with Sgs1-Top3. Genes Dev. 2003;17:1768–1778. doi: 10.1101/gad.1105203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kaliraman V., Brill S.J. Role of SGS1 and SLX4 in maintaining rDNA structure in Saccharomyces cerevisiae. Curr. Genet. 2002;41:389–400. doi: 10.1007/s00294-002-0319-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hennessy K.M., Lee A., Chen E., Botstein D. A group of interacting yeast DNA replication genes. Genes Dev. 1991;5:958–969. doi: 10.1101/gad.5.6.958. [DOI] [PubMed] [Google Scholar]
- 80.Ozenberger B.A., Roeder G.S. A unique pathway of double-strand break repair operates in tandemly repeated genes. Mol. Cell. Biol. 1991;11:1222–1231. doi: 10.1128/mcb.11.3.1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mullen J.R., Kaliraman V., Brill S.J. Bipartite structure of the SGS1 DNA helicase in Saccharomyces cerevisiae. Genetics. 2000;154:1101–1114. doi: 10.1093/genetics/154.3.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Brewer B.J., Fangman W.L. A replication fork barrier at the 3′ end of yeast ribosomal RNA genes. Cell. 1988;55:637–643. doi: 10.1016/0092-8674(88)90222-x. [DOI] [PubMed] [Google Scholar]
- 83.Kobayashi T., Horiuchi T. A yeast gene product, Fob1 protein, required for both replication fork blocking and recombinational hotspot activities. Genes Cells. 1996;1:465–474. doi: 10.1046/j.1365-2443.1996.d01-256.x. [DOI] [PubMed] [Google Scholar]
- 84.Coulon S., Gaillard P.H., Chahwan C., McDonald W.H., Yates J.R. III, Russell P. Slx1-Slx4 are subunits of a structure-specific endonuclease that maintains ribosomal DNA in fission yeast. Mol. Biol. Cell. 2004;15:71–80. doi: 10.1091/mbc.E03-08-0586. [DOI] [PMC free article] [PubMed] [Google Scholar]