Abstract
The reversible fatty acid amide hydrolase (FAAH) inhibitor OL135 reverses mechanical allodynia in the spinal nerve ligation (SNL) and mild thermal injury (MTI) models in the rat. The purpose of this study was to investigate the role of the cannabinoid and opioid systems in mediating this analgesic effect.
Elevated brain concentrations of anandamide (350 pmol g−1 of tissue vs 60 pmol g−1 in vehicle-treated controls) were found in brains of rats given OL135 (20 mg kg−1) i.p. 15 min prior to 20 mg kg−1 i.p. anandamide.
Predosing rats with OL135 (2–60 mg kg−1 i.p.) 30 min before administration of an irreversible FAAH inhibitor (URB597: 0.3 mg kg−1 intracardiac) was found to protect brain FAAH from irreversible inactivation. The level of enzyme protection was correlated with the OL135 concentrations in the same brains.
OL135 (100 mg kg−1 i.p.) reduced by 50% of the maximum possible efficacy (MPE) mechanical allodynia induced by MTI in FAAH+/+mice (von Frey filament measurement) 30 min after dosing, but was without effect in FAAH−/− mice.
OL135 given i.p. resulted in a dose-responsive reversal of mechanical allodynia in both MTI and SNL models in the rat with an ED50 between 6 and 9 mg kg−1. The plasma concentration at the ED50 in both models was 0.7 μM (240 ng ml−1).
In the rat SNL model, coadministration of the selective CB2 receptor antagonist SR144528 (5 mg kg−1 i.p.), with 20 mg kg−1 OL135 blocked the OL135-induced reversal of mechanical allodynia, but the selective CB1 antagonist SR141716A (5 mg kg−1 i.p.) was without effect.
In the rat MTI model neither SR141716A or SR144528 (both at 5 mg kg−1 i.p.), or a combination of both antagonists coadministered with OL135 (20 mg kg−1) blocked reversal of mechanical allodynia assessed 30 min after dosing.
In both the MTI model and SNL models in rats, naloxone (1 mg kg−1, i.p. 30 min after OL135) reversed the analgesia (to 15% of control levels in the MTI model, to zero in the SNL) produced by OL135.
Keywords: FAAH, fatty acid amide hydrolase, fatty acid amide, endocannabinoid, pain, analgesia, cannabinoid receptor, naloxone
Introduction
Pharmacologically active preparations of Cannabis sativa have been recognised since ancient times as having potentially useful therapeutic effects, including analgesia (reviewed in Calixto et al., 2000). With the discovery and cloning of the cannabinoid receptors CB1 (Matsuda et al., 1990) and CB2 (Munro et al., 1993) and the identification of anandamide, the first endogenous substance with agonist activity at both receptors (Devane et al., 1992), a rationale for the analgesic effects of cannabis was developed. CB1 is expressed both in the periphery (Ahluwalia et al., 2000; 2003) and in the central nervous system (CNS), where it is present in areas important for the processing of nociceptive signalling (e.g. spinal cord, periaqueductal grey, and thalamus; Tsou et al., 1998). Inhibition of transmitter release by activation of presynaptic CB1 receptors provides a mechanism by which activation of these receptors in sensory processing pathways could modulate the transmission of nociceptive information (Elphick & Egertova, 2001). CB2 receptors were initially thought to be confined to the periphery, where they are expressed on a wide variety of immune cells. Although they do not appear to be expressed generally on central neurons, they can be expressed on microglia, a class of glial cells which have been implicated in the control of nociceptive transmission in the spinal cord (Franklin & Stella, 2003; Zhang et al., 2003; Nunez et al., 2004; Hua et al., 2005; Tsuda et al., 2005) and one specialised class of neuron, the retinal ganglion cell, may also express this receptor (Lu et al., 2000).
A major limitation on the potential utility of cannabinoid agonists as therapeutic agents is the profile of side effects resulting from the nonselective activation of central CB1 receptors, which includes dysphoria, dizziness, and effects on motor coordination and memory (Carlini, 2004). In addition, there is a significant abuse potential, which has hindered their development as therapeutic agents (Gardner, 2005; Gourlay, 2005).
An alternative approach, which may avoid such side effects, is to manipulate the endogenous cannabinoid system. It has been shown that the neuronal synthesis of anandamide, which itself has analgesic properties, is upregulated by elevated calcium concentrations, such as would occur in active neural pathways (Di Marzo et al., 1994; Cadas et al., 1996; Sugiura et al., 1996). Anandamide has a short half-life, as it is rapidly hydrolysed by the enzyme fatty acid amid hydrolase (FAAH; Giang & Cravatt, 1997; Willoughby et al., 1997; Cravatt et al., 2001), and resting concentrations in the CNS are very low. Inhibition of the enzyme would be expected to prolong the duration of action of endogenously released anandamide, particularly in those pathways in which its synthesis was upregulated as a result of activity, as would occur in nociceptive pathways under conditions of chronic pain. Such potentiation, acting preferentially on active pathways, might be expected to have a reduced risk of psychotropic effects compared with nonselective activation of cannabinoid receptors by exogenously applied agonists.
FAAH knockout mice have been described (Cravatt et al., 2001). They have elevated resting brain concentrations of anandamide, and manifest an analgesic phenotype in several commonly used models of pain. Furthermore, a potent and selective inhibitor of FAAH, OL135, has been described, and treatment of rats with this inhibitor results in an amelioration of pain behaviours (Lichtman et al., 2004a; Leung et al., 2005). The exact mechanism by which FAAH inhibition exerts these effects is not clear. FAAH metabolises at least two other fatty acid amides, palmitoylethanolamine (PEA) and oleamide, and PEA has both analgesic and anti-inflammatory effects (Bisogno et al., 2002; Lambert et al., 2002). Anandamide itself is a weak partial agonist at CB1 receptors, has some affinity for CB2 receptors, and has lower but significant activity at other receptors or channels which have been implicated in nociceptive transmission, including TRPV1, 5HT 2 and 3 receptors, and voltage-gated calcium channels (Mackie et al., 1993; Fan, 1995; Gebremedhin et al., 1999; Barann et al., 2002; Di Marzo et al., 2002; Roberts et al., 2002). We therefore sought to investigate the downstream mechanisms by which FAAH inhibition produces analgesic effects. We first confirmed using two independent assays that OL135 effectively accesses the CNS and inhibits FAAH, with a resultant increase in the levels of brain anandamide. We also demonstrated that the effect of OL135 is mediated exclusively through FAAH, as the compound is ineffective in producing analgesia in FAAH knockout mice. We also found that the downstream mechanisms differ in two different pain models, the mild thermal injury (MTI) model of peripheral tissue damage, and the spinal nerve ligation (SNL) neuropathic pain model. In the former, blockade of neither of the two well-characterised cannabinoid receptors was able to prevent the analgesia produced by FAAH inhibition, whereas selective blockade of CB2 receptors did ameliorate the analgesia produced by FAAH inhibition in the neuropathic model. However, in both models, a substantial opioid involvement is indicated by the ability of naloxone to reverse the analgesic effects of FAAH inhibition.
Methods
Compounds
Anandamide (1-3H-arachidonylethanolamine; [3H]AEA) was obtained from GE Healthcare, Piscataway, NJ, U.S.A. (20 Ci mmol−1). The selective CB1 receptor antagonist SR141716A was purchased from Organix, Inc., Woburn, MA, U.S.A. URB597 was obtained from Cayman Chemical, Ann Arbor, MO, U.S.A. Anandamide standard and deuterated-anandamide internal standard for liquid chromatography mass spectroscopy (LCMS) experiments were provided by Dr Richard Apodaca (Johnson & Johnson Pharmaceutical Research & Development, L.L.C., San Diego, CA, U.S.A), and OL135 and the selective CB2 antagonist SR144528 were also synthesized at Johnson & Johnson Pharmaceutical Research & Development, L.L.C. All other reagents were obtained from the Sigma Chemical Company unless otherwise indicated.
Anandamide was dissolved in Tocrisolve (Tocris Inc., Ellisville, MI, U.S.A.). OL135 was dissolved at 10 mg ml−1 in 1 : 1 : 18 ethanol : alkamuls-620 : 5% dextrose. SR141716A and SR144528 were dissolved at 2.5 mg ml−1 in 1 : 1 : 2 : 16 pharmasolve : Tween 80 : peanut oil : 20% intralipid. URB597 was dissolved at 0.3 mg kg−1 in 5% Pharmasolve dissolved in 20% HP-beta-cyclodextran in water. All compounds were administered i.p. in a volume of 2 ml kg−1 in rat and 10 ml kg−1 in mouse, except for URB597, which was administered through the left ventricle in a volume of 1 ml kg−1.
Animals
Male Sprague–Dawley rats were purchased from Harlan Industries (San Diego, CA, U.S.A.) and maintained on a 12-h reverse light/dark cycle in a climate-controlled room. Rats weighed between 250 and 350 g prior to MTI and between 110 and 130 g prior to SNL. Male C57Bl/6 mice were purchased from Jackson Laboratories (West Sacramento, CA, U.S.A.) and FAAH−/− mice on a C57Bl/6 genetic background (Cravatt et al., 2001) were bred in-house. Mice were housed in a climate-controlled room under a normal light cycle with free access to food and water. Mice weighed between 20 and 30 g prior to testing. All animal procedures were carried out under protocols approved by the Institutional Animal Care and Use Committee.
Anandamide and OL135 determination in rat tissue samples
Male 250 g Sprague–Dawley rats were used in all experiments. Rats received i.p. injections of OL135 (20 mg kg−1). After 15 min, they were injected i.p. with anandamide (10, 20, or 50 mg kg−1) or vehicle, and after a further 30 min they were killed by CO2 asphyxiation. Brains were removed immediately after breathing effort ceased and placed on dry ice covered with foil, then stored at –80°C. In some cases, 2 cm sections of spinal cord were collected at the same time, bisected along the saggital midline, and similarly rapidly frozen. Subsequently, tissue samples were thawed in 2 ml distilled H2O per gram of tissue and homogenised on ice.
OL135 and/or anandamide were extracted in acetonitrile and measured by liquid chromatography/mass spectroscopy (LCMS). Separate samples of each tissue homogenate were assayed for OL135 or for anandamide using similar methods.
For OL135 measurements, dimethyl sulphoxide (DMSO) (containing OL135 standard, as appropriate) was added to 50 μl of brain homogenate, which was then extracted with 150 μl of acetonitrile. The components were mixed, centrifuged for 5 min at 20,800 × g and 100 μl of the supernatant fraction was transferred to an high performance liquid chromatography (HPLC) vial. After diluting the supernatant 1 : 4 with 10 mM ammonium acetate (pH 7.4), the vial was capped, mixed and 5 μl was injected into an Ace C4 2 × 50 mm column (Advanced Chromatography Technologies, Aberdeen, Scotland) running a 10 mM ammonium acetate pH 7 mobile phase with an acetonitrile gradient. The column eluent flowed into an ABI/Sciex 4000 triple quadripole mass spectrometer (Applied Biosystems, Foster City, CA, U.S.A.). Daughter ions were quantitated by comparison with a standard curve by linear regression using the mass spectrometer's software. OL135 levels could be determined between 30 nM and 10 μM using this method. The same analytical methods were used to determine the concentration of OL135 in plasma samples.
Anandamide was similarly measured, in this case by adding anandamide standard (as indicated) and deuterated-anandamide internal standard dissolved in DMSO to 350 μl of each homogenate, which was then extracted with two volumes of acetonitrile. After agitation, the samples were centrifuged for 5 min at 20,800 × g at 4oC. The supernatant was transferred to a brown glass HPLC vial. The sample in the HPLC vial was diluted 1 : 1 with 10 mM ammonium acetate pH 7, then 0.5 ml was injected into the LCMS for quantitation. Liquid chromatography mass spectrometry was performed on a Finnigan LCQ Deca using a 2.1 × 150 mm Ace 5 C18 column (Advanced Chromatography Technologies, Aberdeen, Scotland) running a 10 mM ammonium acetate pH 7 mobile phase with an acetonitrile gradient. Quantitation of the daughter ions was accomplished by selective reaction monitoring. Anandamide counts for each sample were divided by deuterated-anandamide counts and compared against an identically prepared standard curve by linear regression (GraphPad Prism; San Diego, CA, U.S.A.). All samples measured fell within the range of the standard curve (6.7 to 2000 pmol g−1 tissue), the lowest standard of which yielded a minimum signal : noise ratio of 10 : 1. Student's T-tests were used to determine statistical significance for data sets with two treatment groups, while significant differences in experiments with more than two groups were determined using an analysis of variance (ANOVA) with the Dunnett's or Newman–Keul's post-test, as appropriate (GraphPad Prism; San Diego, CA, U.S.A.).
In vivo enzyme protection assay
In order to measure directly the levels of inhibition produced in vivo by a centrally active FAAH inhibitor, we devised an assay in which we measured protection of enzyme active sites against inactivation by an irreversible FAAH inhibitor (URB597; 3′carbamoyl-biphenyl-3-yl cyclohexylcarbamate; Mor et al., 2004) by prior occupation with OL135. We first defined the minimum dose and time of exposure in vivo to the irreversible probe compound required to produce the maximum attainable levels of enzyme inhibition in the subsequent in vitro enzyme assay. The probe compound was applied by intracardiac injection to allow rapid distribution to the brain via the circulation, and this was followed by perfusion with 60 ml of ice-cold phosphate-buffered saline (PBS) given within 30 s of the probe compound to wash it out of the preparation. Under these conditions, we found that without pretreatment with reversible compound, about 80% of the brain FAAH activity could be reproducibly inhibited by a 0.3 mg kg−1 dose of probe compound (data not shown). This level of inhibition was attained within 20–30 s of probe dosing (data not shown). Higher doses did not increase further the level of inhibition, and lower doses produced a lower inhibition. In all subsequent experiments, the probe compound was used under these conditions (0.3 mg kg–1, followed by 60 ml PBS intracardiac within 20–30 s).
Male Sprague–Dawley rats (300±25 g) were assigned to one of five groups (n=8−10 per group). Rats were initially given doses of vehicle (n=4–5 per group) or OL135 (n=4–5 per group) between 2 and 60 mg kg−1 i.p. After 30 min, under deep isoflurane/oxygen anesthesia, they were divided into two subgroups and given an intracardiac (left ventricle) injection of either vehicle or the irreversible probe compound (URB597) at 0.3 mg ml−1, rapidly (30 s) followed by an intracardiac perfusion with PBS (60 ml) to remove the excess probe compound. Brains were removed and rapidly frozen for subsequent ex vivo FAAH activity assay. The ex vivo FAAH activity assay was performed on frozen brain samples, which were thawed in 6 ml PBS per brain and homogenised. Homogenised samples were further diluted in FAAH assay buffer (125 mM Tris-hydroxymethyl methylamine, 1 mM ethylenediamine tetra-acetic acid (EDTA), 0.2% glycerol, 0.02% Triton X-100, 0.4 mM Hepes, pH 8) and used for in vitro FAAH determination by the method of Wilson et al. (2003). The total reaction volume of 100 μl consisted of 50 μl of brain homogenate, 40 μl of 250 nM [3H]AEA for a final tracer concentration of 100 nM, and as appropriate 10 μl of either FAAH inhibitor Cay 10400 to set blank values (Cayman Chemical Co., Ann Arbor, MI, U.S.A.) or 10 μl of buffer. After 60 min at room temperature, 60 μl of the reaction mixture was filtered rapidly through activated charcoal, and the unbound labelled ethanolamine eluates were collected directly into a 96-well plate loaded with liquid scintillant and counted on a Hewlett-Packard TopCount.
The resulting data were processed to yield a figure for percent protection as follows: 100 × ((OL135+probe)−(probe alone)+(control−OL135 only))/control, where probe alone=animals dosed intracardiac with URB597 only; OL135+probe=animals dosed with OL135 followed by URB597; OL135=animals dosed only with OL135 without subsequent URB597; control refers to animals dosed with vehicle only.
The statistical significance of differences in FAAH protection between treatment groups was determined using an analysis of variance (one-way ANOVA) followed by a Newman–Keul's post-test (GraphPad Prism).
Von Frey filament test for mechanical allodynia
Both of the pain models used in this work (MTI and SNL) result in a mechanical allodynia in the hind paw of the affected side. Mechanical (tactile) allodynia was assessed by recording the pressure at which the subject withdrew the affected paw from graded stimuli. Von Frey filaments ranging from 0.41 to 15.8 g were used to test rats and filaments ranging from 0.1 to 4.1 g were used to test mice. Von Frey filaments were applied perpendicularly to the plantar surface of the paw through wire-mesh observation cages. A median paw withdrawal threshold (PWT) was determined using an adaptation of the Dixon up–down method, as described previously (Chaplan et al., 1994).
Mild thermal injury
A first-degree burn injury (erythema without blistering) was produced under brief isoflurane/oxygen anesthesia. In the rat, the plantar surface of the left hind paw was placed on water-dampened 56°C hotplate for 20 s and steady contact was maintained by applying an 84 g weight to the dorsum (Nozaki-Taguchi & Yaksh, 1998). In the mouse, the duration of contact was calibrated to 10 s to avoid blistering, and contact was maintained using a 12 g weight. Animals were allowed to recover. Tactile allodynia developed within 30 min of the burn injury and persisted for at least 3 h.
Spinal nerve ligation model
Surgery for SNL was performed as previously described (Kim & Chung, 1992). Briefly, rats were anesthetised with isoflurane/oxygen. The left lumbar spinal nerves L5 and L6 were isolated and tightly ligated with 6-0 silk suture distal to the dorsal root ganglion and prior to their entrance into the sciatic nerve, as described by Kim & Chung (1992). An additional step, gentle rubbing of the L4 spinal nerve, was added to promote the severity of the allodynia (Lee et al., 2002). The incisions were closed and the rats were allowed to recover for 2 weeks under conditions described above. Rats were included in the study only if they did not exhibit motor dysfunction (e.g., paw dragging).
Drug treatments for pain testing
Immediately after the baseline measurement in either model, test compounds were administered i.p. and the measurement was repeated usually at 0.5 h intervals after administration, usually for a duration of 2 h. All compounds were administered i.p. in a volume of 2 ml kg−1 in rat and 10 ml kg−1 in mouse.
Data treatment and statistics
Since the von Frey filament set was calibrated on a logarithmic scale by the vendor (Stoelting, Wood Dale, IL, U.S.A.) and our selection of nine filaments was also based on near equal logarithmic intervals, data were plotted using a logarithmic scale. Data were expressed as mean±standard error of the mean (s.e.m.). Time course data were analysed using two-way ANOVA (group and time) followed by Bonferroni's multiple comparisons. Other data were analysed using one-way ANOVA followed by Bonferroni's post-tests. The significance threshold was set at 0.05. All analyses were performed using GraphPad Prism.
Results
Central activity of OL135
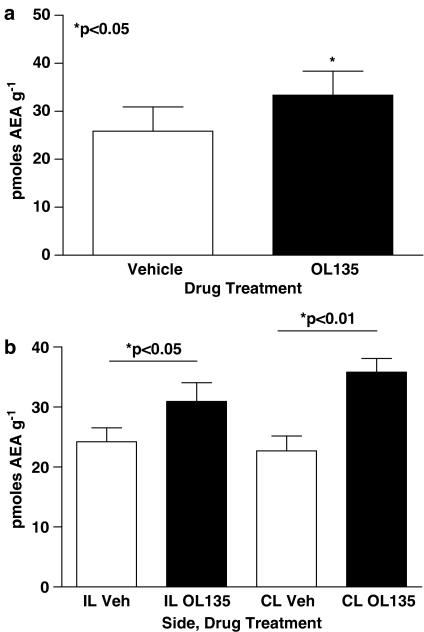
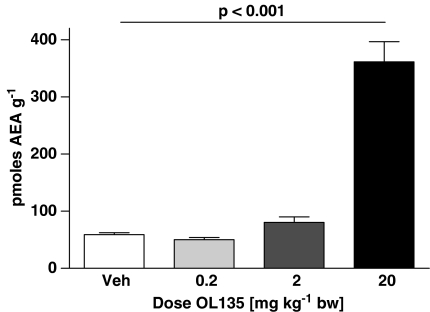
OL135 is an alpha keto heterocyclic compound which reversibly and selectively inhibits FAAH with an IC50 of approximately 5 nM at the rat enzyme (Lichtman et al., 2004a and unpublished observations). It is devoid of activity at CB1, CB2, and mu and delta opioid receptors, and has a low activity at kappa opioid receptors (about 35% inhibition at 10 μM; unpublished observations). We used three approaches to demonstrate that OL135 was able to access the CNS and produce effective inhibition of FAAH in vivo. First, we used a selective and sensitive LCMS method to measure the amount of anandamide present in the brain. In preliminary experiments, we observed low resting concentrations of anandamide in the brains and spinal cords of SNL treated rats of 25 pmol g−1 of tissue. These were slightly but significantly elevated 40 min after dosing the rats with OL135 (20 mg kg−1 i.p.: Figure 1a, brain; Figure 1b, spinal cord). To assess the robustness of this anandamide protection in the face of globally elevated anandamide concentrations, we used an experimental paradigm in which rats were initially dosed with the FAAH inhibitor or vehicle, and after 15 min were further dosed with anandamide (20 mg kg−1 i.p.). At 30 min after anandamide treatment, only low levels (∼60 pmol g−1 tissue) were observed in the brains of rats pretreated with vehicle (Figure 2). Pretreatment with 20 mg kg−1 OL135 produced a significant increase in brain anandamide levels to approximately 350 pmol g−1 tissue 30 min after dosing, indicating that inhibition of the enzyme resulted in a protection of exogenously supplied anandamide against degradation. Lower doses of OL135 were ineffective (Figure 2).
Figure 1.
OL135 treatment increases endogenous anandamide in rats with SNL neuropathic lesions. Rats with a previously induced SNL neuropathic lesion on the ipsilateral (IL; left) side were dosed with 20 mg kg−1 OL135 i.p. and 40 min later brains (a) or lumbar spinal cord segments (b) were collected for assay of anandamide levels. OL135 treatment produces a small but significant enhancement in anandamide levels in brain and in lumbar spinal cord segments. No statistically significant difference was observed between spinal cord anandamide levels contra and ipsilateral to the SNL ligation. n=6 per group.
Figure 2.
OL135 treatment inhibits degradation of exogenously administered anandamide. Rats were dosed initially with OL135 (varying doses i.p.) and subsequently with 20 mg kg−1 anandamide. After 30 min, brains were collected and assayed for anandamide. Significantly elevated levels of anandamide were found after 20 mg kg−1 OL135, but not at lower doses. n=6 per group.
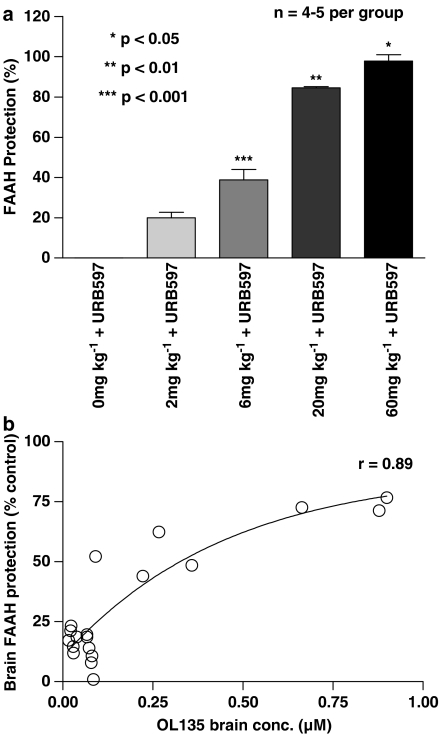
Second, we devised an assay in which we measured protection of enzyme active sites against inactivation by an irreversible FAAH inhibitor (URB597) by prior occupation with OL135. The probe compound URB597 is a rapidly binding irreversible brain penetrant FAAH inhibitor with an IC50 of 4.6 nM (Mor et al., 2004; Fegley et al., 2005). This compound was administered by intracardiac injection to ensure rapid access to the brain via blood circulation. Preliminary experiments were performed to select the lowest dose and time of exposure to this compound that resulted in the highest achievable levels of FAAH inactivation in the subsequent in vitro enzyme assay. These experiments indicated that a dose of 0.3 mg kg−1 of URB597 given by intracardiac injection 30 s before rapid perfusion with PBS was sufficient to give the maximum level of inhibition that were obtainable (which was approximately 80% of uninhibited control levels). We then used this as the standard condition for administering URB597 in subsequent experiments in which rats were initially given varying doses of OL135 i.p. 30 min before application of the probe compound.
Significant levels of enzyme protection were given by 2 mg kg−1 OL135, and there appeared to be a dose-dependent response to increasing levels of OL135 up to 60 mg kg−1 (Figure 3). OL135 dosed at 20 mg kg−1, which was used as the standard in vivo dose for the efficacy studies in rats described below, gave approximately 80% protection of the control activities found in untreated brains (Figure 3). Our third approach was to measure directly the concentrations of OL135 within the brain after dosing with OL135. The data shown in Figure 3b show the concentrations of OL135 determined within the same brains as those used for the enzyme protection experiments. It can be seen that an i.p. dose of 60 mg kg−1 OL135 resulted in concentrations of just under 1 μM in the brain about 30 min after dosing. As expected, the brain concentration varied according to dose, and was well correlated (r=0.89) with the level of enzyme protection observed. Together with the direct determination of OL135 levels in the CNS after dosing, the anandamide and FAAH enzyme protection studies established that OL135 is indeed able to access the CNS and inhibit FAAH. When sufficiently high levels of FAAH inhibition are achieved, this is then seen as an increase in anandamide concentration within the tissue.
Figure 3.
OL135 protects FAAH enzyme activity against an irreversible probe inhibitor in vivo. (a) Rats were injected i.p. with vehicle or OL135 at varying doses. After 30 min, they were deeply anaesthetised and received an intracardiac injection of URB597, an irreversible probe compound (1 mg kg−1) or its vehicle. Excess probe was washed out by a rapid (30 s) intracardiac infusion of 60 ml cold PBS. Brains were removed and assayed for FAAH activity. FAAH activities of control rats were expressed as 100% and those treated with probe alone as 0%. One-way ANOVA with Neumann–Keul multiple comparisons (n=4–5 per group). *P<0.05 vs 0–20 mg kg−1 OL135+URB597; **P<0.01 vs 60 and 0–6 mg kg−1 OL135+URB597; ***P<0.001 vs 0, 2, 20 and 60 mg kg−1 OL135+URB597. (b) Values for OL135 concentrations determined in the same brain samples as used for the FAAH protection assay shown in (a) were correlated with the level of FAAH protection observed (r=0.89 using Prism curve fitting software).
OL135 mediates analgesia by FAAH inhibition alone
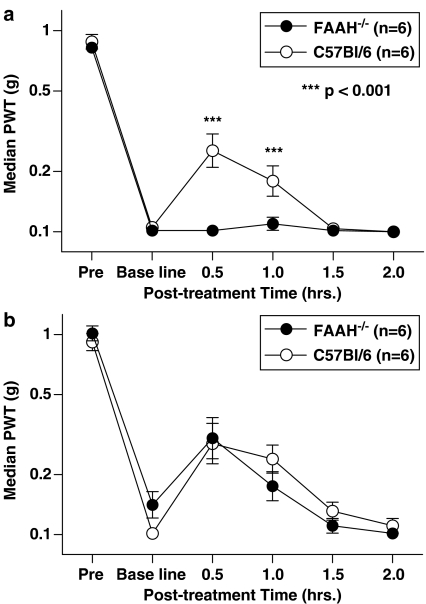
In wild-type C57/Bl6 mice, OL135 given at 100 mg kg−1 i.p. produced a significant reversal of the mechanical allodynia resulting from mild thermal injury (Figure 4a). The effect was maximal at 30 min after dosing. In contrast, the compound at this dose was completely ineffective when given to congenic FAAH−/− mice (Figure 4a). These FAAH−/− mice did not differ significantly at any time point from C57Bl/6 mice in their response to a reference analgesic, ibuprofen, indicating that they are not generally unresponsive to analgesics (Figure 4b). These findings indicate that the analgesic effect of OL135 is indeed mediated exclusively by inhibition of FAAH.
Figure 4.
OL135 analgesia is dependent upon the presence of FAAH. (a) FAAH knockout mice or C57Bl/6 wild-type mice were subjected to a mild thermal injury. When mechanical allodynia was fully established, OL135 (100 mg kg−1 ip) was administered and mechanical allodynia was assessed at various time points, by measuring the median paw withdrawal threshold (PWT) in grams (g) using calibrated von Frey filaments. In the wild-type mice, OL135 produced a significant reversal of mechanical allodynia at 30 min post dosing, but the effect was absent in the knockout mice (two-way ANOVA with Bonferroni's post-tests, ***P<0.001, df=1, F=27.25). (b) FAAH knockout mice were similarly responsive to 100 mg kg−1 i.p. of ibuprofen, a reference analgesic not dependent upon FAAH.
FAAH inhibition evokes analgesia through different mechanisms in two different pain models
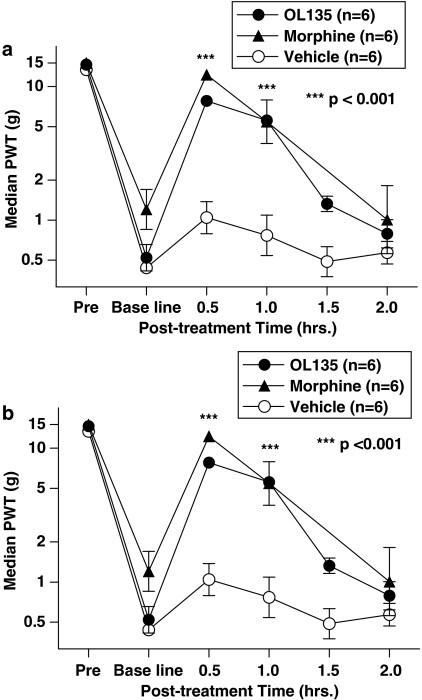
Rats were subjected to mild thermal injury and given 20 mg kg−1 i.p. OL135, a dose at which about 80% of the maximal achievable levels of brain FAAH inhibition is observed, and at which significant protection of exogenously dosed anandamide is seen (Figures 2 and 3). OL135 substantially reversed the mechanical hyperalgesia to a degree comparable with that seen with 1 mg kg−1 morphine (∼8 g von Frey threshold (OL135) vs 10 g (morphine) at 30 min; Figure 5a. The maximum effect was seen at 30 min post dosing, and was still significantly different from controls at 60 min. In the SNL model of neuropathic pain, animals with a well-developed mechanical allodynia also responded to 20 mg kg−1 OL135, with a similar maximum efficacy at 30 min, and a significant difference from controls at 60 min. The efficacy of OL135 at 30 min (∼8 g von Frey threshold) was close to that given by 3 mg kg−1 morphine (∼10 g von Frey threshold) and the duration of action of both compounds was similar (Figure 5b). This level and duration of efficacy was similar to that which we observed in this model using 500 mg kg−1 p.o. gabapentin (data not shown), which is a widely prescribed analgesic with some efficacy in human neuropathic pain (Wiffen et al., 2005).
Figure 5.
OL135 reverses mechanical allodynia in two rat models of pain. (a) Rats were subjected to a mild thermal injury as described, and allodynia was measured using von Frey hairs. OL135 treatment (20 mg kg−1 i.p.) resulted in a reduction of the allodynia that is comparable to 1 mg kg−1 morphine which was maximal at 30 min post treatment and still significant at 60 min post treatment compared with the vehicle (two-way ANOVA with Bonferroni's post-tests, ***P<0.001, df=2, F=30.97). (b) Rats with a fully developed SNL neuropathic lesion were dosed with either morphine (3 mg kg−1 i.p.) or OL135 (20 mg kg−1 i.p.). OL135 reversed the mechanical allodynia with an efficacy similar to that of morphine, and maximal effect at 30 min, which was still significant at 60 min compared to vehicle (two-way ANOVA with Bonferroni's post-tests, *** P<0.001, df=2, F=31.04).
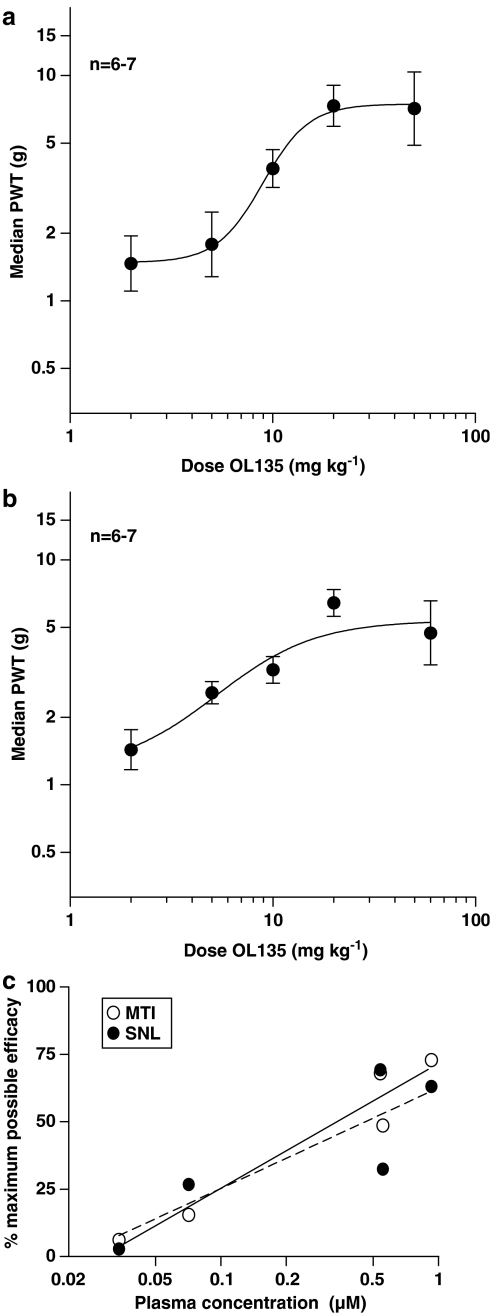
In both models, the behavioral response was dose related when von Frey thresholds were plotted against dose (ED50=8.9 mg kg−1 for MTI and 5.3 mg kg−1 for SNL; Figure 6a and b). The plasma concentration (Cp) of OL135 was determined in the same experiments, and plotting the Cp vs the maximum possible efficacy (MPE) yielded essentially overlapping plots for the two models, with a Cp at the ED50 of 0.7 μM (240 ng ml−1; Figure 6c).
Figure 6.
OL135 reverses mechanical allodynia in a dose-dependent manner. (a) Rats subjected to MTI were given 2, 5, 10, 20, and 50 mg kg−1 OL135 and their allodynic thresholds at 30 min were analysed by nonlinear regression. (b) Rats subjected to SNL were dosed with 2, 5, 10, 20, and 50 mg kg−1 OL135 and their allodynic thresholds at 30 min were analysed by nonlinear regression. (c) The plasma concentration of OL135 at 30 min achieved by doses of 2, 5, 10, 20, and 50 mg kg−1 were determined in cohort rats and correlated with efficacy in the MTI and SNL models. For comparison between the two models, the median PWTs were normalised to generate a percent maximum possible efficacy (MPE) value using the following transformation: %MPE=(PWT(t)−PWT(baseline))/[PWT(pre)−PWT(baseline)), where PWT(t)=PWT at time t post-treatment, PWT(baseline)=PWT at baseline, and PWT(pre)=PWT of naïve rats prior to initiation of pain model; r=0.97 for MTI and 0.86 for SNL.
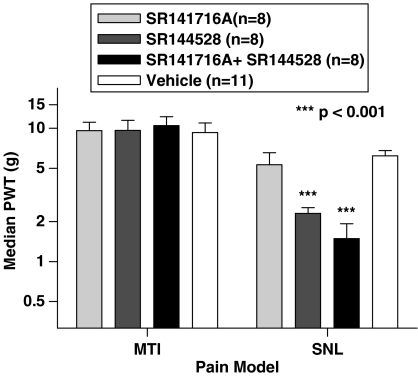
We then sought to investigate the involvement of CB1 and CB2 receptors in the mediation of these analgesic effects by attempting to block the analgesia by the use of selective receptor antagonists. SR141716A, a potent and selective CB1 receptor antagonist, applied at 5 mg kg−1 i.p. 30 min before OL135, failed to attenuate the analgesic effect of FAAH inhibition in either model (Figure 7). This dose of SR141716A was shown previously by us to reverse analgesia produced in the MTI model by the nonspecific CB1/2 agonist WIN55,212-2 (not shown). In contrast, the selective CB2 receptor antagonist SR144528 (5 mg kg−1), while failing to affect the analgesia produced by OL135 in the MTI model, almost completely reversed it in the SNL model (Figure 7). It is possible that a joint activation of both of the well-defined cannabinoid receptors might occur in the MTI model, and that inhibition of either of these alone would fail to reverse the analgesia because of compensation through the alternate receptor. We tested this possibility by giving both antagonists simultaneously to rats which had also been dosed with 20 mg kg−1 of OL135. In the SNL model, the virtually complete reversal of OL135-mediated analgesia by the CB2 antagonist was not affected by the additional inhibition of the CB1 receptor. The simultaneous inhibition of both CB1 and CB2 receptors in the MTI model failed to reverse OL135-mediated analgesia (Figure 7).
Figure 7.
FAAH inhibition produces analgesia by different mechanisms in different pain models. Rats were subjected either to a mild thermal injury or an SNL neuropathic lesion. When the tactile allodynia was fully developed, they were dosed with OL135 (20 mg kg−1) and either SR141716A (CB1 antagonist), SR144528 (CB2 antagonist), a combination of SR141716A and SR144528, or a vehicle. Neither antagonist nor a combination thereof was able to reverse significantly the antiallodynic effect of OL135 in the MTI model. The CB2 antagonist, but not the CB1 antagonist, almost completely reversed the effect of OL135 in the neuropathic rats (one-way ANOVA with Bonferroni's post-tests, ***P<0.001, df=3, F=17.31).
Involvement of opioid mechanisms
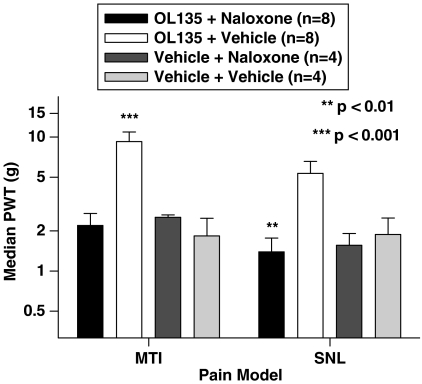
We investigated the possible involvement of opioid mechanisms in mediating the analgesia in the two models by attempting to reverse it using the nonselective opioid antagonist naloxone. In control experiments, 3 mg kg−1 morphine i.p. completely reversed the tactile allodynia produced by MTI, and this effect was itself completely reversed by a subsequent dose of naloxone (1 mg kg−1; data not shown). In both the MTI and the SNL models, this dose of naloxone completely reversed the analgesia mediated by OL135 (Figure 8), indicating that in both models, an opioid mechanism is evoked by FAAH inhibition.
Figure 8.
FAAH inhibition produces analgesia by an opioid-dependent mechanism in both MTI and spinal nerve ligation models. Rats were subjected to a mild thermal injury or spinal nerve ligation. When the tactile allodynia was fully developed, they were given OL135 (20 mg kg−1) and after 10 min, either saline or naloxone (1 mg kg−1). Allodynia was measured using von Frey hairs 30 min after administration of naloxone. Naloxone completely abolished the antiallodynic effect of OL135 in the MTI (one-way ANOVA with Bonferroni's post-tests, ***P<0.001, df=3, F=14.87) and in the SNL models (one-way ANOVA with Bonferroni's post-tests, **P<0.01, df=3, F=7.831). For comparative purposes, administration of either naloxone or vehicle in the absence of OL135 did not produce elevated tactile thresholds.
Discussion
Anandamide and other endogenous fatty acid amides such as PEA have been shown to have analgesic properties in several different animal models of pain. Since hydrolysis by FAAH is the major mechanism terminating the actions of anandamide, and since it can also hydrolyse PEA, inhibition of the enzyme would be expected to enhance the analgesic properties of such endogenous molecules, as they would accumulate to higher levels in the absence of hydrolysis. This was confirmed in the FAAH knockout mice produced by Cravatt et al. (2001). These mice have enhanced levels of anandamide and exhibit a hypoalgesic phenotype in several pain models, including the formalin test, carrageenan thermal hyperalgesia, and thermal nociception measured by hot plate and tail immersion assays. A reversible inhibitor of FAAH, OL135, has also been reported to raise the levels of CNS anandamide and produce analgesia in several rodent pain models (Lichtman et al., 2004a).
We confirmed by three different means that OL135 was able to access the CNS and elevate levels of brain anandamide. In the first approach, we observed that 20 mg kg−1 OL135 (i.p.) produced a slight but significant elevation in the concentration of anandamide in both the brain and spinal cord when this was assessed 40 min later (Figure 1). Larger signals were obtained when systemic anandamide (20 mg kg−1 i.p.) was given in conjunction with OL135 (Figure 2). These experiments thus confirmed that enzyme inhibition protected the exogenously applied substrate within the CNS. Second, by analogy with in vivo receptor-binding radioactive assays, we reasoned that occupation of enzyme inhibitor binding sites by a reversible inhibitor under equilibrium conditions would protect the occupied sites against their irreversible binding (with consequent permanent inhibition of activity) to a subsequently applied irreversible inhibitor. Using the protocol described in the Methods section, we achieved a dose-responsive protection of enzyme activity in a dose range between 2 and 60 mg kg−1 (Figure 3). These data indicated that the compound was not only accessing the CNS but was also active at the molecular target. Within this series of experiments, we also determined directly the concentration of OL135 within the brain after different doses, and this constitutes our third type of evidence for the central activity of the compound. There was a good correlation (r=0.89) between brain concentration of OL135 and the levels of inhibition achieved (Figure 3b). It is noteworthy that we observed significant levels of FAAH protection in the enzyme protection assay at lower doses of OL135 (2 and 6 mg kg−1; Figure 3) which were not reflected in a comparable protection of anandamide (Figure 2). Only when the dose reached 20 mg kg−1 was significant anandamide protection observed, a dose at which 80% of the maximum possible enzyme protection was achieved, indicating that 80% of the enzyme-binding sites were occupied by OL135 during the in vivo exposure. It therefore appears that under normal conditions, there is an excess of FAAH activity available, and inhibition at levels lower than 80% leaves sufficient residual activity to degrade fully the added anandamide.
We used FAAH knockout mice to confirm that the effects of OL135 are indeed dependent upon the expression of FAAH, which is thus identified as the authentic molecular target. In FAAH−/− mice, the compound was without effect at 100 mg kg−1 i.p., a dose which produced a significant level of analgesia in normal congenic mice (Figure 4a). The knockout mice did display analgesia in the same assay in response to ibuprofen, a reference analgesic working through an unrelated mechanism (Figure 4b). The relatively high dose of OL135 used in mice requires comment, especially in view of the fact that OL135 has been reported to be active at doses of 10 mg kg−1 in hot plate and formalin assays (Lichtman et al., 2004a). The mouse MTI model, as presently developed, appears to be a particularly stringent test that, in general, requires higher doses of most classes of compound for significant antiallodynic activity when compared with other models. In addition to this general comment, it is also possible that the differences in the doses required may reflect the different mechanisms in play in these acute nociceptive tests when compared with the mechanical allodynia measured in our studies. This probably includes a differential contribution of C and Aβ fibers in the two types of models. It is well established that C fibres mediate thermal nociception, and it has also been shown that C fibre activity is likely to be a more significant component of the second phase of the formalin assay than Aβ fibre activity (Puig and Sorkin, 1996), whereas Aβ fibres make a more prominent contribution than C fibres to the tactile sensation involved in mechanical allodynia (Ossipov et al., 1999).
In the rat, OL135 produced a substantial degree of analgesia in both the mild thermal injury and the SNL models. At 20 mg kg−1 i.p., the reversal of mechanical allodynia was maximal at 30 min after dosing, and remained significant at 60 min after dosing (Figure 5). At this dose, central FAAH was inhibited by about 80% as measured by the enzyme protection assay. In both behavioural models, the effect was dose responsive with an ED50 in the range of 5–9 mg kg−1 (Figure 6a and b). These data (Figure 6) indicate that in both models the 20 mg kg−1dose shown in the time course experiments in Figure 5, and used as a standard for the mechanistic experiments with cannabinoid receptor antagonists and naloxone, achieves close to the maximum efficacy possible, and increasing the dose to 60 mg kg−1 did not produce a commensurate increase in efficacy.
When plotted against the plasma concentrations, the efficacy data for both models was identical, and suggested that at the ED50, the plasma concentration was 0.7 μM, or 240 ng ml−1 (Figure 6c). The requirement to reach such high plasma concentrations in order to achieve efficacy explains the relatively high doses of OL135 required. The apparent discrepancy between this plasma concentration and the IC50 of the compound at the rat receptor (2.1 nM; Lichtman et al., 2004a) is readily explained by the very high plasma binding exhibited by OL135 (>99% in human, rat, and monkey: our unpublished observations).
Anandamide is a weak partial agonist at CB1 receptors. These receptors are expressed in the nociceptive processing pathway, including sensory neurons, spinal cord, thalamus, and periaqueductal gray. Activation of CB1 can mediate profound antinociceptive and antihyperalgesic effects, as has been established by a substantial body of literature (Edsall et al., 1996; Richardson et al., 1998; Martin et al., 1999; Drew et al., 2000; Monhemius et al., 2001; Kelly & Chapman, 2001). Cravatt et al. (2001) provided direct evidence for the involvement of the CB1 receptor in mediating the analgesic effects of genetic ablation of FAAH in the tail immersion assay of thermal nociception. This was confirmed by Lichtman et al. (2004a), who also showed that CB1 receptor mediated the effects of OL135-mediated FAAH inhibition in the formalin test. In contrast to these data, we found that the CB1 receptor was not involved in mediating the analgesia exhibited by OL135 treatment in either the MTI or the SNL assays of mechanical allodynia. This is discussed further below, but we note here that since different mechanisms downstream of FAAH block (by either genetic or pharmacological means) are involved in these different behavioural models, we would not necessarily expect that they would display the same level of sensitivity to the FAAH blockade.
CB2, the second well-characterised cannabinoid receptor, has also recently been shown to have a potential role in mediating the analgesic effects of endocannabinoids (Malan et al., 2001; 2002). The CB2 receptor is up regulated in the lumbar spinal cord of rats with experimental neuropathic pain, but not inflammatory pain states (Zhang et al., 2003), and several recent papers have focused on the role of CB2 receptors in neuropathic pain states (Ibrahim et al., 2003; Scott et al., 2004; Ibrahim et al., 2005). Thus, AM 1241 was used to demonstrate that CB2 activation inhibits tactile and thermal hyperalgesia in the L5/L6 SNL model in rats and mice (Ibrahim et al., 2003). The compound was equally effective when applied to L5/L6 nerve-ligated mice lacking the CB1 receptor, indicating that this receptor is not necessary for the manifestation of the AM 1241-induced antihyperalgesia. These authors recently proposed that the relevant receptors are expressed on non-neuronal peripheral cells, and that their activation caused release of opioid peptides which mediate the analgesia through mu opioid receptors expressed on the sensory neuron (Ibrahim et al., 2005).
The CB2 receptor has also been implicated as a mediator of analgesia in non-neuropathic models. Hohmann et al. (2004) showed that selective activation of CB2 receptors by the systemic administration of the selective agonist AM 1241 attenuated the thermal and mechanical hyperalgesia evoked by intradermal capsaicin. Intraplantar injections into the capsaicin-treated paw were also effective, implying a peripheral locus for the CB2 receptor target in this case. Lichtman et al. (2004b) presented evidence suggesting a role for CB2 receptors in mediating the analgesia in the carrageenan model in FAAH−/− mice. These data were supported by the effect of pharmacological inhibition of FAAH using the irreversible selective inhibitor URB597 in the carrageenan paw model in rats (Holt et al., 2005).
In our hands, CB2 receptor blockade reversed the analgesia produced by OL135 in the SNL model of neuropathic pain (Figure 7), implying that the effects of endocannabinoids are mediated at least in part through this receptor in this model. In contrast, CB1 receptor block was without effect on OL135-mediated analgesia in the SNL model. These data are clearly congruent with the published studies discussed above which implicate the CB2 receptor as a significant component in the neuropathic models. The involvement of an opioid mechanism evoked by CB2 activation, as suggested by Ibrahim et al. (2005), is supported by the complete reversal of the OL135-mediated analgesia by naloxone (Figure 8).
Neither CB1 nor CB2 receptor antagonists were able to reverse the analgesic effects of OL135 in the rat MTI model. However, the analgesia produced by FAAH inhibition was once again fully reversed by naloxone (Figure 8). Thus, although neither CB1 nor CB2 receptors appear to be responsible for mediating OL135-induced analgesia in this model, an opioid mechanism does appear to be involved. It is unlikely that a direct interaction with opioid receptors by the compound itself is responsible for eliciting the opioid component of the mechanism, since OL135 is devoid of activity at mu and delta receptors, and at high concentrations, it acts as a weak inhibitor of the kappa opioid receptor (35% inhibition at 10 μM; our unpublished data).
Since neither a CB2 receptor antagonist, nor a combination of CB1 and CB2 antagonists could reverse OL135-mediated analgesia in the MTI model, it would appear that in this model, either anandamide or another fatty acid amide is acting through a non-CB receptor mechanism which nevertheless involves an opioid component.
Anandamide is a potent ligand at the TRPV1 ion channel, and has some activity, either direct or indirect, at other molecular targets potentially relevant to nociceptive transmission and its modulation, including 5-HT receptors, voltage-gated calcium channels, and the NMDA receptor (Mackie et al., 1993; Fan, 1995; Twitchell et al., 1997; Hampson et al., 1998; Gebremedhin et al., 1999; Barann et al., 2002; Di Marzo et al., 2002; Roberts et al., 2002; Guo & Ikeda, 2004). It is also possible that the relevant fatty acid amide is not anandamide. PEA is one likely candidate, based on its documented antihyperalgesic and anti-inflammatory actions (Calignano et al., 1998; Jaggar et al., 1998; Lambert et al., 2002). The molecular target of PEA was undefined until recently, when it was shown to mediate its anti-inflammatory actions through activation of peroxisome proliferator activator receptor (PPAR) alpha (Verme et al., 2005). However, a direct modulation of nociceptive signalling is not one of the known functions of PPAR receptors.
In the studies of Calignano et al. (1998, 2001), the antinociceptive activity of PEA was shown to be unaffected by blockade of CB1 receptors, but attenuated by a selective CB2 antagonist (Calignano et al., 1998; 2001). Since PEA does not interact with high affinity at CB2 receptors in vitro, this has given rise to the suggestion that either PEA or a metabolite may act to potentiate the activity of other ligands at CB2 receptors, the so-called ‘entourage effect'. However, this cannot be identical with the mechanism evoked in our MTI studies, which involved neither CB1 nor CB2 receptors.
The situation is thus complex, since FAAH inhibition is likely to increase the levels of at least two relevant mediators PEA and anandamide (Calignano et al., 1998) and several potential downstream targets of these mediators may be involved in manifesting the resulting analgesia. Furthermore, the primary mechanism may differ between different pain models, and in any situation where multiple potential mechanisms exist, they may interact synergistically, additively, or epistatically. In spite of this complexity, the ability to evoke different mechanisms efficacious in different pain syndromes, in addition to the possibility of achieving a synergistic analgesic effect by elevating levels of two agonists, is one of the attractions of FAAH as a target in pain therapeutics.
Acknowledgments
We thank Dr Nigel Shankley for helpful advice and encouragement during development of the FAAH protection assay, Dr Dan Pippel for supply of OL135, Jimmy Liang for the synthesis of SR144528, and Dr Richard Apodaca (all of Johnson & Johnson Pharmaceutical Research & Development, L.L.C) for synthesis of the deuterated standard used in the LCMS experiments. We gratefully acknowledge Kenway Hoey and Anita Everson for their assistance with mass spectrometry, Philip Timmerman for helpful discussions and Michele Rizzolio for help with formulating compounds.
Abbreviations
- DMSO
dimethyl sulphoxide
- EDTA
ethylene diamine tetra-acetic acid
- FAAH
fatty acid amide hydrolase
- 5-HT
5-hydroxytryptamine
- i.p.
intraperitoneal
- MPE
maximum possible efficacy
- MTI
mild thermal injury
- NMDA
N-methyl D-aspartate
- PBS
phosphate-buffered saline
- SNL
spinal nerve ligation
- PWT
paw withdrawal threshold
- TRPV1
transient receptor potential vanilloid 1
References
- AHLUWALIA J., URBAN L., BEVAN S., NAGY I. Anandamide regulates neuropeptide release from capsaicin-sensitive primary sensory neurons by activating both the cannabinoid 1 receptor and the vanilloid receptor 1 in vitro. Eur. J. Neurosci. 2003;17:2611–2618. doi: 10.1046/j.1460-9568.2003.02703.x. [DOI] [PubMed] [Google Scholar]
- AHLUWALIA J., URBAN L., CAPOGNA M., BEVAN S., NAGY I. Cannabinoid 1 receptors are expressed in nociceptive primary sensory neurons. Neuroscience. 2000;100:685–688. doi: 10.1016/s0306-4522(00)00389-4. [DOI] [PubMed] [Google Scholar]
- BARANN M., MOLDERINGS G., BRUSS M., BONISCH H., URBAN B.W., GOTHERT M. Direct inhibition by cannabinoids of human 5-HT3A receptors: probable involvement of an allosteric modulatory site. Br. J. Pharmacol. 2002;137:589–596. doi: 10.1038/sj.bjp.0704829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BISOGNO T., DE PETROCELLIS L., DI MARZO V. Fatty acid amide hydrolase, an enzyme with many bioactive substrates. Possible therapeutic implications. Curr. Pharm. Des. 2002;8:533–547. doi: 10.2174/1381612023395655. [DOI] [PubMed] [Google Scholar]
- CADAS H., GAILLET S., BELTRAMO M., VENANCE L., PIOMELLI D. Biosynthesis of an endogenous cannabinoid precursor in neurons and its control by calcium and cAMP. J. Neurosci. 1996;16:3934–3942. doi: 10.1523/JNEUROSCI.16-12-03934.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CALIGNANO A., LA RANA G., GIUFFRIDA A., PIOMELLI D. Control of pain initiation by endogenous cannabinoids. Nature. 1998;394:277–281. doi: 10.1038/28393. [DOI] [PubMed] [Google Scholar]
- CALIGNANO A., LA RANA G., PIOMELLI D. Antinociceptive activity of the endogenous fatty acid amide, palmitylethanolamide. Eur. J. Pharmacol. 2001;419:191–198. doi: 10.1016/s0014-2999(01)00988-8. [DOI] [PubMed] [Google Scholar]
- CALIXTO J.B., BEIRITH A., FERREIRA J., SANTOS A.R., FILHO V.C., YUNES R.A. Naturally occurring antinociceptive substances from plants. Phytother. Res. Sep. 2000;14:401–418. doi: 10.1002/1099-1573(200009)14:6<401::aid-ptr762>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- CARLINI E.A. The good and the bad effects of (−) trans-delta-9-tetrahydrocannbinol (Delta 9 THC) on humans. Toxicon. 2004;44:461–467. doi: 10.1016/j.toxicon.2004.05.009. [DOI] [PubMed] [Google Scholar]
- CHAPLAN S.R., BACH F.W., POGREL JW SNL J.M., YAKSH T.L. Quantitative assessment of tactile allodynia in the rat paw. J. Neurosci. Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- CRAVATT B.F., DEMAREST K., PATRICELLI M.P., BRACEY M.H., GIANG D.K., MARTIN B.R., LICHTMAN A.H. Supersensitivity to anandamide and enhanced endogenous cannabinoid signaling in mice lacking fatty acid amide hydrolase. Proc. Natl. Acad. Sci. U.S.A. 2001;98:9371–9376. doi: 10.1073/pnas.161191698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEVANE W.A., HANUS L., BREUER A., PERTWEE R.G., STEVENSON L.A., GRIFFIN G., GIBSON D., MANDELBAUM A., ETINGER A., MECHOULAM R. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258:1946–1949. doi: 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- DI MARZO V., DE PETROCELLIS L., FEZZA F., LIGRESTI A., BISOGNO T. Anandamide receptors. Prostaglandins Leukot. Essent. Fatty Acids. 2002;66:377–391. doi: 10.1054/plef.2001.0349. [DOI] [PubMed] [Google Scholar]
- DI MARZO V., FONTANA A., CADAS H., SCHINELLI S., CIMINO G., SCHWARTZ J.C., PIOMELLI D. Formation and inactivation of endogenous cannabinoid anandamide in central neurons. Nature. 1994;372:686–691. doi: 10.1038/372686a0. [DOI] [PubMed] [Google Scholar]
- DREW L.J., HARRIS J., MILLNS P.J., KENDALL D.A., CHAPMAN V. Activation of spinal cannabinoid 1 receptors inhibits C-fibre driven hyperexcitable neuronal responses and increases [35S]GTPgammaS binding in the dorsal horn of the spinal cord of noninflamed and inflamed rats. Eur. J. Neurosci. 2000;12:2079–2086. doi: 10.1046/j.1460-9568.2000.00101.x. [DOI] [PubMed] [Google Scholar]
- EDSALL S.A., KNAPP R.J., VANDERAH T.W., ROESKE W.R., CONSROE P., YAMAMURA H.I. Antisense oligodeoxynucleotide treatment to the brain cannabinoid receptor inhibits antinociception. NeuroReport. 1996;7:593–596. doi: 10.1097/00001756-199601310-00052. [DOI] [PubMed] [Google Scholar]
- ELPHICK M., EGERTOVA M. The neurobiology and evolution of cannabinoid signaling. Phil. Trans. R. Soc. London B. 2001;356:381–408. doi: 10.1098/rstb.2000.0787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAN P. Cannabinoid agonists inhibit the activation of 5-HT3 receptors in rat nodose ganglion neurons. J. Neurophysiol. 1995;73:907–910. doi: 10.1152/jn.1995.73.2.907. [DOI] [PubMed] [Google Scholar]
- FEGLEY D., GAETANI S., DURANTI A., TONTINI A., MOR M., TARZIA G., PIOMELLI D. Characterization of the fatty acid amide hydrolase inhibitor cyclohexyl carbamic acid 3′-carbamoyl-biphenyl-3-yl ester (URB597): effects on anandamide and oleoylethanolamide deactivation. J. Pharmacol. Exp. Ther. 2005;313:352–358. doi: 10.1124/jpet.104.078980. [DOI] [PubMed] [Google Scholar]
- FRANKLIN A., STELLA N. Arachidonylcyclopropylamide increases microglial cell migration through cannabinoid CB2 and abnormal-cannabidiol-sensitive receptors. Eur. J. Pharmacol. 2003;474:195–198. doi: 10.1016/s0014-2999(03)02074-0. [DOI] [PubMed] [Google Scholar]
- GARDNER E.L. Endocannabinoid signaling system and brain reward: emphasis on dopamine. Pharmacol. Biochem. Behav. 2005;81:263–284. doi: 10.1016/j.pbb.2005.01.032. [DOI] [PubMed] [Google Scholar]
- GEBREMEDHIN D., LANGE A.R., CAMPBELL W.B., HILLARD C.J., HARDER D.R. Cannabinoid CB1 receptor of cat cerebral arterial muscle functions to inhibit L-type Ca2+ channel current. Am. J. Physiol. 1999;276:2085–2093. doi: 10.1152/ajpheart.1999.276.6.H2085. [DOI] [PubMed] [Google Scholar]
- GIANG D.K., CRAVATT B.F. Molecular characterization of human and mouse fatty acid amide hydrolases. Proc. Natl. Acad. Sci. U.S.A. 1997;94:2238–2242. doi: 10.1073/pnas.94.6.2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOURLAY D. Addiction and pain medicine. Pain Res. Manag. 2005;10 Suppl A:38A–43A. doi: 10.1155/2005/512653. [DOI] [PubMed] [Google Scholar]
- GUO J., IKEDA S.R. Endocannabinoids modulate N-type calcium channels and G-protein-coupled inwardly rectifying potassium channels via CB1 cannabinoid receptors heterologously expressed in mammalian neurons. Mol. Pharmacol. 2004;65:665–674. doi: 10.1124/mol.65.3.665. [DOI] [PubMed] [Google Scholar]
- HAMPSON A.J., BORNHEIM L.M., SCANZIANI M., YOST C.S., GRAY A.T., HANSEN B.M., LEONOUDAKIS D.J., BICKLER P.E. Dual effects of anandamide on NMDA receptor-mediated responses and neurotransmission. J. Neurochem. 1998;70:671–676. doi: 10.1046/j.1471-4159.1998.70020671.x. [DOI] [PubMed] [Google Scholar]
- HOHMANN A.G., FARTHING J.N., ZVONOK A.M., MAKRIYANNIS A. Selective activation of cannabinoid CB2 receptors suppresses hyperalgesia evoked by intradermal capsaicin. J. Pharmacol. Exp. Ther. 2004;308:446–453. doi: 10.1124/jpet.103.060079. [DOI] [PubMed] [Google Scholar]
- HOLT S., COMELLI F., COSTA B., FOWLER C. Inhibitors of fatty acid amide hydrolase reduce carrageenan-induced hind paw inflammation in pentobarbital-treated mice: comparison with indomethacin and possible involvement of cannabinoid receptors. Br. J. Pharmacol. 2005;146:467–476. doi: 10.1038/sj.bjp.0706348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUA X.Y., SVENSSON C., MATSUI T., FITZSIMMONS B., YAKSH T.L., WEBB M. Intrathecal minocycline attenuates peripheral inflammation-induced hyperalgesia by inhibiting p38 MAPK in spinal microglia. Eur. J. Neurosci. 2005;22:2431–2440. doi: 10.1111/j.1460-9568.2005.04451.x. [DOI] [PubMed] [Google Scholar]
- IBRAHIM M.M., DENG H., ZVONOK A., COCKAYNE D.A., KWAN J., MATA H.P., VANDERAH T.W., LAI J., PORRECA F., MAKRIYANNIS A., MALAN T.P., Jr Activation of CB2 cannabinoid receptors by AM1241 inhibits experimental neuropathic pain: pain inhibition by receptors not present in the CNS. Proc. Natl. Acad. Sci. U.S.A. 2003;100:10529–10533. doi: 10.1073/pnas.1834309100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IBRAHIM M.M., PORRECA F., LAI J., ALBRECHT P.J., RICE F.L., KHODOROVA A., DAVAR G., MAKRIYANNIS A., VANDERAH T.W., MATA H.P., MALAN T.P., Jr CB2 cannabinoid receptor activation produces antinociception by stimulating peripheral release of endogenous opioids. Proc. Natl. Acad. Sci. U.S.A. 2005;102:3093–3098. doi: 10.1073/pnas.0409888102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JAGGAR S.I., HASNIE F.S., SELLATURAY S., RICE A.S. The anti-hyperalgesic actions of the cannabinoid anandamide and the putative CB2 receptor agonist palmitoylethanolamide in visceral and somatic inflammatory pain. Pain. 1998;76:189–199. doi: 10.1016/s0304-3959(98)00041-4. [DOI] [PubMed] [Google Scholar]
- KELLY S., CHAPMAN V. Selective cannabinoid CB1 receptor activation inhibits spinal nociceptive transmission in vivo. J. Neurophysiol. 2001;86:3061–3064. doi: 10.1152/jn.2001.86.6.3061. [DOI] [PubMed] [Google Scholar]
- KIM S.H., CHUNG J.M. An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. Pain. 1992;50:355–363. doi: 10.1016/0304-3959(92)90041-9. [DOI] [PubMed] [Google Scholar]
- LAMBERT D.M., VANDEVOORDE S., JONSSON K.O., FOWLER C.J. The palmitoylethanolamide family: a new class of anti-inflammatory agents. Curr. Med. Chem. 2002;9:663–674. doi: 10.2174/0929867023370707. [DOI] [PubMed] [Google Scholar]
- LEE E.J., SIM J.Y., PARK J.Y., HWANG J.H., PARK P.H., HAN S.M. Intrathecal carbachol and clonidine produce a synergistic antiallodynic effect in rats with a nerve ligation injury. Can. J. Anaesth. 2002;49:178–184. doi: 10.1007/BF03020492. [DOI] [PubMed] [Google Scholar]
- LEUNG D., DU W., HARDOUIN C., CHENG H., HWANG I., CRAVATT B.F., BOGER D.L. Discovery of an exceptionally potent and selective class of fatty acid amide hydrolase inhibitors enlisting proteome-wide selectivity screening: concurrent optimization of enzyme inhibitor potency and selectivity. Bioorg. Med. Chem. Lett. 2005;15:1423–1428. doi: 10.1016/j.bmcl.2004.12.085. [DOI] [PubMed] [Google Scholar]
- LICHTMAN A.H., LEUNG D., SHELTON C.C., SAGHATELIAN A., HARDOUIN C., BOGER D.L., CRAVATT B.F. Reversible inhibitors of fatty acid amide hydrolase that promote analgesia: evidence for an unprecedented combination of potency and selectivity. J. Pharmacol. Exp. Ther. 2004a;311:441–448. doi: 10.1124/jpet.104.069401. [DOI] [PubMed] [Google Scholar]
- LICHTMAN A.H., SHELTON C.C., ADVANI T., CRAVATT B.F. Mice lacking fatty acid amide hydrolase exhibit a cannabinoid receptor-mediated phenotypic hypoalgesia. Pain. 2004b;109:319–327. doi: 10.1016/j.pain.2004.01.022. [DOI] [PubMed] [Google Scholar]
- LU Q., STRAIKER A., LU Q., MAGUIRE G. Expression of CB2 cannabinoid receptor mRNA in adult rat retina. Vis. Neurosci. 2000;17:91–95. doi: 10.1017/s0952523800171093. [DOI] [PubMed] [Google Scholar]
- MACKIE K., DEVANE W.A., HILLE B. Anandamide, an endogenous cannabinoid, inhibits calcium currents as a partial agonist in N18 neuroblastoma cells. Mol. Pharmacol. 1993;44:498–503. [PubMed] [Google Scholar]
- MALAN T.P., Jr, IBRAHIM M.M., DENG H., LIU Q., MATA H.P., VANDERAH T., PORRECA F., MAKRIYANNIS A. CB2 cannabinoid receptor-mediated peripheral antinociception. Pain. 2001;93:239–245. doi: 10.1016/S0304-3959(01)00321-9. [DOI] [PubMed] [Google Scholar]
- MALAN T.P., Jr, IBRAHIM M.M., VANDERAH T.W., MAKRIYANNIS A., PORRECA F. Inhibition of pain responses by activation of CB(2) cannabinoid receptors. Chem Phys. Lipids. 2002;121:191–200. doi: 10.1016/s0009-3084(02)00155-x. [DOI] [PubMed] [Google Scholar]
- MARTIN W.J., LOO C.M., BASBAUM AI spinal cannabinoids are anti-allodynic in rats with persistent inflammation. Pain. 1999;82:199–205. doi: 10.1016/S0304-3959(99)00045-7. [DOI] [PubMed] [Google Scholar]
- MATSUDA L.A., LOLAIT S.J., BROWNSTEIN M.J., YOUNG A.C., BONNER T.I. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature. 1990;346:561–564. doi: 10.1038/346561a0. [DOI] [PubMed] [Google Scholar]
- MONHEMIUS R., AZAMI J., GREEN D.L., ROBERTS M.H. CB1 receptor mediated analgesia from the nucleus reticularis gigantocellularis pars alpha is activated in an animal model of neuropathic pain. Brain Res. 2001;908:67–74. doi: 10.1016/s0006-8993(01)02605-1. [DOI] [PubMed] [Google Scholar]
- MOR M., RIVARA S., LODOLA A., PLAZZI P.V., TARZIA G., DURANTI A., TONTINI A., PIERSANTI G., KATHURIA S., PIOMELLI D. Cyclohexylcarbamic acid 3'- or 4'-substituted biphenyl-3-yl esters as fatty acid amide hydrolase inhibitors: synthesis, quantitative structure–activity relationships and molecular modeling studies. J. Med. Chem. 2004;47:4998–5008. doi: 10.1021/jm031140x. [DOI] [PubMed] [Google Scholar]
- MUNRO S., THOMAS K.L., ABU-SHAAR M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993;365:61–65. doi: 10.1038/365061a0. [DOI] [PubMed] [Google Scholar]
- NOZAKI-TAGUCHI N., YAKSH T.L. A novel model of primary and secondary hyperalgesia after mild thermal injury in the rat. Neurosci. Lett. 1998;254:25–28. doi: 10.1016/s0304-3940(98)00648-x. [DOI] [PubMed] [Google Scholar]
- NUNEZ E., BENITO C., PAZOS M.R., BARBACHANO A., FAJARDO O., GONZALEZ S., TOLON R.M., ROMERO J.C. CB2 receptors are expressed by perivascular microglial cells in the human brain: an immunohistochemical study. Synapse. 2004;53:208–213. doi: 10.1002/syn.20050. [DOI] [PubMed] [Google Scholar]
- OSSIPOV M.H., BIAN D., MALAN T.P., Jr, LAI J., PORRECA F. Lack of involvement of capsaicin-sensitive primary afferents in nerve-ligation injury induced tactile allodynia in rats. Pain. 1999;79:127–133. doi: 10.1016/s0304-3959(98)00187-0. [DOI] [PubMed] [Google Scholar]
- PUIG S., SORKIN L. Formalin-evoked activity in identified primary afferent fibers: systemic lidocaine suppresses phase-2 activity. Pain. 1996;64:345–355. doi: 10.1016/0304-3959(95)00121-2. [DOI] [PubMed] [Google Scholar]
- RICHARDSON J.D., KILO S., HARGREAVES K.M. Cannabinoids reduce hyperalgesia and inflammation via interaction with peripheral CB1 receptors. Pain. 1998;75:111–119. doi: 10.1016/S0304-3959(97)00213-3. [DOI] [PubMed] [Google Scholar]
- ROBERTS L.A., CHRISTIE M.J., CONNOR M. Anandamide is a partial agonist at native vanilloid receptors in acutely isolated mouse trigeminal sensory neurons. Br. J. Pharmacol. 2002;137:421–428. doi: 10.1038/sj.bjp.0704904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCOTT D.A., WRIGHT C.E., ANGUS J.A. Evidence that CB-1 and CB-2 cannabinoid receptors mediate antinociception in neuropathic pain in the rat. Pain. 2004;109:124–131. doi: 10.1016/j.pain.2004.01.020. [DOI] [PubMed] [Google Scholar]
- SUGIURA T., KONDO S., SUKAGAWA A., TONEGAWA T., NAKANE S., YAMASHITA A., WAKU K. Enzymatic synthesis of anandamide, an endogenous cannabinoid receptor ligand, through N-acylphosphatidylethanolamine pathway in testis: involvement of Ca(2+)-dependent transacylase and phosphodiesterase activities. Biochem. Biophys. Res. Commun. 1996;218:113–117. doi: 10.1006/bbrc.1996.0020. [DOI] [PubMed] [Google Scholar]
- TSOU K., BROWN S., SANUDO-PENA M.C., MACKIE K., WALKER J.M. Immunohistochemical distribution of cannabinoid CB1 receptors in the rat central nervous system. Neuroscience. 1998;83:393–411. doi: 10.1016/s0306-4522(97)00436-3. [DOI] [PubMed] [Google Scholar]
- TSUDA M., INOUE K., SALTER M.W. Neuropathic pain and spinal microglia: a big problem from molecules in ‘small' glia. Trends Neurosci. 2005;28:101–107. doi: 10.1016/j.tins.2004.12.002. [DOI] [PubMed] [Google Scholar]
- TWITCHELL W., BROWN S., MACKIE K.J. Cannabinoids inhibit N- and P/Q-type calcium channels in cultured rat hippocampal neurons. J. Neurophysiol. 1997;78:43–50. doi: 10.1152/jn.1997.78.1.43. [DOI] [PubMed] [Google Scholar]
- VERME J.L., FU J., ASTARITA G., LA RANA G., RUSSO R., CALIGNANO A., PIOMELLI D. The nuclear receptor peroxisome proliferator-activated receptor-alpha mediates the anti-inflammatory actions of palmitoylethanolamide. Mol. Pharmacol. 2005;67:15–19. doi: 10.1124/mol.104.006353. [DOI] [PubMed] [Google Scholar]
- WIFFEN P.J., MCQUAY H.J., EDWARDS J.E., MOORE R.A.Gabapentin for acute and chronic pain Cochrane Database Syst. Rev. 200520CD005452Review [DOI] [PubMed] [Google Scholar]
- WILLOUGHBY K.A., MOORE S.F., MARTIN B.R., ELLIS E.F. The biodisposition and metabolism of anandamide in mice. J. Pharmacol. Exp. Ther. 1997;282:243–247. [PubMed] [Google Scholar]
- WILSON S.J., LOVENBERG T.W., BARBIER A.J. A high-throughput-compatible assay for determining the activity of fatty acid amide hydrolase. Anal. Biochem. 2003;318:270–275. doi: 10.1016/s0003-2697(03)00217-3. [DOI] [PubMed] [Google Scholar]
- ZHANG J., HOFFERT C., VU H.K., GROBLEWSKI T., AHMAD S., O'DONNELL D. Induction of CB2 receptor expression in the rat spinal cord of neuropathic but not inflammatory chronic pain models. Eur. J. Neurosci. 2003;17:2750–2754. doi: 10.1046/j.1460-9568.2003.02704.x. [DOI] [PubMed] [Google Scholar]