Abstract
Many studies assume that an increase in brain size is beneficial. However, the costs of producing and maintaining a brain are high, and we argue that brain size should be secondarily reduced by natural selection whenever the costs outweigh the benefits. Our results confirm this by showing that brain size is subject to bidirectional selection. Relative to the ancestral state, brain size in bats has been reduced in fast flyers, while it has increased in manoeuvrable flyers adapted to flight in complex habitats. This study emphasizes that brain reduction and enlargement are equally important, and they should both be considered when investigating brain size evolution.
Keywords: Chiroptera, mammals, encephalization, constraints, energetic costs
1. Introduction
Since Jerison (1973) argued that brain size cumulatively increased over evolutionary time, studies that followed have concentrated on how and why brains become larger (e.g. Harvey & Krebs 1990; Finlay & Darlington 1995; Barton & Harvey 2000). However, the development and maintenance of a large brain are costly (Aschoff et al. 1971). Although the constraint of energetic costs associated with neuronal tissue was recognized, the focus remained on how species maintain and increase overall brain size (Martin 1981; Aiello & Wheeler 1995; Jones & MacLarnon 2004). However, a permanent reduction in brain size or parts of a brain should evolve when the energetic benefits from a reduction of metabolic costs outweigh the loss of neuronal capacities, suggesting that bigger is not always better.
Bats provide an excellent opportunity to investigate brain size evolution, because they are under high selection pressure for increased energetic efficiency owing to their expensive mode of locomotion (Berger & Hart 1974; Tobalske et al. 2003). In flying animals (and bats in particular), wing area (as the most straightforward wing morphological measure) reflects flight performance (Norberg & Rayner 1987; Altshuler & Dudley 2002; Safi & Dechmann 2005). Species that forage in open space rely on speed and have small, narrow wings relative to body mass, resulting in low agility and low manoeuvrability (Norberg & Rayner 1987). These narrow-winged species have low relative costs of flight and thus increased flight efficiency. The opposite extreme of ecomorphological adaptation are bats that forage in highly structured habitats. These species have broad and large wings that render them highly manoeuvrable, but that make flight also more costly (Norberg & Rayner 1987).
Here, we attempt to disentangle the effects of adaptation to habitat structure and flight efficiency on the evolution of mammalian brain size. We estimate ancestral character traits (Pagel 1997, 1999a,b), relate total brain size to habitat complexity and flight efficiency (Felsenstein 1985) and investigate the mode of brain size evolution (Pagel 1997, 1999a,b) to determine whether a reduction of brain size can be adaptive.
2. Methods
We used ln-transformed data for body mass (g), wing area (m2) and total brain size (mg) of 104 bat species from 13 families from the literature (Norberg & Rayner 1987; Baron et al. 1996). As recommended by Garland et al. (1992), we analysed the data on two levels: once on a species level (using standard general linear models or regressions) and once taking phylogeny into account either with a phylogenetic generalized least-squares approach (PGLS) using the software Continuous (Pagel 1994, 1997, 1999b) or a phylogenetic independent contrast approach using the software CAIC (Purvis & Rambaut 1995).
We used a composite molecular phylogeny to infer relationships between species (see Electronic Appendix). Because branch lengths were partly unknown we set them to equal length (Garland et al. 1992).
We tested the appropriateness of equal branch lengths in Continuous using the likelihood ratio test and the scoring parameter kappa (κ), which differentially stretches or compresses individual phylogenetic branch lengths (Pagel 1997). We set κ to zero, i.e. enforced a punctuational mode of evolution (ln-likelihood model=−78.24) and compared it with the maximum-likelihood estimate (MLE=2.9, 95% CI=0–3). This comparison revealed that for all traits included in the model branch length had no effect (ln-likelihood ratio=1.7×10−12, d.f.=1, p=1.0), justifying the use of equal branch length. Another important scoring coefficient is lambda (λ), which reveals whether the phylogeny predicts the pattern of covariance among species (Pagel 1999a). Its value (λ=0.82, 95% CI=0.66–1.0) indicated that species are not independent (if 0<λ≥1) and that phylogenetic correction was required for appropriate statistical testing.
For the phylogenetic independent contrasts in CAIC, the plots of the absolute values of the standardized contrasts versus the standard deviation showed no correlation for all variables. This suggested again that equal branch lengths standardized the contrasts reasonably (Diaz-Uriarte & Garland 1998).
In Continuous, two models of trait evolution are available: the standard constant-variance random walk model (A) and a directional random walk model (B). By comparing their ln-likelihood, ratio models can be tested for their fit to the data. The comparison of the two models of trait evolution (A versus B) revealed no evidence for model B for all investigated traits (ln-likelihood A: −78.24; ln-likelihood B: −77.65; ln-likelihood ratio=0.59, d.f.=4, p=0.88). Consequently, we used model A to estimate correlations between the traits (Pagel 1994, 1997, 1999b).
3. Results
(a) Ancestral state
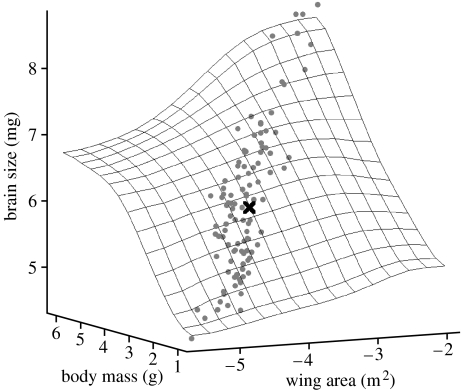
Our MLEs of ancestral states for body mass and wing area indicated that the ancestor of modern bats was intermediate in body, wing and brain sizes (figure 1). The estimates were: body mass=2.96 (antilogged 19.22 g); brain size=6.12 (antilogged=454 mg); wing area=−4.15 (antilogged=0.016 m2).
Figure 1.
Estimate of ancestral (black cross) ln (body mass), ln (brain size) and ln (wing area). Grey dots depict extant species values (n=104).
(b) Correlate of brain size
As expected (Jones & MacLarnon 2004), body mass correlated with brain size (species level: r=0.95, r2=0.914, t=32.911, p<0.0001; slope: 0.81, 95% CI=0.71–0.91; PGLS: r=0.96, r2=0.93, t=35.88, p<0.0001; slope: 0.67, 95% CI=0.50–0.84).
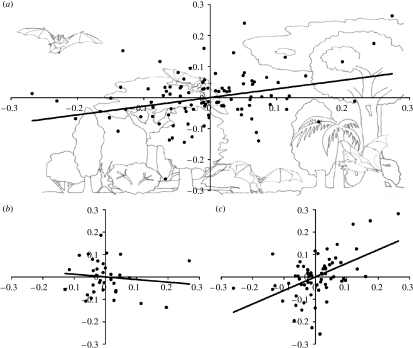
When correcting for phylogeny and body mass, wing area correlated positively with brain size (tables 1 and 2; figure 2; partial correlation correcting for body mass at species level: r=0.07, r2=0.005, t=0.722, p=0.47). Thus, relative to body mass, wing area predicts encephalization in bats after phylogenetic correction (tables 1 and 2; figure 2a).
Table 1.
Partial regression of ln (wing area) and ln (brain size) correcting for ln (body mass). The effect of phylogenetic inertia was corrected using the phylogenetic least-squares approach (PGLS).
| PGLS | |||||
|---|---|---|---|---|---|
| n | r | r2 | t | p | |
| all species | 104 | 0.26 | 0.08 | 2.59 | 0.004 |
| animal-eating species | 68 | 0.46 | 0.21 | 4.16 | <0.0001 |
| plant-eating species | 36 | −0.26 | 0.07 | −1.52 | 0.14 |
Table 2.
The effect of ln (wing area) on ln (brain size) correcting for ln (body mass) (covariate) on the level of phylogenetic independent contrasts.
| phylogenetic independent contrasts | |||||
|---|---|---|---|---|---|
| d.f. | SS3 | F | p | ||
| all species | wing area (m2) | 1 | 0.07 | 12.04 | 0.0008 |
| body mass (g) | 1 | 0.55 | 99.99 | <0.0001 | |
| error | 101 | ||||
| animal-eating species | wing area (m2) | 1 | 0.11 | 21.59 | <0.0001 |
| body mass (g) | 1 | 0.32 | 66.47 | <0.0001 | |
| error | 65 | ||||
| plant-eating species | wing area (m2) | 1 | <0.01 | 0.46 | 0.50 |
| body mass (g) | 1 | 0.24 | 40.28 | <0.0001 | |
| error | 33 | ||||
Figure 2.
Plot of residual contrasts in ln (wing area) (x-axes) and ln (brain size) (y-axes; residuals generated from a least-squares regression of phylogenetic independent contrasts in ln (wing area) and ln (brain size) against ln (body mass)) (a) for all species in this study. (b) The same plot for plant-eating bats, and (c) for animal-eating bats.
Plant-eating bats have larger relative brain sizes than animal-eating bats (Eisenberg & Wilson 1978; Hutcheon et al. 2002; Jones & MacLarnon 2004). When we separated plant-eating species from animal-eating species, we found that relative wing area correlated with relative brain size only in the latter (tables 1 and 2; figure 2b,c).
(c) Mode of brain size evolution
If brain size relative to body mass did increase unidirectionally with progressing evolution, a directional random walk model (model B) should fit the data better than a constant-variance random walk model (model A). However, the PGLS comparison of residuals from a least-squares regression of body mass versus brain size revealed no evidence for a directional mode of brain size evolution in bats (PGLS: log likelihood A=36.35; log likelihood B=36.65; log likelihood ratio=0.30, d.f.=2, p=0.74). There was also no evidence for a directional evolution of brain size relative to body mass for the reduced dataset of either animal-eating or plant-eating bat species (animal-eating bats: model A versus B log likelihood ratio=0.57, d.f.=2, p=0.57; plant-eating bats: model A versus B log likelihood ratio=0.04, d.f.=2, p=0.96).
4. Discussion
Our study demonstrates that brain size evolution is bidirectional and that there are ecological situations where it is beneficial to reduce neuronal mass according to a balance between energetic demands and ecological conditions.
The results show that the ancestral bat was of average body, wing and brain size, which is in line with recent predictions according to the fossil record (Norberg 1989; Simmons & Geisler 1998). We also found that relative wing area correlates with relative brain size, suggesting that these traits co-evolved. Bats foraging in complex environments have broad and large wings relative to body mass rendering them highly manoeuvrable, but inefficient flyers (Norberg & Rayner 1987), while the opposite is true for species foraging in open space. Therefore, brain size either increased to accommodate the neuronal structures necessary for flight in dense habitats, such as improved spatial memory or hearing ability (Safi & Dechmann 2005) or decreased when sensory needs were relaxed to reduce weight and energetic costs as well as improve aerodynamics. Examples are the family Molossidae or the Vespertilionid genus Nyctalus, which are highly adapted to open habitats, whose residual brain size values are distinctly lower than the average of all investigated species. The lack of a correlation between relative wing area and residual brain size in plant-eating bats suggests that neuronal requirements for these species are consistently high. In contrast, animal-eating species forage in all types of habitats, including open space, which results in a much wider range of morphological adaptations and neuronal requirements.
Although the selection for brain size reduction may be particularly strong in flying animals (Brenowitz 2004), a reduction in brain size should be a general property of evolution. Studies that investigate the effect of factors such as social system, diet or gestation length on brain size or parts of the brain should therefore be careful to identify ancestral states without the general assumption that smaller is more primitive. The assumption that larger brains are derived is probably associated with the quest to explain why humans have large brains. Instead, the investigation of both increases and decreases of the brain and/or brain parts is required; both pursuits are equally rewarding when identifying fundamental processes shaping neural structures. We demonstrate that brain size evolution may be bidirectional and that bigger is not always better.
Acknowledgments
We thank T. H. Clutton-Brock, K. E. Jones and C. van Schaik for their invaluable help and comments on earlier versions of this manuscript. We are also grateful to two anonymous referees who contributed to improve our manuscript. K.S. was supported by the ‘Graduiertenkollegium Zürich: Wissensgesellschaft und Geschlechterbeziehungen’.
Supplementary Material
References
- Aiello L.C, Wheeler P. The expensive-tissue hypothesis—the brain and the digestive-system in human and primate evolution. Curr. Anthropol. 1995;36:199–221. [Google Scholar]
- Altshuler D.L, Dudley R. The ecological and evolutionary interface of hummingbird flight physiology. J. Exp. Biol. 2002;205:2325–2336. doi: 10.1242/jeb.205.16.2325. [DOI] [PubMed] [Google Scholar]
- Aschoff J, Günther B, Kramer K. Urban and Schwarzenberg; München: 1971. Energiehaushalt und Temperaturregulation. [Google Scholar]
- Baron G, Stephan H, Frahm H.D. Birkhäuser; Basel: 1996. Comparative neurobiology in Chiroptera. [Google Scholar]
- Barton R.A, Harvey P.H. Mosaic evolution of brain structure in mammals. Nature. 2000;405:1055–1058. doi: 10.1038/35016580. [DOI] [PubMed] [Google Scholar]
- Berger M, Hart J.S. Physiology and energetics of flight. In: Farner D.S, King J.R, editors. Avian biology. vol. IV. Academic Press; New York: 1974. pp. 260–415. [Google Scholar]
- Brenowitz E.A. Plasticity of the adult avian song control system. Ann. NY Acad. Sci. 2004;1016:560–585. doi: 10.1196/annals.1298.006. [DOI] [PubMed] [Google Scholar]
- Diaz-Uriarte R, Garland T. Effects of branch length errors on the performance of phylogenetically independent contrasts. Syst. Biol. 1998;47:654–672. doi: 10.1080/106351598260653. [DOI] [PubMed] [Google Scholar]
- Eisenberg J.F, Wilson D.E. Relative brain size and feeding strategies in the Chiroptera. Evolution. 1978;32:740–751. doi: 10.1111/j.1558-5646.1978.tb04627.x. [DOI] [PubMed] [Google Scholar]
- Felsenstein J. Phylogenies and the comparative method. Am. Nat. 1985;125:1–15. doi: 10.1086/703055. [DOI] [PubMed] [Google Scholar]
- Finlay B.L, Darlington R.B. Linked regularities in the development and evolution of mammalian brains. Science. 1995;268:1578–1584. doi: 10.1126/science.7777856. [DOI] [PubMed] [Google Scholar]
- Garland T, Harvey P.H, Ives A.R. Procedures for the analysis of comparative data using phylogenetically independent contrasts. Syst. Biol. 1992;41:18–32. [Google Scholar]
- Harvey P.H, Krebs J.R. Comparing brains. Science. 1990;249:140–146. doi: 10.1126/science.2196673. [DOI] [PubMed] [Google Scholar]
- Hutcheon J.M, Kirsch J.W, Garland T. A comparative analysis of brain size in relation to foraging ecology and phylogeny in the Chiroptera. Brain Behav. Evol. 2002;60:165–180. doi: 10.1159/000065938. doi:10.1159/000065938 [DOI] [PubMed] [Google Scholar]
- Jerison H.J. Academic Press; New York: 1973. Evolution of the brain and intelligence. [Google Scholar]
- Jones K.E, MacLarnon A.M. Affording larger brains: testing hypotheses of mammalian brain evolution on bats. Am. Nat. 2004;164:E20–E31. doi: 10.1086/421334. [DOI] [PubMed] [Google Scholar]
- Martin R.D. Relative brain size and basal metabolic-rate in terrestrial vertebrates. Nature. 1981;293:57–60. doi: 10.1038/293057a0. [DOI] [PubMed] [Google Scholar]
- Norberg U.M. Ecological determinants of bat wing shape and echolocation call structure with implications for some fossil bats. In: Hanàk V, Horàcek T, Gaisler J, editors. European bat research 1987. Charles University Press; Prague: 1989. pp. 197–211. [Google Scholar]
- Norberg U.M, Rayner J.M.V. Ecological morphology and flight in bats (Mammalia, Chiroptera)—wing adaptations, flight performance, foraging strategy and echolocation. Phil. Trans. R. Soc. B. 1987;316:337–419. [Google Scholar]
- Pagel M. Detecting correlated evolution on phylogenies: a general method for the comparative analysis of discrete characters. Proc. R. Soc. B. 1994;255:37–45. [Google Scholar]
- Pagel M. Inferring evolutionary processes from phylogenies. Zool. Scr. 1997;26:331–348. [Google Scholar]
- Pagel M. Inferring the historical patterns of biological evolution. Nature. 1999a;401:877–884. doi: 10.1038/44766. [DOI] [PubMed] [Google Scholar]
- Pagel M. The maximum likelihood approach to reconstructing ancestral character states of discrete characters on phylogenies. Syst. Biol. 1999b;48:612–622. [Google Scholar]
- Purvis A, Rambaut A. Comparative-analysis by independent contrasts (CAIC)—an Apple-Macintosh application for analyzing comparative data. Comput. Appl. Biosci. 1995;11:247–251. doi: 10.1093/bioinformatics/11.3.247. [DOI] [PubMed] [Google Scholar]
- Safi K, Dechmann D.K.N. Adaptation of brain regions to habitat complexity: a comparative analysis in bats (Chiroptera) Proc. R. Soc. B. 2005;272:179–186. doi: 10.1098/rspb.2004.2924. doi:10.1098/rspb.2004.2924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons N.B, Geisler J.H. Phylogenetic relationships of Icaronycteris, Archaeonycteris, Hassianycteris, and Palaeochiropteryx to extant bat lineages, with comments on the evolution of echolocation and foraging strategies in Microchiroptera. Bull. Am. Mus. Nat. Hist. 1998:4–182. [Google Scholar]
- Tobalske B.W, Hedrick T.L, Dial K.P, Biewener A.A. Comparative power curves in bird flight. Nature. 2003;421:363–366. doi: 10.1038/nature01284. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.