Abstract
We demonstrate by using low-temperature high-resolution spectroscopy that red-shifted mutants of green fluorescent protein are photo-interconverted among three conformations and are, therefore, not photostable “one-color” systems as previously believed. From our experiments we have further derived the energy-level schemes governing the interconversion among the three forms. These results have significant implications for the molecular and cell biological applications of the green fluorescent protein family; for example, in fluorescence resonant energy transfer experiments, a change in “color” on irradiation may not necessarily be due to energy transfer but can also arise from a photo-induced conversion between conformers of the excited species.
The Aequorea victoria green fluorescent protein (GFP) has become a favored marker in molecular and cell biology because of its strong intrinsic visible fluorescence and the feasibility of fusing it to other proteins without affecting their normal functions (1–5). For example, mutants of GFP with different absorption and fluorescence spectra (4–8) are presently used in fluorescence lifetime imaging microscopy and fluorescence resonance energy transfer experiments to study protein-protein interactions, signaling, and trafficking in cellular systems (refs. 3–5 and references therein; refs. 9–12). In all of these studies it is assumed that the color changes observed in the GFP emission are caused by dynamic processes involving the proteins to which GFP is attached and do not arise in the GFP itself. That is, GFP-mutants are generally assumed to be in one conformation (i.e., to emit light of “one color”) and remain in that conformation under laser illumination (i.e., to be photostable), a supposition that we here prove to be incorrect.
The recent determination of the crystal structure of wild-type (wt) GFP and its mutants (13–15) has facilitated a structure-based, rational design of further mutants (3, 5, 16) in which the amino acids exchanged are either directly involved in the cyclization of the chromophore (serine 65, tyrosine 66, and glycine 67) or are located in their vicinity (4, 6–8, 17, 18). Frequently, the purpose of these mutations is to obtain one-color GFP-mutants with a single conformation, in contrast to wt-GFP, which exhibits absorption bands attributed to more than one conformation (3, 4, 19–23).
The photophysics of wt-GFP presents a complex problem, and that of its mutants has not been studied in detail. The room-temperature spectra are broad and rather unstructured (3–7, 18, 19). To determine the energy-level schemes of GFPs it is necessary to go to low temperature, where the spectrum becomes more structured. This has the additional merit that many of the thermally induced conversions are blocked at low temperature and, therefore, discrimination among individual species is facilitated. Energy-level schemes derived from low-temperature experiments put constraints on the interpretation of room-temperature results. A recent study by high-resolution spectroscopy at 1.6 K proved remarkably successful for wt-GFP (23). In that work we discovered that narrow holes could be burnt exclusively at the spectral positions of the 0–0 transitions. Using the hole-burning technique as a diagnostic tool we identified the 0–0 transitions of the three constituent conformations A, B, and I. In addition, we determined vibrational frequencies of the ground and excited states by fluorescence line-narrowing and unraveled the pathways by which these forms interconvert (23). Encouraged by this success, we have investigated three red-shifted mutants for which we demonstrate here that, contrary to current opinion, the “photostable, one color” mutants can also be photo-interconverted between at least three conformations, as in the case of wt-GFP.
Materials and Methods
Sample Preparation.
DNAs encoding for the red-shifted GFP-mutants S65T, RS-GFP, and EYFP were cloned into the expression vector PRSETa (Invitrogen). The mutants carry the following changes in the protein sequence: S65T (Ser-65→Thr), RS-GFP (Phe-64→Met, Ser-65→Gly, Gln-69→Leu) and EYFP (Ser-65→Gly, Val-68→Leu, Ser-72→Ala and Thr-203→Tyr). The recombinant proteins with a 6-histidine tag at the amino terminus were expressed and purified in Göttingen on a Ni-chelating resin (Ni-NTA-Agarose, Qiagen, Hilden, Germany) using standard procedures (24). All proteins were dissolved in 10 mM Na-phosphate buffer (pH 7) containing 50% (vol/vol) spectroscopic grade glycerol. Protein concentrations were ≈20 μM (S65T and RS-GFP) or ≈5 μM (EYFP), as estimated from the respective chromophore extinction coefficients (25).
High-Resolution Spectroscopy.
Absorption, excitation, emission, and hole-burning spectra were recorded in Leiden. The samples were excited with a pulsed N2-pumped (Molectron UV 22) dye-laser (Molectron DL 200, bandwidth ≈1 cm−1) (23). Continuous tunability was achieved between 360 and 535 nm by using 10 dyes. Absorption spectra were taken by scanning the laser and detecting the transmission through the sample with a photomultiplier (type EMI 9658B). For excitation, emission, and hole-burning spectra, the fluorescence signal was monitored at 90° with respect to the excitation beam through a 0.85-m double monochromator (SPEX 1402, resolution 5 cm−1) with the same photomultiplier and an electrometer (Keithley 610 CR). For low-temperature spectroscopic experiments, the samples were placed in a cuvette (thickness 3 mm) and were introduced into a 4He-bath cryostat that had first been filled with liquid N2. The latter was blown out after a few minutes. The cryostat was then filled with liquid He and was pumped down to 1.6 K.
Results and Discussion
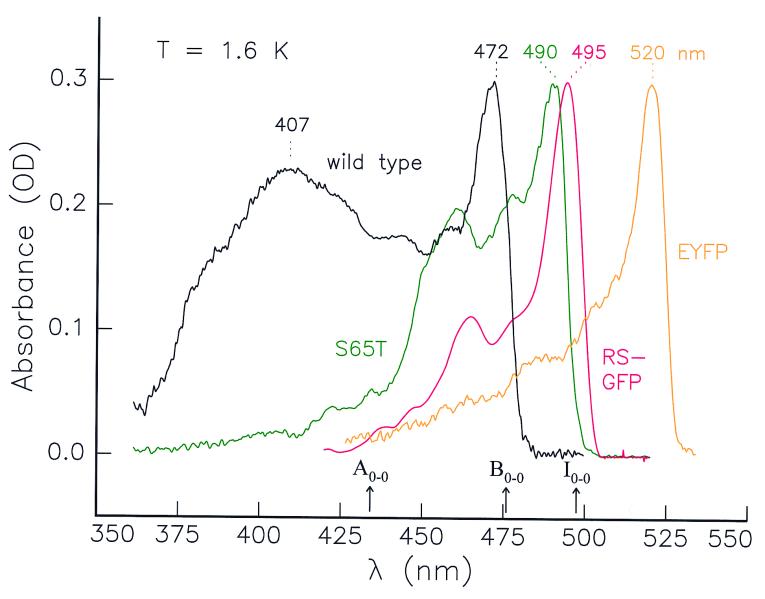
The absorption spectra of wt-GFP and the red-shifted mutants S65T (4, 5, 7), RS-GFP (3, 5, 8), and EYFP (3, 14, 18) at 1.6 K are shown in Fig. 1. They are narrower and more structured than at room temperature (20, 21, 23). At 1.6 K the spectrum of wt-GFP has two strong absorption bands at 407 and 472 nm (23), commonly assigned to a neutral form A and an anionic form B of the chromophore, respectively (3, 4, 6). It was postulated that the characteristic GFP-fluorescence at 508 nm originates from the excited state I* of a third, intermediate form I that results from the reaction A*→I* on excitation of A (20, 21). Before our experiments (23), however, no evidence for the occurrence of the intermediate in its ground state was found. Using hole-burning and high-resolution spectroscopy at 1.6 K and 295 K, we have discovered the absorption band of the I-form and located its 0–0 transition at 495 ± 1 nm (23). It is this metastable I-form of wt-GFP that in the absorption spectrum at 295 K is responsible for the weak feature around 500 nm. Further, we have shown in ref. 23 that the I-form, which in thermodynamic equilibrium is not present at low temperature, can be photoinduced at 1.6 K by exciting either the A- or the B-form. The reactions between A and I, and between I and B are reversible: i.e., A↔I↔B; there is no direct interconversion A↔B, and thus I is a true intermediate.
Figure 1.
Absorption spectra of wild-type GFP and the red-shifted mutants S65T, RS-GFP, and EYFP at T = 1.6 K. The maxima of the absorption bands and the 0–0 transitions of the three conformers of wt-GFP are indicated.
The red-shifted GFP-mutants studied here show common features: a strong absorption maximum at their red edge accompanied by weaker features toward the blue (Fig. 1). Whereas wt-GFP has two principal maxima absorbing further to the blue and assigned to the A- and B-forms, the mutants seem to lack the A-form. Note that S65T and RS-GFP have their maxima in the same spectral region as the I-form of wt-GFP (495 nm) (23). The similarity in wavelengths prompted us to investigate whether these red-shifted mutants are in a conformation corresponding to the B-form, as claimed in the literature for S65T (16, 22), or to the I-form as suggested by our own recent results on wt-GFP (23). We illustrate our approach taking the RS-GFP mutant as an example and summarize the general conclusions obtained for S65T and EYFP as well. Our experiments on the latter two mutants have been described in greater detail elsewhere (ref. 26; data not shown).
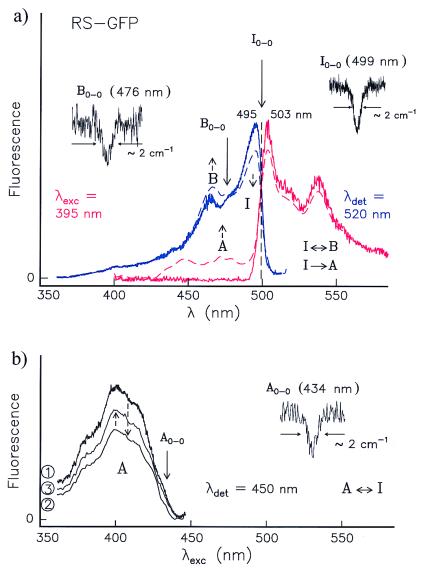
Contrary to our observations for wt-GFP, the emission spectrum of a previously unilluminated RS-GFP sample at 1.6 K (shown in red in Fig. 2a) proved independent of the excitation wavelength λexc. There are two bands at 503 and 537 nm and a shoulder at 517 nm that do not change their relative intensities with λexc, indicating that the emission stems from a single form. This is confirmed by the mirror symmetry of the emission and excitation spectra (Fig. 2a) about their intersection. No further intersections of this kind were observed at other wavelengths. The emission spectrum strongly resembles that of the I-form of wt-GFP (compare Fig. 2a with Fig. 4 b and d of ref. 23); the two bands and the shoulder have nearly identical spectral positions and similar relative intensities. The simplest interpretation of these results is that RS-GFP in these experiments has a conformation that is similar to that of the I-form of wt-GFP. Moreover, the experiments reported below reveal that this conformation of RS-GFP is not photostable but is an intermediate between two other forms. We, therefore, label it as I-form.
Figure 2.
(a) Excitation (blue curve) and emission (red curve) spectra of RS-GFP at 1.6 K before (—) and after (---) burning into I0–0 at 499 ± 1 nm (right hole). Before burning, only the I-form is present. After burning, the vibronic bands of the B-form between 450 and 475 nm increase (blue, ---); thus, I→B. By burning into B0–0 at 476 ± 1 nm, a hole is produced (Left), and the original spectrum recovers; thus, B→I. After burning into the I0–0, the A-form is also photoinduced, which is reflected in the fluorescence spectrum. In the region from 400 to 490 nm there is no signal before burning (red, —), but there is fluorescence after burning (red, ---). (b) Excitation spectra of A with λdet = 450 nm. Optical switching between A and I occurs. (1) The A-form has been produced by burning into I0–0 at 499 ± 1 nm. (2) After burning a hole in the 0–0 transition of A at 434 ± 1 nm, the intensity of the whole A-spectrum decreases whereas that of the I-form increases (not shown). (3) After subsequent burning into I, the population of the A-form increases again.
The proof that the observed intersection in Fig. 2a corresponds to a pure electronic transition, assigned I0–0, is provided by our discovery that at 499 ± 1 nm, but at no other wavelengths, a relatively narrow hole (limited by the laser bandwidth) could be burnt in a previously unilluminated RS-GFP sample. In contrast, holes burned in vibronic bands have a much larger width (by a factor of ≈103) because of the short excited-state lifetimes and are difficult to detect (ref. 27 and references therein; ref. 28). By burning into the I-form, not only does the 0–0 hole appear, but, simultaneously, its vibronic band at ≈495 nm decreases, while the bands between ≈460–480 nm increase (see dashed blue curve in Fig. 2a). This indicates that a second conformation is photoinduced. Because after this illumination a hole could also be burned at 476 ± 1 nm, we assign this wavelength to the 0–0 transition of the B-form (B0–0), again by analogy with wt-GFP (23). Upon exciting I to I* we did not observe any emission from B*, but only from I* (to I). Thus, the reaction I*→B must occur through a radiationless pathway, which may go through a lower lying triplet state (see also the top diagram in Fig. 3). Conversely, when B is excited in the region 460–470 nm at 1.6 K, fluorescence from I* but not from B* is observed, implying that the reaction B*→I* occurs through the first excited singlet state and is barrierless. Moreover, by burning a hole into B0–0 (Fig. 2a Left), the intensity of the vibronic region of I at 495 nm increases (not shown). We conclude that photointerconversion (reversible “optical switching”) between the two conformers I and B occurs along the paths sketched in Fig. 3 Top.
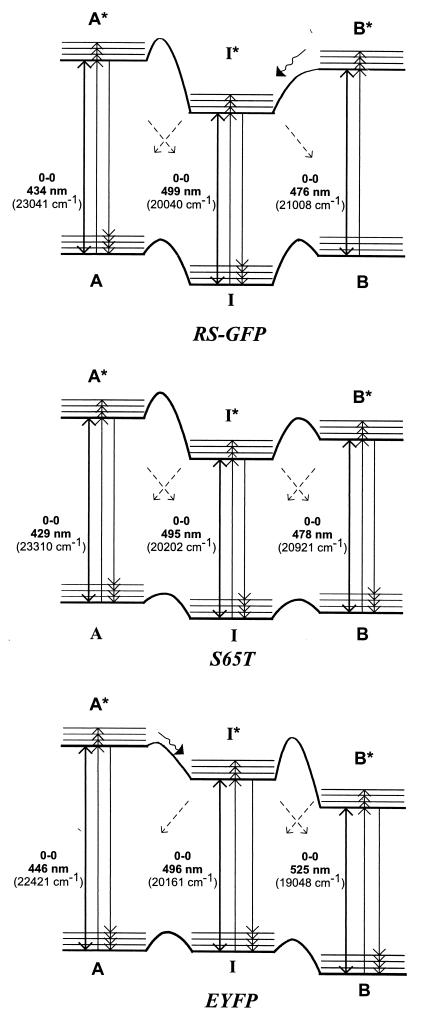
Figure 3.
Energy-level diagrams of the red-shifted mutants RS-GFP (Top), S65T (Middle), and EYFP (Bottom). The forms A, I, and B photointerconvert as indicated by the diagonal arrows. The relative populations of the three forms differ for the various mutants. In RS-GFP only the I-form is populated at 1.6 K before burning; by burning into I, the A- and B-forms are photoinduced. In S65T, all forms are populated at 1.6 K, with the I-form having the lowest ground-state level. In EYFP, the most populated form is B; I and A are significantly less populated at 1.6 K. Their populations can be enhanced by burning into B.
Burning into I generates not only B but also A (Fig. 2b). After burning into I0–0 at 499 nm, the excitation spectrum of A (not present before burning) is obtained when detecting at ≈450 nm, i.e., in the region where emission from A* would be expected (Fig. 2b, spectrum 1). As with I and B, a hole can now be burned on the red wing of A at 434 ± 1 nm, which we associate with A0–0 (Fig. 2b Right). Together with the burned hole, the entire A-band is bleached (spectrum 2), and the I-form is recovered (not shown); i.e., A transforms into I. When burning again into I, the A-band increases (from spectrum 2 to 3), proving that reversible optical switching between the forms I and A occurs. This is confirmed by the fluorescence spectrum obtained by excitation in the A-region at 395 nm before and after burning into I (Fig. 2a, red curves). In the range between 450 and 475 nm, where emission from A* would be expected, no signal is detected before burning (full red line), but after burning into I, two bands at 449 and 473 nm appear (dashed red line), proving that the radiationless reaction I*→A occurs (see Fig. 3 Top). This reaction might take place through the triplet state.
The ground-state levels of the A- and B-forms of RS-GFP, which are photoinduced by illuminating the I-form, have to be at least 100 cm−1 higher in energy than those of the I-form (see Fig. 3 Top) because A and B are not present in a previously unilluminated sample at 1.6 K. Furthermore, because the excitation spectrum detected in the emission region of I at 520 nm (Fig. 2a, blue) does not increase in the region in which the photoinduced A-form absorbs (350–450 nm), we conclude that the reaction A*→I*, in contrast to that in wt-GFP, does not occur. It follows that the barrier between A* and I * has to be high (a few 1,000 cm−1) and that the reaction A*→I proceeds radiationlessly.
We have performed detailed low-temperature spectroscopic experiments, analogous to those carried out on wt-GFP (23) and RS-GFP, also on S65T and EYFP (ref. 26; data not shown). The latter two mutants follow a general pattern with three distinguishable conformers, again as in wt-GFP (23) and RS-GFP. We designate the three conformers as A, I, and B, according to the convention that A absorbs to the blue of B and I acts as an intermediate in the photointerconversions; i.e., there is no direct conversion between A and B at low temperature. We cannot for the moment specify the structural distinctions between the corresponding A-, I-, and B-conformers of the various mutants.
In contrast to RS-GFP, the intersection of the excitation- and emission spectra of S65T depends on the wavelength of excitation and detection. Because we have observed three of such intersections (not shown), we ascribe them to the 0–0 transition region of the I-, B-, and A-forms. The wavelengths coincide with those at which holes could be burned, which proves that the spectra do not stem from one single species. We have further verified that the three forms photointerconvert: i.e., I↔A and I↔B. The most red-shifted EYFP mutant, having only one strong absorption maximum at 520 nm (see Fig. 1), nevertheless exists in three conformations A, I, and B at 1.6 K.
The energy-level schemes and the pathways of photointerconversion for the three mutants are compared in Fig. 3. S65T (Fig. 3 Middle) follows a similar scheme as RS-GFP (Fig. 3 Top) with the I-form being the most populated one. But in contrast to RS-GFP, for which the ground state of the I-form is the only conformation populated at 1.6 K before hole-burning and lies therefore significantly lower than those of the A- and B-forms, in S65T the A- and B-forms are also present at 1.6 K, although in considerably smaller amounts than the I-form. The ground states of A and B in S65T thus must lie at the most a few tens of cm−1 above that of the latter. Singlet excited-state photoconversions between A* and I* and between B* and I* seem to be absent in S65T, but the radiationless reactions, A*→I, I*→A, I*→B, and B*→I, still take place, conceivably through triplet states. Because of the similarity of the wavelengths of the absorption band at ≈490–500 nm and its function as an intermediate between the two other forms A and B, we would associate the structure found for S65T (14) with the I-form of wt-GFP, and not with the B-form, as has been suggested in refs. 16 and 22.
In EYFP (Fig. 3 Bottom), the ground-state of the B-form is now lowest, in contrast to RS-GFP and S65T. Upon excitation into A*, fluorescence is simultaneously observed from A* and I* as in wt-GFP (23), but excitation into B* only leads to B*→B emission and not to emission from I*. When exciting into I*, there is no reaction I*→B*, either. We conclude that I and B can be reversibly switched between each other by optical excitation, but only through radiationless pathways. Our findings for the red-shifted mutants suggest that other GFP-mutants may also exist in several forms and that, most probably, photointerconversion between different forms is a general phenomenon in GFPs.
The pathways for interconversion between the various conformations of GFP are likely dictated by the specific amino acid side chains surrounding the chromophore (22). We may speculate about the structural basis for the barrierless reaction B*→I* found here for RS-GFP (Fig. 3 Top) and the reduced dominant fluorescence lifetime (≈3 ns → ≈1 ns) and associated rotational correlation time of the same mutant in solution (29). These phenomena are presumably the result of the specific amino acid substitutions, particularly S65G and Q69L. The former leads to a smaller and probably more flexible chromophore, while the latter perturbs the hydrogen-bonding network adjacent to the chromophore. Although the S65G change is also present in EYFP, its effect seems to be compensated by the additional T203Y mutation, which is responsible for the π-π stacking between the phenol rings of Tyr-203 and the chromophore (Tyr- 66) (30). This effect might lead to a more rigid chromophore in EYFP and, consequently, to a higher barrier between B* and I* and to the strong red shift of B0–0 (Fig. 3 Bottom).
Our experiments demonstrate that reversible optical switching is not only revealed in single-molecule experiments (18, 31) but can also be observed in an ensemble of GFP-molecules at low temperature. Furthermore, the energy-level diagrams of Fig. 3 allow one to specify the pathways of interconversion (26) that govern the “on/off blinking” and switching in single-molecule experiments at room temperature (18).
The present study bears important implications for GFP-mutants used in molecular and cell biology. For instance, in the study of protein-protein interactions by fluorescence resonance energy transfer, a change in the color of the fluorescence is commonly interpreted as evidence for energy transfer and, therefore, for an interaction between proteins (3–5,9). In the light of the results presented here, such a color change may well arise from a photoinduced intramolecular conversion between conformers of a specific GFP-mutant rather than from an intermolecular interaction.
Acknowledgments
T.M.H.C., A.J.L., and S.V. thank J. H. van der Waals for his continuing encouragement and critical comments. We further acknowledge R. Rivera-Pomar and D. Piston for gifts of GFP plasmids. V.S is a recipient of the Human Frontiers Science Program long-term postdoctoral fellowship. The spectroscopic experiments were financially supported by the Netherlands Foundation for Physical Research (FOM) and the Council for Chemical Research of the Netherlands Organization for Scientific Research (CW-NWO).
Abbreviations
- GFP
green fluorescent protein
- wt
wild-type
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.050365997.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.050365997
References
- 1.Prasher D C, Eckenrode V K, Ward W W, Prendergast F G, Cormier M J. Gene. 1992;111:229–233. doi: 10.1016/0378-1119(92)90691-h. [DOI] [PubMed] [Google Scholar]
- 2.Chalfie M, Tu Y, Euskirchen G, Ward W W, Prasher D C. Science. 1994;263:802–805. doi: 10.1126/science.8303295. [DOI] [PubMed] [Google Scholar]
- 3.Tsien R Y. Annu Rev Biochem. 1998;67:509–544. doi: 10.1146/annurev.biochem.67.1.509. [DOI] [PubMed] [Google Scholar]
- 4.Cubitt A B, Heim R, Adams S R, Boyd A E, Gross L A, Tsien R Y. Trends Biochem Sci. 1995;20:448–455. doi: 10.1016/s0968-0004(00)89099-4. [DOI] [PubMed] [Google Scholar]
- 5.Heim R, Tsien R Y. Curr Biol. 1996;6:178–182. doi: 10.1016/s0960-9822(02)00450-5. [DOI] [PubMed] [Google Scholar]
- 6.Heim R, Prasher D C, Tsien R Y. Proc Natl Acad Sci USA. 1994;91:12501–12504. doi: 10.1073/pnas.91.26.12501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heim R, Cubitt A B, Tsien R Y. Nature (London) 1995;373:663–664. doi: 10.1038/373663b0. [DOI] [PubMed] [Google Scholar]
- 8.Delagrave S, Hawtin R E, Silva C M, Yang M M, Youvan D C. Bio/Technology. 1995;13:151–154. doi: 10.1038/nbt0295-151. [DOI] [PubMed] [Google Scholar]
- 9.Miyawaki A, Llopis J, Heim R, McCaffery J M, Adams J A, Ikura M, Tsien R Y. Nature (London) 1997;388:882–887. doi: 10.1038/42264. [DOI] [PubMed] [Google Scholar]
- 10.Presley J F, Cole N A, Schroer T A, Hirschborn K, Zaal J M, Lippincott-Schwartz J. Nature (London) 1997;389:81–85. doi: 10.1038/38001. [DOI] [PubMed] [Google Scholar]
- 11.Pepperkok R, Squire A, Geley S, Bastiaens P I H. Curr Biol. 1999;9:269–276. doi: 10.1016/s0960-9822(99)80117-1. [DOI] [PubMed] [Google Scholar]
- 12.Miyawaki A, Griesbeck O, Heim R, Tsien R Y. Proc Natl Acad Sci USA. 1999;96:2135–2140. doi: 10.1073/pnas.96.5.2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang F, Moss L G, Philips G N., Jr Nat Biotechnol. 1996;14:1246–1251. doi: 10.1038/nbt1096-1246. [DOI] [PubMed] [Google Scholar]
- 14.Ormö M, Cubitt A B, Kallio K, Gross L A, Tsien R Y, Remington S J. Science. 1996;273:1392–1395. doi: 10.1126/science.273.5280.1392. [DOI] [PubMed] [Google Scholar]
- 15.Wachter R, King B A, Heim R, Kallio K, Tsien R Y, Boxer S G, Remington S J. Biochemistry. 1997;36:9759–9765. doi: 10.1021/bi970563w. [DOI] [PubMed] [Google Scholar]
- 16.Palm G J, Zdanov A, Gaitanaris G A, Stauber R, Pavlakis G N, Wlodawer A. Nat Struct Biol. 1997;4:361–365. doi: 10.1038/nsb0597-361. [DOI] [PubMed] [Google Scholar]
- 17.Niwa H, Inouye S, Hirano T, Matsuno T, Kojima S, Kubota M, Ohashi M, Tsuji F I. Proc Natl Acad Sci USA. 1996;93:13617–13622. doi: 10.1073/pnas.93.24.13617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dickson R M, Cubitt A B, Tsien R Y, Moerner W E. Nature (London) 1997;388:355–358. doi: 10.1038/41048. [DOI] [PubMed] [Google Scholar]
- 19.Ward W W, Prentice H J, Roth A F, Cody C W, Reeves S C. Photochem Photobiol. 1982;35:803–808. [Google Scholar]
- 20.Chattoraj M, King B A, Bublitz G U, Boxer S G. Proc Natl Acad Sci USA. 1996;93:8362–8367. doi: 10.1073/pnas.93.16.8362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lossau H, Kummer A, Heinecke R, Pöllinger-Dammer F, Kompa C, Bieser G, Jonsson T, Silva C M, Yang M M, Youvan D C, Michel-Beyerle M E. Chem Phys. 1996;213:1–16. [Google Scholar]
- 22.Brejc K, Sixma T K, Kitts P A, Kain S R, Tsien R Y, Ormö M, Remington S J. Proc Natl Acad Sci USA. 1997;94:2306–2311. doi: 10.1073/pnas.94.6.2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Creemers T M H, Lock A J, Subramaniam V, Jovin T M, Völker S. Nat Struct Biol. 1999;6:557–560. doi: 10.1038/9335. [DOI] [PubMed] [Google Scholar]
- 24.Janknecht R, de Martijnoff G, Lou J, Hipskind R A, Nordheim A, Stunnenberg G. Proc Natl Acad of Sci USA. 1991;88:8972–8976. doi: 10.1073/pnas.88.20.8972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Patterson G H, Knobel S M, Sharif W D, Kain S R, Piston D W. Biophys J. 1997;73:2782–2790. doi: 10.1016/S0006-3495(97)78307-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Creemers T M H. Ph.D. thesis. Leiden, the Netherlands: Univ. of Leiden; 1999. [Google Scholar]
- 27.Völker S. In: Relaxation Processes in Molecular Excited States. Fünfschilling J, editor. Dordrecht, the Netherlands: Kluwer; 1989. pp. 113–242. [Google Scholar]
- 28.Völker S. Annu Rev Phys Chem. 1989;40:499–530. [Google Scholar]
- 29.Volkmer, A., Subramanian, V., Birch, D. J. S. & Jovin, T. M. (2000) Biophys. J., in press. [DOI] [PMC free article] [PubMed]
- 30.Wachter R M, Elsliger M-A, Kallio K, Hanson G T, Remington S J. Structure (London) 1998;6:1267–1277. doi: 10.1016/s0969-2126(98)00127-0. [DOI] [PubMed] [Google Scholar]
- 31.Jung G, Wiehler J, Göhde W, Tittel J, Basché Th, Steipe B, Bräuchle C. Bioimaging. 1998;6:54–61. [Google Scholar]