Abstract
The tyrosine kinase Src upregulates the activity of the N-methyl-D-aspartate subtype of glutamate receptor (NMDAR) and tyrosine phosphorylation of this receptor is critical for induction of NMDAR-dependent plasticity of synaptic transmission. A binding partner for Src within the NMDAR complex is the protein PSD-95. Here we demonstrate an interaction of PSD-95 with Src that does not require the well-characterized domains of PSD-95. Rather, we show binding to Src through a 12-amino-acid sequence in the N-terminal region of PSD-95, a region not previously known to participate in protein–protein interactions. This region interacts directly with the Src SH2 domain. Contrary to typical SH2 domain binding, the PSD-95–Src SH2 domain interaction is phosphotyrosine-independent. Binding of the Src-interacting region of PSD-95 inhibits Src kinase activity and reduces NMDAR phosphorylation. Intracellularly administering a peptide matching the Src SH2 domain-interacting region of PSD-95 depresses NMDAR currents in cultured neurons and inhibits induction of long-term potentiation in hippocampus. Thus, the PSD-95–Src SH2 domain interaction suppresses Src-mediated NMDAR upregulation, a finding that may be of broad importance for synaptic transmission and plasticity.
Keywords: LTP, NMDA receptor, PSD-95, SH2 domain, Src
Introduction
The non-receptor protein tyrosine kinase Src is expressed widely throughout the central nervous system (CNS) and is present at high levels in neurons of the brain and spinal cord. A major function of Src in the adult CNS is to regulate glutamatergic transmission and synaptic plasticity (Salter and Kalia, 2004). Src acts postsynaptically at glutamatergic synapses to upregulate the activity of the N-methyl-D-aspartate receptor (NMDAR) (Yu et al, 1997), a principal subtype of glutamate receptor crucial for neuronal development, neuroplasticity and excitotoxicity (Dingledine et al, 1999). An increasing body of evidence indicates that Src serves as a point of convergence through which a number of signalling pathways modulate NMDAR activity. These pathways involve G-protein-coupled receptors, Ras, cytokine receptors, receptor protein tyrosine kinases, integrins, insulin and lipoprotein receptors (Salter and Kalia, 2004; Macdonald et al, 2005; Chen et al, 2005; Jones and Leonard, 2005).
By upregulating the activity of NMDARs at Schaffer collateral–CA1 synapses within the hippocampus, Src functions as an intermediary in the induction of long-term potentiation (LTP) (Lu et al, 1998), a form of lasting enhancement of synaptic transmission and the predominant cellular model of learning and memory (Bliss and Collingridge, 1993). Tyrosine phosphorylation of the NMDAR subunit protein NR2B, the most prominent tyrosine phosphorylated protein within postsynaptic densities at glutamatergic synapses (Moon et al, 1994), has been found to increase in LTP in CA1 hippocampus (Nakazawa et al, 2001) and dentate gyrus (Rostas et al, 1996). Tyrosine phosphorylation of NR2B subunits has also been shown to increase in several pathological processes in the CNS, including ischemia, seizure and hyperalgesia (Salter and Kalia, 2004). Thus, the tyrosine kinase Src has emerged as a key regulator of NMDAR function and thereby of excitatory synaptic transmission, plasticity and pathophysiology.
NMDARs are multiprotein complexes comprised of the core NMDAR subunit proteins, which form the ion permeation pathway (Dingledine et al, 1999), together with a variety of molecular scaffolds and signalling enzymes (Husi et al, 2000). Src has been identified as a component of the NMDAR complex (Yu et al, 1997; Husi et al, 2000). We have discovered two proteins within the complex that interact with Src: ND2 (Gingrich et al, 2004) and PSD-95 (Kalia and Salter, 2003). For ND2, it has been determined that a region within its C-terminal domain interacts with the unique domain of Src and that ND2 functions to anchor Src within the NMDAR complex (Gingrich et al, 2004). By contrast, it is unknown how PSD-95 interacts with Src and moreover, the function of this interaction remains obscure. Therefore, here we sought to characterize the interacting regions in PSD-95 and Src, and to determine the functional consequences of this interaction.
Results
SH2 domain of Src interacts with the N-terminal region of PSD-95
We explored the region of Src involved in the association with PSD-95 by testing GST fusion proteins of three main regions of Src: the unique domain, SH3 domain and SH2 domain (Brown and Cooper, 1996; Thomas and Brugge, 1997) (Figure 1A). We found that the Src SH2 domain GST fusion protein pulled down PSD-95 from rat forebrain extracts (Figure 1B). In contrast, PSD-95 was not pulled down by the other GST fusion proteins or by GST alone. Thus, PSD-95 interacts with the SH2 domain of Src but not the Src unique domain or Src SH3 domain.
Figure 1.
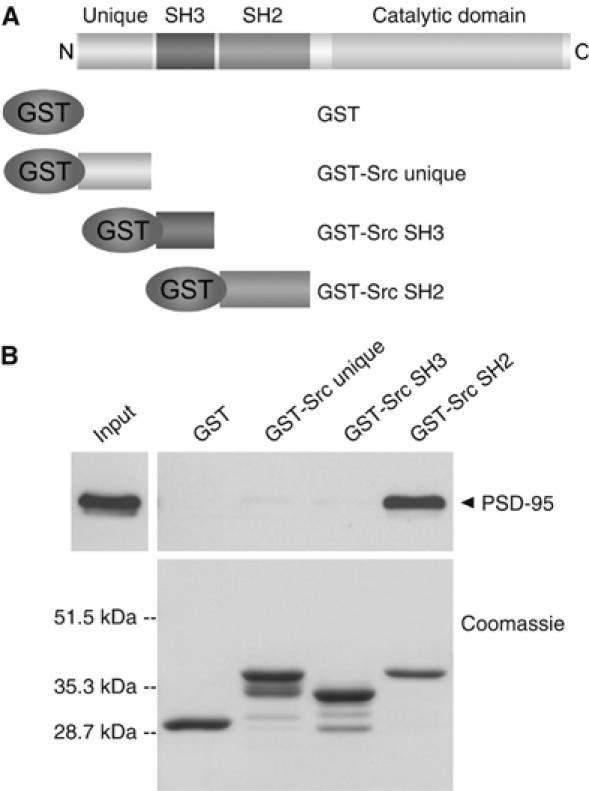
PSD-95 interacts with the SH2 domain of Src. (A) Illustration of GST fusion proteins of Src unique domain, Src SH3 domain and Src SH2 domain. (B) Pull-down assays using rat forebrain extracts (600 μg) with GST fusion proteins shown in panel A. Proteins were probed with anti-PSD-95 (upper). Input was 60 μg of protein from brain extracts. GST fusion proteins were stained with Coomassie blue (lower). Molecular weight markers are indicated on left. Results are representative of five experiments.
We therefore next determined the region of PSD-95 that interacts with the Src SH2 domain. PSD-95 is the lead member of the PSD-95 family of membrane-associated guanylate kinase (MAGUK) proteins (Cho et al, 1992). The domain structure of PSD-95 includes three PDZ domains, one SH3 domain and a guanylate kinase homology (GuK) domain (Figure 2A), each of which have been found to mediate protein–protein interactions (Kim and Sheng, 2004). To investigate the association of PSD-95 with the Src SH2 domain, we used a series of PSD-95 deletion constructs that were individually expressed in HEK293 cells. Each of the deletion constructs lacked one or more of the known protein–protein interaction modules of PSD-95 (Figure 2A). We found that the Src SH2 domain GST fusion protein pulled down PSD-95 from lysates of HEK293 cells transfected with wild-type PSD-95 (Figure 2B). The Src SH2 domain GST fusion protein also pulled down the PSD-95 deletion proteins lacking the PDZ1 and PDZ2 domains (ΔPDZ1/2), the PDZ3 domain (ΔPDZ3) or the SH3 and GuK domains (ΔSH3/GuK). In contrast, GST alone did not pull down any of the PSD-95 proteins (Figure 2B). Thus, removal of each of the known protein–protein interaction domains of PSD-95 failed to eliminate the interaction with the Src SH2 domain.
Figure 2.

Src SH2 domain binds directly to the N-terminal region of PSD-95. (A) Illustration of PSD-95 deletion constructs. (B) Pull-down assays were performed using lysates (300 μg) of HEK293 cells transfected with constructs shown in panel A. Proteins that associated with GST alone or GST-Src SH2 were probed with anti-PSD-95. Input was 30 μg of protein from cell lysates. Arrows indicate positions of corresponding PSD-95 proteins. Results are representative of five experiments. (C) GST alone or GST-Src SH2 was incubated with the PSD-95(1–54) peptide (1.0 μM). PSD-95(1–54) that bound was probed with an anti-PSD-95 antibody raised against aa 18–32 of PSD-95. Input was 10% of starting material. (D) Left: densitometric quantification from three experiments, one of which is represented in panel C. Bars correspond to mean (±s.e.m.) normalized to maximum gray value for each experiment. Right: modified ELISAs to measure pull-down of GST-Src SH2 domain by PSD-95(1–54). Bars correspond to mean (±s.e.m.) normalized to maximum absorbance reading for each experiment and are representative of three experiments.
The only region common to all of the PSD-95 deletion mutants was amino acids 1–54. Because we had observed previously that ΔSH3/GuK PSD-95 is sufficient to bind to Src (Kalia and Salter, 2003), we used ΔSH3/GuK PSD-95 to determine whether amino acids 1–54 are necessary for binding to the Src SH2 domain. To this end, we made a protein (Δ(1–54)/SH3/GuK) in which these residues were deleted from the ΔSH3/GuK PSD-95 construct. We found that the Δ(1–54)/SH3/GuK PSD-95 protein was not pulled down by GST-Src SH2 (Figure 2B), implying that amino acids 1–54 are required for binding to the Src SH2 domain. We previously observed that the SH3 domain of PSD-95 may also interact with Src (Kalia and Salter, 2003), but here we found that the PSD-95 SH3 domain does not interact with the SH2 domain of Src (data not shown). Thus, the region of amino acids 1–54 of PSD-95, but not the PSD-95 SH3 domain, may mediate binding to the SH2 domain of Src. Therefore, for the remainder of the present study, we focused on this region of PSD-95.
To determine whether the region of amino acids 1–54 of PSD-95 interacts directly with the Src SH2 domain, we tested the binding of the Src SH2 domain GST fusion protein and PSD-95(1–54) peptide, which had been cleaved from GST. We found that GST-Src SH2 domain interacted with PSD-95(1–54) in pull-down assays (Figure 2C and D, left) and conversely, that PSD-95(1–54) bound to GST-Src SH2 domain in modified ELISAs (Figure 2D, right). The binding of PSD-95(1–54) was detectable at peptide concentrations ⩾0.1 μM, and the amount of PSD-95(1–54) that was pulled down increased with increasing concentrations of the peptide up to 2.0 μM, the maximum concentration used. The GST-Src SH2 domain interacted with PSD-95(1–54) at all concentrations of the Src SH2 domain tested (0.625–2.5 μM) in modified ELISAs. Taken together, these results imply that PSD-95(1–54) directly interacts with the SH2 domain of Src and that the interaction occurs at low micromolar concentrations of either protein.
We next determined whether PSD-95(1–54) was sufficient to interact with Src (Figure 3). We found that the PSD-95(1–54) GST fusion protein pulled down recombinant Src as well as Src from rat forebrain extracts. In contrast, GST alone, GST-PSD-95 PDZ1 or GST-PSD-95 PDZ2 did not pull down Src. To ensure that the purified recombinant PDZ domains were in their native conformations and hence should have interacted with PDZ ligands, we probed the proteins pulled down by the PDZ domain proteins for NR2A/B subunits of the NMDAR, which have been shown to directly bind to the PDZ1 and PDZ2 domains of PSD-95 (Kornau et al, 1995). We found that GST-PSD-95 PDZ1 and GST-PSD-95 PDZ2 pulled down NR2A/B subunits. Conversely, neither GST alone nor GST-PSD-95(1–54) pulled down NR2A/B. From these results, we conclude that the region comprising the first 54 amino acids of PSD-95 is a protein–protein interaction module in PSD-95. This is a region of PSD-95 not previously suspected as mediating interactions with other proteins.
Figure 3.

N-terminal region of PSD-95 is sufficient for the interaction with Src. (A) Illustration of GST fusion proteins of aa 1–54, PDZ1 domain and PDZ2 domain of PSD-95. (B) Pull-down assays with full-length recombinant Src and GST fusion proteins illustrated in panel A. Src that bound was probed with anti-Src (upper). Pull-down assays from rat forebrain extracts (600 μg) with the GST fusion proteins were performed. Membrane was probed with anti-Src, stripped and then re-probed with anti-NR2A/B (middle). The amount of NR2A/B pulled down by PDZ2 was greater than that by PDZ1 as expected from the reported differences in affinities (Lim et al, 2002). Input was 20% of starting material. GST fusion proteins were stained with Coomassie blue (lower). Molecular weight markers are shown on left. Similar results were seen in each of three experiments.
Interaction between the Src SH2 domain and N-terminal region of PSD-95 is phosphotyrosine-independent
The SH2 domain of Src and SH2 domains of other signalling molecules typically bind ligands containing phosphorylated tyrosine residues (Songyang et al, 1993). The sequence of amino acids 1–54 of PSD-95 contains three tyrosine residues but none is contained in a consensus phosphorylation sequence according to a peptide library-based searching algorithm (Yaffe et al, 2001). The GST fusion proteins and PSD-95(1–54) peptide used in the experiments described above were expressed in Escherichia coli BL21 cells that do not express protein tyrosine kinases and consequently, were not phosphorylated on tyrosine (confirmed by immunoblotting with anti-phosphotyrosine (anti-pY) antibody; data not shown).
It has been reported that Src does not phosphorylate PSD-95 in vitro (Nada et al, 2003) and that PSD-95 lacks tyrosine phosphorylation, as detected by immunoblotting (Tezuka et al, 1999; Nada et al, 2003). To test whether PSD-95 from rat brain extracts used in the present study was tyrosine phosphorylated, we immunoprecipitated PSD-95 and then probed with anti-pY (Figure 4A). Tyrosine phosphorylation of PSD-95 from the extracts was not detected. Thus, we inferred that the pull-down of PSD-95 by GST-Src SH2 as well as the in vitro interaction between the PSD-95(1–54) peptide and Src SH2 domain occurred without tyrosine phosphorylation of the interacting region in PSD-95.
Figure 4.
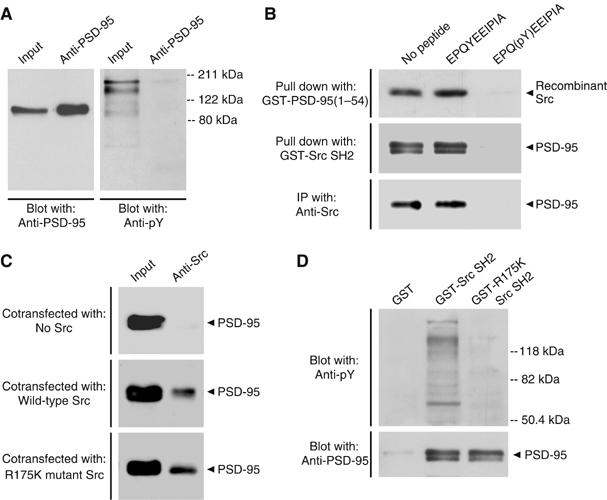
Src SH2 domain binding does not require tyrosine phosphorylation of the N-terminal region of PSD-95. (A) Immunoprecipitations from PSD fractions (100 μg) with anti-PSD-95. Immunoprecipitants were sequentially probed with a different anti-PSD-95 antibody (left) and anti-pY (right). A band corresponding to PSD-95 was not detectable with anti-pY even following longer exposures (data not shown). Input was 8 μg of PSD proteins. Molecular weight markers are shown on right. Similar results were observed in three separate experiments. (B) Upper: pull-down assays with GST-PSD-95(1–54) and recombinant Src preincubated with no peptide, EPQYEEIPIA or EPQ(pY)EEIPIA (1 mM). Src that remained bound to GST-PSD-95(1–54) was probed with anti-Src. Middle: pull-down assays from rat forebrain extracts (600 μg) with GST-Src SH2 preincubated with no peptide, EPQYEEIPIA or EPQ(pY)EEIPIA (1 mM). Proteins that remained associated with GST-Src SH2 were probed with anti-PSD-95. Lower: immunoprecipitations with anti-Src from lysates of HEK293 cells (300 μg) cotransfected with PSD-95 and Src, and incubated with no peptide, EPQYEEIPIA or EPQ(pY)EEIPIA (1 mM). Proteins were probed with anti-PSD-95. Similar results were found in each of three experiments. (C) Immunoprecipitations with anti-Src from lysates of HEK293 cells (300 μg) transfected with PSD-95 and no Src (upper), wild-type Src (middle) or R175K mutant Src (lower). Immunoprecipitants were probed with anti-PSD-95. Input was 30 μg of cell lysate proteins. Similar results were found in each of three experiments. (D) Pull-down assays from forebrain extracts (600 μg) with GST alone, GST-Src SH2 or GST-R175K Src SH2. Proteins were sequentially probed with anti-pY (upper) and anti-PSD-95 (lower). Molecular weight markers are indicated on right. Results are representative of three experiments.
Nevertheless, it is possible that binding of PSD-95 to the Src SH2 domain might depend upon the same binding surface in the Src SH2 domain as that used to bind phosphotyrosine ligands. This possibility was tested in a series of experiments using a phosphotyrosine peptide, EPQ(pY)EEIPIA. This peptide is a high-affinity ligand for the Src SH2 domain (Songyang et al, 1993) and prevents binding of other ligands to the domain (Gilmer et al, 1994). We performed three experiments with this peptide: in vitro pull-down assays with GST-PSD-95(1–54) and recombinant Src (Figure 4B, upper); pull-down assays with GST-Src SH2 domain and forebrain extracts (Figure 4B, middle); and co-immunoprecipitations using lysates from HEK293 cells transiently cotransfected with Src and PSD-95 (Figure 4B, lower). In all three experiments, we found that including EPQ(pY)EEIPIA eliminated the Src–PSD-95 interaction. But when we included no peptide or the non-phosphorylated peptide, EPQYEEIPIA, which does not bind to the Src SH2 domain, the Src–PSD-95 interaction was not affected. The results from this series of experiments demonstrate that the EPQ(pY)EEIPIA peptide precludes the binding between Src and PSD-95, indicating that the Src SH2 domain cannot simultaneously bind a phosphotyrosine ligand and PSD-95(1–54).
Within the binding surface of the Src SH2 domain, the arginine residue at amino-acid position 175 (R175) is involved in an ion-pairing interaction with the phosphate group on tyrosine in the ligand (Waksman et al, 1993). Mutation of this critical arginine residue to lysine (R175K) has been shown to reduce binding of tyrosine phosphorylated peptides to less than 10% of that of wild-type Src SH2 domain (Bibbins et al, 1993). Because binding of PSD-95 to the Src SH2 domain does not require tyrosine phosphorylation of PSD-95, we wondered whether binding might also not require R175 in the SH2 domain. We expressed Src with or without the R175K mutation together with PSD-95 and found that the R175K mutation did not reduce the amount of PSD-95 co-immunoprecipitated with Src (Figure 4C). In addition, we made a GST fusion protein of the R175K Src SH2 domain. We found that the amount of tyrosine phosphorylated proteins pulled down by GST-R175K Src SH2 domain was dramatically less than that pulled down by wild-type Src SH2 domain (Figure 4D, upper), confirming that this mutation had greatly reduced the interaction of the Src SH2 domain with tyrosine phosphorylated proteins. In contrast to the decrease in tyrosine phosphorylated proteins pulled down by GST-R175K Src SH2, the amount of PSD-95 pulled down by this mutant protein was not reduced (Figure 4D, lower). Together these findings indicate that, although PSD-95 is not tyrosine phosphorylated, binding of PSD-95 to the SH2 domain of Src depends upon the binding surface of the Src SH2 domain which is involved in interactions with phosphotyrosine-containing peptides. However, R175 within the Src SH2 domain which mediates binding to phosphotyrosine is not required for the Src SH2 domain–PSD-95 interaction.
Deletion of the N-terminal region of PSD-95 enhances Src-mediated tyrosine phosphorylation of NMDAR subunits
To begin to investigate the functional consequences of the interaction between the Src SH2 domain and N-terminal region of PSD-95, we determined whether this region of PSD-95 regulates the phosphorylation of the NR2B subunit of the NMDAR, a major target of Src (Cheung and Gurd, 2001). NR2B binds directly to PSD-95 via the PDZ1 or PDZ2 domain of PSD-95 (Kornau et al, 1995). Therefore, we reconstituted the NMDAR–PSD-95 complex and expressed recombinant NR1/NR2B NMDARs together with a constitutively active form of Src (Y527F Src) (Brown and Cooper, 1996) in HEK293 cells. Without coexpressing Y527F Src, tyrosine phosphorylation of NR2B was not detectable (data not shown). To test the effect of the N-terminal region of PSD-95 on Src-mediated tyrosine phosphorylation of NR2B, we compared the level of NR2B tyrosine phosphorylation when wild-type PSD-95 was coexpressed versus when a mutant PSD-95 lacking amino acids 14–54 (Δ(14–54) PSD-95) was coexpressed. In the mutant PSD-95, we retained the first 13 amino acids of PSD-95, as they contain the consensus sequence for palmitoylation required for normal trafficking of PSD-95 to the plasma membrane (El Husseini et al, 2000). We found that the tyrosine phosphorylation level of NR2B was enhanced by 1.9-fold when NMDARs were coexpressed with Δ(14–54) PSD-95 as compared with wild-type PSD-95 (Figure 5A). Tyrosine phosphorylation of NR2B was dependent on Src kinase activity because NR2B phosphorylation was undetectable when a catalytically inactive form of Src (K295R Src) (Kamps and Sefton, 1986) was expressed instead of constitutively active Y527F Src. As with NR2B, we found that Src-mediated tyrosine phosphorylation of NR2A was enhanced by Δ(14–54) PSD-95 as compared with wild-type PSD-95 (Supplementary Figure S1).
Figure 5.

Deleting the N-terminal region of PSD-95 increases Src-mediated NMDAR tyrosine phosphorylation. (A) Upper: immunoprecipitations with anti-NR2 from lysates of HEK293 cells (300 μg) cotransfected with NR1/2B, Y527F Src or K295R Src, and wild-type (WT) PSD-95 or Δ(14–54) PSD-95. Immunoprecipitated NR2B was sequentially probed with anti-pY and anti-NR2B. Blots shown are representative of three separate experiments. Lower: densitometric quantification from three experiments, one of which is represented above. Band intensity was quantitated as mean gray value and the ratio of pY NR2B to total NR2B was calculated. Bars correspond to mean (±s.e.m.) ratios normalized to ratios obtained for cotransfection of NR1/2B with Y527F Src and wild-type PSD-95. (*P<0.0005, t-test versus Y527F Src and wild-type PSD-95). Inset: representative 5-point standard curve (R2=0.99, P<0.001) derived from serial dilutions of forebrain proteins. (B) Upper: immunoblots with lysates of HEK293 cells transfected with NR1/2B, Y527F Src, and WT PSD-95 or Δ(14–54) PSD-95 (left). In each lane, 30 μg of protein were loaded. Blots were sequentially probed for NR2B, Src, PSD-95 and GAPDH, which served as a loading control. Results are representative of three separate experiments. Immunoprecipitations were carried out from the cell lysates (300 μg) with anti-NR2 (right). Proteins were sequentially probed with anti-NR2B, anti-Src and anti-PSD-95. Results are representative of three separate experiments. Lower: densitometric quantification from three experiments, one of which is represented above. Bars correspond to mean (±s.e.m.) band intensity, which was quantitated as mean gray value (P>0.4, t-test versus WT PSD-95). (C) Upper: immunoprecipitations with a pan anti-Src antibody from lysates of HEK293 cells (300 μg) transfected with Y527F Src plus no PSD-95, WT PSD-95 or Δ(14–54) PSD-95. Immunoprecipitated Src was immunoblotted with anti-pY416 Src (upper) and anti-Src (lower). Input was 30 μg of proteins from cells cotransfected with Y527F Src and Δ(14–54) PSD-95. Positions of IgG heavy chains are indicated. Results are representative of three separate experiments. Lower: densitometric analysis from three experiments, one of which is represented above. Bars correspond to mean (±s.e.m.) band intensity, which was quantitated as mean gray value (*P<0.01, t-test versus no PSD-95 or versus Δ(14–54) PSD-95).
We found that protein expression of Y527F Src and NR2B in cells expressing wild-type PSD-95 was not significantly different from that in cells expressing Δ(14–54) PSD-95 (Figure 5B). Thus, there were no significant changes in the relative amounts of the kinase or its substrate that could have accounted for the difference in NR2B phosphorylation. Another potential explanation for the lower level of NR2B phosphorylation in cells expressing wild-type PSD-95 might have been that less Y527F Src was associated with the NMDAR complex in these cells. However, we found that the amount of Y527F Src co-immunoprecipitating with anti-NR2B when wild-type PSD-95 was coexpressed was not different from that when Δ(14–54) PSD-95 was coexpressed (Figure 5B). Thus, deleting the 14–54 region of PSD-95 did not alter the association of Y527F Src with the NMDAR complex.
To determine whether the 14–54 region of PSD-95 affects the catalytic activity of Src, we expressed in HEK293 cells constitutively active Y527F Src, alone or together with wild-type PSD-95 or Δ(14–54) PSD-95. We reasoned that, if the interaction of Src with the 14–54 region of PSD-95 suppresses Src catalytic activity, the coexpression of wild-type PSD-95 but not Δ(14–54) PSD-95 should suppress Src activity. We assessed the level of Src activation by using an antibody that recognizes an active form of Src in which tyrosine 416 (Y416) of Src is phosphorylated. Y416 is located within the activation loop of the kinase and phosphorylation of this residue is required for full activity of Src. Therefore, the level of phosphorylated Y416 (pY416) is a surrogate marker of Src tyrosine kinase activity (Brown and Cooper, 1996; Thomas and Brugge, 1997). We immunoprecipitated Src from the cell lysates with a pan anti-Src antibody, which recognizes both Y416 and pY416 forms of Src. By probing with an antibody that recognizes pY416, but not Y416, we found that the degree of Src activation was reduced by coexpressing with wild-type PSD-95 versus no PSD-95 (Figure 5C). In contrast, Src activity was not affected by coexpressing Δ(14–54) PSD-95. The total amount of Y527F Src immunoprecipitated was not affected by coexpression of either PSD-95 or Δ(14–54) PSD-95. Thus, these results indicate that PSD-95 suppresses Src activity and that this suppression depends upon the 14–54 region of PSD-95. Furthermore, these results imply that the increase in Src-mediated tyrosine phosphorylation of NR2B by Δ(14–54) PSD-95, compared with wild-type PSD-95, is due to removal of the suppression of Src catalytic activity.
PSD-95(43–57) binds directly to the SH2 domain and suppresses the tyrosine kinase activity of Src
The above findings imply that there is a stretch of amino acids N-terminal to the first PDZ domain of PSD-95 that depresses the catalytic activity of Src. To map this stretch, we used a partially overlapping series of four 15-mer synthetic peptides. In order that each of these peptides was of the same length, the series collectively spanned amino acids 6–57 of PSD-95 (Figure 6A). The peptides were synthesized without phosphoamino acids. We tested the peptides individually for their ability to disrupt the direct interaction between the PSD-95(1–54) GST fusion protein and recombinant Src in vitro. We observed that when Src was preincubated with the PSD-95(43–57) peptide, the pull-down of Src by GST-PSD-95(1–54) was eliminated (Figure 6B, left). However, preincubating with each of the other three PSD-95 peptides did not reduce the amount of Src that interacted with GST-PSD-95(1–54). The inhibition of the Src–PSD-95(1–54) interaction by the PSD-95(43–57) peptide was concentration-dependent (Figure 6B, right). Moreover, we found that the Src SH2 domain GST fusion protein bound directly to the PSD-95(43–57) peptide (Figure 6C). The amount of GST-Src SH2 that bound to the other PSD-95 peptides was not different from the background binding level in these experiments. Therefore, we concluded that the PSD-95(43–57) peptide directly interacts with the Src SH2 domain.
Figure 6.
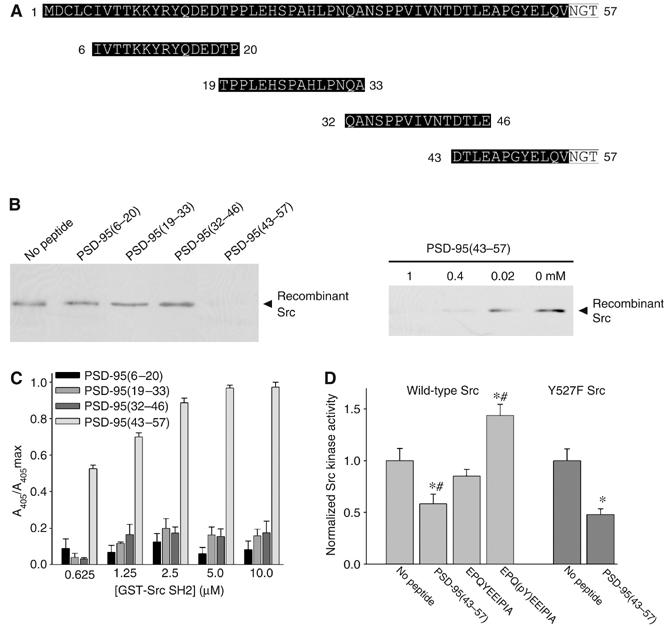
PSD-95(43–57) peptide suppresses Src tyrosine kinase activity. (A) Sequences of four 15-mer peptides spanning aa 6–57 of PSD-95. Highlighted in white are amino acids outside of the sequence of PSD-95(1–54). (B) Left: pull-down assays with GST-PSD-95(1–54) and recombinant Src preincubated with each of the peptides (1 mM) shown in panel A. Src that remained bound to GST-PSD-95(1–54) was detected by immunoblot. Right: pull-down assays with GST-PSD-95(1–54) and recombinant Src preincubated with decreasing concentrations of PSD-95(43–57) as indicated. Similar results were seen in three separate experiments. (C) Modified ELISAs to measure binding of GST-Src SH2 to each of the PSD-95 peptides. Bars correspond to mean (±s.e.m.) normalized to maximum absorbance reading for each experiment and are representative of four separate experiments. (D) Light gray bars: in vitro kinase activity of recombinant Src following incubation with no peptide (n=5), PSD-95(43–57) (n=5), EPQYEEIPIA (n=3) or EPQ(pY)EEIPIA (n=3). Bars correspond to mean (±s.e.m.) Src kinase activity normalized to activity of Src incubated with no peptide (*P<0.05, t-test versus no peptide; #P<0.01; t-test versus EPQYEEIPIA). Dark gray bars: in vitro kinase activity of Y527F Src immunoprecipitated with anti-Src from lysates of HEK293 cells (300 μg) transfected with Y527F Src. Tyrosine kinase activity of immunoprecipitated Y527F Src was measured following incubation with no peptide (n=3) or PSD-95(43–57) (n=3). Bars correspond to mean (±s.e.m.) Src kinase activity normalized to activity of Src incubated with no peptide (*P<0.05, t-test versus no peptide).
To determine whether the PSD-95(43–57) peptide affects Src catalytic activity, we measured the activity of recombinant wild-type Src in phosphorylating an optimum peptide substrate (Cheng et al, 1992) with or without including the PSD-95(43–57) peptide. We found that the activity of Src that was incubated with PSD-95(43–57) was decreased to 58±9% (n=5) of the activity of Src that was incubated with no peptide (n=5; Figure 6D). Conversely, we found that the activity of Src that was incubated with the EPQ(pY)EEIPIA peptide, which binds to the SH2 domain but which is known to activate Src (Brown and Cooper, 1996; Thomas and Brugge, 1997), was increased to 144±11% (n=3) relative to the level with no peptide. The EPQYEEIPIA peptide had no statistically significant effect on Src activity. Similar to its effect on the tyrosine kinase activity of wild-type Src, the PSD-95(43–57) peptide depressed the activity of the constitutively active Src mutant, Y527F Src (n=3; Figure 6D). In contrast, this peptide had no effect on the catalytic activity of another Src family kinase, Fyn (n=4; Supplementary Figure S2).
The single tyrosine contained in PSD-95(43–57) is not within a Src consensus phosphorylation sequence (Yaffe et al, 2001), but it was nevertheless possible that this tyrosine might have been phosphorylated at a slow rate by Src in vitro. In this case, the decrease in Src-mediated phosphorylation of the optimum peptide substrate may have been caused by non-optimal phosphorylation of the PSD-95(43–57) peptide, which occluded phosphorylation of the optimum substrate. However, we found that the PSD-95(43–57) peptide was not phosphorylated by Src (data not shown). Hence, taken together, these results indicate that PSD-95(43–57) binds directly to Src and causes suppression of the kinase activity of Src.
N-terminal region of PSD-95 reduces NMDAR-mediated synaptic currents in cultured hippocampal neurons
We investigated the consequences of the PSD-95–Src SH2 domain interaction on the function of native NMDARs by using cultured hippocampal neurons. These cells are well suited for this purpose because in these cells, basal NMDAR-mediated currents are tonically enhanced by Src (Salter and Kalia, 2004). Therefore, if the N-terminal region of PSD-95 is able to inhibit Src within the NMDAR complex, one predicts that the PSD-95(1–54) peptide would suppress NMDAR currents in these neurons. We examined the effect of PSD-95(1–54) on NMDAR currents by recording miniature excitatory postsynaptic currents (mEPSCs) in the whole-cell patch-clamp configuration (Figure 7). We found that administering the PSD-95(1–54) GST fusion protein intracellularly through the recording pipette led to a progressive decline in the NMDAR-mediated component of mEPSCs: on average, the NMDAR component of mEPSCs recorded 20 min after the start of recording was reduced to 57±6% of the initial value during the first 2 min of recording (n=20 cells). In contrast, GST alone did not significantly alter the NMDAR component of mEPSCs (107±11%; n=8 cells). Moreover, neither GST-PSD-95(1–54) nor GST alone affected the AMPAR-mediated component of mEPSCs (Figure 7). Thus, we conclude that PSD-95(1–54) suppresses NMDAR-mediated synaptic currents in these neurons.
Figure 7.
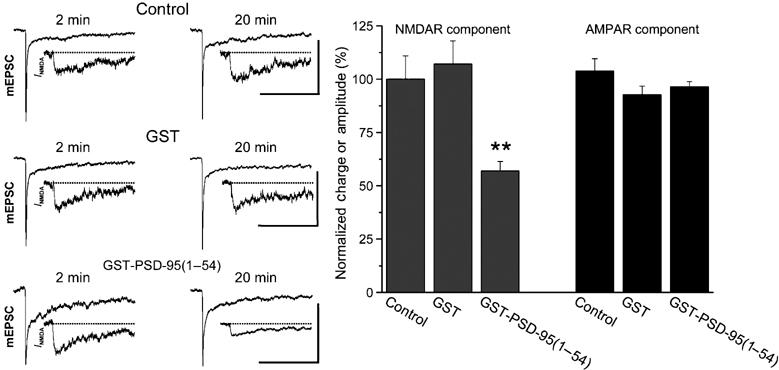
Administering PSD-95(1–54) peptide reduces NMDAR-mediated synaptic currents. Left: traces of representative averaged mEPSCs or NMDAR components (INMDA; inset) obtained at 2 or 20 min after breakthrough with control intracellular solution (ICS) or ICS supplemented with GST alone or GST-PSD-95(1–54) in the recording pipette. Scale bars for mEPSCs are 25 pA/100 ms. Right: mean (±s.e.m.) changes in the NMDAR and AMPAR components of averaged mEPSCs recorded at 20 min after breakthrough expressed as a percentage of control mEPSCs obtained 2 min after breakthrough during recordings with control ICS (n=10 cells), GST alone (n=8 cells) or GST-PSD-95(1–54) (n=20 cells) (**P<0.01, t-test versus control ICS or versus GST alone).
PSD-95(43–57) depresses induction of LTP in CA1 neurons
In CA1 neurons in hippocampal slices, in contrast to cultured hippocampal neurons, synaptic NMDARs are not tonically enhanced by basal Src activity in that suppressing Src in the NMDAR complex in these neurons does not affect NMDAR-mediated synaptic currents. Nevertheless, suppressing Src is known to prevent induction of LTP at Schaffer collateral–CA1 synapses (Salter and Kalia, 2004). Thus, if amino acids 43–57 in PSD-95 are capable of inhibiting Src activity within the NMDAR complex, it is predicted that the PSD-95(43–57) peptide would suppress induction of LTP at Schaffer collateral–CA1 synapses without affecting basal synaptic NMDAR responses. To test this prediction, we made whole-cell recordings from CA1 neurons in acute hippocampal slices and administered the PSD-95(43–57) peptide intracellularly via the patch pipette. We found that PSD-95(43–57) did not affect either the amplitude or voltage dependence of pharmacologically isolated NMDAR EPSCs (Figure 8A and B) nor did it affect basal synaptic excitatory postsynaptic potentials (EPSPs; Figure 8C and D, upper).
Figure 8.
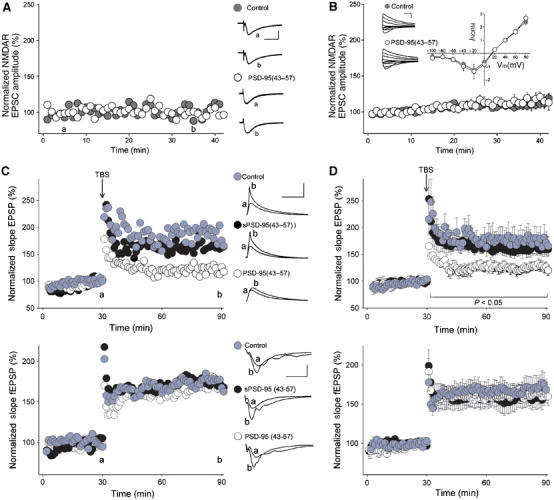
PSD-95(43–57) peptide depresses induction of LTP at Schaffer collateral–CA1 synapses. (A) Scatter plot of pharmacologically isolated NMDAR EPSCs from CA1 neurons in hippocampal slices. Peak amplitude is plotted over time for representative cells recorded with control ICS (•) or ICS supplemented with PSD-95(43–57) (○). Right: traces represent the average of six consecutive EPSCs at the times indicated (a and b). Scale bars are 100 ms/70 pA. (B) Average NMDAR EPSC amplitude (±s.e.m.) over time with control ICS (•; n=12) or PSD-95(43–57) (○; n=7). Inset: current–voltage (I–V) relationships for pharmacologically isolated NMDAR EPSCs with control ICS (•; n=11) or PSD-95(43–57) (○; n=10). Superimposed NMDAR EPSC traces at membrane potentials from −100 to +80 mV are shown for two representative cells. Scale bars are 200 ms/200 pA. (C) Scatter plots of EPSP slope (upper) and simultaneously recorded fEPSP slope (lower) from individual representative experiments with control ICS (•), the scrambled PSD-95(43–57) peptide (sPSD-95(43–57)) (•) or PSD-95(43–57) (○). Right: each trace represents the average of six consecutive EPSPs or fEPSPs recorded at the times indicated (a and b). Scale bars are 10 mV/100 ms for EPSPs and 0.8 mV/10 ms for fEPSPs. (D) Plot of average EPSP slope versus time for experiments with control ICS (•; n=11), sPSD-95(43–57) (•; n=12) or PSD-95(43–57) (○; n=10) (upper). Averaged slope of fEPSPs measured during experiments when whole-cell recordings were performed (lower).
To determine whether the PSD-95(43–57) peptide affects induction of LTP at these synapses, we recorded in whole-cell mode from individual CA1 neurons in hippocampal slices (Figure 8C and D, upper) while simultaneously recording population responses of nearby neurons using field recordings (Figure 8C and D, lower). LTP was induced by theta-burst stimulation (TBS) of the Schaffer collateral input. In control recordings with no added peptide, TBS caused lasting potentiation of whole-cell EPSPs and field EPSPs (fEPSPs), which persisted throughout the recording period: 60 min after TBS, whole-cell EPSP slope was 171±21% (n=11) of the pre-TBS level and fEPSP slope was 168±13% (n=11). By contrast, in cells in which PSD-95(43–57) was administered intracellularly, whole-cell EPSP slope 60 min after TBS was 126±10% (n=10) of the pre-TBS level, which was significantly less than the TBS-induced potentiation in the control cells (P<0.05). In the field recordings from slices in which PSD-95(43–57) was tested, the potentiation produced by TBS was not different from that in the control slices (Figure 8C and D, lower), indicating that effective TBS had been delivered to the slices. In cells in which a control peptide with identical amino-acid composition but a scrambled sequence (sPSD-95(43–57)) was administered intracellularly, EPSP slope was 159±12% (n=12) 60 min after TBS, not significantly different from TBS-induced potentiation in the control cells without peptide (Figure 8C and D, upper). Thus, administering PSD-95(43–57), but not sPSD-95(43–57), into CA1 neurons suppresses induction of LTP by TBS stimulation of Schaffer collaterals.
Discussion
Here we have discovered that within PSD-95, N-terminal to the first PDZ domain, amino acids 43–57 interact directly with the SH2 domain of Src. Previous studies on PSD-95 have focused primarily on the PDZ, SH3 and GuK domains of PSD-95. By contrast, the N-terminal region of PSD-95 has been much less investigated and no previous function has been found for amino acids 43–57. Here, we found that the Src SH2 domain-interacting site was contained within PSD-95(43–57). PSD-95(1–54) bound to the Src SH2 domain, implying that the final three amino acids of the PSD-95(43–57) peptide were not necessary for this binding. Thus, we infer that within PSD-95, amino acids 43–54 mediate the binding to the SH2 domain of Src. Amino acids 43–54 are outside of the well-known protein–protein interaction domains within PSD-95 and hence, we have defined a previously unrecognized protein–protein interaction module in PSD-95. Furthermore, we found that this region of PSD-95 inhibits the catalytic activity of Src, implying that, in addition to functioning as a molecular scaffold, PSD-95 may function directly as a regulator of a key enzyme in the NMDAR complex.
The N-terminal region of PSD-95 is one of three regions of the protein that, in contrast to much of the primary amino-acid sequence, is highly divergent from other MAGUK proteins of the PSD-95 family (Kim et al, 1996); the other two regions are the segment between PDZ2 and PDZ3, and the segment between the SH3 and GuK domains, termed the HOOK domain (Tavares et al, 2001). The highly conserved domains allow for commonality of binding partners for PSD-95 family members (Kim et al, 1996), whereas the regions outside of these conserved domains may confer differences in binding characteristics to the various MAGUK proteins. For example, we have previously found that Src associates with PSD-95 but not with the other members of the PSD-95 family of MAGUK proteins, namely PSD-93, SAP97 and SAP102 (Kalia and Salter, 2003). Indeed, all of the other members of the PSD-95 family show relatively low identity to PSD-95 in the 43–54 region and thus, within the PSD-95 family, this is a region unique for PSD-95. In other PSD-95 family members, the divergent regions have also been reported to be involved in protein binding: the HOOK region of SAP102 binds to calmodulin (Masuko et al, 1999) and the N-terminal region of SAP97 engages in an intramolecular interaction with its SH3 domain (Mehta et al, 2001). Thus, a common theme emerging from these studies is that regions of low sequence homology within PSD-95 family members, such as amino acids 43–54 of PSD-95, do participate in protein–protein interactions and may thereby allow for distinctive binding partners.
Amino acids 43–54 of PSD-95 bound directly to the SH2 domain of Src. SH2 domains typically bind phosphotyrosine-containing peptide ligands and previously described peptide ligands for the Src SH2 domain are phosphorylated on tyrosine (Songyang et al, 1993). But, we found that binding to the Src SH2 domain occurred in the absence of phosphorylation of the PSD-95(1–54) peptide or PSD-95(43–57) peptide. The binding of the PSD-95(43–54)-containing ligands was prevented by the EPQ(pY)EEIPIA peptide, indicating that the Src SH2 domain cannot simultaneously bind a phosphotyrosine-containing ligand and PSD-95(43–54). Thus, it is possible that PSD-95(43–54) binds to the same binding surface as that used to bind phosphotyrosine ligands. Phosphotyrosine-independent ligand binding to the phosphotyrosine binding surface has been demonstrated through crystallographic and NMR studies for the binding of SLAM to the SH2 domain of SAP (Li et al, 1999; Poy et al, 1999).
An alternative possibility is that PSD-95(43–54) binds to a region outside the phosphotyrosine binding surface of the Src SH2 domain and that the PSD-95(43–54) binding site is not accessible when the SH2 domain has a phosphotyrosine ligand bound to it. Binding of ligands to regions of the SH2 domain separate from the phosphotyrosine binding surface have not previously been found for the Src SH2 domain. But, the SH2 domain of Crk has been found to bind the SH3 domain of Abl via the so-called DE loop in the Crk SH2 domain (Donaldson et al, 2002). The DE loop is not a common feature of SH2 domains but, in the Crk SH2 domain, this loop contains a hidden SH3 ligand that is made accessible as a consequence of a large structural rearrangement induced by binding of phosphotyrosine ligands. Whether PSD-95(43–54) binds to the phosphotyrosine binding surface or to another region, this is a previously unknown type of interaction for the Src SH2 domain that is now open for investigation through structural studies.
Although outside the catalytic domain per se, binding of ligands to the SH2 domain of Src critically regulates the kinase activity of Src. The predominant model of Src regulation is that intramolecular interactions between the Src SH2 domain and a phosphorylated tyrosine residue near the C-terminus, and between the SH3 domain and a cryptic SH3 ligand in the linker region, lock Src in an inactive conformation. Accordingly, displacement of these intramolecular interactions by binding of SH2 and/or SH3 ligands in other proteins has been shown to lead to activation of Src (Brown and Cooper, 1996; Thomas and Brugge, 1997). However, binding of ligands to the SH2 domain has also been found to inhibit Src activity (Chang et al, 1998; Shakespeare et al, 2000). In the present study, we found that PSD-95(43–57) inhibited activity of Src in vitro and, conversely, that deletion of amino acids 43–54 in PSD-95 enhanced Src activity in cells. Thus, PSD-95(43–54) appears to be a negative regulator of Src function. Amino acids 43–54 are identical in PSD-95 from mouse, rat and human, suggesting conservation of this function of PSD-95 across mammalian species.
Intermolecular interactions mediated through SH2 domains are a common means for localizing a protein containing an SH2 domain in apposition to the protein containing the ligand. For kinases containing an SH2 domain, such as Src family kinases, SH2 domain-mediated interactions may serve to bring the kinase in close proximity to substrate proteins (Brown and Cooper, 1996). In the case of the Src family kinase Fyn, its SH2 domain has been reported to interact with the PDZ3 domain of PSD-95 (Tezuka et al, 1999). This interaction does not affect Fyn kinase activity and instead recruits Fyn to the NMDAR complex. The region of PSD-95 including amino acids 43–54 has been shown to not be sufficient to bind to Fyn (Tezuka et al, 1999). We found that a PSD-95(43–54)-containing peptide did not affect Fyn catalytic activity but did inhibit the catalytic activity of Src. In addition, we found that removal of the Src SH2 domain-interacting region of PSD-95 did not affect the amount of Src in the NMDAR complex. Thus, in contrast to the interaction of PSD-95 with the Fyn SH2 domain, the interaction with the Src SH2 domain is not required for anchoring the kinase to the NMDAR complex. As the Src SH2 domain interacts with a region of PSD-95 distinct from that with which the Fyn SH2 domain interacts, a single PSD-95 molecule might be capable of binding both kinases. However, the role of each of the SH2-mediated binding interactions is different for each kinase: for Fyn, the SH2 interaction with PSD-95 anchors the kinase in the NMDAR complex without affecting kinase activity (Tezuka et al, 1999), whereas, for Src, the SH2 interaction is not required for localization but rather negatively regulates kinase activity.
A major function for Src within the CNS is regulation of NMDAR-mediated synaptic transmission and plasticity (Salter and Kalia, 2004). We found that intracellularly administering a protein containing the PSD-95(43–54) region depressed synaptic NMDAR currents in cultured hippocampal neurons. These are cells in which tonic Src activity is known to upregulate NMDAR function on an ongoing basis (Yu et al, 1997). In contrast, in CA1 neurons in hippocampal slices, synaptic NMDAR currents are known to not be tonically upregulated by Src (Lu et al, 1998) and we found here that the PSD-95(43–57) peptide had no effect on NMDAR currents in CA1 neurons. Likewise, PSD-95(43–57) did not affect basal AMPAR-mediated synaptic responses in these cells. However, we found that PSD-95(43–57), but not the scrambled peptide, suppressed induction of LTP at Schaffer collateral synapses. By reconstituting NMDAR subunits together with activated Src and wild-type or mutant PSD-95, we found that amino acids 43–54 of PSD-95 suppress the activity of Src within the NMDAR complex. The simplest interpretation of these findings taken together is that, within CA1 neurons, PSD-95 by means of amino acids 43–54 contributes to tonic suppression of Src, which needs to be overcome in order to produce LTP.
Consistent with PSD-95 acting to tonically suppress induction of LTP, mice lacking PSD-95 show a leftward shift in the frequency-response function for induction of LTP and an increase in the maximum level of potentiation (Migaud et al, 1998). Because PSD-95 interacts with multiple signalling proteins (Kim and Sheng, 2004), this enhancement of LTP might be a consequence of the loss of the scaffolding function of PSD-95 and alteration of various proteins within the NMDAR complex. However, our present findings provide a simple explanation that may account, at least in part, for the enhancement of LTP in mice lacking PSD-95: loss of the suppression of Src activity by PSD-95(43–54).
In summary, our present work demonstrates that there is a direct interaction between the Src SH2 domain and PSD-95. This interaction is exceptional in that it is mediated through PSD-95(43–54), a region in PSD-95 not previously known to participate in protein–protein interactions, and the binding to the SH2 domain of Src is phosphotyrosine-independent. PSD-95 negatively regulates, via this interaction, Src catalytic activity within the NMDAR complex and suppresses the induction of LTP. PSD-95 interacts with a number of ion channels and receptors, and Src-mediated phosphorylation is a common means to regulate protein function. Thus, we expect that the Src SH2 domain–PSD-95 interaction may be a widespread mechanism for regulating synaptic transmission and plasticity throughout the CNS.
Materials and methods
Tissue preparation
All animals were used in accordance with the guidelines of the Canadian Council on Animal Care. Forebrain extracts, specifically crude synaptosomal (P2) or PSD fractions, were prepared from adult male rats and solubilized according to previously described protocols (Huang et al, 2001). The following inhibitors were included in all solutions: 20 μg/ml aprotinin, 20 μg/ml pepstatin A, 20 μg/ml leupeptin, 1 mM PMSF and 1 mM sodium orthovanadate.
Recombinant proteins and peptides
DNA constructs used for GST fusion protein expression are detailed in Supplementary data. GST fusion proteins were affinity purified using glutathione Sepharose beads (Amersham) according to the manufacturer's instructions as described in Supplementary data. Recombinant Src and Fyn were purchased from Upstate. The sequence of the scrambled PSD-95(43–57) peptide was LPEQDTTGLNYAVEG. All peptides were synthesized at the Peptide Synthesis Facility, Hospital for Sick Children, Toronto, Canada.
Cell transfection
DNA constructs used for cell transfection are described in Supplementary data. HEK293 cells (ATCC) were transiently transfected with plasmid DNA (maximum 7 μg per 10 cm dish) using a standard calcium phosphate method. For cells expressing recombinant NMDARs, 500 μM APV was included in the media. Cells were lysed 48 h after transfection in modified RIPA buffer with inhibitors listed above for tissue preparation.
Pull-down assay
Equal amounts of GST fusion proteins (20 μg) coupled to glutathione Sepharose beads were incubated overnight at 4°C with proteins solubilized from rat forebrain extracts or HEK293 cell lysates. Beads were then washed 4 × with PBS and re-suspended in 2 × SDS–PAGE sample buffer. Samples were boiled, separated by SDS–PAGE, transferred to nitrocellulose membranes and analyzed by immunoblotting, as described in Supplementary data. To test for direct binding between proteins, GST fusion proteins (5–20 μg) coupled to beads were blocked for 1 h at room temperature in binding buffer (50 mM HEPES pH 7.3, 0.05% (w/v) Triton X-100, 10% (w/v) glycerol, 2 mM DTT) with 0.1% (w/v) BSA. Blocked beads were then incubated overnight at 4°C with recombinant Src (10 ng; Upstate) or increasing amounts of PSD-95(1–54) diluted in binding buffer as indicated. For competition experiments, recombinant Src was first preincubated for 1 h at room temperature with the peptide being tested before incubation with blocked beads. Samples were then washed 4 × , re-suspended and boiled, separated by SDS–PAGE, transferred to a nitrocellulose membrane and analyzed by immunoblotting (see Supplementary data).
Immunoprecipitation
Immunoprecipitation of membrane proteins was performed under weakly denaturing conditions as described previously (Huang et al, 2001). Solubilized proteins from rat forebrain extracts or HEK293 cell lysates were incubated overnight at 4°C with anti-Src (a gift from J Bolen, DNAX, Palo Alto, CA), anti-PSD-95 (Affinity BioReagents, Oncogene) or anti-NR2 (Affinity BioReagents) antibodies. Immune complexes were isolated by the addition of 50 μl of protein G Sepharose beads (Amersham) followed by incubation for 5 h at 4°C. Beads were washed and samples were then separated by SDS–PAGE, transferred to nitrocellulose membranes and analyzed by immunoblotting as detailed in Supplementary data.
SH2 domain ELISA
Direct binding between the GST-Src SH2 domain and PSD-95 peptides was assessed with a modified ELISA using previously described methods (Gilmer et al, 1994). See Supplementary data for further details.
In vitro kinase assay
The Src kinase kit (Upstate) was used to measure Src or Fyn kinase activity in vitro. Kinase assays were performed according to the manufacturer's instructions as detailed in Supplementary data.
Electrophysiological recordings
The methods for miniature EPSC recordings are described in Pelkey et al (2002) and are summarized in Supplementary data. The protocols used for hippocampal slice recordings and for induction of LTP are described in detail in Supplementary data.
Supplementary Material
Supplementary Figures
SUPPLEMENTARY MATERIALS AND METHODS
Acknowledgments
We thank YQ Huang and R Lum for preliminary work on the project; JL Hicks, S Singhroy and D Wong for technical support; J Bolen, S Hanks, A Laudano, J MacDonald, M Sheng, H Umemori and T Yamamoto for reagents; and JD Forman-Kay, JR Glover, WS Trimble and LY Wang for comments and discussions. This work was supported by the Canadian Institutes of Health Research.
References
- Bibbins KB, Boeuf H, Varmus HE (1993) Binding of the Src SH2 domain to phosphopeptides is determined by residues in both the SH2 domain and the phosphopeptides. Mol Cell Biol 13: 7278–7287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss TV, Collingridge GL (1993) A synaptic model of memory: long-term potentiation in the hippocampus. Nature 361: 31–39 [DOI] [PubMed] [Google Scholar]
- Brown MT, Cooper JA (1996) Regulation, substrates and functions of src. Biochim Biophys Acta 1287: 121–149 [DOI] [PubMed] [Google Scholar]
- Chang BY, Conroy KB, Machleder EM, Cartwright CA (1998) RACK1, a receptor for activated C kinase and a homolog of the beta subunit of G proteins, inhibits activity of src tyrosine kinases and growth of NIH 3T3 cells. Mol Cell Biol 18: 3245–3256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Beffert U, Ertunc M, Tang TS, Kavalali ET, Bezprozvanny I, Herz J (2005) Reelin modulates NMDA receptor activity in cortical neurons. J Neurosci 25: 8209–8216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng HC, Nishio H, Hatase O, Ralph S, Wang JH (1992) A synthetic peptide derived from p34cdc2 is a specific and efficient substrate of src-family tyrosine kinases. J Biol Chem 267: 9248–9256 [PubMed] [Google Scholar]
- Cheung HH, Gurd JW (2001) Tyrosine phosphorylation of the N-methyl-D-aspartate receptor by exogenous and postsynaptic density-associated Src-family kinases. J Neurochem 78: 524–534 [DOI] [PubMed] [Google Scholar]
- Cho KO, Hunt CA, Kennedy MB (1992) The rat brain postsynaptic density fraction contains a homolog of the Drosophila discs-large tumor suppressor protein. Neuron 9: 929–942 [DOI] [PubMed] [Google Scholar]
- Dingledine R, Borges K, Bowie D, Traynelis SF (1999) The glutamate receptor ion channels. Pharmacol Rev 51: 7–61 [PubMed] [Google Scholar]
- Donaldson LW, Gish G, Pawson T, Kay LE, Forman-Kay JD (2002) Structure of a regulatory complex involving the Abl SH3 domain, the Crk SH2 domain, and a Crk-derived phosphopeptide. Proc Natl Acad Sci USA 99: 14053–14058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Husseini AE, Craven SE, Chetkovich DM, Firestein BL, Schnell E, Aoki C, Bredt DS (2000) Dual palmitoylation of PSD-95 mediates its vesiculotubular sorting, postsynaptic targeting, and ion channel clustering. J Cell Biol 148: 159–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmer T, Rodriguez M, Jordan S, Crosby R, Alligood K, Green M, Kimery M, Wagner C, Kinder D, Charifson P (1994) Peptide inhibitors of src SH3–SH2-phosphoprotein interactions. J Biol Chem 269: 31711–31719 [PubMed] [Google Scholar]
- Gingrich JR, Pelkey KA, Fam SR, Huang Y, Petralia RS, Wenthold RJ, Salter MW (2004) Unique domain anchoring of Src to synaptic NMDA receptors via the mitochondrial protein NADH dehydrogenase subunit 2. Proc Natl Acad Sci USA 101: 6237–6242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Lu W, Ali DW, Pelkey KA, Pitcher GM, Lu YM, Aoto H, Roder JC, Sasaki T, Salter MW, MacDonald JF (2001) CAKbeta/Pyk2 kinase is a signaling link for induction of long-term potentiation in CA1 hippocampus. Neuron 29: 485–496 [DOI] [PubMed] [Google Scholar]
- Husi H, Ward MA, Choudhary JS, Blackstock WP, Grant SG (2000) Proteomic analysis of NMDA receptor-adhesion protein signaling complexes. Nat Neurosci 3: 661–669 [DOI] [PubMed] [Google Scholar]
- Jones ML, Leonard JP (2005) PKC site mutations reveal differential modulation by insulin of NMDA receptors containing NR2A or NR2B subunits. J Neurochem 92: 1431–1438 [DOI] [PubMed] [Google Scholar]
- Kalia LV, Salter MW (2003) Interactions between Src family protein tyrosine kinases and PSD-95. Neuropharmacology 45: 720–728 [DOI] [PubMed] [Google Scholar]
- Kamps MP, Sefton BM (1986) Neither arginine nor histidine can carry out the function of lysine-295 in the ATP-binding site of p60src. Mol Cell Biol 6: 751–757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim E, Sheng M (2004) PDZ domain proteins of synapses. Nat Rev Neurosci 5: 771–781 [DOI] [PubMed] [Google Scholar]
- Kim E, Cho KO, Rothschild A, Sheng M (1996) Heteromultimerization and NMDA receptor-clustering activity of Chapsyn-110, a member of the PSD-95 family of proteins. Neuron 17: 103–113 [DOI] [PubMed] [Google Scholar]
- Kornau HC, Schenker LT, Kennedy MB, Seeburg PH (1995) Domain interaction between NMDA receptor subunits and the postsynaptic density protein PSD-95. Science 269: 1737–1740 [DOI] [PubMed] [Google Scholar]
- Li SC, Gish G, Yang D, Coffey AJ, Forman-Kay JD, Ernberg I, Kay LE, Pawson T (1999) Novel mode of ligand binding by the SH2 domain of the human XLP disease gene product SAP/SH2D1A. Curr Biol 9: 1355–1362 [DOI] [PubMed] [Google Scholar]
- Lim IA, Hall DD, Hell JW (2002) Selectivity and promiscuity of the first and second PDZ domains of PSD-95 and synapse-associated protein 102. J Biol Chem 277: 21697–21711 [DOI] [PubMed] [Google Scholar]
- Lu YM, Roder JC, Davidow J, Salter MW (1998) Src activation in the induction of long-term potentiation in CA1 hippocampal neurons. Science 279: 1363–1367 [DOI] [PubMed] [Google Scholar]
- Macdonald DS, Weerapura M, Beazely MA, Martin L, Czerwinski W, Roder JC, Orser BA, MacDonald JF (2005) Modulation of NMDA receptors by pituitary adenylate cyclase activating peptide in CA1 neurons requires G alpha q, protein kinase C, and activation of Src. J Neurosci 25: 11374–11384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuko N, Makino K, Kuwahara H, Fukunaga K, Sudo T, Araki N, Yamamoto H, Yamada Y, Miyamoto E, Saya H (1999) Interaction of NE-dlg/SAP102, a neuronal and endocrine tissue-specific membrane-associated guanylate kinase protein, with calmodulin and PSD-95/SAP90. A possible regulatory role in molecular clustering at synaptic sites. J Biol Chem 274: 5782–5790 [DOI] [PubMed] [Google Scholar]
- Mehta S, Wu H, Garner CC, Marshall J (2001) Molecular mechanisms regulating the differential association of kainate receptor subunits with SAP90/PSD-95 and SAP97. J Biol Chem 276: 16092–16099 [DOI] [PubMed] [Google Scholar]
- Migaud M, Charlesworth P, Dempster M, Webster LC, Watabe AM, Makhinson M, He Y, Ramsay MF, Morris RG, Morrison JH, O'Dell TJ, Grant SG (1998) Enhanced long-term potentiation and impaired learning in mice with mutant postsynaptic density-95 protein. Nature 396: 433–439 [DOI] [PubMed] [Google Scholar]
- Moon IS, Apperson ML, Kennedy MB (1994) The major tyrosine-phosphorylated protein in the postsynaptic density fraction is N-methyl-D-aspartate receptor subunit 2B. Proc Natl Acad Sci USA 91: 3954–3958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nada S, Shima T, Yanai H, Husi H, Grant SG, Okada M, Akiyama T (2003) Identification of PSD-93 as a substrate for the Src family tyrosine kinase Fyn. J Biol Chem 278: 47610–47621 [DOI] [PubMed] [Google Scholar]
- Nakazawa T, Komai S, Tezuka T, Hisatsune C, Umemori H, Semba K, Mishina M, Manabe T, Yamamoto T (2001) Characterization of Fyn-mediated tyrosine phosphorylation sites on GluR epsilon 2 (NR2B) subunit of the N-methyl-D-aspartate receptor. J Biol Chem 276: 693–699 [DOI] [PubMed] [Google Scholar]
- Pelkey KA, Askalan R, Paul S, Kalia LV, Nguyen TH, Pitcher GM, Salter MW, Lombroso PJ (2002) Tyrosine phosphatase STEP is a tonic brake on induction of long-term potentiation. Neuron 34: 127–138 [DOI] [PubMed] [Google Scholar]
- Poy F, Yaffe MB, Sayos J, Saxena K, Morra M, Sumegi J, Cantley LC, Terhorst C, Eck MJ (1999) Crystal structures of the XLP protein SAP reveal a class of SH2 domains with extended, phosphotyrosine-independent sequence recognition. Mol Cell 4: 555–561 [DOI] [PubMed] [Google Scholar]
- Rostas JA, Brent VA, Voss K, Errington ML, Bliss TV, Gurd JW (1996) Enhanced tyrosine phosphorylation of the 2B subunit of the N-methyl-D-aspartate receptor in long-term potentiation. Proc Natl Acad Sci USA 93: 10452–10456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salter MW, Kalia LV (2004) Src kinases: a hub for NMDA receptor regulation. Nat Rev Neurosci 5: 317–328 [DOI] [PubMed] [Google Scholar]
- Shakespeare W, Yang M, Bohacek R, Cerasoli F, Stebbins K, Sundaramoorthi R, Azimioara M, Vu C, Pradeepan S, Metcalf C III, Haraldson C, Merry T, Dalgarno D, Narula S, Hatada M, Lu X, van Schravendijk MR, Adams S, Violette S, Smith J, Guan W, Bartlett C, Herson J, Iuliucci J, Weigele M, Sawyer T (2000) Structure-based design of an osteoclast-selective, nonpeptide src homology 2 inhibitor with in vivo antiresorptive activity. Proc Natl Acad Sci USA 97: 9373–9378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Songyang Z, Shoelson SE, Chaudhuri M, Gish G, Pawson T, Haser WG, King F, Roberts T, Ratnofsky S, Lechleider RJ (1993) SH2 domains recognize specific phosphopeptide sequences. Cell 72: 767–778 [DOI] [PubMed] [Google Scholar]
- Tavares GA, Panepucci EH, Brunger AT (2001) Structural characterization of the intramolecular interaction between the SH3 and guanylate kinase domains of PSD-95. Mol Cell 8: 1313–1325 [DOI] [PubMed] [Google Scholar]
- Tezuka T, Umemori H, Akiyama T, Nakanishi S, Yamamoto T (1999) PSD-95 promotes Fyn-mediated tyrosine phosphorylation of the N-methyl-D-aspartate receptor subunit NR2A. Proc Natl Acad Sci USA 96: 435–440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas SM, Brugge JS (1997) Cellular functions regulated by Src family kinases. Annu Rev Cell Dev Biol 13: 513–609 [DOI] [PubMed] [Google Scholar]
- Waksman G, Shoelson SE, Pant N, Cowburn D, Kuriyan J (1993) Binding of a high affinity phosphotyrosyl peptide to the Src SH2 domain: crystal structures of the complexed and peptide-free forms. Cell 72: 779–790 [DOI] [PubMed] [Google Scholar]
- Yaffe MB, Leparc GG, Lai J, Obata T, Volinia S, Cantley LC (2001) A motif-based profile scanning approach for genome-wide prediction of signaling pathways. Nat Biotechnol 19: 348–353 [DOI] [PubMed] [Google Scholar]
- Yu XM, Askalan R, Keil GJ, Salter MW (1997) NMDA channel regulation by channel-associated protein tyrosine kinase Src. Science 275: 674–678 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figures
SUPPLEMENTARY MATERIALS AND METHODS


