Abstract
The survival and growth of squamous epithelial cells require signals generated by integrin-matrix interactions. After conversion to squamous cell carcinoma, the cells remain sensitive to detachment-induced anoikis, yet in tumor cell aggregates, which are matrix-deficient, these cells are capable of suprabasal survival and proliferation. Their survival is enhanced through a process we call synoikis, whereby junctional adhesions between neighboring cells generate specific downstream survival signals. Here we show that in squamous cell carcinoma cells, E-cadherin-mediated cell-cell contacts specifically induce activation of epidermal growth factor receptor (EGFR). EGFR activation in turn triggers the ERK/MAPK signaling module, leading to elevation of anti-apoptotic Bcl-2. After intercellular adhesion, formation of adherens junctions triggers the formation of E-cadherin-EGFR complexes, correlating with EGFR transactivation. Analysis of the process with a dominant-negative EGFR mutant indicated that activation of EGFR is ligand-independent. Our data implicate cell-cell adhesion-induced activation of EGFR as a cooperative mechanism that generates compensatory survival signaling, protecting malignant cells from detachment-induced death.
Survival of normal epithelial cells depends on signals generated by the interaction of these cells with a thin extracellular matrix called the basement membrane. In the absence of these signals, the cells die, exhibiting molecular characteristics of apoptosis.1,2 This form of apoptosis is also called anoikis, or “death of homelessness,” because it is believed to preclude epithelial cells from reattachment and growth outside their proper tissue context.3,4 By contrast, many cancer cells of epithelial origin are anoikis resistant: they can survive in the absence of contact with the basement membrane inside three-dimensional tumor nests and in the absence of matrix attachment during metastasis.5 Therefore, acquisition of anoikis resistance plays an essential role in the progression of certain cancers.
Some of the mechanisms that confer anoikis resistance on epithelial cells during tumor progression have been described.6–9 Our previous work implicated E-cadherin-mediated cell-cell adhesion in protecting oral squamous cell carcinoma (SCC) cells from anoikis.10 However, the exact mechanism of E-cadherin-mediated anoikis resistance is unclear. Because cadherins lack enzymatic activity, their ability to induce cell survival signals may depend on their association with other signaling systems. E-cadherin can physically associate with a number of signaling effectors, such as PI3K and PTP1B, in adherens junctions.11,12 In this study we focused on the potential role of the growth factor receptor tyrosine kinase, epidermal growth factor receptor (EGFR), because it is well known that in most oral SCCs and in cell lines established from these tumors EGFR is overexpressed13 and that EGFR can complex with E-cadherin14 leading to activated EGFR.15 We hypothesized that E-cadherin-mediated cell-cell adhesion transactivates EGFR and that activation of EGFR and the downstream pathways promotes survival and resistance to anoikis.
To test this hypothesis, we used a three-dimensional multicellular model of SCC in which cells are cultured in the absence of adhesive substrate yet form complex cell-cell adhesive interactions.10 We first performed experiments to confirm that EGFR is activated on E-cadherin-mediated cell-cell contact formation in SCC cells and that this EGFR activation is mediated by E-cadherin. Using specific pharmacological inhibitors, we confirmed that activation of EGFR and the downstream MAPK pathway is required for E-cadherin-mediated anoikis resistance in SCC cells. In search of an underlying mechanism(s) by which E-cadherin transactivates EGFR, we found evidence that E-cadherin-mediated EGFR activation is ligand-independent and is related to E-cadherin/EGFR complex formation and clustering. Taken together, our results suggest that E-cadherin-mediated cell-cell adhesion promotes cell survival through ligand-independent activation of EGFR and the downstream MAPK pathway.
Experimental Procedures
Materials
Human recombinant epidermal growth factor (EGF) was from Invitrogen, Carlsbad, CA. Pharmacological inhibitors of EGFR (tyrphostin AG1478) were from Calbiochem (La Jolla, CA), MEK1/2 inhibitor (U0126) from Promega (Madison, WI), and PI3K inhibitor (LY294002) from Cell Signaling Technology, Beverly, MA. A mouse anti-E-cadherin monoclonal antibody (mAb) (HECD-1; obtained from M. Takeichi, Kyoto University, Japan) was used in immunoprecipitation, immunofluorescence staining, and antibody inhibition and clustering experiments. Anti-human E-cadherin (rat mAb, E9; obtained from C. Damsky, University of California, San Francisco, CA) was used in Western blotting. Anti-mouse E-cadherin (rat mAb ECCD-2; Zymed, South San Francisco, CA) was used in fluorescence-activated cell-sorting (FACS) analysis and immunofluorescent staining. Anti-EGFR and tyrosine-phosphorylated EGFR (activated EGFR) (mAb clones 13 and 74, respectively; Transduction Laboratories, Lexington, KY) were used in Western blotting and immunofluorescence staining. Antibodies raised against the extracellular domain of EGFR (Ab-11; NeoMarkers, Freemont, CA) were used in Western blotting. Antibodies against phospho-MAPK (phospho-p44/42-Thr202/Tyr204; Cell Signaling Technology) and MAPK (anti-pan-ERK mAb; Transduction Laboratories) were used in Western blotting. A mouse anti–Bcl-2 mAb (ascites; DAKO, Carpinteria, CA) was used in immunoprecipitation and Western blotting. A polyclonal antibody to Bax (Ab-1; Oncogene Research, Boston, MA) and to Bcl-xL (BD Biosciences) was used in Western blotting. For loading control, a mouse anti-tubulin mAb (Ab-4; NeoMarkers) was used in Western blotting.
Cell Culture
The human oral SCC cell lines HSC-3 and HOC-313, clones D1 and C8, have been described previously.16 Cells were cultured in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum (Gemini, Woodland, CA). HOC-313 cells were cloned by serial dilution of a single-cell suspension plated in 96-well plates. Single-cell clones were randomly selected for further culture.
Cell monolayer cultures were prepared by seeding 6 × 105 cells in tissue culture dishes (10 cm; Falcon). For the culture of HSC-3 and C8 cells in suspension, monolayers were trypsinized the day before and then briefly treated with 5 mmol/L ethylenediaminetetraacetic acid (EDTA) to prepare single cells. To generate multicellular aggregates (MCAs), cells were then plated on polyhydroxylethyl-methacrylate (poly-HEMA)-coated 10-cm dishes (2 × 106 cells/dish) in the presence of Dulbecco’s modified Eagle’s medium supplemented with 0.5% fetal bovine serum. To produce suspended single-cell cultures, cells were suspended in semisolid medium consisting of 0.5% fetal bovine serum/Dulbecco’s modified Eagle’s medium containing 1.5% methylcellulose (Sigma, St. Louis, MO) at 6 × 105 cells per 10-cm poly-HEMA-coated dish. For harvesting of single cells in methylcellulose, cultures were diluted with two parts of phosphate-buffered saline (PBS) and centrifuged at 900 × g.
Recombinant Adenovirus Infection
The infection of HSC-3 cells by recombinant adenoviral vectors was performed as described previously.17 Briefly, the recombinant adenoviral vectors carrying EGFR-CD533 or green fluorescent protein (GFP) (gifts from K. Valerie, Virginia Commonwealth University, Richmond, VA) were added to HSC-3 cells at a multiplicity of infection of 100. Cells were then incubated for 24 hours before further experiments to ensure adequate expression of the genes of interest.
Transfection
We generated the E-cadherin or Bcl-2 expression vectors by taking the full-length mouse E-cadherin cDNA or human Bcl-2 cDNA and subcloning into the pcDNA3 vector (Invitrogen). For constitutive expression of E-cadherin or Bcl-2, 1 × 106 E-cadherin-negative C8 cells or HSC-3 cells were transfected with 4 μg of pcDNA3/E-cadherin vector or pcDNA3/Bcl-2 vector, respectively, using the Lipofectamine Plus kit (Life Technologies, Inc., Grand Island, NY) according to the manufacturer’s protocol. Stable transfected cells were selected in 800 μg/ml of G418 (Life Technologies, Inc.) for 2 weeks. Because the Bcl-2-transfected cells exhibit a slightly higher rate of proliferation (data not shown), we were able to isolate a cell population after several more passages that expressed elevated levels of ectopically expressed Bcl-2, which was confirmed by immunoblot analysis. To enrich for E-cadherin expressing C8 cells, the population of transfected cells was processed for two cycles of FACS after labeling with ECCD-2 anti-E-cadherin mAb.
Immunofluorescence Staining
HSC-3 cells were plated as MCA culture for the indicated times and then transferred onto poly-l-lysine-coated glass coverslips and incubated for 30 minutes at 37°C. After washing with 0.1 mmol/L Na3VO4 in PBS, cells were fixed and permeabilized for 15 minutes at room temperature with 4% paraformaldehyde and 0.1% Triton X-100. Cells were stained with primary antibodies and counterstained by either fluorescein- or rhodamine-labeled secondary antibodies. Analysis was performed with a confocal microscope (Bio-Rad, Richmond, CA).
Immunoprecipitation and Immunoblotting
Immunoprecipitation and immunoblotting were performed as described previously.16 Cells were lysed in RIPA buffer (150 mmol/L NaCl, 50 mmol/L Tris-HCl, pH 7.5, 1% Nonidet P-40, 0.1% sodium dodecyl sulfate, 5 mmol/L EDTA, 2 mmol/L phenylmethyl sulfonyl fluoride, 1 mmol/L aprotinin, 1 mmol/L leupeptin, 10 mmol/L sodium pyrophosphate, 10 mmol/L sodium fluoride, and 1 mmol/L sodium vanadate). Cell lysates were processed for immunoprecipitation with specific antibody. The immunoprecipitates or total cell lysates were fractionated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, transferred to Immobilon membranes, and probed with appropriate antibodies. Immunoreactive bands were visualized using enhanced chemiluminescence (Amersham Biosciences, Piscataway, NJ).
Antibody Clustering Assay
HSC-3 cells grown to near confluence were serum-starved overnight. For antibody-mediated clustering, cells were harvested from culture with 5 mmol/L EDTA, washed with PBS, and plated on poly-HEMA-coated dishes. The cells in suspension were incubated for 30 minutes at 4°C with a predetermined saturating concentration of HECD-1 antibody (20 μg/ml) in serum-free medium. The cells were then incubated with 5 μg/ml of goat anti-mouse IgG for various times at 37°C to induce clustering of E-cadherin. At the end of each time period, the cells were washed with ice-cold PBS containing 10 mmol/L NaF, 10 mmol/L Na4P2O7, and 0.5 mmol/L Na3VO4, and lysed with RIPA buffer, followed by immunoblotting.
Apoptosis and Viability Assays
To assay for intranucleosomal DNA cleavage, cells were grown as monolayers, suspended single cells, or MCAs for the indicated times. Cells were then collected from poly-HEMA dishes by pipetting or from tissue culture dishes by scraping into the medium in which they had been incubated. In monolayer cultures, floating cells were collected and combined with the attached cells before DNA extraction. Harvested cells were then processed for the DNA-laddering assay and a FACS-based terminal dUTP nick-end labeling (TUNEL) assay. For intranucleosomal DNA laddering, genomic DNA was extracted using a Suicide Track DNA Ladder isolation kit (Oncogene Research). Samples were then analyzed in a 1.5% agarose gel. For the TUNEL assay, cells (2 × 106) were fixed for 15 minutes in 1% paraformaldehyde in PBS, followed by 30-minute fixation in 70% ethanol. The fixed cells were then washed, stained with FITC-dUTP, and treated with propidium iodide/RNase using an APO-Direct kit (Pharmingen, La Jolla, CA), followed by flow cytometric analysis using a FACScan Analyzer (Becton Dickinson, Mountain View, CA).
To assess the proportion of cells that not only survived but also regained proliferative ability after suspension, cells from suspension cultures were replated at low cell density on tissue culture plates in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum. The clonogenic and proliferative ability of cells that reattached after plating was determined 3 days later by crystal violet staining and counting of colonies.
Results
E-Cadherin-Mediated Cell-Cell Adhesion Activates EGFR
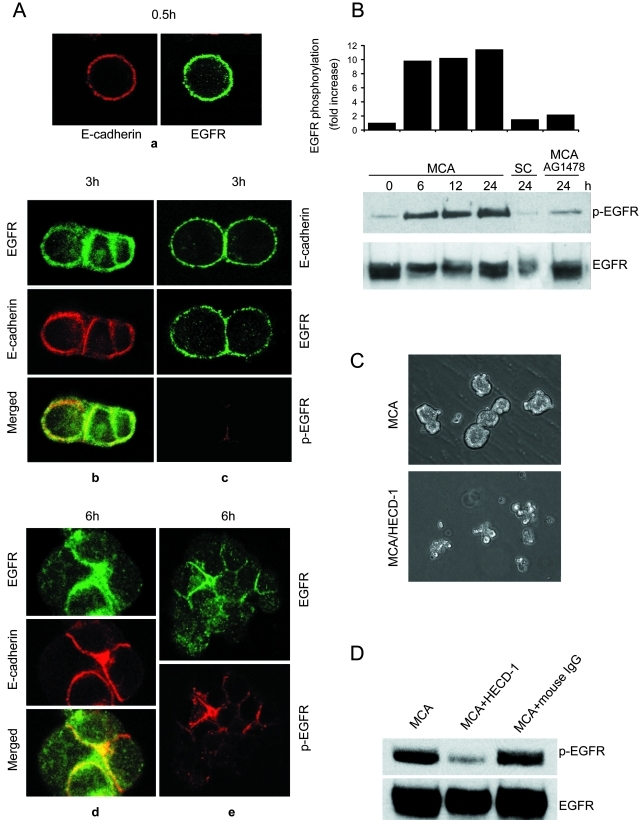
We have previously shown that survival after loss of attachment to the extracellular matrix requires E-cadherin-mediated intercellular adhesion. In the current study, we first examined the dynamics of E-cadherin and EGFR distribution, because the two receptors have been reported to be associated as a complex in SCC cells.14,15 Previously we had reported that when HSC-3 cells were plated on poly-HEMA-coated dishes, the cells gradually formed E-cadherin-mediated aggregates, beginning with single cells that subsequently formed small aggregates of a few cells by 3 hours that had begun to compact by 6 hours, collected into large, irregular clumps of cells by 12 hours, and condensed into spherical MCAs between 15 and 24 hours.10,16 Analysis of the distribution of E-cadherin and EGFR by confocal microscopy showed that in freshly detached cells, both receptors were diffusely and uniformly distributed, with no evidence of patching (Figure 1A, a). As intercellular adhesion proceeded, the receptors began to co-localize at the cell-cell boundaries by as early as 3 hours (Figure 1A, b). However, there was a fraction of cells that had just started to form adhesions in which E-cadherin and EGFR remained evenly dispersed around the cell periphery (Figure 1A, c). In some cases it appeared that E-cadherin was more enriched at the junctional areas compared to EGFR (Figure 1A, b). By 6 hours much of E-cadherin and EGFR had co-distributed at sites of cell-cell adhesion, and had disappeared from cell surface areas that were devoid of cell-cell contact (Figure 1A, d and e).
Figure 1-4260.
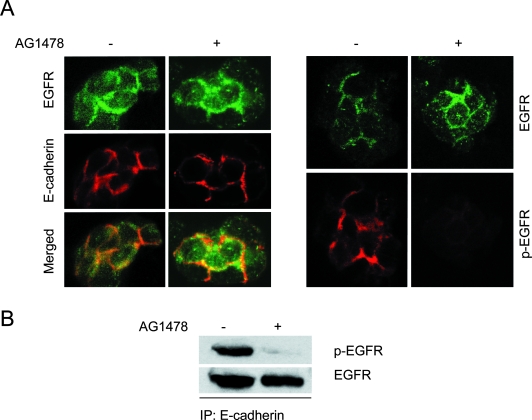
E-cadherin-mediated cell-cell adhesion induces EGFR activation. A: HSC-3 cells were cultured as MCAs for 0.5 hours (a), 3 hours (b, c), or 6 hours (d, e) and then transferred onto glass coverslips and immunolabeled for EGFR, E-cadherin, or activated EGFR (p-EGFR) as indicated. In C, which represents the early stage of cell aggregation, the top frame stained for E-cadherin is of a different cell pair than the others. Specimens were analyzed by confocal microscopy, and representative images are shown. B: Immunoblot analysis of activated EGFR during MCA formation. As controls, HSC-3 cells were cultured in suspension as single cells (SCs) for 24 hours or treated with 1 μmol/L tyrphostin AG1478 for 24 hours in MCA culture. Relative protein densities of phosphorylated EGFR were determined; EGFR kinase activity is expressed as fold induction relative to the HSC-3 MCA at 0 hours. Data reported here are representative of four independent experiments. C: Effect of anti-E-cadherin mAb on HSC-3 MCA formation. HSC-3 cells were plated as MCA culture in the absence or presence of 50 μg/ml anti-E-cadherin mAb HECD-1 for 24 hours. D: HSC-3 cells were plated as MCA culture in the presence of 50 μg/ml anti-E-cadherin mAb HECD-1 or mouse IgG for 24 hours. Equivalent protein was immunoblotted with mAbs to phospho-EGFR and total EGFR.
That EGFR was rapidly concentrated at cell-cell contacts in MCAs prompted us to investigate whether E-cadherin-mediated intercellular adhesion induces EGFR phosphorylation and activation. After cells started to form MCAs, phosphorylated EGFR (p-EGFR), visualized with specific antibodies, was detectable at cell-cell adhesion sites by 3 hours but was the intensity was weak and variable (Figure 1A, c). However, by 6 hours a strong signal for p-EGFR was detected in most cell aggregates (Figure 1A, e).
To confirm that phosphorylated EGFR detected at cell-cell junctions represented transactivation of the receptor, we analyzed the level of activated EGFR during the formation of aggregates by immunoblotting with antibodies specific for tyrosine-phosphorylated EGFR (Figure 1B). At the time of plating, activated EGFR was at basal levels. After the gradual formation of cell aggregates, there was a parallel induction of EGFR activation evident at 6 hours and persisting at even higher levels at the 12- and 24-hour time points. In contrast, phosphorylated EGFR in suspended single cells remained at low levels throughout this time period. To test whether cell-cell adhesion was correlated with intrinsic receptor kinase activity, we used tyrphostin AG1478, a specific inhibitor of EGFR kinase activity.18 Incubation with 1 μmol/L AG1478 abolished EGFR activation in aggregates at 24 hours (Figure 1B). Together, these results suggest that cell-cell adhesion leads to EGFR activation at sites of E-cadherin engagement and that adhesion-mediated EGFR activation requires receptor kinase activity.
To establish the role of E-cadherin in EGFR activation, HSC-3 cells were treated with inhibiting anti-E-cadherin antibody HECD-1 (50 μg/ml) before plating and during MCA formation. In the presence of HECD-1, the formation of the large, compact aggregates was severely inhibited at 24 hours (Figure 1C). As shown in Figure 1D, hindering E-cadherin engagement with blocking antibodies ablated EGFR activation detected in immunoblots, whereas the control IgG was without effect. Therefore, the activation of EGFR after cell-cell adhesion formation is dependent on functional E-cadherin.
E-Cadherin-Mediated EGFR Activation Promotes Anoikis Resistance
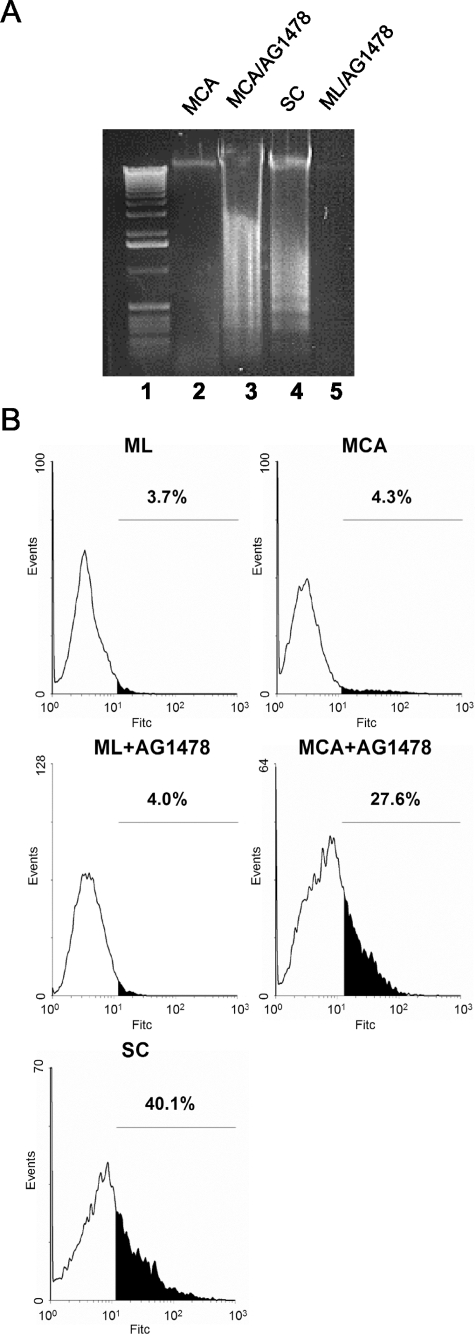
MCAs of HSC-3 cells were resistant to apoptosis, whereas suspended single cells rapidly underwent anoikis, as detected by the extensive DNA laddering typical of apoptotic intranucleosomal cleavage (Figure 2A, lanes 2 and 4). To test whether E-cadherin-dependent EGFR activation provides the survival signal in aggregates, we incubated cells with tyrphostin AG1478. EGFR blockade with 1 μmol/L AG1478 induced apoptosis in HSC-3 cell aggregates cultured for 48 hours, indicated by DNA laddering (Figure 2A, lane 3). The DNA laddering induced by AG1478 was similar to that of HSC-3 single cells cultured in suspension (Figure 2A, lane 4). By contrast, AG1478-treated HSC-3 monolayer cells showed no evidence of apoptosis (Figure 2A, lane 5). Next, we analyzed DNA fragmentation to identify apoptotic cells by TUNEL assay. Again, AG1478 treatment dramatically increased the percentage of TUNEL-positive cells in HSC-3 MCAs at 48 hours (Figure 2B). Control monolayer HSC-3 cells showed low levels of TUNEL-positive cells, whereas, after suspension as single cells for the same time period, more than 40% of the cells were apoptotic.
Figure 2-4260.
Cell-cell adhesion-induced EGFR activation suppresses anoikis. A: DNA fragmentation of HSC-3 cells. HSC-3 cells were plated as MCA culture in the absence or presence of 1 μg/ml AG1478 for 48 hours (lanes 2 and 3). Suspended single cells (SC) alone (lane 4) and monolayers (ML) cultured for 48 hours in the presence of 1 μmol/L AG1478 (lane 5) were used as controls. The DNA laddering assay for intranucleosomal DNA cleavage was performed as described in Experimental Procedures. Lane 1: Standard 100-bp DNA ladder. B: TUNEL analysis of AG1478-treated HSC-3 cells. HSC-3 cells were plated as ML, MCA, or SC culture for 48 hours before TUNEL analysis. AG1478 (1 μmol/L) was added to the culture as indicated. Values represent the apoptotic cell fractions (%) that stained positive with FITC-dUTP.
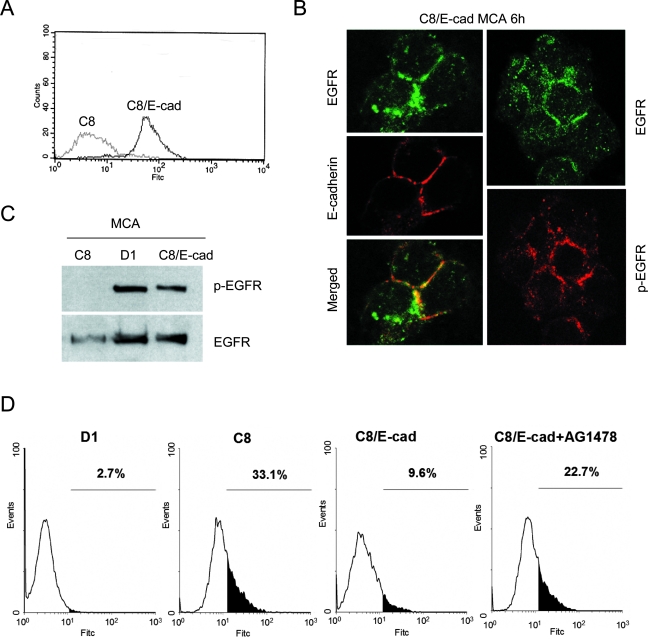
To further test the idea that E-cadherin engagement can induce EGFR activation and rescue cells from apoptosis, we exogenously expressed mouse E-cadherin in E-cadherin-negative C8 cells, a SCC cell line that fails to forms poor intercellular adhesions and undergoes apoptosis in suspension.10 The E-cadherin-transfected C8 (C8/E-cad) cells expressed high levels of the receptor (Figure 3A). To determine whether the exogenously transfected E-cadherin co-localizes with EGFR at cell-cell boundaries, aggregates of C8/E-cad cells were processed for immunofluorescence analysis. Similar to what was observed with HSC-3 MCAs (Figure 1A), we found that in C8/E-cad MCAs the EGFR was also co-localized with E-cadherin and that significant levels of phosphorylated EGFR was detected by immunolabeling at cell-cell junctions (Figure 3B). In parental C8 cells that poorly aggregate, the distribution of EGFR remained uniformly distributed when cells are in suspension (data not shown). Next, EGFR activation was assessed by immunoblotting for EGFR tyrosine phosphorylation in the C8/E-cad cells after they were plated as MCAs for 24 hours. No EGFR activation was detected in MCAs from E-cadherin–negative C8 cells, but strong phosphorylation of EGFR was induced in MCAs from C8/E-cad cells (Figure 3C). The level of EGFR activation was nearly as intense as that generated in high-E-cadherin-expressing D1 cells, a positive control. Subsequently we analyzed the effect of ectopically expressed E-cadherin on survival in suspension by TUNEL assay in which the C8/E-cad cells showed enhanced resistance to anoikis (Figure 3D). Whereas ∼33% of C8 cells cultured in suspension were TUNEL-positive, only 9.6% of the C8/E-cad cells were apoptotic. Again, this enhanced anoikis resistance of C8/E-cad cells was reversed by EGFR kinase blockade with tyrphostin AG1478 (22.7%). Therefore, ectopic expression of E-cadherin is able to induce EGFR activation in C8 MCAs and partially rescue the C8 cells from anoikis.
Figure 3-4260.
Ectopic expression of E-cadherin rescues E-cadherin-negative cells from anoikis. A: FACS analysis of E-cadherin-transfected C8 (C8/E-cad) cells. E-cadherin-negative C8 cells are shown as a control. B: C8/E-cad cells were cultured as MCAs for 6 hours and then transferred onto glass coverslips and immunostained for EGFR (green), and E-cadherin or activated EGFR (p-EGFR) (red). C: Immunoblot analysis of activated EGFR in E-cadherin-transfected C8 MCAs at 24 hours. E-cadherin-positive D1 and E-cadherin-negative C8 MCAs are shown as controls. D: TUNEL analysis of E-cadherin-transfected C8 MCAs at 48 hours. D1 and C8 MCAs are shown as controls.
E-Cadherin-Mediated Adhesion Leads to ERK/MAPK Activation
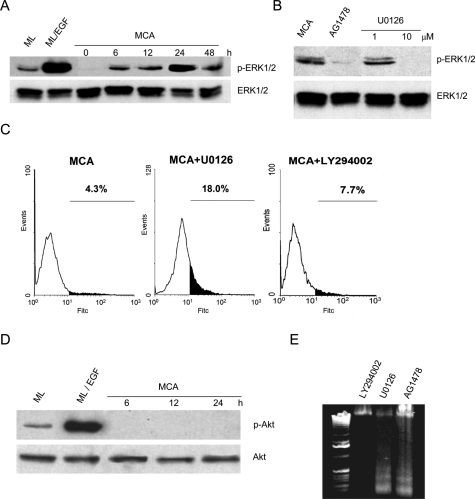
Among downstream pathways of EGFR, MEK/MAPK (ERK1/2) and PI3K/Akt pathways are believed to be the main pathways involved in cell survival.19 We tested whether E-cadherin-dependent phosphorylation of EGFR activates these two pathways. After induction of cell-cell adhesion, there was a progressive and sustained elevation of phosphorylated ERK1/2 that was evident as early as 6 hours and peaked at ∼24 hours (Figure 4A). This time-course activation of ERK1/2 closely paralleled EGFR activation after aggregate formation. Moreover, inhibition of EGFR kinase activity with 1 μmol/L tyrphostin AG1478 abolished ERK1/2 phosphorylation, indicating that ERK1/2 activation in MCAs was induced by EGFR transactivation (Figure 4B). To determine whether downstream MAPK phosphorylation is required for the survival of MCAs in suspension, we used U0126, a specific MEK inhibitor, and measured DNA fragmentation by TUNEL assay to quantify apoptotic cells. As shown in Figure 4B, 10 μmol/L U0126 blocked ERK1/2 phosphorylation in HSC-3 cell aggregates. At this concentration, U0126 also induced apoptosis in HSC-3 aggregates at 48 hours (Figure 4C).
Figure 4-4260.
EGFR-dependent ERK/MAPK activation in HSC-3 cell aggregates. A: ERK1/2-MAPK activation pattern in HSC-3 MCAs was determined as described in Experimental Procedures. B: Effect of AG1478 on ERK1/2 phosphorylation. HSC-3 cells were plated as MCA culture in the absence or presence of 1 μmol/L AG1478 for 24 hours before protein extraction. The MEK inhibitor U0126 at 1 and 10 μmol/L was used as the control. C: TUNEL analysis of 10 μmol/L U0126-treated or 50 μmol/L LY294002-treated HSC-3 MCAs at 48 hours as described in Experimental Procedures. D: Akt activation pattern in HSC-3 MCAs using specific antibodies. E: DNA fragmentation of HSC-3 cells. HSC-3 cells were plated as MCA culture in the presence of 50 μmol/L LY294002, 10 μmol/L U0126, or 1 μg/ml AG1478 for 48 hours.
In contrast to the robust ERK1/2 activation after intercellular adhesion, phosphorylated Akt was not detected at significant levels in MCAs (Figure 4D). However, Akt activation was readily observed in substrate-adherent HSC-3 monolayer cells, and the level of activated Akt was strongly elevated after EGF treatment. The apparent lack of a role for Akt in cell-cell adhesion-induced survival is also suggested by the effect of the specific PI3K inhibitor LY294002, which had no detectable effect on MCA cell survival measured by both TUNEL assay (Figure 4C) and DNA laddering assay (Figure 4E). In contrast, treatment of MCAs with AG1478 to block EGFR, or with U0126 to inhibit MAPK, induced apoptosis as evidenced by extensive DNA laddering. These findings imply that MCA cell survival depends primarily on the E-cadherin/EGFR/MAPK signaling pathway.
Adhesion-Dependent EGFR and MAPK Activation Regulates Bcl-2 Expression
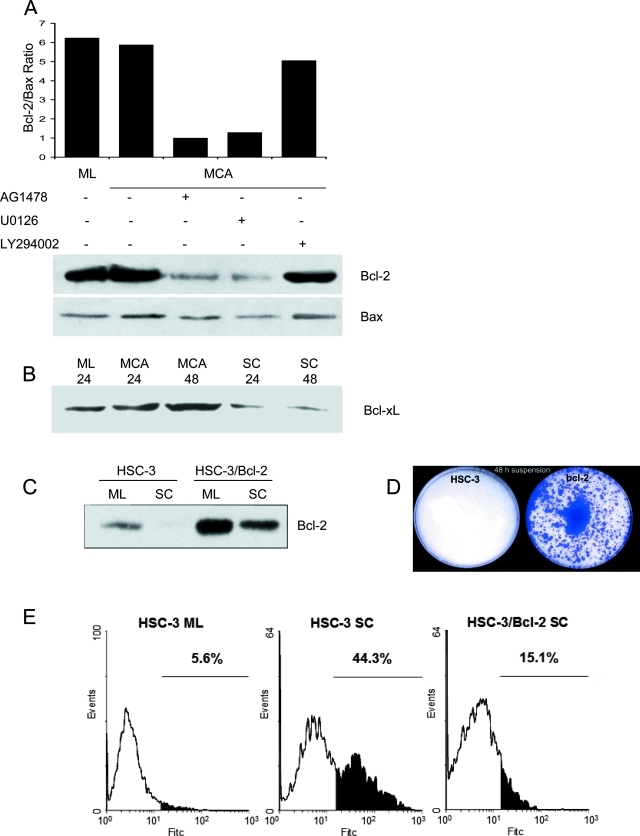
Previously we found that the level of anti-apoptotic Bcl-2 protein is elevated in response to E-cadherin-mediated adhesion.10 These results indicated that the susceptibility to apoptosis in suspended single cells is correlated with the loss of Bcl-2 expression. To test whether E-cadherin-mediated adhesion regulates Bcl-2 expression via the EGFR/MAPK pathway, we treated HSC-3 aggregates with either EGFR inhibitor AG1478, MAPK inhibitor U0126, or PI3K inhibitor LY294002 (Figure 5A). As expected, both monolayers and MCAs exhibited high Bcl-2 levels. But after treatment with either AG1478 or U0126, the level of Bcl-2 decreased drastically, whereas the level of Bax remained relatively constant. The Bcl-2/Bax protein ratio decreased by more than threefold in MCAs after blockade of EGFR or MAPK. Consistent with what we observed above, treatment with LY294002 did not affect the Bcl-2/Bax protein ratio in MCAs, further suggesting that PI3K/Akt pathway was not essential for E-cadherin-mediated cell survival. We also examined the levels of the related Bcl-2 family member, Bcl-xL (Figure 5B). Bcl-xL also showed high levels of expression after MCA formation but was down-regulated in suspended single cells paralleling the response seen for Bcl-2.
Figure 5-4260.
Analysis of Bcl-2 family proteins in HSC-3 cell aggregates. A: HSC-3 cells were plated as MCA culture in the presence of 1 μmol/L AG1478, 10 μmol/L U0126, or 50 μmol/L LY294002 for 24 hours before cell lysates were processed for immunoprecipitation and immunoblotting with anti-Bcl-2 mAb. Equivalent protein was also immunoblotted with anti-Bax antibody, and the relative density of the Bcl-2/Bax protein ratio was determined. Data reported here are representative of three independent experiments. B: Immunoblot analysis of Bcl-xL in HSC-3 cells cultured as monolayers (ML), MCAs, or as suspended single cells (SC) for the indicated times. C: Immunoblot analysis of Bcl-2 in Bcl-2-transfected HSC-3 cells cultured as monolayers (ML) or as suspended single cells (SC). Controls were normal HSC-3 cells cultured as monolayers or as suspended single cells. D: Clonal growth of control HSC-3 cells or of Bcl-2-transfected HSC-3 cells recovered after 48 hours of single-cell suspension culture. E: TUNEL analysis of Bcl-2-transfected HSC-3 cells cultured as suspended single cells for 48 hours. Normal HSC-3 monolayers and suspended single cells were used as controls.
To establish whether elevated Bcl-2 is linked to HSC-3 cell survival, we stably transfected HSC-3 cells with Bcl-2. Overexpression of Bcl-2 in HSC-3 cells was then confirmed by Western blotting (Figure 5C). Compared with control cells, Bcl-2 protein levels were much higher in both monolayer and single-cell suspension culture of Bcl-2-transfected cells. Importantly, in viability assays, when both cell lines were subjected to single-cell suspension for 48 hours and then replated onto culture dishes, no visible colonies of control cells survived (Figure 5D). However, in the Bcl-2-transfected cells, a significant number of cells were able to survive and proliferate to form colonies after replating. Similarly, mock-transfected HSC-3 cells were unable to survive (data not shown). Next, we used a TUNEL assay to analyze the effect of Bcl-2 overexpression on anoikis. As shown in Figure 5E, in contrast to control cells, which progressed to apoptosis as single cells, cells overexpressing Bcl-2 exhibited a marked reduction in anoikis. Taken together, these results suggest that EGFR and MAPK activation is required for an elevated Bcl-2/Bax protein ratio in cell aggregates and that Bcl-2 seems to be sufficient to provide protection from anoikis.
Cross-Linking of E-Cadherin Activates EGFR
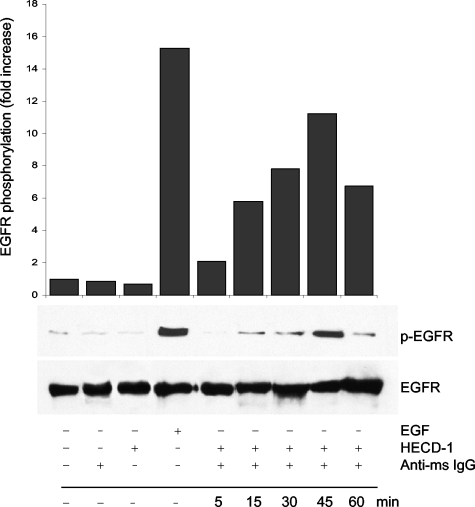
To test the role of E-cadherin in EGFR activation directly, we induced E-cadherin cross-linking by antibody-mediated clustering assay (Figure 6). In these experiments, HSC-3 cells in single-cell suspension culture were first treated with E-cadherin mAb, followed by incubation with a secondary cross-linking antibody. Treatment of suspended single cells with anti-E-cadherin antibodies induced a rapid EGFR activation that was first evident by 15 minutes after cross-linking and plateaued by 45 minutes. After 45 minutes the level of active EGFR appeared to decline, suggesting that antibody-induced EGFR activation is transient, perhaps because of rapid endocytosis of the receptor, which is likely to occur after antibody-induced crosslinking. In contrast, HECD-1 mAb or anti-mouse IgG alone failed to trigger EGFR activation. These findings clearly show that the activation of EGFR can occur in the absence of cell-cell adhesion when E-cadherin is artificially ligated and crosslinked with specific antibody. Obviously this approach does not mimic the complexity that occurs at cell-cell junctional assemblies, but does suggest a mechanism dependent on receptor crosslinking and that this event is sufficient to induce EGFR transactivation.
Figure 6-4260.
EGFR activation induced by E-cadherin cross-linking. HSC-3 cells were detached with 5 mmol/L EDTA and kept in suspension in poly-HEMA-coated dishes. After incubation for 1 hour at 4°C with a saturating concentration of anti-E-cadherin HECD-1 mAb (50 μg/ml), surface clustering of E-cadherin receptors was induced by further incubation with goat anti-mouse IgG (5 μg/ml) for the indicated times at 37°C. Equivalent protein was then immunoblotted with antibodies to activated EGFR (p-EGFR) and total EGFR. Relative protein densities of activated EGFR were determined; EGFR kinase activity is expressed as fold increase relative to the HSC-3 cells in suspension. Data reported here are representative of three independent experiments.
Adhesion-Induced Activation of EGFR Is Ligand-Independent
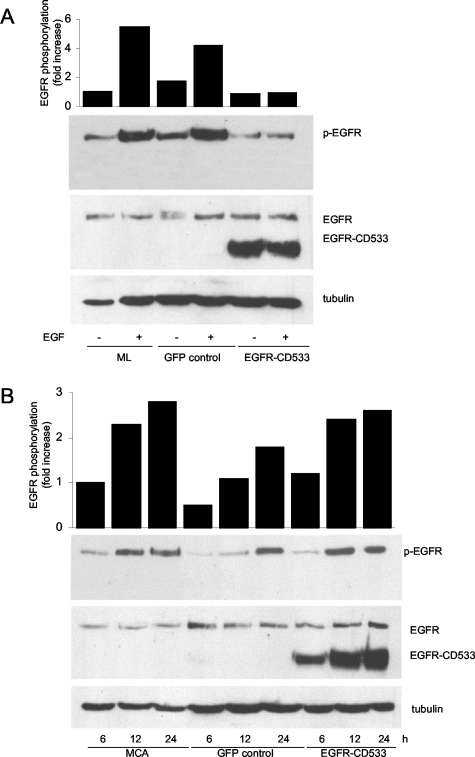
The finding that artificial cross-linking of E-cadherin directly induces EGFR activation suggests that adhesion-induced EGFR activation occurs through a process that does not involve the interaction of EGFR with a ligand. Since it has been shown that EGFR transactivation can occur through cleavage of EGF-like precursors,20 it was important to establish in the current system that EGFR activation proceeds via a ligand-independent event. To test whether such a mechanism was occurring in MCAs, we transduced HSC-3 cells with a recombinant adenoviral vector containing EGFR-CD533. This EGFR truncation mutant, which lacks the cytoplasmic COOH-terminal 533 amino acids that include the kinase domain, functions as a dominant-negative.17,21 At high levels of expression, this mutant should not only compete for ligands but also will abrogate EGFR activation. HSC-3 cells transduced with EGFR-CD533 were shown to abundantly express the mutant by Western blotting with an antibody specific to the extracellular domain of EGFR (Figure 7A). Nontransduced and control GFP-transduced monolayer cells were fully responsive to EGF, but cells overexpressing the EGFR mutant became unresponsive to ligand (Figure 7A). This demonstrated that EGFR-CD533 was acting as an effective dominant-negative. In contrast, when similarly transduced cells were cultured as MCAs, the expression of this dominant-negative EGFR had no effect on the progressive increase of EGFR phosphorylation after MCA formation (Figure 7B). These results strongly suggest that EGFR activation after cell-cell adhesion is ligand-independent and is functionally different from ligand-induced receptor activation.
Figure 7-4260.
Intercellular adhesion induces ligand-independent activation of EGFR. A: Dominant-negative EGFR-CD533 blocked EGF-induced EGFR activation in HSC-3 monolayer. HSC-3 cells were infected with recombinant adenoviruses to express either EGFR-CD533 or control GFP. Twenty-four hours after initial infection, cells were serum-starved overnight and stimulated with 15 ng/ml of EGF for 5 minutes. Data reported here are representative of three independent experiments. B: Dominant-negative EGFR-CD533 failed to inhibit EGFR activation in HSC-3 MCAs. HSC-3 cells were infected with recombinant adenoviruses to express either EGFR-CD533 or control GFP. Twenty-four hours after initial infection, cells were plated as MCA culture for the indicated times. Data reported here are representative of three independent experiments.
EGFR Forms Complexes with E-Cadherin at Cell-Cell Junctions
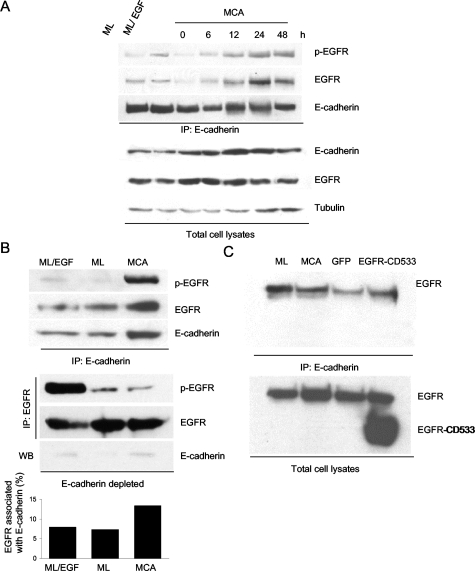
Previous studies have shown that EGFR can be linked via β-catenin to the cytoplasmic tail of E-cadherin.14 To investigate whether E-cadherin and EGFR form stable complexes in MCAs, MCA cell lysates were processed for immunoprecipitation with anti-E-cadherin mAb, followed by immunoblotting with anti-EGFR antibodies. That EGFR was effectively recovered in the anti-E-cadherin immunoprecipitates (Figure 8A) indicates that E-cadherin and EGFR can physically associate. Importantly, although the amount of EGFR co-immunoprecipitating with E-cadherin was low in monolayer cells or when the suspended cells were initially plated, the level of EGFR-cadherin complex formation gradually increased as MCAs were forming (6 to 12 hours) and became maximal at 24 to 48 hours. The increase in EGFR co-immunoprecipitating with E-cadherin paralleled the increase in activated EGFR co-immunoprecipitating with E-cadherin, suggesting that EGFR activation correlates with E-cadherin-EGFR complex formation at cell-cell contacts. The phospho-EGFR/EGFR protein ratio confirmed that the specific activity of the receptors remained fairly constant throughout 48 hours. The reblotting with anti-E-cadherin antibody after stripping of the membrane confirmed that the differences in EGFR association were not caused by the variation in the amount of E-cadherin recovered in the immunoprecipitates. When the total cell lysates were analyzed by immunoblotting, the relative level of E-cadherin was found to increase during MCA formation whereas the EGFR level remained relatively constant. To measure the distribution of activated EGFR between cadherin-bound and cadherin-free pools under different culture conditions, cell lysates were first exhaustively immunoprecipitated with anti-E-cadherin mAb, followed by sequential immunoprecipitation with anti-EGFR antibodies. The E-cadherin and EGFR immunoprecipitates were then blotted with EGFR-specific antibodies. For EGF-stimulated monolayer cells, the majority of the activated EGFR was not associated with E-cadherin but rather was recovered in the E-cadherin-depleted cell lysate fraction. Similarly, the low level of activated EGFR in the control monolayer cells was partitioned in the E-cadherin-depleted fraction (Figure 8B). In contrast, for MCAs, nearly all of the activated EGFR was complexed with E-cadherin, with only a minor fraction recovered in the E-cadherin-depleted fraction. Consistent with this finding was an increase in total EGFR recovered in the E-cadherin immunoprecipitates from MCAs. It was found that the amount of EGFR/E-cadherin complex represented only a small fraction of total cellular EGFR (∼13%) even when the cells had formed mature aggregates (Figure 8B). In contrast, cultures of control monolayers or EGF-treated monolayers, EGFR/E-cadherin complexes represented only ∼7% of total cellular pool of EGFR. These results support the conclusion that the cellular distribution of adhesion-induced and ligand-independent EGFR activation is distinct from that of EGF-induced activation and that as cell-cell adhesion proceeds, the subset of EGFR complexed with E-cadherin is transactivated.
Figure 8-4260.
E-cadherin-EGFR complex formation correlates with EGFR activation during cell-cell adhesion. A: The level of activated EGFR complexed with E-cadherin increased during MCA formation. HSC-3 cells were plated as ML for 24 hours or as MCA for the indicated times. E-cadherin was immunoprecipitated (IP) from total cell lysates with HECD-1 mAb. The association of activated EGFR (p-EGFR) or EGFR with E-cadherin was detected with specific anti-p-EGFR or anti-EGFR mAb. The amount of EGFR and E-cadherin in total cell lysates by immunoblotting is also shown for comparison. B: Activated EGFR in MCAs is preferentially complexed with E-cadherin. HSC-3 cells were plated as ML or MCA culture for 24 hours. Cell extracts from EGF-treated HSC-3 monolayer cells and HSC-3 MCAs were exhaustively immunoprecipitated with anti-E-cadherin mAb to recover E-cadherin. Then the E-cadherin-depleted supernatants were immunoprecipitated with anti-EGFR antibodies. The anti-E-cadherin (top) and anti-EGFR (bottom) immunoprecipitates were processed for immunoblotting with anti-p-EGFR mAb to access levels of activated EGFR. Membranes were stripped and reblotted with anti-EGFR mAb and anti-E-cadherin antibodies (top only). The E-cadherin level in E-cadherin-depleted supernatants is also shown (bottom). C: EGFR cytoplasmic domain is required for association with E-cadherin. HSC-3 cells were infected with recombinant adenoviruses to express either EGFR-CD533 or control GFP. Twenty-four hours after initial infection, cells were plated as ML or MCA culture. E-cadherin was immunoprecipitated from total cell lysates with HECD-1 mAb. The association of EGFR or EGFR-CD533 with E-cadherin was detected with specific anti-EGFR extracellular domain antibodies. The expression of both EGFR and EGFR-CD533 in EGFR-CD533-transduced cells is indicated for total cell lysates.
As stated above, the dominant-negative EGFR mutant EGFR-CD533 was unable to inhibit EGFR activation in MCAs. To test whether EGFR-CD533 could complex with E-cadherin as the wild-type receptor does, HSC-3 cells were transduced with the cytoplasmic domain-truncated mutant and permitted to form MCAs. EGFR is known to associate with the cytoplasmic tail of E-cadherin via β-catenin,14 presumably through a region in the EGFR cytoplasmic domain. This seems to be the case because only the wild-type EGFR, but not EGFR-CD533, could be recovered in the anti-E-cadherin immunoprecipitates (Figure 8C).
To further evaluate the potential role of EGFR activation in EGFR/E-cadherin complex formation and its co-localization at cell-cell junctions, we used tyrphostin AG1478 to specifically block EGFR kinase activity and then analyze the receptor distribution by both immunolabeling and immunoprecipitation. In the presence of AG1478 we found that after 6 hours, HSC-3 cells were able to form compacted cell aggregates to the same extent as control cells. Importantly, the condensation of EGFR and E-cadherin at MCA cell-cell boundaries in the presence or absence of AG1478 was comparable (Figure 9A, left). The extensive EGFR phosphorylation at cell-cell junctions of MCAs that was readily detected in control aggregates was effectively inhibited in the presence of AG1478 (Figure 9A, right). Next, we found that the amount of EGFR that was co-immunoprecipitated with E-cadherin was similar in control and AG1478-treated MCAs (Figure 9B). However, the level of phosphorylated EGFR associated with E-cadherin was severely reduced in AG1478-treated MCAs. These results strongly suggest that EGFR kinase activity is not required for its ability to complex with E-cadherin or to localize at cell-cell junctions.
Figure 9-4260.
Assembly and co-localization of the EGFR/E-cadherin complex is independent of EGFR kinase activity. A: HSC-3 cells were cultured as MCAs for 6 hours in the absence or presence of AG1478 (1 μmol/L) and then transferred onto glass coverslips and immunolabeled for EGFR (green) and E-cadherin or activated EGFR (p-EGFR) (red). Specimens were analyzed by confocal microscopy, and representative images are shown. B: HSC-3 cells were plated as MCA for 12 hours in the absence or presence of AG1478 (1 μmol/L). E-cadherin was immunoprecipitated (IP) from total cell lysates with HECD-1 mAb. The presence of activated EGFR (p-EGFR) or total EGFR in the immunoprecipitate was detected with specific anti-p-EGFR or anti-EGFR mAb.
Discussion
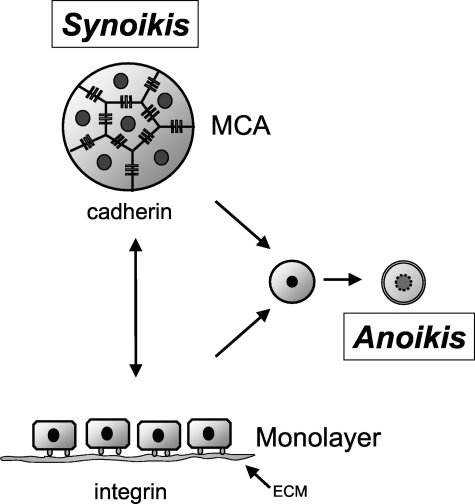
We have shown that SCC cells cultured in suspension are able to avoid apoptosis and terminal differentiation when they form tissue-like MCAs. Thus, cell-cell contacts promote cell survival in the absence of interactions with the extracellular matrix and create a permissive environment for cell proliferation. This behavior is similar to how SCC cells are organized in vivo, where they proliferate suprabasally to form nests of three-dimensional aggregates with extensive cell-cell junctions. We propose the use of the Greek term “synoikis” to describe this process of requiring neighboring associations of cells for survival (Figure 10). The current data provide evidence that cell-cell adhesion triggers cadherin-dependent activation of EGFR and its associated downstream signaling pathways that promote cell survival.
Figure 10-4260.
Adhesion-mediated cell survival. SCC cells require either integrin or cadherin receptor-based adhesion for survival but can switch from one to the other. However, loss of both leads to rapid onset of cell death through anoikis. We have termed the process of cells surviving together in a cooperative colony as synoikis.
It has long been accepted that a cell’s survival depends on signals provided by its surroundings. As pointed out by Frish and Francis,3 survival signals can be produced by several activities including when ligands activate surface receptors, when cells adhere to the extracellular matrix, or when cells form intercellular adhesions. EGFR has emerged as an important modulator of cell survival. EGFR mediates survival of colorectal carcinoma cells22 and the receptor confers resistance to anoikis in suspension to normal epidermal keratinocytes23 and mammary epithelial cells,24 suggesting that growth factor receptors can provide complementary survival signals to epithelial cells when cells are deprived of cell-matrix interaction. Jost and collaborators8,25 showed that in keratinocyte cell lines that EGFR activation and downstream signaling by the MAPK pathway could promote survival of suspended cells. Consistent with these findings is the observation that EGF stimulates anchorage-independent growth of oral SCC cell lines.26 Cadherins have also been implicated in regulating cell survival in granulosa cells,27 oral SCC cells,10 and prostate carcinoma cells.9 In the current study, we were able to link two different kinds of survival signal generators—the intercellular adhesion and cell surface growth factor receptors—and we propose that different cell survival machineries can act synergistically to amplify survival signals in carcinoma cells.
There seem to be multiple mechanisms by which cadherins can modulate cell survival. In prostate and mammary epithelial cells, E-cadherin-mediated aggregation promotes cell survival through the Rb cell-cycle control pathway.28 In prostate carcinoma cells, N-cadherin stimulates the PI3K/Akt pathway thereby up-regulating Bcl-2,9 which is supported by the fact that E-cadherin has also been shown to activate the PI3K/Akt pathway.29 Our results indicate that E-cadherin promotes SCC cell survival through activation of EGFR and the downstream MAPK pathway, which is also supported by a previous study demonstrating that E-cadherin-mediated cell-cell contact formation activates the MAPK pathway using a calcium switch approach in monolayer cultures.15 The finding that E-cadherin-mediated cell-cell adhesion induces activation of Gab-1, a docking protein that signals through ras/MAPK and PI3K/Akt pathways, suggests that both mechanisms may be operational.30 Although the specific mechanism by which E-cadherin modulates cell survival may be cell type-specific, the possible mechanisms are not mutually exclusive and may function simultaneously.
In the current studies, the normally low level of Akt activation observed in HSC-3 cell monolayers was strongly enhanced after EGF stimulation. However, we were unable to detect significant levels of Akt phosphorylation in cells grown in suspended single cell culture, which is similar to the recent findings of Chan and colleagues.31 Consistent with the apparent lack of Akt activation in cell aggregates, inhibition of the PI3K/Akt pathway with LY294002 had little effect on cell survival in MCA, in contrast to inhibitors of EGFR or the ERK/MAPK pathway. We conclude that in the case of SCC HSC-3 cells, the primary signaling pathway essential to E-cadherin-mediated survival is through EGFR activation of the MAPK pathway. Interestingly, inhibition of ERK/MAPK pathway with specific inhibitors produced a modest induction of anoikis compared to that induced by EGFR kinase inhibition. This suggests that additional signaling pathways downstream of EGFR activation may also be involved in survival (eg, STAT3, JNK, and PLCγ).32–34 We are currently investigating these possibilities.
Because commitment to apoptosis is usually defined by the relative balance between pro- and anti-apoptotic members of the Bcl-2 protein family, cadherin-mediated cell survival signaling is likely to regulate members of this family. In our study, Bcl-2 levels are reduced on detachment and that EGFR activation blocks this down-regulation, which is consistent with our previous report.10 In contrast, the levels of proapoptotic Bax remained constant; thus, the Bcl-2/Bax ratio increased after E-cadherin-mediated adhesion. It is well known that cell survival is favored by a high Bcl-2/Bax protein ratio35 and overexpression of Bcl-2 can protect cells against anoikis.3 Because Bcl-xL showed high levels of expression after MCA formation and was down-regulated in suspended single cells, this effector may also play a role in the survival of MCAs. A relevant finding is that in keratinocyte-related cells, it has been shown that EGFR activation promotes Bcl-xL expression and cell survival.8,25 In a breast epithelial cell line, it was found that Bcl-xL up-regulation was induced after activation of EGFR and this was associated with anchorage-independent cell survival.36 Previously, it was found that N-cadherin’s up-regulation of Bcl-2 through the PI3K/Akt pathway is performed by phosphorylation of the proapoptotic protein Bad.9 In our study, the phosphorylation level of Bad remained stable after cell-cell adhesion (data not shown), although it has been shown that ERK/MAPK can increase the level of Bcl-2 through the phosphorylation of Bad.37 Presumably, the increase in Bcl-2 protein is because of up-regulation of the Bcl-2 gene by ERK/MAPK.38 Recent studies have shown that in breast epithelial cells, loss of cell-matrix adhesion and signaling by EGFR regulates expression of Bim, a proapoptotic protein.39 This finding may be relevant to the current results in which cadherin and EGFR are involved in survival.
EGFR transactivation has been reported to take place in various systems by both ligand-dependent and ligand-independent mechanisms. For example, G-protein-coupled receptors activate EGFR through a ligand-dependent mechanism by promoting the cleavage of EGF-like precursors and the production of soluble ligand.40,41 In contrast, integrin adhesion receptors have been proposed to regulate EGFR through a ligand-independent activation pathway.42,43 Our results indicate that EGFR transactivation after E-cadherin-mediated adhesion is ligand-independent.
It will be interesting to compare the result of ligand-dependent and ligand-independent activation of EGFR. In our study, as in others,42 the level of EGFR phosphorylation was less intense than that produced by acute activation with EGF, suggesting that the number or extent of phosphorylation sites in the EGFR autophosphorylation domain differs from that induced after ligand-triggered activation. However, we showed that cell-cell adhesion-mediated EGFR activation, as well as downstream MAPK signaling, are long lived. These attenuated but sustained signals generated via adhesion receptors presumably yield a unique set of signals that are distinct from the acute ligand-induced transient activation. As summarized by Marshall,44 transient or sustained MAPK activation leads to different cellular responses (eg, proliferation, differentiation), including changes in gene expression. Thus, the attenuated but sustained MAPK signaling appears to activate anti-apoptosis pathways, resulting in enhanced cell survival.
It remains unclear exactly how E-cadherin-mediated cell-cell adhesion transactivates EGFR. It is likely that activation of EGFR in cell aggregates proceeds via a two-step process that includes the initial linkage of EGFR with E-cadherin followed by E-cadherin homodimerization. The formation of the EGFR/E-cadherin complex does not appear to be dependent on extensive cell-cell adhesion because it is present in monolayer cells at lower levels where cell junction formation is limited. It seems that the EGFR/E-cadherin complexes are pre-existing, possibly formed after receptor synthesis. When cells begin to assemble new junctional adhesions, E-cadherin alone and EGFR/E-cadherin complexes are somehow recruited to these nascent zonula adherens junctions presumably by interactions with the cytoskeleton. More studies are needed to define the mechanism of this process. Apparently it is the juxtaposition and oligomerization of neighboring EGF receptors through the catenin linkage to the cytoskeleton that triggers the transactivation after recruitment of the EGFR/E-cadherin complex to junctional contacts Because EGFR kinase activity does not appear to be required for the formation of an EGFR/E-cadherin complex, it is likely that an important event in the EGFR activation occurs after recruitment of the complex at the cell-cell junction. Interestingly, Moro and colleagues 42 showed that EGFR kinase activity is required for the assembly of integrin-EGFR complex, which suggests that integrin and E-cadherin may use a different mechanism to transactivate EGFR.
The HSC-3 cells used in the present study are poorly differentiated with a scattered morphology and tend to form limited cell-cell adhesions in monolayer culture.16 This type of phenotype is consistent with the low-level EGFR activation seen in monolayer culture (Figure 8B) and the infrequent co-localization of E-cadherin and p-EGFR under these conditions (data not shown). The minor level of phosphorylated EGFR detected in monolayer culture is consistent with these findings and may reflect activation via integrin signaling.42
There is substantial evidence that E-cadherin is important during the induction and progression of epithelial cancers.45 The loss of E-cadherin or its dysfunction leads to increased invasiveness and frequently increased proliferation of carcinoma cells.46 It is interesting that in some tumors, cadherins attenuate cell growth but in others appear to promote not only growth but also survival. Recently, it was shown that intercellular adhesion is not required for inhibiting invasion but that the suppression instead reflects a mechanism in which an unidentified β-catenin binding protein is involved.47 Importantly, in some cancers expression of E-cadherin may be maintained, as is the case of invasive ovarian carcinoma which frequently displays strong expression of cadherins.48 Furthermore, in well- and moderately differentiated head and neck SCCs, studies have found that, in general, modest but variable expression of E-cadherin is preserved as lesions advance through premalignant to invasive and metastatic stages.49–51 For example, Islam and colleagues52 found that in 47 head and neck SCCs, all lesions uniformly showed positive staining for E-cadherin and a fraction also showed co-staining for N-cadherin. In some studies, there is extensive cytoplasmic E-cadherin localization along with lower expression levels. It is possible that for head and neck SCC, the organization of tumor cells as aggregates in tumor nests may provide a survival benefit as this carcinoma is known to be anchorage-dependent.53 This is in contrast with other tumors (eg, breast, colon, and prostate) that tend to be anchorage-independent.
Previous studies have shown that human cancers such as SCCs grow as multicellular masses in vitro or in vivo, and that cell-cell adhesion promotes resistance to treatment strategies, including traditional chemotherapy and radiotherapy.54–56 Of note, some studies have shown that resistance to chemotherapy and radiotherapy can be pharmacologically reversed by using treatment that disrupts either cell-cell adhesion58 or EGFR function.59,60 Our results may provide a clue to the mechanism by which cell-cell adhesion renders tumor cells resistant to therapy because the major target is to induce apoptosis. As mentioned above, in oral SCCs modest E-cadherin expression is preserved in lesions advancing from premalignant to invasive and even metastatic stages.49,50,52,57 We speculate that at least during the early stages of tumor progression, SCC may depend on cell-cell adhesion for suprabasal survival and proliferation and that E-cadherin-mediated cell survival via overexpressed EGFR may render cancer cells resistant to treatment.
In summary, our results suggest a novel mechanism for adhesion-mediated cell survival that we call synoikis (Figure 10). E-cadherin-mediated cell-cell adhesion induces ligand-independent EGFR activation, which triggers the ERK/MAPK signaling module, blocking the down-regulation of anti-apoptotic Bcl-2. The exact mechanism of E-cadherin-mediated EGFR transactivation is unclear, but it is likely correlated with EGFR-E-cadherin complex formation at cell-cell junctions and receptor oligomerization. The activation of EGFR appears to effectively compensate for the loss of integrin signals, thereby suppressing anoikis. It is possible that the intercellular signaling via cadherins and activation of EGFR may have important consequences for tumor cell growth in head and neck cancers. The current results suggest that additional studies should be done to explore the potential linkage between cadherin-mediated EGFR phosphorylation and patient survival.
Acknowledgments
We thank Drs. Caroline Damsky and Rik Derynck for helpful discussions and for their critical review of this manuscript.
Footnotes
Address reprint requests to Randall H. Kramer, Ph.D., Departments of Stomatology and Anatomy, Schools of Medicine and Dentistry, University of California at San Francisco, Box 0512, Room HSW-604, San Francisco, CA 94143-0512. E-mail: rkramer@itsa.ucsf.edu.
Supported by the National Institutes of Health (grants DE11436 and DE13904 to R.K.) and the Graduate Program in Oral and Craniofacial Sciences (to X.S.).
References
- Ruoslahti E, Reed JC. Anchorage dependence, integrins, and apoptosis. Cell. 1994;77:477–478. doi: 10.1016/0092-8674(94)90209-7. [DOI] [PubMed] [Google Scholar]
- Frisch SM, Ruoslahti E. Integrins and anoikis. Curr Opin Cell Biol. 1997;9:701–706. doi: 10.1016/s0955-0674(97)80124-x. [DOI] [PubMed] [Google Scholar]
- Frisch SM, Francis H. Disruption of epithelial cell-matrix interactions induces apoptosis. J Cell Biol. 1994;124:619–626. doi: 10.1083/jcb.124.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith JE, Jr, Winitz S, Lewis JM, Hess S, Ren XD, Renshaw MW, Schwartz MA. The regulation of growth and intracellular signaling by integrins. Endocr Rev. 1996;17:207–220. doi: 10.1210/edrv-17-3-207. [DOI] [PubMed] [Google Scholar]
- Frisch SM, Screaton RA. Anoikis mechanisms. Curr Opin Cell Biol. 2001;13:555–562. doi: 10.1016/s0955-0674(00)00251-9. [DOI] [PubMed] [Google Scholar]
- Khwaja A, Rodriguez-Viciana P, Wennstrom S, Warne PH, Downward J. Matrix adhesion and Ras transformation both activate a phosphoinositide 3-OH kinase and protein kinase B/Akt cellular survival pathway. EMBO J. 1997;16:2783–2793. doi: 10.1093/emboj/16.10.2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen K, Rak J, Leung T, Dean NM, Kerbel RS, Filmus J. Activated Ras prevents downregulation of Bcl-X(L) triggered by detachment from the extracellular matrix A mechanism of Ras-induced resistance to anoikis in intestinal epithelial cells. J Cell Biol. 2000;149:447–456. doi: 10.1083/jcb.149.2.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jost M, Huggett TM, Kari C, Rodeck U. Matrix-independent survival of human keratinocytes through an EGF receptor/MAPK-kinase-dependent pathway. Mol Biol Cell. 2001;12:1519–1527. doi: 10.1091/mbc.12.5.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran NL, Adams DG, Vaillancourt RR, Heimark RL. Signal transduction from N-cadherin increases Bcl-2 Regulation of the phosphatidylinositol 3-kinase/Akt pathway by homophilic adhesion and actin cytoskeletal organization. J Biol Chem. 2002;277:32905–32914. doi: 10.1074/jbc.M200300200. [DOI] [PubMed] [Google Scholar]
- Kantak SS, Kramer RH. E-cadherin regulates anchorage-independent growth and survival in oral squamous cell carcinoma cells. J Biol Chem. 1998;273:16953–16961. doi: 10.1074/jbc.273.27.16953. [DOI] [PubMed] [Google Scholar]
- Arregui C, Pathre P, Lilien J, Balsamo J. The nonreceptor tyrosine kinase fer mediates cross-talk between N-cadherin and beta1-integrins. J Cell Biol. 2000;149:1263–1274. doi: 10.1083/jcb.149.6.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs EM, Ali RG, McCormack AJ, Yap AS. E-cadherin homophilic ligation directly signals through Rac and phosphatidylinositol 3-kinase to regulate adhesive contacts. J Biol Chem. 2002;277:6708–6718. doi: 10.1074/jbc.M109640200. [DOI] [PubMed] [Google Scholar]
- Todd R, Wong DT. Epidermal growth factor receptor (EGFR) biology and human oral cancer. Histol Histopathol. 1999;14:491–500. doi: 10.14670/HH-14.491. [DOI] [PubMed] [Google Scholar]
- Hoschuetzky H, Aberle H, Kemler R. Beta-catenin mediates the interaction of the cadherin-catenin complex with epidermal growth factor receptor. J Cell Biol. 1994;127:1375–1380. doi: 10.1083/jcb.127.5.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pece S, Gutkind JS. Signaling from E-cadherins to the MAPK pathway by the recruitment and activation of epidermal growth factor receptors upon cell-cell contact formation. J Biol Chem. 2000;275:41227–41233. doi: 10.1074/jbc.M006578200. [DOI] [PubMed] [Google Scholar]
- Kawano K, Kantak SS, Murai M, Yao CC, Kramer RH. Integrin alpha3beta1 engagement disrupts intercellular adhesion. Exp Cell Res. 2001;262:180–196. doi: 10.1006/excr.2000.5083. [DOI] [PubMed] [Google Scholar]
- Reardon DB, Contessa JN, Mikkelsen RB, Valerie K, Amir C, Dent P, Schmidt-Ullrich RK. Dominant negative EGFR-CD533 and inhibition of MAPK modify JNK1 activation and enhance radiation toxicity of human mammary carcinoma cells. Oncogene. 1999;18:4756–4766. doi: 10.1038/sj.onc.1202849. [DOI] [PubMed] [Google Scholar]
- Levitzki A, Gazit A. Tyrosine kinase inhibition: an approach to drug development. Science. 1995;267:1782–1788. doi: 10.1126/science.7892601. [DOI] [PubMed] [Google Scholar]
- Datta SR, Brunet A, Greenberg ME. Cellular survival: a play in three Akts. Genes Dev. 1999;13:2905–2927. doi: 10.1101/gad.13.22.2905. [DOI] [PubMed] [Google Scholar]
- Carpenter G. EGF receptor transactivation mediated by the proteolytic production of EGF-like agonists. Sci STKE. 2000;2000:PE1. doi: 10.1126/stke.2000.15.pe1. [DOI] [PubMed] [Google Scholar]
- Kashles O, Yarden Y, Fischer R, Ullrich A, Schlessinger J. A dominant negative mutation suppresses the function of normal epidermal growth factor receptors by heterodimerization. Mol Cell Biol. 1991;11:1454–1463. doi: 10.1128/mcb.11.3.1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Fan Z, Masui H, Rosen N, Mendelsohn J. Apoptosis induced by an anti-epidermal growth factor receptor monoclonal antibody in a human colorectal carcinoma cell line and its delay by insulin. J Clin Invest. 1995;95:1897–1905. doi: 10.1172/JCI117871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodeck U, Jost M, DuHadaway J, Kari C, Jensen PJ, Risse B, Ewert DL. Regulation of Bcl-xL expression in human keratinocytes by cell-substratum adhesion and the epidermal growth factor receptor. Proc Natl Acad Sci USA. 1997;94:5067–5072. doi: 10.1073/pnas.94.10.5067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore AP, Valentijn AJ, Wang P, Ranger AM, Bundred N, O’Hare MJ, Wakeling A, Korsmeyer SJ, Streuli CH. Activation of BAD by therapeutic inhibition of epidermal growth factor receptor and transactivation by insulin-like growth factor receptor. J Biol Chem. 2002;277:27643–27650. doi: 10.1074/jbc.M108863200. [DOI] [PubMed] [Google Scholar]
- Jost M, Huggett TM, Kari C, Boise LH, Rodeck U. Epidermal growth factor receptor-dependent control of keratinocyte survival and Bcl-xL expression through a MEK-dependent pathway. J Biol Chem. 2001;276:6320–6326. doi: 10.1074/jbc.M008210200. [DOI] [PubMed] [Google Scholar]
- Lee K, Tanaka M, Shigeno C, Yamamoto I, Ohta S, Rikimaru K, Hatanaka M, Konishi J. Epidermal growth factor stimulates the anchorage-independent growth of human squamous cell carcinomas overexpressing its receptors. Biochem Biophys Res Commun. 1990;168:905–911. doi: 10.1016/0006-291x(90)91114-8. [DOI] [PubMed] [Google Scholar]
- Peluso JJ, Pappalardo A, Trolice MP. N-cadherin-mediated cell contact inhibits granulosa cell apoptosis in a progesterone-independent manner. Endocrinology. 1996;137:1196–1203. doi: 10.1210/endo.137.4.8625889. [DOI] [PubMed] [Google Scholar]
- Day ML, Zhao X, Vallorosi CJ, Putzi M, Powell CT, Lin C, Day KC. E-cadherin mediates aggregation-dependent survival of prostate and mammary epithelial cells through the retinoblastoma cell cycle control pathway. J Biol Chem. 1999;274:9656–9664. doi: 10.1074/jbc.274.14.9656. [DOI] [PubMed] [Google Scholar]
- Pece S, Chiariello M, Murga C, Gutkind JS. Activation of the protein kinase Akt/PKB by the formation of E-cadherin-mediated cell-cell junctions Evidence for the association of phosphatidylinositol 3-kinase with the E-cadherin adhesion complex. J Biol Chem. 1999;274:19347–19351. doi: 10.1074/jbc.274.27.19347. [DOI] [PubMed] [Google Scholar]
- Shinohara M, Kodama A, Matozaki T, Fukuhara A, Tachibana K, Nakanishi H, Takai Y. Roles of cell-cell adhesion-dependent tyrosine phosphorylation of Gab-1. J Biol Chem. 2001;276:18941–18946. doi: 10.1074/jbc.M100909200. [DOI] [PubMed] [Google Scholar]
- Chan TO, Rodeck U, Chan AM, Kimmelman AC, Rittenhouse SE, Panayotou G, Tsichlis PN. Small GTPases and tyrosine kinases coregulate a molecular switch in the phosphoinositide 3-kinase regulatory subunit. Cancer Cell. 2002;1:181–191. doi: 10.1016/s1535-6108(02)00033-8. [DOI] [PubMed] [Google Scholar]
- Song L, Turkson J, Karras JG, Jove R, Haura EB. Activation of Stat3 by receptor tyrosine kinases and cytokines regulates survival in human non-small cell carcinoma cells. Oncogene. 2003;22:4150–4165. doi: 10.1038/sj.onc.1206479. [DOI] [PubMed] [Google Scholar]
- Lamb JA, Ventura JJ, Hess P, Flavell RA, Davis RJ. JunD mediates survival signaling by the JNK signal transduction pathway. Mol Cell. 2003;11:1479–1489. doi: 10.1016/s1097-2765(03)00203-x. [DOI] [PubMed] [Google Scholar]
- Miao JY, Kaji K, Hayashi H, Araki S. Inhibitors of phospholipase promote apoptosis of human endothelial cells. J Biochem (Tokyo) 1997;121:612–618. doi: 10.1093/oxfordjournals.jbchem.a021629. [DOI] [PubMed] [Google Scholar]
- Mackey TJ, Borkowski A, Amin P, Jacobs SC, Kyprianou N. bcl-2/bax ratio as a predictive marker for therapeutic response to radiotherapy in patients with prostate cancer. Urology. 1998;52:1085–1090. doi: 10.1016/s0090-4295(98)00360-4. [DOI] [PubMed] [Google Scholar]
- Schulze A, Lehmann K, Jefferies HB, McMahon M, Downward J. Analysis of the transcriptional program induced by Raf in epithelial cells. Genes Dev. 2001;15:981–994. doi: 10.1101/gad.191101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheid MP, Duronio V. Dissociation of cytokine-induced phosphorylation of Bad and activation of PKB/akt: involvement of MEK upstream of Bad phosphorylation. Proc Natl Acad Sci USA. 1998;95:7439–7444. doi: 10.1073/pnas.95.13.7439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YZ, Boxer LM, Latchman DS. Activation of the Bcl-2 promoter by nerve growth factor is mediated by the p42/p44 MAPK cascade. Nucleic Acids Res. 1999;27:2086–2090. doi: 10.1093/nar/27.10.2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reginato MJ, Mills KR, Paulus JK, Lynch DK, Sgroi DC, Debnath J, Muthuswamy SK, Brugge JS. Integrins and EGFR coordinately regulate the pro-apoptotic protein Bim to prevent anoikis. Nat Cell Biol. 2003;5:733–740. doi: 10.1038/ncb1026. [DOI] [PubMed] [Google Scholar]
- Prenzel N, Zwick E, Daub H, Leserer M, Abraham R, Wallasch C, Ullrich A. EGF receptor transactivation by G-protein-coupled receptors requires metalloproteinase cleavage of proHB-EGF. Nature. 1999;402:884–888. doi: 10.1038/47260. [DOI] [PubMed] [Google Scholar]
- Gschwind A, Hart S, Fischer OM, Ullrich A. TACE cleavage of proamphiregulin regulates GPCR-induced proliferation and motility of cancer cells. EMBO J. 2003;22:2411–2421. doi: 10.1093/emboj/cdg231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moro L, Dolce L, Cabodi S, Bergatto E, Erba EB, Smeriglio M, Turco E, Retta SF, Giuffrida MG, Venturino M, Godovac-Zimmermann J, Conti A, Schaefer E, Beguinot L, Tacchetti C, Gaggini P, Silengo L, Tarone G, Defilippi P. Integrin-induced epidermal growth factor (EGF) receptor activation requires c-Src and p130Cas and leads to phosphorylation of specific EGF receptor tyrosines. J Biol Chem. 2002;277:9405–9414. doi: 10.1074/jbc.M109101200. [DOI] [PubMed] [Google Scholar]
- Liu D, Aguirre Ghiso J, Estrada Y, Ossowski L. EGFR is a transducer of the urokinase receptor initiated signal that is required for in vivo growth of a human carcinoma. Cancer Cell. 2002;1:445–457. doi: 10.1016/s1535-6108(02)00072-7. [DOI] [PubMed] [Google Scholar]
- Marshall CJ. Specificity of receptor tyrosine kinase signaling: transient versus sustained extracellular signal-regulated kinase activation. Cell. 1995;80:179–185. doi: 10.1016/0092-8674(95)90401-8. [DOI] [PubMed] [Google Scholar]
- Cavallaro U, Christofori G. Cell adhesion and signalling by cadherins and Ig-CAMs in cancer. Nat Rev Cancer. 2004;4:118–132. doi: 10.1038/nrc1276. [DOI] [PubMed] [Google Scholar]
- Margulis A, Andriani F, Fusenig N, Hashimoto K, Hanakawa Y, Garlick JA. Abrogation of E-cadherin-mediated adhesion induces tumor cell invasion in human skin-like organotypic culture. J Invest Dermatol. 2003;121:1182–1190. doi: 10.1046/j.1523-1747.2003.12523.x. [DOI] [PubMed] [Google Scholar]
- Wong AS, Gumbiner BM. Adhesion-independent mechanism for suppression of tumor cell invasion by E-cadherin. J Cell Biol. 2003;161:1191–1203. doi: 10.1083/jcb.200212033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundfeldt K. Cell-cell adhesion in the normal ovary and ovarian tumors of epithelial origin; an exception to the rule. Mol Cell Endocrinol. 2003;202:89–96. doi: 10.1016/s0303-7207(03)00068-6. [DOI] [PubMed] [Google Scholar]
- Andrews NA, Jones AS, Helliwell TR, Kinsella AR. Expression of the E-cadherin-catenin cell adhesion complex in primary squamous cell carcinomas of the head and neck and their nodal metastases. Br J Cancer. 1997;75:1474–1480. doi: 10.1038/bjc.1997.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowie GL, Caslin AW, Roland NJ, Field JK, Jones AS, Kinsella AR. Expression of the cell-cell adhesion molecule E-cadherin in squamous cell carcinoma of the head and neck. Clin Otolaryngol. 1993;18:196–201. doi: 10.1111/j.1365-2273.1993.tb00829.x. [DOI] [PubMed] [Google Scholar]
- Kawano T, Nakamura Y, Yanoma S, Kubota A, Furukawa M, Miyagi Y, Tsukuda M. Expression of E-cadherin, and CD44s and CD44v6 and its association with prognosis in head and neck cancer. Auris Nasus Larynx. 2004;31:35–41. doi: 10.1016/j.anl.2003.09.005. [DOI] [PubMed] [Google Scholar]
- Islam S, Carey TE, Wolf GT, Wheelock MJ, Johnson KR. Expression of N-cadherin by human squamous carcinoma cells induces a scattered fibroblastic phenotype with disrupted cell-cell adhesion. J Cell Biol. 1996;135:1643–1654. doi: 10.1083/jcb.135.6.1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rheinwald JG, Beckett MA. Defective terminal differentiation in culture as a consistent and selectable character of malignant human keratinocytes. Cell. 1980;22:629–632. doi: 10.1016/0092-8674(80)90373-6. [DOI] [PubMed] [Google Scholar]
- Green SK, Frankel A, Kerbel RS. Adhesion-dependent multicellular drug resistance. Anticancer Drug Des. 1999;14:153–168. [PubMed] [Google Scholar]
- St. Croix B, Sheehan C, Rak JW, Florenes VA, Slingerland JM, Kerbel RS. E-Cadherin-dependent growth suppression is mediated by the cyclin-dependent kinase inhibitor p27(KIP1) J Cell Biol. 1998;142:557–571. doi: 10.1083/jcb.142.2.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damiano JS, Hazlehurst LA, Dalton WS. Cell adhesion-mediated drug resistance (CAM-DR) protects the K562 chronic myelogenous leukemia cell line from apoptosis induced by BCR/ABL inhibition, cytotoxic drugs, and gamma-irradiation. Leukemia. 2001;15:1232–1239. doi: 10.1038/sj.leu.2402179. [DOI] [PubMed] [Google Scholar]
- Ziober BL, Silverman SS, Jr, Kramer RH. Adhesive mechanisms regulating invasion and metastasis in oral cancer. Crit Rev Oral Biol Med. 2001;12:499–510. doi: 10.1177/10454411010120060401. [DOI] [PubMed] [Google Scholar]
- St. Croix B, Man S, Kerbel RS. Reversal of intrinsic and acquired forms of drug resistance by hyaluronidase treatment of solid tumors. Cancer Lett. 1998;131:35–44. doi: 10.1016/s0304-3835(98)00199-2. [DOI] [PubMed] [Google Scholar]
- Raben D, Bianco C, Helfrich B, Weng E, Ciardiello F, Harari P. Interference with EGFR signaling: paradigm for improving radiation response in cancer treatment. Expert Rev Anticancer Ther. 2002;2:461–471. doi: 10.1586/14737140.2.4.461. [DOI] [PubMed] [Google Scholar]
- Bonner JA, De Los Santos J, Waksal HW, Needle MN, Trummel HQ, Raisch KP. Epidermal growth factor receptor as a therapeutic target in head and neck cancer. Semin Radiat Oncol. 2002;12:11–20. doi: 10.1053/srao.2002.34864. [DOI] [PubMed] [Google Scholar]