Abstract
Inflammatory fibrosis is a characteristic feature of myocarditis, dilated cardiomyopathy (DCM), and congestive heart failure. Th1-type immune responses, mediated by interleukin (IL)-12-induced interferon (IFN)-γ, are believed to exacerbate autoimmune diseases including myocarditis. In this study, we examined the effect of IL-12Rβ1 and IFN-γ deficiency on the development of chronic CB3-induced myocarditis using knockout mice. We found increased chronic CB3-induced myocarditis (14.1 to 43.1%, P < 0.001); pericarditis (1.5 to 7.6%, P < 0.001); fibrosis (9.7 to 27.4%, P < 0.05); and the profibrotic cytokines transforming growth factor-β1, IL-1β, and IL-4 in the hearts of IFN-γ-deficient mice. All mice infected with CB3 developed DCM, but IFN-γ-deficient mice developed a fibrous, adhesive pericarditis associated with increased numbers of degranulating mast cells (MCs) in the pericardium (26.6 to 45.9%, P < 0.01), increased histamine levels (716 to 1930 ng/g of heart, P < 0.01), and reduced survival (100 to 43%). In contrast, IL-12Rβ1 deficiency did not significantly alter the development of chronic myocarditis. Thus, IFN-γ protects against the development of severe chronic myocarditis, pericarditis, and DCM after CB3 infection by reducing MC degranulation, fibrosis, and the profibrotic cytokines transforming growth factor-β1, IL-1β, and IL-4 in the heart.
The development of chronic fibrosis is an important feature of a number of pathological conditions such as pulmonary or hepatic disease and rheumatoid arthritis (RA).1 Fibrosis also contributes significantly to the development of congestive heart failure. Cytokines play an essential role in tissue remodeling by stimulating fibroblast proliferation and collagen deposition. Transforming growth factor-β (TGF-β) increases fibrosis by stimulating fibroblast proliferation and the production of collagen while at the same time inhibiting matrix metalloproteinase-induced degradation of collagen.1–4 Angiotensin II (AngII), a component of the cardiac renin-angiotensin-aldosterone system, indirectly increases TGF-β levels in response to mechanical stretch or damage.3,5 Other profibrotic cytokines including tumor necrosis factor-α (TNF-α), interleukin (IL)-1β, and IL-4 also increase fibroblast proliferation resulting in greater collagen synthesis and fibrosis.2,6,7
Regardless of the origin, injury to the heart can result in cardiomyocyte hypertrophy, fibroblast proliferation, fibrosis, and cell death.6 Inflammatory cardiomyopathy because of viral infection is a frequent cause of sudden death in young adults and often progresses to dilated cardiomyopathy (DCM), a major cause of heart failure.8 Coxsackievirus B3 (CB3) infection of susceptible mice results in disease similar to that observed in humans, with the development of acute myocarditis from days 7 to 14 after infection, which later progresses to a chronic phase of disease from day 28 to at least day 58 after infection.9 During the chronic phase, no infectious virus can be recovered from the heart.9 Cytokines released during the innate response to CB3 infection are critical in the progression to chronic autoimmune disease, because exogenous administration of TNF-α or IL-1β along with CB3 induces chronic myocarditis in resistant strains of mice.10,11 We have recently shown that increased IL-1β and IL-18 levels in the heart directly correlate with increased acute myocardial inflammation after CB3 infection, and that these cytokines are modulated via IL-12 receptor (R) β1 and toll-like receptor 4 signaling.12 In contrast, we found that interferon (IFN)-γ is important in protecting against viral replication but does not significantly alter acute myocardial inflammation after CB3 infection.12 However, the role of IL-12-induced IFN-γ in the progression to chronic myocarditis after CB3 infection has not been previously examined.
IL-12 produced during the early phase of a bacterial or viral infection promotes the differentiation of T cells to a Th1 phenotype, which supports cell-mediated immunity, cytotoxic T cell generation, activation of phagocytic cells, and eventual eradication of intracellular pathogens.13 Th1-mediated immune responses are also associated with a number of autoimmune diseases, such as inflammatory bowel disease, type I diabetes, multiple sclerosis, RA, and myocarditis.14–16 Signaling by IL-12 requires co-expression of the IL-12Rβ1 and IL-12Rβ2 chains for high-affinity IL-12p70 binding and maximal IFN-γ production. In the mouse, IL-12R signaling activates signal transducer and activator of transcription (STAT)1, STAT3, and STAT4, with STAT4 being responsible for most of the biological activities of IL-12p70 through the production of IFN-γ.17,18 Although IFN-γ has been shown to exacerbate some Th1-mediated autoimmune diseases by expanding autoreactive T cells, IFN-γ also prevents chronic fibrosis by inhibiting the activation of fibroblasts and by antagonizing the effects of TGF-β.19–22 Thus, a better understanding of the mechanisms involved in the development of chronic myocarditis and DCM after viral infection is needed to treat or prevent chronic inflammatory heart disease.
In this study, we examined the role of IL-12-induced IFN-γ on the development of chronic, autoimmune myocarditis triggered by CB3 infection using mice deficient in IL-12Rβ1 or IFN-γ. We found that IFN-γ was crucial in protecting mice from the development of severe chronic myocarditis, pericarditis, and fibrosis. Increased cardiac fibrosis in IFN-γ-deficient mice was associated with areas of increased inflammation, pericardial thickening, and increased TGF-β1, IL-1β, and IL-4 levels in the heart. Although all wild-type and knockout mice infected with CB3 developed DCM, disease in IFN-γ-deficient mice was more severe and accompanied by increased numbers of degranulating pericardial mast cells (MCs), increased histamine levels, and reduced survival. In contrast, IL-12Rβ1 deficiency did not alter chronic inflammation or fibrosis. Thus, rather than exacerbating chronic myocarditis, IFN-γ was found to protect against the development of severe myocardial inflammation, pericardial thickening, and DCM after CB3 infection by reducing fibrosis, MC degranulation, and the profibrotic cytokines TGF-β1, IL-1β, and IL-4 in the heart.
Materials and Methods
Mice
IL-12Rβ1- and IFN-γ-deficient mice on a BALB/c genetic background and wild-type BALB/c controls were obtained from the Jackson Laboratory (Bar Harbor, ME). Mice were maintained under pathogen-free conditions in the animal facility at Johns Hopkins School of Medicine, and approval was obtained from the Animal Care and Use Committee of the Johns Hopkins University for all procedures.
Histology
Individual experiments were conducted three to five times with 7 to 10 mice per group. Mice, 6 to 8 weeks of age, were inoculated intraperitoneally with a heart-passaged stock of CB3 (Nancy strain) originally obtained from the American Type Culture Collection (Manassas, VA). CB3 was diluted in sterile phosphate-buffered saline (PBS) and 103 plaque-forming units (PFU) injected intraperitoneally on day 0 and tissues collected on days 12, 21, 28, 35, or 45 after infection. Age-matched, mock-treated (PBS or uninfected heart homogenate), uninfected BALB/c, IL-12Rβ1-deficient, or IFN-γ-deficient mice were inoculated on day 0 and analyzed at days 35 or 45 after inoculation. Hearts were cut longitudinally and fixed in 10% phosphate-buffered formalin and embedded in paraffin. Five-μm- thick sections were cut at various depths in the tissue section and stained with hematoxylin and eosin (H&E) to determine the level of inflammation and DCM, and with Masson’s trichrome to detect collagen deposition. Sections were examined by two independent investigators in a blinded manner and myocarditis with necrosis, pericarditis, or fibrosis was assessed as the percentage of the heart section with inflammation or fibrosis compared to the overall size of the heart section, with the aid of a microscope eyepiece grid. The development of DCM was assessed by gross observation at low magnification from histology sections. Heart sections were stained with toluidine blue to detect MC granules. The number of MCs in 50 fields of view was counted with the aid of a microscope eyepiece grid. MCs were assessed as degranulating when purple-stained granule droplets could be observed adjacent to MCs under the microscope at high power.
Cytokine and Histamine Measurements
Splenocyte cultures were performed essentially as previously described.23 Briefly, viable splenocytes from individual mice were cultured at 1 × 107 cells/well of a 24-well plate in RPMI 1640 medium supplemented with 10% fetal bovine serum, 15 mmol/L HEPES, 1% l-glutamine, 1% minimum essential medium vitamins, 1% nonessential amino acid, 0.1 mmol/L β-mercaptoethanol, 1% sodium pyruvate, and 100 U/ml of penicillin-streptomycin (Life Technologies, Inc., Carlsbad, CA) for 48 hours without stimulation. Two days in culture is not the optimal time point for all cytokines, but was chosen as a time when the cytokines of interest could be detected.
Heart samples were weighed and frozen with dry ice immediately after collection and stored at −80°C until homogenized. Both homogenized and culture supernatants were stored at −80°C until used in enzyme-linked immunosorbent assays (ELISAs). Cytokine levels were measured in homogenized heart supernatants or cultured spleen supernatants using Quantikine cytokine ELISA kits purchased from R&D Systems (Minneapolis, MN), according to the manufacturer’s instructions. Active, rather than latent, TGF-β1 levels were assessed. The limit of detection for the cytokine kits was as follows: IFN-γ, 2 pg/ml; TGF-β1, 1.6 pg/ml; TNF-α, 5.1 pg/ml; IL-1β, 3 pg/ml; and IL-4, 2 pg/ml. Histamine levels were measured in homogenized heart supernatants using histamine ELISA kits purchased from ImmunoBiological Laboratories (RE59221; Hamburg, Germany), according to the manufacturer’s instructions. Heart cytokine and histamine levels were expressed as pg/g of heart tissue.
Plaque Assay
The level of infectious virus was determined in individual homogenates by plaque assay according to standard procedures.24,25 Briefly, samples were frozen with dry-ice immediately after collection and stored at −80°C until homogenized. Tissues were homogenized at 10% w/v in 2% minimal essential medium and supernatants were stored at −80°C until used in the plaque assay. Dilutions of tissue supernatants were incubated on confluent Vero cell (American Type Culture Collection) monolayers for 1 hour at 37°C and 5% CO2 to allow viral attachment and then incubated for 3 days to allow plaque formation. Virus titers are expressed as the mean PFU/g tissue ± SEM and the limit of detection was 10 PFU/g of tissue.
Statistical Analysis
Normally distributed data were analyzed by the Student’s t-test, otherwise the Mann-Whitney U-test was used. Significant differences were obtained by comparing CB3-infected knockout mice with infected BALB/c controls. Test values with a P < 0.05 were considered significantly different from control values (*, P < 0.05; **, P < 0.01; ***, P < 0.001).
Results
Chronic Myocarditis Is Increased in IFN-γ-Deficient Mice after CB3 Infection
IFN-γ-mediated Th1 responses are believed to exacerbate many autoimmune diseases, including myocarditis.16 We previously found that IFN-γ protects CB3-infected mice by reducing viral replication in the heart during acute myocarditis (day 12 after infection), but that IFN-γ does not significantly alter myocardial inflammation during acute myocarditis.12 However, the role of IFN-γ in the development of the chronic phase of CB3-induced myocarditis has not been studied. To investigate the effect of IFN-γ on the development of chronic CB3-induced myocarditis, we examined BALB/c mice deficient in IL-12Rβ1 or IFN-γ and compared the level of myocarditis at days 35 and 45 after CB3 infection, during the peak of chronic myocarditis,9 to wild-type BALB/c controls. IL-12Rβ2-deficient mice were not available on a BALB/c genetic background, and so were not examined in this study.
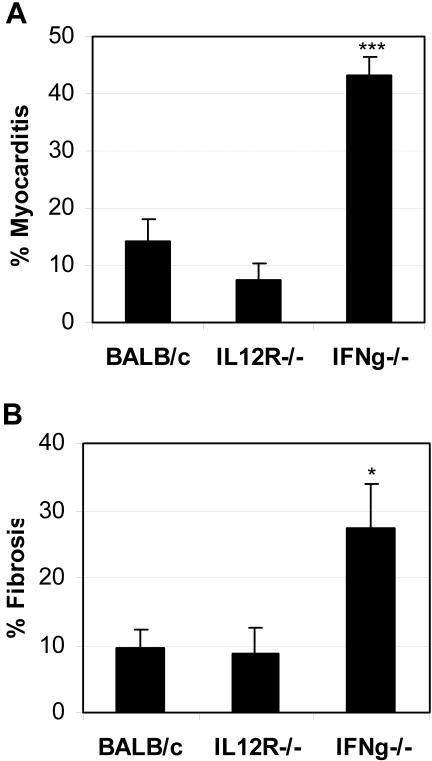
We found that IL-12Rβ1 deficiency did not significantly alter the level of chronic myocarditis (inflammation and necrosis) at day 35 after infection (Figure 1A) or 45 after infection (data not shown) compared to wild-type BALB/c mice. On the other hand, IFN-γ deficiency resulted in a significant increase in chronic myocarditis compared to controls (Figure 1A). The level of inflammation at day 45 was very similar to day 35 (Figure 1A) in IFN-γ-deficient hearts, but with increased areas of necrosis at day 45 after infection (data not shown). Inflammation or necrosis was not observed in the hearts at day 35 in age-matched, mock PBS-treated, uninfected BALB/c, IL-12Rβ1-deficient, or IFN-γ-deficient mice, or in BALB/c mice inoculated with uninfected heart homogenates (data not shown). Thus, IFN-γ was found to protect against the development of chronic CB3-induced myocarditis resulting in reduced inflammation and necrosis in the heart independently of IL-12Rβ1 signaling.
Figure 1.
Chronic myocarditis and fibrosis is increased in IFN-γ-deficient mice. Mice deficient in IL-12Rβ1 (IL12R−/−) or IFN-γ (IFNg−/−) were compared to wild-type BALB/c control mice for the level of myocarditis and fibrosis in the heart 35 days after CB3 infection. Mice received 103 PFU of CB3 or PBS intraperitoneally on day 0 and hearts were collected on day 35 after infection. Myocarditis or fibrosis were not observed in the heart at day 35 of age-matched uninfected, mock PBS-treated BALB/c, IL-12Rβ1-deficient, or IFN-γ-deficient mice, or in BALB/c mice inoculated with uninfected heart homogenates. Myocarditis was assessed as the percentage of the heart with inflammation and necrosis compared to the overall size of the heart section. Fibrosis was assessed as the percentage of the heart section with collagen deposition, which stains bright blue with Masson’s trichrome stain. Data show one representative experiment of five conducted with 7 to 10 mice per group. Data are presented as the mean ± SEM. *, P < 0.05; ***, P < 0.001.
Infectious Virus Is Not Reactivated in the Heart or Pancreas of IL-12Rβ1- or IFN-γ-Deficient Mice
Detection of infectious virus by plaque assay occurs as early as 12 hours to day 10 after infection in the pancreas, and from 24 hours to day 14 after infection in the hearts of CB3-infected BALB/c mice.9,26 After day 14 after infection, infectious virus cannot be detected by plaque assay in the heart or pancreas.9 Because IFN-γ is important in protecting against CB3 replication,12,27 we wanted to know whether removing IFN-γ would prevent clearance or allow reactivation of virus during the chronic phase, thus potentially accounting for the increased chronic myocarditis observed in IFN-γ-deficient mice (Figure 1A). Therefore, we examined the heart and pancreas of IL-12Rβ1-deficient and IFN-γ-deficient and wild-type BALB/c mice for the presence of virus by plaque assay at days 21, 28, 35, and 45 after CB3 infection. No plaques were detected in the heart or pancreas of knockout or control mice at any time point (data not shown), indicating that infectious virus was not present. These results show that the increased inflammation observed in IFN-γ-deficient hearts is because of factors other than active viral replication. Although the possibility exists that the presence of latent, nonreplicating virus in the heart may be recognized by the immune system leading to chronic inflammation,25 the best evidence that persistent virus is not necessary for the progression to chronic myocarditis is the fact that susceptible mice develop chronic myocarditis after inoculation with only cardiac myosin and adjuvant. Thus, active infection is likely to be needed only to release cardiac myosin (self) during the acute response.
Cardiac Fibrosis Is Increased in IFN-γ-Deficient Mice during Chronic Myocarditis
Fibrosis involves proliferation of fibroblasts and deposition of extracellular matrix proteins such as collagen. Even though myocytes account for more than 90% of the volume of the heart, they comprise only ∼25% of the total number of cardiac cells, with the remainder being primarily fibroblasts.6,22 Thus, it is not surprising that fibrosis is an important contributor to the development of congestive heart failure and DCM, and a key determinant of the clinical outcome of chronic heart disease.6 However, the role of fibrosis in the progression to chronic myocarditis and DCM after CB3 infection has not been previously investigated.
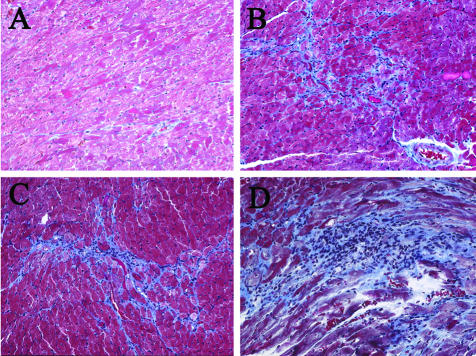
To study the role of IL-12-induced IFN-γ on the development of cardiac fibrosis, heart sections from CB3-infected IL-12Rβ1- or IFN-γ-deficient mice were stained with Masson’s trichrome to detect collagen deposition and fibrosis and compared to wild-type BALB/c controls (Figure 1B and Figure 2). Fibrosis was not observed in the heart at days 35 or 45 in age-matched, mock PBS-treated, uninfected BALB/c (Figure 2A), IL-12Rβ1-deficient, or IFN-γ-deficient mice (data not shown), or in BALB/c mice inoculated with uninfected heart homogenates (data not shown). Similar to the role for IL-12Rβ1 signaling in chronic inflammation (Figure 1A), IL-12Rβ1 deficiency did not significantly alter the development of fibrosis after CB3 infection compared to wild-type BALB/c controls (Figure 1B; Figure 2, B and C). We found that fibrosis was significantly increased in IFN-γ-deficient hearts compared to wild-type BALB/c mice at day 35 after infection (Figure 1B) as indicated by bright blue staining for collagen deposition (Figure 2D). Areas of fibrosis were predominantly associated with regions of inflammation and necrosis for all strains of mice infected with CB3 (Figure 2). These results indicate that the protective effect of IFN-γ in reducing the development of chronic CB3-induced myocarditis relates to the role of IFN-γ in reducing fibrosis in the heart.
Figure 2.
Increased fibrosis is associated with areas of inflammation in the heart. Uninfected, mock-treated BALB/c (A), IL-12Rβ1-deficient, or IFN-γ-deficient mice did not develop cardiac fibrosis. Mice deficient in IL-12Rβ1 (C) or IFN-γ (D) were compared to wild-type BALB/c (B) mice for the level of fibrosis and inflammation in the heart 35 days after CB3 infection. Mice received 103 PFU of CB3 or PBS intraperitoneally on day 0 and hearts were collected on day 35 after infection. Fibrosis was assessed as the area of the heart section with collagen deposition, which stains bright blue with Masson’s trichrome stain. Individual experiments were conducted five times with 7 to 10 mice per group, with one representative heart shown for each group. Original magnifications, ×64.
Pericarditis Is Increased in IFN-γ-Deficient Mice during Chronic Myocarditis
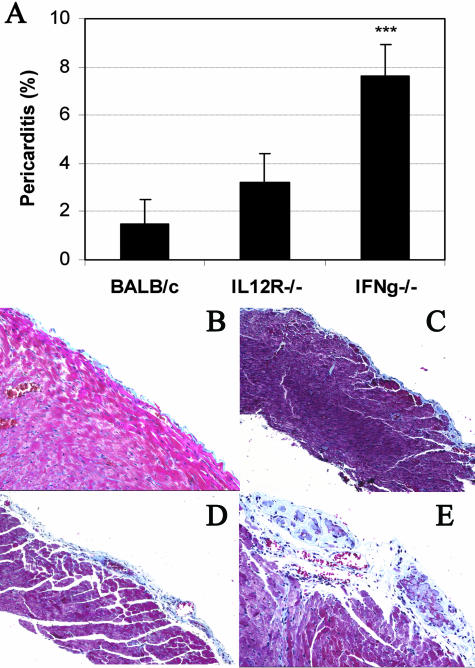
Pericarditis usually develops subsequent to other cardiac-associated diseases such as rheumatic fever, systemic lupus erythematosus, systemic sclerosis, and bacterial or viral myocarditis.22 Most causes of pericarditis result in an acute, serous pericarditis, while the chronic, fibrous form of pericarditis is less common. We have found that pericarditis develops after infection with either murine cytomegalovirus or CB3.9 However, the role of IFN-γ in the development of chronic pericarditis after CB3 infection has not been previously examined. Uninfected BALB/c (Figure 3B), IL-12Rβ1-deficient, or IFN-γ-deficient mice (data not shown) did not develop pericarditis unless they were infected with virus. We found that IL-12Rβ1 deficiency did not alter the development of chronic pericarditis, which was present at low levels in both IL-12Rβ1-deficient and wild-type BALB/c controls after infection (Figure 3; A, C, D). However, IFN-γ-deficient mice developed increased pericarditis at day 35 after infection (data not shown) and day 45 after infection compared to BALB/c mice as assessed by H&E staining (data not shown) and Masson’s trichrome to detect fibrosis (Figure 3; A, C, E). IFN-γ-deficient mice developed a thickened fibrous pericarditis after CB3 infection that was associated with calcification (Figure 3E). The pericarditis induced in IFN-γ-deficient hearts was particularly severe and associated with obliteration of the pericardial sac and fibrous adhesion to the surrounding structures. Thus, IFN-γ is important in preventing the development of the adhesive, fibrous form of pericarditis after CB3 infection.
Figure 3.
Pericarditis is increased in IFN-γ-deficient mice after CB3 infection. Mice deficient in IL-12Rβ1 (IL12R−/−) (A, D) or IFN-γ (IFNg−/−) (A, E) were compared to wild-type BALB/c mice (A, C) for the level of pericarditis (A) and pericardial fibrosis (B–E) in the heart 45 days after CB3 infection. Mice received 103 PFU of CB3 or PBS intraperitoneally on day 0 and hearts were collected on day 45 after infection. Uninfected, mock-treated BALB/c (B), IL-12Rβ1-deficient, or IFN-γ-deficient mice did not develop pericarditis. Pericarditis was assessed as the percentage of the heart with pericarditis and pericardial fibrosis compared to the overall size of the heart section. Fibrosis was assessed as the area of the heart section with collagen deposition, which stains bright blue with Masson’s trichrome stain. Data show one representative experiment of three conducted with 7 to 10 mice per group. Data are presented as the mean ± SEM. ***, P < 0.001. Original magnifications: ×64 (B and E); ×40 (C and D).
IFN-γ Deficiency Increases DCM and Mortality during Chronic CB3-Induced Myocarditis
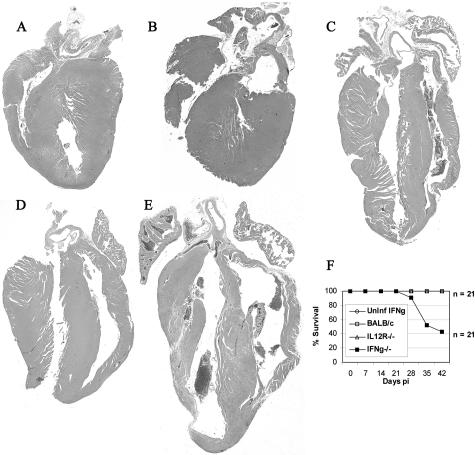
DCM is characterized by progressive cardiac hypertrophy, dilation, and contractile (systolic) dysfunction often developing subsequent to myocarditis.8,22 Because the majority of mice that are infected with CB3 develop DCM during the chronic phase of myocarditis,28 we were interested in the role of the IL-12R/IFN-γ pathway in this process. DCM is not usually observed in BALB/c mice at day 12 after infection, during acute CB3-induced myocarditis, but develops by day 35 after infection.28 However, hypertrophy of the myocardium, which is often a precursor to the development of DCM, can be observed during acute CB3 myocarditis in BALB/c mice (day 12 after infection) (Figure 4A). Age-matched, uninfected BALB/c, IL-12Rβ1-deficient (data not shown), or IFN-γ-deficient mice (Figure 4B) did not develop a dilated phenotype. However, all strains of mice infected with CB3, including wild-type and knockout mice, developed chronic DCM with increased chamber dilation, and either hypertrophy or thinning of the ventricular walls (Figure 4; C to E). However, DCM was generally more severe in IFN-γ-deficient mice (Figure 4E). All mouse strains except for IFN-γ-deficient mice survived the development of chronic CB3-induced myocarditis, but deaths occurred in IFN-γ-deficient mice during the chronic phase of myocarditis from day 28 onwards (Figure 4F), suggesting progression to congestive heart failure in this strain. Because IFN-γ deficiency resulted in the development of a fibrous, adhesive form of pericarditis (Figure 3E), these mice may have developed more severe DCM because of, at least in part, the increased strain placed on the heart by adhesion of the pericardium to the surrounding tissues. Thus, wild-type BALB/c mice develop DCM with a 100% prevalence after CB3 infection that is not prevented by IL-12Rβ1 or IFN-γ deficiency. However, IFN-γ reduces the development of severe DCM, heart failure, and death by preventing the fibrous, adhesive form of pericarditis.
Figure 4.
IFN-γ deficiency increases DCM and mortality during chronic myocarditis. A: Hypertrophy of the myocardium is observed in CB3-infected BALB/c mice during acute myocarditis at day 12 after infection. B: Age-matched, uninfected, mock-treated BALB/c, IL-12Rβ1-deficient, or IFN-γ-deficient mice do not develop DCM at day 35. CB3-infected mice deficient in IL-12Rβ1 (D) or IFN-γ (E) were compared to control BALB/c (C) mice for the level of DCM in the heart 35 days after infection. F: Survival was assessed in CB3-infected and uninfected mouse strains by observing mice from day 0 to day 45 after infection. No deaths were observed in uninfected, mock-treated IFN-γ-deficient, or CB3-infected BALB/c, or IL-12Rβ1-deficient mice. Mice received 103 PFU of CB3 or PBS intraperitoneally on day 0 and hearts were collected on day 12 or 35 after infection. Hearts were cut longitudinally and assessed for dilation by gross inspection of H&E-stained sections at low power. Individual experiments were conducted five times with 7 to 10 mice per group, with one representative heart shown for each group. Original magnifications, ×2.5.
TGF-β1 and IL-4 Are Increased in Splenocyte Cultures from IFN-γ-Deficient Mice during Chronic Myocarditis
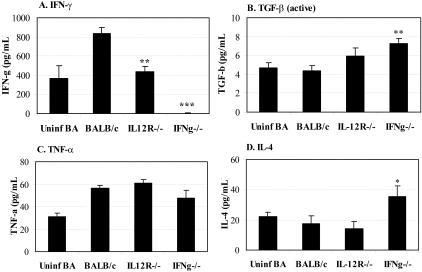
Collagen accumulation during fibrosis depends on the balance between stimulation of collagen synthesis and degradation by matrix metalloproteinase, and is regulated by cytokines and growth factors such as platelet-derived growth factor, fibroblast growth factor, TGF-β1, IFN-γ, TNF-α, IL-1β, and IL-4.1,6 We therefore examined the levels of IFN-γ, TGF-β1, TNF-α, IL-1β, and IL-4 production from splenocytes (Figure 5) and the heart (Figure 6) of CB3-infected IL-12Rβ1- and IFN-γ-deficient mice compared to uninfected and CB3-infected controls.
Figure 5.
TGF-β1 and IL-4 levels are increased in splenocyte cultures from IFN-γ-deficient mice during chronic myocarditis. Mice deficient in IL-12Rβ1 (IL12R−/−) or IFN-γ (IFNg−/−) were compared to wild-type BALB/c mice for the level of IFN-γ (A), active TGF-β1 (B), TNF-α (C), or IL-4 (D) in unstimulated splenocyte cultures during CB3-induced chronic myocarditis. Unstimulated splenocyte cultures from uninfected, mock-treated BALB/c mice were evaluated for comparison of uninfected cytokine levels. Mice received 103 PFU of CB3 or PBS intraperitoneally on day 0 and splenocytes were collected on day 35 after infection and culture supernatants analyzed using cytokine ELISA kits. Individual experiments were conducted three times and data presented as the mean ± SEM of pg/ml of 7 to 10 mice per group. Significant differences were obtained by comparing CB3-infected knockout mice with infected BALB/c controls. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
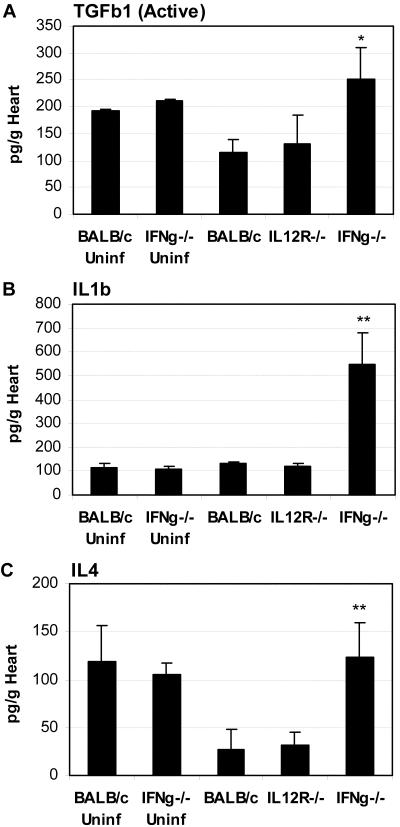
Figure 6.
TGF-β1, IL-1β, and IL-4 levels are increased in the hearts of IFN-γ-deficient mice during chronic myocarditis. Mice deficient in IL-12Rβ1 (IL-12R−/−) or IFN-γ (IFNg−/−) were compared to wild-type BALB/c mice for the level of active TGF-β1 (A), IL-1β (B), or IL-4 (C) in the heart during chronic CB3-induced myocarditis. Uninfected, mock-treated BALB/c and IFN-γ-deficient mice were evaluated for comparison of uninfected cytokine levels in the heart. Mice received 103 PFU of CB3 or PBS intraperitoneally on day 0 and hearts were collected on day 35 after infection and homogenized supernatants analyzed for cytokine levels using ELISA kits. Individual experiments were conducted three times and data presented as the mean ± SEM of pg/g of heart tissue of 7 to 10 mice per group. Significant differences were obtained by comparing CB3-infected knockout mice with infected BALB/c controls. *, P < 0.05; **, P < 0.01.
IFN-γ-deficient mice developed splenomegaly during the chronic phase of CB3-induced myocarditis (data not shown), indicating that deficiency in IFN-γ results in increased proliferation of splenocyte populations. However, the same number of splenocytes was used for analysis of cytokines in all strains of mice. We found that IFN-γ production from splenocytes was significantly reduced after CB3 infection of IL-12Rβ1- or IFN-γ-deficient mice compared to infected BALB/c controls (Figure 5A), as has been previously reported for these mouse strains.14,29,30 As expected, IFN-γ was not detected in IFN-γ-deficient splenocyte cultures after CB3 infection (Figure 5A). We found that active levels of TGF-β1 were significantly increased in IFN-γ-deficient splenocytes (Figure 5B), although at very low levels. IL-1β was not detected in splenocyte culture supernatants during chronic myocarditis (data not shown). Although we found no differences between strains in TNF-α levels (Figure 5C), we did observe significantly greater levels of IL-4 in IFN-γ-deficient splenocyte cultures (Figure 5D). Although IFN-γ levels were decreased in IL-12Rβ1-deficient cultures, this did not result in increased TGF-β1 or IL-4 levels in this strain (Figure 5), suggesting that the effects of IFN-γ on the production of these profibrotic cytokines in the spleen were not mediated through IL-12Rβ1. These results indicate that a profibrotic environment exists in the spleen that may be characteristic of the inflammatory cells in the heart that lead to fibrosis.
TGF-β1, IL-1β, and IL-4 Are Increased in the Heart of IFN-γ-Deficient Mice during Chronic Myocarditis
Because the local cytokine environment is key in determining whether fibrosis develops, we examined the levels of IFN-γ, TGF-β1, TNF-α, IL-1β, and IL-4 production in the hearts of IL-12Rβ1- and IFN-γ-deficient mice infected with CB3 35 days earlier compared to BALB/c controls (Figure 6). We also examined the level of these cytokines in the heart of uninfected BALB/c or IFN-γ-deficient mice for comparison of pre-existing cytokine levels (Figure 6). TGF-β occurs in three isoforms, TGF-β1, TGF-β2, and TGF-β3, with TGF-β1 being the most important for mediating fibrosis.31,32 TGF-β1 is released from many different cells such as macrophages and cardiomyocytes in an inactive latent form that must be activated to mediate the effects of the TGF-R.33,34 We were surprised to find that bioactive TGF-β1 was present at relatively high levels in the hearts of uninfected BALB/c mice, but there was no significant difference in the level of TGF-β1 in the hearts of uninfected mice deficient in IFN-γ compared to uninfected BALB/c mice (Figure 6A). However, during chronic myocarditis we found that TGF-β1 levels were significantly increased in the hearts of IFN-γ-deficient mice compared to CB3-infected BALB/c controls (Figure 6A), consistent with the increase we observed in fibrosis in this strain (Figure 1B and Figure 2D). TGF-β1 is closely associated with increased fibrosis because of its ability to inhibit matrix metalloproteinase while increasing collagen deposition.2,4 TNF-α, IL-1β, and IL-6 can also contribute to increased fibrosis by augmenting AngII and AngII receptors in the heart.7,35,36 In contrast to splenocytes (Figure 5), neither TNF-α nor IFN-γ was detected in the heart during chronic myocarditis (data not shown). However, the hearts of IFN-γ-deficient mice had significantly elevated levels of the profibrotic cytokines IL-1β and IL-4 (Figure 6, B and C). Thus, IFN-γ protects against the development of severe chronic fibrosis by influencing the production of the profibrotic cytokines TGF-β1, IL-1β, and IL-4 in the heart.
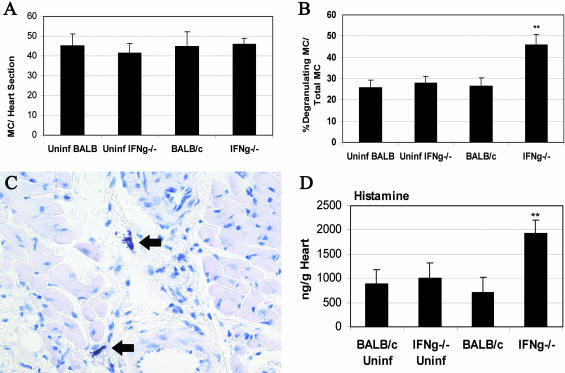
MC Degranulation Is Increased in the Pericardium of IFN-γ-Deficient Mice during Chronic Myocarditis
There is increasing evidence that MCs participate in angiogenesis as well as tissue remodeling and fibrosis. Mediators released from degranulating MCs such as histamine, tryptase, chymase, and cytokines stimulate vascular tube formation, fibroblast proliferation, collagen deposition, and matrix metalloproteinase degradation.37–40 MCs are found in the human heart in increased numbers during cardiovascular disease and congestive heart failure.41–43 Furthermore, studies using MC-deficient mice have confirmed a role for MCs in increasing fibrosis leading to heart failure.44 Since we had observed elevated levels of TGF-β1, IL-1β, and IL-4 in IFN-γ-deficient hearts (Figure 6), cytokines that are produced by MCs,38 we looked for MCs in the hearts of IFN-γ-deficient mice compared to BALB/c controls at day 35 after infection using toluidine blue staining for MC granules. We found abundant, but similar numbers of, MCs in the heart during chronic myocarditis (Figure 7A). A similar number of MCs was also present in uninfected, mock-treated BALB/c or IFN-γ-deficient hearts (Figure 7A). Interestingly, IFN-γ-deficient mice had significantly increased numbers of degranulating MCs compared to the total number of degranulating and intact MCs (Figure 7B). Intact and degranulating MCs were primarily observed surrounding vessels and the pericardium, but could also be found dispersed within the myocardium (Figure 7C). However, degranulating MCs were almost exclusively located in the pericardium (92.5 ± 5.5%) compared to the myocardium. The high number of degranulating MCs in the pericardium of IFN-γ-deficient mice suggests that increased levels of mediators released from MCs may be responsible for the fibrous, adhesive form of pericarditis and increased DCM observed in this strain. To confirm that MCs were degranulating at higher levels in the hearts of IFN-γ-deficient mice, we examined the level of histamine in the heart. We found that histamine levels were significantly increased in the hearts of IFN-γ-deficient mice during chronic myocarditis compared to wild-type BALB/c controls (Figure 7D). These results suggest a role for IFN-γ in regulating MC degranulation after viral infection and provide a mechanism to explain how MCs can exacerbate cardiovascular disease leading to heart failure.
Figure 7.
MC degranulation is increased in the pericardium of IFN-γ-deficient mice during chronic myocarditis. Mice deficient in IFN-γ (IFNg−/−) were compared to wild-type BALB/c mice for the total number of MCs (A) or the percentage of degranulating MCs compared to total degranulating and intact MCs (B). C: Degranulating MCs (arrows) observed in IFN-γ-deficient heart. D: Increased histamine levels in the heart of IFN-γ-deficient mice confirmed MC degranulation. Uninfected, mock-treated BALB/c, and IFN-γ-deficient mice were evaluated for comparison to infected mice. Heart sections were stained with toluidine blue to detect MC granules. The number of MCs in 50 fields of view was counted with the aid of a microscope eyepiece grid. MCs were assessed as degranulating when purple-stained granule droplets (by toluidine blue stain) could be observed adjacent to MCs under the microscope. Histamine levels were measured using ELISA kits and data presented as the mean ± SEM of pg/g of heart tissue of 7 to 10 mice per group. Significant differences were obtained by comparing CB3-infected knockout mice with infected BALB/c controls. **, P < 0.01. Original magnifications, ×140 (C).
Discussion
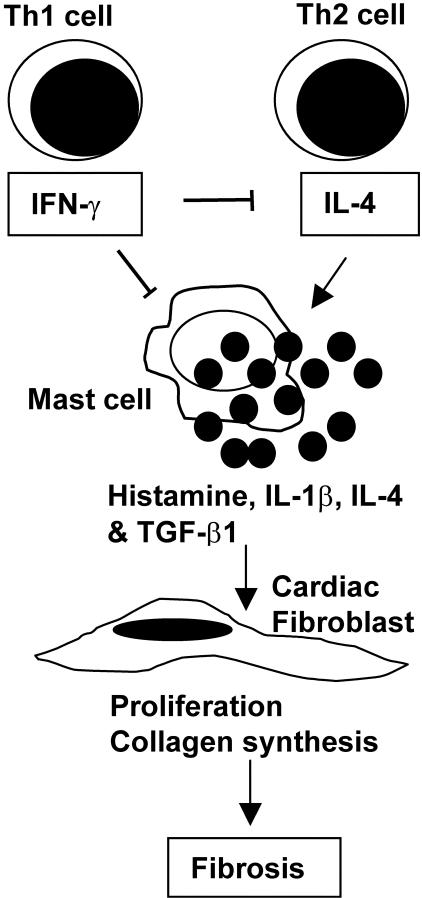
In this study, we examined the role of IL-12-induced IFN-γ on the development of chronic CB3-induced myocarditis. We report here that, rather than increasing autoimmune inflammation, IFN-γ protects against the development of severe chronic myocarditis triggered by CB3 infection by reducing cardiac inflammation, fibrosis, and the profibrotic cytokines TGF-β1, IL-1β, and IL-4 in the heart. Infectious virus is not present during the development of chronic myocarditis, and is not reactivated by IFN-γ deficiency. MCs are abundantly present in the heart during chronic CB3 myocarditis and may contribute to fibrosis by releasing the profibrotic mediators histamine, IL-4, and bioactive TGF-β and IL-1β (Figure 8). Importantly, IFN-γ may protect against the development of DCM, heart failure, and death by reducing MC degranulation in the pericardium and preventing the adhesive, fibrous form of pericarditis (Figure 8). Furthermore, deficiency in IL-12Rβ1 did not influence chronic CB3 myocarditis or DCM, indicating that the effect of IFN-γ on chronic heart disease is not mediated through IL-12Rβ1 signaling. Overall, these results show the important role for IFN-γ in preventing the development of severe chronic myocarditis and DCM by reducing MC degranulation, profibrotic cytokine production, and fibrosis in the heart.
Figure 8.
Possible mechanisms involved in the reduction of chronic CB3-induced fibrosis and pericarditis by IFN-γ. Th1 (IFN-γ) and Th2 (IL-4) cytokines are elevated in the heart after CB3 infection.26 Elevated levels of IL-4 (and IL-1β) in susceptible mouse strains may stimulate release of profibrotic mediators such as histamine, TGF-β1, IL-1β, and IL-4 from MCs in the heart during chronic myocarditis. Release of these profibrotic mediators from MCs and macrophages during chronic CB3 myocarditis stimulates cardiac fibroblast proliferation, collagen deposition, and fibrosis. IFN-γ directly and/or indirectly regulates the severity of chronic fibrosis and pericarditis by transcriptionally inhibiting IL-4 production from Th2 cells and MCs, by down-regulating MC activation and mediator production, and by down-regulating fibroblast proliferation and collagen production. By reducing MC degranulation in the pericardium IFN-γ may prevent development of the fibrous, adhesive form of pericarditis that leads to heart failure.
IL-12-mediated Th1 responses are believed to augment inflammatory autoimmune diseases such as inflammatory bowel disease, type I diabetes, and RA.15 Phagocytic and antigen-presenting cells produce IL-12, which promotes the differentiation of T cells to a Th1 phenotype associated with IFN-γ production. IL-12 receptors are primarily expressed on activated NK and T cells, and signaling requires co-expression of the IL-12Rβ1 and IL-12Rβ2 chains for the generation of high-affinity IL-12p70 binding and maximal IFN-γ production.29 Recently, we examined the role of IL-12-induced IFN-γ on the development of acute myocarditis (day 12) after CB3 infection.12 We found that mice lacking IL-12Rβ1 had decreased levels of IL-1β and IL-18 in the heart, which was directly associated with decreased myocarditis. However, there was no alteration in the severity of acute myocarditis in IFN-γ-deficient mice after CB3 infection.12,28 Overall, our studies show that while IL-12Rβ1 exacerbates acute myocarditis by increasing the proinflammatory cytokines IL-1β and IL-18,12 IFN-γ protects against acute and chronic CB3 myocarditis by reducing viral replication and fibrosis, respectively.28
We found a similar role for IL-12Rβ1 and IFN-γ in the cardiac myosin-induced experimental autoimmune myocarditis model.28,30 In experimental autoimmune myocarditis, in which virus is not involved in the pathogenesis of disease, IL-12Rβ1-deficient mice do not develop myocarditis and have significantly reduced levels of IL-1β in splenocyte cultures,30 indicating the importance of IL-12Rβ1 signaling in promoting the development of autoimmune myocarditis. In contrast, using the experimental autoimmune myocarditis model IFN-γ- or IFN-γR-deficient mice or mice treated with antibody to neutralize IFN-γ develop large, inflamed hearts and congestive heart failure.30,45,46 Thus, the role for IL-12-induced IFN-γ and a Th1-type immune response in exacerbating autoimmune disease needs to be re-evaluated.28,47
It may be that the ability of IL-12Rβ1 signaling to increase proinflammatory cytokines such as TNF-α, IL-1β, and IL-18 has been mistakenly attributed to a Th1-type response. Increased IFN-γ is used as the hallmark for a Th1 response, and yet TNF-α, IL-1β, and IL-18 can increase IFN-γ levels independent of classical IL-12R signaling.12,28 Furthermore, it has recently been shown that other cytokines that also bind to IL-12Rβ1, such as IL-23, may be more important than IL-12p70 in promoting autoimmune disease.12,28,48–51 Similarly, we have found that acute CB3 myocarditis is unaltered in IL-12p35-deficient mice, which lack IL-12p70, compared to wild-type BALB/c controls.28 We did not examine the effect of IL-12Rβ2 signaling in this study because this strain is not yet available on a BALB/c genetic background. However, we have previously shown that the proinflammatory cytokines TNF-α or IL-1β are all that is needed to induce chronic autoimmune disease after CB3 infection in mice that normally do not develop chronic myocarditis after infection.9–11 We also observe significantly increased levels of TNF-α and IL-1β in the heart of susceptible BALB/c mice compared to resistant C57BL/6 mice during acute CB3-induced myocarditis (day 12 after infection).52 Furthermore, the role for proinflammatory cytokines in exacerbating other autoimmune diseases such as inflammatory bowel disease, experimental allergic encephalomyelitis, and RA is well established.14,48,53,54 The fact that studies comparing IL-12p35- or IL-12p40-deficient mice have shown that these cytokines have unique functions28,48,55 further suggests that cytokines other than IL-12p70 critically impact the development of autoimmune disease. In this study, we also found a distinct role for IL-12Rβ1 compared to IFN-γ in the development of chronic myocarditis, DCM, and fibrosis after CB3 infection. Because the proinflammatory cytokines TNF-α, IL-1β, and IL-18 are known to play critical roles in angiogenesis and fibrosis after injury, their increased levels in susceptible mice after CB3 infection has important implications for the development of chronic, autoimmune heart disease in susceptible individuals.
Fibrosis is a complication of the wound-healing process observed in many chronic inflammatory conditions including autoimmune diseases. The development of chronic fibrosis is key in determining whether a patient will recover from inflammatory heart disease.6 Accumulation of collagen stiffens the ventricles and impedes both contraction and relaxation. A number of factors are known to increase fibroblast proliferation, collagen deposition, and cardiac fibrosis including TGF-β1, fibroblast growth factor, AngII, TNF-α, IL-1β, and IL-4. AngII primarily increases fibrosis by indirectly increasing TGF-β1 levels,5 while IL-1β and TNF-α have long-lasting effects by up-regulating and maintaining TGF-β1 transcription for as long as 6 to 10 weeks.6,22 Perhaps the higher level of TNF-α and IL-1β that we observe in the heart of susceptible mice during acute CB3 myocarditis52 leads to long-term activation of TGF-β1 and the progression to chronic myocarditis in susceptible mice. This is plausible because administration of TNF-α or IL-1β after CB3 infection induces chronic myocarditis in resistant mouse strains.10,11 Although we did not observe increased levels of TNF-α in splenocyte cultures of IFN-γ-deficient mice (Figure 5C), we did observe significant increases in IL-1β levels in the heart (Figure 6B) suggesting that IL-1β levels may be influenced by IFN-γ levels in the heart during chronic CB3-induced myocarditis.
TGF-β1, on the other hand, is the primary factor responsible for exacerbating fibrosis. TGF-β is important for growth and development, inflammation and repair, and host immunity.32 At least 23 distinct genes code for the TGF-β superfamily and there are at least five TGF-β isoforms, with TGF-β1 being the most important in the heart.31,32 TGF-β1 is produced by all types of leukocytes including lymphocytes, macrophages, dendritic cells, and MCs.33 Activation of latent TGF-β1 has been linked to the fibrotic complications associated with chronic disorders including autoimmune diseases.33 Macrophages and MCs, in particular, are associated with exacerbating fibrosis because of release of profibrotic cytokines.33,56 In addition to cytokines such as TNF-α, IL-1β, IL-4, and TGF-β1, MC degranulation releases enzymes such as tryptase and chymase, and histamine that stimulate fibroblast proliferation and collagen synthesis.37–40 Furthermore, MCs have been shown to contribute to a number of chronic inflammatory conditions such as RA, Crohn’s disease, scleroderma, and multiple sclerosis in addition to heart failure.2,41,43,44,57–60 Recently, we found that susceptible BALB/c mice have significantly more MCs and increased TNF-α, IL-1β, and IL-4 levels in the heart and spleen early after CB3 infection compared to resistant C57BL/6 mice.26 In the current study, we observed abundant numbers of degranulating MCs in the pericardium (Figure 7) that were associated with increased histamine levels (Figure 7D) and increased levels of TGF-β1, IL-1β, and IL-4 (Figure 6) in the hearts of IFN-γ-deficient mice compared to infected BALB/c controls. Because degranulating MCs are an important source of profibrotic mediators they may therefore contribute directly to the fibrosis observed after CB3 infection. This study shows that direct or indirect regulation of MC degranulation by IFN-γ is particularly important in preventing fibrous, adhesive pericarditis and the progression to heart failure.
IFN-γ not only reduces viral replication after CB3 infection,12 but also reduces TGF-β1, IL-1β, and IL-4 inflammation and fibrosis well after infectious virus has been cleared from the heart. IFN-γ inhibits transcription of TGF-β1 and IL-4 by direct transcriptional modification, via STAT1 for example.21 IL-4 and IL-1β are known to increase activation and degranulation of MCs,60–62 and thus the regulation of MC degranulation by IFN-γ reported in this study may also occur indirectly by transcriptional reduction of IL-4 levels from Th2 or MCs in the heart (Figure 8). In vitro exposure of MCs to IFN-γ inhibits proliferation and activation, resulting in the expression of the high-affinity receptor for IgG (FcγRI) on MCs.63,64 IFN-γ also prevents fibrosis directly by inhibiting fibroblast proliferation and collagen synthesis (Figure 8), and facilitates wound healing by increasing integrin expression.20,65,66 The absence of IFN-γ in knockout mice and increase in TGF-β1, IL-1β, IL-4, and fibrosis in this study indicates a similar mechanism of action for IFN-γ during the development of chronic CB3-induced myocarditis and pericarditis as in other fibrotic diseases.
Sustained activation of autoreactive T cells has been put forward as an explanation for the role of IFN-γ in initiating autoimmune disease.67 The increased level of inflammation in the heart in the absence of IFN-γ in this study (Figure 1A) argues against that explanation after CB3 infection. IFN-γ has also been shown to regulate apoptosis in T cells,68 which may explain why autoreactive T-cell populations were expanded in splenocytes from IFN-γ-deficient mice in experimental autoimmune myocarditis30 and experimental allergic encephalomyelitis.69 Because lymphocyte populations are not increased in the heart during acute CB3 myocarditis (day 12 after infection) in IFN-γ-deficient mice,12 the affect of IFN-γ on regulating T-cell populations appears to occur later during the pathogenesis of chronic disease. The findings of this study show that a primary role for IFN-γ in protecting against the development of severe chronic myocarditis and DCM after CB3 infection is to inhibit MC degranulation and reduce fibrosis (Figure 8). Although IFN-γ was not detected in the hearts of infected BALB/c mice (Figure 6), systemic IFN-γ (possibly from the spleen; Figure 5A) could readily influence perivascular MCs located in the heart, similar to previous findings.70 Overall, we found that IFN-γ protects against the development of severe chronic CB3-induced myocarditis and pericarditis, suggesting that IFN-γ may be a good therapeutic agent in preventing the development of severe fibrosis, DCM, and heart failure. However, administration of IFN-γ to mice and in clinical trials to humans has shown that IFN-γ therapy can induce adverse side affects that ultimately exacerbate disease or even lead to death.66,71 Thus, a greater understanding of the role of IFN-γ in the development of chronic autoimmune disease and fibrosis after viral infection is needed before successful treatments can be developed. This study broadens our understanding of the mechanisms by which IFN-γ protects against the development of severe chronic fibrosis and heart failure by regulating cytokine production and MC degranulation.
Acknowledgments
We thank Daniela Cihakova, Rajni Sharma, and Jennifer Nyland for a critical reading of the manuscript; and Norman Barker for excellent photography.
Footnotes
Address reprint requests to Dr. Noel R. Rose, Department of Pathology, Johns Hopkins Medical Institutions, 720 Rutland Ave., Baltimore, MD 21205. E-mail: nrrose@jhsph.edu.
Supported by the National Institutes of Health (grants HL67290, HL70729, and AI51835).
References
- Cotran RS. Tissue repair: cellular growth, fibrosis, and wound healing. Cotran RS, Kumar V, Collins T, editors. Philadelphia: Saunders Co.,; Robbins Pathologic Basis of Disease. 1999:pp 89–112. [Google Scholar]
- Lee CG, Homer RJ, Zhu Z, Lanone S, Wang X, Koteliansky V, Shipley JM, Gotwals P, Noble P, Chen Q, Senior RM, Elias JA. Interleukin-13 induces tissue fibrosis by selectively stimulating and activating transforming growth factor β1. J Exp Med. 2001;194:809–821. doi: 10.1084/jem.194.6.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz JEJ, Witt SA, Glascock BJ, Nieman ML, Reiser PJ, Nix SL, Kimball TR, Doetschman T. TGF-β1 mediates the hypertrophic cardiomyocytes growth induced by angiotensin II. J Clin Invest. 2002;109:787–796. doi: 10.1172/JCI14190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwahara F, Kai H, Tokuda K, Kai M, Takeshita A, Egashira K, Imaizumi T. Transforming growth factor-β function blocking prevents myocardial fibrosis and diastolic dysfunction in pressure-overloaded rats. Circulation. 2002;106:130–135. doi: 10.1161/01.cir.0000020689.12472.e0. [DOI] [PubMed] [Google Scholar]
- Bouzegrhane F, Thibault G. Is angiotensin II a proliferative factor of cardiac fibroblasts? Cardiovasc Res. 2002;53:304–312. doi: 10.1016/s0008-6363(01)00448-5. [DOI] [PubMed] [Google Scholar]
- Manabe I, Shindo T, Nagai R. Gene expression in fibroblasts and fibrosis: involvement in cardiac hypertrophy. Circ Res. 2002;91:1103–1113. doi: 10.1161/01.res.0000046452.67724.b8. [DOI] [PubMed] [Google Scholar]
- Peng J, Gurantz D, Tran V, Cowling RT, Greenberg BH. Tumor necrosis factor-α-induced AT1 receptor upregulation enhances angiotensin II-mediated cardiac fibroblast responses that favor fibrosis. Circ Res. 2002;91:1119–1126. doi: 10.1161/01.res.0000047090.08299.d5. [DOI] [PubMed] [Google Scholar]
- Feldman AM, McNamara D. Myocarditis. N Engl J Med. 2000;343:1388–1398. doi: 10.1056/NEJM200011093431908. [DOI] [PubMed] [Google Scholar]
- Fairweather D, Kaya Z, Shellam GR, Lawson CM, Rose NR. From infection to autoimmunity. J Autoimmun. 2001;16:175–186. doi: 10.1006/jaut.2000.0492. [DOI] [PubMed] [Google Scholar]
- Lane JR, Neumann DA, LaFond-Walker A, Herskowitz A, Rose NR. Interleukin 1 or tumor necrosis factor can promote Coxsackievirus B3-induced myocarditis in resistant B10.A mice. J Exp Med. 1992;175:1123–1129. doi: 10.1084/jem.175.4.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane JR, Neumann DA, LaFond-Walker A, Herskowitz A, Rose NR. Role of IL-1 and tumor necrosis factor in Coxsackievirus-induced autoimmune myocarditis. J Immunol. 1993;151:1682–1690. [PubMed] [Google Scholar]
- Fairweather D, Yusung S, Frisancho-Kiss S, Barrett M, Gatewood S, Steele R, Rose NR. IL-12Rβ1 and TLR4 increase IL-1β and IL-18-associated myocarditis and Coxsackievirus replication. J Immunol. 2003;170:4731–4737. doi: 10.4049/jimmunol.170.9.4731. [DOI] [PubMed] [Google Scholar]
- Ma X, Trinchieri G. Regulation of interleukin-12 production by antigen-presenting cells. Adv Immunol. 2001;79:55–92. doi: 10.1016/s0065-2776(01)79002-5. [DOI] [PubMed] [Google Scholar]
- Segal BM, Klinman DM, Shevach EM. Cutting edge: microbial products induce autoimmune disease by an IL-12-dependent pathway. J Immunol. 1997;158:5087–5090. [PubMed] [Google Scholar]
- Caspi RR. IL-12 in autoimmunity. Clin Immunol Immunopathol. 1998;88:4–13. doi: 10.1006/clin.1998.4540. [DOI] [PubMed] [Google Scholar]
- Huber S, Shi C, Budd RC. Gammadelta T cells promote a Th1 response during Coxsackievirus B3 infection in vivo: role of Fas and Fas ligand. J Virol. 1998;76:6487–6494. doi: 10.1128/JVI.76.13.6487-6494.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Garra A. Cytokines induce the development of functionally heterogeneous T helper cell subsets. Immunity. 1998;8:275–283. doi: 10.1016/s1074-7613(00)80533-6. [DOI] [PubMed] [Google Scholar]
- Moser M, Murphy KM. Dendritic cell regulation of Th1-Th2 development. Nat Immunol. 2000;1:199–205. doi: 10.1038/79734. [DOI] [PubMed] [Google Scholar]
- Gurujeyalakshmi G, Giri SN. Molecular mechanisms of antifibrotic effect of interferon gamma in bleomycin-mouse model of lung fibrosis: down-regulation of TGF-beta and procollagen I and III gene expression. Exp Lung Res. 1995;21:791–808. doi: 10.3109/01902149509050842. [DOI] [PubMed] [Google Scholar]
- Oldroyd SD, Thomas GL, Gabbiani G, El Nahas AM. Interferon-γ inhibits experimental renal fibrosis. Kidney Int. 1999;56:2116–2127. doi: 10.1046/j.1523-1755.1999.00775.x. [DOI] [PubMed] [Google Scholar]
- Eickelberg O, Pansky A, Koehler E, Bihl M, Tamm M, Hildebrand P, Perruchoud AP, Kashgarian M, Roth M. Molecular mechanisms of TGF-(beta) antagonism by interferon (gamma) and cyclosporin A in lung fibroblasts. FASEB J. 2001;15:797–806. doi: 10.1096/fj.00-0233com. [DOI] [PubMed] [Google Scholar]
- Schoen FJ. The heart. Cotran RS, Kumar V, Collins T, editors. Philadelphia: Saunders Co.,; Robbins Pathologic Basis of Disease. 1999:pp 543–599. [Google Scholar]
- Kaya Z, Afanasyeva M, Wang Y, Dohmen KM, Schlichting J, Tretter T, Fairweather D, Holers VM, Rose NR. Contribution of the innate immune system to autoimmune myocarditis: a role for complement. Nat Immunol. 2001;2:739–745. doi: 10.1038/90686. [DOI] [PubMed] [Google Scholar]
- Rose NR, Hill SL, Neumann DA. Experimental myocarditis. San Diego: Academic Press,; Autoimmune Disease ModelsA Guidebook. 1994:pp 175–189. [Google Scholar]
- Lenzo JC, Fairweather D, Cull V, Shellam GR, Lawson CM. Characterisation of murine cytomegalovirus myocarditis: cellular infiltration of the heart and virus persistence. J Mol Cell Cardiol. 2002;34:629–640. doi: 10.1006/jmcc.2002.2003. [DOI] [PubMed] [Google Scholar]
- Fairweather D, Frisancho-Kiss S, Gatewood S, Njoku D, Steele R, Barrett M, Rose NR. Mast cells and innate cytokines are associated with susceptibility to autoimmune heart disease following Coxsackievirus B3 infection. Autoimmunity. 2004;37:131–145. doi: 10.1080/0891693042000196200. [DOI] [PubMed] [Google Scholar]
- Fairweather D, Rose NR. Type I diabetes: virus infection or autoimmune disease? Nat Immunol. 2002;3:338–340. doi: 10.1038/ni0402-338. [DOI] [PubMed] [Google Scholar]
- Fairweather D, Afanasyeva M, Rose NR. Cellular immunity: a role for cytokines. Doria A, Pauletto P, editors. Amsterdam: Elsevier Science,; The Handbook of Systemic Autoimmune DiseasesThe Heart in Systemic Autoimmune Diseases. 2004:pp 3–17. [Google Scholar]
- Wu C-Y, Faerrante J, Gately MK, Magram J. Characterization of IL-12 receptor β1 chain (IL-12Rβ1)-deficient mice: IL-12Rβ1 is an essential component of the functional mouse IL-12 receptor. J Immunol. 1997;159:1658–1665. [PubMed] [Google Scholar]
- Afanasyeva M, Wang Y, Kaya Z, Stafford EA, Dohmen KM, Sadighi Akha AA, Rose NR. Interleukin-12 receptor/STAT4 signaling is required for the development of autoimmune myocarditis in mice by an interferon-γ-independent pathway. Circulation. 2001;104:3145–3151. doi: 10.1161/hc5001.100629. [DOI] [PubMed] [Google Scholar]
- Li J-M, Brooks G. Differential protein expression and subcellular distribution of TGFβ1, β2 and β3 in cardiomyocytes during pressure overload-induced hypertrophy. J Mol Cell Cardiol. 1997;29:2213–2224. doi: 10.1006/jmcc.1997.0457. [DOI] [PubMed] [Google Scholar]
- Clark DA, Coker R. Molecules in focus: transforming growth factor-beta (TGF-β). Int J Biochem Cell Biol. 1998;30:293–298. doi: 10.1016/s1357-2725(97)00128-3. [DOI] [PubMed] [Google Scholar]
- Letterio JJ, Roberts AB. Regulation of immune responses by TGF-β. Annu Rev Immunol. 1998;16:137–161. doi: 10.1146/annurev.immunol.16.1.137. [DOI] [PubMed] [Google Scholar]
- Attisano L, Wrana JL. Signal transduction by the TGF-β superfamily. Science. 2002;296:1646–1647. doi: 10.1126/science.1071809. [DOI] [PubMed] [Google Scholar]
- Li YY, McTiernan CF, Feldman AM. Proinflammatory cytokines regulate tissue inhibitors of metalloproteinases and disintegrin metalloproteinase in cardiac cells. Cardiovasc Res. 1999;42:162–172. doi: 10.1016/s0008-6363(98)00297-1. [DOI] [PubMed] [Google Scholar]
- Sano M, Fukuda K, Kodama H, Pan J, Saito M, Matsuzaki J, Takahashi T, Makino S, Kato T, Ogawa S. Interleukin-6 family of cytokines mediate angiotensin II-induced cardiac hypertrophy in rodent cardiomyocytes. J Biol Chem. 2000;275:29717–29723. doi: 10.1074/jbc.M003128200. [DOI] [PubMed] [Google Scholar]
- Blair RJ, Meng H, Marchese MJ, Ren S, Schwartz LB, Tonnesen MG, Gruber BL. Human mast cells stimulate vascular tube formation: tryptase is a novel, potent angiogenic factor. J Clin Invest. 1997;99:2691–2700. doi: 10.1172/JCI119458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cairns JA, Walls AF. Mast cell tryptase stimulates the synthesis of type I collagen in human lung fibroblasts. J Clin Invest. 1997;99:1313–1321. doi: 10.1172/JCI119290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He S, Gaca MDA, Walls AF. The activation of synovial mast cells: modulation of histamine release by tryptase and chymase and their inhibitors. Eur J Pharmacol. 2001;412:223–229. doi: 10.1016/s0014-2999(01)00734-8. [DOI] [PubMed] [Google Scholar]
- Garbuzenko E, Nagler A, Pickholtz D, Gillery P, Reich R, Maquart F-X, Levi-Schaffer F. Human mast cells stimulate fibroblast proliferation, collagen synthesis and lattice contraction: a direct role for mast cells in skin fibrosis. Clin Exp Allergy. 2002;32:237–246. doi: 10.1046/j.1365-2222.2002.01293.x. [DOI] [PubMed] [Google Scholar]
- Dvorak AM. Mast cell degranulation in human hearts. N Engl J Med. 1986;315:969–970. doi: 10.1056/nejm198610093151515. [DOI] [PubMed] [Google Scholar]
- Marone G, deCrescenzo G, Adt M, Patella V, Arbustini E, Genovese A. Immunological characterization and functional importance of human heart mast cells. Immunopharmacology. 1995;31:1–18. doi: 10.1016/0162-3109(95)00037-3. [DOI] [PubMed] [Google Scholar]
- Arbustini E, Gavazzi A, Dal Bello B, Morbini P, Campana C, Diegli M, Grasso M, Fasani R, Banchieri N, Porcu E, Pilotto A, Ponzetta M, Bellin O, Lucreziotti S, Vigano M. Ten-year experience with endomyocardial biopsy in myocarditis presenting with congestive heart failure: frequency, pathologic characteristics, treatment and follow-up. G Ital Cardiol. 1997;27:209–223. [PubMed] [Google Scholar]
- Hara M, Ono K, Hwang M-W, Iwasaki A, Okada M, Nakatani K, Sasayama S, Matsumori A. Evidence for a role of mast cells in the evolution to congestive heart failure. J Exp Med. 2002;195:375–381. doi: 10.1084/jem.20002036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afanasyeva M, Wang Y, Kaya Z, Park S, Zilliox MJ, Schofield BH, Hill SL, Rose NR. Experimental autoimmune myocarditis in A/J mice is an interleukin-4-dependent disease with a Th2 phenotype. Am J Pathol. 2001;159:193–203. doi: 10.1016/S0002-9440(10)61685-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson U, Kurrer MO, Bingisser R, Eugster HP, Saremaslani P, Follath F, Marsch S, Widmer U. Lethal autoimmune myocarditis in interferon-gamma receptor-deficient mice: enhanced disease severity by impaired inducible nitric oxide synthase induction. Circulation. 2001;103:18–21. doi: 10.1161/01.cir.103.1.18. [DOI] [PubMed] [Google Scholar]
- Gor DO, Rose NR, Greenspan NS. Th1-Th2: a procrustean paradigm. Nat Immunol. 2003;4:503–505. doi: 10.1038/ni0603-503. [DOI] [PubMed] [Google Scholar]
- Camoglio L, Juffermans NP, Peppelenbosch M, te Velde AA, ten Kate FJ, van Deventer SJH, Kopf M. Contrasting roles of IL-12p40 and IL-12p35 in the development of hapten-induced colitis. Eur J Immunol. 2002;32:261–269. doi: 10.1002/1521-4141(200201)32:1<261::AID-IMMU261>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Zhang G-X, Gran B, Yu S, Li J, Siglienti I, Chen X, Kamoun M, Rostami A. Induction of experimental autoimmune encephalomyelitis in IL-12 receptor-β2-deficient mice: IL-12 responsiveness is not required in the pathogenesis of inflammatory demyelination in the central nervous system. J Immunol. 2003;170:2153–2160. doi: 10.4049/jimmunol.170.4.2153. [DOI] [PubMed] [Google Scholar]
- Cua DJ, Sherlock J, Chen Y, Murphy CA, Joyce B, Seymour B, Lucian L, To W, Kwan S, Churakova T, Zurawski S, Wiekowski M, Lira SA, Gorman D, Kastelein RA, Sedgwick JD. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature. 2003;421:744–748. doi: 10.1038/nature01355. [DOI] [PubMed] [Google Scholar]
- Watford WT, O’Shea JJ. A case of mistaken identity. Nature. 2003;421:706–708. doi: 10.1038/421706a. [DOI] [PubMed] [Google Scholar]
- Fairweather D, Rose NR. Women and autoimmune diseases. Emerg Inf Dis. 2004;10:2005–2011. doi: 10.3201/eid1011.040367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello CA. Interleukin-1, interleukin-1 receptors and interleukin-1 receptor antagonist. Int Rev Immunol. 1998;16:457–499. doi: 10.3109/08830189809043005. [DOI] [PubMed] [Google Scholar]
- Joosten LA, Helsen MM, Saxne T, van De Loo FA, Heinegard D, van Den Berg WB. IL-1 alpha/beta blockade prevents cartilage and bone destruction in murine type II collagen-induced arthritis, whereas TNF-alpha blockade only ameliorates joint inflammation. J Immunol. 1999;163:5049–5055. [PubMed] [Google Scholar]
- Piccotti JR, Li K, Chan SY, Ferrante J, Magram J, Eichwald EJ, Bishop DK. Alloantigen-reactive Th1 development in IL-12-deficient mice. J Immunol. 1998;160:1132–1138. [PubMed] [Google Scholar]
- Frangogiannis NG, Smith CW, Entman ML. The inflammatory response in myocardial infarction. Cardiovasc Res. 2002;53:31–47. doi: 10.1016/s0008-6363(01)00434-5. [DOI] [PubMed] [Google Scholar]
- Hawkins RA, Claman HN, Clark RA, Steigerwald JC. Increased dermal mast cell populations in progressive systemic sclerosis: a link in chronic fibrosis? Ann Intern Med. 1985;102:182–186. doi: 10.7326/0003-4819-102-2-182. [DOI] [PubMed] [Google Scholar]
- Secor VH, Secor WE, Gutekunst C-A, Brown MA. Mast cells are essential for early onset and severe disease in a murine model of multiple sclerosis. J Exp Med. 2000;191:813–821. doi: 10.1084/jem.191.5.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley DE, Tetlow LC. Mast cell activation and its relation to proinflammatory cytokine production in the rheumatoid lesion. Arthritis Res. 2000;2:65–74. doi: 10.1186/ar70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorentz A, Schuppan D, Gebert A, Manns MP, Bischoff SC. Regulatory effects of stem cell factor and interleukin-4 on adhesion of human mast cells to extracellular matrix proteins. Blood. 2002;99:966–972. doi: 10.1182/blood.v99.3.966. [DOI] [PubMed] [Google Scholar]
- Subramanian N, Bray MA. Interleukin 1 releases histamine from human basophils and mast cells in vitro. J Immunol. 1987;138:271–275. [PubMed] [Google Scholar]
- Strait RT, Morris SC, Smiley K, Urban JF, Jr, Finkelman FD. IL-4 exacerbates anaphylaxis. J Immunol. 2003;170:3835–3842. doi: 10.4049/jimmunol.170.7.3835. [DOI] [PubMed] [Google Scholar]
- Boyce JA. Mast cells: beyond IgE. J Allergy Clin Immunol. 2003;111:24–32. doi: 10.1067/mai.2003.60. [DOI] [PubMed] [Google Scholar]
- Prussin C, Metcalfe DD. IgE, mast cells, basophils, and eosinophils. J Allergy Clin Immunol. 2003;111:S486–S494. doi: 10.1067/mai.2003.120. [DOI] [PubMed] [Google Scholar]
- Ahdieh M, Vandenbos T, Youakin A. Lung epithelial barrier function and wound healing are decreased by IL-4 and IL-13 and enhanced by IFN-γ. Am J Physiol. 2001;281:C2029–C2038. doi: 10.1152/ajpcell.2001.281.6.C2029. [DOI] [PubMed] [Google Scholar]
- Selman M. A dark side of interferon-γ in the treatment of idiopathic pulmonary fibrosis? Am J Respir Crit Care Med. 2003;167:945–947. doi: 10.1164/rccm.2301004. [DOI] [PubMed] [Google Scholar]
- Wheeler DS, Kooy NW. A formidable challenge: the diagnosis and treatment of viral myocarditis in children. Crit Care Clin. 2003;19:365–391. doi: 10.1016/s0749-0704(03)00006-x. [DOI] [PubMed] [Google Scholar]
- Rafaeli Y, Van Parijs L, Alexander SI, Abbas AK. Interferon gamma is required for activation-induced death of T lymphocytes. J Exp Med. 2002;196:999–1005. doi: 10.1084/jem.20020666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu CQ, Wittmer S, Dalton DK. Failure to suppress the expansion of the activated CD4 T cell population in interferon gamma-deficient mice leads to exacerbation of experimental autoimmune encephalomyelitis. J Exp Med. 2000;192:123–128. doi: 10.1084/jem.192.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz MS, La Cava A, Fine C, Rodriguez E, Ilic A, Sarvetnick N. Pancreatic expression of interferon-γ protects mice from lethal coxsackievirus B3 infection and subsequent myocarditis. Nat Med. 2000;6:693–697. doi: 10.1038/76277. [DOI] [PubMed] [Google Scholar]
- Honore I, Nunes H, Groussard O, Kambouchner M, Chambellan A, Aubier M, Valeyre D, Crestani B. Acute respiratory failure after interferon-γ therapy of end-stage pulmonary fibrosis. Am J Resp Crit Care Med. 2003;167:953–957. doi: 10.1164/rccm.200208-818CR. [DOI] [PubMed] [Google Scholar]