Abstract
The genome of Bunyamwera virus (BUN; family Bunyaviridae, genus Orthobunyavirus) consists of three segments of negative-sense RNA. The smallest segment, S, encodes two proteins, the nonstructural protein NSs, which is nonessential for viral replication and transcription, and the nucleocapsid protein N. Although a precise role in the replication cycle has yet to be attributed to NSs, it has been shown that NSs inhibits the induction of alpha/beta interferon, suggesting that it plays a part in counteracting the host antiviral defense. A defense mechanism to limit viral spread is programmed cell death by apoptosis. Here we show that a recombinant BUN that does not express NSs (BUNdelNSs) induces apoptotic cell death more rapidly than wild-type virus. Screening for apoptosis pathways revealed that the proapoptotic transcription factor interferon regulatory factor 3 (IRF-3) was activated by both wild-type BUN and BUNdelNSs infection, but only wild-type BUN was able to suppress signaling downstream of IRF-3. Studies with a BUN minireplicon system showed that active replication induced an IRF-3-dependent promoter, which was suppressed by the NSs protein. In a cell line (P2.1) defective in double-stranded RNA signaling due to low levels of IRF-3, induction of apoptosis was similar for wild-type BUN and BUNdelNSs. These data suggest that the BUN NSs protein can delay cell death in the early stages of BUN infection by inhibiting IRF-3-mediated apoptosis.
The Bunyaviridae family contains mostly arthropod-borne viruses that share certain biological characteristics and is divided into five genera: Orthobunyavirus, Phlebovirus, Nairovirus, Hantavirus, and Tospovirus. Several members of the family are important human and animal pathogens, causing encephalitis or hemorrhagic fever (e.g., Hantaan, Crimean-Congo hemorrhagic fever, La Crosse, and Rift Valley fever viruses) and are examples of the so-called “emerging infections,” often arising in response to ecological changes. Bunyavirus particles are spherical and enveloped and contain a genome comprising three segments of single-stranded RNA of negative polarity. The largest segment, named L, codes for an RNA-dependent RNA polymerase (L protein). The M segment codes for a precursor to two virion glycoproteins (G1 and G2) and, in some cases, for a nonstructural protein (NSm) whose role is not yet understood. The S segment codes for the nucleoprotein N, and, in most genera, also for a nonstructural protein called NSs. The N protein associates with the genomic and antigenomic RNA segments to form helical nucleocapsids. Genome replication and transcription take place in the cytoplasm, while virus budding generally occurs at the Golgi apparatus (13, 14).
The role of NSs in the viral replication cycle is poorly understood, and NSs proteins vary considerably in sequence and in their coding strategy between genera. Indeed, even within a genus, NSs proteins display greater sequence variation than that found between the N proteins (10). Recent studies have given important hints about the function(s) of NSs. With a minireplicon system for Bunyamwera virus (BUN), a member of the Orthobunyavirus genus and the prototype of the family, we showed that while the L and N proteins are necessary and sufficient for replication and transcription (11), these activities can be inhibited by NSs (61). Shutoff of host cell protein synthesis, a hallmark of BUN infection of mammalian cells, was shown to be strongly reduced during infection with a Bunyamwera virus that does not express NSs (BUNdelNSs), pointing towards a role of NSs in inhibition of host protein synthesis (6). In addition, and in contrast to wild-type BUN, BUNdelNSs was shown to be an inducer of the beta interferon (IFN-β) promoter (6), and we have recently demonstrated that NSs is directly responsible for counteracting the induction of α/β-IFN (60). This study also suggested that double-stranded RNA (dsRNA) was responsible for inducing IFN during infection with BUNdelNSs. A mutant of Rift Valley fever phlebovirus that has a large deletion in the S segment that affects the majority of the NSs coding region was shown to be avirulent, and further studies showed that the ability to inhibit α/β-IFN production correlated with virulence (4, 55). Thus, these reports suggest that bunyavirus NSs proteins in general function as α/β-IFN antagonists.
IFNs are upregulated through the coordinate activation of a transcription-regulating protein such as NF-κB or the interferon regulatory factors (IRFs). They constitute the first defense mechanisms activated in virus-infected cells, and in most cell types IFN-β is secreted first, which in turn activates the production of α-IFN in an autocrine and paracrine manner (9, 15). Many DNA and RNA viruses activate the IFN regulatory factor IRF-3, which upregulates a specific subset of genes that lead to apoptosis of the infected cell. It is indeed of major importance to the host that infected cells contain the infection and avoid dissemination of the virus (46). It has been shown previously that bunyaviruses are able to induce apoptosis in infected cells. In the case of La Crosse virus, which belongs to the Orthobunyavirus genus and is the principal cause of pediatric encephalitis in several geographic areas of North America, apoptosis was detected after infection of both newborn mice and a neuronal cell line, whereas another neuronal cell line was resistant to apoptosis or other cytopathic effects (42). More recently, it has also been shown that La Crosse virus can induce apoptosis in BHK-21 cells but not the mosquito cell line C6/36 (3).
As the NSs proteins are apparently involved in antagonism of host defense mechanisms, we investigated whether BUN-induced apoptosis was also affected. Here we show that BUNdelNSs induces apoptosis earlier than the NSs-expressing wild-type BUN and that both viruses activate IRF-3. With a minireplicon system that mimics BUN RNA synthesis, we found that replication and transcription of the minireplicon are sufficient to induce IRF-3 activity. Coexpression of NSs in the minireplicon system decreases IRF-3 activity, suggesting that NSs has an antiapoptotic effect by inhibiting some downstream effect of IRF-3 activation.
MATERIALS AND METHODS
Cells and viruses.
293 cells were maintained in Dulbecco's modified Eagle's medium supplemented with 10% fetal calf serum. 2fTGH cells, which have an intact interferon system, and 2f/SV5-V cells, which are deficient in interferon signaling (1), were maintained in Dulbecco's modified Eagle's medium supplemented with 10% fetal calf serum in the absence or presence of 400 μg of geneticin per ml as necessary. U4C cells, which are unresponsive to all interferons, and P2.1 cells, which are derived from U4C and are deficient in their response to dsRNA (32, 43), were kindly supplied by G. R. Stark and maintained in Dulbecco's modified Eagle's medium supplemented with 10% fetal calf serum. BSR-T7/5 cells, which stably express T7 RNA polymerase (7), were a kind gift of K.-K. Conzelmann and grown in Glasgow minimal essential medium supplemented with 10% tryptose phosphate broth, 10% fetal calf serum, and 1 mg of geneticin per ml. Working stocks of wild-type BUN and BUNdelNSs viruses were grown in BHK-21 cells at 33°C, and titers were determined by plaque assays on BHK-21 cells as previously described (5, 57).
Plasmids.
Plasmids pTM1-BUNN, pTM1-BUNL, pT7riboBUNMREN(−), pTM1-BUNNSs, and pTM1-firefly luciferase have been described previously (61). Plasmid pRL-SV40 (Promega) contains a Renilla luciferase gene under the control of a simian virus 40 promoter. Plasmid pTM1-BUNLmut contains point mutations inactivating the highly conserved polymerase motif E (39), changing the sequence at residues 1214 to 1217 from TCKEFVSLFN (conserved motif underlined) to TCKAGVALFN (mutated residues in bold). Mutation was carried out with the QuikChange procedure (Stratagene) with plasmid pUC119-L(E/P) (26) as the template. This plasmid contains a fragment of the L gene (nucleotides 3145 to 3811) encoding motif E. Mutations were checked by automated sequencing, and the fragment containing the mutated motif E was introduced into pTM1-BUNL.
Reporter plasmids used for signal transduction assays contained either promoter elements responsive to activity of the transcription factors p53, pG13pyLuc (12), or IRF-3, p55C1BLuc (62), or promoters of genes encoding proteins involved in apoptotic pathways: bcl-2, pLB322 (36); bcl-Xl, pGl2-Bcl-Xl (20); bax, pMBAXPF (24); fas, pGL3/FasP-Luc (34); and fasL, pFasLHsLuc (29).
Transfection, minireplicon reconstitution, and reporter gene assays.
Reporter gene assays were carried out in 293 cells grown in 35-mm dishes as previously described (60) by transfecting 1 μg of firefly luciferase reporter plasmid with 5 μg of DAC-30 (Oswell, Southampton, United Kingdom). As an internal control, 0.1 μg of plasmid pRL-SV40 encoding Renilla luciferase were also transfected. After 5 h at 37°C, cells were infected with virus at a multiplicity of infection (MOI) of 1. Cells were lysed 16 h later, and luciferase activities were measured in 20 μl of cell extract with a dual luciferase assay (Promega). Firefly luciferase activities were normalized to the corresponding Renilla luciferase activities to calculate the level of induction.
The minireplicon system has been described before (61). Briefly, BSR-T7/5 cells were transfected with 1 μg of pTM1-BUNL, 0.5 μg of pTM1-BUNN, 0.5 μg of pT7riboBUNMREN(−), and 1 μg of reporter plasmid with 5 μg of DAC-30. When plasmids were omitted from the mix, the overall DNA amounts were kept constant by including empty vector pTM1 DNA.
IRF-3 dimerization assay.
The IRF-3 dimerization assay followed that described previously (25, 30). Briefly, at 24 h postinfection, 2fTGH or 2f/SV5-V cells were resuspended in a buffer containing 50 mM Tris-HCl (pH 7.5), 150 mM NaCl, 1 mM EDTA, 1% Nonidet P-40, protease inhibitors (Complete Protease Inhibitor; Roche), and phosphatase inhibitors (Phosphatase Inhibitor Cocktail II; Calbiochem), vortexed, incubated on ice for 10 min, and then centrifuged at 4°C for 5 min at 10,000 × g. Cell extracts were then analyzed by electrophoresis in a 10% nondenaturing polyacrylamide gel. IRF-3 monomers and dimers were detected by Western blot analysis with polyclonal anti-IRF-3 antibody FL-425 (Santa Cruz).
IRF-3 nuclear translocation assay.
Endogenous cellular IRF-3 was detected with the tyramide signal amplification method (Perkin Elmer). Vero cells were grown on coverslips to 30 to 50% confluency and infected with wild-type BUN or BUNdelNSs at an MOI of 5. After incubation at 37 for 8 h, the cells were fixed with 3% paraformaldehyde and permeabilized with 0.5% Triton X-100 in phosphate-buffered saline (PBS). Cells were washed three times with PBS and incubated with the primary antibodies, mouse monoclonal 742 against BUN G1 protein (31) and polyclonal rabbit anti-IRF-3 (Santa Cruz Biotechnology), each diluted 1:500 in TNB buffer (Perkin Elmer). After incubation at room temperature for 1 h, the coverslips were washed three times in PBS and then treated with the secondary antibodies, fluorescein isothiocyanate-conjugated goat anti-mouse immunoglobulin G (Sigma), and biotin-conjugated anti-rabbit immunoglobulin (Perkin Elmer) at a dilution of 1:200. Cells were again washed three times in PBS and incubated for 30 min with streptavidin-conjugated horseradish peroxidase (Perkin Elmer) at a dilution of 1:100. After being washed in PBS, cells were incubated for 5 min in Fluorophore Tyramide amplification reagent (Perkin Elmer) and then washed in PBS and mounted with Fluorsave solution (Calbiochem). Stained cell samples were examined with a Leica confocal laser scanning microscope with a 63× NA 1.4 objective.
DNA fragmentation analysis.
The procedure used to detect DNA fragmentation has been described previously (63). Briefly, cells attached to the bottom of the culture dish were scraped with a rubber policeman and pooled with detached cells in the supernatant. After centrifugation, cells were resuspended in 80 μl of PBS and lysed in 300 μl of buffer containing 10 mM Tris-HCl (pH 7.6), 10 mM EDTA, and 0.6% sodium dodecyl sulfate. After addition of 100 μl of 5 M NaCl, the mixtures were incubated overnight at 4°C and then centrifuged at 14,000 rpm for 20 min at 4°C. Supernatants were treated sequentially with RNase A (1 mg/ml) and proteinase K (0.2 mg/ml) for 20 min each at 37°C. DNA was precipitated by adding 2 volumes of ethanol and incubating overnight at −20°C. After centrifugation, DNA was dissolved in TE buffer (10 mM Tris-HCl [pH 8], 1 mM EDTA) and analyzed by electrophoresis in a 1.5% agarose-Tris borate-EDTA gel, followed by staining with ethidium bromide.
Analysis of apoptosis by flow cytometry.
Flow cytometry was performed with a FACScalibur flow cytometer (Becton Dickinson). Annexin V-phycoerythrin and 7-amino actinomycin D (7-AAD) staining (annexin V-phycoerythrin apoptosis detection kit; BD Pharmingen) was used to distinguish cells in various stages of apoptosis. Cells were infected at an MOI of 1, and cells from the supernatant and those attached to the dish were pooled for analysis. Cells treated with puromycin at a final concentration of 5 μg/ml were used as a positive control for apoptosis. Immunofluorescence analysis and Western blotting with anti-BUN N antibodies (61) were performed to verify the infection status of the cells used for the various assays.
RESULTS
Induction of apoptotic cell death by BUNs.
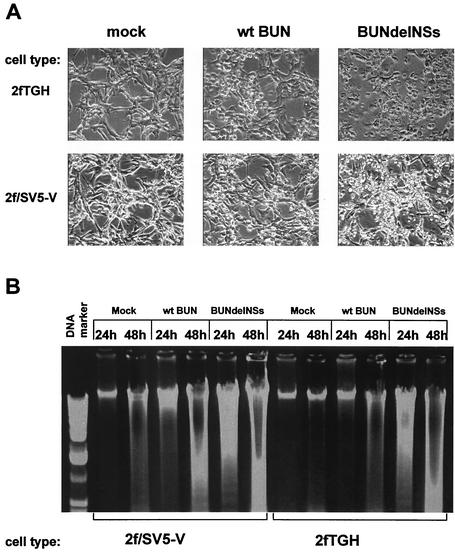
We first investigated whether any differences in cell morphology could be observed in 2fTGH and 2f/SV5-V cells infected with wild-type BUN or BUNdelNSs. 2fTGH cells have an intact IFN system, while 2f/SV5-V cells are deficient in IFN signaling (1). Cell monolayers were examined at various times after infection. At 24 h postinfection, cell shrinkage and detachment from the support matrix could be observed in BUNdelNSs-infected cells and to a lesser extent in wild-type BUN-infected cells (Fig. 1A). No cytopathic effect was observed in mock-infected cells. Chromatin condensation and fragmentation is a marker of apoptotic cell death, and we analyzed this phenomenon (also known as DNA laddering) in 2fTGH and 2f/SV5-V cells at 24 h and 48 h postinfection. As shown in Fig. 1B, DNA fragmentation could be detected in BUNdelNSs-infected cells as early as 24 h postinfection, whereas no fragmentation was detected in wild-type BUN-infected cells at this time. DNA fragmentation was, however, detected in wild-type BUN-infected cells at 48 h postinfection. Similar observations were made for both cell types, indicating that apoptosis induction is independent of IFN signaling. This suggests that BUNdelNSs induces apoptotic cell death more quickly than its wild-type counterpart, implying that the NSs protein has antiapoptotic activity in the early stages of infection. Similar observations were made in other cell lines, including CV1, BHK, 293, Caki-1, and Caki-2 (data not shown).
FIG. 1.
Induction of cell death in cells infected with wild-type (wt) BUN or BUNdelNSs. Cells were infected at an MOI of 1 PFU/cell. (A) Shrinkage and death of 2fTGH and 2f/SV5-V at 24 h postinfection. Cells were examined by phase contrast microscopy. (B) DNA fragmentation in 2fTGH and 2f/SV5-V cells at 24 h and 48 h postinfection. Low-molecular-weight DNA was isolated as described under Materials and Methods, and DNA fragmentation was analyzed by electrophoresis in a 1.5% agarose gel followed by ethidium bromide staining.
Annexin V and 7-AAD staining of BUN-infected cells.
Flow cytometry and annexin V and 7-AAD staining were used to investigate further BUN-induced cell death in a second, independent assay. Annexin V has a high affinity for phosphatidylserine, a membrane phospholipid that is translocated from the inner to the outer membrane leaflet in the early stages of apoptosis and thus serves as a marker for early apoptosis. 7-AAD is a vital stain. Cells that stain annexin V positive and 7-AAD negative are in the early stages of apoptosis, whereas cells staining positive for both markers are in the late stages of apoptosis or dead.
2fTGH and 2f/SV5-V cells were infected with wild-type BUN or BUNdelNSs at an MOI of 1, and at 18 or 24 h postinfection cells from the supernatant were pooled with cells attached to the support matrix and stained as described under Materials and Methods. Infected cells were analyzed by indirect immunofluorescence with anti-N antibodies to confirm that all cells were infected (not shown). As positive control, uninfected cells treated with puromycin were analyzed. Usually between 35% and 50% of the cell population was apoptotic after 24 h (data not shown).
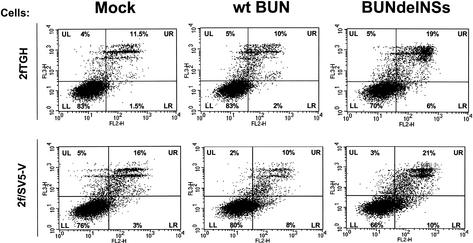
Dot plots of analyzed cell populations of the 24-h-postinfection samples, divided in quadrants (see below), are shown in Fig. 2. The lower left quadrant indicates cells that were alive and nonapoptotic and thus stained with neither 7-AAD nor annexin V. The lower right quadrant indicates cells that were annexin V positive and 7-AAD negative (early apoptotic), whereas the upper right quadrant indicates cells that stained positive for both markers (late apoptotic and dead cells). The upper left quadrant indicates dead cells that stained positive for 7-AAD only.
FIG. 2.
Annexin V and 7-AAD staining and flow cytometric analysis of infected cells. 2fTGH cells (upper panels) and 2f/SV5-V cells (lower panels) were infected with wild-type (wt) BUN or BUNdelNSs (MOI of 1 PFU/cell) and harvested at 24 h postinfection. Dot plots are divided into quadrants LL (lower left; cells staining negative for both 7-AAD and annexin V), UL (upper left; cells staining positive for 7-AAD but negative for annexin V), LR (lower right; staining annexin V positive and 7-AAD negative, representing early apoptotic cells), and UR (upper right; staining positive for both 7-AAD and annexin V, representing late apoptotic/dead cells).
When 2fTGH cells infected with wild-type BUN were analyzed, about 3% of cells at 18 h postinfection and 12% of cells at 24 h postinfection were found to be in the early apoptotic or late apoptotic/dead stage (upper right and lower right combined). Similarly, when 2f/SV5-V cells were infected with wild-type BUN, the figures were 10% at 18 h postinfection and 18% at 24 h postinfection, and 13% of uninfected 2fTGH and 19% of uninfected 2f/SV5-V cells were also found to be in the early apoptotic or late apoptotic/dead stage.
Cells infected with BUNdelNSs showed a different apoptotic pattern. At 18 h postinfection, 11% of infected 2fTGH and 17.5% of infected 2fSV5-V cells were in the early apoptotic or late apoptotic/dead stage, while at 24 h postinfection, these figures rose to 25% and 31%, respectively. The data demonstrate a marked increase in the number of early apoptotic and late apoptotic/dead cells in both cell lines infected with BUNdelNSs virus at early times after infection. These results support the earlier finding that BUNdelNSs induces apoptotic cell death more rapidly then wild-type BUN.
Activation of apoptotic pathways.
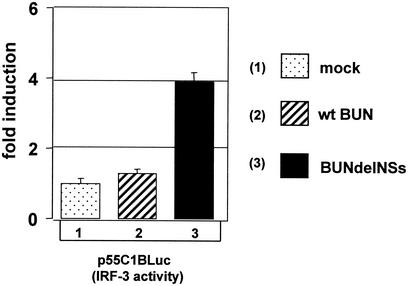
Little is known about the apoptotic pathways activated during infection by members of the Bunyaviridae family, the notable exception being the downregulation of the antiapoptotic protein Bcl-2 seen during Hantaan virus infection (27). Therefore we carried out a screen involving several well-characterized molecules and pathways in order to detect if differences exist between wild-type BUN and BUNdelNSs. To this aim, we used reporter plasmids that encoded the firefly luciferase gene under control of either a promoter responsive to an apoptosis-related transcription factor or a promoter of a gene encoding a protein involved in apoptosis (see Materials and Methods). 293 cells were transfected with the reporter plasmids and then infected at an MOI of 1 with wild-type BUN or BUNdelNSs; cells were harvested 16 h later, and luciferase activity was determined. No activation of a p53-responsive promoter or the fas, fasL, bax, bcl-2, or bcl-Xl promoter could be detected. By contrast, the promoter used to detect activity of the proapoptotic protein IRF-3 was activated up to fourfold following infection with BUNdelNSs, whereas IRF-3 activity in wild-type BUN-infected 293 cells was only stimulated 1.2-fold (Fig. 3).
FIG. 3.
Induction of IRF-3 activity during BUN infection. 293 cells were either mock infected or infected with wild-type (wt) BUN or BUNdelNSs (MOI, 1 PFU/cell), as indicated, and transfected with the IRF-3-responsive plasmid p55C1BLuc. Firefly luciferase activity was measured 16 h posttransfection, and the induction over the levels in mock-infected cells was plotted.
The plasmid used in these experiments, p55CIBluc, contains multiple positive regulatory domain I elements and has been shown to be activated in response to virus infection and potentiated by IRF-3 expression, whereas other IRFs such as IRF-2, IRF-4, interferon consensus sequence binding protein, and interferon-stimulated gene factor 3γ did not affect this reporter (62). These data indicate that BUNdelNSs activates an IRF-3-dependent proapoptotic pathway, while the wild-type virus fails to do so.
Both wild-type BUN and BUNdelNSs activate IRF-3.
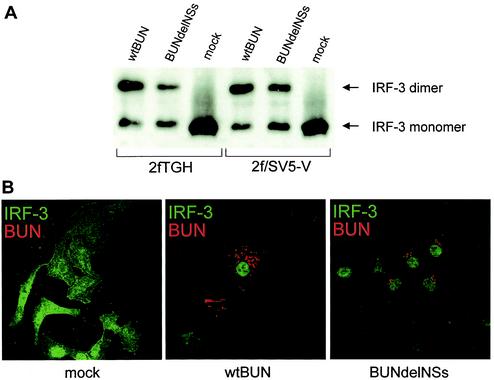
IRF-3 is a ubiquitously expressed protein of about 55 kDa that is present as a latent form in the cytoplasm. Following virus infection, IRF-3 is activated by phosphorylation by an as yet unidentified kinase, resulting in homodimerization and translocation to the nucleus (35, 40, 59, 62). We investigated the activation of IRF-3 following BUN infection, first by analysis of proteins on nondenaturing polyacrylamide gels. 2fTGH or 2f/SV5-V cells were infected with either wild-type BUN or BUNdelNSs or mock infected, and cell extracts were prepared 24 h later. After gel electrophoresis on a native gel, the proteins were blotted onto a nitrocellulose membrane and reacted with an anti-IRF-3 antibody. As shown in Fig. 4A, a single broad band corresponding to the monomeric form of IRF-3 was observed in mock-infected cells. In contrast, in both sets of infected cells, an additional slower-migrating band was seen that corresponds to the dimeric, activated form of IRF-3.
FIG. 4.
Activation of IRF-3 in infected cells. (A) IRF-3 homodimerization assay. 2fTGH and 2f/SV5-V cells were infected with 1 PFU of wild-type (wt) BUN or BUNdelNSs virus per cell or mock infected. At 24 h postinfection, cellular extracts were prepared as described under Materials and Methods and analyzed by nondenaturing polyacrylamide gel electrophoresis and Western blotting with an anti-IRF-3 antibody. IRF-3 monomers and dimers are indicated by arrows. (B) Nuclear transport of IRF-3. Vero cells were either mock infected or infected with 5 PFU of wild-type BUN or BUNdelNSs virus per cell. At 24 h postinfection, cells were fixed and analyzed by double immunofluorescence with antibodies against IRF-3 (green) and BUN (red).
To confirm that the dimeric form translocated to the nucleus, cells were subjected to immunofluorescent analysis with anti-IRF-3 and anti-BUN G1 antibodies (Fig. 4B). In mock-infected cells, IRF-3 staining was both cytoplasmic and nuclear, and obviously no BUN (red) staining was evident. In contrast, in both wild-type BUN- and BUNdelNSs-infected cells (as evidenced by red staining of BUN G1), IRF-3 staining was almost exclusively nuclear. Taken together, the data in Fig. 4 indicate that both wild-type BUN and BUNdelNSs activate IRF-3.
Induction of apoptotic cell death in a cell line expressing low levels of IRF-3.
The above results demonstrate IRF-3 activation in infected cells that is blocked downstream by NSs. To investigate a possible link between IRF-3 and induction of apoptotic cell death, we used the human U4C and P2.1 cell lines (32, 43). The Janus-associated kinase (JAK)-negative U4C cell line was derived from 2fTGH cells through mutagenesis and is unresponsive to all IFNs, while the P2.1 line was derived from U4C and is unresponsive to dsRNA, an effect that is partially due to decreased levels of IRF-3 (about 10 times lower than in the parental U4C line), probably because of a higher rate of degradation of IRF-3 in P2.1 cells (30, 39).
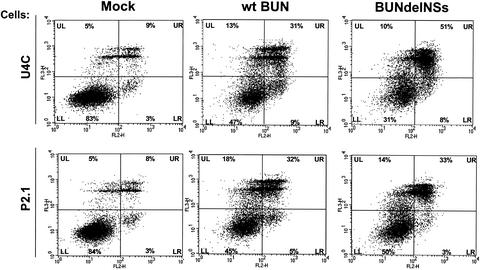
To determine if there was any difference in induction of apoptotic cell death by wild-type BUN and BUNdelNSs, U4C and P2.1 cells were infected at an MOI of 1, followed by annexin V and 7-AAD staining. At 24 h postinfection, cells from the supernatant were pooled with cells attached to the support matrix and stained as described under Materials and Methods. Dot plots of analyzed cell populations are shown in Fig. 5. In the case of U4C cells, only a small number of cells were found to be annexin V positive and 7-AAD negative (early apoptotic) or annexin V positive and 7-AAD positive (late apoptotic/dead). Indeed, cells in the lower and upper right quadrants together accounted for only 12% of the cell population. In the case of wild-type BUN-infected cells, the percentage of cells in the lower and upper right quadrants increased to 40%, but in the case of BUNdelNSs, an even greater increase was observed, up to 59%. These results indicate that while there was an increase in the number of apoptotic cells following wild-type BUN infection, induction of cell death was significantly more prominent at this early time after BUNdelNSs infection.
FIG. 5.
Annexin V and 7-AAD staining and flow cytometric analysis of infected cells. U4C cells (upper panels) and P2.1 cells (lower panels) were infected with wild-type (wt) BUN or BUNdelNSs virus (MOI, 1 PFU/cell) and harvested at 24 h postinfection. Dot plots are divided into quadrants LL (lower left; cells staining negative for both 7-AAD and annexin V), UL (upper left; cells staining positive for 7-AAD but negative for annexin V), LR (lower right; staining annexin V positive and 7-AAD negative, representing early apoptotic cells), and UR (upper right; staining positive for both 7-AAD and annexin V, representing late apoptotic/dead cells).
In the dsRNA-signaling defective P2.1 cells (Fig. 5), again only a low percentage of uninfected control cells were found to be in the early apoptotic or late apoptotic/dead stage (lower and upper right quadrants combined; 11%). Interestingly, for wild-type BUN- and BUNdelNSs-infected cells, the numbers of early apoptotic and late apoptotic/dead cells were found to be similar (lower and upper right quadrants combined; 37% and 36%). Thus, while an increase in apoptotic cell death could be observed with both viruses, BUNdelNSs did not seem to further increase the number of apoptotic P2.1 cells compared to wild-type BUN infection. This suggests that the early onset of apoptosis induced by BUNdelNSs was dependent on IRF-3 and that NSs was able to interfere with it.
Induction of IRF-3 activity by BUN polymerase activity.
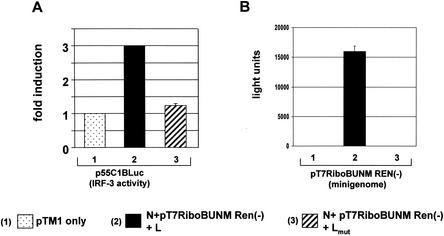
To define how the IRF-3-dependent pathway is activated during BUN infection, we assessed IRF-3 activity in a reconstituted minireplicon system (54). This comprises transfection of BSRT7/5 cells with plasmids that express the BUN L and N proteins and a plasmid expressing a BUN genome analogue, pT7RiboBUNMRen(−), that encodes Renilla luciferase. IRF-3 activity was monitored by cotransfecting reporter plasmid p55C1BLuc. As shown in Fig. 6A, a basal level of firefly luciferase activity was detected when cells were transfected with empty expression plasmid alone (Fig. 6A, column 1). When the BUN minireplicon was transcribed and replicated, up to threefold induction of the IRF-3-sensitive reporter was observed (Fig. 6A, column 2). However, an induction of only 1.2-fold was seen when a mutated L protein (Lmut)-expressing plasmid was substituted for the wild-type L-expressing plasmid (Fig. 6A, column 3). The corresponding Renilla luciferase activities, to monitor replication and transcription of the minigenome, are shown in Fig. 6B. These data indicate that active replication of the minigenome was required for measurable IRF-3 activity and that expression of a mutant L protein had no significant effect.
FIG. 6.
Induction of IRF-3 activity by a reconstituted minireplicon system for BUN. BSR-T7/5 cells were transfected with minireplicon plasmids or pTM1 alone as indicated. Plasmid p55C1BLuc, which contains repeated IRF-3 binding elements, was cotransfected as a reporter construct for IRF-3 activation, indicated by firefly luciferase synthesis. Renilla luciferase activity indicated transcription and replication of the BUN minigenome. Overall DNA amounts were kept constant by adding empty pTM1 plasmid. (A) Detection of IRF-3 activity. Bars: 1, relative IRF-3 activity in cells transfected with pTM1 only; 2, relative IRF-3 activity in cells transfected with pTM1-BUNL (active L clone), pTM1-BUNN, and pT7RiboBUNMREN(−); 3, relative IRF-3 activity in cells transfected with pTM1-BUNLmut (inactive L clone), pTM1-BUNN, and pT7RiboBUNMREN(−). (B) Corresponding Renilla luciferase activities, indicating transcription and replication of the RenM(−) minigenome.
NSs protein inhibits IRF-3 activity.
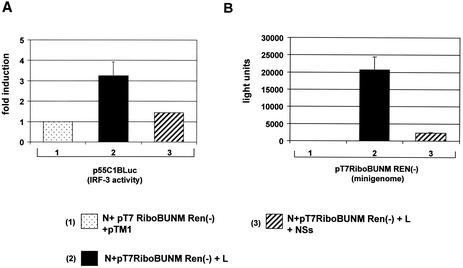
Since the BUN NSs protein has been shown to counteract α/β-IFN induction (60) and to be pivotal for inhibition of early apoptosis in infected cells, we investigated whether NSs could inhibit IRF-3-dependent activity in the minireplicon system. Cells were cotransfected with a vector that expressed NSs, and IRF-3-dependent activity was again assessed by measurement of firefly luciferase generated by reporter plasmid p55C1BLuc. The level of induction of firefly luciferase is presented relative to firefly luciferase activity in cells transfected only with the minigenome reporter and N expression plasmid (Fig. 7A, column 1). Only a 1.4-fold induction of the IRF-3-dependent reporter was detected when the NSs expression plasmid was cotransfected with minireplicon plasmids (Fig. 7A, column 3), compared to 3-fold induction in the absence of NSs (column 2). The corresponding Renilla luciferase activities are given in Fig. 7B, showing that, as expected (61), minigenome activity was strongly reduced when NSs was expressed (compare columns 2 and 3).
FIG. 7.
Inhibition of IRF-3 activity by NSs in the BUN minireplicon system. BSR-T7/5 cell were transfected with minireplicon plasmids and p55C1BLuc as described for Fig. 6 and additionally with the indicated NSs-expressing plasmid. Overall amounts of DNA transfected were kept constant by adding empty pTM1 plasmid. (A) Detection of IRF-3. Bars: 1, relative IRF-3 activity in cells transfected with pTM1, pTM-BUNN, and pT7RiboBUNMREN(−); 2, relative IRF-3 activity in cells transfected with pTM-BUNL, pTM-BUNN, and pT7RiboBUNMREN(−); 3, relative IRF-3 activity in cells transfected with pTM-BUNL, pTM-BUNN, pT7RiboBUNMREN(−), and pTM-BUNNSs. (B) Corresponding Renilla luciferase activities, indicating transcription and replication of the RenM(−) minigenome.
DISCUSSION
In this study, we examined the contribution of NSs to delaying the induction of cell death in BUN-infected cells. Our results indicate that BUNdelNSs induces cell death earlier than its NSs-expressing wild-type counterpart. Morphological features, DNA fragmentation, and phosphatidylserine exposure (21) suggest that BUNdelNSs virus-induced cell death is apoptotic. This occurs independently of the IFN system, as similar results were observed in 2fTGH and 2f/SV5-V cells. The DNA fragmentation data suggested that only late in infection do wild-type BUN-infected cells undergo apoptosis.
Apoptotic cell death induced by viruses of the Bunyaviridae family has been described previously. Hantaan and Prospect Hill hantaviruses have been shown to induce apoptosis in cultured cells (27), and, importantly, Hantaan virus activates IRF-3 (52). Similarly, La Crosse orthobunyavirus has been shown to induce apoptosis in a particular neuronal cell line and in BHK-21 cells (3, 42). It should be noted that we were unable to detect BUN-induced apoptosis in the mosquito cell line C3/36, which is in accordance with a recent finding with La Crosse virus (3). Little is known about the mechanisms and effector molecules involved in bunyavirus-induced apoptosis, but levels of the antiapoptotic protein Bcl-2 levels seemed to be decreased in Hantaan virus-infected cells (27). However, while molecules such as Bax and Bcl-2 intervene in a pathway involving mitochondria and release of cytochrome c (50), other negative-strand viruses such as respiratory syncytial virus and influenza A virus (17, 41) seem to involve a death receptor pathway, the Fas/Fas ligand system.
BUNdelNSs, which grows to lower titers than wild-type virus and has a small-plaque phenotype (6), offers the possibility of studying differences in BUN-induced apoptosis and the pathways involved. It has been shown before that the NSs protein plays a role in counteracting induction of α/β-IFN and thus is involved in viral pathogenesis. Indeed, BUNdelNSs killed mice more slowly than wild-type BUN, and the virus spread more slowly in the brain (6). We suggest that the more rapid induction of apoptosis by BUNdelNSs contributed to containing the infection. BUNdelNSs was shown to be impaired for growth in IFN-competent cells (at least 500-fold), while a lesser effect (about 10-fold) was detected in cells lacking an IFN system (60). In this respect, the BUN NSs protein appears functionally similar to the NS1 protein of influenza A virus, which acts as an IFN antagonist and has been shown to inhibit NF-κB and IRF-3 activation (18, 53, 56). BUNdelNSs induced apoptosis earlier than wild-type BUN in 2fTGH cells as well as in the IFN-signaling impaired 2f/SV5-V cells. We also showed that both wild-type BUN and BUNdelNSs infection activated IRF-3 (Fig. 4). It should be noted that although the simian virus 5 V protein has been reported to block IRF-3 activation, the amount of V required is much higher than that expressed in the 2f/SV5-V cells (22, 44), thus accounting for the apparent anomalous observation seen in the infected 2f/SV5-V cells.
IRF-3 is constitutively expressed in most cells and belongs to a group of IRFs, together with IRF-5 and IRF-7, that are activated immediately in response to virus infection. IRF-3, IRF-8, and IRF-1 are known to induce apoptosis, and their organization and mechanism have been reviewed extensively (2, 38, 46). Upon stimulation by dsRNA or virus infection, IRF-3 is phosphorylated by an as yet unidentified kinase (48, 49) and translocates into the nucleus, where it drives transcription of α/β-IFN genes and other targets as part of a complex with CBP/p300 (19, 58). Moreover, DNA damage can induce IRF-3 phosphorylation by DNA-dependent protein kinase (28), and it seems that IRF-3 activation occurs independently of NF-κB and Janus kinase (43).
RNA viruses (such as measles, Sendai, Newcastle disease, vesicular stomatitis, Hantaan, sin nombre, and respiratory syncytial viruses) and DNA viruses (human cytomegalovirus and herpes simplex virus type 1) are known to activate IRF-3 (8, 40, 45, 48, 52). For Sendai virus, it has been shown that IRF-3 mediates virus-induced apoptosis, as it is only activated during virus infection, and also allows stimulation of the antiviral response and elimination of the infected cell, thus preventing viral replication and spread (23). IFN- and p53-independent activation of IRF-3 have also been implicated in Newcastle disease virus-induced apoptosis (58). The results that we obtained in 2fTGH and the IFN-signaling defective 2f/SV5-V cells are in accord with those previous findings.
We also found that while wild-type BUN can induce apoptotic cell death to some extent in U4C cells, apoptosis is strongly increased in BUNdelNSs-infected U4C cells (an IFN-unresponsive, 2fTGH-derived cell line). In the dsRNA-signaling deficient P2.1 cells, which have 10-fold lower IRF-3 levels than U4C cells (32, 43), induction of apoptotic cell death by both wild-type BUN and BUNdelNSs was found to be similar. This strongly suggests that IRF-3 is indeed a major component of apoptotic pathways induced during BUN infection and is counteracted by NSs.
Recent data obtained with measles virus suggest that the nucleocapsid protein is sufficient to drive activation of IRF-3, putting in question the assumption that dsRNA is always necessary for IRF-3 activation (54). With a BUN minireplicon system, we found that active replication and transcription of the minigenome was necessary to induce an IRF-3-dependent reporter efficiently. Similarly, activation of IRF-3 by a hepatitis C virus subgenomic replicon has been described (16). A mutant BUN L protein as well as different combinations and concentrations of components of the minireplicon system (data not shown) failed to induce the IRF-3-dependent reporter significantly. However, it is possible that the viral protein concentrations were not high enough to exceed a certain threshold. While the measles virus nucleocapsid protein has been shown to interact with IRF-3 (54), thus far we have been unable to detect an interaction between either BUN N or NSs and IRF-3. It should also be noted that work on influenza viruses has shown that UV-inactivated influenza A virus fails to activate IRF-3, while influenza B virus does (30). It has been suggested that viral mRNA synthesis was required for IRF-3 activation by influenza A virus, suggesting that different mechanisms of activation are possible (30).
Results obtained from the BUN minireplicon system and by analyzing IRF-3 activity in infected cells suggest that NSs can inhibit IRF-3 activity, which could be a mechanism to delay cell death. Previously we showed that expression of NSs inhibited viral RNA synthesis in a minireplicon system (61). This might suggest that BUNdelNSs produces less dsRNA during infection, resulting in the differential responses observed in wild-type BUN- and BUNdelNSs-infected cells. However, the amounts of dsRNA made during infection are not known. In addition, we recently showed that both wild-type BUN and BUNdelNSs activate protein kinase R to similar extents (51), suggesting that the viruses produce roughly equivalent amounts of dsRNA (33). In addition, Fig. 4A shows a similar level of IRF-3 activation by both wild-type BUN and BUNdelNSs. Hence it seems likely that NSs exerts its effects after IRF-3 activation and nuclear translocation, perhaps by interfering with DNA binding of the IRF-3/CBP/p300 transcription complex. Experiments to address this are in progress.
In the case of influenza A virus, the role of NS1 in virus-induced apoptosis is not yet clear. One paper reported that influenza A delNS1 virus induces cell death faster then wild-type virus, implying that NS1 downregulates apoptosis (63), whereas another paper reported that transiently expressed NS1 induces apoptosis (47). Previous data from our laboratory indicate that the NSs protein is involved in virus-mediated shutoff of host protein synthesis, as little shutoff is seen in BUNdelNSs-infected mammalian cells (6), and it has been suggested before that shutoff of host protein synthesis by viruses is a measure to counteract expression of cellular antiviral genes (37).
In summary, our results show that the BUNdelNSs virus induces cell death more rapidly then its wild-type counterpart and that IRF-3 seems to be an important component of BUN-activated apoptotic pathways. However, since wild-type BUN and BUNdelNSs activate IRF-3 similarly, it appears that the NSs protein inhibits IRF-3 action after its activation. Thus, not only does NSs counteract the host IFN response early in infection, it also appears to influence a mechanism which is meant to limit viral dissemination, i.e., virus-induced cell death.
Acknowledgments
We thank D. Green (La Jolla Institute for Allergy and Immunology, San Diego, Calif.), B. Vogelstein (John Hopkins University School of Medicine, Baltimore, Md.), D. Latchman (Windeyer Institute of Medical Sciences, University College London, London, United Kingdom), G. Nunez (University of Michigan Medical School, Ann Arbor, Mich.), T. Fujita (Tokyo Institute of Medical Science, Tokyo, Japan), T. Sakai (Kyoto Prefectural University of Medicine, Kyoto, Japan), and X. Xu (Rush Presbyterian St Luke's Medical Center, Chicago, Ill.) for sharing plasmids, K.-K. Conzelmann (Max von Pettenkofer Institute, Munich, Germany) for the BSR-T7/5 cell line, and G. R. Stark (Lerner Research Institute, The Cleveland Clinic Foundation, Cleveland, Ohio) for the U4C and P2.1 cell lines. We also thank J. Fazakerley for helpful discussions when this project was initiated and G. Sourvinos for help with microscopy.
F.W. was supported by grants We 2616/1-1 and We 2616/1-2 from the Deutsche Forschungsgemeinschaft. Work in R.M.E.'s laboratory is funded by the Wellcome Trust (grants 065121 and 058356).
REFERENCES
- 1.Andrejeva, J., D. F. Young, S. Goodbourn, and R. E. Randall. 2002. Degradation of STAT1 and STAT2 by the V proteins of simian virus 5 and human parainfluenza virus type 2, respectively: consequences for virus replication in the presence of alpha/beta and gamma interferons. J. Virol. 76:2159-2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barnes, B., B. Lubyova, and P. M. Pitha. 2002. On the role of IRF in host defense. J. Interferon Cytokine Res. 22:59-71. [DOI] [PubMed] [Google Scholar]
- 3.Borucki, M. K., B. J. Kempf, B. J. Blitvich, C. D. Blair, and B. J. Beaty. 2002. La Crosse virus: replication in vertebrate and invertebrate hosts. Microbes Infect. 4:341-350. [DOI] [PubMed] [Google Scholar]
- 4.Bouloy, M., C. Janzen, P. Vialat, H. Khun, J. Pavlovic, M. Huerre, and O. Haller. 2001. Genetic evidence for an interferon-antagonistic function of Rift Valley fever virus nonstructural protein NSs. J. Virol. 75:1371-1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bridgen, A., and R. M. Elliott. 1996. Rescue of a segmented negative-strand RNA virus entirely from cloned complementary DNAs. Proc. Natl. Acad. Sci. USA 93:15400-15404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bridgen, A., F. Weber, J. K. Fazakerley, and R. M. Elliott. 2001. Bunyamwera bunyavirus nonstructural protein NSs is a nonessential gene product that contributes to viral pathogenesis. Proc. Natl. Acad. Sci. USA 98:664-669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buchholz, U. J., S. Finke, and K. K. Conzelmann. 1999. Generation of bovine respiratory syncytial virus (BRSV) from cDNA: BRSV NS2 is not essential for virus replication in tissue culture, and the human RSV leader region acts as a functional BRSV genome promoter. J. Virol. 73:251-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Casola, A., N. Burger, T. Liu, M. Jamaluddin, A. R. Brasier, and R. P. Garofalo. 2001. Oxidant tone regulates RANTES gene expression in airway epithelial cells infected with respiratory syncytial virus. Role in viral-induced interferon regulatory factor activation. J. Biol. Chem. 276:19715-19722. [DOI] [PubMed] [Google Scholar]
- 9.Deonarain, R., A. Alcami, M. Alexiou, M. J. Dallman, D. R. Gewert, and A. C. Porter. 2000. Impaired antiviral response and alpha/beta interferon induction in mice lacking beta interferon. J. Virol. 74:3404-3409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dunn, E. F., D. C. Pritlove, and R. M. Elliott. 1994. The S RNA genome segments of Batai, Cache Valley, Guaroa, Kairi, Lumbo, Main Drain and Northway bunyaviruses: sequence determination and analysis. J. Gen. Virol. 75:597-608. [DOI] [PubMed] [Google Scholar]
- 11.Dunn, E. F., D. C. Pritlove, H. Jin, and R. M. Elliott. 1995. Transcription of a recombinant bunyavirus RNA template by transiently expressed bunyavirus proteins. Virology 211:133-143. [DOI] [PubMed] [Google Scholar]
- 12.el-Deiry, W. S., S. E. Kern, J. A. Pietenpol, K. W. Kinzler, and B. Vogelstein. 1992. Definition of a consensus binding site for p53. Nat. Genet. 1:45-49. [DOI] [PubMed] [Google Scholar]
- 13.Elliott, R. M. 1996. The Bunyaviridae: concluding remarks and future prospects, p. 295-332. In R. M. Elliott (ed.), The Bunyaviridae. Plenum Press, New York, N.Y.
- 14.Elliott, R. M. 1997. Emerging viruses: the Bunyaviridae. Mol. Med. 3:572-577. [PMC free article] [PubMed] [Google Scholar]
- 15.Erlandsson, L., R. Blumenthal, M. L. Eloranta, H. Engel, G. Alm, S. Weiss, and T. Leanderson. 1998. Interferon-beta is required for interferon-alpha production in mouse fibroblasts. Curr. Biol. 8:223-226. [DOI] [PubMed] [Google Scholar]
- 16.Fredericksen, B., G. R. Akkaraju, E. Foy, C. Wang, J. Pflugheber, Z. J. Chen, and M. Gale, Jr. 2002. Activation of the interferon-beta promoter during hepatitis C virus RNA replication. Viral Immunol. 15:29-40. [DOI] [PubMed] [Google Scholar]
- 17.Fujimoto, I., T. Takizawa, Y. Ohba, and Y. Nakanishi. 1998. Coexpression of Fas and Fas-ligand on the surface of influenza virus-infected cells. Cell Death Differ. 5:426-431. [DOI] [PubMed] [Google Scholar]
- 18.Garcia-Sastre, A. 2001. Inhibition of interferon-mediated antiviral responses by influenza A viruses and other negative-strand RNA viruses. Virology 279:375-384. [DOI] [PubMed] [Google Scholar]
- 19.Grandvaux, N., M. J. Servant, B. tenOever, G. C. Sen, S. Balachandran, G. N. Barber, R. Lin, and J. Hiscott. 2002. Transcriptional profiling of interferon regulatory factor 3 target genes: direct involvement in the regulation of interferon-stimulated genes. J. Virol. 76:5532-5539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grillot, D. A., M. Gonzalez-Garcia, D. Ekhterae, L. Duan, N. Inohara, S. Ohta, M. F. Seldin, and G. Nunez. 1997. Genomic organization, promoter region analysis, and chromosome localization of the mouse bcl-x gene. J. Immunol. 158:4750-4757. [PubMed] [Google Scholar]
- 21.Hay, S., and G. Kannourakis. 2002. A time to kill: viral manipulation of the cell death program. J. Gen. Virol. 83:1547-1564. [DOI] [PubMed] [Google Scholar]
- 22.He, B., R. G. Paterson, N. Stock, J. E. Durbin, R. K. Durbin, S. Goodbourn, R. E. Randall, and R. A. Lamb. 2002. Recovery of paramyxovirus simian virus 5 with a V protein lacking the conserved cysteine-rich domain: the multifunctional V protein blocks both interferon-β induction and interferon signaling. Virology 303:15-32. [DOI] [PubMed] [Google Scholar]
- 23.Heylbroeck, C., S. Balachandran, M. J. Servant, C. DeLuca, G. N. Barber, R. Lin, and J. Hiscott. 2000. The IRF-3 transcription factor mediates Sendai virus-induced apoptosis. J. Virol. 74:3781-3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Igata, E., T. Inoue, N. Ohtani-Fujita, Y. Sowa, Y. Tsujimoto, and T. Sakai. 1999. Molecular cloning and functional analysis of the murine bax gene promoter. Gene 238:407-415. [DOI] [PubMed] [Google Scholar]
- 25.Iwamura, T., M. Yoneyama, K. Yamaguchi, W. Suhara, W. Mori, K. Shiota, Y. Okabe, H. Namiki, and T. Fujita. 2001. Induction of IRF-3/-7 kinase and NF-kappaB in response to double-stranded RNA and virus infection: common and unique pathways. Genes Cells 6:375-388. [DOI] [PubMed] [Google Scholar]
- 26.Jin, H., and R. M. Elliott. 1992. Mutagenesis of the L protein encoded by Bunyamwera virus and production of monospecific antibodies. J. Gen. Virol. 73:2235-2244. [DOI] [PubMed] [Google Scholar]
- 27.Kang, J. I., S. H. Park, P. W. Lee, and B. Y. Ahn. 1999. Apoptosis is induced by hantaviruses in cultured cells. Virology 264:99-105. [DOI] [PubMed] [Google Scholar]
- 28.Karpova, A. Y., M. Trost, J. M. Murray, L. C. Cantley, and P. M. Howley. 2002. Interferon regulatory factor-3 is an in vivo target of DNA-PK. Proc. Natl. Acad. Sci. USA 99:2818-2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kasibhatla, S., T. Brunner, L. Genestier, F. Echeverri, A. Mahboubi, and D. R. Green. 1998. DNA damaging agents induce expression of Fas ligand and subsequent apoptosis in T lymphocytes via the activation of NF-kappa B and AP-1. Mol. Cell 1:543-551. [DOI] [PubMed] [Google Scholar]
- 30.Kim, M.-J., A. G. Latham, and R. M. Krug. 2002. Human influenza viruses activate an interferon-independent transcription of cellular antiviral genes: outcome with influenza A virus is unique. Proc. Natl. Acad. Sci. USA 99:10096-10101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lappin, D. F., G. W. Nakitare, J. W. Palfreyman, and R. M. Elliott. 1994. Localisation of Bunyamwera bunyavirus G1 glycoprotein to the Golgi requires association with G2 but not NSm. J. Gen. Virol. 75:3441-3451. [DOI] [PubMed] [Google Scholar]
- 32.Leaman, D. W., A. Salvekar, R. Patel, G. C. Sen, and G. R. Stark. 1998. A mutant cell line defective in response to double-stranded RNA and in regulating basal expression of interferon-stimulated genes. Proc. Natl. Acad. Sci. USA 95:9442-9447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leitner, W. W., L. N. Hwang, M. J. deVeer, A. Zhou, R. H. Silverman, B. R. G. Williams, T. W. Dubensky, H. Ying, and N. P. Restifo. 2003. Alphavirus-based DNA vaccine breaks immunological tolerance by activating innate antiviral pathways. Nat. Med. 9:33-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li, X. R., A. S. Chong, J. Wu, K. A. Roebuck, A. Kumar, J. E. Parrillo, U. R. Rapp, R. P. Kimberly, J. W. Williams, and X. Xu. 1999. Transcriptional regulation of Fas gene expression by GA-binding protein and AP-1 in T cell antigen receptor.CD3 complex-stimulated T cells. J. Biol. Chem. 274:35203-35210. [DOI] [PubMed] [Google Scholar]
- 35.Lin, R., C. Heylbroeck, P. M. Pitha, and J. Hiscott. 1998. Virus-dependent phosphorylation of the IRF-3 transcription factor regulates nuclear translocation, transactivation potential, and proteasome-mediated degradation. Mol. Cell. Biol. 18:2986-2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu, Y. Z., L. M. Boxer, and D. S. Latchman. 1999. Activation of the Bcl-2 promoter by nerve growth factor is mediated by the p42/p44 MAPK cascade. Nucleic Acids Res. 27:2086-2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lyles, D. S. 2000. Cytopathogenesis and inhibition of host gene expression by RNA viruses. Microbiol. Mol. Biol. Rev. 64:709-724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mamane, Y., C. Heylbroeck, P. Genin, M. Algarte, M. J. Servant, C. LePage, C. DeLuca, H. Kwon, R. Lin, and J. Hiscott. 1999. Interferon regulatory factors: the next generation. Gene 237:1-14. [DOI] [PubMed] [Google Scholar]
- 39.Muller, R., O. Poch, M. Delarue, D. H. Bishop, and M. Bouloy. 1994. Rift Valley fever virus L segment: correction of the sequence and possible functional role of newly identified regions conserved in RNA-dependent polymerases. J. Gen. Virol. 75:1345-1352. [DOI] [PubMed] [Google Scholar]
- 40.Navarro, L., K. Mowen, S. Rodems, B. Weaver, N. Reich, D. Spector, and M. David. 1998. Cytomegalovirus activates interferon immediate-early response gene expression and an interferon regulatory factor 3-containing interferon-stimulated response element-binding complex. Mol. Cell. Biol. 18:3796-3802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.O'Donnell, D., R., L. Milligan, and J. M. Stark. 1999. Induction of CD95 (Fas) and apoptosis in respiratory epithelial cell cultures following respiratory syncytial virus infection. Virology 257:198-207. [DOI] [PubMed] [Google Scholar]
- 42.Pekosz, A., J. Phillips, D. Pleasure, D. Merry, and F. Gonzalez-Scarano. 1996. Induction of apoptosis by La Crosse virus infection and role of neuronal differentiation and human bcl-2 expression in its prevention. J. Virol. 70:5329-5335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Peters, K. L., H. L. Smith, G. R. Stark, and G. C. Sen. 2002. IRF-3-dependent, NFkappa B- and JNK-independent activation of the 561 and IFN-beta genes in response to double-stranded RNA. Proc. Natl. Acad. Sci. USA 99:6322-6327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Poole, E., B. He, R. A. Lamb, R. E. Randall, and S. Goodbourn. 2002. The V proteins of simian virus 5 and other paramyxoviruses inhibit induction of interferon-β. Virology 303:33-46. [DOI] [PubMed] [Google Scholar]
- 45.Preston, C. M., A. N. Harman, and M. J. Nicholl. 2001. Activation of interferon response factor-3 in human cells infected with herpes simplex virus type 1 or human cytomegalovirus. J. Virol. 75:8909-8916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reich, N. C. 2002. Nuclear/cytoplasmic localization of IRFs in response to viral infection or interferon stimulation. J. Interferon Cytokine Res. 22:103-109. [DOI] [PubMed] [Google Scholar]
- 47.Schultz-Cherry, S., N. Dybdahl-Sissoko, G. Neumann, Y. Kawaoka, and V. S. Hinshaw. 2001. Influenza virus ns1 protein induces apoptosis in cultured cells. J. Virol. 75:7875-7881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Servant, M. J., B. ten Oever, C. LePage, L. Conti, S. Gessani, I. Julkunen, R. Lin, and J. Hiscott. 2001. Identification of distinct signaling pathways leading to the phosphorylation of interferon regulatory factor 3. J. Biol. Chem. 276:355-363. [DOI] [PubMed] [Google Scholar]
- 49.Smith, E. J., I. Marie, A. Prakash, A. Garcia-Sastre, and D. E. Levy. 2001. IRF3 and IRF7 phosphorylation in virus-infected cells does not require double-stranded RNA-dependent protein kinase R or Ikappa B kinase but is blocked by vaccinia virus E3L protein. J. Biol. Chem. 276:8951-8957. [DOI] [PubMed] [Google Scholar]
- 50.Strasser, A., L. O'Connor, and V. M. Dixit. 2000. Apoptosis signaling. Annu. Rev. Biochem. 69:217-245. [DOI] [PubMed] [Google Scholar]
- 51.Streitenfeld, H., A. Boyd, J. K. Fazakerley, A. Bridgen, R. M. Elliott, and F. Weber. 2003. Activation of protein kinase R by Bunyamwera virus is independent of the viral interferon antagonist NSs. J. Virol. 77:5507-5511. [DOI] [PMC free article] [PubMed]
- 52.Sundstrom, J. B., L. K. McMullan, C. F. Spiropoulou, W. C. Hooper, A. A. Ansari, C. J. Peters, and P. E. Rollin. 2001. Hantavirus infection induces the expression of RANTES and IP-10 without causing increased permeability in human lung microvascular endothelial cells. J. Virol. 75:6070-6085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Talon, J., C. M. Horvath, R. Polley, C. F. Basler, T. Muster, P. Palese, and A. Garcia-Sastre. 2000. Activation of interferon regulatory factor 3 is inhibited by the influenza A virus NS1 protein. J. Virol. 74:7989-7996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.tenOever, B. R., M. J. Servant, N. Grandvaux, R. Lin, and J. Hiscott. 2002. Recognition of the measles virus nucleocapsid as a mechanism of IRF-3 activation. J. Virol. 76:3659-3669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vialat, P., A. Billecocq, A. Kohl, and M. Bouloy. 2000. The S segment of rift valley fever phlebovirus (Bunyaviridae) carries determinants for attenuation and virulence in mice. J. Virol. 74:1538-1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang, X., M. Li, H. Zheng, T. Muster, P. Palese, A. A. Beg, and A. Garcia-Sastre. 2000. Influenza A virus NS1 protein prevents activation of NF-κB and induction of alpha/beta interferon. J. Virol. 74:11566-11573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Watret, G. E., C. R. Pringle, and R. M. Elliott. 1985. Synthesis of bunyavirus-specific proteins in a continuous cell line (XTC-2) derived from Xenopus laevis. J. Gen. Virol. 66:473-482. [DOI] [PubMed] [Google Scholar]
- 58.Weaver, B. K., O. Ando, K. P. Kumar, and N. C. Reich. 2001. Apoptosis is promoted by the dsRNA-activated factor (DRAF1) during viral infection independent of the action of interferon or p53. FASEB J. 15:501-515. [DOI] [PubMed] [Google Scholar]
- 59.Weaver, B. K., K. P. Kumar, and N. C. Reich. 1998. Interferon regulatory factor 3 and CREB-binding protein/p300 are subunits of double-stranded RNA-activated transcription factor DRAF1. Mol. Cell. Biol. 18:1359-1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Weber, F., A. Bridgen, J. K. Fazakerley, H. Streitenfeld, N. Kessler, R. E. Randall, and R. M. Elliott. 2002. Bunyamwera bunyavirus nonstructural protein NSs counteracts the induction of alpha/beta interferon. J. Virol. 76:7949-7955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Weber, F., E. F. Dunn, A. Bridgen, and R. M. Elliott. 2001. The Bunyamwera virus nonstructural protein NSs inhibits viral RNA synthesis in a minireplicon system. Virology 281:67-74. [DOI] [PubMed] [Google Scholar]
- 62.Yoneyama, M., W. Suhara, Y. Fukuhara, M. Fukuda, E. Nishida, and T. Fujita. 1998. Direct triggering of the type I interferon system by virus infection: activation of a transcription factor complex containing IRF-3 and CBP/p300. EMBO J. 17:1087-1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhirnov, O. P., T. E. Konakova, T. Wolff, and H. D. Klenk. 2002. NS1 protein of influenza A virus downregulates apoptosis. J. Virol. 76:1617-1625. [DOI] [PMC free article] [PubMed] [Google Scholar]