Abstract
The 1.2-kb DNA fragment of the Rickettsia conorii outer membrane protein B gene (OmpB451-846) was subcloned using site-specific PCR primers and expressed as six smaller fragments: OmpB458-652, OmpB595-744, OmpB595-654, OmpB645-692, OmpB689-744, and OmpB739-848. NCTC cells transfected with a mammalian expression vector expressing the fragments OmpB689-744 and OmpB739-848 stimulated immune anti-R. conorii CD8 T lymphocytes, suggesting the presence of CD8 T-lymphocyte-stimulating epitopes on these fragments. In order to further characterize the CD8 T-lymphocyte-stimulatory elements, CD8 T-lymphocyte epitopes on OmpB689-744 and OmpB739-848 were mapped by overlapping synthetic peptides. The ability of these synthetic peptides to stimulate immune CD8 T lymphocytes was determined by gamma interferon (IFN-γ) production and cell proliferation after incubation with simian virus 40-transformed murine vascular endothelial cells in the presence of a 20 μM solution of each synthetic peptide. Five synthetic peptides, SKGVNVDTV (OmpB708-716), ANVGSFVFN (OmpB735-743), IVSGTVGGQ (OmpB749-757), ANSTLQIGG (OmpB789-797), and IVEFVNTGP (OmpB812-820), induced secretion of IFN-γ at significantly higher levels than the controls. Three of these five peptides, SKGVNVDTV (OmpB708-716), ANSTLQIGG (OmpB789-797), and IVEFVNTGP (OmpB812-820), also stimulated the proliferation of immune CD8 T lymphocytes. Significantly higher levels of specific cytotoxic T-lymphocyte killing were observed with the same three synthetic peptides, SKGVNVDTV (OmpB708-716), ANSTLQIGG (OmpB789-797), and IVEFVNTGP (OmpB812-820).
Rickettsiae are small (0.3 by 1.0 μm), obligately intracellular bacteria that reside in arthropod hosts in at least part of their life cycle. Tick-transmitted Rocky Mountain spotted fever and louse-borne typhus fever are among the most virulent human infections known. All adequately studied Rickettsia species have a quantitatively major, immunodominant S-layer cell wall protein (OmpB), which is encoded as a 168-kDa precursor that undergoes posttranslational protease modification to the mature 135-kDa molecule (18). The spotted fever group rickettsiae differ from typhus group rickettsiae by possessing an additional major, immunodominant surface protein (OmpA) and have antigenic differences from typhus group rickettsiae in the lipopolysaccharides. OmpA contains a large, central, hydrophilic domain of 6 to 15 nearly identical tandem repeat units comprising 72 to 75 amino acids (aa) each.
We have developed mouse models that conform better to the target cells (endothelium) and pathology (disseminated vascular injury) of rickettsial diseases than previously available models, and they offer opportunities for major important advances in the study of rickettsial immunity (16, 45, 46). Previous studies provided evidence that T lymphocytes are critical in the development of immune protection in rickettsial diseases (9, 23, 26, 28, 29, 43). Recently performed research showed that adoptive transfer of immune CD4 or CD8 T lymphocytes protects mice against lethal disseminated endothelial infection with Rickettsia conorii (15). Depletion of CD8, but not CD4, T lymphocytes converts infection with an ordinarily sublethal dose of R. conorii into an overwhelming fatal rickettsial infection in the majority of animals and a persistent symptomatic infection in the survivors. Thus, the evidence that T lymphocytes, particularly those of the CD8 subset, are crucial effectors of immunity applies broadly within the genus Rickettsia (45). The potential importance of CD4 and CD8 T lymphocytes in human rickettsial infections is emphasized by the presence of large numbers of these cells in eschars of patients with R. conorii infection at the time of immune clearance of the rickettsiae (20).
Vaccination with recombinant OmpA has protected guinea pigs against Rickettsia rickettsii and R. conorii, and purified native OmpB has protected vaccinated guinea pigs against Rickettsia typhi (2, 11, 38, 40). Immunization of mice with recombinant Mycobacterium vaccae expressing rompA3006-3960 or rompA2331-3976 and boosted with the homologous proteins (OmpA755-1301 or OmpA703-1288, respectively) stimulated partial protection against lethal challenge with R. conorii (10). The fragments OmpA703-1288, OmpA1644-2213, OmpB451-846, and OmpB754-1308 stimulated T-lymphocyte proliferation and secretion of gamma interferon (IFN-γ) after primary immunization with DNA and booster immunization with the homologous recombinant proteins, and 100% of mice were protected against lethal challenge by immunization with the combination of the four fragments (12). Based on these findings, it was hypothesized that OmpB contains protective elements and that some such elements are able to stimulate immune CD8 T lymphocytes. The specific aims of this study were to define the CD8 T-lymphocyte-stimulating epitopes of OmpB451-846 of R. conorii at the oligopeptide level and identify the epitopes processed intracellularly and presented on the cell membrane by endothelial cells for the activation of CD8 T lymphocytes. The health impact of this research is that it will establish the knowledge and principles to enable the production of effective vaccines against rickettsial diseases.
MATERIALS AND METHODS
Rickettsiae.
R. conorii (Malish 7 strain), a human isolate from South Africa with an unknown number of passages in yolk sacs of specific-pathogen-free embryonated chicken eggs (CE), was obtained from the American Type Culture Collection (ATCC; Manassas, Va.) and has the following passage history in our laboratory: plaque purification in Vero cells, 30 CE passages, four Vero cell passages, and two further CE passages. Rickettsial suspensions were prepared by homogenization of infected yolk sacs with a Waring blender and diluted to a 10% suspension in sucrose-phosphate-glutamate buffer (SPG; 0.218 M sucrose, 0.0038 M KH2PO4, 0.0072 M K2HPO4, 0.0049 M monosodium l-glutamic acid, pH 7.0). Aliquots of the stock were kept frozen at −80°C. Rickettsiae were prepared by Renografin density gradient purification from infected Vero cells (African green monkey kidney cells; ATCC) (19). After purification, rickettsiae were killed by exposure to 100°C for 10 min.
Mice.
Six- to eight-week-old male C3H/HeN (H-2k) mice were purchased from Harlan Sprague-Dawley (Indianapolis, Ind.). These mice were seronegative for mouse hepatitis virus and were kept in a P-3 biohazard containment-level animal room after inoculation with rickettsiae.
Cells.
The simian virus 40-transformed murine vascular endothelial cell (SVEC) line SVEC4-10 was kindly provided by Michael Edidin (Johns Hopkins University, Baltimore, Md.) (30). The NCTC clone 2555 fibroblast cell line (ATCC) was derived from a C3H/HeN mouse with the same major histocompatibility complex (MHC) class I (H-2k) haplotype. These cells were maintained in vitro in Dulbecco's modified essential medium (DMEM; GIBCO/BRL, Grand Island, N.Y.) containing 10% bovine calf serum (BCS; HyClone Inc., Logan, Utah), 2 mM l-glutamine, and 0.01 M HEPES buffer. The cells were passaged twice weekly.
Plasmid DNA constructs.
A 1.2-kb OmpB451-846 fragment was PCR amplified from p120R-19, a generous gift from Robert Gilmore (17). p120R-19 contains the majority of the open reading frame for OmpB of R. rickettsii. R. rickettsii and R. conorii share homology in rompB genes of greater than 95%. The OmpB451-846 fragment was further subcloned by using site-specific PCR primers (Table 1). These fragments were ligated to the mammalian expression vector backbone pcDNA3.1/CT-GFP-TOPO (Invitrogen, Carlsbad, Calif.) and transformed into competent Escherichia coli TOP10 cells according to the manufacturer's instructions. Transformants bearing plasmids containing rickettsial DNA fragments were identified by restriction endonuclease analysis. Orientation and frame relative to the cytomegalovirus promoter were confirmed by DNA sequence analysis with an ABI Prism 377 DNA sequencer (Perkin-Elmer Applied Biosystems, Foster City, Calif.). All plasmid constructs were maintained in the E. coli transformants under ampicillin selection, and large-scale concentrated plasmid preparations (1.5 to 2.2 mg/ml) of these constructs were generated using EndoFree Plasmid Mega kits (Qiagen, Valencia, Calif.) according to the manufacturer's instructions.
TABLE 1.
Site-specific PCR primers of the OmpB451-846 fragment
| Peptide | Forward Primer | Reverse primer |
|---|---|---|
| OmpB458-652 | 5′-ATGATTACAGCAATTGAAGCATCAGGTG-3′ | 5′-CGTCATTTCCAATAACTAACTCGT-3′ |
| OmpB595-744 | 5′-ATGACAGGCGTTGTTGATGCGAGTAG-3′ | 5′-CGGCATTAAAGACGAAAGAACCT-3′ |
| OmpB739-848 | 5′-ATGTTCGTCTTTAATGCCGGTGGAA-3′ | 5′-CAATCACTACGTTACCGGGACCAGAA-3′ |
| OmpB595-654 | 5′-ATGGGCGTTGTTGATGGGAGTAG-3′ | 5′-CTGCACCGTCATTTCCAATAACTA-3′ |
| OmpB645-692 | 5′-ATGGAGTTAGTTATTGGAAATGACG-3′ | 5′-TTGTACCGGCTGCAAGAGTTGTA-3′ |
| OmpB689-744 | 5′-ATGGCCGGTACAAATTTAGGTAGTG-3′ | 5′-CGGCATTAAAGACGAAAGAAC-3′ |
Lipofectamine2000 transfection.
NCTC fibroblast cells were transfected by suspending 3 × 104 cells/well in 100 μl of DMEM containing 10% BCS in a 96-well plate. After 0.32 μg of each diluted recombinant plasmid DNA was added with the diluted Lipofectamine2000 (GIBCO/BRL), the mixtures were incubated at room temperature for 20 min to allow DNA-Lipofectamine2000 complexes to form. Then, the DNA-Lipofectamine2000 complexes (100 μl) were added directly to each well and mixed gently by rocking the plate back and forth. After 48 h, transfected cells were selected by growth in DMEM containing 10% BCS and 800 μg of Geneticin (G418; GIBCO/BRL)/ml. NCTC cells transfected with the recombinant plasmids were used to evaluate the activation of CD8 T lymphocytes.
R. conorii-specific CD8 T-lymphocyte cell line.
A CD8 T-lymphocyte cell line with high specificity for rickettsial antigens was developed by serial in vitro antigen stimulations. Five 6- to 8-week-old C3H/HeN male mice were inoculated intravenously with a sublethal dose of R. conorii (5 × 102 PFU). All mice became ill and recovered by day 9 postinoculation. On that day, the murine spleens were harvested. Cell lines were derived from whole-spleen suspensions and from CD8 T lymphocytes purified from the same spleen suspensions. CD8 T lymphocytes were purified using selective columns (R & D Systems, Minneapolis, Minn.). In vitro stimulation was carried out in six-well plates containing a monolayer of paraformaldehyde-fixed, R. conorii-infected SVECs. Three milliliters of purified CD8 T lymphocytes at a concentration of 6 × 105 cells/ml or 3 ml of spleen suspension at a concentration of 6 × 106 cells/ml was added to each well. Three milliliters containing 6 × 106 gamma-irradiated (2,300-rad) syngeneic naïve splenocytes/ml were added to each well as feeder cells. Plates were incubated at 37°C in a 5% CO2 atmosphere. Lymphocytes were restimulated every week. Human recombinant interleukin-2 (Roche Molecular Biochemicals, Indianapolis, Ind.) was added to each well at a concentration of 10 U/ml from the second passage onwards.
Lymphocyte responses.
T-lymphocyte responses were determined by measurement of IFN-γ secretion and cell proliferation after antigen stimulation. IFN-γ secretion was measured by use of a quantitative sandwich enzyme-linked immunosorbent assay kit (R & D Systems) according to the manufacturer's instructions. Results reported were the averages of triplicate samples. Standard deviations were determined by using the Microsoft Excel program. Differences among data sets were considered significant if P was ≤0.05; P values were determined by Student's t test with Microsoft Excel. The standard thymidine incorporation assay was used to quantify lymphocyte proliferation. The R. conorii-specific CD8 T-lymphocyte cell line was adjusted to a concentration of 106 cells/ml, and 100 μl per well was aliquoted into 96-well plates. On day 5, 1 μCi of [3H]thymidine was added per well, and the plates were incubated overnight at 37°C. Cells were harvested into GF/B (Packard Instruments Co., Meriden, Conn.) plates. After evaporation to dryness, 30 μl of scintillation fluid was added to each well. [3H]thymidine incorporation was determined in a beta counter (Top Count; Packard Instruments Co.). Lymphocyte proliferation was considered significant if the stimulation index (counts per minute of sample stimulated with antigen/counts per minute of sample without antigen) was greater than 3. These experiments were repeated three times.
Cytotoxic T-lymphocyte (CTL) assays.
CTL assays were performed according to the standard method reported previously (3). Since the major target cells of rickettsial infection in humans and in the C3H/HeN mouse model are the endothelial cells, the R. conorii-infected MHC class I-matched endothelial cell line, SVEC4-10 (H-2k), was chosen as the target cells in the CTL assay (46). The effector cells were a CD8 T-lymphocyte cell line reactive against R. conorii. The percentage of target cell lysis was calculated by the following formula: [(counts per minute of the experiment − counts per minute spontaneously released)/(counts per minute of the maximum release − counts per minute spontaneously released)] × 100.
CD8 T-cell epitope mapping of OmpB451-846 by using synthetic peptides.
The amino acid sequences of polypeptides that stimulate CD8 T-cell activation were further mapped by using overlapping synthetic peptides. Each peptide contained nine amino acid residues, and two adjacent peptides overlapped by four or five residues. The peptides were custom synthesized (Bio-Synthesis Inc., Lewisville, Tex.). We have established a murine CD8 T-cell line reactive against the antigens of R. conorii. These cells were used for T-cell epitope mapping of OmpB. The mouse CD8 T lymphocytes were adjusted to 106 cells/ml, and 0.1 ml was aliquoted into each well of 96-well plates. The synthetic peptides were diluted and added to duplicate wells at a 20 μM concentration. Each well also contained 105 antigen-presenting cells (SVECs) and interleukin-2 (one unit per well). The ability of these peptides to activate CD8 T lymphocytes in vitro was tested by lymphocyte proliferation, IFN-γ production, and CTL assay.
RESULTS
Cloning and expression of rickettsial OmpB451-846 gene fragments.
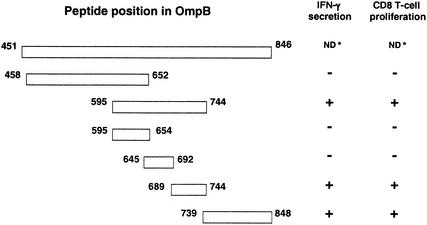
Overlapping fragments of OmpB451-846 were amplified by using site-specific PCR primers as three smaller fragments: OmpB458-652, OmpB595-744, and OmpB739-848. The OmpB595-744 fragment was further subcloned by using site-specific PCR primers into three smaller fragments: OmpB595-654, OmpB645-692, and OmpB689-744 (Fig. 1). Their sizes were as expected. These PCR products were cloned into pcDNA3.1/CT-GFP-TOPO vector. Clones containing these fragments in the right orientation were selected and used to transfect NCTC fibroblast cells.
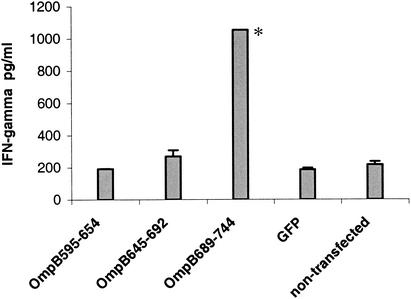
FIG. 1.
Stimulation of CD8 T-cell proliferation and IFN-γ secretion by NCTC cells expressing recombinant proteins derived from OmpB451-846 of R. conorii. ND, not done.
Transfection and selection of antigen-expressing fibroblast cells.
Plasmids containing rickettsial OmpB451-846 gene fragments were purified and successfully transfected with Lipofectamine2000 into NCTC fibroblast cells, which were derived from C3H/HeN mice with the same MHC class I haplotype (H-2k). The plasmid vector pcDNA3.1/CT-GFP-TOPO contains a neomycin resistance cassette that allows the selection of transfected mammalian cells with a closely related antibiotic, Geneticin (G418). Mammalian cells are more sensitive to Geneticin than to neomycin. The sensitivity varies among cell lines, and the killing dose for NCTC cells was determined as 800 μg/ml. These Taq polymerase-amplified PCR products were expressed as C-terminal fusion products with the green fluorescent protein (GFP). Expression of rickettsial fragments was determined by detection of fluorescent cells with a filter set with a maximum excitation of 450 nm and a maximum emission of 508 nm (Omega Optical Inc., Brattleboro, Vt.).
Establishment of a CD8 T-lymphocyte cell line reactive against R. conorii.
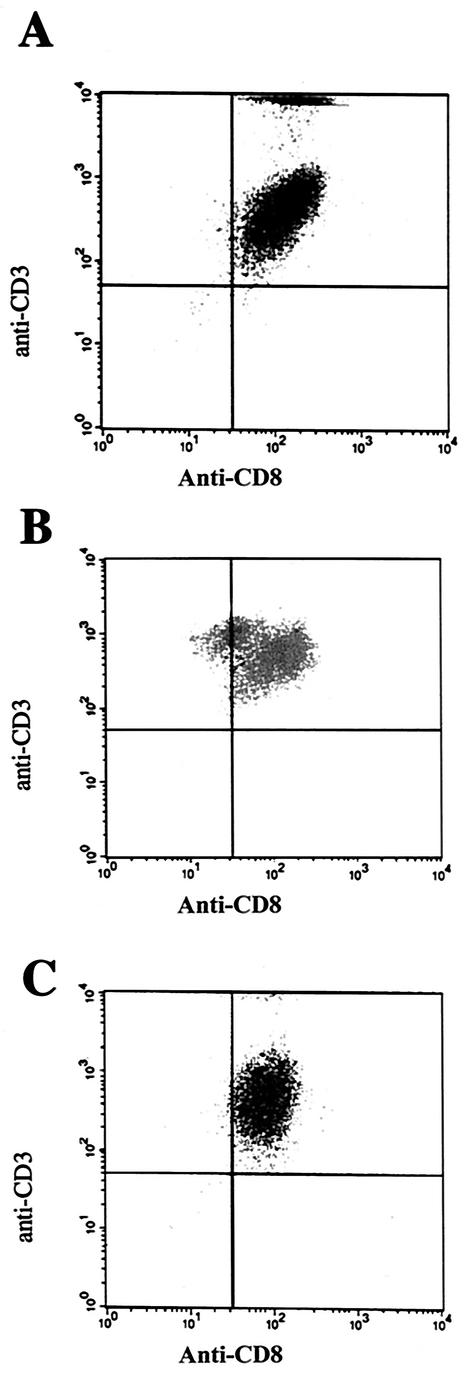
The R. conorii-specific CD8 T-lymphocyte populations derived from whole-spleen suspensions reached 95% purity (the other 5% may be CD4 lymphocytes from the irradiated feeder cells) after four passages in vitro (Fig. 2). This cell line is a very useful tool for the identification of the rickettsial CD8 T-lymphocyte epitopes.
FIG. 2.
Flow cytometric analysis of double-stained lymphocyte populations with anti-CD3 antibody conjugated to peridinin chlorophyll-α protein and anti-CD8 antibody conjugated to fluorescein isothiocyanate. (A) Population of purified CD8 T lymphocytes after two cycles of in vitro stimulation. (B and C) Splenocyte suspensions in which the population of CD8 T lymphocytes was enhanced by in vitro stimulation after two (B) and four (C) cycles. The CD8 T-lymphocyte population derived from whole-spleen suspensions reached a purity of 95% after four in vitro passages (C).
Presentation of fragments representing ompB1550-2739 by rickettsial gene-transfected fibroblast cells.
The ability of transfected NCTC cells to activate immune CD8 T lymphocytes was determined. Significant proliferation of immune CD8 T lymphocytes was observed after stimulation by NCTC cells transfected with OmpB595-744 (stimulation index, 3.848 ± 0.170) or OmpB739-848 (stimulation index, 4.784 ± 0.158). NCTC cells transfected with OmpB595-744 or OmpB739-848 stimulated the secretion of IFN-γ by immune CD8 T lymphocytes at significantly higher (P ≤ 0.05) levels than those of the controls (Fig. 1 and 3). Transfected NCTC cells expressing OmpB458-652, nontransfected NCTC cells, or GFP-transfected NCTC cells did not stimulate lymphocyte proliferation or IFN-γ secretion.
FIG. 3.
IFN-γ secretion by immune CD8 T lymphocytes after incubation with NCTC cells transfected with the vector control pcDNA3.1/CT-GFP-TOPO (GFP) or the vector expressing the polypeptide OmpB458-652, OmpB595-744, or OmpB739-848. The positive control is the IFN-γ secretion by immune CD8 T lymphocytes after incubation with R. conorii-infected NCTC cells. *, P ≤ 0.05 compared with stimulation by cells transfected with vector only.
The epitopes of OmpB595-744 were further mapped by subcloning the fragment into three smaller fragments. Proliferation of immune CD8 T lymphocytes was observed after stimulation by NCTC cells transfected with OmpB689-744 (stimulation index, 3.297 ± 0.040). NCTC cells transfected with OmpB689-744 stimulated the secretion of IFN-γ by immune CD8 T lymphocytes at significantly higher (P ≤ 0.05) levels than those of the controls (Fig. 1 and 4). Transfected NCTC cells expressing subclone OmpB595-654 or OmpB645-692, nontransfected NCTC cells, and GFP-transfected NCTC cells did not stimulate lymphocyte proliferation or IFN-γ secretion.
FIG. 4.
IFN-γ secretion by immune CD8 T lymphocytes after incubation with NCTC cells transfected with the vector control pcDNA3.1/CT-GFP-TOPO (GFP) or the vector expressing the polypeptide OmpB595-654, OmpB645-692, or OmpB689-744. *, P ≤ 0.05 compared with stimulation by cells transfected with vector only.
CD8 T-cell epitope mapping of OmpB with synthetic peptides.
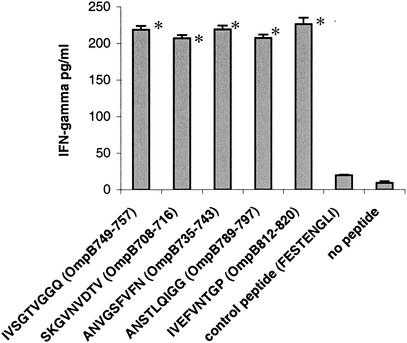
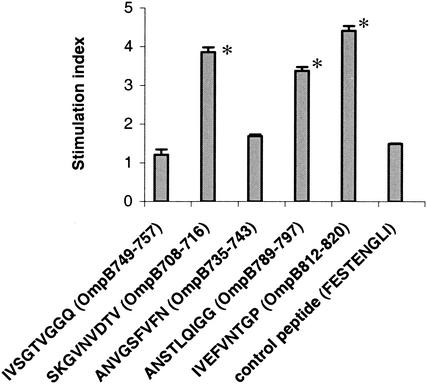
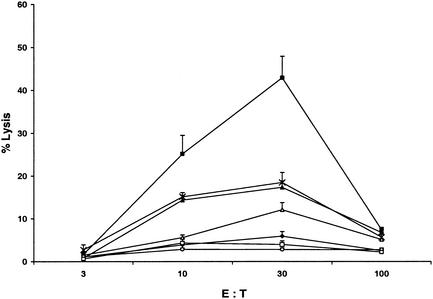
NCTC cells transfected with a mammalian expression vector expressing the fragments OmpB689-744 and OmpB739-848 stimulated immune CD8 T lymphocytes, suggesting the presence of CD8 T-lymphocyte-stimulating epitopes. CD8 T-lymphocyte epitopes were mapped on OmpB689-744 and OmpB739-848 by using overlapping synthetic peptides. The ability of each of these synthetic peptides to activate CD8 T lymphocytes was tested in vitro by IFN-γ secretion, lymphocyte proliferation, and CTL assay. Five synthetic peptides, SKGVNVDTV (OmpB708-716), ANVGSFVFN (OmpB735-743), IVSGTVGGQ (OmpB749-757), ANSTLQIGG (OmpB789-797), and IVEFVNTGP (OmpB812-820), incubated with SVECs induced secretion of IFN-γ at significantly higher levels (P ≤ 0.05) than did the controls (Fig. 5). Three of these five peptides, SKGVNVDTV (OmpB708-716), ANSTLQIGG (OmpB789-797), and IVEFVNTGP (OmpB812-820), also stimulated the proliferation of immune CD8 T lymphocytes (Fig. 6). Significantly higher levels of specific CTL killing were observed with the same three synthetic peptides, SKGVNVDTV (OmpB708-716), ANSTLQIGG (OmpB789-797), and IVEFVNTGP (OmpB812-820) (Fig. 7).
FIG. 5.
IFN-γ secretion by immune CD8 T lymphocytes after stimulation with SVECs incubated with the synthetic peptides. FESTENGLI, an H-2k-restricted epitope from the nucleoprotein of influenza virus, was used as a negative control. *, P ≤ 0.05 compared with stimulation by control peptide.
FIG. 6.
Cell proliferation of immune CD8 T lymphocytes after stimulation with SVECs incubated with the 20 μM solution of synthetic peptides. FESTENGLI, an H-2k-restricted epitope from the nucleoprotein of influenza virus, was used as a negative control. *, P ≤ 0.05 compared with stimulation by control peptide.
FIG. 7.
CTL activity directed against SVECs at effector-to-target cell (E:T) ratios of 3, 10, 30, and 100, measured as the percentage of lysis of target cells. Effector cells were an R. conorii-specific CD8 cell line. Target cells are indicated as follows: ▪, R. conorii-infected SVECs, no peptide; ♦, uninfected SVECs, with synthetic peptide IVSGTVGGQ (OmpB749-757); ▵, uninfected SVECs, with synthetic peptide ANSTLQIGG (OmpB789-797); ×, uninfected SVECs, with synthetic peptide IVEFVNTGP (OmpB812-820); ▴, uninfected SVECs, with synthetic peptide SKGVNVDTV (OmpB708-716); ○, uninfected SVECs, with synthetic peptide ANVGSFVFN (OmpB735-743); □, uninfected SVECs, no peptide.
DISCUSSION
Boutonneuse fever caused by R. conorii has a high prevalence in southern Europe, parts of western Asia, and Africa. Fatalities occur particularly in patients who are elderly or who have other host-dependent risk factors (33, 42). Infection with spotted fever group rickettsial organisms elicits protective immunity that is solid and long-lasting (34). The immune mechanisms depend largely on T lymphocytes (9, 15, 21, 23, 26, 28, 29, 43).
This report represents for the first time the identification of three CD8 T-lymphocyte epitopes in OmpB of R. conorii. These epitopes illustrate an approach to selecting candidate antigens for rickettsial subunit vaccine development. Currently, there is no rickettsial vaccine available that is close to being effective and acceptable. Three vaccines have been developed for prevention of Rocky Mountain spotted fever: a killed rickettsial preparation of infected ticks developed in 1924, a killed rickettsial vaccine from infected yolk sacs of embryonated hen's eggs developed in 1938, and a killed rickettsial vaccine developed from cell culture in the 1970s (7, 8, 22, 37). However, all these vaccines failed to confer effective immune protection (6, 13). The use of molecular approaches to development of rickettsial vaccines is quite clearly making significant progress. Rickettsial vaccine development has been thwarted by the difficulty of manipulating these organisms outside viable host cells. Knowledge of the protective immune effector mechanisms and how to stimulate them is a rational prerequisite to an ultimately successful vaccine regimen (41). Development of rickettsial vaccines will have a significant effect on the prevention of these life-threatening, diagnostically difficult diseases.
The main goal of this study was to identify CD8 T-lymphocyte epitopes of R. conorii. Because CD8 T cells play important roles in resistance to infection with rickettsiae, effective vaccines against rickettsiosis will likely require identification of antigens that contain CD8 T-cell epitopes. CD8 T-cell epitopes consist of 8- or 9-aa peptides that are selected for presentation based on MHC class I allele-specific binding motifs and their ability to survive proteolytic digestion in the host cell. The subunit strategy for development of an effective rickettsial vaccine relies on identification of antigens and the precise epitope recognized by the protective CD8 T cells. In this study, the plasmid vector pcDNA3.1/CT-GFP-TOPO (Invitrogen) was used for the expression of the OmpB fragments in NCTC fibroblast cells. This vector system is associated with fewer toxic effects of intact bacterial proteins in the host cells, since the approach is designed for expression of gene fragments.
So far, identification of CD8 T-lymphocyte epitopes has been focused mainly on facultatively intracellular bacteria. Four different Listeria monocytogenes epitopes are known to be presented to CTLs by MHC class I H-2Kd molecules (4, 31, 32). These epitopes are derived from bacterial virulence factors, listeriolysin (LLO 91-99), murein hydrolase p60 (p60 217-225 and p60 449-457), and metalloprotease (mpl 84-92). A novel HLA-B*35-restricted CD8 T-cell epitope in Mycobacterium tuberculosis Rv2903c (aa 201 to 209) was identified based on a reverse immunogenetics approach (24). Klein et al. identified three HLA-B*35-restricted CD8 T-cell epitopes, Ag85A (aa 267 to 275), Ag85B (aa 264 to 272), and Ag85C (aa 204 to 212), in the M. tuberculosis antigen 85 complex (25). Smith et al. identified two human HLA-A*0201-restricted CD8 T-cell epitopes, P48-56 and P242-250, by spanning the mycobacterial major secreted protein Ag85A (36). Six HLA-A*0201-restricted CTL epitopes were identified by Charo et al. in heat shock protein 65 (hsp65) of mycobacteria (5): Mhsp65(9416), Mhsp65(9362), Mhsp65(9369), Mhsp65(1097), Mhsp65(11500), and Mhsp65(921). Lalvani et al. identified two CD8 T-cell-specific epitopes, ES13 (aa 82 to 90) and ES12 (aa 69 to 76), in the early secretory antigenic target 6 during active tuberculosis (27). Very few studies of CD8 T-lymphocyte epitopes have been reported for obligately intracellular bacteria. Saren et al. (35) identified three Chlamydia pneumoniae CD8 epitopes that were processed and presented on the infected cells. Wizel et al. (47) generated 18 H-2b binding peptides representing sequences from 12 C. pneumoniae antigen-sensitized target cells for MHC class I-restricted lysis by CD8 CTLs from the spleen and lungs of infected mice. During this study, we combined genetic and biochemical means in an attempt to identify CD8 T-lymphocyte epitopes from OmpB of R. conorii. Our results showed that three synthetic peptides of OmpB451-846, SKGVNVDTV (OmpB708-716), ANSTLQIGG (OmpB789-797), and IVEFVNTGP (OmpB812-820), induced secretion of IFN-γ and stimulated the proliferation of immune CD8 T lymphocytes and specific CTL killing at significantly higher levels than the controls did. In our study, at effector/target ratios of 3, 10, and 30, increasing levels of epitope-specific CTL lysis were observed (Fig. 7). Protective immunity against rickettsiae is achieved by a complex interaction of responses mediated by CD4 and CD8 T lymphocytes, macrophages, NK cells, antibodies, cytokines, and chemokines (1, 39). CTL activity, which is expressed by immune CD8 T lymphocytes, appears to be crucial to recovery from rickettsial infection (44). Depletion of CD8 T lymphocytes results in the establishment of persistent R. conorii infection in mice associated with increased mortality after an ordinarily sublethal dose of rickettsiae (15). IFN-γ secretion by and CTL activity of immune CD8 T lymphocytes are involved, but the specifics are yet to be determined (14).
This research extends the knowledge of the role of OmpB as an immunogen and the relevance of the response of CD8 T lymphocytes to such antigens. Epitopes with strong CD8 stimulatory capability will represent good candidates for rickettsial vaccine development because they stimulate a relevant arm of the immune response against these intracellular bacteria. The identification of potential CD8 T-lymphocyte epitopes at the oligopeptide level was addressed for the H-2k haplotype. Future progress in subunit vaccine development will require identification of CD8 T-lymphocyte epitopes that stimulate human CD8 T lymphocytes via MHC class I presentation.
Acknowledgments
This work was supported by a grant from the National Institute of Allergy and Infectious Diseases (AI 21242).
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Billings, A. N., H. M. Feng, J. P. Olano, and D. H. Walker. 2001. Rickettsial infection in murine models activates an early anti-rickettsial effect mediated by NK cells and associated with production of gamma interferon. Am. J. Trop. Med. Hyg. 65:52-56. [DOI] [PubMed] [Google Scholar]
- 2.Bourgeois, A. L., and G. A. Dasch. 1981. The species-specific surface protein antigen of Rickettsia typhi: immunogenicity and protective efficacy in guinea pigs, p. 71-80. In W. Burgdorfer and R. L. Anacker (ed.), Rickettsiae and rickettsial diseases. Academic Press, New York, N.Y.
- 3.Brunner, K. T., H. D. Engers, and J. C. Grottini. 1976. The 51Cr-release assay as used for the quantitative measurement of cell-mediated cytolysis in vitro, p. 423. In B. R. Bloom and J. R. David (ed.), In vitro methods in cell-mediated and tumor immunity. Academic Press, New York, N.Y.
- 4.Busch, D. H., and E. G. Pamer. 1998. MHC class 1/peptide stability: implications for immunodominance, in vitro proliferation, and diversity of responding CTL. J. Immunol. 160:4441-4448. [PubMed] [Google Scholar]
- 5.Charo, J., A. Geluk, M. Sundback, B. Mirzai, A. D. Diehl, K.-J. Malmberg, A. Achour, S. Huriguchi, K. E. van Meijgaarden, J.-W. Drijfhout, N. Beekman, P. van Veelen, F. Ossendorp, T. H. M. Ottenhoff, and R. Kiessling. 2001. The identification of a common pathogen-specific HLA class I A*0201-restricted cytotoxic T cell epitope encoded within the heat shock protein 65. Eur. J. Immunol. 31:3602-3611. [DOI] [PubMed] [Google Scholar]
- 6.Clements, M. L., C. L. Wisseman, Jr., T. E. Woodward, P. Fiset, J. S. Dumler, W. McNamee, R. E. Black, J. Rooney, T. P. Hughes, and M. M. Levine. 1983. Reactogenicity, immunogenicity, and efficacy of a chick embryo cell-derived vaccine for Rocky Mountain spotted fever. J. Infect. Dis. 148:922-930. [DOI] [PubMed] [Google Scholar]
- 7.Cox, H. R. 1938. Use of yolk sac of developing chick embryo as medium for growing rickettsiae of Rocky Mountain spotted fever and typhus groups. Public Health Rep. 53:2241-2247. [Google Scholar]
- 8.Cox, H. R. 1948. The preparation and standardization of rickettsial vaccines, p. 203-214. In F. R. Moulton (ed.), Rickettsial diseases of man. American Association for the Advancement of Science, Washington, D.C.
- 9.Crist, A. E., Jr., C. L. Wisseman, Jr., and J. R. Murphy. 1984. Characteristics of lymphoid cells that adoptively transfer immunity to Rickettsia mooseri infection in mice. Infect. Immun. 44:55-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crocquet-Valdes, P. A., C. M. Diaz-Montero, H. M. Feng, H. Li, A. D. T. Barrett, and D. H. Walker. 2002. Immunization with a portion of rickettsial outer membrane protein A stimulates protective immunity against spotted fever rickettsiosis. Vaccine 20:979-988. [DOI] [PubMed] [Google Scholar]
- 11.Dasch, G. A., and A. L. Bourgeois. 1981. Antigens of the typhus group of rickettsiae: importance of the species-specific surface protein antigens in eliciting immunity, p. 61-69. In W. Burgdorfer and R. L. Anacker (ed.), Rickettsiae and rickettsial diseases. Academic Press, Inc., New York, N.Y.
- 12.Diaz-Montero, C. M., H.-M. Feng, P. A. Crocquet-Valdes, and D. H. Walker. 2001. Identification of protective components of two major outer membrane proteins of spotted fever group rickettsiae. Am. J. Trop. Med. Hyg. 65:371-378. [DOI] [PubMed] [Google Scholar]
- 13.Dupont, H. L., R. B. Hornick, A. T. Dawkins, G. G. Heiner, I. B. Fabrikant, C. L. Wisseman, Jr., and T. E. Woodward. 1973. Rocky Mountain spotted fever: a comparative study of the active immunity induced by inactivated and viable pathogenic Rickettsia rickettsii. J. Infect. Dis. 128:340-344. [DOI] [PubMed] [Google Scholar]
- 14.Feng, H.-M., V. L. Popov, and D. H. Walker. 1994. Depletion of gamma interferon and tumor necrosis factor alpha in mice with Rickettsia conorii-infected endothelium: impairment of rickettsicidal nitric oxide production resulting in fatal, overwhelming rickettsial disease. Infect. Immun. 62:1952-1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feng, H.-M., V. L. Popov, G. Yuoh, and D. H. Walker. 1997. Role of T-lymphocyte subsets in immunity to spotted fever group rickettsiae. J. Immunol. 158:5314-5320. [PubMed] [Google Scholar]
- 16.Feng, H.-M., J. Wen, and D. H. Walker. 1993. Rickettsia australis infection: a murine model of a highly invasive vasculopathic rickettsiosis. Am. J. Pathol. 142:1471-1482. [PMC free article] [PubMed] [Google Scholar]
- 17.Gilmore, R. D., Jr., W. Cieplak, Jr., P. F. Policastro, and T. Hackstadt. 1991. The 120 kilodalton outer membrane protein (rOmp B) of Rickettsia rickettsii is encoded by an unusually long open reading frame: evidence for protein processing from a large precursor. Mol. Microbiol. 5:2361-2370. [DOI] [PubMed] [Google Scholar]
- 18.Hackstadt, T., R. Messer, W. Cieplak, and M. G. Peacock. 1992. Evidence for proteolytic cleavage of the 120-kilodalton outer membrane protein of rickettsiae: identification of an avirulent mutant deficient in processing. Infect. Immun. 60:159-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hanson, B. A., C. L. Wisseman, Jr., A. Waddell, and D. J. Silverman. 1981. Some characteristics of heavy and light bands of Rickettsia prowazekii on Renografin gradients. Infect. Immun. 34:596-604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Herrero-Herrero, J. I., D. H. Walker, and R. Ruiz-Beltran. 1987. Immunohistochemical evaluation of the cellular immune response to Rickettsia conorii in taches noires. J. Infect. Dis. 155:802-805. [DOI] [PubMed] [Google Scholar]
- 21.Jerrells, T. R. 1988. Mechanisms of immunity to Rickettsia species and Coxiella burnetii, p. 79-100. In D. H. Walker (ed.), Biology of rickettsial diseases. CRC Press, Inc., Boca Raton, Fla.
- 22.Kenyon, R. H., and C. E. Pedersen, Jr. 1975. Preparation of Rocky Mountain spotted fever vaccine suitable for human immunization. J. Clin. Microbiol. 1:500-503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kenyon, R. H., and C. E. Pedersen, Jr. 1980. Immune responses to Rickettsia akari infection in congenitally athymic nude mice. Infect. Immun. 28:310-313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klein, M. R., A. S. Hammond, S. M. Smith, A. Jaye, P. T. Lukey, and K. P. W. J. McAdam. 2002. HLA-B*35*-restricted CD8+-T-cell epitope in Mycobacterium tuberculosis Rv 2903c. Infect. Immun. 70:981-984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klein, M. R., S. M. Smith, A. S. Hammond, G. S. Ogg, A. S. King, J. Vekemans, A. Jaye, P. T. Lukey, and K. P. McAdam. 2001. HLA-B*35-restricted CD8 T cell epitopes in the antigen 85 complex of Mycobacterium tuberculosis. J. Infect. Dis. 183:928-934. [DOI] [PubMed] [Google Scholar]
- 26.Kokorin, I. N., E. A. Kabanova, E. M. Shirokova, E. G. Abrosimova, N. N. Rybkina, and V. I. Pushkareva. 1982. Role of T lymphocytes in Rickettsia conorii infection. Acta Virol. 26:91-97. [PubMed] [Google Scholar]
- 27.Lalvani, A., R. Brookes, R. J. Wilkinson, A. S. Malin, A. A. Pathan, P. Andersen, H. Dockrell, G. Pasvol, and A. V. S. Hill. 1998. Human cytolytic and interferon γ-secreting CD8+ T lymphocytes specific for Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. USA 95:270-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Montenegro, N. R., D. H. Walker, and B. C. Hegarty. 1984. Infection of genetically immunodeficient mice with Rickettsia conorii. Acta Virol. 28:508-514. [PubMed] [Google Scholar]
- 29.Murphy, J. R., C. L. Wisseman, Jr., and P. Fiset. 1978. Mechanisms of immunity in typhus infection: some characteristics of intradermal Rickettsia mooseri infection in normal and immune guinea pigs. Infect. Immun. 22:810-820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.O'Connell, K. A., and M. Edidin. 1990. A mouse lymphoid endothelial cell line immortalized by simian virus 40 binds lymphocytes and retains functional characteristics of normal endothelial cells. J. Immunol. 144:521-525. [PubMed] [Google Scholar]
- 31.Pamer, E. G. 1994. Direct sequence identification and kinetic analysis of an MHC class I-restricted Listeria monocytogenes CTL epitope. J. Immunol. 152:686-694. [PubMed] [Google Scholar]
- 32.Pamer, E. G., J. T. Harty, and M. J. Bevan. 1991. Precise prediction of a dominant class I MHC-restricted epitope of Listeria monocytogenes. Nature 353:852-855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Raoult, D., P. Zuchelli, P. J. Weiller, C. Charrel, J. L. San Marco, H. Gallais, and P. Casanova. 1986. Incidence, clinical observations and risk factors in the severe form of Mediterranean spotted fever among patients admitted to hospital in Marseilles 1983-1984. J. Infect. 12:111-116. [DOI] [PubMed] [Google Scholar]
- 34.Ricketts, H. T., and L. Gomez. 1908. Studies on immunity in Rocky Mountain spotted fever. First communication. J. Infect. Dis. 5:221-244. [Google Scholar]
- 35.Saren, A., S. Pascolo, S. Stevanovic, T. Dumrese, M. Puolakkainen, M. Sarvas, H.-G. Rammensee, and J. M. Vuola. 2002. Identification of Chlamydia pneumoniae-derived mouse CD8 epitopes. Infect. Immun. 70:3336-3343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smith, S. M., R. Brookes, M. R. Klein, A. S. Malin, P. T. Lukey, A. S. King, G. S. Ogg, A. V. S. Hill, and H. M. Dockrell. 2000. Human CD8+ CTL specific for the mycobacterial major secreted antigen 85A. J. Immunol. 165:7088-7095. [DOI] [PubMed] [Google Scholar]
- 37.Spencer, R. R., and R. R. Parker. 1925. Rocky Mountain spotted fever: vaccination of monkeys and man. Public Health Rep. 40:2159-2167. [Google Scholar]
- 38.Sumner, J. W., K. G. Sims, D. C. Jones, and B. E. Anderson. 1995. Protection of guinea-pigs from experimental Rocky Mountain spotted fever by immunization with baculovirus-expressed Rickettsia rickettsii rOmpA protein. Vaccine 13:29-35. [DOI] [PubMed] [Google Scholar]
- 39.Valbuena, G., H.-M. Feng, and D. H. Walker. 2002. Mechanisms of immunity against rickettsiae. New perspectives and opportunities offered by unusual intracellular parasites. Microbes Infect. 4:625-633. [DOI] [PubMed] [Google Scholar]
- 40.Vishwanath, S., G. A. McDonald, and N. G. Watkins. 1990. A recombinant Rickettsia conorii vaccine protects guinea pigs from experimental boutonneuse fever and Rocky Mountain spotted fever. Infect. Immun. 58:646-653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Walker, D. H. 1988. Role of the composition of rickettsiae in rickettsial immunity: typhus and spotted fever groups, p. 101-109. In D. H. Walker (ed.), Biology of rickettsial diseases. CRC Press, Inc., Boca Raton, Fla.
- 42.Walker, D. H. 1990. The role of host factors in the severity of spotted fever and typhus rickettsioses. Ann. N. Y. Acad. Sci. 590:10-19. [DOI] [PubMed] [Google Scholar]
- 43.Walker, D. H., and F. W. Henderson. 1978. Effect of immunosuppression on Rickettsia rickettsii infection in guinea pigs. Infect. Immun. 20:221-227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Walker, D. H., J. P. Olano, and H.-M. Feng. 2001. Critical role of cytotoxic T lymphocytes in immune clearance of rickettsial infection. Infect. Immun. 69:1841-1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Walker, D. H., V. L. Popov, and H.-M. Feng. 2000. Establishment of a novel endothelial target mouse model of a typhus group rickettsiosis: evidence for critical roles for gamma interferon and CD8 T lymphocytes. Lab. Investig. 80:1361-1372. [DOI] [PubMed] [Google Scholar]
- 46.Walker, D. H., V. L. Popov, J. Wen, and H.-M. Feng. 1994. Rickettsia conorii infection of C3H/HeN mice: a model of endothelial-target rickettsiosis. Lab. Investig. 70:358-368. [PubMed] [Google Scholar]
- 47.Wizel, B., B. C. Starcher, B. Samten, Z. Chroneos, P. F. Barnes, J. Dzuris, Y. Higashimoto, E. Appella, and A. Sette. 2002. Multiple Chlamydia pneumoniae antigens prime CD8+ Tc1 responses that inhibit intracellular growth of this vacuolar pathogen. J. Immunol. 169:2524-2535. [DOI] [PubMed] [Google Scholar]