Abstract
Dendritic cells are potent antigen-presenting cells that are present in the gastrointestinal tract and are required for the induction of a Th1 T-cell acquired immune response. Since infection with the gastric pathogen Helicobacter pylori elicits a Th1 cell response, the interaction of these organisms with dendritic cells should reflect the Th1 bias. We incubated H. pylori with cultured human dendritic cells and measured the cytokine induction profile, comparing the response to that induced by Salmonella enterica serovar Typhimurium. We found that H. pylori induced little interleukin 6 (IL-6) and essentially no IL-10 in contrast to S. enterica. However, H. pylori induced levels of IL-12 that were 30% of those induced by S. enterica, indicating a Th1 response. An isogenic cagE mutant of H. pylori lost about 50% of its IL-12-inducing ability, suggesting a role for the cag type IV secretion system in the stimulation of dendritic cells.
Helicobacter pylori produces a chronic gastric infection that is widespread in the human population and is associated with severe disease sequelae including gastritis, peptic ulcers, mucoa-associated lymphoid tissue lymphoma, and gastric adenocarcinoma (reviewed in reference 10). Even in people without symptoms, H. pylori produces histologic changes of gastritis, indicating a continuous inflammatory reaction of the gastric mucosa. Although H. pylori elicits both innate and acquired immune responses, the host is unable to eliminate the organism from the gastric mucosal surface, and indefinite infection is the usual outcome. In fact, this ineffective immune response is postulated to contribute substantially to the development of long-term disease syndromes (reviewed in reference 18). The innate immune response is driven, at least in part, by bacterial virulence factors such as the cag pathogenicity island (PAI) that stimulate production of proinflammatory mediators from the gastric epithelium (reviewed in reference 16). The acquired immune response includes both antibody- and cell-mediated components. A strong Th1 T-cell component has been described and has been implicated in perpetuating the inflammatory changes that lead to disease (18). Recent evidence indicates that antigen presentation by dendritic cells is required to initiate a primary Th1 T-cell immune response (5, 12). Furthermore, the Th1-biased response is facilitated by IL-12 elaboration in the context of the antigen presentation process. IL-12 is upregulated in the gastric mucosa of dyspeptic patients and is associated with presence of the cag PAI (9). These immunologic principles strongly suggest that H. pylori must interact with dendritic cells and elicit IL-12 secretion during infection of the gastric mucosa in order to induce the Th1 acquired immune response.
Dendritic cells are a group of antigen-presenting cells that are widely distributed in tissues, including the gastrointestinal mucosa (5, 12, 20). Immature dendritic cells in the mucosa-associated lymphoid tissue lymphoma, are phagocytic and specialize in antigen capture. Dendritic cells are capable of migrating through epithelial tight junctions to gain access to the gastrointestinal lumen (14, 15). Upon exposure to whole bacteria or bacterial components, immature dendritic cells differentiate into mature, antigen-presenting cells with upregulated expression of major histocompatibility complex class I and II, costimulatory, and adhesion molecules (5, 12).
Dendritic cells are derived from myeloid precursors and can be cultured in vitro by differentiation of human peripheral blood monocytes treated with IL-4 and granulocyte-macrophage colony-stimulating factor (GM-CSF) (5, 17). The resulting immature dendritic cells are phagocytic and express Toll-like receptors capable of interaction with various bacterial components. The resulting signal transduction events stimulate cytokine release and differentiation into mature dendritic cells. For example, activation of Toll-like receptor 2 results in preferential secretion of IL-12 rather than IL-10 by human dendritic cells exposed to mycobacterial lipoprotein (17). We postulated that H. pylori would interact with immature dendritic cells to produce a characteristic cytokine pattern that also includes selective production of IL-12.
Human dendritic cells were derived and cultured in vitro from peripheral blood monocytes according to standard procedures (7, 17). Briefly, monocytes were isolated from the peripheral blood mononuclear cell fraction of healthy donors by adherence to plasma-coated plastic flasks. Monocytes were seeded into six-well plates and cultured in RPMI medium containing 10% fetal calf serum with IL-4 (1,000 U/ml) and GM-CSF (1,000 U/ml). After 5 days, the nonadherent cells were harvested by washing, resuspended in fresh RPMI medium containing 10% fetal calf serum with GM-CSF, and seeded into a 24-well plate at a concentration of 2 × 105 cells per well.
The bacterial strains used for infection were H. pylori SD4 (a clinical isolate described previously and shown to induce IL-8 secretion in epithelial cells [4]) and Salmonella enterica serovar Typhimurium 14028s (7). Cultures were maintained on Columbia agar plates containing 5% laked sheep or horse blood and 1% fungizone (amphotericin B; Omega Scientific), as described previously (4). Bacteria were maintained in an atmosphere of 10% CO2, 5% O2, and 85% N2. For dendritic cell experiments, bacteria from 1- or 2-day-old plates were resuspended in RPMI medium and counted with a Petroff-Hausser chamber.
The cagE mutation in H. pylori SD4 was constructed by insertion of a chloramphenicol resistance gene (cat, originally isolated from Campylobacter coli, obtained from D. Taylor [19]) in the EcoRI site, 756 bp from the 5′ start site of the published cagE gene sequence from strain NCTC 11638 (3). The 1.2-kb cagE gene was obtained by PCR from H. pylori strain SD14 (4). The resulting construct, on plasmid PCR-Script (Stratagene, La Jolla, Calif.), was transformed into H. pylori SD4 by standard procedures (8). Briefly, bacteria were inoculated as a patch on Columbia blood agar plates, and 5 μg of plasmid DNA prepared from E. coli DH5α was spread over the patch. After 24 h of incubation at 37°C in an atmosphere of 5% O2, 10% CO2, and 85% N2, bacteria were streaked for single-colony isolation to plates containing 20 μg of chloramphenicol per ml and incubated further. Single colonies were isolated, expanded, and checked for proper insertion of the cat gene by PCR, performed by using one primer located in the cagE gene and one the cat gene. Mutants were confirmed by identification of an appropriate-sized band by Southern hybridization.
All bacterial strains were inoculated into tissue culture wells at a ratio of 10 organisms per dendritic cell. This inoculum was chosen on the basis of preliminary dose-response experiments indicating that 10:1 was the optimal ratio for cytokine secretion. For the dual infections, H. pylori SD4 was added 1 h before the S. enterica. Infected cultures were centrifuged at 200 × g for 5 min and incubated for 1 h at 37°C in 5% CO2. Subsequently, gentamicin was added at 20 μg/ml and the incubation was continued for 48 h. At this time, the supernatant was aspirated, centrifuged, and frozen in aliquots at −70°C until used for the cytokine assays. H. pylori infection of AGS gastric epithelial cells was done as described previously (4). Cytokine assays were performed by using commercial enzyme-linked immunosorbent assay (ELISA) kits for IL-6, IL-8, IL-10, and IL-12 p70.
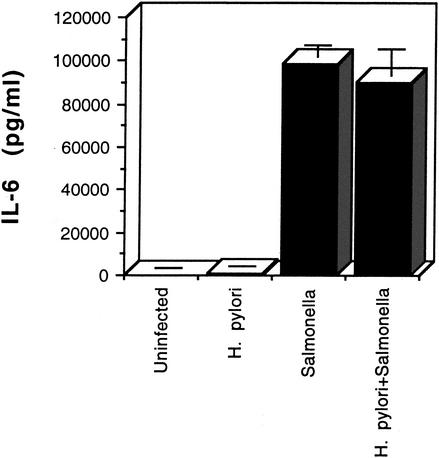
Initially, we examined the production of IL-6 by dendritic cells infected with equal numbers of H. pylori or S. enterica serovar Typhimurium cells. Figure 1 shows that serovar Typhimurium was a potent stimulator of IL-6 secretion, while by comparison, H. pylori induced little IL-6 release. To determine whether H. pylori was actively inhibiting IL-6 secretion by dendritic cells, we infected the cells first with H. pylori and then with Salmonella. As seen in Fig. 1, preinfection with H. pylori had no effect on the IL-6 levels induced by serovar Typhimurium, indicating the lack of an inhibitory activity in H. pylori.
FIG. 1.
IL-6 secretion by human dendritic cells infected with H. pylori or Salmonella enterica. IL-6 levels in the supernatants collected after 48 h from triplicate culture wells were measured by ELISA for each of the infection conditions. Error bars represent standard deviations.
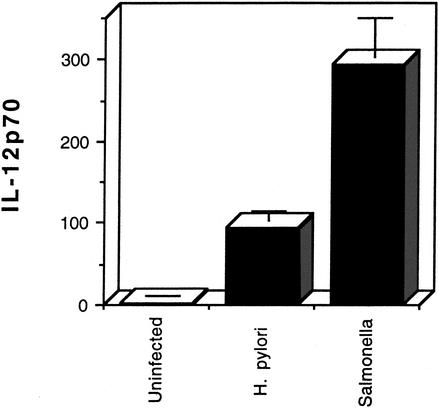
Next, we tested the ability of H. pylori and S. enterica to stimulate IL-12 p70 secretion by dendritic cells, as shown in Fig. 2. In contrast to IL-6, H. pylori infection produced levels of IL-12 that were approximately 30% of those induced by S. enterica. These results suggest that H. pylori stimulates differential cytokine production by dendritic cells.
FIG. 2.
IL-12 p70 (picograms per milliliter) produced by human dendritic cells after infection with H. pylori or Salmonella enterica by using the same samples as shown in Fig. 1. Error bars represent standard deviations.
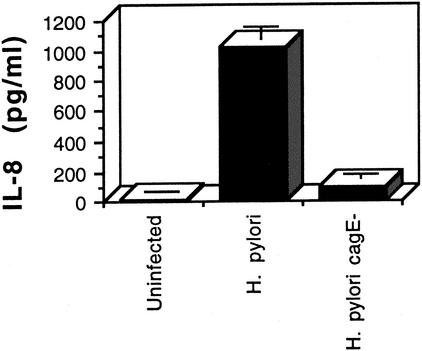
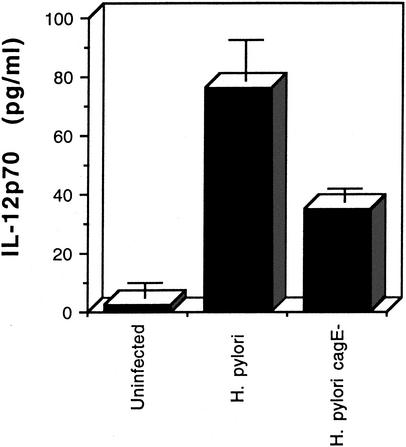
To determine the role of the cag PAI type IV protein secretion system in the interaction of H. pylori with dendritic cells, we constructed a cagE knockout mutation in strain SD4. We verified that strain SD4 stimulated IL-8 secretion in gastric epithelial cells by a cag type IV secretion-system-dependent mechanism. As shown in Fig. 3, AGS cells were infected with either wild-type H. pylori SD4 or its isogenic cagE-negative mutant, and IL-8 secretion was determined 24 h after infection. The results indicate that SD4 stimulation of IL-8 secretion from gastric epithelial cells is largely dependent on cagE. We then infected dendritic cells with wild-type H. pylori or the cagE mutant. As shown in Fig. 4, the cagE mutant was about 50% less active at inducing IL-12 than the wild type. Similar results were obtained with another cagE mutant isolate of H. pylori SD4. This study indicates that H. pylori stimulates IL-12 secretion by both cagE-dependent and cagE-independent mechanisms.
FIG. 3.
IL-8 secretion by human gastric epithelial cells infected with H. pylori SD4 or an isogenic cagE mutant defective in type IV protein secretion function. IL-8 levels in the culture supernatants from triplicate wells collected 24 h after infection were measured by ELISA. Error bars represent standard deviations.
FIG. 4.
Effect of a cagE mutation on IL-12p70 secretion by human dendritic cells infected with H. pylori. Supernatants were collected from triplicate wells 48 h after infection and assayed by ELISA. Error bars represent standard deviations. The difference in IL-12 production by cells infected with the wild-type strain compared to the cagE mutant is significant at P < 0.01 (t test)
Results reported in studies of the inhibition of growth and intracellular survival of H. pylori in human monocyte-derived macrophages have varied, depending on the presence or absence of the cag PAI (1, 11, 13). We examined whether there was a difference in survival of the wildtype and cagE mutant in our assay system. Therefore, we incubated SD4 and SD4cagE with dendritic cells as above (multiplicity of infection, 10), treated the cultures with gentamicin (20 μg/ml) for 1 h, added saponin (0.1%), sonicated the cells for 30 s in a water bath, and plated the resulting lysed cells for intracellular bacteria. We were unable to recover viable bacteria from the gentamicin-treated cultures, while in the absence of gentamicin we recovered approximately equal numbers of H. pylori SD4 and SD4cagE, representing 1 to 2% of the original inocula. The results indicate that H. pylori survives poorly under these assay conditions, but no significant difference was found between wild-type and cagE mutants.
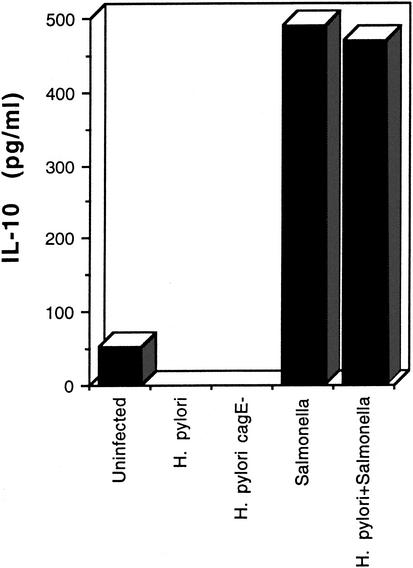
Differential secretion of IL-12 compared to IL-10 has been reported for dendritic cells treated with bacterial lipoprotein (13). Accordingly, we examined the ability of H. pylori and S. enterica to induce IL-10 secretion in dendritic cells. The results are shown in Fig. 5. Neither strain SD4 nor its cagE mutant induced detectable levels of IL-10 in the dendritic cell cultures. In contrast, IL-10 was readily detected in cultures infected with S. enterica. Similar to the IL-6 result, preinfection with H. pylori did not inhibit the activity of S. enterica in this system, indicating that the lack of IL-10 induction by H. pylori is not due to an inhibitory effect.
FIG. 5.
IL-10 secretion by human dendritic cells infected with H. pylori or Salmonella enterica. Values represent the means of culture supernatants collected from duplicate wells and assayed by ELISA. The experiment was repeated independently with similar results.
These results demonstrate that H. pylori induces IL-12 secretion from monocyte-derived human dendritic cells but does not elicit IL-10 production. Secretion of IL-6 in response to H. pylori was detectable but occurred only at low levels. In contrast, an equal number of S. enterica bacteria induced secretion of all three cytokines from dendritic cells. Compared to S. enterica, H. pylori was much more active in eliciting an IL-12 response than in eliciting an IL-6 response (see Fig. 2 and 3). We chose S. enterica serovar Typhimurium as a positive control in these studies since it has been used extensively in cytokine induction experiments, including those performed with dendritic cells (7, 20). For a comparison of this organism to H. pylori, we infected cells with equal numbers of bacteria. Although this clearly means that the amount of any bacterial component, such as protein or lipopolysaccharide (LPS), will be different in the inocula, we reasoned that the organism load is a relevant biological parameter in an infection. We recognize that for any given cytokine, comparisons between different organisms are difficult to interpret. However, the data clearly indicate that H. pylori preferentially stimulates IL-12 secretion over that of IL-6 and IL-10 from human dendritic cells.
The results suggest that H. pylori components interact with dendritic cells to selectively induce the IL-12 secretion pathway, while S. enterica lacks this selectivity. The difference between H. pylori and Salmonella may be partly explained by the structures and relative biological effects of their LPS. H. pylori has an atypical lipid A that is markedly less immunostimulatory than Salmonella LPS (2). It is likely that much of the cytokine-inducing activity of S. enterica in these studies is due to its LPS, since purified Salmonella LPS has been shown to induce these responses in human dendritic cells. The relatively poor ability of H. pylori to induce IL-6 and IL-10 may be due to the low endotoxin activity of its LPS. This explanation implies that IL-12 secretion is due to a different bacterial stimulus than that causing secretion of IL-6 or IL-10 in the case of H. pylori. Our data suggest that a component of this stimulus is the cag PAI-encoded type IV secretion system. We found that a cagE knockout mutant has lost about 50% of its IL-12-inducing ability. The CagE protein is absolutely required for the activity of the type IV system, as evidenced by the marked loss of IL-8-inducing activity in epithelial cell infections (Fig. 4). The cag PAI is known to activate both the mitogen-activated protein kinase and NF-κB signal transduction pathways in host cells (16). However, the cag PAI is only partially responsible for IL-12 induction, since residual activity remains with the cagE mutant. The evidence suggests that IL-12 induction by H. pylori is multifactorial.
The selective IL-12 secretion from dendritic cells during H. pylori infection is consistent with the ability of H. pylori to elicit a Th1-type acquired cellular immune reaction. IL-12 and IL-18 appear to be key cytokines produced by antigen-presenting cells to preferentially stimulate a Th1 T-cell response (6, 17). In contrast, IL-10 has immunosuppressive effects and can downregulate the Th1 response (17). Therefore, the lack of IL-10 induction by H. pylori would also favor a Th1 response. The presence of bacterial factors that stimulate IL-12 production in an organism with low endotoxin activity may account in part for the propensity of H. pylori to modulate the acquired immune response toward a Th1 bias.
Acknowledgments
This work was supported by PHS grant R01 DK53649.
Editor: F. C. Fang
REFERENCES
- 1.Allen, L.-A., L. S. Schlesinger, and B. Kang. 2000. Virulent strains of Helicobacter pylori demonstrate delayed phagocytosis and stimulate homotypic phagosome fusion in macrophages. J. Exp. Med. 191:115-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bliss, Jr., C. M., D. T. Golenbock, S. Keates, J. K. Linevsky, and C. P. Kelly. 1998. Helicobacter pylori lipopolysaccharide binds to CD14 and stimulates release of interleukin-8, epithelial neutrophil-activating peptide 78, and monocyte chemotactic protein 1 by human monocytes. Infect. Immun. 66:5357-5363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Censini, S., C. Lange, Z. Xiang, J. E. Crabtree, P. Ghiara, M. Borodovsky, R. Rappuoli, and A. Covacci. 1996. cag, a pathogenicity island of Helicobacter pylori, encodes type I-specific and disease-associated virulence factors. Proc. Natl. Acad. Sci. USA 93:14648-14653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cole, S. P., D. Cirillo, M. F. Kagnoff, D. G. Guiney, and L. Eckmann. 1997. Coccoid and spiral Helicobacter pylori differ in their abilities to adhere to gastric epithelial cells and induce interleukin-8 secretion. Infect. Immun. 65:843-846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cutler, C. W., R. Jotwani, and B. Pulendran. 2001. Dendritic cells: immune saviors or Achilles' heel? Infect. Immun. 69:4703-4708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Saint-Vis, B., I. Fugier-Vivier, C. Massacrier, C. Gaillard, B. Vanbervliet, S. Ait-Yahia, J. Banchereau, Y.-J. Liu, S. Lebecque, and C. Caux. 1998. The cytokine profile expressed by human dendritic cells is dependent on cell subtype and mode of activation. J. Immunol. 160:1666-1676. [PubMed] [Google Scholar]
- 7.Dreher, D., M. Kok, L. Cochand, S. G. Kiama, P. Gehr, J.-C. Pechere, and L. P. Nicod. 2001. Genetic background of attenuated Salmonella typhimurium has profound influence on infection and cytokine patterns in human dendritic cells. J. Leukoc. Biol. 69:583-589. [PubMed] [Google Scholar]
- 8.Ge, Z., and D. E. Taylor. 1997. H. pylori DNA transformation by natural competence and electroporation. In C. L. Clayton and H. L. T. Mobley (ed.), Helicobacter pylori protocols. Humana Press, Totowa, N.J. [DOI] [PubMed]
- 9.Hida, N., T. Shimoyama, Jr., P. Neville, M. F. Dixon, A. T. R. Axon, T. Shimoyama, Sr., and J. E. Crabtree. 1999. Increased expression of IL-10 and IL-12 (p40) mRNA in Helicobacter pylori infected gastric mucosa: relation to bacterial cag status and peptic ulceration. J. Clin. Pathol. 52:658-664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mitchell, H. M. 2001. Epidemiology of infection, p. 7-18. In H. L. T. Mobley, G. L. Mendz, and S. L. Hazell (ed.), Helicobacter pylori: physiology and genetics. ASM Press, Washington, D.C. [PubMed]
- 11.Odenbreit, S., B. Gebert, J. Puls, W. Fisher, and R. Haas. 2001. Interaction of Helicobacter pylori with professional phagocytes: role of the cag pathogenicity island and translocation, phosphorylation, and processing of CagA. Cell. Microbiol. 3:21-31. [DOI] [PubMed] [Google Scholar]
- 12.Palucka, K., and J. Banchereau. 2002. How dendritic cells and microbes interact to elicit or subvert protective immune responses. Curr. Opin. Immunol. 14:420-431. [DOI] [PubMed] [Google Scholar]
- 13.Ramarao, N., and T. F. Meyer. 2001. Helicobacter pylori resists phagocytosis by macrophages: quantitative assessment by confocal microscopy and fluorescence-activated cell sorting. Infect. Immun. 69:2604-2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rescigno, M., M. Urbano, B. Valzasina, M. Francolini, G. Rotta, R. Bonasio, F. Granucci, J.-P. Kraehenbuhl, and P. Ricciardi-Castagnoli. 2001. Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nat. Immunol. 2:361-367. [DOI] [PubMed] [Google Scholar]
- 15.Scheinecker, C., R. McHugh, E. M. Shevach, and R. N. Germain. 2002. Constitutive presentation of a natural tissue autoantigen exclusively by dendritic cells in the draining lymph node. J. Exp. Med. 196:1079-1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stein, M., R. Rappuoli, and A. Covacci. 2001. The cag pathogenicity island, p. 345-353. In H. L. T. Mobley, G. L. Mendz, and S. L. Hazell (ed.), Helicobacter pylori physiology and genetics. ASM Press, Washington, D.C.
- 17.Thoma-Uszynski, S., S. M. Kiertscher, M. T. Ochoa, D. A. Bouis, M. V. Norgard, K. Miyake, P. J. Godowski, M. D. Roth, and R. L. Modlin. 2000. Activation of Toll-like receptor 2 on human dendritic cells triggers induction of IL-12, but not IL-10. J. Immunol. 165:3804-3810. [DOI] [PubMed] [Google Scholar]
- 18.Wang, J., T. G. Blanchard, and P. B. Ernst. 2001. Host inflammatory response to infection, p. 471-480. In H. L. T. Mobley, G. L. Mendz, and S. L. Hazell (ed.), Helicobacter pylori: physiology and genetics. ASM Press, Washington, D.C. [PubMed]
- 19.Wang, Y., and D. E. Taylor. 1990. Chloramphenicol resistance in Campylobacter coli: nucleotide sequence, expression, and cloning vector construction. Gene 94:23-28. [DOI] [PubMed] [Google Scholar]
- 20.Wick, M. J. 2002. The role of dendritic cells during Salmonella infection. Curr. Opin. Immunol. 14:437-443. [DOI] [PubMed] [Google Scholar]