Abstract
CD44 has been implicated in immune and inflammatory processes. We have analyzed the role of CD44 in the outcome of Listeria monocytogenes infection in murine bone marrow-derived macrophages (BMM). Surprisingly, a dramatically decreased intracellular survival of L. monocytogenes was observed in CD44−/− BMM. CD44−/− heart or lung fibroblast cultures also showed reduced bacterial levels. Moreover, livers from CD44−/−-infected mice showed diminished levels of L. monocytogenes. In contrast, intracellular growth of Salmonella enterica serovar Typhimurium was the same in CD44−/− and control BMM. The CD44-mediated increased bacterial proliferation was not linked to altered BMM differentiation or to secretion of soluble factors. CD44 did not mediate listerial uptake, and it played no role in bacterial escape from the primary phagosome or formation of actin tails. Furthermore, CD44-enhanced listerial proliferation occurred in the absence of intracellular bacterial spreading. Interestingly, coincubation of BMM with hyaluronidase or anti-CD44 antibodies that selectively inhibit hyaluronan binding increased intracellular listerial proliferation. Treatment of cells with hyaluronan, in contrast, diminished listerial growth and induced proinflammatory transcript levels. We suggest that L. monocytogenes takes advantage of the CD44-mediated signaling to proliferate intracellularly, although binding of CD44 to certain ligands will inhibit such response.
CD44 glycoprotein is found on the surface of many cell types, including lymphocytes, macrophages, and epithelial cells. Expression levels vary depending on origin and activation status of the cell. CD44-dependent processes are known to include organ development, neuronal axon guidance, hematopoiesis, and numerous immune functions. Among the latter, CD44 participates in lymphocyte adhesion to inflamed endothelium, lymphocyte homing, and tumor metastasis (31). Hyaluronan (HA), a main carbohydrate component of the extracellular matrix, is the principal but not sole ligand of CD44. CD44 is also the major receptor for HA. HA is normally a glycosaminoglycan of high molecular weight. At sites of inflammation, low-molecular-weight (LMW) HA accumulates, most likely due to the presence of hyaluronidases (HA'ses) and/or reactive oxygen species. Binding of LMW HA to CD44 can induce expression of cytokines, chemokines, and adhesion and effector molecules and can induce translocation of transcription factors in cell lines or primary cell cultures (2, 22-24, 26, 28, 29, 41). Thus, besides tethering cells to extracellular ligands, CD44 has broader functions in cellular signaling cascades. CD44 also provides a link between the plasma membrane and the actin cytoskeleton. CD44 can have coreceptor functions mediating the signaling of receptor tyrosine kinases, such as Met.
The impact of CD44 in the regulation of immune responses and inflammation has been broadly studied (27, 31, 40, 42), but few studies have addressed the potential role of CD44 in the control of pathogens (4, 12, 13, 15, 39).
The gram-positive bacterium Listeria monocytogenes is a human pathogen that causes severe disease in immunocompromised individuals and will induce abortions in pregnant women. L. monocytogenes is known to invade a variety of cells, including macrophages. After cellular uptake, the bacterium escapes from the primary phagosome into cytoplasm, where it starts to multiply and then spread to nearby cells (45). The presence of an inducible listerial hexose phosphate transporter mediating rapid intracellular replication has been recently described (17). In the cytoplasm L. monocytogenes expresses ActA protein, a cofactor for the nucleation of actin filaments. The bacterium polymerizes actin filaments around itself, creating a long actin tail. Such tails will propel listeria to the cell membrane, where projections involved in listerial cell-to-cell spread will be formed (11).
Immune resistance to L. monocytogenes depends on the ability of the host to mount a Th1-like immune response (43). Cytokines such as gamma interferon (IFN-γ) will activate macrophage bactericidal mechanisms, which play a crucial role in the control of listerial infection in vivo (20, 32).
We initially hypothesized that signals through HA and CD44 could inhibit the intracellular growth of L. monocytogenes by upregulating the expression of inflammatory genes and by controlling the cytoskeleton rearrangements. Instead, our studies revealed that L. monocytogenes makes use of CD44 signaling to grow efficiently intracellularly.
MATERIALS AND METHODS
Reagents.
Anti-CD44 (KM 703, KM 81), anti-CD4, and anti-major histocompatibility complex (MHC) class I monoclonal antibodies were purified from the supernatant of hybridomas CRL-1896, TIB-241, L3T4, and HB51, respectively (American Type Culture Collection, Manassas, Va.), by using protein G-Sepharose (Amersham-Pharmacia, Uppsala, Sweden). Hyaluronidase (HA'se) from Streptomyces species was purchased from Calbiochem (San Diego, Calif.). HA’se type III from sheep testes, chondroitinase ABC from Proteus vulgaris, heparinase (HS’se) III from Flavobacterium heparinum, and heparan sulfate (HS) from bovine kidneys were all purchased from Sigma-Aldrich (St. Louis, Mo.). Hyaluronans (HA), with the sizes of 190 and 250 kDa, were kindly provided by Ove Vik (Q-Med AB, Uppsala, Sweden).
Mice.
C57Bl/6 mice were bred under specific-pathogen-free conditions. A mutant mouse strain without CD44 was generated by homologous recombination in embryonic stem cells and was backcrossed with C57Bl/6 (36).
Bacteria.
L. monocytogenes wild-type (WT) strain EGD (BUG600, serotype 1/2a) and the ΔplcB2 mutant (35) with a defective lecithinase were used.The actA-defective mutants, deficient in actin polymerization strain LO28ΔActA (BUG 875) (18) and the BUG314 strain, containing a Tn917 transposon inserted in actA (25) and the parental control strain LO28, all obtained from the Pasteur Institute (Paris, France), were used. To study intracellular bacterial localization of L. monocytogenes NF-L357, which contains a transcriptional fusion between actA-plcB and the green fluorescent protein gene (gfp) was used (16). In all cases the bacteria were grown in brain heart infusion (BHI) broth and BHI agar (Difco, Becton Dickinson, Md.) at 37°C. Before infection bacteria were grown at 37°C in BHI broth to late-exponential phase (optical density at 600 nm, 0.8). The bacteria were washed once with phosphate-buffered saline (PBS), resuspended in BHI broth with 15% glycerol, plated on duplicate BHI agar plates, and quantified after an overnight incubation at 37°C. Bacterial suspensions were stored at −70°C until use.
The plasmid pAUL-A (8), which replicates at 30°C but not at 37°C and possesses an erythromycin resistance gene, was used to distinguish replication rate from death rate of L. monocytogenes in bone marrow-derived macrophages (BMM). L. monocytogenes EGD was transformed with pAUL-A by electroporation and was grown at 30°C in BHI medium containing 5 μg of erythromycin per ml overnight. To produce cultures containing less than one copy of the plasmid per bacterium, 20 ml of the culture was inoculated in 180 ml of BHI and was grown at 37°C for 6 h. Numbers of total and plasmid-containing L. monocytogenes and the total number of plasmids were calculated during this incubation by plating bacteria on BHI agar in the presence or absence of erythromycin or by further incubating bacteria at 42°C to stationary phase before plating on BHI agar in the presence of erythromycin. An initial 5.3-fold increase in the number of plasmid-containing bacteria during the first 4.5 h in culture was determined. The mean copy number at start was calculated to be 3.6 plasmids per bacterium, and growth of plasmid-containing bacteria represents the segregation of preexisting plasmids into daughter bacteria. After 4.5 h the total number of plasmid-containing bacteria stopped increasing and was identical to the total number of plasmids. Moreover, the total number of plasmids was not altered during the 5-h culture period. Thus, after 4.5 h each plasmid-containing bacteria had one copy of the plasmid. At this time, 20 to 30% of total bacteria contained pAUL-A. Aliquots of the culture grown for 4.5 h were frozen at −70°C and were used later for infection of BMM. BMM were infected as described below, and CFU in lysates were measured in plates containing 5 μg of erythromycin/ml (measuring plasmid-containing bacteria) and in plates not containing erythromycin (measuring total number of bacteria). The relative proportion of pAUL-A-containing bacteria is a measurement of the growth rate, while the total number of bacteria with pAUL-A is a measurement of killing by the host cell. A similar strategy has been previously used to evaluate toxicity during in vitro and in vivo infection with Salmonella enterica serovar Typhimurium (3, 21).
Differentiation of BMM.
BMM were established as described previously (33). In brief, mice were killed by cervical dislocation, and femurs and tibias of the hind legs were dissected and flushed with 5 ml of ice-cold, sterile PBS. Bone marrow cells (typically 2 × 107 to 3 × 107 per mouse) were washed and resuspended in Dulbecco's minimal essential medium (DMEM) containing glucose and supplemented with 10% fetal calf serum (FCS), 20% L929 cell-conditioned medium (as a source of macrophage-colony stimulating factor), 100 μg of streptomycin/ml, 100 U of penicillin/ml, and 10 mM HEPES buffer. The cells were cultured at 5 × 104 cells per well in 24-well plates for 7 days at 37°C, 5% CO2. BMM cultures were then created by washing vigorously with PBS to remove nonadherent cells. Adherent cells were also harvested and counted by trypan blue exclusion. Typically, the yield of bone marrow cells was 2.0 × 105 to 4.0 × 105 BMM per well (24-well plates) after 7 days in culture, which is when they were infected.
Fibroblast primary cultures.
Fibroblasts were obtained by enzymatic digestion of hearts and lungs from 6- to 10-week-old CD44−/− or WT mice as described previously (7). In brief, organs were minced in Iscove's modified medium (IMDM) supplemented with 10% FCS, 100 μg of streptomycin/ml, 100 U of penicillin/ml, and 10 mM HEPES buffer and were incubated in IMDM containing 1 mg of collagenase (type IV; Sigma, St. Louis, Mo.) per ml at 37°C for 10 min. Released cells in the supernatant were collected, and undigested tissue was further enzymatically treated for two more cycles. Recovered cells were pooled and plated, and highly enriched cultures of adherent cardiac and pulmonar cells were recovered during the plating procedure. After the third passage, nearly all cells appeared to be fibroblasts by morphology, as also previously determined by immunostaining (7). Such cultures were trypsinized and resuspended in IMDM, and 5 × 104 cells per well were seeded on a 24-well plate. Infection was performed 24 h after plating.
Infection and infectivity assay.
BMM and fibroblasts cultured in 24-well plates were cocultured with L. monocytogenes for 1 h at 37°C, 5% CO2. Cells were then extensively washed with PBS to remove the extracellular bacteria. Triplicate wells were used for all conditions. To prevent extracellular bacterial growth, cells were then cultivated in DMEM containing 5% FCS and 5 μg of gentamicin per ml. At different time points after infection cells were washed with PBS and lysed with 0.1% Triton X-100 in PBS, and aliquots of lysates were plated on BHI agar plates. Bacteria in two or three tenfold dilutions of the lysates were quantified. Plates were incubated overnight at 37°C and CFU were determined.
BMM were also infected in parallel with S. enterica serovar Typhimurium strain 14028 (American Type Culture Collection). S. enterica serovar Typhimurium was grown in Luria broth (LB) agar plates overnight. For this purpose, bacterial colonies were diluted in PBS at a concentration of 5 × 108 bacteria per ml. Ninety microliters of bacteria was coincubated with 10 μl of C57Bl/6 normal mouse serum for 30 min at 37°C. Bacteria were further diluted at 106 per ml in DMEM-5% FCS. BMM were cocultured with S. enterica serovar Typhimurium and were centrifuged at 500 × g for 5 min at room temperature. Cells were incubated for 1 h at 37°C and were extensively washed with PBS, and DMEM containing 5 μg of gentamicin per ml was then added. Cells were lysed at the indicated time points after infection as described above, and CFU in cell lysates were quantified on LB agar plates after overnight incubation.
Immunostaining and flow cytometry.
BMM were phenotypically characterized by fluorescence-activated cell sorter analysis. For this purpose, BMM were detached from plates by using a cell scraper and were incubated with PBS containing 10% normal goat serum (Sigma) to block unspecific binding of the secondary antibody. Cells were then incubated with purified monoclonal rat antibody to F4/80, Mac-3, CD80, or GR-1 for 30 min on ice. Cells were then washed and incubated with biotinylated goat anti-rat serum for 30 min on ice. Anti-CD44, -CD19, -CD3, and -CD8 antibodies were directly labeled with fluorescein isothiocyanate. Anti-CD4, -CD14, and -NK1.1 antibodies were directly labeled with phycoerythrin. Anti-MHC class II (Ia) and -ICAM-1 (MALA-2) antibodies were biotinylated. Isotype-matched control rat immunoglobulins (Igs) directly labeled with fluorescein isothiocyanate, phycoerythrin, or biotin were used as controls. When staining with biotinylated or directly labeled antibodies, BMM were preincubated with anti-CD16/CD32 antibodies to block Fcγ receptors. After incubation with biotinylated antibodies, cells were washed, stained with neutroavidin Alexa (Molecular Probes, Eugene, Oreg.), and analyzed with a FACScan (Becton-Dickinson). All primary antibodies were purchased from Pharmingen (San Diego, Calif.), except for F4/80 (Serotec, Raleigh, N.C.).
Immunostaining of intracellular bacteria.
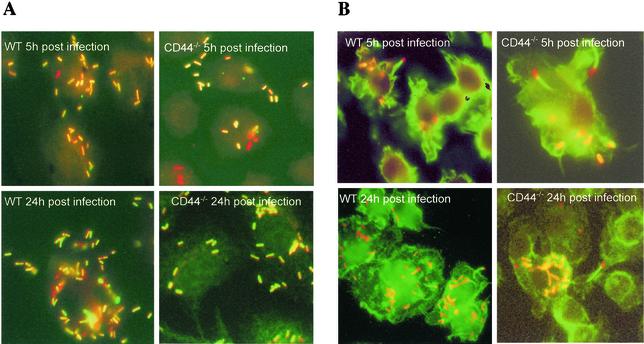
To analyze intracellular bacterial localization, BMM were infected with the L. monocytogenes NF-L357 strain, which contains a chromosomal actA-gfp-plcB transcriptional fusion, and were immunostained with anti-L. monocytogenes serum. actA/plcB expression (and, therefore, gfp) occurs shortly after L. monocytogenes gains access to the host cell cytosol (16). BMM grown on 13-mm2-diameter coverslides were infected with strain NF-L357 as described above. At different time points after infection cells were washed with PBS and fixed with 4% paraformaldehyde in PBS for 15 min, and the cell membrane was lysed with 0.5% Triton X-100 in PBS for 15 min at room temperature. Thereafter cells were washed with PBS, and unspecific binding was blocked by incubation with PBS containing 5% bovine serum albumin (BSA) and 0.5% Triton X-100. The cultures were then incubated with rabbit anti-L. monocytogenes serum (Difco Laboratories, Detroit, Mich.) diluted 1:800 in PBS supplemented with 1% BSA, 0.5% Triton X-100, and 0.01% NaN3 for 1 h at room temperature. The cells were then washed with PBS and were stained with a Texas-Red-conjugated donkey anti-rabbit IgG (H+L) (Jackson ImmunoResearch Laboratories, West Baltimore Pike, Pa.) diluted 1:100 in PBS containing 1% BSA for 1 h at room temperature and were washed again. Cover slides were analyzed with a fluorescent microscope.
Fluorescent staining of actin filaments.
When residing in cytoplasm, L. monocytogenes generates an actin comet tail which confers movement to the bacteria, enabling it to enter adjacent cells. Listerial actin tails were stained with fluorochrome-labeled phalloidin. In brief, BMM grown on 13-mm2-diameter coverslides and infected with L. monocytogenes were used. At indicated time points after infection cells were washed with PBS and were fixed with PBS containing 4% formaldehyde, and the cell membrane was lysed with PBS containing 0.5% Triton X-100. Cells were washed and incubated with anti-L. monocytogenes serum as described above, washed, and stained with Alexa Fluor 594-labeled goat anti-rabbit IgG (H+L) (Molecular Probes). The cells were then washed and stained with Alexa Fluor 488-labeled phalloidin (Molecular Probes) for 20 min at room temperature, and coverslides were read with a fluorescent microscope.
RT-PCR assay.
Tumor necrosis factor alpha (TNF-α), IFN-γ, IFN-α, interleukin-1 (IL-1), IL-6, IL-12p40, inducible nitric oxide synthase (iNOS), and β-actin mRNA in freshly extracted RNA from L. monocytogenes-infected or HA-treated WT or CD44−/− BMM were visualized by reverse transcription (RT)-PCR assays as previously described (34).
Reactions were carried out for 37 cycles (38 cycles for IL-12 and 30 cycles for β-actin) in a thermal cycler with an annealing step at 60°C (67°C for IL-12).
The primer sequences for the amplification of the cDNA are listed in Table 1. IFN-α primer sequences were obtained from Clontech. These primers recognize a consensus sequence present in IFN-α1, IFN-α2, and IFN-α7 genes.
TABLE 1.
Primer sequences used in this study
| Primer | Sequence |
|---|---|
| Sense iNOS | 5′ CCC TTC CGA AGT TTC TGG CAG CAG CAG C3′ |
| Antisense iNOS | 5′ GGC TGT CAG AGC CTC GTG GCT TTG G3′ |
| Sense IFN-γ | 5′ TGG ACC TGT GGG TTG TTG ACC TCA AAC TTG GC3′ |
| Antisense IFN-γ | 5′ TCG ATC TTG GCT TTG CAG CTC TTC CTC ATG GC3′ |
| Sense IL-12 p40 | 5′ CGT GCT CAT GGC TGG TGC AAA G3′ |
| Antisense IL-12 p40 | 5′ CTT CAT CTG CAA GTT CTT GGG C3′ |
| Sense IL-1α | 5′ ATG GCC AAA GTT CCT GAC TTG TTT3′ |
| Antisense IL-1α | 5′ C CTT CAG CAA CAC GGG CTG GTC3′ |
| Sense IL-6 | 5′ ATG AAG TTC CTC TCT GCA AGA GAC T3′ |
| Antisense IL-6 | 5′ CA CTA GGT TTG CCG AGT AGA TCT C3′ |
| Sense TNF-α | 5′ ATG AGC ACA GAA AGC ATG ATC CGC3′ |
| Antisense TNF-α | 5′ CC AAA GTA GAC CTG CCC GGA CTC3′ |
| Sense IFN-α | 5′ GAC TCA TCT GCT GCT TGG AAT GCA ACC CTC3′ |
| Antisense IFN-α | 5′ GAC TCA CTC CTT CTC CTC ACT CAG TCT TGC C3′ |
| Sense β-actin | 5′ GTG GGC CGC TCT AGG CAC CAA3′ |
| Antisense β-actin | 5′ CTC TTT GAT GTC ACG CAC GAT TTC3′ |
RESULTS
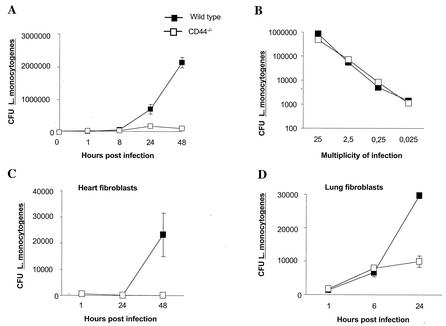
We first compared the outcome of L. monocytogenes infection in CD44−/− and WT BMM. Surprisingly, CD44−/− BMM showed a strikingly reduced growth of intracellular L. monocytogenes compared to that of WT cells (Fig. 1A). The reduced numbers of L. monocytogenes in CD44−/− BMM was not due to a diminished bacterial uptake by the mutant cells, since similar numbers of intracellular L. monocytogenes were measured in mutant and WT BMM at 1 h after infection (Fig. 1B). By using L. monocytogenes containing one copy of the temperature-sensitive, erythromycin-resistant gene containing plasmid pAUL-A per bacteria, it was shown that the reduced intracellular number of L. monocytogenes in CD44−/− BMM was due to increased rate of death rather than to reduced growth rate of the bacteria (Table 2).
FIG. 1.
Reduced intracellular growth of L. monocytogenes in CD44−/− BMM, lung, and heart fibroblasts. (A) CD44−/− and WT BMM were infected with L. monocytogenes at a multiplicity of infection of 0.2 bacteria per cell. One hour after infection BMM were extensively washed with PBS, and DMEM supplemented with 5% FCS containing 5 μg gentamicin per ml was added. BMM were lysed at the indicated time points, and CFU were quantified after plating. The experiment shown is representative of at least 10 independent experiments. (B) BMM from CD44−/− and WT mice were infected with different numbers of L. monocytogenes. One hour after infection cells were extensively washed to remove extracellular bacteria and were lysed; CFU were then measured. Similarly, CFU were measured for L. monocytogenes-infected BMM further incubated with or without 5 μg of gentamicin per ml for 15 min, a dose that was lethal for extracellular L. monocytogenes (data not shown). Thus, the depicted CFU originate from intracellular L. monocytogenes. One representative of several independent experiments is shown. (C and D) Fibroblasts derived from heart (C) and lung (D) from CD44−/− and WT mice were infected with L. monocytogenes as described in Materials and Methods. Cells were washed and lysed at the indicated times after infection, and the amounts of CFU were quantified. An MOI of 1:1 was used in the depicted experiments. Independent experiments that used MOIs of 10:1 and 2:1 also showed similar results. Bars represent the standard errors.
TABLE 2.
Growth and death rates of BMM infected with L. monocytogenesa
| Infection characteristic | Growth rate for:
|
||||||
|---|---|---|---|---|---|---|---|
| MOI | H after infection | WT BMM
|
CD44−/− BMM
|
||||
| Total CFU | CFU with pAUL-A | Propor- tion with pAUL-A | Total CFU | CFU with pAUL-A | Propor- tion with pAUL-A | ||
| 0.32 | 1 | 4,050 | 1,300 | 0.32 | 4,939 | 1,600 | 0.33 |
| 0.32 | 24 | 190,000 | 340 | 0.0018 | 106,500 | 230 | 0.0022 |
| 0.32 | 48 | 255,000 | 0 | 0 | 14,650 | 0 | 0 |
| 3.2 | 1 | 40,500 | 15,300 | 0.38 | 58,075 | 16,800 | 0.29 |
| 3.2 | 24 | 1,160,000 | 2,320 | 0.0020 | 665,000 | 1,330 | 0.0020 |
BMM were infected with L. monocytogenes containing pAUL-A as described in Materials and Methods. At the indicated time points after infection total CFU and CFU containing pAUL-A (bacteria grown in presence of erythromycin) were measured. The proportion of L. monocytogenes carrying pAUL-A was calculated. The proportion of bacteria maintaining pAUL-A is a measurement of the growth rate, while the total number of bacteria with pAUL-A is a measurement of killing by the host cell. Listerial growth is thus the outcome of bacterial death and proliferation of few surviving, in agreement with previous electron microscopy data (30). The total number of bacteria results from the balance between growth rate and killing. Note that although after 48 h of infection CD44−/− BMM contain 17-fold less L. monocytogenes than WT BMM, neither CD44−/− nor WT BMM contain any pAUL-A, indicating that the reduced number of bacteria in CD44−/− BMM is the result of diminished survival and is not the consequence of a decreased growth rate. One representative of two independent experiments is shown.
To test for the presence of CD44-facilitated listerial growth in cell types other than BMM, we studied L. monocytogenes infection in primary lung and heart fibroblast cultures from CD44−/− and WT mice. Listerial number was again lower in CD44−/− cells from both sources (Fig. 1C and D).
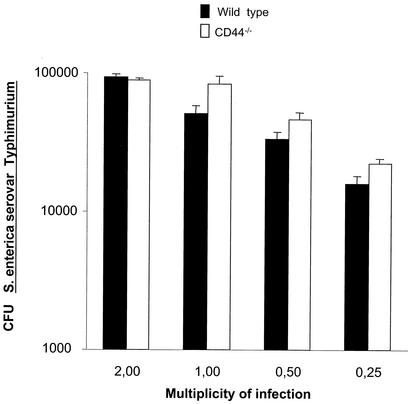
Next we analyzed whether CD44 is a general supporter of intracellular growth of bacterial pathogens. However, no differences in uptake (data not shown) or intracellular growth of S. enterica serovar Typhimurium in CD44−/− and WT BMM were found (Fig. 2).
FIG. 2.
CD44 does not mediate intracellular growth of S. enterica serovar Typhimurium. CD44−/− and WT BMM were infected with S. enterica serovar Typhimurium at an MOI of 0.25, 0.5, 1.0, and 2.0 bacteria per cell. One hour after infection cells were extensively washed and incubated in DMEM-5% FCS containing 5 μg of gentamicin per ml. CFU were measured 24 h after infection. Differences in the uptake of S. enterica serovar Typhimurium by BMM after 1 h were measured and were the same in both WT and CD44−/− samples (data not shown).
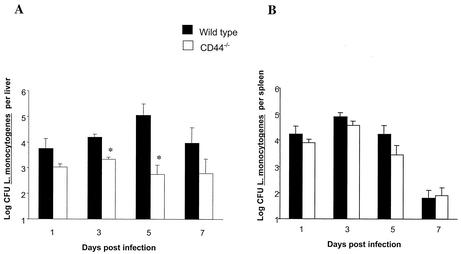
We next explored whether reduced in vitro growth of L. monocytogenes in CD44−/− BMM related to a diminished susceptibility of CD44−/− mice to bacterial infection in vivo. WT and CD44−/− mice were infected intraperitoneally with 105 L. monocytogenes cells, and bacterial numbers were quantified in spleen and liver at different time points after infection. The livers from CD44−/− mice showed a significantly diminished number of L. monocytogenes compared to that of WT controls, whereas bacterial numbers recovered from the spleens of both mouse strains were similar (Fig. 3).
FIG. 3.
Diminished susceptibility of CD4−/− mice to infection with L. monocytogenes. CD44−/− and WT mice were infected intraperitoneally with 105 CFU of L. monocytogenes (0.1 50% lethal infective dose). Mice (eight animals per group) were sacrificed at the indicated time points after infection, and bacterial loads (CFU) were measured in spleens and livers. Mortality was not observed in WT or mutant mouse strains. Asterisks indicate that differences in log10 CFU versus that of WT controls are significant (P < 0.05, Student's t test).
We found no variation in phenotype of CD44−/− BMM compared to that of WT BMM that could be related to the diminished growth of L. monocytogenes. CD44−/− and WT BMM both expressed high levels of Mac-3, GR-1, F4/80, and MHC class II, expressed moderate levels of CD80 and CD14, and were negative for T, B, or NK cell markers, such as CD3, CD4, CD8, CD19, and NK1.1 as measured by fluorescence-activated cell sorting (data not shown).
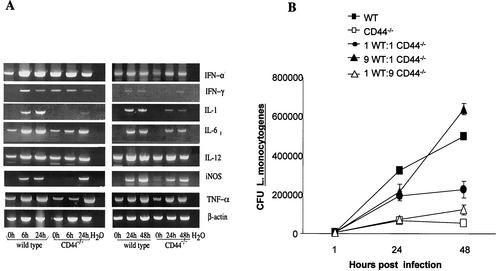
We then studied if different levels of cellular activation could account for the diminished intracellular growth of L. monocytogenes in CD44−/− BMM. For this purpose RNA was extracted from BMM before and at different time points after infection, and the presence of transcripts for inflammatory cytokines was evaluated by RT-PCR. The levels of IFN-γ, IFN-α, IL-12p40, IL-1, IL-6, iNOS, and TNF-α mRNA were all increased in L. monocytogenes-infected WT and CD44−/− BMM. CD44−/− BMM contained equal or lower levels of all transcripts than WT BMM (Fig. 4A). Thus, the enhanced death rate of L. monocytogenes in CD44−/− BMM is not linked to the presence of higher levels of inflammatory cytokine transcripts.
FIG. 4.
Role for soluble factors in the reduced intracellular survival of L. monocytogenes in CD44−/− BMM. (A) Total RNA was extracted from L. monocytogenes-infected CD44−/− and WT BMM at the indicated time points after infection. The accumulation of IFN-α, IFN-γ, IL-1, IL-6, IL-12, iNOS, TNF-α, and β-actin in these samples was visualized by RT-PCR. Two independent experiments are shown. (B) WT and CD44−/− BMM were coincubated at the indicated ratios, and the coculture was infected with L. monocytogenes. Controls containing only CD44−/− or WT BMM were also included. All wells contained 2 × 105 total BMM. CFU were measured at the indicated time points as described in Materials and Methods. A representative from two independent experiments is shown.
We next analyzed intracellular bacterial growth by using various cocultures of CD44−/− and WT BMM. We hypothesized that an increased secretion of inflammatory cytokines from CD44−/− BMM or the secretion of soluble factors promoting bacterial growth by WT BMM could modify the listerial numbers in either direction in the coculture. No shift in the expected bacterial numbers in these cocultures was detected (Fig. 4B), suggesting that soluble factors account neither for the enhanced listerial growth in the presence of CD44 nor for the increased bacterial death rate in its absence.
CD44 might be necessary for escape of L. monocytogenes from phagosome to cytoplasm. To study this, BMM were infected with the NF-L357 strain of L. monocytogenes containing a gfp gene only expressed when bacteria localize in the cytoplasm (16). Staining gfp-expressing bacteria with anti-L. monocytogenes sera, we found similar ratios of gfp-expressing and -nonexpressing bacteria in CD44−/− and WT BMM. WT BMM showed higher total numbers of bacteria than CD44−/− BMM. Thus, CD44 plays no detectable role in the escape of L. monocytogenes from the phagosome into the cytoplasm (Fig. 5A).
FIG. 5.
Role of CD44 in the listerial escape into the cytoplasm and actin tail formation. (A) CD44−/− and WT BMM were infected as indicated in the text with L. monocytogenes NF-L357, which contains gfp that is expressed in the cytoplasm of the infected cell. At the indicated time points after infection BMM were washed, fixed, incubated with antilisteria sera, and stained with Texas-Red-conjugated donkey anti-rabbit IgG as described in Materials and Methods. Bacteria expressing GFP (in green or yellow) or not expressing GFP (in red) were detected in both WT and CD44−/− BMM. No qualitative difference in the ratio of GFP-positive and -negative bacteria in CD44−/− and WT BMM was observed. On the other hand, lower amounts of L. monocytogenes were visualized in CD44−/− BMM. Magnification, 400×. (B) L. monocytogenes-infected CD44−/− and WT BMM were washed, fixed, and stained with antilisteria-specific antibodies (as indicated in the legend to Fig. 4A) and with Alexa Fluor 488-labeled phalloidin to visualize actin tails. Actin tails were observed on bacteria infecting both CD44−/− and WT BMM. Magnification, 400×.
CD44 may participate in the formation of actin tails by L. monocytogenes, thereby reducing bacterial growth in CD44−/− BMM. However, intracellular L. monocytogenes with actin tails was observed both in CD44−/− and in WT BMM (Fig. 5B).
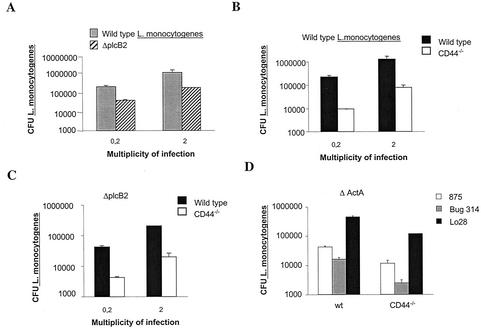
The possible importance of cell-to-cell spread in CD44-enhanced growth of L. monocytogenes was next examined. For this purpose cells were infected with ΔplcB2 or ΔactA L. monocytogenes mutants (showing reduced or abolished cell-to-cell spread). As expected, WT BMM showed lower numbers of bacteria when infected with the ΔplcB2 or the ΔactA mutants than with the respective WT strains of L. monocytogenes (Fig. 6A and D). CD44−/− BMM showed lower levels of ΔplcB2, LO28ΔactA, or BUG375 ΔactA L. monocytogenes compared to those shown by WT BMM (Fig. 6C and D). Thus, the diminished bacterial growth in CD44−/− BMM is unlikely to be due to a reduction in bacterial cell-to-cell spreading.
FIG. 6.
Role of plcB2 and actA in the reduced bacterial survival in CD44−/− BMM. WT (A, B, and C) and CD44−/− (B and C) BMM were infected with the ΔplcB2 mutant (A and C) or the EGD parental strain (A and B) of L. monocytogenes at an MOI of 2 and 0.2 bacteria per cell, respectively. WT and CD44−/− BMM were infected with the ΔactA mutants BUG314 or BUG875 or the LO28 parental strain (D). Twenty-four hours after infection cells were lysed and the CFU in cell lysates were quantified. A representative of two independent experiments is shown.
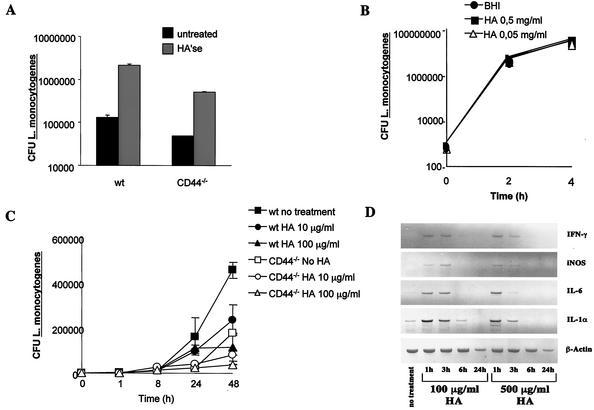
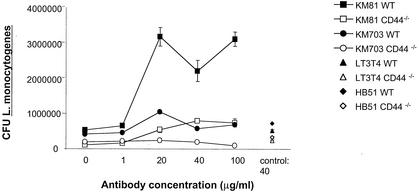
Secretion of HA by infected BMM might mediate the CD44-dependent enhanced intracellular listerial growth. BMM were thus treated with HA'se before and after infection with L. monocytogenes (Fig. 7A). Coincubation of WT BMM with HA'se, however, increased the intracellular proliferation of L. monocytogenes. In agreement with this result, coincubation of BMM with HA reduced intracellular bacterial growth (Fig. 7C) while enhancing accumulation of iNOS, IL-1, IL-6, and IFN-γ mRNA (Fig. 7D). Control experiments showed that HA as such did not affect extracellular growth of L. monocytogenes (Fig. 7B). However, HA and HA'se also affected listerial proliferation in CD44−/− BMM in a manner similar to that of WT BMM (Fig. 7A and C). Thus, hyaloadherins other than CD44 participate in HA-mediated control of listerial infection. To analyze whether CD44 plays a role in modulation of intracellular bacterial growth by HA, BMM were treated with monoclonal antibodies neutralizing (KM 81) or not neutralizing (KM 703) the HA binding ability of CD44 (47). Anti-MHC class I and anti-CD4 monoclonal antibodies were used as controls. Incubation of WT but not CD44−/− BMM with KM 81 resulted in an increased intracellular proliferation of L. monocytogenes, whereas no such consequence was observed after incubation of BMM with KM 703 or the control monoclonal antibodies (Fig. 8). Thus, whereas signaling via CD44 facilitates listerial growth, addition of HA can restrict growth occurring in part via binding to CD44.
FIG. 7.
Effect of addition or depletion of HA in the intracellular growth of L. monocytogenes in BMM. (A) CD44−/− and WT BMM were coincubated with 2 U of HA'se per ml of DMEM-5% FCS at 37°C, 5% CO2 1 h before and during infection with L. monocytogenes. The bacterial load (CFU) of treated and control BMM was quantified 24 h after infection. A representative of four independent experiments is depicted. Addition of HA (250 kDa) to cocultures of HA'se and BMM completely inhibited the enzyme-mediated enhancement of bacterial proliferation, confirming the specificity of the enzymatic reaction (data not shown). (B) L. monocytogenes was grown at 37°C in BHI medium in the presence of 50 and 500 μg of HA (190 kDa) or in the absence of HA. CFU were recorded in culture aliquots at the indicated time points of incubation. (C) WT and CD44−/− BMM were treated with 10 and 100 μg of HA (190 kDa) per ml 1 day before and during infection with L. monocytogenes. The bacterial load (CFU) of treated and control BMM was quantified at the indicated time points after infection. A representative from two independent experiments is shown. (D) WT BMM were treated with 100 and 500 μg of HA/ml (190 kDa) diluted in DMEM-5% FCS. The presence of IFN-γ, iNOS, IL-6, IL-1α, and β-actin mRNA in treated and control BMM was determined by RT-PCR at the indicated hours after treatment. A representative from two independent experiments is shown.
FIG. 8.
Increased intracellular listerial growth in BMM coincubated with a monoclonal antibody specifically neutralizing the HA binding ability of CD44. CD44−/− and WT BMM were treated with different concentrations of the anti-CD44 monoclonal antibodies KM 81 and KM 703 1 h before and during infection with L. monocytogenes. Anti-CD4 (L3T4) and anti-MHC class I (HB51) antibodies were used as controls. The bacterial load (CFU) of treated and control BMM was quantified after 24 h of infection.
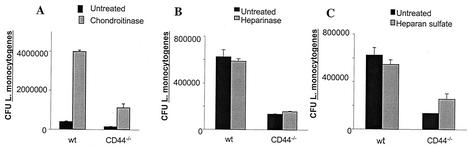
Proteoglycan forms of CD44 also exhibit affinity for molecules, such as chondroitin sulfate, HS, fibronectin, and osteopontin (31). Treatment of BMM with chondroitin sulfatase also enhanced intracellular listerial growth (Fig. 9A), whereas no effect on the outcome of infection was observed upon adding HS or HS'se (Fig. 9B and C).
FIG. 9.
Effect of treatment with chondroitinase, HS'se, and HS on the intracellular growth of L. monocytogenes. CD44−/− and WT BMM were coincubated with 0.01 of U chondroitinase (A), 0.5 U of HS'se (B), or 20 μg of HS (C) per ml of DMEM-5% FCS at 37°C 1 h before and during infection with L. monocytogenes. The bacterial load (CFU) of treated and control BMM was quantified at 24 h (A) and at the indicated time points (B and C) after infection. A representative from two independent experiments for each condition is shown.
DISCUSSION
Herein we demonstrate that the presence of CD44 facilitates intracellular growth of L. monocytogenes in murine systems. Changes in bacterial proliferation did not relate to altered bacterial phagocytosis or secretion of proinflammatory cytokines in CD44−/− BMM. The presence of CD44 increased bacterial survival without altering growth rate and was not required for bacterial escape from primary phagosome into cytoplasm or for actin tail formation. CD44-facilitated listerial growth does not depend on functional cell-to-cell spreading by L. monocytogenes. These results altogether suggest that CD44 signaling affects survival of L. monocytogenes in the cytoplasm of the primary infected cell. In agreement with this suggestion, inhibitors of tyrosine protein kinases, phosphatidylinositol 3-kinase, and actin polymerization enzymes that mediate CD44 signaling all reduced listerial growth in WT macrophages (data not shown). These inhibitors, however, do not uniquely affect CD44 activation. To our knowledge, such a role for CD44 in determining the intracellular fate of bacteria has not been described. This CD44-enhanced intracellular listerial survival was observed both in cultures of primary macrophages and fibroblasts and is likely to be directly related to diminished L. monocytogenes numbers in livers of CD44−/−-infected mice. The presence of different immune effector cells (10) participating in listerial control in spleen and liver could account for the dissimilar involvement of CD44 in listerial control in these organs. Alternatively, CD44-mediated intracellular listerial proliferation could be more prevalent in liver compared to that in spleen cell populations.
On the other hand, CD44 did not affect the intracellular growth of another intracellular pathogen, S. enterica serovar Typhimurium.
We plan to use cells transfected with CD44 lacking intracellular protein domains necessary for different signaling pathways to further dissect the role of this cell receptor in listerial growth. CD44 undergoes alternative splicing (encompassing 10 variant exons) giving rise to isoforms that probably show differences in their biological functions (31), and it could relate to the regulatory function of the receptor during infection with L. monocytogenes. Alternative splicing will not take place in the transfected molecule. Affinity of CD44 for ligands is dependent on posttranslational modifications, such as glycosylation, that are cell type and growth condition specific (38), and it could be also altered by listerial infection. CD44 has been suggested to act as a functional coreceptor for hepatocyte growth factor/scatter factor by binding and presenting growth factor to the receptor tyrosine kinase c-Met (44). The bacterial surface protein InlB promotes not only internalization (5, 14) but also the escape into the cytoplasm of L. monocytogenes in mammalian cells (19). In turn, the Met receptor mediates InIB-dependent internalization of L. monocytogenes (37). However, mutants lacking InlB (as well as InlA and InlA/B) showed diminished intracellular growth in CD44−/− BMM compared to that of WT BMM (data not shown).
There is indirect evidence that the ability to replicate within host cells requires specific adaptations in the microbe. A hexose phosphate transporter (hpt) has been identified as a virulence factor involved in the listerial replication phase (9, 17). Whether listerial hpt and other virulence factor(s) specifically interact with CD44 signaling and promote listerial growth will be further studied.
CD44 signaling has been indicated to affect the outcome of other extracellular and intracellular infections. Binding of the HA capsule of Streptococcus pyogenes to CD44 mediates cytoskeletal rearrangements (12, 13). Such rearrangements disrupt the intercellular junctions and permit bacterial passage between epithelial cells (13). Interaction of the Shigella flexneri IpaB-IpaC-secreted complex with CD44 has also been suggested to be involved in the cellular uptake of shigella and in shigella-induced cytoskeletal rearrangements (39).
Inflammatory stimuli promote local accumulation of LMW HA fragments (6, 42). We confirmed previous reports (22, 23, 26, 29, 41) indicating that coincubation of cells with LMW HA increased proinflammatory transcripts and demonstrate further that such coincubation diminished listerial growth in macrophages. Eliminating HA via HA'se treatment accelerated listerial proliferation, which is in line with the possibility that macrophage-derived HA acts by binding to hyaloadherins and diminishes listerial growth. A role for CD44 in HA-mediated control of listerial growth was further suggested by our finding of an accelerated listerial growth in WT BMM treated with a monoclonal antibody that selectively interferes with HA binding to CD44 (47). However, and in line with the fact that CD44 is not the sole receptor reacting with HA (1, 42, 46), other cellular receptors also participate in the control of listerial infection, since HA'se enhanced listerial growth in CD44−/− BMM and coincubation with HA diminished listerial growth in these cells. Adding complexity to this analysis is the fact that CD44 not only binds HA but also binds HS, chondroitin sulfate, collagen, fibronectin, and osteopontin (42). In this study we found that treatment of BMM with chondroitin sulfatase but not HS'se increased listerial intracellular growth, a finding we will further explore.
Altogether our results suggest a dual role of CD44 in the outcome of infection with L. monocytogenes; whereas a quiescent receptor (or low levels of ligands) will deliver signals that will facilitate intracellular listerial growth, ligands binding to CD44 will diminish such response.
Acknowledgments
This work was supported by the Karolinska Institute; The Foundation for Knowledge and Competence Development, Stockholm, Sweden; The Swedish Cancer Society; The Swedish Medical Research Council; and Q-Med AB, Uppsala, Sweden.
We thank M. Rhen, Microbiology & Tumorbiology Center, Karolinska Institute, for providing the S. enterica serovar Typhimurium bacterial strain and for critical analysis of the manuscript; O. Wik, Q-Med AB, for providing the HA preparation used; and T. Mak, Amgen, Ontario, Canada, for providing the CD44−/− mice. We are grateful to P. Cossart, Unite des Ineractions Bacteries-Cellules, Institut Pasteur, Paris, France; N. Freitag, Wayne State University, School of Medicine, Detroit, Mich.; and T. Chakraborty, Institut für Medizinische Mikrobiologie, Justus-Liebig-Universität Gieβen, Gieβen, Germany, for their kind gifts of the L. monocytogenes strains. We thank Berit Olsson for excellent technical assistance.
Editor: B. B. Finlay
REFERENCES
- 1.Banerji, S., J. Ni, S. X. Wang, S. Clasper, J. Su, R. Tammi, M. Jones, and D. G. Jackson. 1999. LYVE-1, a new homologue of the CD44 glycoprotein, is a lymph-specific receptor for hyaluronan. J. Cell Biol. 144:789-801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beck-Schimmer, B., B. Oertli, T. Pasch, and R. P. Wuthrich. 1998. Hyaluronan induces monocyte chemoattractant protein-1 expression in renal tubular epithelial cells. J. Am. Soc. Nephrol. 9:2283-2290. [DOI] [PubMed] [Google Scholar]
- 3.Benjamin, W. H., Jr., P. Hall, S. J. Roberts, and D. E. Briles. 1990. The primary effect of the Ity locus is on the rate of growth of Salmonella typhimurium that are relatively protected from killing. J. Immunol. 144:3143-3151. [PubMed] [Google Scholar]
- 4.Blass, S. L., E. Pure, and C. A. Hunter. 2001. A role for CD44 in the production of IFN-gamma and immunopathology during infection with Toxoplasma gondii. J. Immunol. 166:5726-5732. [DOI] [PubMed] [Google Scholar]
- 5.Braun, L., H. Ohayon, and P. Cossart. 1998. The InIB protein of Listeria monocytogenes is sufficient to promote entry into mammalian cells. Mol. Microbiol. 27:1077-1087. [DOI] [PubMed] [Google Scholar]
- 6.Cao, H. J., H. S. Wang, Y. Zhang, H. Y. Lin, R. P. Phipps, and T. J. Smith. 1998. Activation of human orbital fibroblasts through CD40 engagement results in a dramatic induction of hyaluronan synthesis and prostaglandin endoperoxide H synthase-2 expression. Insights into potential pathogenic mechanisms of thyroid-associated ophthalmopathy. J. Biol. Chem. 273:29615-29625. [DOI] [PubMed] [Google Scholar]
- 7.Castanos-Velez, E., S. Maerlan, L. M. Osorio, F. Aberg, P. Biberfeld, A. Orn, and M. E. Rottenberg. 1998. Trypanosoma cruzi infection in tumor necrosis factor receptor p55-deficient mice. Infect. Immun. 66:2960-2968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chakraborty, T., M. Leimeister-Wachter, E. Domann, M. Hartl, W. Goebel, T. Nichterlein, and S. Notermans. 1992. Coordinate regulation of virulence genes in Listeria monocytogenes requires the product of the prfA gene. J. Bacteriol. 174:568-574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chico-Calero, I., M. Suarez, B. Gonzalez-Zorn, M. Scortti, J. Slaghuis, W. Goebel, and J. A. Vazquez-Boland. 2002. Hpt, a bacterial homolog of the microsomal glucose-6-phosphate translocase, mediates rapid intracellular proliferation in Listeria. Proc. Natl. Acad. Sci. USA 99:431-436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Conlan, J. W. 1999. Early host-pathogen interactions in the liver and spleen during systemic murine listeriosis: an overview. Immunobiology 201:178-187. [DOI] [PubMed] [Google Scholar]
- 11.Cossart, P., and H. Bierne. 2001. The use of host cell machinery in the pathogenesis of Listeria monocytogenes. Curr. Opin. Immunol. 13:96-103. [DOI] [PubMed] [Google Scholar]
- 12.Cywes, C., I. Stamenkovic, and M. R. Wessels. 2000. CD44 as a receptor for colonization of the pharynx by group A Streptococcus. J. Clin. Investig. 106:995-1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cywes, C., and M. R. Wessels. 2001. Group A Streptococcus tissue invasion by CD44-mediated cell signalling. Nature 414:648-652. [DOI] [PubMed] [Google Scholar]
- 14.Dramsi, S., I. Biswas, E. Maguin, L. Braun, P. Mastroeni, and P. Cossart. 1995. Entry of Listeria monocytogenes into hepatocytes requires expression of inIB, a surface protein of the internalin multigene family. Mol. Microbiol. 16:251-261. [DOI] [PubMed] [Google Scholar]
- 15.Eugene, E., I. Hoffmann, C. Pujol, P. O. Couraud, S. Bourdoulous, and X. Nassif. 2002. Microvilli-like structures are associated with the internalization of virulent capsulated Neisseria meningitidis into vascular endothelial cells. J. Cell Sci. 115:1231-1241. [DOI] [PubMed] [Google Scholar]
- 16.Freitag, N. E., and K. E. Jacobs. 1999. Examination of Listeria monocytogenes intracellular gene expression by using the green fluorescent protein of Aequorea victoria. Infect. Immun. 67:1844-1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goetz, M., A. Bubert, G. Wang, I. Chico-Calero, J. A. Vazquez-Boland, M. Beck, J. Slaghuis, A. A. Szalay, and W. Goebel. 2001. Microinjection and growth of bacteria in the cytosol of mammalian host cells. Proc. Natl. Acad. Sci. USA 98:12221-12226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gouin, E., P. Dehoux, J. Mengaud, C. Kocks, and P. Cossart. 1995. iactA of Listeria ivanovii, although distantly related to Listeria monocytogenes actA, restores actin tail formation in an L. monocytogenes actA mutant. Infect. Immun. 63:2729-2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gregory, S. H., A. J. Sagnimeni, and E. J. Wing. 1997. Internalin B promotes the replication of Listeria monocytogenes in mouse hepatocytes. Infect. Immun. 65:5137-5141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guleria, I., and J. W. Pollard. 2001. Aberrant macrophage and neutrophil population dynamics and impaired Th1 response to Listeria monocytogenes in colony-stimulating factor 1-deficient mice. Infect. Immun. 69:1795-1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gulig, P. A., T. J. Doyle, M. J. Clare-Salzler, R. L. Maiese, and H. Matsui. 1997. Systemic infection of mice by wild-type but not Spv- Salmonella typhimurium is enhanced by neutralization of gamma interferon and tumor necrosis factor alpha. Infect. Immun. 65:5191-5197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haslinger, B., S. Mandl-Weber, A. Sellmayer, and T. Sitter. 2001. Hyaluronan fragments induce the synthesis of MCP-1 and IL-8 in cultured human peritoneal mesothelial cells. Cell Tissue Res. 305:79-86. [DOI] [PubMed] [Google Scholar]
- 23.Hodge-Dufour, J., P. W. Noble, M. R. Horton, C. Bao, M. Wysoka, M. D. Burdick, R. M. Strieter, G. Trinchieri, and E. Pure. 1997. Induction of IL-12 and chemokines by hyaluronan requires adhesion-dependent priming of resident but not elicited macrophages. J. Immunol. 159:2492-2500. [PubMed] [Google Scholar]
- 24.Horton, M. R., C. M. McKee, C. Bao, F. Liao, J. M. Farber, J. Hodge-DuFour, E. Pure, B. L. Oliver, T. M. Wright, and P. W. Noble. 1998. Hyaluronan fragments synergize with interferon-gamma to induce the C-X-C chemokines mig and interferon-inducible protein-10 in mouse macrophages. J. Biol. Chem. 273:35088-35094. [DOI] [PubMed] [Google Scholar]
- 25.Kocks, C., E. Gouin, M. Tabouret, P. Berche, H. Ohayon, and P. Cossart. 1992. L. monocytogenes-induced actin assembly requires the actA gene product, a surface protein. Cell 68:521-531. [DOI] [PubMed] [Google Scholar]
- 26.McKee, C. M., C. J. Lowenstein, M. R. Horton, J. Wu, C. Bao, B. Y. Chin, A. M. Choi, and P. W. Noble. 1997. Hyaluronan fragments induce nitric-oxide synthase in murine macrophages through a nuclear factor κB-dependent mechanism. J. Biol. Chem. 272:8013-8018. [DOI] [PubMed] [Google Scholar]
- 27.Noble, P. W. 2002. Hyaluronan and its catabolic products in tissue injury and repair. Matrix Biol. 21:25-29. [DOI] [PubMed] [Google Scholar]
- 28.Noble, P. W., F. R. Lake, P. M. Henson, and D. W. Riches. 1993. Hyaluronate activation of CD44 induces insulin-like growth factor-1 expression by a tumor necrosis factor-alpha-dependent mechanism in murine macrophages. J. Clin. Investig. 91:2368-2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oertli, B., B. Beck-Schimmer, X. Fan, and R. P. Wuthrich. 1998. Mechanisms of hyaluronan-induced up-regulation of ICAM-1 and VCAM-1 expression by murine kidney tubular epithelial cells: hyaluronan triggers cell adhesion molecule expression through a mechanism involving activation of nuclear factor-kappa B and activating protein-1. J. Immunol. 161:3431-3437. [PubMed] [Google Scholar]
- 30.Portnoy, D. A., R. D. Schreiber, P. Connelly, and L. G. Tilney. 1989. Gamma interferon limits access of Listeria monocytogenes to the macrophage cytoplasm. J. Exp. Med. 170:2141-2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pure, E., and C. A. Cuff. 2001. A crucial role for CD44 in inflammation. Trends Mol. Med. 7:213-221. [DOI] [PubMed] [Google Scholar]
- 32.Rosen, H., S. Gordon, and R. J. North. 1989. Exacerbation of murine listeriosis by a monoclonal antibody specific for the type 3 complement receptor of myelomonocytic cells. Absence of monocytes at infective foci allows Listeria to multiply in nonphagocytic cells. J. Exp. Med. 170:27-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rothfuchs, A. G., D. Gigliotti, K. Palmblad, U. Andersson, H. Wigzell, and M. E. Rottenberg. 2001. IFN-alpha beta-dependent, IFN-gamma secretion by bone marrow-derived macrophages controls an intracellular bacterial infection. J. Immunol. 167:6453-6461. [DOI] [PubMed] [Google Scholar]
- 34.Rottenberg, M. E., A. Gigliotti Rothfuchs, D. Gigliotti, M. Ceausu, C. Une, V. Levitsky, and H. Wigzell. 2000. Regulation and role of IFN-gamma in the innate resistance to infection with Chlamydia pneumoniae. J. Immunol. 164:4812-4818. [DOI] [PubMed] [Google Scholar]
- 35.Schluter, D., E. Domann, C. Buck, T. Hain, H. Hof, T. Chakraborty, and M. Deckert-Schluter. 1998. Phosphatidylcholine-specific phospholipase C from Listeria monocytogenes is an important virulence factor in murine cerebral listeriosis. Infect. Immun. 66:5930-5938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schmits, R., J. Filmus, N. Gerwin, G. Senaldi, F. Kiefer, T. Kundig, A. Wakeham, A. Shahinian, C. Catzavelos, J. Rak, C. Furlonger, A. Zakarian, J. J. Simard, P. S. Ohashi, C. J. Paige, J. C. Gutierrez-Ramos, and T. W. Mak. 1997. CD44 regulates hematopoietic progenitor distribution, granuloma formation, and tumorigenicity. Blood 90:2217-2233. [PubMed] [Google Scholar]
- 37.Shen, Y., M. Naujokas, M. Park, and K. Ireton. 2000. InIB-dependent internalization of Listeria is mediated by the Met receptor tyrosine kinase. Cell 103:501-510. [DOI] [PubMed] [Google Scholar]
- 38.Skelton, T. P., C. Zeng, A. Nocks, and I. Stamenkovic. 1998. Glycosylation provides both stimulatory and inhibitory effects on cell surface and soluble CD44 binding to hyaluronan. J. Cell Biol. 140:431-446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Skoudy, A., J. Mounier, A. Aruffo, H. Ohayon, P. Gounon, P. Sansonetti, and G. Tran Van Nhieu. 2000. CD44 binds to the Shigella IpaB protein and participates in bacterial invasion of epithelial cells. Cell Microbiol. 2:19-33. [DOI] [PubMed] [Google Scholar]
- 40.Tammi, M. I., A. J. Day, and E. A. Turley. 2002. Hyaluronan and homeostasis: a balancing act. J. Biol. Chem. 277:4581-4584. [DOI] [PubMed] [Google Scholar]
- 41.Termeer, C. C., J. Hennies, U. Voith, T. Ahrens, J. M. Weiss, P. Prehm, and J. C. Simon. 2000. Oligosaccharides of hyaluronan are potent activators of dendritic cells. J. Immunol. 165:1863-1870. [DOI] [PubMed] [Google Scholar]
- 42.Turley, E. A., P. W. Noble, and L. Y. Bourguignon. 2002. Signaling properties of hyaluronan receptors. J. Biol. Chem. 277:4589-4592. [DOI] [PubMed] [Google Scholar]
- 43.Unanue, E. R. 1997. Why listeriosis? A perspective on cellular immunity to infection. Immunol. Rev. 158:5-9. [DOI] [PubMed] [Google Scholar]
- 44.van der Voort, R., T. E. Taher, V. J. Wielenga, M. Spaargaren, R. Prevo, L. Smit, G. David, G. Hartmann, E. Gherardi, and S. T. Pals. 1999. Heparan sulfate-modified CD44 promotes hepatocyte growth factor/scatter factor-induced signal transduction through the receptor tyrosine kinase c-Met. J. Biol. Chem. 274:6499-6506. [DOI] [PubMed] [Google Scholar]
- 45.Vazquez-Boland, J. A., M. Kuhn, P. Berche, T. Chakraborty, G. Dominguez-Bernal, W. Goebel, B. Gonzalez-Zorn, J. Wehland, and J. Kreft. 2001. Listeria pathogenesis and molecular virulence determinants. Clin. Microbiol. Rev. 14:584-640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang, B., B. L. Yang, R. C. Savani, and E. A. Turley. 1994. Identification of a common hyaluronan binding motif in the hyaluronan binding proteins RHAMM, CD44 and link protein. EMBO J. 13:286-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zheng, Z., S. Katoh, Q. He, K. Oritani, K. Miyake, J. Lesley, R. Hyman, A. Hamik, R. M. Parkhouse, A. G. Farr, et al. 1995. Monoclonal antibodies to CD44 and their influence on hyaluronan recognition. J. Cell Biol. 130:485-495. [DOI] [PMC free article] [PubMed] [Google Scholar]