Abstract
Apoptotic host cell death is a critical determinant in the progression of microbial infections and outcome of resultant diseases. The potentially fatal human infection caused by Rickettsia rickettsii, the etiologic agent of Rocky Mountain spotted fever, involves the vascular endothelium of various organ systems of the host. Earlier studies have shown that survival of endothelial cells (EC) during this infection depends on their ability to activate the transcription factor nuclear factor κB (NF-κB). Here, we investigated the involvement of caspase cascades and associated signaling pathways in regulation of host cell apoptosis by NF-κB. Infection of cultured human EC with R. rickettsii with simultaneous inhibition of NF-κB induced the activation of apical caspases 8 and 9 and also the executioner enzyme, caspase 3, whereas infection alone had no significant effect. Inhibition of either caspase-8 or caspase-9 with specific cell-permeating peptide inhibitors caused a significant decline in the extent of apoptosis, confirming their importance. The peak caspase-3 activity occurred at 12 h postinfection and led to cleavage of poly(ADP-ribose) polymerase, followed by DNA fragmentation and apoptosis. However, the activities of caspases 6 and 7, other important downstream executioners, remained unchanged. Caspase-9 activation was mediated through the mitochondrial pathway of apoptosis, as evidenced by loss of transmembrane potential and cytoplasmic release of cytochrome c. These findings suggest that activation of NF-κB is required for maintenance of mitochondrial integrity of host cells and protection against infection-induced apoptotic death by preventing activation of caspase-9- and caspase-8-mediated pathways. Targeted inhibition of NF-κB may therefore be exploited to enhance the clearance of infections with R. rickettsii and other intracellular pathogens with similar survival strategies.
Endothelial cells (EC), an important component of the vessel wall, occupy a strategic location between the blood and extravascular space and participate in the regulation of diverse functions, including vascular tone, angiogenesis, inflammatory responses, and maintenance of normal hemostasis. The rickettsial disease Rocky Mountain spotted fever is caused by the obligatory intracellular bacterium Rickettsia rickettsii, and the pathologic sequelae are primarily due to infection-induced changes in EC properties (43). During rickettsioses caused by R. rickettsii and Rickettsia conorii, EC display an “activated” phenotype, with changes in von Willebrand factor release and adhesive properties for platelets (31, 33), gene expression for proinflammatory and procoagulant proteins (9, 16, 34-36), and activation of NF-κB (37).
NF-κB is a ubiquitous transcriptional factor that regulates cell growth, cell-to-cell communication, migration, and amplification or spreading of primary pathogenic signals (3, 40). A number of viral and bacterial products induce the expression of early response genes in their eukaryotic host cells through the NF-κB family of transcription factors (15, 21, 24, 25). NF-κB is retained in the cytoplasm by binding to inhibitors of κB (IκB) proteins, of which IκBα is the most abundant and most thoroughly characterized. The classical signaling pathway for NF-κB activation encompasses phosphorylation of IκBα on two specific serine residues (Ser 32 and Ser 36) by the IκB kinase complex, followed by polyubiquitination and degradation by the 26S proteasome. Increasing evidence suggests that NF-κB can serve as either a pro- or antiapoptotic mediator (17). In this context, it plays an essential role in protection from apoptosis induced by tumor necrosis factor alpha (TNF-α), radiation, and chemotherapy (5, 44, 47). With an in vitro model system, our laboratory has shown that NF-κB-dependent inhibition of apoptosis is critical for the survival of host cells during R. rickettsii infection, enabling the cell to persist as a site for bacterial replication (8).
The caspase family of proteases mediate many features of apoptosis. These enzymes are synthesized and maintained intracellularly as catalytically inert proenzymes and comprise a large and a small subunit with a variable amino-terminal prodomain. Their activation requires loss of the prodomain by catalytic cleavage of a carboxyl-terminal aspartate, followed by heterodimerization of the large and small subunits into an active enzyme (27). Many apoptotic responses are initiated by activation of apical caspase-8 or caspase-9, the former by the tumor necrosis factor receptor family or Fas (6, 26), and the latter by release of cytochrome c following mitochondrial damage (39). Activity of either results in activation of downstream or effector caspases, such as caspase-3, caspase-6, and caspase-7. These in turn cause cellular disassembly by cleavage of other death substrates and nucleoproteins, including poly(ADP-ribose) polymerase (PARP) (20), lamin (28), and the caspase-activated DNase/DNA fragmentation factor (CAD/DFF) complex (22). The result is fragmentation of cellular DNA and cell death (42).
The present study was undertaken to characterize the specific apoptotic mechanisms activated in response to R. rickettsii and to investigate their regulation by infection-induced NF-κB. The results suggest that proapoptotic signaling during R. rickettsii infection involves activation of effector caspase-3 via upstream activation of caspases 8 and 9. Also, the antiapoptotic functions of activated NF-κB appear to be mediated through suppression of the caspase cascade and preservation of mitochondrial integrity.
MATERIALS AND METHODS
Cell culture.
Cultures of human umbilical vein EC were established as previously described (13, 45). Cells were grown at 37°C in antibiotic-free McCoy's 5a medium (Flow Laboratories, McLean, Va.), supplemented with fetal bovine serum (20%, vol/vol), endothelial cell growth supplement (50 μg/ml; Collaborative Research Inc., Bedford, Mass.), l-glutamine, and heparin (100 μg/ml; Sigma Chemical Co., St. Louis, Mo.). Near-confluent cultures of EC at second passage were used for various experiments. Wild-type and IκBα dominant negative mutant-expressing T24 bladder carcinoma cells were grown in a humidified 5% CO2 incubator at 37°C until ≈80% confluent. The cells were cultured in RPMI 1640 medium (Life Technologies Inc., Rockville, Md.) supplemented with l-glutamine, geneticin (400 μg/ml; Life Technologies Inc.), and heat-inactivated fetal bovine serum (10%). Geneticin was withdrawn from the medium about 24 h prior to infection and/or treatment.
R. rickettsii infection and treatment of cultured cells.
A plaque-purified seed stock (1 × 107 to 5 × 107 PFU of R. rickettsii [Sheila Smith strain] per ml) was prepared from infected Vero cells as described earlier (8, 37). The endothelial or T24 carcinoma cells were infected with approximately 5 × 104 to 6 × 104 PFU of R. rickettsii diluted in 80 μl of culture medium for every square centimeter of cell culture area. After incubation for 2 h, the medium containing infective organisms was aspirated, and the cell monolayer was washed twice to remove Vero cell debris and then retained in fresh culture medium for the remainder of infection. Infection of EC plated on Thermanox coverslips (Nalge-Nunc International, Naperville, Ill.) was monitored in parallel by staining with polyclonal antirickettsia serum (Centers for Disease Control and Prevention, Atlanta, Ga.) by indirect immunofluorescence as described before (35, 45). Each cell displaying positive staining for at least two adherent and/or intracellular rickettsiae was counted as infected, and in a typical experiment, about 60 to 80% of the EC were infected at 6 h. The proteasome inhibitor MG132 (carbobenzoxy-l-leucyl-l-leucyl-l-leucinal) was from Peptide International (Louisville, Ky.). EC were exposed to MG132 (25 μM) for 30 min prior to infection. As reported previously, treatment with up to 50 μM MG132 had no significant effect on the initial rate or extent of infection (8, 29). In some experiments, incubation with staurosporine (2 μM) or etoposide (300 μM) was included as a positive control for induction of apoptosis.
Caspase activity assays.
EC were left uninfected or infected for different durations in the presence or absence of MG132 or treated with staurosporine or etoposide (positive controls for caspase assays). Cells were washed with ice-cold phosphate-buffered saline (PBS) total protein extracts were prepared, and protein contents of lysates were determined by the Bradford assay (Bio-Rad, Hercules, Calif.). Activity assays for caspase-3, caspase-6, and caspase-8 were performed with colorimetric assay kits (Calbiochem, San Diego, Calif.).
Western blot analyses.
Total protein extracts from EC or T24 bladder carcinoma cells were prepared in lysis buffer (50 mM HEPES [pH 7.0], 250 mM NaCl, 0.1% Nonidet P-40, 5 mM EDTA, 1 mM dithiothreitol, and 1 mM phenylmethylsulfonyl fluoride) supplemented with protease and phosphatase inhibitor cocktails (Sigma). After 15 min of incubation at 4°C, the lysates were cleared by centrifugation at 13,000 × g for 10 min. Equal amounts of total protein were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) on 12% gels and then transferred to nitrocellulose membranes (Bio-Rad). The membranes were probed with primary antibodies specific for human caspase-3, caspase-7, and cleaved PARP (Cell Signaling Technology, Beverly, Mass.). The protein-antibody complexes were visualized with peroxidase-conjugated secondary antibodies (Santa Cruz Biotechnology Inc., Santa Cruz, Calif.) and enhanced chemiluminescence detection system (Perkin Elmer Life Sciences, Boston, Mass.). For a loading control, the blots were subsequently stripped and probed with mouse monoclonal anti-α-tubulin antibody (human-reactive clone B-5-1-2; Accurate Chemical and Scientific Corporation, Westbury, N.Y.).
Immunofluorescent staining.
EC plated on gelatin-coated glass coverslips were infected with R. rickettsii in the presence or absence of NF-κB inhibitor or treated with an apoptosis-inducing agent. The cells were then washed with ice-cold PBS and fixed with either 3.7% (vol/vol) formaldehyde or 4% (wt/vol) paraformaldehyde for 20 min at 25°C. Following permeabilization with 0.5% Triton X-100 for 20 min at 25°C, cells were incubated with primary antibodies at 37°C for 30 min in a humidified chamber for specific detection of cleaved caspase-3, cleaved PARP, cleaved caspase-8, cleaved caspase-9 (1:50 dilution; Cell Signaling Technology), and cytochrome c (1:200 dilution; BD Pharmingen, San Diego, Calif.). The antibodies were routinely diluted in a blocking solution containing 2% ovalbumin in PBS. The immunofluorescent detection was performed by incubating with a rhodamine-conjugated goat anti-rabbit or anti-mouse IgG (Calbiochem, San Diego, Calif.) or Texas Red-conjugated anti-mouse IgG (Southern Biotechnology Associates Inc., Birmingham, AIla.), which were used at a dilution of 1:100. Finally, the coverslips were mounted on a glass slide with Biomedia gel mount and Fluoromount G (Electron Microscopy Sciences, Fort Washington, Pa.) and examined under a fluorescent microscope (Nikon Eclipse E-800) attached to a Spot digital image system. The images were analyzed with Windows-based Spot software, version 3.04 (Diagnostic Instruments Inc., Sterling Heights, Mich.).
In situ detection of DNA fragmentation.
The extent of apoptosis of EC was determined by a terminal deoxynucleotidyl transferase-mediated digoxigenin-dUTP nick-end labeling (TUNEL) assay with the ApopTag-Fluorescein in situ apoptosis detection kit (Intergen Company, Purchase, N.Y.) as described earlier (8). Briefly, the cells were fixed in 3.7% formaldehyde, rinsed with PBS, incubated with terminal deoxynucleotidyl transferase and digoxigenin-dUTP, and stained with fluorescein-conjugated antidigoxigenin antibody. The coverslips were mounted on a slide with gel mount solution, after which propidium iodide counterstain (3 μg/ml) was applied. The number of positively stained nuclei was determined by analysis of digitized images of TUNEL staining, which were captured with Spot image software and stored as TIFF files in Adobe Photoshop version 5.0. A minimum of 500 cells located in multiple, randomly selected fields were counted for each condition in three different sets of experiments, and data were calculated as the arithmetic mean values of percent TUNEL-positive cells.
Live-dead assay.
Viable and apoptotic cells were distinguished by the live-dead double staining kit (Oncogene Research Products, San Diego, Calif.) following the manufacturer's instructions. After infection with R. rickettsii or exposure to apoptosis inducers, the cells on coverslips were stained and observed under a fluorescent microscope to detect healthy cells, which capture the cell-permeating cytodye, fluorescing green, and the apoptotic cells, which capture both the cell-permeating cytodye and the nonpermeating propidium iodide (red).
Caspase-8 and caspase-9 inhibition studies.
EC were exposed for 30 min to the cell-permeating caspase-8 inhibitor Ac-Ala-Ala-Val-Ala-Leu-Leu-Pro-Ala-Val-Leu-Leu-Ala-Leu-Leu-Ala-Pro-Ile-Glu-Thr-Asp-CHO or the caspase-9 inhibitor Ac-Ala-Ala-Val-Ala-Leu-Leu-Pro-Ala-Val-Leu-Leu-Ala-Leu-Leu-Ala-Pro-Leu-Glu-His-Asp-CHO (Calbiochem), where Ac is acetyl and CHO is aldehyde. Following this, cells were infected with R. rickettsii in the presence or absence of MG132 and maintained in the presence of the inhibitor for caspase-8 or caspase-9 for the duration of the experiment. The cells were then fixed, and the extent of apoptosis was determined by TUNEL staining as described above.
Mitochondrial transmembrane potential change detection.
The Mito-Capture mitochondrial apoptosis detection kit (Alexis Biochemicals, San Diego, Calif.) was used to detect changes in the mitochondrial membrane potential of EC during infection and treatment with inducers of apoptosis. This assay is based on differential uptake of a cationic dye in the mitochondria of healthy versus apoptotic cells. Multiple, randomly selected fields for each experimental condition were analyzed as detailed above.
Statistical analysis.
Most of the data sets in this study were calculated as a percentage of the corresponding control levels. t test analysis was performed with two-tailed distribution and two-sample unequal variance, and the results were considered statistically significant at P ≤ 0.05.
RESULTS
Cleavage of downstream caspases.
The cell death pathway induced by R. rickettsii and the protective functions of NF-κB activation in host cell apoptosis were investigated with primary cultures of EC. MG132, a peptide aldehyde compound and competitive inhibitor of proteasome activity, was used because it specifically and completely inhibits the early phase of the Rickettsia-induced NF-κB response, and the onset of endothelial apoptosis due to this blockade is dependent on intracellular infection (8, 29). Although proteasome inhibitors are known to induce apoptosis in tumor cells by activating specific caspases (50, 51), it remains largely unknown whether similar or different interactions occur between the ubiquitin-proteasome pathway and components of the caspase cascade in cultured human EC. Therefore, we initially examined the contribution of downstream executioner caspases in EC infected with R. rickettsii (≈6 × 104 PFU/cm2) for 6, 12, 18, and 24 h in the presence and absence of MG132. Cell extracts were analyzed for the levels of caspase-3 and caspase-6 activity with Ac-DEVD-para-nitroaniline (pNA) and VEID-pNA as the respective specific chromogenic substrates.
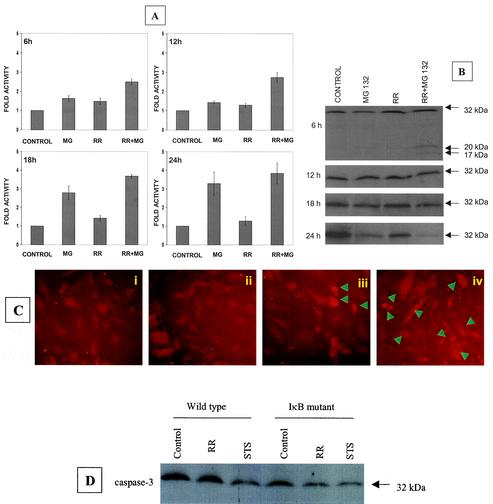
Although R. rickettsii alone caused a minimal induction of caspase-3 activity, incubation with MG132 resulted in a statistically significant increase in caspase-3 activity, most evident at 18 and 24 h. At 6 and 12 h post infection, the presence of MG132 resulted in a higher activity of caspase-3 in comparison to treatment with MG132 (P = 0.017 and 0.027, respectively) or infection with R. rickettsii alone (P = 0.013 and 0.016, respectively) (Fig. 1A). Western blot analysis with an antibody to detect both the native (procaspase) and active forms of caspase-3 revealed the presence of ≈20-kDa and ≈17-kDa forms of active caspase-3 after 6 h of R. rickettsii infection with MG132 (Fig. 1B). Depletion of the ≈32-kDa procaspase-3 was also evident at later times during infection and appeared to correspond with the induction of caspase-3 activity. These findings were supported by an in situ immunofluorescent staining assay with an antibody specific for cleaved caspase-3 (Fig. 1C).
FIG. 1.
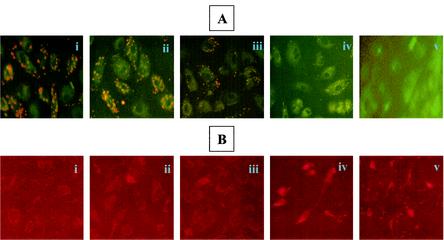
Activation of caspase-3 during R. rickettsii-induced apoptosis. EC were infected with R. rickettsii (≈5 × 104 PFU/cm2) for 2 h, after which extracellular bacteria were removed by washing and the cells were incubated further as indicated. NF-κB activation was attenuated by incubating with MG132 (25 μM), an inhibitor of proteasome function, for 30 min prior to and during the infection. (A) Analysis of caspase-3 activity in EC extracts, with DEVD-pNA as the chromogenic substrate. EC were left untreated (control), treated with MG132 (MG), or infected in the absence (RR) or presence (RR + MG) of MG132. Measurements of caspase activity were performed at 6 h, 12 h, 18 h, and 24 h. The baseline values (mean ± SEM) for untreated-uninfected cells at these times were 0.010 ± 0.001, 0.018 ± 0.003, 0.022 ± 0.004, and 0.025 ± 0.005 absorbance units, respectively. For comparison, the activity level in controls was assigned a value of 1, and the results are presented as mean ± SEM from three independent experiments. (B) Western blot analysis of caspase-3 at different times postinfection. (C) Indirect immunofluorescent staining for detection of cleaved caspase-3 at 12 h. The conditions were (i) uninfected EC, (ii) EC exposed to MG132, (iii) EC infected with R. rickettsii, and (iv) EC infected with R. rickettsii in the presence of MG132. Arrowheads indicate cells that are positive for the cleaved caspase-3 fragment and exhibit morphologic features of apoptosis. (D) Western blot analysis of cell extracts from wild-type (empty LXSN vector) and IκBα mutant-expressing T-24 cells, showing processing of procaspase-3 after 6 h of infection. Exposure of cells to staurosporine (STS) was included as a positive control. Equal amounts of protein were resolved on 12% polyacrylamide gels, blotted to a nitrocellulose membrane, probed with an anticaspase-3 antibody, and visualized by chemiluminescent detection.
Assay of enzymatic activity at 6, 12, 18, and 24 h postinfection and immunoblot analysis indicated no alteration in the activation pattern of caspase-6 during R. rickettsii infection. Also, cleavage of native caspase-7 to its active form was not noted during infection in either the presence or absence of NF-κB activation (not shown). The involvement of NF-κB activation in R. rickettsii-induced cell apoptosis was further confirmed with T24 bladder carcinoma cells, which express a phosphorylation mutant of IκBα. Figure 1D shows significant depletion of native caspase-3 in IκBα mutant cells after infection for 6 h with R. rickettsii. Probing with anti-α-tubulin antibody confirmed no significant variations in the loading of protein among different samples (not shown). Together, these results indicate activation of caspase-3 as the major effector mechanism responsible for apoptotic signaling triggered by Rickettsia infection in cells rendered incapable of NF-κB activation.
Cleavage of PARP
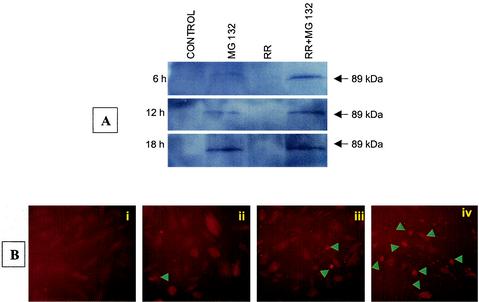
Caspase-3-mediated cleavage of the 116-kDa polypeptide PARP to its characteristic 89-kDa fragment prevents futile DNA repair cycles and is considered a distinct marker of apoptosis (20). Western blot analysis with an antibody specific for the 89-kDa fragment of PARP revealed that EC infection for up to 18 h had no effect on PARP cleavage. Treatment with MG132 (25 μM) resulted in minimal cleavage only after 12 h. Proteolysis of native PARP, however, was clearly evident in the cells infected for 6 and 12 h in the presence of MG132 (Fig. 2A). Densitometric analysis showed a significant increase in proteolysis of PARP in lysates from EC infected with R. rickettsii in combination with the inhibition of NF-κB activation. This pattern of PARP cleavage was consistent with the kinetics of induction of caspase-3 activity. Staining with Hoechst 33342 dye and microscopic analysis confirmed that infection of cells with simultaneous exposure to MG132 caused nuclear condensation, chromatin margination, and oligonucleosomal DNA fragmentation (not shown). Further studies revealed elevation in the accumulation of cleaved PARP, as evidenced by enhanced staining of the 89-kDa fragment of PARP in NF-κB-blocked and R. rickettsii-infected cells (Fig. 2B).
FIG. 2.
Cleavage of PARP during R. rickettsii infection of EC. (A) Western blot analysis for detection of cleaved PARP at different times after infection with R. rickettsii in the presence and absence of the NF-κB inhibitor MG132. (B) Immunofluorescent staining of cleaved PARP at 12 h in (i) uninfected EC, (ii) MG132-treated cells, (iii) cells infected with R. rickettsii, and (iv) R. rickettsii-infected cells in the presence of MG132. Arrowheads point to cells positive for PARP cleavage and morphological changes characteristic of apoptotic death.
Detection of apoptotic cells.
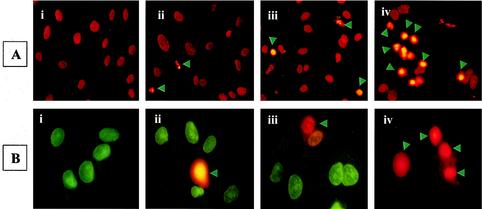
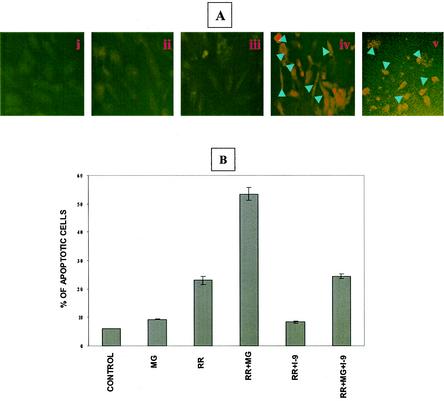
TUNEL analysis was used to further evaluate the extent of apoptosis (8), revealing that R. rickettsii infection resulted in extensive apoptosis in the presence of MG132 (Fig. 3A, panel iv). Quantitation demonstrated a significant increase in apoptotic cells during R. rickettsii infection in the presence of MG132 in comparison to those treated with MG132 or infected with R. rickettsii alone (P < 0.001). Double staining with a membrane-permeating cytodye and impermeant propidium iodide to discriminate between live and dead cells also yielded similar results (Fig. 3B). Analysis of data obtained from this study indicated that infection with continued blockade of NF-κB activation caused a significant population (ranging from about 32 to 38%) of EC to acquire both dyes, indicating loss of viability.
FIG. 3.
Induction of EC apoptosis during R. rickettsii infection. EC were infected for 12 h with or without concurrent inhibition of NF-κB response, followed by in situ detection of apoptosis. Panels represent (i) no treatment, (ii) incubation with MG132, (iii) infection with R. rickettsii, and (iv) infection in the presence of MG132. (A) TUNEL staining showing apoptotic nuclei with a condensed appearance (arrowheads). (B) Live-dead assay distinguishes normal and apoptotic cells. Healthy cells acquire green cell-permeating cytodye, while apoptotic cells (indicated by arrowheads) capture both the cytodye and the nonpermeating propidium iodide, resulting in orange fluorescence.
Involvement of caspase-8.
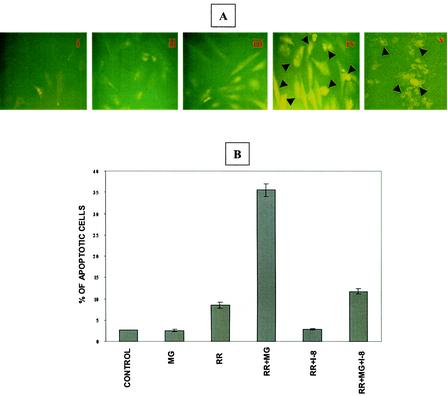
To determine whether activation of caspase-8 contributes to infection-induced apoptosis, in situ detection was carried out by immunofluorescent microscopy with an antibody specific for the 10-kDa fragment of cleaved caspase-8 (Fig. 4A). Caspase-8 was activated in EC infected in the presence of MG132 but not in cells either infected or exposed to MG132 alone. Morphologic evidence of apoptosis and positive staining for cleaved caspase-8 were evident in infected cells treated with MG132 and in positive controls treated with etoposide (Fig. 4A, panels iv and v).
FIG. 4.
Activation of caspase-8 during R. rickettsii-induced EC apoptosis. (A) Immunofluorescent detection of caspase-8 activation. The conditions in panels i through iv are identical to those in Fig. 3. Panel v is a positive control showing activation of caspase-8 in EC exposed to etoposide (300 μM). Arrowheads point to activated caspase-8-positive cells. (B) Effect of a specific inhibitor of caspase-8, IETD-CHO, on infection-induced apoptosis. EC were treated with 10 μM IETD-CHO (I-8) in the presence or absence of MG132, followed by infection with R. rickettsii. Quantitation of apoptosis was performed by TUNEL analysis as described in the text. A total of 500 cells were enumerated for each condition. Results are expressed as the mean percentage of apoptotic cells ± SEM from three independent experiments.
To confirm the involvement of this enzyme, a cell-permeating peptide inhibitor of caspase-8, IETD-CHO, was used. Quantitation following TUNEL staining showed that R. rickettsii infection caused apoptosis of 9% ± 1% and 36% ± 2% of total EC in the absence and presence of MG132, respectively (P < 0.005). The presence of the caspase-8 inhibitor, however, reduced the percentage of apoptotic cells under similar conditions to mean values of 3% and 12%, respectively (P < 0.005). In cells infected in the presence of MG132, caspase-8 activity as measured with IETD-pNA as a specific substrate, was found to be increased by 84% ± 37% in comparison to those treated with MG132 alone and by 70% ± 20% over R. rickettsii infection alone (not shown). Taken together, these results demonstrate that activation of caspase-8 is an important upstream signaling event for the induction of EC apoptosis during Rickettsia infection.
Involvement of mitochondria.
Mitochondria play an important role in programmed cell death, and many inducers of apoptosis trigger the release of mitochondrial cytochrome c into the cytoplasm (39). To explore if mitochondrial integrity was compromised during infection-induced apoptosis, we investigated changes in transmembrane potential (Δψm) by mitocapture staining. R. rickettsii infection for 12 h with concurrent inhibition of NF-κB activation resulted in the disruption of Δψm, whereas either infection or inhibition of NF-κB alone had no significant effect (Fig. 5A). As a positive control, EC were exposed to staurosporine (1 μM for 6 h), and this treatment caused nearly complete depolarization of the mitochondrial membrane (Fig. 5A, panel v).
FIG. 5.
Mitochondrial alterations during R. rickettsii-induced endothelial apoptosis. (A) Mitocapture staining to determine the status of mitochondrial transmembrane potential (Δψm) during EC infection. Representative images of (i) untreated and uninfected, (ii) MG132-treated, (iii) R. rickettsii-infected, (iv) infected and MG132-treated cells, and (v) staurosporine-treated (positive control) cells. The mitocapture dye yields fluorescence and accumulates in mitochondria of healthy cells. Note the absence of dye uptake in panels iv and v, indicating disruption of Δψm. (B) Immunofluorescence analysis of cytochrome c distribution under similar experimental conditions. Diffuse staining in iv and v indicates release of cytochrome c from mitochondria, whereas the punctate staining in panels i, ii, and iii indicates mitochondrial localization of cytochrome c.
The mitochondrial damage was also monitored by in situ staining for the release of cytochrome c into the cytoplasm, with identical treatment with staurosporine as a positive control. In comparison to conditions wherein cells were either infected or treated with MG132 alone, a significant amount of cytochrome c release occurred when EC were infected with R. rickettsii in the presence of MG132 (Fig. 5B). These findings indicate the involvement of mitochondrial signal mechanisms during apoptosis triggered by R. rickettsii infection with concurrent inhibition of NF-κB.
Activation of caspase-9.
The participation of mitochondria in apoptotic mechanisms often leads to the recruitment of procaspase-9 and activation of caspase-9 via the cytochrome c apoptosis protease-inducing factor-1 (Apaf-1) apoptosome pathway (7, 39). Therefore, we next investigated whether caspase-9 is involved in EC apoptosis due to infection with R. rickettsii. with a specific antibody against the 37-kDa fragment of cleaved caspase-9, EC subjected to MG132 treatment or infection with R. rickettsii alone exhibited minimal activation of caspase-9, whereas cleaved caspase-9 accumulated in EC infected in the presence of MG132 (Fig. 6A). The extent of caspase-9 activation was similar to that caused by staurosporine exposure, which was included as a positive control (Fig. 6A, panel v). We then investigated the effect of LEHD-CHO, a cell-permeating inhibitor of caspase-9, on the extent of apoptosis by scoring TUNEL-positive cells following inhibition of caspase-9 (Fig. 6B). LEHD-CHO caused a 30% reduction (P < 0.001) in the population of apoptotic EC infected in the presence of MG132.
FIG. 6.
Activation of caspase-9 during R. rickettsii-induced EC apoptosis. (A) Indirect immunofluorescence-based detection of caspase-9 activation. The experimental conditions in panels i through iv are identical to those in Fig. 3. Panel v represents a positive control showing activation of caspase-9 in EC exposed to staurosporine (2 μM). Arrowheads point to cells with activated caspase-9. (B) Effect of a specific inhibitor of caspase-9, LEHD-CHO, on infection-induced apoptosis. EC were treated with 10 μM LEHD-CHO (I-9) in the presence of absence of MG132, followed by infection with R. rickettsii. Quantitation of apoptosis was performed by TUNEL analysis of at least 500 cells for each condition. Results are expressed as the mean percentage of apoptotic cells ± SEM from three independent experiments.
DISCUSSION
The regulation of cellular apoptosis is a complex physiological process involving a multitude of trigger signals and regulatory proteins (7). Indeed, the activation or prevention of apoptosis may serve as a crucial determinant in the outcome of an infection. Recent efforts to understand the molecular basis of pathogenicity have led to the realization of unique capabilities of infectious bacteria to establish intricate interactions with the components of host cell signaling pathways and subvert normal host defense responses (11, 40). For example, manipulation of the host cell apoptotic machinery to ensure survival is critically important for an obligate parasite dependent on the viable host cell environment for its growth and replication (14). Organisms belonging to the spotted fever group Rickettsia species infect EC and alter their phenotype to subvert normal apoptotic mechanisms which could eliminate the intracellular infection (8).
The transcription factor NF-κB modulates the expression of genes involved in cell proliferation and programmed cell death, generally inhibiting apoptosis induced by cytokines and chemotherapeutic agents (5, 17, 21, 44, 47). We have previously reported that R. rickettsii infection of EC activates NF-κB, and pharmacologic or genetic inhibition of this host response results in enhanced apoptosis (8, 37). To identify the mechanisms underlying the antiapoptotic functions of NF-κB during Rickettsia infection, we focused on the role of apical and effector caspases. The executioner caspases 3, 6, and 7 perform distinct, nonredundant roles during the demolition phase of apoptosis (32). The results presented project caspase-3 as the likely candidate to mediate infection-induced apoptosis of EC, as evidenced by both increased protease activity and proteolytic cleavage of the proenzyme to yield p20 and p17 subunits (Fig. 1 and 2). In agreement with the kinetics of apoptosis induction, the activity of caspase-3 appears to peak in cells infected with R. rickettsii for about 12 h and is accompanied by cleavage of PARP, an enzyme responsible for conversion of NAD to nicotinamide and protein-linked ADP-ribose polymers (20). Cleavage of procaspase-3 in T24 cells expressing a dominant negative mutant of IκBα but not in wild-type cells transfected with the empty vector further confirms activation of caspase-3 as the terminal event and the participation of NF-κB in the prevention of apoptosis.
Depending on the stimulus, the activation of caspase-3 during apoptosis can be regulated by multiple distinct pathways, culminating in the activation of apical caspase-8 or caspase-9. Ligation of death receptors such as Fas/CD95 initiates one such mechanism that governs the processing of procaspase-8 to caspase-8 (6, 7, 26). Another distinct pathway is mediated by alteration of mitochondrial integrity with disruption of membrane potential (Δψm) leading to the cytoplasmic release of apoptosis-inducing proteins, including cytochrome c. Binding of cytochrome c to Apaf-1 then stimulates the processing of procaspase-9 to yield active caspase-9 (7, 39). Our findings suggest that inhibition of NF-κB during Rickettsia infection results in the disruption of Δψm, causing generation of active caspase-9 and simultaneous induction of caspase-8 activity. Although inhibition of these caspases reduced the extent of apoptosis, complete inhibition could not be achieved by either the caspase-8- or caspase-9-specific inhibitor alone. Therefore, it appears that both of these upstream mechanisms contribute to the activation of caspase-3 subsequent to infection with NF-κB blockade.
The ability of microbes to modulate apoptotic mechanisms may be a frequent and important contributor to pathologic effects and virulence. Several pathogenic bacteria and secreted toxins, e.g. Helicobacter pylori and staphylococcal alpha toxin, trigger apoptosis of host cells in vitro and in vivo via caspase-8 and caspase-9, followed by cleavage and activation of caspase-3 (2, 4, 30). Conversely, the ability of intracellular pathogens to establish strategies for the inhibition of host cell apoptosis mechanisms have also been recognized. Viruses reportedly carry genes encoding products capable of interference with the cellular apoptosis system (41). Recent studies have shown that the facultative intracellular bacterium Salmonella enterica serovar Typhimurium activates the Akt prosurvival pathway in epithelial cells via effector SigD, and lipoarabinomannan from virulent Mycobacterium tuberculosis upregulates a signaling pathway involved in the protection of macrophages during infection (23, 38). Similarly, intracellular infection with human granulocytic ehrlichiosis agent inhibits apoptosis of neutrophils (49), and the antiapoptotic activity of gram-negative Bartonella enables escape from host defenses, providing important clues to their ability to induce vascular proliferation in vivo (19).
The detailed mechanisms through which infecting organisms cause alterations in apoptosis likely vary but have been carefully examined for obligate intracellular bacteria belonging to the genus Chlamydia. Cells infected with C. trachomatis are profoundly resistant to exogenous and immunological agents capable of inducing apoptosis, and this protection correlates with the inhibition of mitochondrial cytochrome c release and downstream caspase activation (10). Furthermore, rescue of human monocytic Mono Mac-6 cells during C. pneumoniae infection requires activation of NF-κB (46), whereas no NF-κB-dependent factors are involved in the protection of HeLa cells against infection-induced apoptosis (12). Thus, different species of chlamydiae possess the ability to inhibit host cell apoptosis. It is very interesting, however, that the outcome of infection is dependent, at least in part, on specific characteristics of the cell type, including the state of transcriptional activation (14).
The molecular interactions between caspases and NF-κB play an integral role in the cellular response to soluble inflammatory stimuli, including TNF-α. Binding of TNF to its receptor stimulates both the caspase cascade and translocation of NF-κB to the nucleus (1), but inhibition of NF-κB activity results in a marked increase in TNF-α-induced cytotoxicity (5, 44, 47). Integration of alpha or beta interferon-induced NF-κB into a signaling pathway that promotes cell survival against a variety of apoptotic stimuli has also been observed (48), and induction of NF-κB translocation by human T-lymphotropic virus type 1 Tax protein contributes to inhibition of the caspase cascade (18). In the unique setting of intracellular parasitism, which utilizes infection of host cells by R. rickettsii to induce both proapoptotic and antiapoptotic signals, our results are in agreement with prior observations and indicate regulation of caspase cascade activation and mitochondrial apoptosis pathway by NF-κB. Furthermore, these results support the emerging concept of existence of a complex interrelationship between activities of regulatory transcription factors and survival mechanisms during host-parasite interactions.
In conclusion, the present study demonstrates that inhibition of NF-κB during R. rickettsii infection sensitizes host endothelial cells to apototic death. The mechanisms for this phenomenon involve activation of effector caspase-3 through upstream caspases 8 and 9, along with the loss of mitochondrial polarity and cytosolic release of cytochrome c. Further detailed investigations to identify and characterize the bacterial component(s) responsible and determination of the balance between host cell proapoptotic and antiapoptotic factors should provide new information useful towards understanding the progression of rickettsial infections. This may lead to the identification of special targets for the development of new and supplemental therapeutic interventions in human disease.
Acknowledgments
We thank Loel Turpin, Lisa Santucci, and Li Hua Rong for excellent technical assistance and Dawn Clifton for helpful discussions in the initial stages of the study.
This work was supported in part by grants HL 30616, AI 40689, and AI 17416 from the National Institutes of Health, Bethesda, Md.
Editor: J. N. Weiser
REFERENCES
- 1.Aggarwal, B. B. 2000. Apoptosis and nuclear factor-κB: a tale of association and dissociation. Biochem. Pharmacol. 60:1033-1039. [DOI] [PubMed] [Google Scholar]
- 2.Ashktorab, H., M. Neapolitano, C. Bomma, C. Allen, A. Ahmed, A. Dubois, T. Naab, and D. T. Smoot. 2002. In vivo and in vitro activation of caspase-8 and -3 associated with Helicobacter pylori infection. Microbes Infect. 4:713-722. [DOI] [PubMed] [Google Scholar]
- 3.Baldwin, A. S., Jr. 1996. The NF-κB and IκB proteins: new discoveries and insights. Annu. Rev. Immunol. 14:649-681. [DOI] [PubMed] [Google Scholar]
- 4.Bantel, H., B. Sinha, W. Domschke, G. Peters, K. Schulze-Osthoff, and R. U. Jänicke. 2001. α-Toxin is a mediator of Staphylococcus aureus-induced cell death and activates caspases via the intrinsic death pathway independently of death receptor signaling. J. Cell Biol. 155:637-647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beg, A. A., and D. Baltimore. 1996. An essential role for NF-κB in preventing TNF-α-induced cell death. Science 274:782-784. [DOI] [PubMed] [Google Scholar]
- 6.Boldin, M. P., T. M. Goncharov, Y. V. Goltseve, and D. Wallach. 1996. Involvement of MACH, a novel MORT1/FADD-interacting protease, in Fas/APO-1 and TNF receptor-induced cell death. Cell 85:803-815. [DOI] [PubMed] [Google Scholar]
- 7.Budihardjo, I., H. Oliver, M. Lutter, X. Luo, and X. Wang. 1999. Biochemical pathways of caspase activation during apoptosis. Annu. Rev. Cell Dev. Biol. 15:269-290. [DOI] [PubMed] [Google Scholar]
- 8.Clifton, D. R., R. A. Goss, S. K. Sahni, D. Van Antwerp, R. B. Baggs, V. J. Marder, D. J. Silverman, and L. A. Sporn. 1998. NF-κB-dependent inhibition of apoptosis is essential for host cell survival during Rickettsia rickettsii infection. Proc. Natl. Acad. Sci. USA 95:4646-4651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Drancourt, M., M. C. Alessi, P. Y. Levy, I. Juhan-Vague, and D. Raoult. 1990. Secretion of tissue-type plasminogen activator and plasminogen activator inhibitor by Rickettsia conorii- and Rickettsia rickettsii-infected cultured endothelial cells. Infect. Immun. 58:2459-2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fan, T., H. Lu, H. Hu, L. Shi, G. A. McClarty, D. M. Nance, A. H. Greenberg, and G. Zhong. 1998. Inhibition of apoptosis in Chlamydia-infected cells: blockade of mitochondrial cytochrome c release and caspase activation. J. Exp. Med. 187:487-496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Finlay, B. B., and P. Cossart. 1997. Exploitation of mammalian host cell functions by bacterial pathogens. Science 276:718-725. [DOI] [PubMed] [Google Scholar]
- 12.Fischer, S. F., C. Schwarz, J. Vier, and G. Häcker. 2001. Characterization of antiapoptotic activities of Chlamydia pneumoniae in human cells. Infect. Immun. 69:7121-7129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gimbrone, M. A., Jr., R. S. Cotran, and J. Folkman. 1974. Human vascular endothelial cells in culture. Growth and DNA synthesis. J. Cell Biol. 60:673-680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Häcker, G., and S. F. Fischer. 2002. Bacterial anti-apoptotic activities. FEMS Microbiol. Lett. 211:1-6. [DOI] [PubMed] [Google Scholar]
- 15.Hiscott, J., H. Kwon, and P. Génin. 2001. Hostile takeovers: viral appropriation of the NF-κB pathway. J. Clin. Investig. 107:143-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaplanski, G., N. Teysseire, C. Farnarier, S. Kaplanski, J. C. Lissitsky, J. M. Durand, J. Soubeyrand, C. A. Dinarello, and P. Bongrand. 1995. IL-6 and IL-8 production from cultured human endothelial cells stimulated by infection with Rickettsia rickettsii via a cell-associated IL-1α-dependent pathway. J. Clin. Investig. 96:2839-2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karin, M., and A. Lin. 2002. NF-κB at the crossroads of life and death. Nat. Immunol. 3:221-227. [DOI] [PubMed] [Google Scholar]
- 18.Kawakami, A., T. Nakashima, H. Sakai, S. Urayama, S. Yamasaki, A. Hida, M. Tsuboi, H. Nakamura, H. Ida, K. Migita, Y. Kawabe, and K. Eguchi. 1999. Inhibition of caspase cascade by HTLV-I-tax through induction of NF-κB nuclear translocation. Blood 94:3847-3854. [PubMed] [Google Scholar]
- 19.Kirby, J. E., and D. M. Nekorchuk. 2002. Bartonella-associated endothelial proliferation depends on inhibition of apoptosis. Proc. Natl. Acad. Sci. USA 99:4656-4661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lazebnik, Y. A., S. H. Kaufmann, S. Desnoyers, G. G. Poirier, and W. C. Earnshaw. 1994. Cleavage of poly(ADP-ribose) polymerase by a proteinase with properties like ICE. Nature 371:346-347. [DOI] [PubMed] [Google Scholar]
- 21.Li, Q., and I. M. Verma. 2002. NF-κB regulation in the immune system. Nat. Rev. Immunol. 2:725-734. [DOI] [PubMed] [Google Scholar]
- 22.Liu, X., H. Zou, C. Slaughter, and X. Wang. 1997. DFF, a heterodimeric protein that functions downstream of caspase-3 to trigger DNA fragmentation during apoptosis. Cell 89:175-184. [DOI] [PubMed] [Google Scholar]
- 23.Maiti, D., A. Bhattacharyya, and J. Basu. 2001. Lipoarabinomannan from Mycobacterium tuberculosis promotes macrophage survival by phosphorylating Bad through a phosphatidylinositol 3-kinase/ Akt pathway. J. Biol. Chem. 276:329-333. [DOI] [PubMed] [Google Scholar]
- 24.May, M. J., and S. Ghosh. 1997. Re1/NF-κB and IκB proteins: an overview. Semin. Cancer Biol. 8:63-73. [DOI] [PubMed] [Google Scholar]
- 25.Müller, J. M., H. W. Löms Ziegler-Heitbrock, and P. A. Baeuerle. 1993. Nuclear factor-kappa B, a mediator of lipopolysaccharide effects. Immunobiology 187:233-256. [DOI] [PubMed] [Google Scholar]
- 26.Muzio, M., A. M. Chinnaiyan, F. C. Kischkel, K. O'Rourke, A. Shevchenko, J. Ni, C. Scaffidi, J. D. Bretz, M. Zhang, R. Gentz, M. Mann, P. H. Krammer, M. E. Peter, and V. M. Dixit. 1996. FLICE, a novel FADD-homologous ICE/ CED-3-like protease, is recruited to the CD95 (Fas/APO-1) death-inducing signaling complex. Cell 85:817-827. [DOI] [PubMed] [Google Scholar]
- 27.Nicholson, D. W., and N. A. Thornberry. 1997. Caspases: killer proteases. Trends Biochem. Sci. 22:299-306. [DOI] [PubMed] [Google Scholar]
- 28.Oberhammer, F. A., K. Hochegger, G. Froschl, R. Tiefenbacher, and M. Pavelka, 1994. Chromatin condensation during apoptosis is accompanied by degradation of lamin A + B, without enhanced activation of cdc2 kinase. J. Cell Biol. 126:827-837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sahni, S. K., D. J. Van Antwerp, M. E. Eremeeva, D. J. Silverman, V. J. Marder, and L. A. Sporn. 1998. Proteasome-independent activation of nuclear factor κB in cytoplasmic extracts from human endothelial cells by Rickettsia rickettsii. Infect. Immun. 66:1827-1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shibayama, K., Y. Doi, N. Shibata, T. Yagi, T. Nada, Y. Iinuma, and Y. Arakawa. 2001. Apoptotic signaling pathway activated by Helicobacter pylori infection and increase of apoptosis-inducing activity under serum-starved conditions. Infect. Immun. 69:3181-3189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Silverman, D. J. 1986. Adherence of platelets to human endothelial cells infected by Rickettsia rickettsii. J. Infect. Dis. 153:694-700. [DOI] [PubMed] [Google Scholar]
- 32.Slee, E. A., C. Adrain, and S. J. Martin. 2001. Executioner caspase-3, -6, and -7 perform distinct, non-redundant roles during the demolition phase of apoptosis. J. Biol. Chem. 276:7320-7326. [DOI] [PubMed] [Google Scholar]
- 33.Sporn, L. A., R. J. Shi, S. O. Lawrence, D. J. Silverman, and V. J. Marder. 1991. Rickettsia rickettsii infection of cultured endothelial cells induces release of large von Willebrand factor multimers from Weibel-Palade bodies. Blood 78:2595-2602. [PubMed] [Google Scholar]
- 34.Sporn, L. A., S. O. Lawrence, D. J. Silverman, and V. J. Marder. 1993. E-selectin-dependent neutrophil adhesion to Rickettsia rickettsii-infected endothelial cells. Blood 81:2406-2412. [PubMed] [Google Scholar]
- 35.Sporn, L. A., P. J. Haidaris, R. J. Shi, Y. Nemerson, D. J. Silverman, and V. J. Marder. 1994. Rickettsia rickettsii infection of cultured human endothelial cells induces tissue factor expression. Blood 83:1527-1534. [PubMed] [Google Scholar]
- 36.Sporn, L. A., and V. J. Marder. 1996. Interleukin-1α production during Rickettsia rickettsii infection of cultured endothelial cells: potential role in autocrine cell stimulation. Infect. Immun. 64:1609-1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sporn, L. A., S. K. Sahni, N. B. Lerner, V. J. Marder, D. J. Silverman, L. C. Turpin, and A. L. Schwab. 1997. Rickettsia rickettsii infection of cultured human endothelial cells induces NF-κB activation. Infect. Immun. 65:2786-2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Steele-Mortimer, O., L. A. Knodler, S. L. Marcus, M. P. Scheid, B. Goh, C. G. Pfeifer, V. Duronio, and B. B. Finlay. 2000. Activation of Akt/protein kinase B in epithelial cells by the Salmonella typhimurium effector sigD. J. Biol. Chem. 275:37718-37724. [DOI] [PubMed] [Google Scholar]
- 39.Szewczyk, A., and L. Wojtczak. 2002. Mitochondria as a pharmacological target. Pharmacol. Rev. 54:101-127. [DOI] [PubMed] [Google Scholar]
- 40.Tato, C. M., and C. A. Hunter. 2002. Host-pathogen interactions: subversion and utilization of the NF-κB pathway during infection. Infect. Immun. 70:3311-3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thomson, B. J. 2001. Viruses and apoptosis. Int. J. Exp. Pathol. 82:65-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thornberry, N. A., and Y. Lazebnik. 1998. Caspases: enemies within. Science 281:1312-1316. [DOI] [PubMed] [Google Scholar]
- 43.Valbuena, G., H. M. Feng, and D. H. Walker. 2002. Mechanisms of immunity against rickettsiae: New perspectives and opportunities offered by unusual intracellular parasites. Microbes Infect. 4:625-633. [DOI] [PubMed] [Google Scholar]
- 44.Van Antwerp, D. J., S. J. Martin, T. Kafri, D. R. Green, and I. M. Verma. 1996. Suppression of TNF-α-induced apoptosis by NF-κB. Science 274:787-789. [DOI] [PubMed] [Google Scholar]
- 45.Wagner, D. D., J. B. Olmsted, and V. J. Marder. 1982. Immunolocalization of von Willebrand protein in Weibel-Palade bodies of human endothelial cells. J. Cell Biol. 95:355-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wahl, C., F. Oswald, U. Simnacher, S. Weiss, R. Marre, and A. Essig. 2001. Survival of Chlamydia pneumoniae-infected Mono Mac 6 cells is dependent on NF-κB binding activity. Infect. Immun. 69:7039-7045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang, C.-Y., M. W. Mayo, and A. S. Baldwin, Jr. 1996. TNF- and cancer therapy-induced apoptosis: potentiation by inhibition of NF-κB. Science 274:784-787. [DOI] [PubMed] [Google Scholar]
- 48.Yang, C. H., A. Murti, S. R. Pfeffer, L. Basu, J. G. Kim, and L. M. Pfeffer. 2000. IFN α/β promotes cell survival by activating NF-κB. Proc. Natl. Acad. Sci. USA 97:13631-13636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yoshiie, K., H. Y. Kim, J. Mott, and Y. Rikihisa. 2000. Intracellular infection by the human granulocytic ehrlichiosis agent inhibits human neutrophil apoptosis. Infect. Immun. 68:1125-1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yu, R., S. G. Ren, and S. Melmed. 2002. Proteasome inhibitors induce apoptois in growth hormone- and prolactin-secreting rat pituitary tumor cells. J. Endocrinol. 174:379-386. [DOI] [PubMed] [Google Scholar]
- 51.Zhang, X. M., H. Lin, C. Chen, and B. D. Chen. 1999. Inhibition of ubiquitin-proteasome pathway activates a caspase-3 like protease and induces Bc1-2 cleavage in human M-07e leukaemic cells. Biochem. J. 340:127-133. [PMC free article] [PubMed] [Google Scholar]