Abstract
The transcriptional induction of the GAL genes of Saccharomyces cerevisiae occurs when galactose and ATP interact with Gal3p. This protein-small molecule complex associates with Gal80p to relieve its inhibitory effect on the transcriptional activator Gal4p. Gal3p shares a high degree of sequence homology to galactokinase, Gal1p, but does not itself possess galactokinase activity. By constructing chimeric proteins in which regions of the GAL1 gene are inserted into the GAL3 coding sequence, we have been able to impart galactokinase activity upon Gal3p as judged in vivo and in vitro. Remarkably, the insertion of just two amino acids from Gal1p into the corresponding region of Gal3p confers galactokinase activity onto the resultant protein. The chimeric protein, termed Gal3p+SA, retains its ability to efficiently induce the GAL genes. Kinetic analysis of Gal3p+SA reveals that the Km for galactose is similar to that of Gal1p, but the Km for ATP is increased. The chimeric enzyme was found to have a decreased turnover number in comparison to Gal1p. These results are discussed in terms of both the mechanism of galactokinase function and that of transcriptional induction.
The yeast Saccharomyces cerevisiae utilizes galactose by means of the enzymes of the Leloir pathway. When yeast are grown in the absence of galactose, the genes encoding the enzymes of the pathway (the GAL genes) are transcriptionally inert (reviewed in refs. 1–3). If the cells are switched to medium in which galactose is the sole carbon source, then the GAL genes are rapidly induced and transcribed at high levels (4). The induction of the GAL genes is controlled by the interplay of three proteins—a transcriptional activator, Gal4p, a repressor, Gal80p, and an inducer, Gal3p. Induction appears to occur as a result of a galactose- and ATP-dependent interaction between Gal3p and Gal80p (5–8). This association results in the formation of a transcriptionally active Gal4p-Gal80p-Gal3p complex (9). It has been suggested that the association of Gal3p with Gal80p results in the movement of Gal80p from the activation domain of Gal4p to a different part of the protein (10). The location of this second site of Gal80p interaction on Gal4p remains unclear.
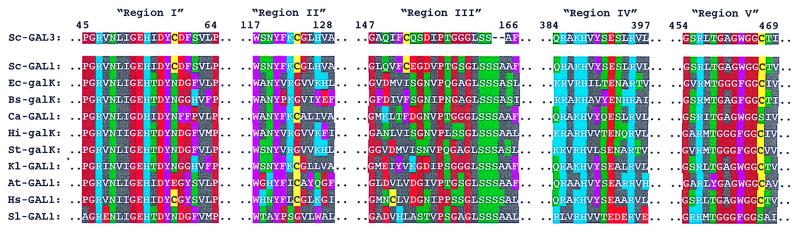
The first step of the Leloir pathway is the conversion of galactose to galactose-1-phosphate by galactokinase by Gal1p (11), the product of the GAL1 gene. Gal1p and Gal3p are highly homologous proteins (73% identity and 92% homology at the amino acid level). Unlike Gal1p, Gal3p does not possess a galactokinase activity (12). Gal1p is bifunctional in that it has galactokinase activity and is able to induce the expression of the GAL genes both in vivo (13) and in vitro (9), although approximately 40-fold less efficiently than Gal3p (9). Galactokinases are relatively well conserved throughout nature. For instance, Gal1p and Escherichia coli galactokinase, galK, share 50% amino acid homology. Galactokinases are, however, extremely highly conserved in five regions (Fig. 1). The functions of these regions have not been defined experimentally, but presumably they could be involved in galactose or ATP binding, or in promoting the catalytic reaction. All functional galactokinases contain an invariant GLSSSA(A/S)(F/L/I) motif within homology region III (Fig. 1). Based on sequence alignments alone, it appears that Gal3p has a truncated version of this motif, with GLSSAF being found at amino acids 161–166 of the protein.
Figure 1.
Sequence comparison of galactokinase molecules. The numbers at the top refer to the Gal3p amino acid sequence. Sc, Saccharomyces cerevisiae; Ec, Escherichia coli; Bs, Bacillus subtilis; Ca, Candida albicans; Hi, Haemophilus influenzae; St, Salmonella typhimurium; Kl, Kluyveromyces lactis; At, Arabidopsis thaliana; Hs, Homo sapiens; St, Streptomyces lividans. Amino acids have been colored according to their properties. Blue indicates positively charge amino acids (H, K, R), red indicates negatively charged residues (D, E), green indicates polar neutral residues (S, T, N, Q), grey indicates nonpolar aliphatics (A, V, L, I, M), and purple indicates nonpolar aromatic residues (F, Y, W). Brown is used to indicate proline and glycine, whereas yellow indicates cysteine.
To delineate the various functions of Gal3p (galactose binding, ATP binding, and interaction with Gal80p), we first created a series of deletion mutations of the protein and tested their ability to complement a yeast strain deleted for Gal3p function. We found that we were unable to remove more than 28 amino acids from the amino-terminal end, or 9 amino acids from the carboxyl-terminal end of the molecule without loss of function. We therefore swapped regions of Gal1p into Gal3p in an attempt to restore galactokinase function to this transcriptional inducer. Surprisingly, we found that the insertion of just two amino acids from Gal1p into Gal3p imparted galactokinase activity onto Gal3p. This chimeric protein still retains the ability to induce transcription of the GAL genes both in vivo and in vitro. We discuss these results both in terms of galactokinase function and the implications for transcriptional induction.
Materials and Methods
Strains and Media.
E. coli strain DH5α was used for all DNA manipulations. The following S. cerevisiae strains were used; yeast nuclear extract was prepared from BJ2168 (MATα ura3, leu2, trp1, gal2, prb1, pep4, prc1) grown in yeast extract/peptone/dextrose media (14), proteins were overproduced in strain MC2 (MATa trp1 ura3–52 leu2–3 prc1–407 prb1-112 pep4-3), and genetic analysis was performed in strain JPY5 (MATα ura3–52 his3Δ200 leu2Δ1 trp1Δ63 lys2Δ385) (15). Disruption of GAL1, GAL3, and GAL80 from JPY5 was achieved by using PCR-generated blaster cassettes (16).
Plasmid Construction.
All plasmid manipulations were performed as described by Sambrook et al. (17). GAL3, GAL1, and all derivatives expressed in vivo from the GAL3 promoter and terminator (−606 to −1 and +1,564 to +2,019) were carried on a yeast 2 μ plasmid with the HIS3 auxotrophic marker (pJP139). Deletion derivatives of GAL3 were all prepared from the parental vector pAP25. The Δ2–28, 2–40, 180–223, 198–223, 198–243, 428–520, 500–520, and 511–520 GAL3 deletions were all constructed by PCR-mediated mutagenesis (oligonucleotide sequences are available upon request). All other deletion derivatives of the GAL3 gene were constructed by using naturally occurring restriction sites (details available upon request). GAL3, GAL1, and all derivatives expressed in vivo from the GAL1 promoter were cloned into the 2 μ, HIS3 expression vector pYX223 (CLONTECH). All GAL3 and GAL1 chimeras were constructed by recombinant PCR (oligonucleotide sequences are available upon request). For high-level protein expression and purification from yeast cells, genes were cloned into pYEX-BX as described (9). All plasmids were sequenced to confirm the fidelity of the manipulations and the PCR (data not shown).
Northern and Western Blotting.
Total RNA was isolated from 5 ml of yeast cells grown in the appropriate medium to an optical density (A600) of 1.0 by using an RNAeasy extraction kit (Qiagen, Chatsworth, CA). RNA was then run on a formamide-agarose gel prior to alkaline transfer to Zetaprobe (Bio-Rad) according to manufacturer's instructions. Blots were hybridized sequentially with a GAL10 probe (+1 to +2,077) and an 18S RNA probe (+279 to +1,405). Double-stranded DNA probes were prepared by using random-primed labeling as described (18).
For Western blotting, 5 ml of yeast cells were grown in the appropriate medium to an optical density (A600) of 1.0. Cells were harvested, washed with 1 ml of sterile water, and the pellet placed in a boiling water bath for 1 min. The pellet was resuspended in 0.2 ml of Laemmli buffer, and 0.2 g of glass beads were added. The tube was vortexed for 90 s and boiled for 3 min twice. The supernatant was recovered, and 5 μl run on 12% SDS/PAGE. Proteins were transferred to nitrocellulose membranes that were subsequently incubated with either rabbit polyclonal Gal3p antibodies or a mouse-derived monoclonal antibody.
Galactokinase Assays.
Galactokinase activity was measured by using an enzyme-linked assay system (19). Briefly, reaction mixtures (150 μl) were set up in microtiter wells containing the following: 20 mM Hepes (pH 8.0), 150 mM NaCl, 5 mM MgCl2, 400 μM phosphoenol pyruvate, 1 mM DTT, 1 mM NADH, 1.1 units pyruvate kinase, 1.5 units lactic dehydrogenase (PK/LDH; Sigma). Reactions were supplemented with various concentrations of galactose (0.01–5 mM), ATP (0.005–5 mM), and Gal1p, Gal3p, or Gal3p+SA (0.72–680 nM). The plates were incubated at 30°C, and the decrease in absorbance at 340 nm was measured by using a Multiskan Ascent plate reader.
Results
Gal3p Is an Essentially Globular Protein.
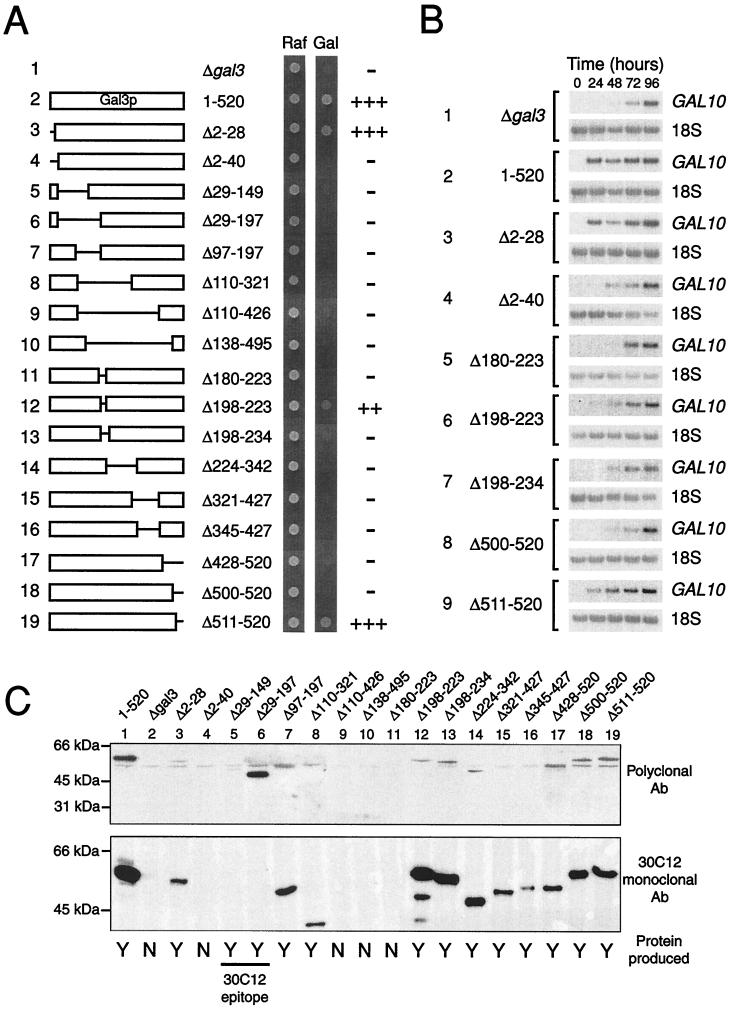
A series of deletion derivatives of Gal3p were constructed by using either naturally occurring restriction sites within the GAL3 gene, or by means of PCR-based mutagenesis. Each of these proteins was produced in a yeast strain in which the GAL3 gene had been deleted from the natural GAL3 promoter contained on a 2 μ plasmid. We had previously determined that the kinetics of galactose induction were impaired in comparison to wild-type yeast if the GAL3 gene was contained on an ARS-CEN plasmid (data not shown). Each of the deletion mutations was tested for its ability to grow on medium containing either raffinose or galactose as the sole carbon source (Fig. 2A). The phenotype of a gal3 deletion is slow growth on galactose. Therefore, growth was scored 2 days after plating. At this time, all of the deletion plasmids permitted growth on raffinose, but yeasts containing the empty plasmid (Fig. 2A, line 1) showed little growth on galactose. Plasmids bearing the wild-type GAL3 gene were able to grow well on galactose after 2 days (Fig. 2A, line 2). When incubated for 5 days, the growth of GAL3 or gal3 yeasts was indistinguishable, presumably because of leaky expression of GAL1 (13).
Figure 2.
The activity of Gal3p deletion mutations. (A) Plasmids expressing the wild-type Gal3p protein or the deletion derivative indicated were transformed into a yeast strain in which the GAL3 gene had been disrupted. Each of the strains were then grown on either raffinose (Raf) or galactose (Gal) as the sole carbon source, and cell growth was monitored after 3 days of growth at 30°C. (B) The effect of various Gal3p deletion derivatives on the expression of GAL10. Northern blot analysis of GAL10 and 18S RNA expression was monitored daily after the cells were transferred to a galactose-containing medium. (C) Western blot analysis of Gal3p and the deletion derivatives. A yeast strain deleted for GAL1, GAL3, and GAL80 was transformed with the appropriate Gal3p expression vector and grown on galactose for 2 days. Western blot analysis was performed on whole cell extracts using either Gal3p polyclonal antibodies or 30C12, a mouse monoclonal antibody raised against Gal3p.
Expression of deletion derivatives in which the amino-terminal 28 amino acids or the carboxyl-terminal 9 amino acids of Gal3p were removed gave rise to proteins that were able to complement the gal3 deletion as well as the wild-type protein (Fig. 2A, lines 3 and 19). Deletions further into the sequence resulted in proteins that were unable to complement the gal3 deletion (Fig. 2A, lines 4 and 18). We also found that a small internal deletion of Gal3p (the removal of amino acids 198–223) resulted in the formation of a partially functional protein (Fig. 2A, line 12). Extending this deletion either toward the amino- or carboxyl-terminal end of the molecule again resulted in a nonfunctional protein (Fig. 2A, lines 11 and 13). All other deletion mutations that we tested were unable to complement the gal3 deletion.
A number of the deletion mutations were tested for their ability to induce GAL10 mRNA expression by Northern blot analysis (Fig. 2B). Wild-type Gal3p induced full expression of GAL10 within 24 hr of induction by galactose (Fig. 2B, line 2). An empty plasmid that failed to complement the gal3 mutation (Fig. 2A, line 1) only induced GAL10 expression to its maximal level 4 days after induction (Fig. 2B, line 1). We found a correlation between the ability of a protein to complement the gal3 mutation (Fig. 2A) and its ability to rapidly induce GAL10 expression (Fig. 2B).
To show that the deletion derivatives outlined above were produced within the cell, we performed Western blot analysis (Fig. 2C). The detection of Gal3p by this method is complicated by the high degree of homology between Gal3p and Gal1p. Our own polyclonal antibodies raised against Gal3p, and those described previously (6), both showed a high degree of crossreactivity with Gal1p (data not shown). We therefore analyzed the production of the Gal3p deletion derivatives in a yeast stain deleted for the GAL1, GAL3, and GAL80 genes. The additional deletion of GAL80 ensured constitutive induction of the GAL3 promoter. We also performed Western blot analysis with a Gal3p monoclonal antibody (30C12) that showed no crossreactivity with Gal1p (data not shown). The production of the Δ2–40, Δ110–426, Δ138–495, and Δ180–223 Gal3p deletion derivatives were not detected in yeast cells (Fig. 2C, lanes 4, 9–11), and thus the inability of these proteins to complement the gal3 mutation can be explained by a lack of protein. All of the other deletion derivatives were expressed, although their accumulation proved to be variable (Fig. 2C). For instance, wild-type Gal3p and Gal3pΔ2–28 both complemented the gal3 mutation to the same extent (Fig. 2A, lines 2 and 3), yet the deletion derivative appears to be expressed at 10-fold lower levels than the wild-type protein (Fig. 2C, lanes 1 and 3). It is therefore not clear whether this difference is a true reflection of the levels of each protein or an artifact of performing Western blot analysis in a triple mutant strain. However, the inability of the majority of the deletion derivatives to complement the gal3 mutation cannot simply be explained as a result of lack of protein. We therefore conclude that Gal3p is an essentially globular protein that is sensitive to the removal of all but the smallest amino acid stretches.
Converting Gal3p into a Galactokinase.
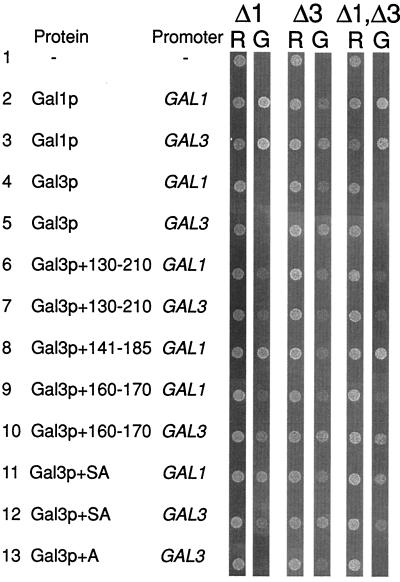
Given the lack of success in isolating smaller, functional versions of Gal3p, we attempted to address the issue of why Gal3p, which is so homologous to Gal1p, does not possess a galactokinase activity itself. We noted that Gal3p is the only galactokinase-like molecule that deviates within the highly conserved GLSSSAA motif of homology region III (Fig. 1). We therefore concentrated on this region of the protein as a potential area to explain the differences in activity of the two proteins. We constructed a series of chimeric genes in which regions of the GAL1 coding sequence were inserted into the GAL3 gene. These chimeras were then tested for their ability to act as a galactokinase (by complementing a gal1 deletion), as a transcriptional inducer (by complementing a gal3 deletion) or as bifunctional proteins (by complementing a gal1, gal3 double deletion). Proteins were either expressed from the GAL1 promoter or from the GAL3 promoter to eliminate the possibility that differing protein levels were interfering with the assays. The results of this analysis are shown in Fig. 3. Wild-type Gal3p, expressed either from its own promoter or from the GAL1 promoter, was unable to complement the gal1 deletion or the gal1, gal3 double deletion (Fig. 3, lines 4 and 5). Gal3p expressed from its own promoter was better able to complement the gal3 deletion than the same protein produced from the GAL1 promoter (Fig. 3, lines 4 and 5). Gal1p, on the other hand, was able to complement the mutations in all three deletion strains, again showing the bifunctional nature of this protein when it is overproduced (Fig. 3, lines 2 and 3). As observed for Gal3p, Gal1p expressed from the GAL3 promoter is better able to complement the gal3 deletion.
Figure 3.
The insertion of two amino acids into Gal3p converts it into a galactokinase. Plasmids expressing Gal1p, Gal3p, or the indicated chimera were transformed into yeast strains lacking GAL1 (Δ1), GAL3 (Δ3), or both GAL1 and GAL3 (Δ1,Δ3). Cells were then plated onto media containing either raffinose (R) or galactose (G) as the sole carbon source. Growth was monitored after 3 days incubation at 30°C. The chimeras are numbered to indicate the amino acids from Gal3p that have been replaced by the corresponding sequences from Gal1p.
We found that the insertion of homology region III from Gal1p into Gal3p was sufficient to grant the resulting chimeric protein with galactokinase activity (Fig. 3, lines 6–10). Indeed, the insertion of the two amino acids “missing” from Gal3p in homology region II (the insertion of a serine and an alanine residue at position 164 of Gal3p) allows the resultant protein to complement the gal1, the gal3, and the gal1, gal3 deletions (Fig. 3, lines 11 and 12). The insertion of only an alanine residue at position 164 of Gal3p resulted in a protein that still functioned as Gal3p but was unable to complement the gal1 mutations (Fig. 3, line 13). We also inserted either a serine residue at position 164 of Gal3p or two alanine residues. Neither of these proteins was able to complement the deletion in gal1, but they also did not complement the mutation in gal3, suggesting that these insertions may compromise the overall structure of the protein or are simply unstable (data not shown).
Activity of Gal3p+SA in Vitro.
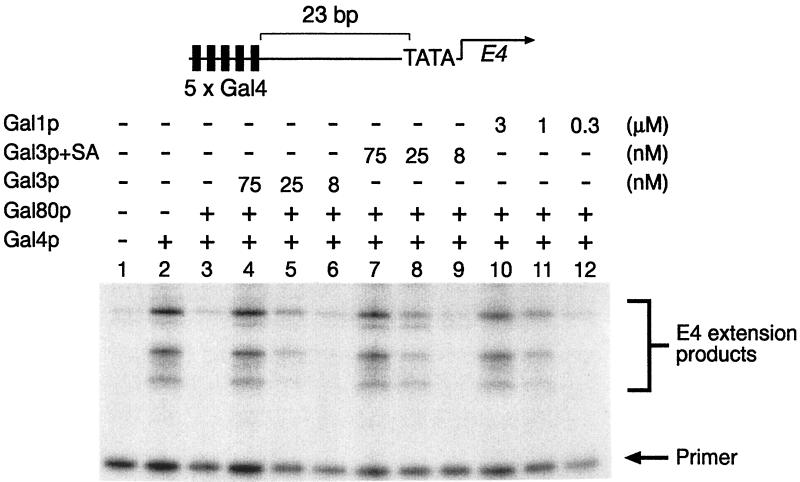
We overproduced Gal3p+SA in yeast cells and purified it to homogeneity (data not shown). We then tested the ability of this protein to function either as a transcriptional inducer in vitro (Fig. 4) or as a galactokinase (Table 1). Using in vitro transcription assays, Gal4p (amino acids 1–93 fused to 768–881) was able to induce transcription approximately 10-fold from a template containing five Gal4p-binding sites upstream of the E4 TATA-box (Fig. 4, lanes 1 and 2). This activation was completely inhibited by an equimolar amount of Gal80p (Fig. 4, lane 3). As observed previously (9), Gal3p or Gal1p are able to restore Gal4p-mediated transcription even in the presence of Gal80p (Fig. 4, lanes 4–6 and 10–12) with approximately 40-fold more Gal1p required to induce transcription to the same level as Gal3p. In these assays, Gal3p+SA behaved indistinguishably from wild-type Gal3p (Fig. 4, lanes 7–9), suggesting that the chimeric protein retained its ability to efficiently induce the Gal4p-Gal80p complex.
Figure 4.
The activity of Gal3p+SA in vitro. In vitro transcription and primer extension reactions were performed as described previously (9). All reactions contained 1.5 mM galactose and 1 mM ATP and, where indicated, 3 nM Gal4p (amino acids 1–93 fused to 768–881), 3 nM Gal80p and either Gal3p (75, 25, and 8 nM in lanes 4, 5, and 6, respectively), Gap3p+SA (75, 25, and 8 nM in lanes 7, 8, and 9, respectively), or Gal1p (3,000, 1,000, and 333 nM in lanes 10, 11, and 12 respectively).
Table 1.
The galactokinase activity of Gal1p, Gal3p, and Gal3p+SA
| Protein |
Km (mM)
|
Turnover number (s−1) | |
|---|---|---|---|
| Galactose | ATP | ||
| Gal1p | 0.25 ± 0.09 | 0.020 ± 0.003 | 20 |
| Gal3p | ND | ND | 0 |
| Gal3p+SA | 0.21 ± 0.06 | 0.17 ± 0.02 | 0.05 |
The galactokinase activity of each protein was measured by using an enzyme-linked assay described in Materials and Methods. Kinetic parameters were determined by using both double reciprocal and Hanes plots (22) and represent the average of at least four separate experiments. ND, no detectable activity.
We investigated the galactokinase activity of Gal1p, Gal3p, and Gal3p+SA. Using an enzyme-linked assay, described in Materials and Methods, both Gal1p and Gal3p+SA displayed Michaelis–Menten kinetics (data not shown). The values we obtained for the Km of our histidine-tagged Gal1p for either galactose and ATP are similar to those reported for the wild-type enzyme (0.25 mM and 0.02 mM for galactose and ATP, respectively, compared with 0.6 mM and 0.15 mM, respectively, previously reported (11). Consistent with previous data (12), we have been unable to detect galactokinase activity of Gal3p using this or other assays (Table 1, and data not shown). The Km for galactose of Gal3p+SA is very similar to that observed for Gal1p (Table 1). However, the Km for ATP is greatly increased for the chimeric protein (0.17 mM compared with 0.02 mM for Gal1p; Table 1). The calculated turnover number for the chimeric protein is considerably smaller than that calculated for Gal1p (Table 1). We calculated the turnover number of Gal1p to be 20 per second, while the chimeric protein had a turnover rate some 400-fold less at 0.05 per second.
Discussion
S. cerevisiae contains two galactokinase-like molecules, Gal1p and Gal3p, which share large regions of amino acid sequence identity and similarity (Fig. 1). The function of each protein is related, but distinct. Gal1p catalyzes the formation of galactose-1-phosphate from galactose and ATP, whereas Gal3p participates in a galactose- and ATP-dependent interaction with Gal80p to induce the GAL genetic switch (5–7, 9). Gal1p is somewhat bifunctional in that it can, when overproduced, also induce GAL gene expression (13), albeit weakly (9). Gal3p, on the other hand, possesses no galactokinase activity (12). Closely related yeasts, e.g., Kluyveromyces lactis, contain a single galactokinase-like molecule that functions both as a galactokinase and as a transcriptional inducer (20).
In efforts to assign function to, and possibly identify domains of, Gal3p, we initially undertook a deletion analysis of the protein (Fig. 2). We found that all but the smallest deletions from the amino- or carboxyl-terminal ends of the protein rendered the protein nonfunctional in terms of its ability to complement a gal3 deletion. Internal deletions were also largely nonfunctional, with the exception of the removal of amino acids 198–223 from the full-length protein, which resulted in the formation of a partially active Gal3p molecule (Fig. 2, line 12). Attempts to construct larger deletion derivatives based around the functional Δ198–223 deletion also proved unsuccessful and resulted in the formation of nonfunctional protein. We therefore conclude that Gal3p is likely to be essentially a globular protein whose structure is intolerant to deletions.
Given the high degree of homology between Gal3p and Gal1p, we utilized chimeric molecules to assign function to regions of each protein. Gal3p is not a galactokinase, but shares a high degree of homology to both Gal1p and other galactokinases (Fig. 1). Galactokinases are particularly well conserved in, what we term, homology region III. In particular, galactokinases contain a highly conserved GLSSSAA motif (amino acids 167–173 in Gal1p). Sequence alignments have shown that Gal3p, the only molecule of this set not to have galactokinase activity, contains an apparent deletion of two amino acids in this highly conserved motif (Fig. 1). The insertion of sequence segments from homology region III of Gal1p into the corresponding amino acid sequence of Gal3p resulted in the formation of chimera with galactokinase activity (Fig. 3, lanes 6–10). That is, the chimeric proteins were able to complement a deletion of gal1, a deletion of gal3, or a double deletion. This in vivo assay easily identified proteins possessing galactokinase activity through complementation of the gal1 deletion by a plasmid-borne copy of the chimeric gene. However, the isolation of the Gal3p induction phenotype by this method has proved difficult because a plasmid-borne copy of GAL1 will complement both the gal1 and gal3 deletions (Fig. 3, lines 3 and 4). This effect was observed when GAL1 was expressed from either the GAL3 or the GAL1 promoter and suggests that relatively modest levels of Gal1p overproduction will allow the protein to act as a transcriptional inducer.
The insertion of the missing two amino acids of homology region III from Gal1p into Gal3p (Gal3p+SA) converts Gal3p into a galactokinase. Gal3p+SA is able to complement gal1 and gal3 deletions in vivo (Fig. 3, lines 11 and 12) and is able to activate GAL gene expression in vitro indistinguishably from wild-type Gal3p (Fig. 4). During in vitro transcription assays, Gal3p+SA behaves like Gal3p in that a 25-fold molar excess over Gal80p is required to activate GAL gene expression, as seen previously (9). A much higher level of Gal1p was required to achieve the same level of transcriptional induction (Fig. 4, lanes 10–12). This shows that Gal3p+SA retains its ability to efficiently activate transcription while at the same time possessing a galactokinase activity. The insertion of a single alanine residue in place of SA (Gal3p+A) is insufficient to promote galactokinase function (Fig. 3, lane 13), but the protein is still able to complement the gal3 deletion in vivo. Our attempts to analyze a single serine insertion or that of two alanines have been hampered by the fact that these proteins do not complement the gal3 deletion, suggesting that they are misfolded or unstable.
Assays of galactokinase activity have revealed that Gal3p+SA is a relatively inefficient enzyme when compared with Gal1p (Table 1). We note, however, that the Km for galactose is very similar for Gal1p and Gal3p+SA. The Km for ATP is, however, greatly increased for the Gal3p+SA protein when compared with Gal1p (Table 1). This suggests that homology region III of Gal1p may be involved in ATP-binding or catalysis. Both Gal1p and Gal3p require both galactose and ATP for their mode of action. Although they perform seemingly different functions, our data suggest that Gal1p and Gal3p act through a similar mechanism. The mechanism of galactokinase action is not known, but it is reasonable to assume that binding of galactose and ATP, either as separate events or in concert, are followed by a catalysis step and then finally the release of products. Gal3p appears to be defective in its ability to perform the catalysis step of the reaction. What is the role of the serine and alanine residues in the catalysis reaction? Further kinetic and structural analyses are currently being used to address this question.
Several attempts have been made to map the interaction between Gal3p and Gal80p by searching for constitutive mutations of Gal3p (8, 21). The position of such mutations have been spread throughout the protein and have thus failed to identify a specific region of Gal3p responsible for the interaction with Gal80p. It is tempting to speculate that the binding of galactose and ATP to Gal1p and Gal3p induces a conformational change in the proteins. In the case of Gal1p, this is required for the catalysis reaction, perhaps by bringing the SA residues close to the site of catalysis, whereas in Gal3p the conformational change is required to promote association with Gal80p. Our data suggest that homology region III is required for the galactokinase reaction and that another region of the protein is responsible for the interaction with Gal80p.
Acknowledgments
We thank Cristina Merlotti and David Timson for many useful discussions and comments on the manuscript. We also thank Tony Maxwell (The University of Leciester) for advice on enzyme assays and Terri Attwood for help with the sequence alignment. This work was supported by grants from the Biotechnology and Biological Sciences Research Council and The Leverhulme Trust.
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Johnston M. Microbiol Rev. 1987;51:458–476. doi: 10.1128/mr.51.4.458-476.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lohr D, Venkov P, Zlantanova J. FASEB J. 1995;9:777–787. doi: 10.1096/fasebj.9.9.7601342. [DOI] [PubMed] [Google Scholar]
- 3.Reece R J, Platt A. BioEssays. 1997;19:1001–1010. doi: 10.1002/bies.950191110. [DOI] [PubMed] [Google Scholar]
- 4.St. John T P, Davis R W. J Mol Biol. 1981;152:285–315. doi: 10.1016/0022-2836(81)90244-8. [DOI] [PubMed] [Google Scholar]
- 5.Zenke F T, Engles R, Vollenbroich V, Meyer J, Hollenberg C P, Breunig K D. Science. 1996;272:1662–1665. doi: 10.1126/science.272.5268.1662. [DOI] [PubMed] [Google Scholar]
- 6.Suzuki-Fujimoto T, Fukuma M, Yano K-I, Sakurai H, Vonika A, Johnston S A, Fukasawa T. Mol Cell Biol. 1996;16:2504–2508. doi: 10.1128/mcb.16.5.2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yano K I, Fukasawa T. Proc Natl Acad Sci USA. 1997;94:1721–1726. doi: 10.1073/pnas.94.5.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blank T E, Woods M P, Lebo C M, Xin P, Hopper J E. Mol Cell Biol. 1997;17:2566–2575. doi: 10.1128/mcb.17.5.2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Platt A, Reece R J. EMBO J. 1998;17:4086–4091. doi: 10.1093/emboj/17.14.4086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sil A K, Alam S, Xin P, Ma L, Morgan M, Lebo C M, Woods M P, Hopper J E. Mol Cell Biol. 1999;19:7828–7840. doi: 10.1128/mcb.19.11.7828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schell M A, Wilson D B. J Biol Chem. 1977;252:1162–1166. [PubMed] [Google Scholar]
- 12.Bhat P J, Oh D, Hopper J E. Genetics. 1990;125:281–291. doi: 10.1093/genetics/125.2.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bhat P J, Hopper J E. Mol Cell Biol. 1992;12:2701–2707. doi: 10.1128/mcb.12.6.2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ohashi Y, Brickman J M, Furman E, Middleton B, Carey M. Mol Cell Biol. 1994;13:2731–2739. doi: 10.1128/mcb.14.4.2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu Y, Reece R J, Ptashne M. EMBO J. 1996;15:3951–3963. [PMC free article] [PubMed] [Google Scholar]
- 16.Lorenz M C, Muir R S, Lim E, McElver J, Weber S C, Heitman J. Gene. 1995;158:113–117. doi: 10.1016/0378-1119(95)00144-u. [DOI] [PubMed] [Google Scholar]
- 17.Sambrook J, Fritsch E F, Maniatis T. Molecular Cloning: A Laboratory Manual. Plainview, NY: Cold Spring Harbor Lab. Press; 1989. [Google Scholar]
- 18.Feinberg A P, Vogelstein B. Anal Biochem. 1984;137:266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- 19.Ali J A, Jackson A P, Howells A J, Maxwell A. Biochemistry. 1993;32:2717–2724. doi: 10.1021/bi00061a033. [DOI] [PubMed] [Google Scholar]
- 20.Meyer J, Walker-Jonah A, Hollenberg C P. Mol Cell Biol. 1991;11:5454–5461. doi: 10.1128/mcb.11.11.5454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vollenbroich V, Meyer J, Engels R, Cardinali G, Menezes R A, Hollenberg C P. Mol Gen Genet. 1999;261:495–507. doi: 10.1007/s004380050993. [DOI] [PubMed] [Google Scholar]
- 22.Cornish-Bowden A. Fundamentals of Enzyme Kinetics. London: Portland Press; 1995. [Google Scholar]