Abstract
The intracellular mechanism of vasoactive intestinal peptide (VIP)-induced, charybdotoxin (ChTx)-sensitive relaxation of longitudinal muscle of the distal colon of Wistar-ST rats was studied.
A single pulse or 100 pulses at 10 Hz of electrical field stimulation (EFS) induced rapid transient relaxation or that with a subsequent contraction of the longitudinal muscle in the presence of atropine and guanethidine, respectively.
Rp-8 bromo cAMPS, an inhibitor of cyclic AMP dependent protein kinase (PKA), at 30 μM inhibited the relaxations induced by EFS with a single or 100 pulses maximally by about 80 or 60%, respectively. It also inhibited VIP (300 nM)-induced relaxation by 82%.
VIP (100 nM–1 μM) increased the cyclic AMP content of longitudinal muscle myenteric plexus preparations obtained from the distal colon.
ChTx at 100 nM almost completely inhibited 8 bromo cyclic AMP-induced relaxation of the distal segments.
EFS with two or three pulses at 10 Hz induced inhibitory junction potentials consisting of two phases, rapid and subsequent slow hyperpolarization in the membrane potential of longitudinal smooth muscle cells. Rp-cAMPS, another inhibitor of PKA, inhibited the delayed slow hyperpolarization. It also inhibited the exogenously added VIP-induced hyperpolarization of the cell membrane.
Thus, the present study suggests that activation of PKA via activation of VIP receptors is associated with activation of ChTx-sensitive K+ channels in relaxation of longitudinal muscle of the distal colon of Wistar-ST rats.
Keywords: VIP, cyclic AMP, ChTx-sensitive K+ channel, nonadrenergic noncholinergic relaxation, rat distal colon
Introduction
We have suggested that the vasoactive intestinal peptide (VIP) and pituitary adenylate cyclase activating peptide (PACAP) are involved in nonadrenergic noncholinergic inhibitory responses of longitudinal muscle of the distal colon of Wistar-ST rats via activation of charybdotoxin (ChTx)- and apamin-sensitive K+ channels, respectively (Kishi et al., 1996). Recently we have also suggested that the activation of tyrosine kinase is involved in PACAP-induced relaxation of the muscle, ‘upstream of' the activation of apamin-sensitive K+ channels (Takeuchi et al., 1999). However, the intracellular mechanism of activation of ChTx-sensitive K+ channels after activation of VIP receptors is unknown.
It has been widely accepted that there is a correlation between an elevated cyclic AMP content and smooth muscle relaxation mediated by β-adrenoceptor stimulation (for review, see Kamm & Stull, 1985). In the gastrointestinal tract, the calcitonin gene related peptide (CGRP) was suggested to mediate relaxation of smooth muscle via elevation of the cyclic AMP content in isolated ileal cells (Rekik et al., 1997) and longitudinal muscle myenteric plexus preparations (Sun & Benishin, 1995) of guinea-pigs, and the internal anal sphincter of opossums (Rattan et al., 1991). VIP was also suggested to induce relaxation with a concomitant increase in cyclic AMP content in rat stomach (Ito et al., 1990) and dispersed stomach muscle cells (Bitar & Makhlouf, 1982; Murthy et al., 1993; Jin et al., 1993; Murthy & Makhlouf, 1995), in the lower oesophageal sphincter (Torphy et al., 1986) and in the internal anal sphincter of the opossum (Chakder & Rattan, 1993). Although the sequence of VIP-cyclic AMP-relaxant response was also suggested in dispersed ileal smooth muscle cells of guinea-pigs (Rekik et al., 1996), there is no direct evidence for this in the rat intestine.
Cyclic AMP dependent protein kinase (PKA) was suggested to activate Ca2+-activated K+ channels in myocytes from the circular layer of canine colon (Carl et al., 1991) and in Xenopus oocytes which expressed Drosophila Slo Kca channels (Esguerra et al., 1994) and in the myometrial cell line PHM1-41 (Meera et al., 1995). PKA was also suggested to activate ATP-sensitive K+ channels in coronary arterial smooth muscle cells (Wellman et al., 1998) and to activate the (4-aminopyridine-sensitive voltage-gated) delayed rectifier current in rabbit portal vein smooth muscle cells (Aiello et al., 1995). The aim of the present study was to determine whether cyclic AMP-PKA system is involved in VIP-mediated relaxation in the distal colon of Wistar-ST rats. Here we report the sequential coupling of activation of the VIP receptor, PKA and ChTx-sensitive K+ channels in relaxation of longitudinal muscle of the distal colon.
Methods
Male Wistar-ST strain rats (8–9 weeks-old) were purchased from Nippon SLC (Shizuoka, Japan). We indicated the strain of rats used simply as Wistar in previous papers (Suthamnatpong et al., 1993; 1994; Kishi et al., 1996), Wistar-ST rats belonging to a subclass of the Wistar strain, were used in those studies. The rats were lightly anaesthetized with ether and then stunned by a blow on the head and bled via the carotid arteries. The colon was then removed and placed in Tyrode solution consisting of (in mM): NaCl 137, KCl 2.7, CaCl2 1.8, MgCl2 1.1, NaH2PO4 0.42, NaHCO3 11.9 and glucose 5.6. The contents of the excised segments were gently flushed out with Tyrode solution. The descending colon which is attached by a mesentery to the small intestine was defined as the distal region.
Recording of responses of longitudinal muscle of rat distal colon to electrical field stimulation (EFS)
Segments of the distal colon (2.5–3.0 cm in length) were suspended in an organ bath containing 5 ml of Tyrode solution maintained at 37°C and bubbled with 95% O2:5% CO2. After an equilibration period of 30 min, responses of the longitudinal muscle to EFS with a single or trains of 100 pulses of 0.5-ms width at 30 V, 10-Hz frequency in the presence of atropine (1 μM) and guanethidine (5 μM), were recorded isotonically with a 10-min interval between tests. These concentrations of atropine and guanethidine did not have any significant effect on the tone or on the spontaneous contractile activity of the segments. The longitudinal muscle was subjected to a resting load of 0.75 g. The responses of the segments to EFS were nearly constant throughout the experiments of 120 min. EFS and drug-treatment were always started at the most relaxed state of resting spontaneous contractile activity: i.e. the bottom of the trace of resting activity.
In the experiments to examine the effect of Rp-8-bromo cAMPS on EFS-induced relaxation, the drug was added into an organ bath 30 min before EFS, since the magnitude of inhibition induced by 1 h-incubation was similar to that induced by 30 min period.
The extent of relaxation was expressed as the area under the line of resting tone that was drawn on the bottom of resting activity (Figure 1 dotted lines). The extent of relaxation induced by exogenous VIP was expressed as the depth of the relaxation.
Figure 1.
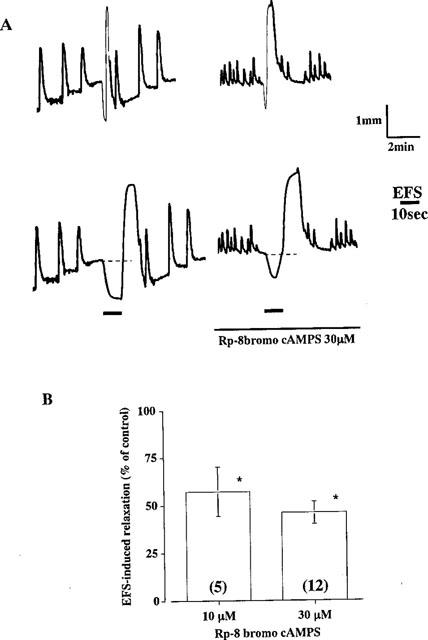
Effect of Rp-8-bromo cAMPS on EFS-induced relaxation of longitudinal muscle in the rat distal colon. (A) Relaxation was induced by EFS (100 pulses at 10 Hz) before and after treatment of the segments with 30 μM Rp-8-bromo cAMPS for 10 min. The continuous line indicates the presence of the drug (upper traces). After recording normal spontaneous movements, the chart was run fast immediately before the stimulation to make the relaxant response clear, (lower traces). Bold black lines indicate the duration of EFS for 10 s. (B) Relaxations induced by EFS (100 pulses) in the presence of Rp-8 bromo cAMPS are expressed as percentages of the control response before addition of the drug. Columns and bars represent means with standard errors in 5 (10 μM) or 9 (30 μM) experiments. *Significantly different from control, P<0.05 (Stutent's t-test).
Measurement of cyclic AMP content of longitudinal muscle myenteric plexus preparations
Longitudinal muscle preparations with adhering myenteric plexus were prepared from the distal colon as described by Paton & Zar (1968). The preparations (10–20 mg wet weight) were equilibrated for 20 min with Tyrode solution at 37°C and aerated with 95% O2 and 5% CO2. The preparations were then incubated with various concentrations of VIP (100 nM–1 μM) for 5 min. In another series of experiments to determine the effect of EFS on the cyclic AMP content, either end of the preparation was tied with threads allowing it to be hand held. This allowed easy mounting of the preparations between two platinum plate electrodes in a specially designed laboratory dish-type organ bath and quick removal from the electrodes at the end of EFS. The parameters for electrical field stimulation were the same as those used for measurement of the mechanical response. After the incubation or stimulation period, preparations were quickly frozen within a few seconds by putting them between two frozen metal plates on dry ice. Frozen preparations were then homogenized in 2 ml of 6.0 N trichloroacetic acid (TCA) solution. After removal of TCA with ether, the cyclic AMP contents were determined with a cyclic AMP assay kit (Amersham Japan, Tokyo). All determinations of cyclic AMP were carried out in the absence of phosphodiesterase inhibitor.
Recording of inhibitory junction potentials (i.j.ps) in longitudinal muscle cells of distal colon induced by EFS
The segments of the distal colon were mounted in a 1.5 ml organ bath maintained at 30°C and perfused continuously with Tyrode solution at a rate of 3 ml min−1. Atropine (1 μM) and guanethidine (5 μM) were added to the bathing solution throughout the experiment to block cholinergic and adrenergic responses, respectively. Membrane potentials were recorded with a conventional glass microelectrode filled with 3 M KCl with a resistance of 50–80 MΩ. The electrode was impaled into the longitudinal muscle cells of the superficial layer from the serosal side (Takewaki & Ohashi, 1977). Intramural nerves within the segment were stimulated with a pair of Ag wire electrodes, one on the serosal surface 1–2 mm from the impaled glass microelectrode and the other in the solution. The distance between the two electrodes was approximately 20 mm.
Drugs
Charybdotoxin, VIP, VIP10–28, pituitary adenylate cyclase activating peptide (PACAP) and PACAP6–38 were from the Peptide Institute, Osaka, Japan. Atropine sulphate was from Wako Pure Chemical, Osaka, Japan. 8-Bromoadenosine-3′,5′-cyclic monophosphate (8 bromo cAMP) and its Rp isomer, Rp-8 bromoadenosine-3′,5′-cyclic monophosphorothioate (Rp-8 bromo cAMPS) and Rp adenosine-3′,5′-cyclic monophosphorothioate (Rp-cAMPS) were from BIOLOG Life Sci, Inst., Bremen, Germany. Drugs were added to the organ bath in a volume of less than 1.0% of the bathing solution. These volumes of the vehicle of the drugs in redistilled water did not affect the spontaneous contractile activity or muscle tone.
Statistical analysis
Data are expressed as means±s.e.means. Statistical analysis of the data for two groups was carried out with a Student's t-test and for more than three groups with a one-way analysis of variance and Bonferroni's test. P values of less than 0.05 were regarded as significant.
Results
Effects of an inhibitor of PKA on EFS- or VIP-induced relaxation of longitudinal muscle in the distal colon of Wistar-ST rats
The distal colonic segments exhibited moderate spontaneous contractile activity and very small relaxation on EFS in the presence of 1 μM atropine and 5 μM guanethidine. However, the resting tone of the longitudinal muscle gradually increased during successive trains of EFS at 10 Hz at 10 min intervals. With progressive increase in the tone in this way, the segments began to exhibit clear relaxation on a single pulse of EFS and rapid transient relaxation with a subsequent contraction on pulse trains at 1–50 Hz for 10 s. Rp-8 bromo cAMPS, an inhibitor of PKA, inhibited relaxation induced by pulse trains at 10 Hz and inhibited it maximally by about 60% at 30 μM (Figure 1). Amplitude and duration of the relaxation were significantly inhibited, but the basal tone of the segments and induced contraction was not affected (Figure 1A). The rapid relaxation induced by a single pulse was also inhibited by the antagonist (78.2±1.8% inhibition at 30 μM Rp-8 bromo cAMPS, n=5). However, the contraction subsequent to the relaxation was not affected.
VIP (300 nM) induced gradual slow relaxation of longitudinal muscle of the segments and inhibited the spontaneous contractile activity. Rp-8 bromo cAMPS at 30 μM significantly inhibited 300 nM VIP-induced relaxation by 82.4±5.3% (n=5; Figure 2). Since these results suggest that VIP-mediated relaxation in the distal colon is associated with activation of PKA, we next examined whether the cyclic AMP content of the longitudinal muscle was elevated by EFS or VIP application.
Figure 2.
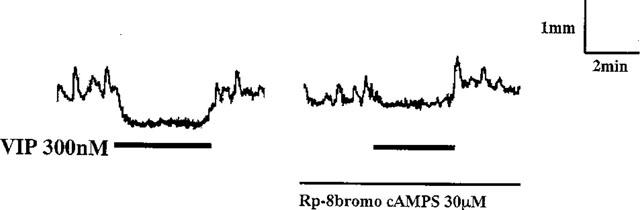
Effect of Rp-8 bromo cAMPS on VIP-induced relaxation. Relaxation was induced by 300 nM VIP before and after treatment of the segments with 30 μM Rp-8-bromo cAMPS for 10 min. The continuous line indicates the presence of the drug. Bold black lines indicate the duration of the presence of VIP.
Effects of VIP, PACAP and EFS on the cyclic AMP content of longitudinal muscle of the distal colon of Wistar-ST rats
Although whole segments of the distal colon were used to record mechanical activity, only the longitudinal muscle with the myenteric plexus was used for measurement of cyclic AMP; this approach was used to avoid contamination by circular smooth muscle. VIP increased the cyclic AMP content of preparations in a concentration dependent manner (Figure 3). EFS at 10 Hz for 10 s also increased the cyclic AMP content from 165.0±1.6 to 239.2±11.3 fmol mg−1 wet tissue and the increase was abolished in the presence of 3 μM VIP10–28 (Figure 3).
Figure 3.
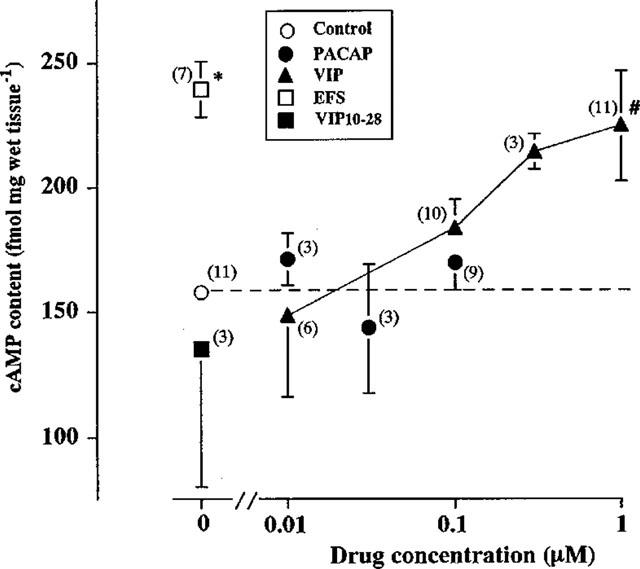
Effects of VIP, PACAP and EFS on the cyclic AMP content of longitudinal muscle myenteric plexus (LMMP) preparations of rat distal colon. LMMP preparations were incubated without or with the indicated concentrations of VIP or PACAP for 5 min, or stimulated with electrical pulses at 10 Hz for 10 s in the absence or presence of 3 μM VIP10–28. Values are means with standard errors for the numbers of experiments shown in parentheses. *,#Significance of difference from the value in the absence of agonist, P<0.05 (*Stutent's t-test, #Bonferroni's test). For further details, see Methods.
PACAP was suggested to mediate the relaxation of longitudinal muscle of the distal colon partially via activation of apamin-sensitive K+ channels (Kishi et al., 1996). So, we next examined the correlation of the cyclic AMP content with PACAP-induced relaxation of the LMMP preparations. Although VIP and EFS increased the cyclic AMP content of LMMP reparations, PACAP at 100 nM which induced a maximal relaxant response in the distal segments did not affect the content (Figure 3).
EFS-induced relaxation was partially inhibited by a PKA inhibitor (Figure 1) and the relaxation which persisted after the PKA inhibitor-treatment was further inhibited by apamin but not ChTx (Figure 4). 8-Bromo cyclic AMP-induced relaxation was not affected by apamin (Table 1). Thus, it seems that cyclic AMP is involved only in the VIP-mediated component of the NANC relaxation.
Figure 4.
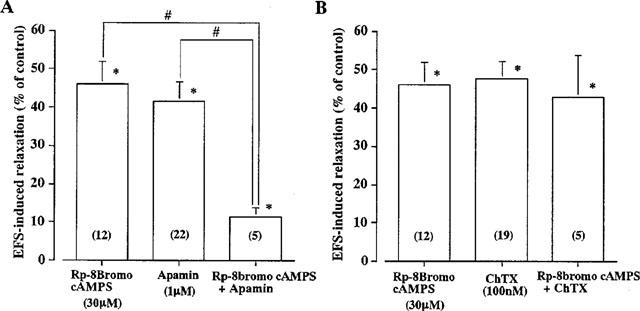
Effects of Rp-8 bromo cAMPS, ChTx and apamin on EFS-induced relaxation of longitudinal muscle in distal colon of Wistar-ST rats. (A) Relaxations induced by EFS (100 Pulses) in the presence of 30 μM Rp-8 bromo cyclic AMPS or 1 μM apamin, or both drugs are expressed as percentages of that obtained before addition of drugs (control). (B) Relaxations were induced by EFS in the presence of Rp-8 bromo cyclic AMPS or 100 nM ChTx, or both drugs. Columns and bars represent means with standard errors of 5–22 experiments shown in parentheses. *Significantly different from the control value, P<0.05 and #Significant difference between the values at P<0.05 (Student's t-test).
Table 1.
Effects of ChTx and apamin on 8-bromo cyclic AMP=induced relaxation
Effect of ChTx on 8 bromo cyclic AMP-induced relaxation of longitudinal muscle of the distal colon
8-Bromo cyclic AMP, a membrane permeable analogue of cyclic AMP, induced slow relaxation of longitudinal muscle of the segments. Since ChTx-sensitive K+ channels were suggested to be involved in the VIP-mediated relaxation of the preparation in our previous study (Kishi et al., 1996), the effect of ChTx on the 8-bromo cyclic AMP-induced relaxation was examined. ChTx at 100 nM, which exhibited a maximal inhibitory effect on the EFS-induced relaxation of the segments (Kishi et al., 1996) almost completely inhibited the 8-bromo cyclic AMP-induced relaxation (Table 1).
Effects of Rp-8 bromo cyclic AMPS on the changes in membrane potentials induced by EFS or VIP in the longitudinal muscle cells of the rat distal colon
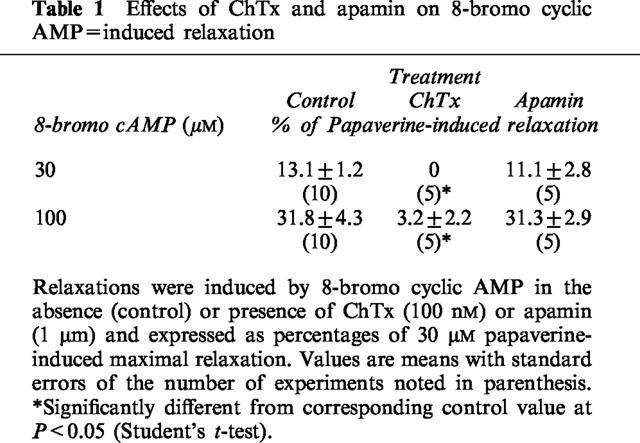
The resting membrane potential of longitudinal muscle cells of the rat distal colon was around −60 mV as reported previously (Kishi et al., 1996). In the presence of 1 μM atropine and 5 μM guanethidine, EFS with a single pulse, or two or three pulses at 10 Hz induced inhibitory junction potentials (i.j.ps). The i.j.ps induced by two or three pulses at 10 Hz often consisted of two phases, rapid and subsequent slow hyperpolarization. But the latter was rarely recorded on a single pulse. The rapid and slow hyperpolarizations were suggested to be mediated by PACAP and VIP, respectively, in a previous study (Kishi et al., 1996). Rp-cyclic AMPS at 50 μM, another inhibitor of PKA, inhibited only the delayed phase, slow hyperpolarization, suggesting that VIP induced the delayed phase of i.j.ps via a cyclic AMP–PKA pathway (Figure 5).
Figure 5.
Effect of Rp-cAMPS on i.j.ps induced by EFS in longitudinal muscle cells of the distal colon of Wistar-ST rats. (A) I.j.ps induced by EFS with 2 pulses at 10 Hz were recorded before and 30 min after application of 50 μM Rp-cAMPS. (B) The chart was run fast to make the i.j.ps clear. Note that i.j.ps consist of rapid and slow hyperpolarization. Atropine (1 μM) and guanethidine (5 μM) were added to the bathing fluid throughout the experiment.
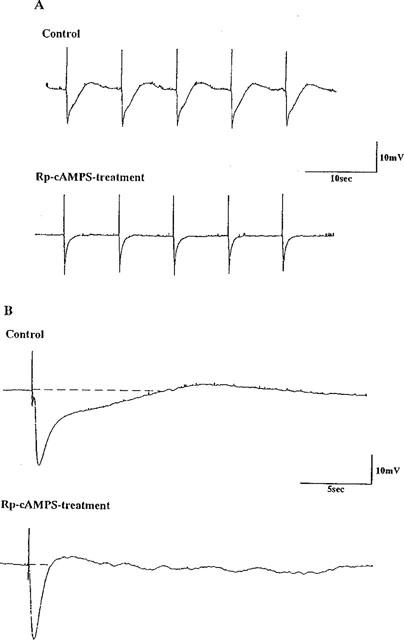
Bath application of VIP induced slow hyperpolarization of membrane potentials in longitudinal muscle cells. Rp-8 bromo cyclic AMPS at 30 μM completely inhibited 100 nM VIP-induced hyperpolarization, 2.2±0.8 mV (n=6; Figure 6A) and significantly inhibited 300 nM VIP-induced hyperpolarization from 6.5±1.1 mV to 1.7±0.9 mV (n=5; Figure 6B).
Figure 6.
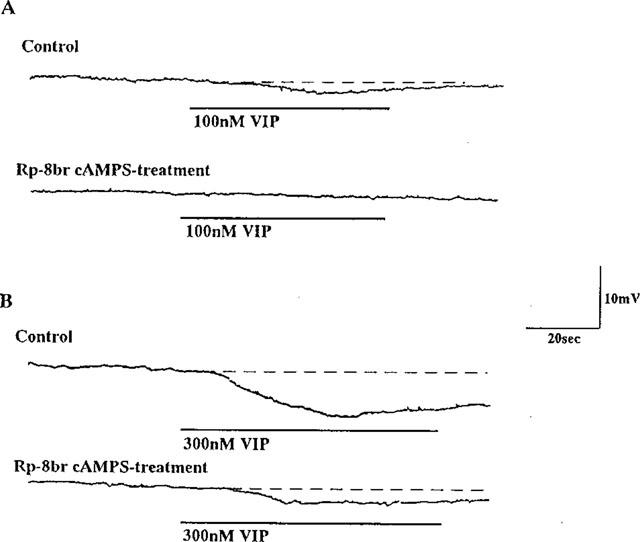
Effect of Rp-8 bromo cAMPS on VIP-induced inhibitory junction potentials of the longitudinal muscle cell membrane. Hyperpolarizations were induced by exogenously added 100 (A) or 300 (B) nM VIP before and 30 min after treatment of 30 μM Rp-8 bromo cAMPS. Note that VIP-induced slow hyperpolarization was inhibited by Rp-8 bromo cyclic AMPS-treatment.
Discussion
All layers of every region of the gut contain vasoactive intestinal peptide (VIP)- immunoreactive nerve fibres (for reviews, see Gillespie, 1982; Fahrenkrug, 1991). However, the role of VIP as a mediator of the inhibitory response has been shown in only rather restricted regions of the gut: the lower oesophageal sphincter of the opossum (Goyal et al., 1980) and rabbit (Biancani et al., 1984), the stomach of the dog (Angel et al., 1983), guinea-pig (Grider et al., 1985a) and rat (Kamata et al., 1988; De Beurme & Lefebvre, 1988), and the taenia coli of the guinea-pig (Grider et al., 1985b). The role of VIP has not been shown in many regions of the intestine: the colon of mice (Fontaine et al., 1986), the ileocolonic junction of dogs (Boeckxstaens et al., 1991), the jejunum (Niioka et al., 1997), proximal colon (Suthamnatpong et al., 1993) and rectum (Takeuchi et al., 1998) of Wistar-ST rats. However, the rat colon is exceptional. Namely, in this region VIP was also suggested to mediate descending relaxation (Grider & Makhlouf, 1986; Grider & Rivier, 1990). Our previous study also suggested that VIP and PACAP mediates about half NANC relaxation of longitudinal muscle of the distal colon of Wistar-ST rats via activating ChTx- and apamin-sensitive K+ channels, respectively (Kishi et al., 1996). The present results suggest that a cyclic AMP–PKA–ChTx-sensitive K+ channel pathway is involved in the VIP-induced relaxation and that this pathway is independent of PACAP-mediated relaxation via activation of apamin sensitive K+ channels. First we found that exogenous VIP and EFS but not exogenous PACAP increased the cyclic AMP content of the longitudinal muscle; second, that a PKA inhibitor inhibited exogenous VIP- but not PACAP-induced relaxation; third, that ChTx but not apamin inhibited 8 bromo cyclic AMP-induced relaxation; fourth, that a PKA inhibitor inhibited EFS-induced relaxation additively with apamin but not ChTx; fifth, that a PKA inhibitor inhibited EFS-induced slow hyperpolarization of longitudinal muscle cell membranes which were inhibited by a VIP antagonist and also inhibited VIP-induced hyperpolarization of the membranes. This suggests a sequence of VIP–cyclic AMP–PKA–ChTx-sensitive K+ channels–relaxant response in the gut.
Although exogenously added VIP (100 nM–1 μM) increased the cyclic AMP content of LMMP preparations, the extent of the increase was less than that in EFS (Figure 3). This inconsistency may be due to a different mode of action of VIP. Namely, VIP released toward the postsynaptic area by EFS induced fast elevation of the cyclic AMP content as a physiological response, while VIP applied exogenously diffuses into the LMMP preparation. Indeed, exogenous VIP induced a slow relaxation which took 5 min to reach a maximum in most cases.
PACAP (10–100 nM) did not increase cyclic AMP content of the preparations. We have suggested that PACAP induced relaxation of longitudinal muscle of the colonic segments via activation of tyrosine kinase-apamin sensitive K+ channel pathway (Takeuchi et al., 1999). These results indicate that relaxant action of 100 nM PACAP which induced the maximal relaxation of the segments is not associated with an increase in cyclic AMP content.
EFS for 10 s induced relaxation with concomitant increase in the cyclic AMP content. For measurement of the cyclic AMP content, LMMP preparations were quickly frozen within a few second (see Methods). However, colonic segments contracted immediately after the stimulation, suggesting an increased cyclic AMP content in spite of muscle contraction. The contraction subsequent to NANC relaxation was not affected by treatments with Rp-8 bromo cAMPS (Figure 1), VIP10–28 or ChTx (Kishi et al., 1996). Therefore, the contraction seems to be induced by turn off-stimulation, independent of the cyclic AMP content.
In guinea-pig trachea smooth muscle, activation of ChTx-sensitive K+ channels was suggested to be involved in PACAP-induced bronchodilation via a cyclic AMP–PKA pathway (Hiramatsu et al., 1994; 1995). In intestinal smooth muscle, ChTx-sensitive K+ channel was also suggested to be constitutively activated for modulation of the activity of ileal smooth muscle cells of guinea-pig: ChTx increased spontaneous activity and inhibited the nerve stimulation-evoked hyperpolarization of the cell membrane (Hong et al., 1997). Moreover, activation of ChTx-sensitive K+ channels is thought to be involved in cyclic AMP-induced hyperpolarization of cell membranes of canine colonic circular muscle (Du et al., 1994), and its activation in setting the membrane potential, and in determining the excitability in longitudinal layer, and in limiting the responses to excitatory agonists in the circular layer of canine colon (Carl et al., 1995). As mentioned in the Introduction, several reports suggest activation of Ca2+-activated K+ channels by PKA (Carl et al., 1991; Esguerra et al., 1994; Meera et al., 1995). The present results also suggest that PKA activates ChTx-sensitive K+ channels in longitudinal muscle of the rat distal colon.
In summary, an inhibitor of PKA inhibited EFS-induced NANC relaxation and exogenous VIP-induced relaxation. The inhibitor also inhibited the delayed slow hyperpolarization of i.j.ps induced by EFS and exogenous VIP-induced hyperpolarization. Thus, the present results suggest that PKA is associated with activation of ChTx-sensitive K+ channels in longitudinal muscle of the distal colon, although the precise mechanism by which it activates them is unknown.
Acknowledgments
This work was supported in part by a Grant-in-Aid for Scientific Research from the Ministry of Education, Science, Sports and Culture of Japan, and by scholarships from Nippon Boehringer Ingelheim and Ono Pharmaceutical Company.
Abbreviations
- 8-bromo cAMP
8-bromo adenosine-3′,5′-cyclic monophosphate
- ChTx
charybdotoxin
- EFS
electrical field stimulation
- H-89
N-[2-(p-bromocinnamylamino)ethyl]-5-isoquinolinesulphonamide dihydrochloride;i.j.ps, inhibitory junction potentials; LMMP, longitudinal muscle-myenteric plexus; PACAP, pituitary adenylate cyclase activating peptide; PKA, cyclic AMP dependent protein kinase; Rp-cAMPS, Rp adenosine-3′,5′-cyclic monophosphorothioate; Rp-8-bromo cAMPS, Rp-8 bromoadenosine-3′,5′-cyclic monophosphorothioate; TCA, trichloroacetic acid; VIP, vasoactive intestinal peptide
References
- AIELLO E.A., WALSH M.P., COLE W.C. Phosphorylation by protein kinase A enhances delayed rectifier K+ current in rabbit vascular smooth muscle cells. Am. J. Physiol. 1995;268:H926–H934. doi: 10.1152/ajpheart.1995.268.2.H926. [DOI] [PubMed] [Google Scholar]
- ANGEL F.V., GO L.W., SCHMALZ P.F., SZURSZEWSKI J.H. Vasoactive intestinal polypeptide. A putative neurotransmitter in the canine gastric muscularis mucosa. J. Physiol. 1983;341:641–645. doi: 10.1113/jphysiol.1983.sp014830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BIANCANI P., WALSH J.H., BEHAR J. Vasoactive intestinal peptide: a neurotransmitter for lower oesophageal sphincter relaxation. J. Clin. Invest. 1984;73:963–967. doi: 10.1172/JCI111320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BITAR K.N., MAKHLOUF G.M. Relaxation of isolated gastric smooth muscle cells by vasoactive intestinal peptide. Science. 1982;216:531–533. doi: 10.1126/science.6176025. [DOI] [PubMed] [Google Scholar]
- BOECKXSTAEN G.E., PELCKMANS P.A., RUYTJENS I.F., BULT H., DE MAN J.G., HERMAN A.G., VAN MAERCKE Y.M. Bioassay of nitric oxide released upon stimulation of non-adrenergic non-cholinergic nerves in the canine ileocolonic junction. Br. J. Pharmacol. 1991;103:1085–1091. doi: 10.1111/j.1476-5381.1991.tb12304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CARL A., BAYGUINOV O., SHUTTLEWORTH C.W., WARD S.M., SANDERS K.M. Role of Ca2+-activated K+ channels in electrical activity of longitudinal and circular muscle layers of canine colon. Am. J. Physiol. 1995;268:C619–C627. doi: 10.1152/ajpcell.1995.268.3.C619. [DOI] [PubMed] [Google Scholar]
- CARL A., KENYON J.L., UEMURA D., FUSETANI N., SANDERS K.M. Regulation of Ca2+-activated K+ channels by protein kinase A and phosphatase inhibitors. Am. J. Physiol. 1991;261:C387–C392. doi: 10.1152/ajpcell.1991.261.2.C387. [DOI] [PubMed] [Google Scholar]
- CHAKDER S., RATTAN S. Involvement of cAMP and cGMP in relaxation of internal anal sphincter by neural stimulation, VIP and NO. Am. J. Physiol. 1993;264:G702–G707. doi: 10.1152/ajpgi.1993.264.4.G702. [DOI] [PubMed] [Google Scholar]
- DE BEURME F.A., LEFEBVRE R.A. Vasoactive intestinal polypeptide as possible mediator of relaxation in the rat gastric fundus. J. Pharm. Pharmacol. 1988;40:711–715. doi: 10.1111/j.2042-7158.1988.tb07000.x. [DOI] [PubMed] [Google Scholar]
- DU C., CARL A., SMITH T.K., SANDERS K.M., KEEF K.D. Mechanism of cyclic AMP-induced hyperpolarization in canine colon. J. Pharmacol. Exp. Ther. 1994;268:208–215. [PubMed] [Google Scholar]
- ESGUERRA M., WANG J., FOSTER C.D., ADELMAN J.P., NORTH R.A., LEVITAN I.B. Cloned Ca2+-dependent K+ channel modulated by a functionally associated protein kinase. Nature. 1994;369:563–565. doi: 10.1038/369563a0. [DOI] [PubMed] [Google Scholar]
- FAHRENKRUG J. Vasoactive intestinal peptide (VIP) and autonomic neurotransmission Novel Peripheral NeuroTransmitters 1991Pergamon Press: New York; Bell, C. (ed.) [Google Scholar]
- FONTAINE J., GRIVEGNEE A.R., ROBBERECHT P. Evidence against VIP as the inhibitory transmitter in non-adrenergic, non-cholinergic nerves supplying the longitudinal muscle of the mouse colon. Br. J. Pharmacol. 1986;89:599–602. doi: 10.1111/j.1476-5381.1986.tb11161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GILLESPIE J.S. Non-adrenergic non-cholinergic inhibitory control of gastrointestinal motility Motility of the Digestive Tract 1982Raven Press: New York; In: Weinbeck, M. (ed.) [Google Scholar]
- GOYAL R.K., RATTAN S., SAID S.I. VIP as possible neurotransmitter of non-cholinergic non-adrenergic inhibitory neurons. Nature. 1980;288:378–380. doi: 10.1038/288378a0. [DOI] [PubMed] [Google Scholar]
- GRIDER J.R., CABLE M.B., BITAR K.N., SAID S.I., MAKHLOUF G.M. Relaxant neurotransmitter in taenia coli of the guinea-pig. Gastroenterology. 1985a;89:36–42. [PubMed] [Google Scholar]
- GRIDER J.R., CABLE M.B., SAID S.I., MAKHLOUF M. Vasoactive interstinal peptide as a neural mediator of gastric relaxation. Am. J. Physiol. 1985b;248:G73–G78. doi: 10.1152/ajpgi.1985.248.1.G73. [DOI] [PubMed] [Google Scholar]
- GRIDER J.R., MAKHLOUF G. Colonic peristaltic reflex: identification of vasoactive intestinal peptide as mediator of descending relaxation. Am. J. Physiol. 1986;251:G40–G45. doi: 10.1152/ajpgi.1986.251.1.G40. [DOI] [PubMed] [Google Scholar]
- GRIDER J.R., RIVIER J.R. Vasoactive intestinal peptide (VIP) as transmitter of inhibitory neurons of the gut: evidence from use of selective VIP antagonists and VIP antiserum. J. Pharmacol. Exp. Ther. 1990;253:738–742. [PubMed] [Google Scholar]
- HIRAMATSU T., KUME H., KOTLIKOFF M.I., TAKAGI K. Role of calcium-activated potassium channels in the relaxation of tracheal smooth muscles by forskolin. Clin. Exp. Pharmacol. Physiol. 1994;21:367–375. doi: 10.1111/j.1440-1681.1994.tb02529.x. [DOI] [PubMed] [Google Scholar]
- HIRAMATSU T., KUME H., YAMAKI K., TAKAGI K. Inhibition of pituitary adenylate cyclase activating polypeptide induced relaxation of guinea-pig tracheal smooth muscle by charybdotoxin. Arzneimittelforschung. 1995;45:689–692. [PubMed] [Google Scholar]
- HONG S.J., ROAN Y.F., CHANG C.C. Spontaneous activity of guinea pig ileum longitudinal muscle regulated by Ca2+-activated K+ channel. Am. J. Physiol. 1997;272:G962–G971. doi: 10.1152/ajpgi.1997.272.5.G962. [DOI] [PubMed] [Google Scholar]
- ITO S., KUROKAWA A., OHGA A., OHTA T., SAWABE K. Mechanical, electrical and cyclic nucleotide responses to peptide VIP and inhibitory nerve stimulation in rat stomach. J. Physiol. 1990;430:337–353. doi: 10.1113/jphysiol.1990.sp018294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JIN J.G., MURTHY K.S., GRIDER J.R., MAKHLOUF G.M. Activation of distinct cAMP-and cGMP-dependent pathways by relaxant agents in isolated gastric muscle cells. Am. J. Physiol. 1993;264:G470–G477. doi: 10.1152/ajpgi.1993.264.3.G470. [DOI] [PubMed] [Google Scholar]
- KAMATA K., SAKAMOTO A., KASUYA Y. Similarities between relaxations induced by vasoactive intestinal peptide and by stimulation of the non-adrenergic non-cholinergic neurons in the rat stomach. Naunyn-Schmiedeberg's Arch. Pharmacol. 1988;338:401–406. doi: 10.1007/BF00172117. [DOI] [PubMed] [Google Scholar]
- KAMM K.E., STULL J.T. The function of myosin and myosin light chain kinase phosphorylation in smooth muscle. Ann. Rev. Pharmacol. Toxicol. 1985;25:593–620. doi: 10.1146/annurev.pa.25.040185.003113. [DOI] [PubMed] [Google Scholar]
- KISHI M., TAKEUCHI T., SUTHAMNATPONG N., ISHII T., NISHIO H., HATA F., TAKEWAKI T. VIP-and PACAP-mediated nonadrenergic, noncholinergic inhibition in longitudinal muscle of rat distal colon: involvement of activation of charybdotoxin-and apamin-sensitive K+ channels. Br. J. Pharmacol. 1996;119:623–630. doi: 10.1111/j.1476-5381.1996.tb15719.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MEERA P., ANWER K., MONGA M., OBERTI C., STEFANI E., TORO L., SANBORN B.M. Relaxin stimulates myometrial calcium-activated potassium channel activity via protein kinase A. Am. J. Physiol. 1995;269:C312–C317. doi: 10.1152/ajpcell.1995.269.2.C312. [DOI] [PubMed] [Google Scholar]
- MURTHY K.S., MAKHLOUF G.M. Interaction of cA-kinase and cG-kinase in mediating relaxation of dispersed smooth muscle cells. Am. J. Physiol. 1995;268:C171–C180. doi: 10.1152/ajpcell.1995.268.1.C171. [DOI] [PubMed] [Google Scholar]
- MURTHY K.S., ZHANG K.M., JIN J.G., GRIDER J.R., MAKHLOUF G.M. VIP-mediated G protein-coupled Ca2+ influx activates a constitutive NOS in dispersed gastric muscle cells. Am. J. Physiol. 1993;265:G660–G671. doi: 10.1152/ajpgi.1993.265.4.G660. [DOI] [PubMed] [Google Scholar]
- NIIOKA S., TAKEUCHI T., KISHI M., ISHII T., NISHIO H., TAKEWAKI T., HATA F. Nonadrenergic, noncholinergic relaxation in longitudinal muscle of rat jejunum. Jpn. J. Pharmacol. 1997;73:155–161. doi: 10.1254/jjp.73.155. [DOI] [PubMed] [Google Scholar]
- PATON W.D.M., ZAR M.A. The origin of acetylcholine released from guinea-pig intestine and longitudinal muscle strips. J. Physiol. 1968;194:13–33. doi: 10.1113/jphysiol.1968.sp008392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RATTAN S., MOUMMI C., CHAKDER S. CGRP and ANF cause relaxation of opossum internal anal sphincter via different mechanisms. Am. J. Physiol. 1991;260:G764–G769. doi: 10.1152/ajpgi.1991.260.5.G764. [DOI] [PubMed] [Google Scholar]
- REKIK M., DELVAUX M., FREXINOS J., BUENO L. The calcitonin gene-related peptide activates both cAMP and NO pathways to induce relaxation of circular smooth muscle cells of guinea-pig ileum. Peptides. 1997;18:1517–1522. doi: 10.1016/s0196-9781(97)00246-5. [DOI] [PubMed] [Google Scholar]
- REKIK M., DELVAUX M., TACK I., FREXINOS J., BUENO L. VIP-induced relaxation of guinea-pig intestinal smooth muscle cells: sequential involvement of cyclic AMP and nitric oxide. Br. J. Pharmacol. 1996;118:477–484. doi: 10.1111/j.1476-5381.1996.tb15428.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SUN Y.D., BENISHIN C.G. Effects of calcitonin gene-related peptide on cyclic AMP production and relaxation of longitudinal muscle of guinea pig ileum. Peptides. 1995;16:293–297. doi: 10.1016/0196-9781(94)00189-8. [DOI] [PubMed] [Google Scholar]
- SUTHAMNATPONG N., HATA F., KANADA A., TAKEUCHI T., YAGASAKI O. Mediators of nonadrenergic, noncholinergic inhibition in the proximal, middle and distal regions of rat colon. Br. J. Pharmacol. 1993;108:348–355. doi: 10.1111/j.1476-5381.1993.tb12808.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SUTHAMNATPONG N., HOSOKAWA M., TAKEUCHI T., HATA F., TAKEWAKI T. Nitric oxide-mediated inhibitory response of rat poroximal colon: independence from changes in membrane potential. Br. J. Pharmacol. 1994;112:676–682. doi: 10.1111/j.1476-5381.1994.tb13129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAKEUCHI T., KISHI M., HIRAYAMA N., ISHII T., NISHIO H., HATA F., TAKEWAKI T. Involvement of activation of tyrosine kinase in the NANC inhibitory response in longitudinal muscle of rat distal colon. J. Physiol. 1999;514:177–188. doi: 10.1111/j.1469-7793.1999.177af.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAKEUCHI T., NIIOKA S., KISHI M., ISHII T., NISHIO H., HATA F., TAKEWAKI T., TAKATSUJI K. Nonadrenergic, noncholinergic relaxation mediated by nitric oxide with concomitant change in Ca2+ level in rectal circular muscle of rats. Eur. J. Pharmacol. 1998;353:67–74. doi: 10.1016/s0014-2999(98)00351-3. [DOI] [PubMed] [Google Scholar]
- TAKEWAKI T., OHASHI H. Non-cholinergic, excitatory transmission to intestinal smooth muscle cells. Nature. 1977;268:749–750. doi: 10.1038/268749a0. [DOI] [PubMed] [Google Scholar]
- TORPHY T.J., FINE C.F., BURMAN M., GARNETTE M.S., ORMSBEE H.S. Lower esophageal sphincter relaxation is associated with increased cyclic nucleotide content. Am. J. Physiol. 1986;251:G786–G793. doi: 10.1152/ajpgi.1986.251.6.G786. [DOI] [PubMed] [Google Scholar]
- WELLMAN G.C., QUAYLE J.M., STANDEN N.B. ATP-sensitive K2+ channel activation by calcitonin gene-related peptide and protein kinase A in pig coronary arterial smooth muscle. J. Physiol. 1998;507:117–129. doi: 10.1111/j.1469-7793.1998.117bu.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


