Abstract
Latent membrane protein 1 (LMP1) is an Epstein–Barr virus (EBV)-encoded, ligand-independent receptor that mimics CD40. We report here that LMP1 signals principally from intracellular compartments. LMP1 associates simultaneously with lipid rafts and with its signaling molecules, tumor necrosis factor-receptor (TNF-R)-associated factors (TRAFs) and TNF-R1-associated death domain protein (TRADD) intracellularly, although it can be detected at low levels at the plasma membrane, indicating that most of LMP1’s signaling complex resides in intracellular compartments. LMP1’s signaling is independent of its accumulation at the plasma membrane in different cells, and as demonstrated by a mutant of LMP1 which has significantly reduced localization at the plasma membrane yet signals as efficiently as does wild-type LMP1. The fusion of the transmembrane domain of LMP1 to signaling domains of CD40, TNF-R1 and Fas activates their signaling; we demonstrate that a fusion of LMP1 with CD40 recruits TRAF2 intracellularly. Our results imply that members of the TNF-R family can signal from intracellular compartments containing lipid rafts and may do so when they act in autocrine loops.
Keywords: CD40/lipid rafts/LMP1/TNF-R
Introduction
Members of the tumor necrosis factor receptor (TNF-R) family initiate signaling by binding their extracellular ligands at the plasma membrane. The receptor–ligand interaction for CD40, a member of the TNF-R family important in both development of B-cells and humoral immune responses (van Kooten and Banchereau, 1997; Grewal and Flavell, 1998; van Kooten, 2000), induces several events leading to the initiation of signaling: it increases CD40’s affinity for its signaling molecules, TNF-receptor-associated factors (TRAFs); it redistributes CD40 into membrane microdomains termed lipid rafts at the plasma membrane thought to be the site where CD40 signals; and it leads to the recruitment of TRAFs into lipid rafts (Pullen et al., 1999; Hostager et al., 2000; Vidalain et al., 2000).
Latent membrane protein 1 (LMP1) encoded by Epstein–Barr virus (EBV) mimics CD40 in many aspects, although, strikingly, LMP1 signals in the apparent absence of a ligand (Martin and Sugden, 1991; Gires et al., 1997). Both LMP1 and CD40 activate overlapping signaling pathways including NF-κB-, AP-1- and STAT-mediated transcription (Berberich et al., 1994; Mitchell and Sugden, 1995; Sakata et al., 1995; Hanissian and Geha, 1997; Eliopoulos and Young, 1998; Gires et al., 1999). They both bind some of the same signaling molecules such as TRAFs through their cytoplasmic tails, although their binding affinities differ (Sandberg et al., 1997; Pullen et al., 1998, 1999). LMP1 but not CD40 also binds to TNF-R1-associated death domain protein (TRADD) (Izumi and Kieff, 1997). LMP1, when expressed in B cells of CD40-null mice, can partially restore their defects (Uchida et al., 1999). On the other hand, CD40 can substitute for LMP1 in maintaining proliferation of EBV-infected B cells (Kilger et al., 1998). As a ligand-independent receptor-like molecule, a portion of LMP1 associates with lipid rafts at steady state, and LMP1 both interacts readily with TRAFs and recruits them to lipid rafts (Devergne et al., 1996; Ardila-Osorio et al., 1999; Higuchi et al., 2001; Kaykas et al., 2001). Targeting LMP1’s signaling domain to lipid rafts has been shown to be required for its efficient signaling (Kaykas et al., 2001).
The mechanism by which LMP1 signals in a ligand-independent manner has been studied intensively. It is known that LMP1 can be cleaved in some live cells by externally added chymotrypsin (Liebowitz et al., 1986; Martin and Sugden, 1991), indicating that a portion of LMP1 can localize to the plasma membrane. It is also clear that the six membrane-spanning domain of LMP1 mediates the aggregation of LMP1 molecules and the assembly of a signaling complex (Gires et al., 1997; Kaykas et al., 2001). The role of the membrane-spanning domains of LMP1 in assembling a ligand-independent signaling complex has been demonstrated by studies of an LMP1–CD40 chimera that has the N-terminus and the membrane-spanning domains of LMP1 fused to the cytoplasmic tail of CD40 and activates CD40’s signals in the absence of CD40L (Gires et al., 1997). Finally, the membrane-spanning domains of LMP1 have been shown to mediate association of LMP1 with lipid rafts (Higuchi et al., 2001; Coffin et al., 2003). Because LMP1 mimics CD40 in many aspects, it has been hypothesized that LMP1 signals at the plasma membrane by forming aggregates through its transmembrane domains.
In this study, we demonstrate that LMP1 is able to signal from intracellular sites containing lipid rafts. We have found that LMP1 co-localizes both with the lipid raft marker cholera toxin and with its signaling molecule TRADD in perinuclear structures, and actively recruits TRAFs there. Using treatment of live cells with chymotrypsin and electron microscopy, we demonstrate that little LMP1 localizes to the plasma membrane. We find also that LMP1’s signaling in different host cells is unrelated to its localization at the plasma membrane. Furthermore, the fraction of LMP1 that co-precipitates with TRAFs is significantly greater than that found at the plasma membrane at steady state. Lastly, a mutant of LMP1, in which three adjacent leucines in its C-terminal cytoplasmic domain are substituted with alanines, is not accessible to digestion by chymotrypsin but signals as efficiently as does wild-type LMP1. Collectively, these results demonstrate that in different cells, much or all of LMP1’s signaling complex resides in intracellular compartments.
The fusion of the N-terminus and the six membrane-spanning domain of LMP1 to signaling domains of TNF-R2, CD40 and Fas activates their signaling in the absence of ligands (Gires et al., 1997; Hatzivassiliou et al., 1998; Kaykas et al., 2001; A.Kaykas and B.Sugden, unpublished results). In extending our study on LMP1 to other members of the TNF-R family, we have found that the LMP1–CD40 chimera localizes similarly to LMP1 in cells and recruits TRAF2 to intracellular sites. Several members of the TNF-R family including CD40, Fas and TNF-R can be co-expressed with their ligands in individual cells and act in autocrine loops in those cells (Dhein et al., 1995; Barker et al., 2001; Pham et al., 2002). Our results imply that members of the TNF-R family have the ability to signal from intracellular compartments containing lipid rafts and may do so when they act in autocrine loops.
Results
LMP1 co-localizes with internalized cholera toxin
Targeting LMP1 to lipid rafts is required for efficient signaling of LMP1 (Kaykas et al., 2001). In EBV-transformed lymphoblastic cells and EBV-negative 293 or BJAB cells expressing LMP1, a relatively constant fraction of LMP1 has been detected in detergent-resistant membranes, the biochemically isolated fraction known to contain lipid rafts (Higuchi et al., 2001; Kaykas et al., 2001; Rothenberger et al., 2002). To characterize where LMP1 associates with lipid rafts, we performed confocal microscopy to examine co-localization of LMP1 with either surface-bound or internalized cholera toxin B subunit (CTxB). CTxB binds to the glycosphingolipid GM1 and has been widely used as a marker for lipid rafts. The CTxB–GM1 complex originating at the cell surface is internalized to endosomes and further to Golgi in a lipid raft-dependent pathway at 37°C (Shogomori and Futerman, 2001; Wolf et al., 2002).
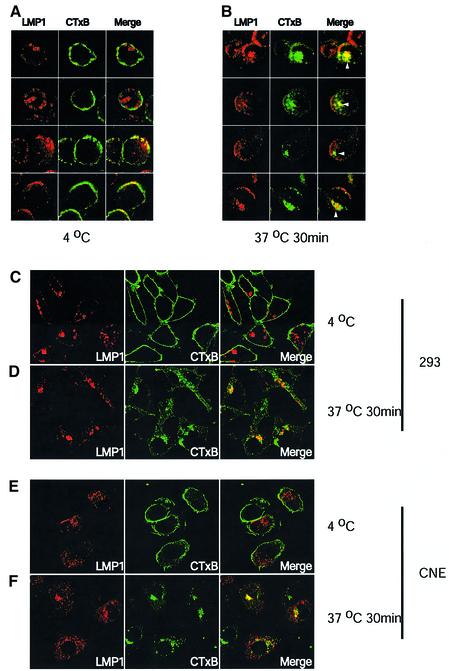
In the 721 cell line, which is an EBV-immortalized lymphoblastic cell clone, and two other EBV-infected primary B-cell clones (data not shown), LMP1’s subcellular localization and levels of expression were unexpectedly heterogeneous among cells. In the majority of cells, LMP1 was detected in punctate vesicular-like and perinuclear structures (Figure 1A). In ∼16% of examined 721 cells (24/135 and 30/202 in two assays), LMP1 localized in cap-like structures at the cell surface (Figure 1A, the third and fourth rows). Only 20% of 721 cells could be stained with CTxB to permit their visualization by confocal microscopy. At 4°C, CTxB bound to the cell surface showed either patches or a nearly homogeneous annulus of staining. LMP1 in cap structures consistently co-localized with surface-bound CTxB (Figure 1A). After the cells were shifted to 37°C to allow surface-bound CTxB to be internalized, LMP1 was also found to co-localize with internalized CTxB in perinuclear regions (Figure 1B).
Fig. 1. LMP1 co-localizes with internalized cholera toxin. 721 cells, 293 or CNE cells transiently expressing LMP1 were incubated with 2.5 µg/ml FITC-conjugated cholera toxin (green) at 4°C for 30 min. Cells were washed twice with cold medium and either fixed, or shifted to 37°C for 30 min then fixed. The fixed cells were then stained for LMP1 (red). Yellow indicates co-localization of LMP1 and CTxB. At 4°C, most of LMP1 did not co-localize with CTxB in 721 cells (A, the first and second rows). In ∼16% of 721 cells, LMP localized to the cap-like structures which co-localized with surface-bound CTxB (A, third and fourth rows, see text). LMP1 also co-localized with internalized CTxB in 721 cells (B). White arrowheads in (B) indicate co-localization of LMP1 and CTxB at the perinuclear structures after CTxB had moved intracellularly on incubating live cells at 37°C for 30 min. In 293 and CNE cells, co-localization of LMP1 and surface-bound CTxB was rarely detected (C and E). LMP1 at the perinuclear and vesicular-like structures did co-localize with internalized CTxB (D and F).
In contrast to 721 cells, the staining pattern of LMP1 was homogeneous among EBV-negative 293 cells. The cap-like staining of LMP1 was not detected in these cells. All of the LMP1 was seen in punctate vesicular-like and perinuclear structures (Figure 1C). A fraction of the perinuclear structures co-localized with AP-1 and GGA3, which are proteins known to localize at the trans-Golgi network, indicating that they may be Golgi (data not shown). The identities of the vesicular-like structures are unknown. LMP1 did not co-localize detectably with either an early endosomal marker EEA1 or a lysosomal marker LAMP-1 in the vesicular-like structures (data not shown). At 4°C, CTxB homogeneously bound to the surface of 293 cells (Figure 1C). LMP1 generally failed to co-localize with the surface-bound CTxB. However, after shifting the cells to 37°C, CTxB and LMP1 co-localized in a fraction of the perinuclear and vesicular structures (Figure 1D). These studies, when combined with those using the biochemical fractionation of detergent-resistant membranes rafts (Higuchi et al., 2001; Kaykas et al., 2001; Rothenberger et al., 2002), indicate that LMP1 partitions into lipid rafts in various cellular compartments. In 293 cells, in which 30% of LMP1 is found in lipid raft fractions, almost all of this partitioning occurs intracellularly. We extended our studies to cells that can be hosts for EBV. CNE cells are presumably descendants of EBV-positive nasopharyngeal carcincoma cells because these tumors are generally EBV positive in vivo but often lose the virus in culture. BJAB cells are derived from an EBV-negative Burkitt’s lymphoma and have been used often to study LMP1. The staining patterns of LMP1 in EBV-negative CNE (Figure 1E) and BJAB (data not shown) cells were similar to those in 293 cells. No heterogeneity and cap-like structures were detected. In CNE cells, LMP1 co-localized with internalized but not with surface-bound CTxB (Figure 1E and F). We were unable to monitor the possible partitioning of LMP1 into intracellular lipid rafts with CTxB in BJAB cells because this cell line only marginally stained with CTxB (data not shown). Detection of LMP1’s association with lipid rafts in intracellular compartments is consistent with a surprising hypothesis that LMP1 can signal from inside cells. Different studies have found LMP1 to be at the plasma membrane (Liebowitz et al., 1986; Martin and Sugden, 1991; Coffin et al., 2001). However, our results indicate that little LMP1 localizes at the plasma membrane in 293, CNE and BJAB cells. We therefore reassessed LMP1’s distribution in both EBV-positive and -negative 293, CNE and BJAB cells to determine if the distribution of this CD40-mimic is consistent with this hypothesis.
The portion of LMP1 susceptible to cleavage by chymotrypsin differs among cell types
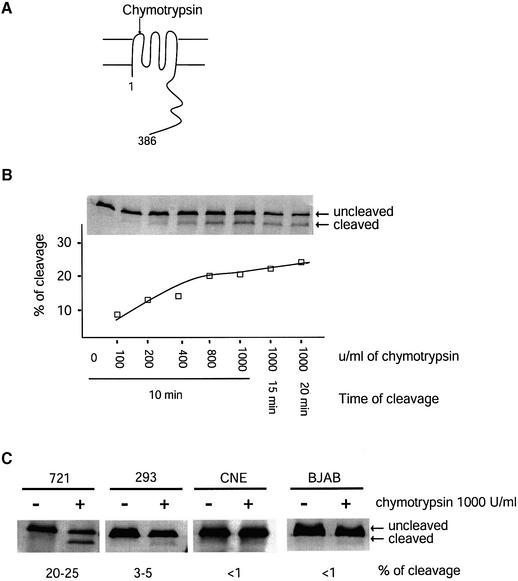
Liebowitz et al. (1986) have found that chymotrypsin cleaves LMP1 at its first extracellular loop in live cells (Figure 2A). We found that the portion of LMP1 cleaved by chymotrypsin at the plasma membrane differed dramatically among cell types. In EBV-positive 721 cells, 20–25% of LMP1 was maximally accessible to digestion by chymotrypsin (Figure 2B). The fraction of LMP1 cleaved was dose dependent and plateaued when treated with 800 U/ml chymotrypsin for a 10 min digestion at room temperature. Increasing either the concentration of chymotrypsin or the time of digestion did not increase significantly the amount of LMP1 digested. Only 3–5% of LMP1 could be cleaved in 293 cells and <1% could be cleaved in CNE and BJAB cells when transfected such that these cells expressed levels of LMP1 comparable with that expressed in 721 cells (Figure 2C). The kinetics of digestion of LMP1 by chymotrypsin in 293, CNE and BJAB cells were similar to those in 721 cells, with 800 U/ml chymotrypsin for 10 min giving maximal digestion (data not shown). In one experiment, 721 cells were labeled with [35S]methionine for 10 min, chased for 0, 10, 20, 40, 60 and 120 min, digested with chymotrypsin and the level of digestion of LMP1 measured. At no time did more than 20% of the labeled LMP1 become digested (data not shown), consistent with our steady-state measurement in 721 cells. The results of cleavage with chymotrypsin are in agreement with the confocal microscopic observation and indicate that only low levels of LMP1 localize to the plasma membrane at the steady state in 293, CNE and BJAB cells. Although little LMP1 localized to the plasma membrane in these cells, LMP1 signals efficiently in all three cell lines (Mitchell and Sugden, 1995; Kieser et al., 1997; Sandberg, 1999; Kwok Fung Lo et al., 2001). These observations support the unexpected hypothesis that LMP1 is able to signal from intracellular compartments.
Fig. 2. The fraction of LMP1 accessible to cleavage in live cells by chymotrypsin differs among cell types. (A) The first extracellular loop of LMP1 can be cleaved by chymotrypsin when LMP1 is at the cell surface. The cleaved product can be separated from intact LMP1 by SDS–PAGE and identified by western blotting using an antibody against the C-terminal cytoplasmic domain of LMP1 (Liebowitz et al., 1986). (B) Live 721 cells were treated with different concentrations of chymotrypsin for different periods of time as indicated and as described in Materials and methods. The chymotrypsin-treated cells were lysed in 1× RIPA. Cell lysates from 5 × 104 cells for each sample were separated by SDS–PAGE and transferred to a nitrocellulose membrane. The LMP1 was detected with a polyclonal rabbit anti-LMP1 antibody followed by 35S-labeled goat anti-rabbit antibodies. The cleaved LMP1 and the uncleaved LMP1 were quantified. The percentage of cleavage was calculated as [cleaved LMP1/(cleaved LMP1 + uncleaved LMP1)] and plotted. Shown is a representative of three independent experiments. (C) Live 721 cells, and 293, CNE and BJAB cells transiently expressing LMP1 were treated with 1000 U/ml chymotrypsin for 10 min at room temperature. The fractions of LMP1 being cleaved were calculated as described in (B).
Electron microscopy confirms that little LMP1 localizes to the plasma membrane
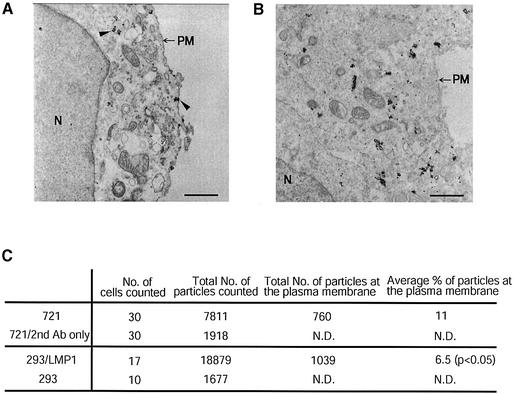
In order to validate the results obtained from treating live cells with chymotrypsin, we used immuno-gold electron microscopy to visualize the subcellular localization of LMP1 in both 721 and 293 cells. The specificity of LMP1 staining was controlled by staining 721 cells with secondary antibodies only and by staining 293 cells not expressing LMP1 with anti-LMP1 antibodies. As shown in Figure 3A and B, in both 721 cells and 293 cells transiently expressing LMP1, the majority of immuno-gold staining for LMP1 resided inside cells associated with the Golgi and other membrane structures. We quantified the gold-tagged LMP1 at the plasma membrane by counting the gold particles in cells randomly chosen throughout ultra-thin sections (Figure 3C). The electron microscopic measurements confirmed those derived from treating cells with chymotrypsin; the majority of LMP1 did not localize to the plasma membrane, but more LMP1 localized at the plasma membrane in 721 cells than in 293 cells. The subcellular localization of LMP1 in EBV-positive and EBV-negative cells is consistent with the hypothesis that it can signal from intracellular lipid rafts.
Fig. 3. Electron microscopy confirms that the majority of LMP1 does not localize at the plasma membrane. LMP1’s subcellular localization was examined in 721 (A) and 293 (B) cells by immunogold electron microscopy as described in Materials and methods. Arrowheads in (A) indicate examples of staining of LMP1 at the plasma membrane and at intracellular compartments. N, nuclear; PM, plasma membrane; scale bar = 1 µm. The fraction of LMP1 at the cell surface was quantified by counting the number of particles from randomly chosen cells (C). The difference between 721 and 293 cells was significant (Wilcoxon one-sided test, P < 0.05). ND, not determined. The silver enhancement of the fine gold particles leads to their dimensions varying. Each deposition was counted once independently of its size.
LMP1 associates with its signaling molecules inside cells
We next examined whether LMP1 interacts with its signaling molecules intracellularly. Because TRAFs and TRADD bind directly to LMP1 and these interactions have been found to be necessary for LMP1’s signaling (Mosialos et al., 1995; Kaye et al., 1996; Izumi and Kieff, 1997; Sandberg et al., 1997; Devergne et al., 1998; Kieser et al., 1999), we were particularly interested in finding where LMP1 associates with these mediators of its signaling.
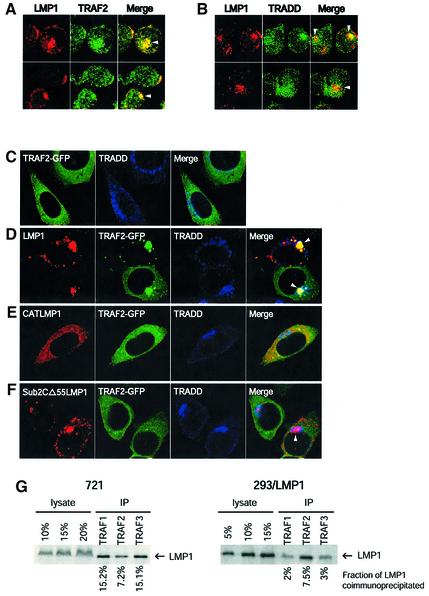
In 721 cells, LMP1 co-localized with endogenous TRAF2 in both cap-like structures at the plasma membrane and perinuclear structures (Figure 4A). LMP1 appeared to recruit TRAF2 to the sites where LMP1 localized. In cells expressing higher levels of LMP1, TRAF2 was more concentrated in regions where LMP1 localized, whereas in cells expressing lower levels of LMP1, TRAF2 was distributed more homogeneously throughout the cells. Endogenous TRADD in 721 cells was detected in perinuclear structures and throughout the cells in speckles. LMP1 co-localized with TRADD most apparently in the perinuclear region (Figure 4B). In 293 cells, endogenous TRADD mainly localized in perinuclear structures similar to what has been described previously (Morgan et al., 2002) (Figure 4C). A portion of LMP1 at the perinuclear structures co-localized with TRADD (Figure 4D). Expression of LMP1 did not affect TRADD’s subcellular localization. We used TRAF2– green fluorescent protein (GFP) to facilitate visualization of TRAF2 and to monitor co-localization of the three proteins. In the absence of LMP1, TRAF2–GFP homogeneously distributed in the the cytoplasm. TRAF2–GFP also localized to speckles in addition to its otherwise homogeneous distribution in the cytoplasm when it was expressed at high levels (Figure 4C). When LMP1 and TRAF2–GFP were co-expressed in cells, LMP1 actively recruited TRAF2–GFP to perinuclear structures and to some but not all of the vesicular-like structures (Figure 4D). This recruitment was so robust that in cells expressing low levels of TRAF2–GFP, all of TRAF2–GFP relocalized to the sites in which LMP1 resided (Figure 4D). Co-localization of LMP1 simultaneously with both TRAF2 and TRADD was only detected inside cells at the perinuclear structures. TRAF3, which is the TRAF molecule that binds LMP1 most strongly, was recruited to LMP1 at the intracellular sites, as was TRAF2 in 293 cells (data not shown).
Fig. 4. LMP1 associates with its signaling molecules intracellularly. 721 cells were stained for LMP1 (red) and TRAF2 (green) (A) and for LMP1 (red) and TRADD (green) (B). Yellow indicates co-localization of red and green. Arrowheads in (A) and (B) indicate co-localization of LMP1 with TRAF2 and TRADD at the perinuclear structures. 293 cells transiently expressing TRAF2–GFP (green) (C), TRAF2–GFP and LMP1 (D), TRAF2–GFP and CATLMP1 (E), and TRAF2–GFP and Sub2CΔ55LMP1 (F) were stained for LMP1 (red) and TRADD (blue). White indicates co-localization of the three colors. Arrowheads in (D) indicate the perinuclear structures where LMP1 recruited TRAF2–GFP and co-localized with TRADD. In cells expressing lower levels of TRAF2–GFP, LMP1 recruited all TRAF2–GFP to its own sites (compare the upper cell in D with those in C). Co-localization of the three proteins was only observed at these perinuclear structures. CATLMP1, which can bind TRAF2–GFP and TRADD but lacks its membrane-spanning domains (E), localizes homogeneously in the cytoplasm but did not affect the distribution of TRAF2–GFP. Sub2CΔ55LMP1 (F), which has a mutated TRAF-binding site and lacks a TRADD-binding site, localized similarly to wild-type LMP1 but did not recruit TRAF2–GFP to the perinuclear structures (arrowhead) and vesicular-like structures. Both CATLMP1 and Sub2CΔ55LMP1 are defective in signaling. (G) The fraction of LMP1 co-precipitated with TRAFs was measured in 721 and 293 cells. Cell lysates were immunoprecipitated with antibodies against TRAF1, 2 or 3 as described in Materials and methods. The immunoprecipitates were separated by SDS–PAGE, transferred to nitrocellulose and detected with antibodies to LMP1. Different amounts of the cell lysate used for each immunoprecipitation were loaded as standard for calculation of the fraction of LMP1 being co-precipitated with TRAFs.
The recruitment of TRAF2 to perinuclear and vesicular-like structures required both the localization of LMP1 to those structures and binding of TRAF2 to LMP1, as demonstrated by two derivatives of LMP1. One derivative of LMP1 (CATLMP1) in which the cytoplasmic tail of LMP1 is fused to chloramphenicol acetyltransferase (CAT) such that the LMP1 moiety is trimerized does not localize to the cellular membranes and fails to signal (Kaykas et al., 2001). CATLMP1 did not recruit TRAF2 into distinct membrane structures. Both CATLMP1 and TRAF2–GFP distributed throughout the cytoplasm (Figure 4E). Another derivative of LMP1 in which its TRAF-binding domain is mutated and its TRADD-binding site is deleted (Sub2CΔ55LMP1) (Sandberg et al., 1997) localized in vesicular-like and perinuclear structures similarly to wild-type LMP1, but also did not affect the subcellular localization of TRAF2–GFP (Figure 4F). We did notice that some small speckles of TRAF2–GFP co-localized with Sub2CΔ55LMP1 in perinuclear structures. The mutated TRAF-binding site does not bind TRAF2 detectably but has ∼10% of wild-type activity to bind TRAF1 (Sandberg et al., 1997). Because TRAF1 and TRAF2 form hetero-oligomers (Pullen et al., 1998), such co-localization was probably mediated by the mutant’s residual TRAF-binding activity.
We also quantified LMP1’s association with endogenous TRAFs in order to measure the fraction of all LMP1 engaged in a signaling complex at steady state. When cell lysates were precipitated with antibodies against TRAF1, 2 and 3, 40% of LMP1 was co-precipitated in 721 cells, and ∼12% of LMP1 was co-precipitated in 293 cells transiently expressing LMP1 (Figure 4G). The fractions of LMP1 found to be co-precipitated with TRAFs were underestimates because the efficiencies of precipitation for TRAF1, 2 and 3 cannot have been complete and the antibodies to TRAF1, 2 and 3 did not allow an estimate of their precipitation efficiency. However, this underestimated fraction of LMP1 associating with TRAFs as measured by their co-immunoprecipitation surpassed that at the plasma membrane at steady state, indicating that LMP1 must associate with endogenous TRAFs intracellularly.
Both the binding to TRAFs and its localization to lipid rafts are important for LMP1’s signaling. In 721 cells, the two events were detected at both the plasma membrane and intracellular compartments. In 293 cells, the two events occurred predominantly in intracellular compartments. LMP1’s signaling also requires its binding to TRADD; LMP1’s association with TRADD occurred detectably only intracellularly in both 721 and 293 cells. Moreover, simultaneous association of LMP1 with TRAF and TRADD was detected only inside cells. Collectively, these observations support LMP1 signaling intracellularly.
A mutant of LMP1 that cannot be cleaved by chymotrypsin in live cells signals as efficiently as does wild-type LMP1
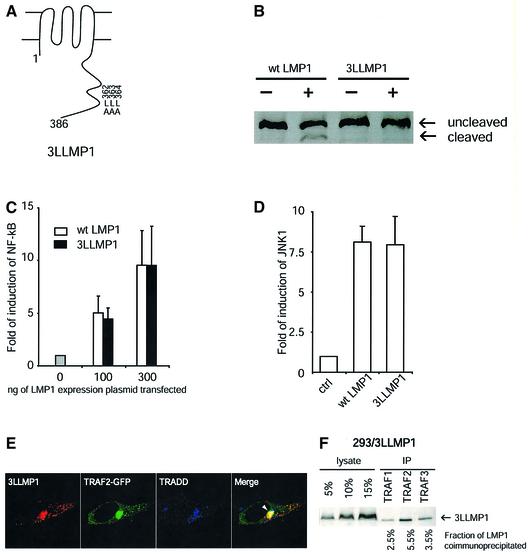
We have found that derivatives of LMP1 with deletions of the entire or the last 55 amino acid of its C-terminal cytoplasmic tail could not be cleaved detectably by chymotrypsin in live cells, indicating that the last 55 amino acids contribute to LMP1’s localization at the plasma membrane (data not shown). Further genetic analysis allowed us to identify a derivative of LMP1 that was resistant to cleavage by extracellularly added chymotrypsin but had wild-type activity in its signaling. The derivative, termed 3LLMP1, had leucine to alanine substitutions at amino acids 362, 363 and 364 (Figure 5A). 3LLMP1 was not cleaved detectably by chymotrypsin in live 293 (Figure 5B) and BJAB (data not shown) cells, even though this assay detected as little as 0.1% of LMP1 being cleaved (data not shown). Electron microscopy indicated that, on average, ∼3.1% (total of 9260 enhanced gold particles counted from 15 cells) of 3LLMP1 was at or close to the plasma membrane, which was significantly less than wild-type LMP1 (P < 0.005, Wilcoxon one-sided test). The result of electron microscopy on 3LLMP1 was an overestimation because the number of gold particles at the plasma membrane in 293 cells not expressing LMP1 (seven particles/cell) was at least one-third of that stained for 3LLMP1(20 particles/cell). 3LLMP1 activated both NF-κB and JNK1 signaling pathways in 293 cells as efficiently as did wild-type LMP1 (Figure 5C and D). As expected, 3LLMP1 also co-localized with internalized CTxB, TRADD and recruited TRAF2 in intracellular compartments (Figure 5E; data not shown). Approxi mately 12% of 3LLMP1 can be co-precipitated with TRAF1, 2 and 3 in 293 cells, as can wild-type LMP1 (Figure 5F, compare with Figure 4G). The efficient signaling of this derivative of LMP1 indicates that the fraction of LMP1 at the plasma membrane at steady state does not correlate with its ability to signal, and further illustrates LMP1’s capacity to signal from inside 293 cells.
Fig. 5. 3LLMP1 which is not accessible detectably to cleavage by chymotrypsin signals as efficiently as does wild-type LMP1. (A) A diagram shows the leucine to alanine substitutions in 3LLMP1. (B) 293 cells transiently expressing LMP1 or 3LLMP1 were treated with 1000 U/ml chymotrypsin for 10 min at room temperature as described in Materials and methods. LMP1 was analyzed by SDS–PAGE and western blotting. The blot was overdeveloped in order to detect as little as 0.1% of LMP1 being cleaved. The signals therefore are not linear. Activation of NF-κB-mediated transcription (C) and JNK (D) by both wild-ype LMP1 and 3LLMP1 was assayed in 293 cells. For both assays, the basal activities in the absence of LMP1 were set as 1. The fold of induction of activities by LMP1 is shown on the y-axis. (E) 3LLMP1 (red) recruited TRAF2–GFP (green) and co-localized with TRADD (blue) at the perinuclear structures (white arrowhead) in 293 cells. (F) The fraction of 3LLMP1 co-precipitated with TRAFs was measured as described in Figure 4G and was found to be similar to that of wild-type LMP1.
An LMP1–CD40 chimera associates with TRAF2 intracellularly
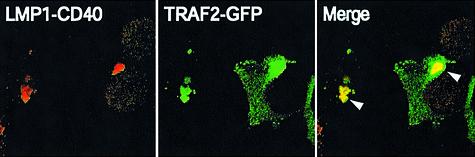
The N-terminus and transmembrane domains of LMP1 probably mediate its localization to intracellular compartments because a derivative of LMP1 lacking its entire C-terminal cytoplasmic tail localized to perinuclear structures similarly to wild-type LMP1 (data not shown). Because a chimera that has a fusion of the N-terminus and the membrane-spanning domain of LMP1 to CD40’s cytoplasmic domain activates CD40 signaling efficiently (Gires et al., 1997; Hatzivassiliou et al., 1998; Kaykas et al., 2001), we asked whether this derivative associates with its signaling molecules inside cells. Most of the LMP1–CD40 chimera localized at the perinuclear structures in 293 cells (Figure 6). A small fraction of LMP1–CD40 was also detected in vesicular-like structures. When co-expressed with TRAF2–GFP, LMP1– CD40 recruited TRAF2–GFP to the perinuclear structures similarly to LMP1. This observation indicates that the LMP1–CD40 chimera also associated with one of its signaling partners inside 293 cells.
Fig. 6. An LMP1–CD40 chimera recruits TRAF2 to intracellular compartments. 293 cells expressing TRAF2–GFP (green) and LMP1–CD40 (red), which has the N-terminus and the transmembrane domain of LMP1 fused to the cytoplasmic tail of CD40, were fixed and stained with anti-CD40 antibodies. Arrowheads indicate co-localization of TRAF2–GFP and LMP1–CD40 at the perinuclear structures (yellow). TRAF2–GFP was recruited to sites where LMP1–CD40 localized (compare TRAF2-GFP’s localization in this figure with that in Figure 4C).
Discussion
LMP1 co-localized with both internalized CTxB and TRADD in perinuclear structures, and actively recruited TRAFs there in EBV-negative 293 cells which support LMP1’s signaling efficiently (Figures 1, 3 and 4). Localizing to lipid rafts and binding to TRAFs and TRADD are necessary for LMP1’s signaling. All of these events were detected in intracellular compartments in 293 cells, indicating that LMP1 resides in its signaling complex at intracellular sites in these cells. The use of chymotrypsin to assay the LMP1 accessible to cleavage in live cells coupled with electron microscopy demonstrated that little LMP1 is at the plasma membrane at steady state in 293, CNE and BJAB cells, consistent with its signaling intracellularly (Figures 2 and 3). The efficient signaling of the 3LLMP1 derivative that accumulates much less at the plasma membrane than does wild-type LMP1 further supports this conclusion. Although the association of LMP1 with TRAF2 and lipid rafts was detected mostly intracellularly in 721 cells and that of LMP1 with TRADD only intracellularly in these cells, we did detect LMP1 co-localized with CTxB and TRAF2 at the plasma membrane in a small fraction of cells, indicating that LMP1 may signal from both intracellular sites and the plasma membrane. Our results cannot rule out that in 293, CNE and BJAB cells, a small fraction of LMP1 signals at the plasma membrane; rather the data clearly demonstrate that at steady state much of LMP1’s signaling is independent of its localization at the plasma membrane.
We have measured where the LMP1 signaling complex accumulates at steady state throughout the study. One caveat of this measurement is that it cannot exclude the possibility that all LMP1 may need to go to the plasma membrane first in order to assemble a signaling complex. Such a scenario is unlikely because there is no evidence supporting that LMP1 at the plasma membrane behaves differently from intracellular LMP1. Furthermore, the localization of LMP1 at perinuclear structures is probably a result of direct targeting of LMP1 to those structures. In a pulse–chase experiment, we have found that most LMP1 synthesized during a 10 min pulse remained resistant to cleavage by chymotrypsin sampled six times during a 2 h chase period (data not shown), indicating that the majority of LMP1 is localized intracellularly. The half-life of LMP1 is 2–4 h. We have also found that in BJAB cells in which LMP1 is inducibly expressed by tetracycline, disruption of the actin cytoskeleton with latrunculin B resulted in the accumulation of newly synthesized LMP1 only at perinuclear structures (unpublished data). Because actin has been shown to be involved in endocytosis (Jeng and Welch, 2001; McPherson, 2002; Schafer, 2002), the experiment probably indicates that LMP1 is targeted to the perinuclear structure without first trafficking to the plasma membrane.
In 721 cells and other clones of EBV-infected lymphoblastic cells (data not shown), we have found that the subcellular localization of LMP1 is heterogeneous. This heterogeneity was characterized by the presence of LMP1 in cap-like structures at the plasma membrane in a fraction of cells. The cap-like structure co-localized with CTxB and TRAF2, indicating that it may be a site where LMP1 signals. However, this membrane ‘capping’ was present in a minority of the cells (16% of 721 cells) and absent from epithelial and EBV-negative B cells. We speculate that this heterogeneity is caused by EBV’s transformation of infected cells, because LMP2, the only other viral membrane protein known to be expressed in EBV-positive lymphoblastic cells, neither interacts with nor affects subcellular localization of LMP1 (Dawson et al., 2001).
Some studies have found previously that chymotrypsin can cleave almost all of LMP1 in mouse Balb/3T3 cells and in transfected 293 cells (Martin and Sugden, 1991; Coffin et al., 2001). We have found that the chymotrypsin must be inactivated prior to cell lysis by adding the protease inhibitor phenylmethylsulfonyl fluoride (PMSF) and washing the cells twice with phosphate-buffered saline (PBS) in the presence of PMSF in order to prevent cleavage of intracellular LMP1. This treatment enabled us to generate a dose-dependent curve for digestion with the protease and to establish criteria for its plateau. Indeed, using the new treatment, we have found that only a small fraction of LMP1 is accessible to cleavage in Balb/3T3 cells (data not shown). Our results with 721 cells are consistent with those of previously published studies in which ∼30% of LMP1 was cleaved in an EBV-positive Burkitt lymphoma cell line Jijoye (Liebowitz et al., 1986). Most importantly, analyses with both electron and confocal microscopy have confirmed the results of the assay with chymotrypsin under our present conditions.
Both activated CD40 and LMP1 recruit TRAFs to lipid rafts. Upon binding to its ligand, CD40 translocates to lipid rafts at the plasma membrane and recruits TRAFs there. In contrast, association of LMP1 with lipid rafts and TRAFs occurs mainly within 293 and BJAB cells at sites distinct from the plasma membrane. In addition to the plasma membrane, lipid rafts have been shown to be present in Golgi, late endosomes and recycling endosomes (Heino et al., 2000; Chatterjee et al., 2001; Ikonen, 2001; Fivaz et al., 2002). LMP1 probably associates with lipid rafts in several of those intracellular compartments because it co-localized with internalized CTxB in both vesicular-like and perinuclear Golgi-like structures (Figures 1 and 4). It is fascinating though puzzling that although the fraction of LMP1 that associates with lipid rafts remains relatively constant from cell type to cell type, only in EBV-infected lymphoblastic cells does this association occur detectably at the plasma membrane. Studying the intracellular trafficking of LMP1 further should illuminate both the trafficking of lipid rafts per se and the mechanism by which LMP1 signals ligand independently.
Our findings with the chimera LMP1–CD40 indicate that CD40 signaling partners can associate in intracellular compartments containing lipid rafts to signal efficiently. The fusions of the amino/transmembrane domain of LMP1 to signaling domains of other members of the TNF-R family including TNF-R2 and Fas also lead to the activation of their signaling (Gires et al., 1997; A.Kaykas and B.Sugden, unpublished results), indicating that members of the TNF-R family are capable of associating with their signaling partners and signaling in intracellular sites as is LMP1. The ability of Fas to signal from intracellular compartments has been demonstrated in several prostate cancer cell lines that are resistant to Fas-mediated killing when treated extracellularly with FasL but become susceptible when these cells express FasL themselves (Hyer et al., 2000). It has been shown that several members of the TNF-R family including CD40, Fas and TNF-R can be co-expressed with their ligands in individual cells and act in autocrine loops in those cells (Dhein et al., 1995; Barker et al., 2001; Pham et al., 2002). We propose that these receptor–ligand pairs, when expressed simultaneously, interact to initiate signaling intracellularly. Targeting receptors to the plasma membrane therefore would occur for the purpose of sensing their extracellular ligands.
Materials and methods
Cell culture and plasmids
721 is an EBV-immortalized lymphoblastic cell line, BJAB is an EBV-negative Burkitt lymphoma cell line, 293 is a human embryonic kidney cell line and CNE is an EBV-negative nasopharyngeal carcinoma cell line. 721, BJAB and CNE cells were grown in RPMI 1640 supplemented with 10% fetal bovine serum (FBS). 293 cells were grown in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% FBS. All cell culture media were supplemented with 200 U/ml penicillin and 200 µg/ml streptomycin, and all cells were grown at 37°C in a humidified 5% CO2 atmosphere.
The expression plasmid for LMP1was generated by cloning the cDNA of LMP1 from the B95-8 strain of EBV into the pSG5 vector. The plasmid encoding 3LLMP1 was generated from a wild-type LMP1 expression plasmid by using PCR-mediated mutagenesis. The plasmid encoding TRAF2–GFP was generated by fusing the enhanced GFP (EGFP) from pEGFPC-1 (Clontech) in-frame to a TRAF2 expression vector. The expression plasmid for CATLMP1 was described before (Kaykas et al., 2001). The plasmid encoding the Sub2CΔ55 derivative of LMP1was generated from an expression plasmid for Sub2LMP1 (Sandberg et al., 1997). Sub2CΔ55LMP1 has substitutions of H203A, D209A and D210A, and a deletion of the last 55 amino acid of LMP1. The plasmid encoding LMP1–CD40 was described before (Kaykas et al., 2001).
Treatment of live cells with chymotrypsin
BJAB and CNE cells (5 × 106) were transfected with 1 µg of an expression plasmid for LMP1 by electroporation. 293 cells at ∼20–30% confluency in 6-well plates were transfected with 100 ng of an expression plasmid for LMP1 in each well by calcium phosphate precipitation. Transfected cells were analyzed 24 or 48 h after transfection. 721 or BJAB cells growing exponentially were harvested and washed twice with PBS. One million cells were then incubated with different concentrations of chymotrypsin diluted in RPMI 1640 in a volume of 300 µl. For 293 and CNE cells, all reactions were performed in 6-well plates 24 or 48 h after transfection. A 300 µl aliquot of chymotrypsin at different concentrations was added to each well. The cell–chymotrypsin mixtures were incubated at room temperature for 10 min or for different periods of time, as indicated in the figures legends. All reactions were stopped by adding 300 µl of cold PBS + 2 mM PMSF and two more washings with cold PBS + 2 mM PMSF. The cells were then lysed with 1× RIPA buffer (20 mM Tris–HCl pH 7.5, 150 mM NaCl, 1% Triton X-100, 0.5% deoxycholate, 0.5% SDS). The cell lysates were analyzed by SDS–PAGE and western blotting.
Immunogold electron microscopy
293 cells at 20–30% confluency in a 10 cm dish were transfected with 1 µg of an expression vector for LMP1 and harvested 48 h after transfection. All procedures were carried out at room temperature. 721 or 293 cells were fixed with 4% paraformaldehyde (PFA). Ten million cells were permeabilized with 5 ml of 0.0015% Triton X-100 (10% of Triton X-100’s critical micell concentration) + 25 µg/ml digitonin in PBS for 15 min, blocked with 1 ml of 5% goat serum + 5% calf serum + 0.1% acetylated bovine serum albumin (Aurion) + 0.1% cold water fish skin gelatin in PBS for 30 min, stained with 1 ml of CS1-4 monoclonal anti-LMP1 antibodies (DAKO) at 1:200 in PBS for 3 h, and then stained with 1 ml of ULTRA-SMALL gold-labeled goat anti-mouse antibodies (Aurion) at 1:80 in PBS for at least 4 h. After staining with antibodies, the cells were washed three times for 2 h each time, fixed with 200 µl of 4% PFA for 10 min, silver-enhanced with 300 µl of freshly prepared silver-enhancement reagent R-GENT SE-EM (Aurion), fixed with 1% glutaraldehyde for 30 min, fixed with 0.1% osmium tetroxide for 15 min, and finally embedded into Spurr’s resin. The silver enhancement allowed detection of the fine gold particles which were used to maximize penetration of the permeablized cells.
Ultra-thin sections of 70–90 µm of the embedded cells were examined on a Philips CM120 electron microscope.
Staining cells with cholera toxin
Exponentially growing 721 cells, transfected 293 or CNE cells growing on a coverslip 24 h after transfection were chilled on ice for 5 min. Cells were incubated on ice with 2.5 µg/ml fluorescein isothiocyanate (FITC)-conjugated cholera toxin in cell culture medium + 2% FBS at a concentration of 4 × 106 cells/ml. Unbound cholera toxin was removed by washing cells three times with cold cell culture medium. The cells were either fixed or shifted to 37°C. At 30 min after the temperature shift, the cells were fixed with 4% PFA and processed for immunostaining.
Immunofluorescent staining
293 cells transiently expressing LMP1 were transfected as described above. For experiments using TRAF2–GFP, 100 ng of an expression plasmid encoding TRAF2–GFP were transfected into each well of 6-well plates. For the LMP1–CD40 experiment, 250 ng of an expressing plasmid encoding LMP1-CD40 were transfected into each well of 6-well plates. Transfected cells were harvested 24–36 h after transfection. 721 cells harvested in a microcentrifuge tube, or 293 cells growing on a coverslip were fixed with 4% PFA–PBS solution. The fixed cells were permeabilized with 0.04% Triton X-100 in PBS and washed twice with PBS. The cells were blocked with 5% FBS in PBS, stained with primary antibodies for 1 h, and then stained with secondary antibodies for 1 h. The cells were washed three times with PBS + 5% FBS after staining with both primary and secondary antibodies. The following dilutions were used for each primary antibody: affinity purified rabbit anti-LMP1, 1:500; mouse monoclonal anti-LMP1 (Dako; CS1-4), 1:500; mouse monoclonal anti-TRADD (BD Transduction Lab), 1:250; rabbit anti-TRAF2 (Santa Cruz; H-249), 1:300; mouse monoclonal anti-AP1(Sigma), 1:250; mouse monoclonal anti-EEA1 (BD Transduction Lab), 1:250; mouse monoclonal anti-LAMP-1 (BD Transduction Lab), 1:250; rabbit anti-CD40 (Santa Cruz; C-20), 1:300. All secondary antibodies were used at 1:500 dilution. For double staining, AlexaFluor-488- and AlexaFluor-594-conjugated secondary antibodies (Molecular Probes) were used. For triple staining with TRAF2–GFP, AlexaFluor-594- and Cy5-conjugated secondary antibodies were used. The cells were mounted on slides with 50% glycerol and examined.
Confocal microscopy
Confocal microscopy was performed on a Bio-Rad MRC 1024 laser scanning confocal microscope as described (Kaykas et al., 2001).
Immunoprecipitation
293 cells transiently expressing LMP1 were harvested 24 h after transfection. 721 cells and 293 cells were lysed with 1× NET (50 mM Tris pH 7.5, 150 mM NaCl, 1 mM EDTA) + 0.5% Triton X-100 + 1 mM PMSF at a concentration of 2 × 107 cells/ml at room temperature for 5 min. The cell lysate was homogenized further by passing it through a 23-gauge needle six times. The lysate was cleared by centrifugation. The supernatant (150 µl, ∼3 × 106 cell equivalents) was precipitated with 2 µg of rabbit antibodies recognizing the N-terminus of TRAF1 (Santa Cruz H-132), TRAF2 (Santa Cruz N-19 and H-249) or TRAF3 (Santa Cruz, H-248). The precipitate was washed four times with 1× NET + 0.5% Triton X-100, resuspended in sample buffer, separated by SDS–PAGE and finally probed with mouse monoclonal anti-LMP1(DAKO, CS1-4) by western blotting. In a typical experiment, one-tenth of each precipitate (3 × 105 cell equivalents) was loaded on a gel. To calculate the amount of LMP1 being precipitated, 1.5 × 104 (5%), 3 × 104 (10%) and 6 × 104 (15%) cell equivalents of total cell lysate were also loaded as standards. LMP1 was quantified using ImageQuant software.
Assay for NF-κB and JNK activities
To assay the activation of NF-κB by LMP1, 293 cells were grown to 20–50% confluency in 6-well plates. Each well was transfected with a total of 6 µg of DNA with 10 ng of NF-κB-luciferase reporter plasmid, 5 ng of expression plasmid for Renilla luciferase, 0–300 ng of expression plasmid for LMP1 or 3LLMP1, and pSG5 vector via calcium phosphate precipitation. At 48 h after transfection, cells were harvested. Half of the cells were used for SDS–PAGE/western blot analysis. About 1 × 105 cells were assayed for luciferase activities using a Dual Luciferase Assay Kit (Promega) according to the manufacturer’s instructions. Transfection efficiencies were normalized by Renilla luciferase activities.
To assay JNK activities, 293 cells at 60–80% confluency in 6-well plates were transfected with LIPOFECTAMINE 2000 reagent (Invitrogen) in DMEM + 1% FBS according to the manufacturer’s instructions. Each well was transfected with a total of 6 µg of DNA with 1 µg of expression vector for hemagglutinin (HA)-tagged JNK1, 0 or 0.5 µg of expression plasmid for LMP1 or 3LLMP1, 30 ng of pEGFP, and pSG5 vector. Cells were kept in DMEM + 1% FBS throughout the transfection. At 24 h after the transfection, the cells were harvested and a quarter of them were used for SDS–PAGE/western blot analysis. The rest of the cells were lysed in kinase lysis buffer [20 mM Tris pH 7.5, 1% Triton X-100, 150 mM NaCl, 2 mM EGTA, 2 mM EDTA, 2 mM sodium vanadate, 1 mM dithiothreitol (DTT), 0.5 mM NaF, 0.5 mM β-glycerophosphate]. The cell lysates were immunoprecipitated with anti-HA covalently linked to Sepharose (Corvex). The precipitates were washed three times with kinase assay buffer (20 mM Tris pH 7.5, 2 mM β-glycerophosphate, 10 mM MgCl2, 1 mM DTT, 50 µM sodium vanadate, 20 mM NaCl). Half of the precipitates was then incubated with 1 µg of GST–JUN (Calbiochem) and 10 µCi of [γ-32P]ATP, 20 µM ATP in kinase assay buffer in a total of 20 µl at 30°C for 30 min. The kinase reactions were stopped by adding 20 µl of SDS–PAGE loading buffer and boiled at 95°C for 5 min. A 20 µl aliquot of each reaction was loaded on a 12% polyacrylamide gel. Phosphorylated GST–Jun was quantified by using ImageQuant software. Transfection efficiencies were monitored by GFP-positive cells and western blottings to quantify HA-JNK1 from total cell lysates.
SDS–PAGE and western blotting
Cell lysates were separated on 10% denaturing polyacrylamide gels and transferred to nitrocellulose membranes. The blots were blocked with 5% non-fat milk, and probed with affinity-purified rabbit anti-LMP1 antibodies at 1:500 dilution, and followed either by alkaline phosphatase-conjugated goat anti-rabbit antibodies or, for quantitative western blotting, by 35S-labeled goat anti-rabbit antibodies. For quantitative western blotting, different amounts of lysate were loaded on each gel to establish a standard curve. The signals were quantified by using ImageQuant software.
Acknowledgments
Acknowledgements
We would like to thank Randall Massey, Ben August and Michael Schwartz for their help on electron microscopy, and Paul Ahlquist, Alan Rapraeger and John Young for their critical reviews of the manuscript. This work is supported by NIH grants CA22443, CA07175, CA09135 and CA70723. B.S. is an American Cancer Society Research Professor.
References
- Ardila-Osorio H., Clausse,B., Mishal,Z., Wiels,J., Tursz,T. and Busson,P. (1999) Evidence of LMP1–TRAF3 interactions in glycosphingolipid-rich complexes of lymphoblastoid and nasopharyngeal carcinoma cells. Int. J. Cancer, 81, 645–649. [DOI] [PubMed] [Google Scholar]
- Barker V., Middleton,G., Davey,F. and Davies,A.M. (2001) TNFα contributes to the death of NGF-dependent neurons during development. Nat. Neurosci., 4, 1194–1198. [DOI] [PubMed] [Google Scholar]
- Berberich I., Shu,G.L. and Clark,E.A. (1994) Cross-linking CD40 on B cells rapidly activates nuclear factor-κB. J. Immunol., 153, 4357–4366. [PubMed] [Google Scholar]
- Chatterjee S., Smith,E.R., Hanada,K., Stevens,V.L. and Mayor,S. (2001) GPI anchoring leads to sphingolipid-dependent retention of endocytosed proteins in the recycling endosomal compartment. EMBO J., 20, 1583–1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffin W.F. III, Erickson,K.D., Hoedt-Miller,M. and Martin,J.M. (2001) The cytoplasmic amino-terminus of the latent membrane protein-1 of Epstein–Barr virus: relationship between transmembrane orientation and effector functions of the carboxy-terminus and transmembrane domain. Oncogene, 20, 5313–5330. [DOI] [PubMed] [Google Scholar]
- Coffin W.F. III, Geiger,T.R. and Martin,J.M. (2003) Transmembrane domains 1 and 2 of the latent membrane protein 1 of Epstein–Barr virus contain a lipid raft targeting signal and play a critical role in cytostasis. J. Virol., 77, 3749–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson C.W., George,J.H., Blake,S.M., Longnecker,R. and Young,L.S. (2001) The Epstein–Barr virus encoded latent membrane protein 2A augments signaling from latent membrane protein 1. Virology, 289, 192–207. [DOI] [PubMed] [Google Scholar]
- Devergne O., Hatzivassiliou,E., Izumi,K.M., Kaye,K.M., Kleijnen,M.F., Kieff,E. and Mosialos,G. (1996) Association of TRAF1, TRAF2 and TRAF3 with an Epstein–Barr virus LMP1 domain important for B-lymphocyte transformation: role in NF-κB activation. Mol. Cell. Biol., 16, 7098–7108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devergne O., Cahir McFarland,E.D., Mosialos,G., Izumi,K.M., Ware,C.F. and Kieff,E. (1998) Role of the TRAF binding site and NF-κB activation in Epstein–Barr virus latent membrane protein 1-induced cell gene expression. J. Virol., 72, 7900–7908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhein J., Walczak,H., Baumler,C., Debatin,K.M. and Krammer,P.H. (1995) Autocrine T-cell suicide mediated by APO-1/(Fas/CD95). Nature, 373, 438–441. [DOI] [PubMed] [Google Scholar]
- Eliopoulos A.G. and Young,L.S. (1998) Activation of the cJun N-terminal kinase (JNK) pathway by the Epstein–Barr virus-encoded latent membrane protein 1 (LMP1). Oncogene, 16, 1731–1742. [DOI] [PubMed] [Google Scholar]
- Fivaz M., Vilbois,F., Thurnheer,S., Pasquali,C., Abrami,L., Bickel,P.E., Parton,R.G. and Van Der Goot,F.G. (2002) Differential sorting and fate of endocytosed GPI-anchored proteins. EMBO J., 21, 3989–4000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gires O., Zimber-Strobl,U., Gonnella,R., Ueffing,M., Marschall,G., Zeidler,R., Pich,D. and Hammerschmidt,W. (1997) Latent membrane protein 1 of Epstein–Barr virus mimics a constitutively active receptor molecule. EMBO J., 16, 6131–6140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gires O. et al. (1999) Latent membrane protein 1 of Epstein–Barr virus interacts with JAK3 and activates STAT proteins. EMBO J., 18, 3064–3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grewal I.S. and Flavell,R.A. (1998) CD40 and CD154 in cell-mediated immunity. Annu. Rev. Immunol., 16, 111–135. [DOI] [PubMed] [Google Scholar]
- Hanissian S.H. and Geha,R.S. (1997) Jak3 is associated with CD40 and is critical for CD40 induction of gene expression in B cells. Immunity, 6, 379–387. [DOI] [PubMed] [Google Scholar]
- Hatzivassiliou E., Miller,W.E., Raab-Traub,N., Kieff,E. and Mosialos,G. (1998) A fusion of the EBV latent membrane protein-1 (LMP1) transmembrane domains to the CD40 cytoplasmic domain is similar to LMP1 in constitutive activation of epidermal growth factor receptor expression, nuclear factor-κB and stress-activated protein kinase. J. Immunol., 160, 1116–21. [PubMed] [Google Scholar]
- Heino S., Lusa,S., Somerharju,P., Ehnholm,C., Olkkonen,V.M. and Ikonen,E. (2000) Dissecting the role of the Golgi complex and lipid rafts in biosynthetic transport of cholesterol to the cell surface. Proc. Natl Acad. Sci. USA, 97, 8375–8380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuchi M., Izumi,K.M. and Kieff,E. (2001) Epstein–Barr virus latent-infection membrane proteins are palmitoylated and raft-associated: protein 1 binds to the cytoskeleton through TNF receptor cytoplasmic factors. Proc. Natl Acad. Sci. USA, 98, 4675–4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hostager B.S., Catlett,I.M. and Bishop,G.A. (2000) Recruitment of CD40 and tumor necrosis factor receptor-associated factors 2 and 3 to membrane microdomains during CD40 signaling. J. Biol. Chem., 275, 15392–15398. [DOI] [PubMed] [Google Scholar]
- Hyer M.L., Voelkel-Johnson,C., Rubinchik,S., Dong,J. and Norris,J.S. (2000) Intracellular Fas ligand expression causes Fas-mediated apoptosis in human prostate cancer cells resistant to monoclonal antibody-induced apoptosis. Mol. Ther., 2, 348–358. [DOI] [PubMed] [Google Scholar]
- Ikonen E. (2001) Roles of lipid rafts in membrane transport. Curr. Opin. Cell Biol., 13, 470–477. [DOI] [PubMed] [Google Scholar]
- Izumi K.M. and Kieff,E.D. (1997) The Epstein–Barr virus oncogene product latent membrane protein 1 engages the tumor necrosis factor receptor-associated death domain protein to mediate B lymphocyte growth transformation and activate NF-κB. Proc. Natl Acad. Sci. USA, 94, 12592–12597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeng R.L. and Welch,M.D. (2001) Cytoskeleton: actin and endocytosis—no longer the weakest link. Curr. Biol., 11, R691–R694. [DOI] [PubMed] [Google Scholar]
- Kaye K.M., Devergne,O., Harada,J.N., Izumi,K.M., Yalamanchili,R., Kieff,E. and Mosialos,G. (1996) Tumor necrosis factor receptor associated factor 2 is a mediator of NF-κB activation by latent infection membrane protein 1, the Epstein–Barr virus transforming protein. Proc. Natl Acad. Sci. USA, 93, 11085–11090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaykas A., Worringer,K. and Sugden,B. (2001) CD40 and LMP-1 both signal from lipid rafts but LMP-1 assembles a distinct, more efficient signaling complex. EMBO J., 20, 2641–2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieser A., Kilger,E., Gires,O., Ueffing,M., Kolch,W. and Hammerschmidt,W. (1997) Epstein–Barr virus latent membrane protein-1 triggers AP-1 activity via the c-Jun N-terminal kinase cascade. EMBO J., 16, 6478–6485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieser A., Kaiser,C. and Hammerschmidt,W. (1999) LMP1 signal transduction differs substantially from TNF receptor 1 signaling in the molecular functions of TRADD and TRAF2. EMBO J., 18, 2511–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilger E., Kieser,A., Baumann,M. and Hammerschmidt,W. (1998) Epstein–Barr virus-mediated B-cell proliferation is dependent upon latent membrane protein 1, which simulates an activated CD40 receptor. EMBO J., 17, 1700–1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwok Fung Lo A., Liu,Y., Wang,X., Wong,Y.C., Kai Fai Lee,C., Huang,D.P. and Tsao,S.W. (2001) Identification of downstream target genes of latent membrane protein 1 in nasopharyngeal carcinoma cells by suppression subtractive hybridization. Biochim. Biophys. Acta, 1520, 131–140. [DOI] [PubMed] [Google Scholar]
- Liebowitz D., Wang,D. and Kieff,E. (1986) Orientation and patching of the latent infection membrane protein encoded by Epstein–Barr virus. J. Virol., 58, 233–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin J. and Sugden,B. (1991) The latent membrane protein oncoprotein resembles growth factor receptors in the properties of its turnover. Cell Growth Differ., 2, 653–660. [PubMed] [Google Scholar]
- McPherson P.S. (2002) The endocytic machinery at an interface with the actin cytoskeleton: a dynamic, hip intersection. Trends Cell Biol., 12, 312–315. [DOI] [PubMed] [Google Scholar]
- Mitchell T. and Sugden,B. (1995) Stimulation of NF-κB-mediated transcription by mutant derivatives of the latent membrane protein of Epstein–Barr virus. J. Virol., 69, 2968–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan M., Thorburn,J., Pandolfi,P.P. and Thorburn,A. (2002) Nuclear and cytoplasmic shuttling of TRADD induces apoptosis via different mechanisms. J. Cell Biol., 157, 975–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosialos G., Birkenbach,M., Yalamanchili,R., VanArsdale,T., Ware,C. and Kieff,E. (1995) The Epstein–Barr virus transforming protein LMP1 engages signaling proteins for the tumor necrosis factor receptor family. Cell, 80, 389–399. [DOI] [PubMed] [Google Scholar]
- Pham L.V., Tamayo,A.T., Yoshimura,L.C., Lo,P., Terry,N., Reid,P.S. and Ford,R.J. (2002) A CD40 signalosome anchored in lipid rafts leads to constitutive activation of NF-κB and autonomous cell growth in B cell lymphomas. Immunity, 16, 37–50. [DOI] [PubMed] [Google Scholar]
- Pullen S.S., Miller,H.G., Everdeen,D.S., Dang,T.T., Crute,J.J. and Kehry,M.R. (1998) CD40–tumor necrosis factor receptor-associated factor (TRAF) interactions: regulation of CD40 signaling through multiple TRAF binding sites and TRAF hetero-oligomerization. Biochemistry, 37, 11836–11845. [DOI] [PubMed] [Google Scholar]
- Pullen S.S., Labadia,M.E., Ingraham,R.H., McWhirter,S.M., Everdeen,D.S., Alber,T., Crute,J.J. and Kehry,M.R. (1999) High-affinity interactions of tumor necrosis factor receptor-associated factors (TRAFs) and CD40 require TRAF trimerization and CD40 multimerization. Biochemistry, 38, 10168–10177. [DOI] [PubMed] [Google Scholar]
- Rothenberger S., Rousseaux,M., Knecht,H., Bender,F.C., Legler,D.F. and Bron,C. (2002) Association of the Epstein–Barr virus latent membrane protein 1 with lipid rafts is mediated through its N-terminal region. Cell. Mol. Life Sci., 59, 171–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakata N., Patel,H.R., Terada,N., Aruffo,A., Johnson,G.L. and Gelfand,E.W. (1995) Selective activation of c-Jun kinase mitogen-activated protein kinase by CD40 on human B cells. J. Biol. Chem., 270, 30823–30828. [DOI] [PubMed] [Google Scholar]
- Sandberg M. (1999) Characterization of the domains of LMP1 of Epstein–Barr virus. PhD Thesis, McArdle Laboratory for Cancer Research, University of Wisconsin-Madison, Madison, WI.
- Sandberg M., Hammerschmidt,W. and Sugden,B. (1997) Characterization of LMP-1’s association with TRAF1, TRAF2 and TRAF3. J. Virol., 71, 4649–4656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer D.A. (2002) Coupling actin dynamics and membrane dynamics during endocytosis. Curr. Opin. Cell Biol., 14, 76–81. [DOI] [PubMed] [Google Scholar]
- Shogomori H. and Futerman,A.H. (2001) Cholesterol depletion by methyl-β-cyclodextrin blocks cholera toxin transport from endosomes to the Golgi apparatus in hippocampal neurons. J. Neurochem., 78, 991–999. [DOI] [PubMed] [Google Scholar]
- Uchida J., Yasui,T., Takaoka-Shichijo,Y., Muraoka,M., Kulwichit,W., Raab-Traub,N. and Kikutani,H. (1999) Mimicry of CD40 signals by Epstein–Barr virus LMP1 in B lymphocyte responses. Science, 286, 300–303. [DOI] [PubMed] [Google Scholar]
- van Kooten C. (2000) Immune regulation by CD40–CD40-l interactions—2; Y2K update. Front. Biosci., 5, D880–D893. [DOI] [PubMed] [Google Scholar]
- van Kooten C. and Banchereau,J. (1997) Functions of CD40 on B cells, dendritic cells and other cells. Curr. Opin. Immunol., 9, 330–337. [DOI] [PubMed] [Google Scholar]
- Vidalain P.O., Azocar,O., Servet-Delprat,C., Rabourdin-Combe,C., Gerlier,D. and Manie,S. (2000) CD40 signaling in human dendritic cells is initiated within membrane rafts. EMBO J., 19, 3304–3313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf A.A., Fujinaga,Y. and Lencer,W.I. (2002) Uncoupling of the cholera toxin–G(M1) ganglioside receptor complex from endocytosis, retrograde Golgi trafficking and downstream signal transduction by depletion of membrane cholesterol. J. Biol. Chem., 277, 16249–16256. [DOI] [PubMed] [Google Scholar]