Abstract
Dysregulation of extracellular matrix turnover is an important feature of many inflammatory processes. Rat renal mesangial cells express high levels of matrix metalloproteinase 9 (MMP-9) in response to inflammatory cytokines such as interleukin-1 beta. We demonstrate that NO does strongly destabilize MMP-9 mRNA, since different luciferase reporter gene constructs containing the MMP-9 3′ untranslated region (UTR) displayed significant reduced luciferase activity in response to the presence of NO. Moreover, by use of an in vitro degradation assay we found that the cytoplasmic fractions of NO-treated cells contained a higher capacity to degrade MMP-9 transcripts than those obtained from control cells. An RNA electrophoretic mobility shift assay demonstrated that three of four putative AU-rich elements present in the 3′ UTR of MMP-9 were constitutively occupied by the mRNA-stabilizing factor HuR and that the RNA binding was strongly attenuated by the presence of NO. The addition of recombinant glutathione transferase-HuR prevented the rapid decay of MMP-9 mRNA, whereas the addition of a neutralizing anti-HuR antibody caused an acceleration of MMP-9 mRNA degradation. Furthermore, the expression of HuR mRNA and protein was significantly reduced by exogenously and endogenously produced NO. These inhibitory effects were mimicked by the cGMP analog 8-bromo-cGMP and reversed by LY-83583, an inhibitor of soluble guanylyl cyclase. These results demonstrate that NO acts in a cGMP-dependent mechanism to inhibit the expression level of HuR, thereby reducing the stability of MMP-9 mRNA.
Remodeling of extracellular matrix (ECM) is an important feature of normal growth and developmental processes. Consequently, an imbalance of ECM synthesis and degradation is associated with many diseases. Although changes in the synthesis of ECM may play a certain role in dysregulation of matrix turnover, recent studies have underlined the paramount role of ECM-degradative systems. The main proteases regulating physiological degradation of ECM are the matrix metalloproteinases (MMPs), a family of zinc-dependent enzymes, including the interstitial collagenases, stromelysins, elastases, membrane-type MMPs, and gelatinases (8, 38, 61, 62). Tight regulation of most of these proteases is accomplished by different mechanisms, including the regulation of MMP gene expression, the processing and conversion of the inactive zymogens by other proteases, and, finally, the inhibition of active MMPs by endogenous inhibitors of MMPs, the tissue inhibitors of metalloproteinases (for a review, see reference 38).
Cultured mesangial cells (MC) respond to proinflammatory cytokines such as tumor necrosis factor alpha (TNF-α) or interleukin-1 beta (IL-1β) with the production of several MMPs, including MMP-9 (gelatinase-B), mainly by an increase of gene expression (20, 65). In addition to MMPs, MC exposed to cytokines produce high levels of NO through the expression of the inducible NO synthase (iNOS) gene (30, 42). Whereas the early and rapid actions of NO signaling affect posttranslational modifications of cellular proteins, long-term regulation is executed primarily on the level of gene transcription (45).
In MC, interestingly, NO can regulate the expression of a variety of ECM-related genes, including those encoding secreted protein acidic and rich in cysteine (55), tissue inhibitor of metalloproteinase 1 (19), tissue plasminogen activator (21), and MMP-9 (19). However, the detailed mechanisms of these events are still unknown. By examining a 1.8-kb region of the rat MMP-9 promoter by means of reporter gene assays, Eberhardt et al. were able to demonstrate that NO, either given by exogenous NO donors or endogenously produced after induction of the iNOS, has minimal effect on cytokine-induced MMP-9 promoter activity (23). Moreover, in rat MC none of the candidate NO-sensitive transcription factors, including nuclear factor κB (NF-κB) and activated protein 1 (AP-1) (31, 46, 54), are altered by NO, thus demonstrating that the NO-mediated effects on MMP-9 expression are mainly due to posttranscriptional effects. Posttranscriptional regulation of mRNA in the cytoplasm is recognized as an important control point in mRNA turnover and includes the localization and stability of mRNA but also translation of mRNA (18, 40, 57). The control of selective mRNA degradation of many inducible genes, including those for cytokines, nuclear transcription factors, and proto-oncogenes, seems to be localized within the 3′ untranslated regions (UTRs) of genes by sequence motifs which contain AU-rich sequences also designated AU-rich elements (AREs) (12, 40, 49, 58). The 3′ untranslated region of the rat MMP-9 gene bears several copies of AUUUA motifs, thus indicating that MMP-9, in similarity to a variety of other genes expressed during inflammation, may also be regulated on the level of mRNA stability.
By use of RNA-binding assays, several studies have identified proteins specifically binding to AREs (most interestingly, proteins which play a role in stabilizing mRNAs). Most prominent among these are members of the embryonic lethal abnormal vision (ELAV) protein family (for reviews, see references 3 and 7), but factors which can destabilize mRNA, such as members of the AUF1 and hnRNPD families (24, 59), were also identified. Members of both families have therefore been implicated in the regulation of mRNA turnover (28, 67).
Eberhardt et al. recently reported that activators of the peroxisome proliferator-activated receptor α indirectly (via increased induction of NO synthesis) and significantly reduce the stability of MMP-9 mRNA (23). With regard to the presence of several AREs in the 3′ UTR of MMP-9, we have now elucidated the involvement of the RNA-stabilizing factor HuR (HuA), a member of the ELAV family, in the modulation by NO of MMP-9 mRNA stability.
MATERIALS AND METHODS
Reagents.
Human recombinant IL-1β was from Cell Concept (Umkirch, Germany). The NO donors DETA NONOate and S-nitroso-N-acetyl-d,l-penicillamine (SNAP) were from Alexis Biochemicals (Grünberg, Germany). The NOS inhibitor NG-monomethyl-l-arginine (L-NMMA) and actinomycin D (from Streptomyces species) were purchased from Alexis Biochemicals. Ribonucleotides and restriction enzymes as well as modifying enzymes were purchased from Roche Diagnostics GmbH (Mannheim, Germany). RNA oligonucleotides were synthesized from Whatman-Biometra (Göttingen, Germany).
Cell culture.
Rat glomerular MC were characterized as described previously (43) and grown in RPMI 1640 supplemented with 10% fetal calf serum, 2 mM glutamine, 5 ng of insulin/ml, 100 U of penicillin/ml, and 100 μg of streptomycin/ml. Serum-free preincubations were performed for 24 h in Dulbecco's modified Eagle's medium supplemented with 0.1 mg of fatty acid-free bovine serum albumin/ml before cytokine treatment. For experiments, 3.0 × 106 to 5.0 × 106 MC per 10-cm-diameter culture dish were used between passages 8 and 19. All cell culture media and supplements were purchased from GIBCO/BRL-Life Technologies (Eggenstein, Germany).
cDNA clones and plasmids.
cDNA inserts for rat MMP-9 were generated as described recently (19). A 1.6-kb ApaI fragment of HuR9 (34) (containing the entire coding sequence of HuR) was cloned in sense orientation into pZeoSV2(-) to generate pZeoSV2-HuR sense as described recently (48). The plasmid pGEX-HuR (encompassing a cDNA encoding residues 2 to 326 of HuR) was generated as described previously (34). A glyceraldehyde-3-phosphate dehydrogenase (GAPDH) cDNA clone was generated using internal primers of coding sequences of rat GAPDH mRNA (GenBank/EMBL databases, accession no. NM 017008). A cDNA insert from mouse 18S rRNA was from Ambion (Austin, Tex.).
Generation of reporter plasmids and transient transfection of MC.
The luc-MMP-9 reporter gene pGL3-MMP-9 1.3kb, encompassing a 1.3-kb fragment of the 5′-flanking region of the rat MMP-9 gene, was cloned as described previously (22). The plasmid 3′-UTR-MMP-9 pGL3-MMP-9-1.3 kb was generated by cloning a 662-bp fragment from the 3′ UTR of rat MMP-9 mRNA into the pGL3-MMP-9-1.3kb promoter plasmid. Using the XbaI-flanked (underlined) forward primer 5′-TATTCTAGACCAACCTTTACCAGCTACTCGAA-3′ and BamHI-flanked (underlined) reverse primer 5′-TATGGATCCATTCATTTATTTAAAAAAGAGTGT-3, corresponding to regions from nucleotide 2315 to 2338 and 2954 to 2977 of the rat MMP-9 cDNA (GenBank accession no. U24441), the 3′ UTR sequence of the rat MMP-9 gene was generated by PCR from reverse transcriptase products. The PCR products subsequently were digested with XbaI and BamHI restriction enzymes and cloned into the BamHI/XbaI-cut pGL3-MMP-9-1.3kb plasmid, thereby allowing a forced insertion of the 3′ UTR of rat MMP-9 mRNA at the 3′ end of the luc+ coding region of pGL3-MMP-9-1.3kb. Similarly, the BamHI/XbaI-cut PCR fragment was cloned into an empty pGL3 control vector to generate a 3′-UTR-MMP-9 pGL3 control. This vector was used parallel in luciferase assays to test the influence of the 3′ UTR of MMP-9 on the stability of luciferase mRNA under the control of a non-MMP-9-related promoter sequence.
Transient transfections of MC were performed using Effectene reagent (Qiagen, Hilden, Germany). The transfections were performed according to the manufacturer's instructions. Transfections with pRL-CMV coding for Renilla luciferase activities were measured with a dual reporter gene system (Promega Corp., Mannheim, Germany) by use of an automated chemiluminescence detector (Berthold, Bad Wildbad, Germany).
Northern blot analysis.
Total cellular RNA was extracted from MC with Tri reagent (Sigma, Deisenhofen, Germany), and RNA was hybridized following standard procedures as described previously (19).
Preparation of cytoplasmic extracts.
Cytoplasmic lysates were separated as described previously (22). Briefly, cytoplasmic lysates were prepared from rat MC by lysis in a hypotone extraction buffer containing 10 mM HEPES (pH 7.9), 10 mM KCl, 0.1 mM EDTA, 0.1 mM EGTA, 10 μg of aprotinin/ml, 10 μg of leupeptin/ml, and 100 μg phenylmethylsulfonyl fluoride/ml. Nuclei were removed by centrifugation (1,200 × g for 15 min), and the supernatants were used as cytoplasmic fractions. To achieve RNase-free conditions, all buffers used were prepared with diethyl pyrocarbonate-treated water.
RNA electrophoretic mobility shift assay (EMSA) and supershift analysis.
Using T4 polynucleotide kinase, radiolabeled single-stranded RNA oligonucleotides were prepared by kinase reaction. The labeled oligonucleotides were subsequently separated by nick-Sephadex columns (Amersham Pharmacia Biotech, Freiburg, Germany). The sequences for gene-specific oligonucleotides are summarized in Table 1. The radiolabeled RNA oligonucleotides (30,000 cpm/reaction) were incubated with 6 μg of cytoplasmic extract and incubated at room temperature for 15 min in a buffer containing 10 mM HEPES (pH 7.6), 3 mM MgCl2, 40 mM KCl, 2 mM dithiothreitol, 5% glycerol, and 0.5% Nonidet P-40. To reduce nonspecific binding, total Saccharomyces cerevisiae RNA (final concentration, 200 ng/ml) was added. The total volume of each reaction was 10 μl. RNA-protein complexes were separated in 6% nondenaturating polyacrylamide gels and run in Tris-borate-EDTA buffer.
TABLE 1.
Oligonucleotides used in EMSA and derived from the region encompassing AUUUA-rich motifs from the 3′ UTR of the rat MMP-9 genea
| Oligonucleotide | Starting ntb | Sequence | Ending nt |
|---|---|---|---|
| UTR-ARE-1 | 2500 | 5′-CCCUUUUAUUUAUUAUGUAUG-3′ | 2520 |
| UTR-ARE-1mut | 2500 | 5′-CCCUUUUGGGCCUGAUGUAUG-3′ | 2520 |
| UTR-ARE-2 | 2536 | 5′-ACAUGUAUUUAACCUAUAGAA-3′ | 2556 |
| UTR-ARE-3 | 2683 | 5′-CAUCAAACAUUUAUUGUGAGC-3′ | 2704 |
| UTR-ARE-4 | 2742 | 5′-CAGAGGAAUUUAUUGGAUGUU-3′ | 2762 |
The nucleotide positions are those corresponding to the rat MMP-9 gene (GenBank accession no. U24441). UTR-ARE-1mut was used for competition assays. The consensus sequences are indicated by boldface. Mutated bases changed in the UTR-ARE-1mut oligonucleotide are underlined.
nt, nucleotide.
Supershift analysis was done by addition of 200 ng of antibody 15 min after the addition of the radioactively labeled RNA oligonucleotide and incubation for a further 15 min at room temperature. All antibodies used for supershift analysis were purchased from Santa Cruz Biotechnology (Heidelberg, Germany).
Purification of GST-HuR protein.
Glutathione S-transferase (GST)-HuR fusion protein was prepared by using the plasmid pGEX-HuR as described previously (34). The purification procedure was performed as described previously (48).
In vitro degradation assay.
Degradation assays were performed as described previously (33) but with the following modifications. Instead of using in vitro-transcribed radioactively labeled MMP-9 mRNA, we used preparations from cytokine-induced total RNAs, thereby yielding a large amount of MMP-9 mRNA at a steady-state level. A total of 20 μg of total RNA from one pool was aliquoted and subsequently incubated at room temperature with 130 μg of cytoplasmic extract derived from untreated or, alternatively, from NO-treated MC for different times. At each time point indicated, the RNA from these incubations was isolated by standard procedures and assessed by Northern blot analysis by means of a 32P-labeled cDNA insert specific for rat MMP-9 (19). The remaining (undegraded) MMP-9 hybridized signals were visualized and quantified densitometrically using an automated detector system (BAS 1500) from Fujifilm (Raytest, Struabenhardt, Germany).
HuR neutralization experiments.
The impact of HuR on the degradation of MMP-9 mRNA was tested by the addition of neutralizing anti-HuR antibodies (Santa Cruz Biotechnology). Antibodies specifically raised against different Hu family members (HuA, HuB, HuC, and HuD) of humans were purchased from Santa Cruz. A total of 0.4 μg of the antibody was added to the cytoplasmic extracts and preincubated at room temperature for 1 h before addition of the RNA samples. To exclude any unspecific inhibitory effects induced by the serum, we tested normal mouse serum in parallel.
To further test the effects of purified recombinant HuR on the degradation of MMP-9 mRNA in the in vitro degradation assay, 200 ng of GST-HuR or, alternatively, GST protein alone was added to the cytoplasmic extract from untreated MC and simultaneously incubated with the RNA samples for 2 h at room temperature.
Western blot analysis.
The total cellular level of HuR protein was analyzed by Western blot analysis using total cellular extracts (50 μg) and probed with specific antibodies. A monoclonal antibody specifically raised against full-length human HuR was obtained from Santa Cruz Biotechnologies. A polyclonal antibody specific for human β-actin was purchased from Chemicon (Hofheim, Germany).
Statistical analysis.
Results are expressed as means ± standard errors of the means (SEM). The data are presented as severalfold induction compared to control conditions or compared to IL-1β-stimulated values. Statistical analysis was performed using Student's t test and an analysis of variance test for significance. P values of <0.05 and <0.01 were considered significant as described for Fig. 3.
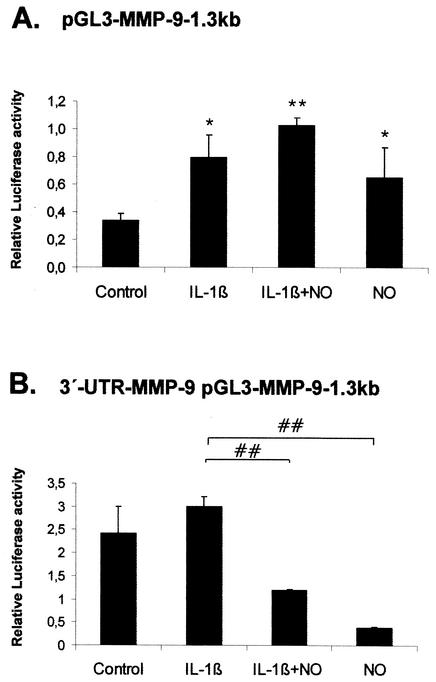
FIG. 3.
Influence of the 3′ UTR of MMP-9 on MMP-9 promoter-driven luciferase activities. Subconfluent MC were transiently cotransfected with 0.4 μg of pGL-MMP-9-1.3kb (A) or alternatively with 0.4 μg of 3′-UTR-MMP-9 pGL-MMP-9-1.3kb (B) containing in addition the 3′ UTR of MMP-9 downstream of the luciferase coding sequence. For each transfection, 0.1 μg of pRL-CMV coding for Renilla luciferase was simultaneously added. After an overnight transfection, MC were treated for 24 h with vehicle (control) or with IL-1β (2 nM) in the presence or absence of DETA NONOate (500 μM) as indicated. The values for beetle luciferase were related to values for Renilla luciferase and are depicted as relative luciferase activities. Data (means ± SEM) represent the results of three different experiments. P values were 0.05 and 0.01 versus control conditions (∗ and ∗∗, respectively) and 0.01 versus IL-1β-stimulated conditions (##).
RESULTS
Nitric oxide reduces the mRNA stability of MMP-9.
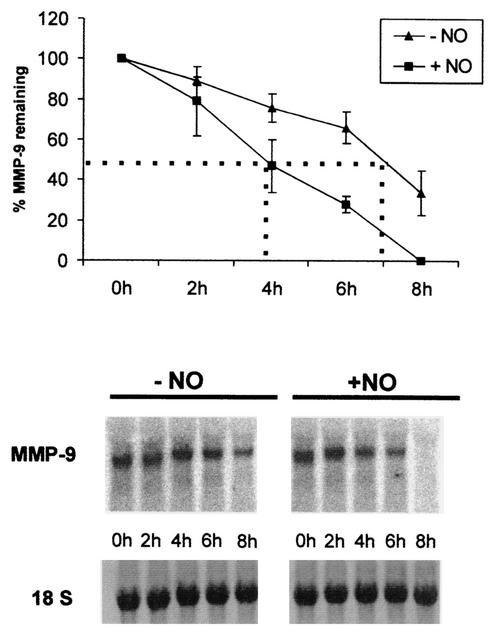
MMP-9 is synthesized in response to diverse stimuli, including cytokines, growth factors, and hormones. Most importantly, the induced expression of MMP-9 can be modulated by oxidative and nitrosative stress conditions (19, 20). We have now demonstrated that the inhibitory effects of NO on the cytokine-induced levels of MMP-9 mRNA and gelatinolytic content of rat glomerular mesangial cells are mainly due to a reduction of the stability of MMP-9 mRNA, since actinomycin D experiments indicated a potent acceleration in the MMP-9 mRNA degradation as demonstrated by Northern blot analysis (Fig. 1). MC were stimulated for 20 h with IL-1β (2 nM) before being treated with the NO donor DETA NONOate (500 μM). The reduction of MMP-9 mRNA in the absence of NO occurred with a half-life of ∼7 h. Interestingly, the addition of DETA NONOate markedly decreased MMP-9 mRNA stability, with a half-life of ∼4 h (Fig. 1, lower panels). The decrease in mRNA stability is most impressively documented by the complete loss of MMP-9 transcripts 8 h after the addition of the NO donor (Fig. 1). Similarly, Eberhardt et al. found that the endogenous NO level produced by the induction of iNOS is sufficient to stimulate MMP-9 mRNA degradation (23). Concomitantly, the presence of DETA NONOate does not affect either the cytokine-induced or basal promoter activity of a luciferase reporter gene encompassing 1.8 kb of the 5′-flanking region of the rat MMP-9 gene (23). These data strongly suggest the involvement of posttranscriptional mechanisms in the regulation of MMP-9 expression by NO.
FIG. 1.
Accelerated degradation of cytokine-induced MMP-9 mRNA by DETA NONOate. Quiescent MC were treated for 20 h with IL-1β (2 nM) and then washed twice before the addition of actinomycin D (5 μg/ml). After a short preincubation of 30 min, cells were additionally treated for the indicated time points without (−NO) or with (+NO) DETA NONOate (500 μM) before being harvested and extracted for total cellular RNA. For Northern blot analysis, 20 μg of total cellular RNA was hybridized to a 32P-labeled MMP-9 probe. The equivalent loading of RNA was ascertained by subsequent hybridization with an 18S rRNA probe. The results of a representative experiment are shown in the lower part of the figure. The upper panel shows means ± standard deviations (n = 3) of three independent experiments and depicts the percentages of remaining MMP-9 transcripts after addition of DETA NONOate (NO).
The 3′ UTR of MMP-9 mRNA confers NO responsiveness to the stability of different mRNAs.
An important paradigm for posttranscriptional regulation is the control of cytoplasmic mRNA stability, which is mediated by the 3′ UTR of mRNAs. Therefore, we focused on the 3′ UTR of the rat MMP-9 mRNA which contains four copies of AUUUA pentameric motifs potentially involved in the regulation of MMP-9 mRNA turnover (Fig. 2). The impact of this region on changes in mRNA stability by NO was investigated by transient transfection of a luciferase reporter gene driven by a 1.3-kb fragment of the rat MMP-9 promoter containing either the 3′ UTR of MMP-9 downstream of the luciferase reporter gene (3′-UTR-MMP-9pGL3MMP-9-1.3 kb) or no additional 3′-regulatory sequences (pGL3-MMP-9-1.3 kb). Therefore, the final luciferase activity measured in MC transiently transfected with luciferase constructs bearing the 3′ UTR of MMP-9 was not only the result of the activity of the passenger MMP-9 promoter but in addition depended on the stability of luciferase mRNA regulated by the 3′ UTR of MMP-9. Transient transfection of MC with pGL3MMP-9-1.3 kb was followed by a 24-h treatment with one of the following vehicles: IL-1β (2 nM), DETA NONOate (500 μM), or IL-1β plus DETA NONOate.
FIG. 2.
Sequence of the 3′ UTR of the rat MMP-9 gene (GenBank accession no. U24441). Potential ARE binding sites involved in the posttranscriptional regulation of MMP-9 mRNA are depicted in boxes. The numbers of AREs were used to distinguish between the different ARE motifs within the MMP-9 UTR. The underlined sequences indicate the regions encompassed by the RNA oligonucleotides used for EMSA.
Stimulation of MC with IL-1β (2 nM) led to a significant increase of MMP-9 promoter activity (2.4-fold; P ≤ 0.05), while NO had only weak modulatory effects on either cytokine-stimulated (3.1-fold; P ≤ 0.01) or basal (1.8-fold; P ≤ 0.05) MMP-9 promoter activities (Fig. 3A). Most interestingly, the same promoter construct under the additional control of the 3′ UTR of MMP-9 displayed a strong reduction on MMP-9 promoter activity, since the basal (6.2-fold reduction; P ≤ 0.01) as well as cytokine-induced (2.1-fold reduction; P ≤ 0.01) MMP-9 promoter activity was markedly reduced by addition of the NO donor (Fig. 3B). It is important that the basal promoter activity was higher in the 3′-UTR-MMP-9 pGL construct (2.42 ± 0.54 compared to 0.32 ± 0.02 relative light units), which presumably resulted from the increase in mRNA stability conferred by this regulatory region. The stronger inhibitory effects of NO on basal and IL-1β-mediated luciferase activity suggest that NO can counteract the positive effects of IL-1β on MMP-9 promoter activity only in the presence of the MMP-9-specific 3′ UTR. The negative posttranscriptional effects also exerted by endogenously produced NO observed upon IL-1β stimulation may explain the weak induction of 3′-UTR-MMP-9-pGL3MMP-9-1.3 kb by IL-1β in comparison to that observed with the pGL3MMP-9-1.3 kb construct (Fig. 3).
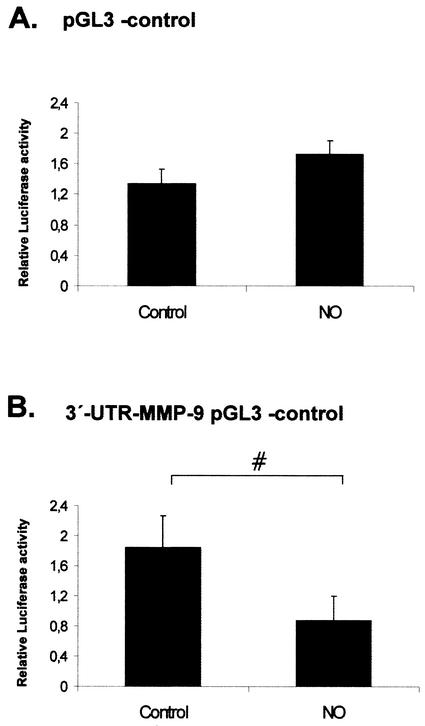
Furthermore, we tested whether the 3′ UTR of MMP-9 under control of a foreign promoter was still able to confer a NO-dependent negative regulatory function on luciferase gene expression. To this end, we chose the pGL3 control vector which contains the luciferase gene under the control of the constitutively active simian virus 40 promoter and enhancer sequences, conferring a strong expression of luciferase when transfected into mammalian cells. A main advantage of this plasmid is that it displays a significant basal promoter activity without responding to cytokine stimuli. Therefore, this reporter gene is most useful for monitoring the contribution of 3′ UTR regions to mRNA stability in the absence of any influence of an inducible enhancer region. Transient transfection of MC with a 3′-UTR-MMP-9 pGL3 control (comprising 662 bp of the MMP-9 3′ UTR downstream from the luciferase coding region) followed by 24 h of treatment with DETA NONOate (500 μM) resulted in a significant reduction of the basal promoter activity (53% reduction; P ≤ 0.05) of the 3′-UTR-MMP-9 pGL3 control promoter (Fig. 4B), whereas the basal promoter activity of the same promoter (pGL3 control) but without the 3′ UTR of MMP-9 was not significantly affected by DETA NONOate (Fig. 4A). Taken together, these data indicate that the 3′ UTR of MMP-9 is a target of NO-mediated mRNA destabilization.
FIG. 4.
Influence of the 3′ UTR of MMP-9 on simian virus 40-driven luciferase activities. Subconfluent MC were transiently cotransfected with 0.4 μg of empty pGL3 control vector (A) or with 0.4 μg of 3′-UTR-MMP-9 pGL3 control (B) additionally containing the 3′ UTR of MMP-9 downstream of the luciferase coding sequence. Each plasmid was cotransfected with 0.1 μg of pRL-CMV used to equilibrate differences in transfection efficiencies. After transfection, MC were treated for 24 h with vehicle or with DETA NONOate (500 μM) as indicated. Values for beetle luciferase were related to values for Renilla luciferase and are depicted as relative luciferase activities. Data (means ± SEM) represent the results of three different experiments. P was 0.05 versus control conditions (#).
Nitric oxide inhibits constitutive complex formation of ARE motifs within the 3′ UTR of MMP-9.
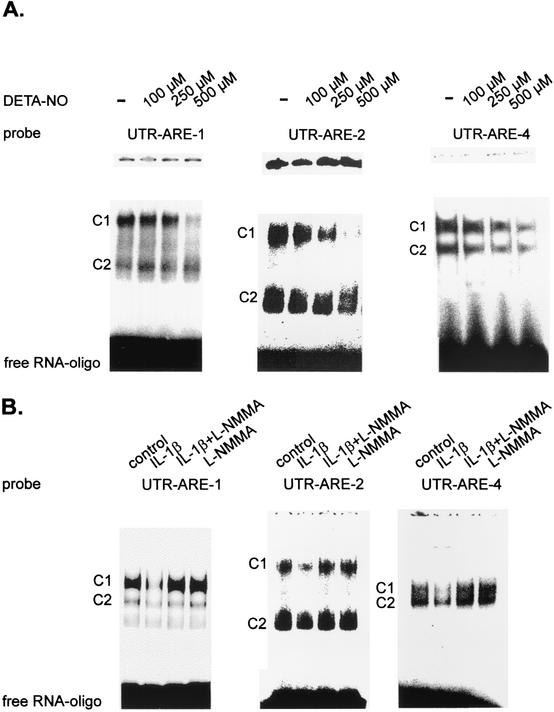
The stability of most short-lived mRNAs is modulated through the specific binding of proteins to the ARE motifs present in the 3′ UTR of these mRNAs (10, 26, 49). To elucidate whether (i) the motifs within the 3′ UTR of MMP-9 display any RNA binding capacity and (ii) RNA binding could be affected by treatment of MC with NO, we performed RNA gel shift assays using 32P-labeled RNA oligonucleotides comprising the different AREs of MMP-9 (designated ARE-1, ARE-2, ARE-3, and ARE-4) (Table 1). MC were either left untreated or treated for 4 h with different concentrations of DETA NONOate before being lysed for preparation of cytosolic fractions. We found that the cytosolic extracts of MC contained proteins which formed two major constitutive complexes of high electrophoretic mobility (Fig. 5) with the ARE-1-, ARE-2-, and ARE-4-containing oligonucleotides but no complex binding to the oligonucleotide encompassing ARE-3 (data not shown). Although each of the four motifs consists of a single AUUUA pentanucleotide core sequence, the compositions of the 5′-flanking bases differ. Whereas ARE-1, ARE-2, and ARE-4 are flanked by an upstream uracil or adenosine, ARE-3 at the 3′ position is flanked by the pyrimidine base cytosine (Table 1).
FIG. 5.
(A) DETA NONOate (DETA-NO) inhibits the constitutive RNA binding of cytoplasmic complexes to different ARE motifs from the 3′ UTR of MMP-9. RNA binding was analyzed by EMSAs using gene-specific oligonucleotides (Table 1). MC were either left untreated (−) or stimulated for 4 h with the indicated concentrations of DETA NONOate before being lysed for preparation of cytoplasmic extracts. Equal amounts of cytoplasmic extracts were incubated with a 32P-labeled RNA probe derived from the corresponding AU-rich region of the 3′ UTR of rat MMP-9 (UTR-ARE-1, UTR-ARE-2, and UTR-ARE-4), and RNA binding was assessed by using 6.0% native polyacrylamide electrophoresis gels. C1 and C2 indicate two main complexes constitutively binding to UTR-ARE-1, UTR-ARE-2, and UTR-ARE-4. The results shown in each panel are representative of three independent experiments giving similar results. (B) Cytokine-mediated inhibition of constitutive RNA binding to different MMP-9-specific AREs is attenuated in the presence of L-NMMA. MC were either left untreated (control) or stimulated for 24 h with IL-1β (2 nM), L-NMMA (2 mM), or both in combination as indicated before being collected for extraction. Equal amounts of cytoplasmic extracts were incubated with the 32P-labeled RNA probes specific for the 3′ UTR of rat MMP-9 depicted in Table 1. The conditions used for EMSA were as described for panel A. The results shown represent one experiment out of three giving similar results.
A recent study elucidating the polymorphism of the 3′ UTR of TNF-α mRNA has demonstrated the complexity of different HuR-binding motifs (16). Most interestingly, treatment of MC with DETA NONOate dose dependently attenuated the constitutive RNA binding to all MMP-9-specific ARE motifs, and with a DETA NONOate concentration of 100 μM, a weak but significant reduction was seen already. Maximal inhibition was observed with a DETA NONOate concentration of 500 μM (Fig. 5A). Similar results were obtained with the NO donor SNAP (data not shown). To further evaluate whether the observed inhibitory effects of NO on ARE binding are of physiological relevance, MC (in addition to NO-donating compounds) were alternatively treated with IL-1β (2 nM), a potent activator of iNOS expression in rat MC (42). Therefore, MC were incubated in the presence or absence of the NOS inhibitor L-NMMA. MC were stimulated for 24 h to ensure production by the cells of a high level of nitric oxide in response to IL-1β (30). Treatment with IL-1β (2 nM) resulted in a marked attenuation of constitutive RNA binding of the cytosolic fractions to ARE-1, ARE-2, and ARE-4 motifs (Fig. 5B). In contrast, the two main complexes C1 and C2 from MC, simultaneously treated with IL-1β and L-NMMA, retained a high constitutive RNA binding to ARE-1, ARE-2, and ARE-4 motifs, whereas L-NMMA alone had no effect on the basal constitutive RNA-binding capacities of any of the MMP-9-derived ARE probes (Fig. 5B). These data indicate that NO (either given from an exogenous source or endogenously produced by the iNOS) potently inhibits the constitutive binding of complexes formed with AUUUA motifs from the 3′ UTR of the rat MMP-9 gene.
Complexes interacting with the ARE motifs of the 3′ UTR of MMP-9 contain the ELAV-like protein HuR (HuA) but no other members of the Hu protein family.
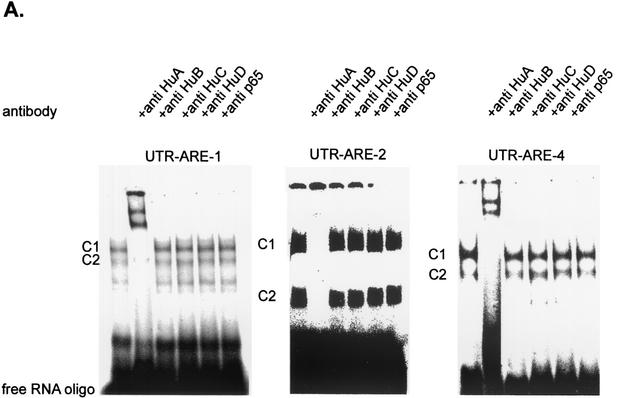
In searching for potential proteins possibly involved in the regulation of mRNA turnover, we focused on the ELAV-like RNA-binding protein HuR (HuA), which is able to specifically interact with AREs found in the 3′ UTRs of many genes (34). To test whether members of the Hu protein family were present in the complexes bound to the ARE motifs of the 3′ UTR of MMP-9, we performed supershift analysis using antibodies recognizing specific epitopes within the murine and rat Hu proteins. Furthermore, to exclude the possibility that the complex seen in the EMSA resulted from unspecific binding of a protein present in the extracts, we additionally tested the effects of an antibody raised against the p65 subunit of the DNA-binding transcription factor NF-κB. Most interestingly, addition of the anti-HuR antibody resulted in a complete disappearance of the complexes C1 and C2 and the concomitant formation of two supershifted complexes (UTR-ARE-1; Fig. 6A). In contrast, addition of anti-HuB, anti-HuC, anti-HuD, or anti-p65 (NF-κB) antibodies did not affect the constitutive RNA binding of C1 and C2 complexes (Fig. 6A). Supershift analysis with the UTR-ARE-2- and UTR-ARE-4-specific oligonucleotides, in similarity to the supershifts obtained with UTR-ARE-1, caused a complete disappearance of both constitutive bound complexes upon addition of the anti-HuR antibody (UTR-ARE-2 and UTR-ARE-4; Fig. 6A), although incubation with the UTR-ARE-2-containing oligonucleotide in some experiments caused no supershifted bands (UTR-ARE-2; Fig. 6A). In similarity to the findings for the supershifts obtained with the UTR-ARE-1 oligonucleotide, addition of anti-HuB, anti-HuC, anti-HuD, or anti-p65 (NF-κB) did not affect the RNA binding to UTR-ARE-2- or UTR-ARE-4-specific probes (Fig. 6A). These data indicate that in MC, the ubiquitously expressed ELAV-protein HuR, but not the Hu family relatives HuB, HuC, and HuD, is the main constituent of complexes binding to ARE motifs of the 3′ UTR of MMP-9.
FIG. 6.
HuR (HuA) is the main constituent of RNA-binding complexes within the cytoplasmic extracts of MC and binds with a high affinity to AREs of the 3′ UTR of MMP-9. (A) For supershift analysis, the 32P-radiolabeled UTR-ARE-1-, UTR-ARE-2-, and UTR-ARE-4-comprising oligonucleotides were incubated with 6 μg of cytoplasmic extract derived from resting MC in the presence or absence of different supershift antibodies as indicated. The antibodies were added 15 min after the addition of the radiolabeled oligonucleotide and incubatedfor a further 15 min at room temperature. (B) Competition capacities of wild-type and mutant UTR-ARE-1 oligonucleotides. The gel shows the results of a representative competition study using different dilutions (as indicated) of either unlabeled wild-type (UTR-ARE-1wt) or unlabeled mutated ARE-1 (UTR-ARE-1mut) oligonucleotides (the sequences are shown in Table 1). The unlabeled competitor oligonucleotides were added 60 min before the addition of 32P-labeled UTR-ARE-1 wild-type oligonucleotide. (C and D) GST-HuR (C) but not GST (D) binds with a high affinity to AREs of the 3′ UTR of MMP-9. The results of EMSA analysis are shown, demonstrating that recombinant GST-HuR protein binds with high affinity to AREs of the 3′-UTR of MMP-9 (C) whereas GST alone has only a weak unspecific RNA binding affinity (D). For EMSA, 200 ng of purified crude protein (GST-HuR or, alternatively, GST) was incubated with the indicated oligonucleotides specific for different AREs of MMP-9 as described in Materials and Methods. For supershift analysis, the 32P-radiolabeled UTR-ARE-1-, UTR-ARE-2-, and UTR-ARE-4-comprising oligonucleotides were incubated with the identical amounts of GST-HuR protein and 200 ng of supershift antibody as indicated. The antibodies were added 15 min after addition of the radiolabeled oligonucleotide and incubated for a further 15 min at room temperature. Similar results were obtained in three independent experiments.
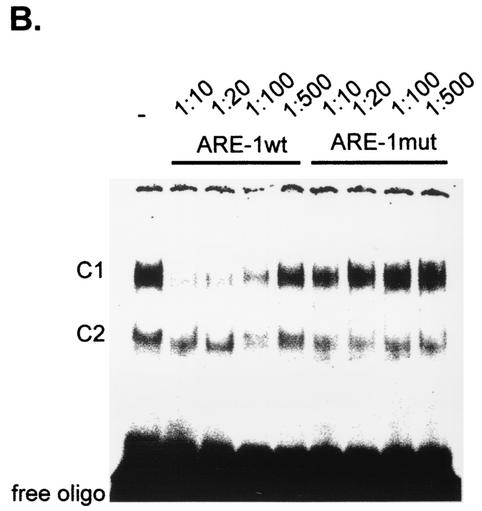
Furthermore, the specificity of RNA-bound complexes present in the cytoplasmic extracts of MC was underlined by competition assays using wild-type or mutant UTR-ARE-1 oligonucleotides (Table 1). For competition analysis, different dilutions (1:10, 1:20, 1:100, or 1:500) of either unlabeled wild-type or mutant UTR-ARE-1 oligonucleotides were added to the binding reaction 1 h prior to the addition of the radiolabeled UTR-ARE-1 probe. As shown in Fig. 6B, competition with the nonlabeled wild-type oligonucleotide (UTR-ARE-1wt) prevented binding of complex C1 when dilutions of 1:10, 1:20, and 1:100 of competitor wild-type oligonucleotide were added whereas binding of C2 was only weakly competed against. In contrast, competitions with the unlabeled mutant oligonucleotide (UTR-ARE-1mut) prevented RNA binding to the radioactively labeled wild-type oligonucleotide in none of the dilutions tested. These data demonstrate that the AUUUA motif is necessary for retention of a full competition capacity in RNA binding. In similarity to findings for UTR-ARE-1 RNA, binding to UTR-ARE-2 and UTR-ARE-4 probes was competed against by addition of cold wild-type ARE-1 competitor oligonucleotide, suggesting a high redundancy between the different ARE motifs of the 3′ UTR of MMP-9 (data not shown).
Recombinant HuR protein strongly binds to ARE motifs of the 3′ UTR of MMP-9.
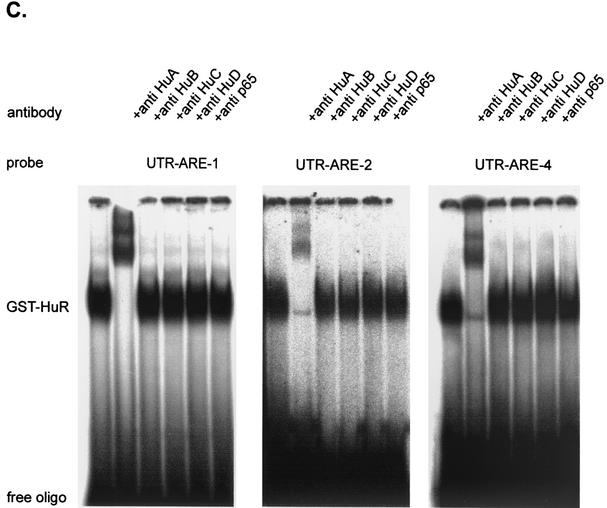
In the next experiment, we tested the RNA-binding capacity of recombinant GST-coupled HuR protein for different ARE motifs of the 3′ UTR of MMP-9. For EMSA, 100 ng of GST-HuR was incubated with the radioactively labeled UTR-ARE-1, UTR-ARE-2, or UTR-ARE-4 oligonucleotides. As shown in Fig. 6C, GST-HuR formed one major complex whose migration properties were the same regardless of which UTR-ARE oligonucleotide was used in the binding reaction.
In similarity to the findings for EMSAs with cytoplasmic fractions, addition of an HuR-specific antibody caused a complete disappearance of the RNA-bound complex and appearance of two supershifted complexes with reduced migration properties whereas antibodies specifically raised against HuB, HuC, HuD, or p65 (NF-κB) did not affect the RNA binding of GST-HuR (Fig. 6C). These data document the high level of binding affinity of HuR to AREs of the 3′ UTR of MMP-9.
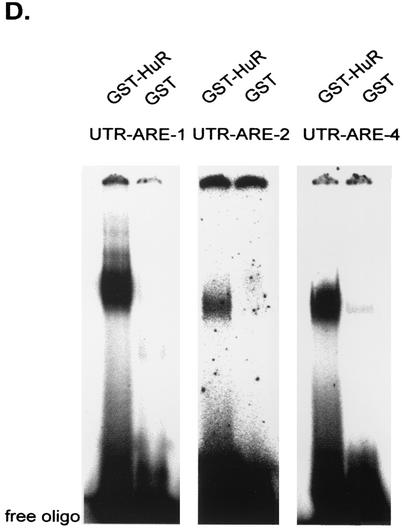
In contrast to findings for GST-HuR, the same amount of GST alone displayed only very weak RNA binding independent of the ARE motif used as the probe (Fig. 6D).
In summary, these data demonstrate that the ELAV-like protein HuR can strongly bind to AREs within the 3′ UTR of MMP-9.
The cytoplasmic fractions of NO-treated MC affect MMP-9 mRNA turnover.
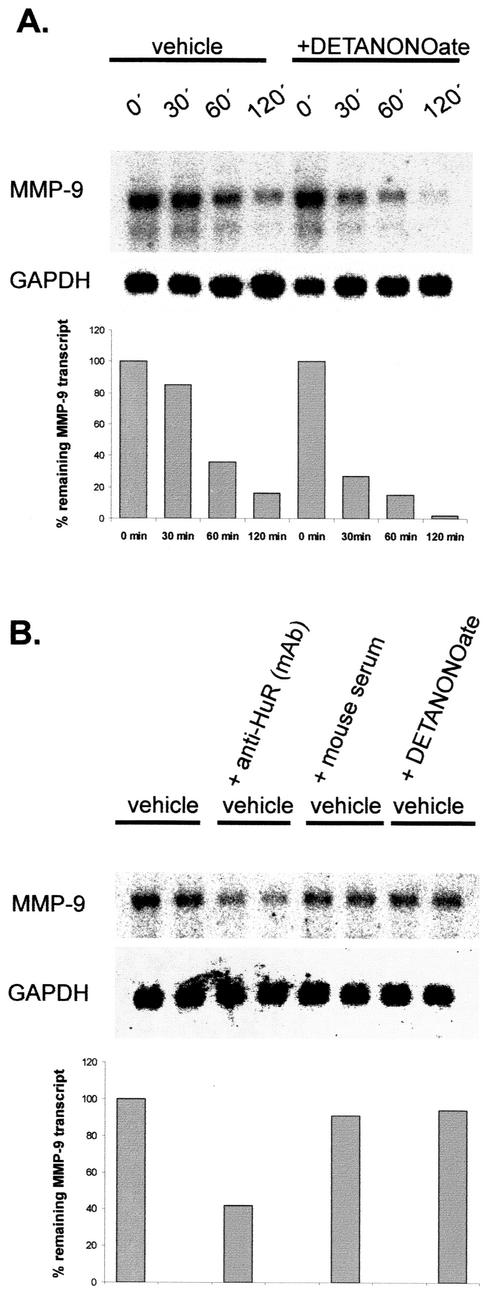
To further prove that treatment of MC with exogenous NO correlates with increased MMP-9 mRNA turnover, we performed in vitro degradation assays. In this assay, the time course of MMP-9 mRNA degradation exhibited by any trans-acting factor accumulated in the cytoplasmic extracts of MC treated for 4 h with DETA NONOate (500 μM) was compared to the degradation profile derived from the incubation with cytoplasmic extracts from untreated MC. To this end, similar volumes of each extract (with a total protein content of 130 μg) were incubated with equal amounts of total RNA isolated from a common pool of cytokine-treated MC. Northern blot analysis performed after a second RNA isolation step revealed that primary undegraded MMP-9 transcripts exposed to the cytoplasmic fractions from NO-treated MC displayed an accelerated degradation compared to the transcripts which had been exposed to cytoplasmic extracts from untreated cells (Fig. 7A). The differences in MMP-9 mRNA stability are clearly shown by the different time course of MMP-9 mRNA decay (Fig. 7A). Additionally, the blot was rehybridized with a GAPDH-specific cDNA probe since the rat GAPDH mRNA contains no typical AREs within its 3′ UTR. In contrast to MMP-9, the level of GAPDH mRNA remained unchanged, thus indicating that the changes in mRNA stability were specific for MMP-9 mRNA (Fig. 7A). Furthermore, these data suggest that endonucleolytic cleavage of MMP-9 transcripts is specifically attributable to factors predominantly present (or absent) in the cytoplasmic extracts of NO-treated MC.
FIG.7.
HuR protects MMP-9 mRNA from rapid decay within the cytoplasmic fractions of MC. (A) For in vitro degradation assays, portions of 20 μg of total RNA from a common pool of total RNA isolated from cytokine-stimulated MC were mixed with 130 μg of cytoplasmic extract derived from either untreated (vehicle) MC or, alternatively, MC treated for 4 h with DETA NONOate (+DETANONOate) (500 μM) as indicated. At different time points as indicated, incubations were stopped by isolation of total RNA. RNA samples were collected and assessed for the remaining amounts of MMP-9 mRNA by Northern blot analysis using a 32P-labeled cDNA insert specific for rat MMP-9. Furthermore, the blots were rehybridized with a 32P-labeled cDNA from GAPDH to prove the presence of equivalent mRNA amounts. The lower panel shows a densitometric analysis of MMP-9-specific signals. Similar results were obtained in three independent experiments. (B) The degradation of cytokine-induced MMP-9 transcripts is accelerated by a neutralizing anti-HuR antibody. Portions of 20 μg of total RNA from a common pool of total RNA isolated from cytokine-stimulated MC were mixed with 130 μg of cytoplasmic extract derived from untreated cells. The cytoplasmic extracts were either kept untreated (vehicle) or, alternatively, were pretreated for 1 h with 0.4 μg of a monoclonal anti-HuR antibody [vehicle + anti-HuR (mAb)], with the same volume of vehicle (vehicle + mouse serum), or with DETA NONOate (500 μM) (vehicle + DETANONOate) before the cytoplasmic extracts were incubated with the total RNA portions. Incubation with the RNA was stopped after 60 min before RNA was extracted for further Northern blot analysis. Samples derived from one cytoplasmic extract were subjected to RNA in duplicates. Furthermore, the blots were rehybridized with a 32P-labeled cDNA from GAPDH to prove the presence of equivalent mRNA amounts. The lower panel shows a densitometric analysis of MMP-9-specific signals. The results shown are representative of one experiment out of three giving similar results. (C) GST-HuR protein protects MMP-9 mRNA from rapid decay. Total RNA (20 μg) from cytokine-stimulated MC was mixed with 130 μg of cytoplasmic extract derived from untreated MC in the absence (vehicle) or presence of 200 ng of GST-HuR or GST. At the indicated time points (0 h and 2 h), incubations were stopped by isolation of total RNA. Subsequently, the RNA was assessed by Northern blot analysis using an MMP-9-specific probe. Furthermore, to prove the specificity of the decay of MMP-9 mRNA, blots were rehybridized with a 32P-labeled cDNA from GAPDH. (D) Neutralization of HuR but not that of other Hu family members leads to a reduction in MMP-9 mRNA stability. Portions of 20 μg of total RNA isolated from cytokine-stimulated MC were mixed with 130 μg of cytoplasmic extract derived from untreated cells. The cytoplasmic extracts were kept either untreated (vehicle) or pretreated for 1 h with 0.4 μg of the Hu-specific antibodies as indicated before the cytoplasmic extracts were incubated with the total RNA portions. Incubation with the RNA was stopped after 60 min before RNA was extracted for Northern blot analysis. Samples derived from one cytoplasmic extract were subjected to RNA in duplicates. To prove the specificity of the effects on MMP-9 mRNA, blots were rehybridized with a 32P-labeled cDNA from GAPDH. Similar results were obtained in two independent experiments.
Neutralization of HuR leads to an accelerated decay of MMP-9 mRNA in an in vitro degradation assay.
As shown by RNA EMSAs, the mRNA-stabilizing protein HuR is a main constituent of the complexes constitutively bound to MMP-9-specific ARE motifs. To prove the functional relevance of HuR in MMP-9 mRNA stabilization, we used an in vitro degradation assay to investigate whether the half-life of MMP-9 mRNA is modulated by addition of a neutralizing anti-HuR antibody. To this end, cytoplasmic fractions from untreated MC were preincubated for 1 h with a monoclonal anti-HuR antibody (a total amount of 400 ng of antiserum) and mixed with the total RNA from cytokine-treated MC for a further 60 min. As a negative control, the same volume of mouse control serum was added to the cytoplasmic fractions instead of the antiserum and was also pretreated for 60 min. Additionally, to check for direct effects of the NO donors, we tested whether cytoplasmic extracts derived from untreated MC but treated with NO after extraction procedures affected the stability of MMP-9 mRNA. As demonstrated in Fig. 7B, the degree of MMP-9 mRNA decay was increased when the cytoplasmic extracts had been pretreated with the neutralizing anti-HuR antibody (Fig. 7B), whereas the mouse control serum on its own had no modulatory effect on MMP-9 mRNA decay (Fig. 7B). Similarly, extracts isolated from untreated cells which had been incubated with DETA NONOate after extraction had no modulatory effect on the MMP-9 mRNA amount (Fig. 7B).
Furthermore, to exclude possible protective effects exerted by other Hu family members, we tested for any neutralizing effects by antibodies specifically raised against HuB, HuC, and HuD proteins (Fig. 7D). In contrast to the neutralizing effects observed with the HuR antiserum, none of the tested Hu antibodies induced degradation of MMP-9 mRNA (Fig. 7D), thus indicating that protective effects on MMP-9 mRNA were exclusively conferred by HuR and not by other proteins of the ELAV family.
Finally, we tested whether the addition of recombinant HuR protein could modulate the stability of MMP-9 transcripts in the in vitro degradation assay. As shown in Fig. 7C, addition of GST-HuR protein (200 ng) markedly inhibited the mRNA decay of MMP-9, whereas the addition of GST alone (200 ng, 2 h) had no protective effects on MMP-9 mRNA decay.
Nitric oxide reduces HuR protein and mRNA levels.
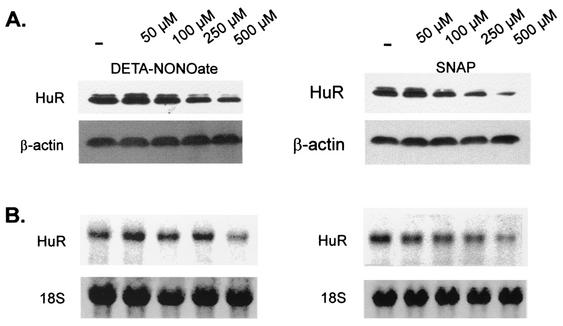
To further evaluate whether the reduction of HuR RNA binding caused by NO was due to a decrease in the amount of total HuR protein, we performed Western blot analysis using the same monoclonal anti-HuR antibody used for supershift analysis. In accordance with the time course of NO-dependent MMP-9 mRNA decay (Fig. 1), MC were stimulated for 4 h with different concentrations of the NO donor DETA NONOate before cells were lysed for Western blot analysis. MC were found to express constitutively high levels of HuR protein. Interestingly, in addition to detecting a major band migrating at 34 kDa, the antibody detected a second minor band at 32 kDa, which indicates a possible posttranslational modification of HuR. Expression of HuR was dose dependently reduced by the presence of DETA NONOate, with a maximal inhibition seen with 500 μM DETA NONOate (Fig. 8A, left panel). In similarity to findings for DETA NONOate, treatment of MC with SNAP, a chemically different NO donor, caused a dose-dependent reduction in the HuR-reactive bands (Fig. 8A, right panel). Furthermore, to determine whether the reduction of HuR protein was due to a decrease in the amount of the corresponding HuR mRNA, we performed Northern blot analysis using a probe from the human HuR cDNA (34). As shown in Fig. 8B, both NO donors, DETA NONOate (left panel) and SNAP (right panel), dose dependently attenuated the basal steady-state HuR mRNA level with a maximal inhibition achieved with 500 μM (each) NO donor (Fig. 8B). These data demonstrate that the NO-mediated alterations in HuR RNA binding predominantly result from the changes in the HuR expression levels.
FIG. 8.
Inhibition of HuR expression in MC by different NO donors. MC were treated for 4 h with different concentrations of either DETA NONOate (left panel) or SNAP (right panel) before cells were lysed for Western blot analysis (A) or for extraction of total RNA (B). (A) Total protein (50 μg) was subjected to Western blot analysis using a monoclonal antibody raised against full-length HuR. Migration properties were determined using standard molecular weight markers. The Western blots shown in panel A were stripped and rehybridized to assess total β-actin levels as a control for sample loading and protein transfer. Similar results were obtained in two independent experiments. (B) Northern blot analysis demonstrating a dose-dependent modulation of HuR mRNA steady-state levels by different NO donors. Total cellular RNA (20 μg) was hybridized to a 32P-labeled cDNA insert from the plasmid HuR9 and analyzed by Northern blot analysis. Equivalent loading of RNA was ascertained by rehybridization to an 18S ribosomal probe. The blot shown is representative of two independent experiments giving similar results.
Endogenously produced nitric oxide inhibits the steady-state mRNA and protein levels of HuR.
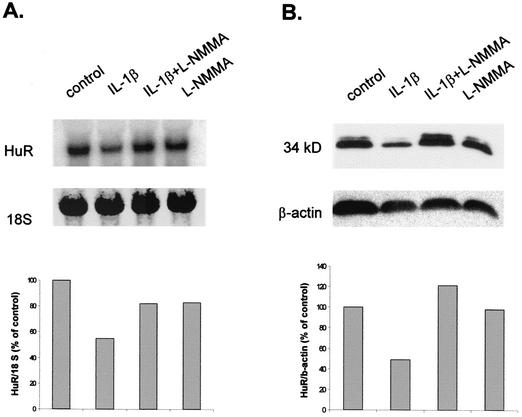
Eberhardt et al. recently demonstrated that NO (either given by exogenous sources or endogenously produced by cytokine-evoked iNOS expression) can reduce the cytokine-induced MMP-9 level (19). To test whether endogenously produced NO is also able to inhibit the constitutive level of HuR, MC were treated for 24 h with IL-1β in the presence or absence of the NOS inhibitor L-NMMA. Measurement of nitrite (the stable end product of NO formation) in cell supernatants has revealed that the amounts of NO produced by the IL-1β-stimulated MC are in the range of that released by the NO donors DETA NONOate and SNAP (19). Stimulation of MC with IL-1β caused a marked reduction of the basal HuR protein and mRNA levels, as shown in Fig. 9A and B, respectively. The reduction in HuR mRNA levels caused by the presence of IL-1β was abolished in the presence of the NOS inhibitor, which by itself had no effect on basal HuR levels (Fig. 9). These findings demonstrate that the cytokine-mediated attenuation of HuR expression depends on mesangial iNOS activity.
FIG. 9.
Cytokine-mediated inhibition of steady-state level of HuR mRNA (A) and protein (B) is blocked in the presence of the NOS inhibitor L-NMMA. Quiescent MC were treated for 24 h with vehicle (control) or with IL-1β (2 nM) or L-NMMA (2 mM) or both in combination as indicated before being harvested for either total RNA isolation (A) or Western blot analysis (B). (A) Total RNA (20 μg) was hybridized to a 32P-labeled cDNA insert from the plasmid HuR9 and analyzed by Northern blot analysis. Equivalent loading of RNA was ascertained by rehybridization to an 18S ribosomal probe. The blot shown is representative of two independent experiments giving similar results. (B) Total protein (50 μg) was subjected to Western blot analysis using a monoclonal HuR-specific antibody. The Western blot shown in panel B was stripped and rehybridized to assess the total β-actin levels as a control for sample loading and protein transfer.
NO-mediated effects on HuR expression are mimicked by cGMP and depend on endogenous guanylyl cyclase activity.
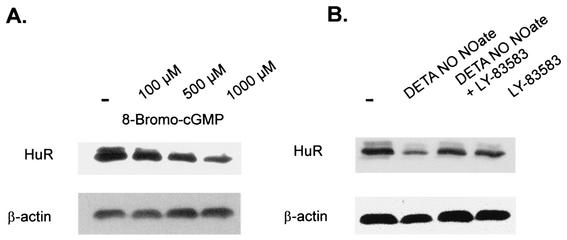
A broad spectrum of physiologic actions exerted by NO depends on the generation of cGMP and is mechanistically linked to activation of guanylyl cyclase through the direct binding of NO to the enzyme's prosthetic heme group (for a review, see reference 50). To elucidate whether the inhibition of HuR expression by NO is mediated by cGMP, we tested for effects of the membrane-permeable analog 8-bromo-cGMP. Similar to the NO donors, 8-bromo cGMP reduced the total protein content of HuR in a dose-dependent manner, with the maximal effect observed with 1 mM 8-bromo-cGMP (Fig. 10A). Concomitantly, 8-bromo-cGMP dose dependently reduced the steady-state level of HuR mRNA (data not shown).
FIG. 10.
(A) Suppression of cellular HuR level by 8-bromo-cGMP. MC were treated for 4 h with vehicle (control; −) or with the different concentrations of the cGMP analog 8-bromo-cGMP as indicated before cells were lysed. Total protein (50 μg) was subjected to Western blot analysis using a monoclonal antibody raised against full-length HuR. Migration properties were determined using standard molecular weight markers. (B) The reduction of HuR protein levels by DETA NONOate is antagonized in the presence of the guanylyl cyclase inhibitor LY-83583. Quiescent MC were treated for 4 h with vehicle (control; −) or with DETA NONOate (500 μM) or LY-83583 (500 nM) or both in combination as indicated before being harvested for preparation of total protein extracts. The level of total cellular HuR content was assessed with a monoclonal anti-HuR antibody. All Western blots were finally stripped and rehybridized to assess total β-actin levels as a control for sample loading and protein transfer. Similar results were obtained in two independent experiments.
Finally, we elucidated whether the NO-mediated inhibitory effects on HuR expression depend on cGMP generation. Therefore, we tested whether NO could still reduce HuR expression in the presence of LY-83583 (500 nM), a specific inhibitor of soluble guanylyl cyclase. As shown in Fig. 10B, the reduction of HuR in DETA NONOate-treated cells was strongly antagonized when cells were pretreated with LY-83583. The presence of LY-83583 had no effects on the basal HuR protein level (Fig. 10B). In summary, these data demonstrate that the reduction of HuR mRNA and protein steady-state levels by NO primarily depends on the activity of guanylyl cyclase and the subsequent increase in cellular cGMP level.
DISCUSSION
MMP-9 is a protease which is critically involved in the regulation of ECM turnover, and imbalanced regulation is functionally correlated with numerous pathological conditions associated with altered matrix turnover such as atherosclerosis, rheumatoid arthritis, and fibrosis of lung, skin, and kidney (for reviews, see references 8, 9, 14, and 32). Inflammatory cytokines such as IL-1β and TNF-α are among the most potent transcriptional inducers of MMP-9 gene expression, mainly through signaling via the mitogen-activated protein kinase (MAPK) pathway and triggering the activation of NF-κB and AP-1 transcription factors (20). The free radical gas NO has been shown to modulate the expression and activity of different MMPs, including MMP-9 (19, 36, 44, 52, 66). In this study we demonstrated that posttranscriptional mechanisms account for the NO-mediated inhibition of MMP-9 expression in rat MC. Using actinomycin D experiments, Eberhardt et al. found that exogenously applied NO as well as endogenously produced NO significantly accelerates degradation of MMP-9 mRNA (23). Furthermore, we have demonstrated that cytoplasmic extracts derived from NO-treated MC displayed increased degradative properties with MMP-9 mRNA transcripts compared to cytosolic fractions from untreated cells. In similarity to findings for MMP-9, a recent study has demonstrated a NO-dependent attenuation of basal and angiotensin II-stimulated expression of transforming growth factor β3 by increased destabilization of the corresponding mRNA (1). However, the underlying mechanisms of NO-evoked mRNA decay had not been further characterized.
Increasing evidence has accumulated demonstrating that selective mRNA turnover plays an important role in the tight control of eukaryotic gene expression (7, 12, 25, 27, 34, 58). Regulation of mRNA stability has been demonstrated to contribute to the strong and rapid changes in RNA abundance observed mainly for genes of the inflammatory- and stress-induced response, among them mRNAs coding for TNF-α (5, 15), IL-2 (6, 28), IL-3 (39), IL-8 (51, 53), c-Fos (11, 40), c-Myc (13, 47), cyclin A and B1 (56), granulocyte-macrophage colony-stimulating factor (2, 11), vascular endothelial cell-derived growth factor (33), cyclooxygenase-2 (COX-2) (17, 63), and iNOS (41, 48), just to name a few. The complex processes of transcript degradation in most cases include a poly(A)-shortening endonucleolytic cleavage which is controlled by trans-acting factors binding to the cis-acting elements within the 3′ UTR of mRNAs (10, 27, 49, 64). Therefore, the half-lives of most mRNAs are influenced by this region. We demonstrate that the 3′ UTR of MMP-9 confers a NO-dependent reduction of basal and cytokine-induced MMP-9-driven luciferase activity when fused downstream of the luciferase reporter gene. Similarly, the 3′ UTR of MMP-9 was able to convey a negative NO responsiveness to a constitutively active luciferase gene when fused downstream of the luciferase coding sequence. From these data it becomes evident that the reduction of luciferase activities induced by NO is conferred by the 3′ UTR of MMP-9.
A common feature of most short-lived mRNAs is the presence of AREs, which are considered cis-regulatory determinants of RNA turnover (10, 35, 64). Also, the 3′ UTR of MMP-9 contains several copies of the pentameric AUUUA motif. This sequence motif was initially described as a destabilizing determinant in the 3′ UTR of granulocyte-macrophage colony-stimulating factor (2, 11) and the immediate-early genes c-fos and c-myc (11, 47). Meanwhile, numerous ARE-binding proteins have been implicated in mRNA turnover, among them proteins which can destabilize mRNA (such as AUF1) (67) but, interestingly, also proteins which can reduce mRNA degradation, most prominently members of the ELAV protein family (33, 34, 40). By native RNA EMSA we identified HuR, a ubiquitously expressed member of the family of ELAV RNA-binding proteins, exhibiting a strong constitutive RNA binding to three different ARE motifs (MMP-9-ARE-1, MMP-9-ARE-2, and MMP-9-ARE-4) within the 3′ UTR of rat MMP-9. Interestingly, a fourth AUUUA repeat (MMP-9-ARE-3) did not display any binding affinity. Sequence comparison of the bases directly flanking the AUUUA pentamer indicated that the presence of a neighboring uracil or adenosine base at the 3′ end of MMP-9-AREs seems critical for RNA binding. In line with these observations, previous studies have suggested that AREs alone are necessary but not sufficient for mRNA destabilization, thereby underlining the importance of neighboring sequences (48). In similarity to findings for externally expressed HuR protein, the crude cytoplasmic extracts of MC displayed a strong constitutive RNA binding to MMP-9-specific AREs. The identity of these complexes was confirmed by supershift analysis using antibodies raised against different members of the Hu protein family. Interestingly, only addition of anti-HuR resulted in the shifting of all complexes and the appearance of two supershifted complexes, whereas antibodies against HuB, HuC, and HuD had no effect. We observed the appearance of two supershifted complexes independent of the source of protein (recombinant versus MC derived) used in the binding reaction. Obviously, HuR was the sole protein in both supershifted complexes and the different migration properties resulted from HuR oligomerization rather than from complexation with other ARE-binding proteins.
Furthermore, we demonstrated that constitutive RNA binding to MMP-9 ARE motifs by the HuR-containing complexes is drastically reduced in cells treated with different NO donors. Most importantly, these experiments displayed a dose-dependent reduction in RNA binding, thus suggesting that RNA binding to the 3′ UTR of MMP-9 is susceptible even to low NO concentrations. Moreover, we proved the physiological relevance of these modulatory effects of NO on HuR binding, as in similarity to findings with exogenous NO, cytokine-treated MC exhibited a reduction in RNA binding dependent on endogenous NO formation.
So far, little is known about the mechanisms by which HuR can regulate mRNA stability. Unlike the other members of the ELAV family (HuB, HuC, and HuD), which show tissue-specific expression, HuR is ubiquitously expressed and shows predominantly nuclear distribution (4, 15, 40). However, a change of distribution between the nuclear and cytoplasmic compartments is considered the main mechanism by which HuR can modulate mRNA decay (29, 40). A previous study has demonstrated a downregulation of HuA in quiescent 3T3 fibroblasts but upregulated HuA levels after serum stimulation (4), thus corroborating the important roles of ELAV proteins during cell growth and differentiation. Since we chose serum-free preincubations for all experimental conditions, it seems most unlikely that the rapid negative effects on HuR expression caused by the presence of NO or cGMP are indirectly due to negative growth effects.
Recent studies have highlighted the role of the p38 MAPK pathway in the regulation of cytoplasmic HuR abundance (60). Using confocal fluorescence microscopy, we found that HuR is most abundant in the nucleus and that nuclear distribution is not changed by treatment of MC with NO (W. Eberhardt and A. Huwiler, unpublished observations). In contrast, EMSA revealed that the RNA-binding properties exhibited by crude nuclear extracts were several times weaker than those exhibited by cytoplasmic fractions. These data suggest that although HuR is mainly localized in the nucleus, NO-mediated alterations in mRNA binding predominantly occur in the cytoplasmic compartment but are not attributable to a modulation of the nucleocytoplasmic shuttling properties of HuR. In this context, the recent finding that the AMP-activated kinase regulates the cytoplasmic HuR and consequently modulates the binding of HuR to its target mRNAs (57) is of special interest. Whether the activation of the AMP-activated kinase or MAPK pathways is also involved in the regulation of MMP-9 mRNA turnover is currently under investigation in our laboratory.
Here we present an alternative mechanism by which a reduction of RNA binding is caused by the altered expression of HuR. We demonstrated that treatment of MC with different NO donors resulted in a significant reduction of the cellular HuR protein content. Furthermore, we found that the reduction in HuR protein is due to a reduction in the corresponding steady-state mRNA level. Similarly, a 24-h exposure of cells to IL-1β caused a significant reduction of HuR on both the protein and mRNA level and this reduction could be blocked by the NOS inhibitor L-NMMA, thus indicating that the cytokine-mediated inhibition of HuR expression is triggered by NO. These data demonstrate that the reduced RNA binding of HuR predominantly results from a decrease in the HuR expression levels. Consistent with our findings, alterations of HuR expression also determine the half-life of COX-2 mRNA and change COX-2 synthesis in colon cancer cells (17). Furthermore, an aberrant expression of HuR was demonstrated in human teratocarcinoma cells (3) and malignant brain tumors (37).
The most common intracellular messenger system activated by NO is the soluble guanylyl cyclase/cGMP system, which is activated by a direct binding of NO to the prosthetic heme group of the enzyme (50). We demonstrated that the NO-mediated reduction of constitutive HuR expression level can be mimicked by the cGMP analog 8-bromo cGMP. Furthermore, we found that the NO-dependent reduction of HuR is blocked in the presence of the specific guanylyl cyclase inhibitor LY-83583, thus demonstrating that the NO-mediated decrease in HuR expression depends on the activation of guanylyl cyclase and thus complements the mainly transcriptional regulation driven by NO in a cGMP-independent manner (44, 50).
In conclusion, the experiments presented here demonstrate for the first time that the expression of MMP-9 (in addition to transcriptional regulation) is controlled by posttranscriptional mechanisms, including the modulation of mRNA stability by the mRNA-stabilizing factor HuR. Moreover, NO reduces the expression of HuR in a cGMP-dependent manner. Whether the decrease of HuR mRNA levels is in turn due to transcriptional or posttranscriptional mechanisms remains to be elucidated.
The excessive accumulation of ECM is an important feature in the progression of fibrotic pathologies and is most likely due to a reduced expression of matrix-metabolizing enzymes. In this context, the increased production of NO often accompanying inflammatory processes may have a causative role in the progression of fibrotic processes by altering matrix turnover via transcriptional as well as posttranscriptional mechanisms.
Acknowledgments
This work was supported by the Deutsche Forschungsgemeinschaft (SFB 553 and PF 361/1-1), the Stiftung Verum für Gesundheit und Umwelt, and a grant from the Stiftung “Nachlässe der Held und Hecker Stiftung 2002.” E.-S. Akool was supported by a grant of the Ministry of Education of the Arab Republic of Egypt.
We thank Andrew P. Levy (Technion-Israel Institute of Technology, Haifa, Israel) for kindly providing the expression plasmid pZeoSV2-HuR containing the entire coding sequence of HuR and Henry Furneaux (Memorial Sloan Kettering Cancer Center, New York, N.Y.) for kindly providing the expression plasmid pGEX-HuR. Furthermore, we thank Roswitha Müller for excellent technical assistance.
REFERENCES
- 1.Abdelaziz, N., F. Colombo, I. Mercier, and A. Calderone. 2001. Nitric oxide attenuates the expression of transforming growth factor-β (3) mRNA in rat cardiac fibroblasts via destabilization. Hypertension 38:261-266. [DOI] [PubMed] [Google Scholar]
- 2.Aharon, T., and R. J. Schneider. 1993. Selective destabilization of short-lived mRNAs with the granulocyte-macrophage colony-stimulating factor AU-rich 3′ noncoding region is mediated by a cotranslational mechanism. Mol. Cell. Biol. 13:1971-1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Antic, D., N. Lu, and J. D. Keene. 1999. ELAV tumor antigen, Hel-N1, increases translation of neurofilament M mRNA and induces formation of neurites in human teratocarcinoma cells. Genes Dev. 13:449-461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Atasoy, U., J. Watson, D. Patel, and J. D. Keene. 1998. ELAV protein HuA (HuR) can redistribute between nucleus and cytoplasm and is upregulated during serum stimulation and T cell activation. J. Cell Sci. 111:3145-3156. [DOI] [PubMed] [Google Scholar]
- 5.Biragyn, A., and S. A. Nedospasov. 1995. Lipopolysaccharide-induced expression of TNFα gene in the macrophage cell line ANA-1 is regulated at the level of transcription processivity. J. Immunol. 155:674-683. [PubMed] [Google Scholar]
- 6.Bohjanen, P. R., B. Petryniak, C. H. June, C. B. Thompson, and T. Lindsten. 1991. An inducible cytoplasmic factor (AU-B) binds selectively to AUUUA multimers in the 3′ untranslated region of lymphokine mRNA. Mol. Cell. Biol. 11:3288-3295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brennan, C. M., and J. A. Steitz. 2001. HuR and mRNA stability. Cell. Mol. Life Sci. 58:266-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brinckerhoff, C. E., and L. M. Matrisian. 2002. Matrix metalloproteinases: a tail of a frog that became a prince. Nat. Rev. Mol. Cell Biol. 3:207-214. [DOI] [PubMed] [Google Scholar]
- 9.Cawston, T. 1998. Matrix metalloproteinases and TIMPs: properties and implications for the rheumatic diseases. Mol. Med. Today 4:130-137. [DOI] [PubMed] [Google Scholar]
- 10.Chen, C.-Y., and A.-B. Shyu. 1994. Selective degradation of early-response-gene mRNAs: functional analysis of sequence features of the AU-rich elements. Mol. Cell. Biol. 14:8471-8482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen, C.-Y., N. Xu, and A.-B. Shyu. 1995. mRNA decay mediated by two distinct AU-rich elements from c-fos and granulocyte-macrophage colony-stimulating factor transcripts: different deadenylation kinetics and uncoupling from translation. Mol. Cell. Biol. 15:5777-5788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen, C. Y., and A. B. Shyu. 1995. AU-rich elements: characterization and importance in mRNA degradation. Trends Biochem. Sci. 20:465-470. [DOI] [PubMed] [Google Scholar]
- 13.Dani, C., N. Mechti, M. Piechaczyk, B. Lebleu, P. Jeanteur, and J. M. Blanchard. 1985. Increased rate of degradation of c-myc mRNA in interferon-treated Daudi cells. Proc. Natl. Acad. Sci. USA 82:4896-4899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davies, M., J. Martin, G. J. Thomas, and D. H. Lovett. 1992. Proteinases and glomerular matrix turnover. Kidney Int. 41:671-678. [DOI] [PubMed] [Google Scholar]
- 15.Dean, J. L., R. Wait, K. R. Mahtani, G. Sully, A. R. Clark, and J. Saklatvala. 2001. The 3′ untranslated region of tumor necrosis factor alpha mRNA is a target of the mRNA-stabilizing factor HuR. Mol. Cell. Biol. 21:721-730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Di Marco, S., Z. Hel, C. Lachance, H. Furneaux, and D. Radzioch. 2001. Polymorphism in the 3′-untranslated region of TNFα mRNA impairs binding of the post-transcriptional regulatory protein HuR to TNFα mRNA. Nucleic Acids Res. 29:863-871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dixon, D. A., N. D. Tolley, P. H. King, L. B. Nabors, T. M. McIntyre, G. A. Zimmerman, and S. M. Prescott. 2001. Altered expression of the mRNA stability factor HuR promotes cyclooxygenase-2 expression in colon cancer cells. J. Clin. Investig. 108:1657-1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dreyfuss, G., M. Hentze, A. I. Lamond. 1996. From transcript to protein. Cell 85:963-972. [DOI] [PubMed] [Google Scholar]
- 19.Eberhardt, W., T. Beeg, K. F. Beck, S. Walpen, S. Gauer, H. Bohles, and J. Pfeilschifter. 2000. Nitric oxide modulates expression of matrix metalloproteinase-9 in rat mesangial cells. Kidney Int. 57:59-69. [DOI] [PubMed] [Google Scholar]
- 20.Eberhardt, W., A. Huwiler, K. F. Beck, S. Walpen, and J. Pfeilschifter. 2000. Amplification of IL-1 β-induced matrix metalloproteinase-9 expression by superoxide in rat glomerular mesangial cells is mediated by increased activities of NF-κ B and activating protein-1 and involves activation of the mitogen-activated protein kinase pathways. J. Immunol. 165:5788-5797. [DOI] [PubMed] [Google Scholar]
- 21.Eberhardt, W., K. F. Beck, and J. Pfeilschifter. 2002. Cytokine-induced expression of tPA is differentially modulated by NO and ROS in rat mesangial cells. Kidney Int. 61:20-30. [DOI] [PubMed] [Google Scholar]
- 22.Eberhardt, W., M. Schulze, C. Engels, E. Klasmeier, and J. Pfeilschifter. 2002. Glucocorticoid-mediated suppression of cytokine-induced matrix metalloproteinase-9 expression in rat mesangial cells: involvement of nuclear factor-κB and Ets transcription factors. Mol. Endocrinol. 16:1752-1766. [DOI] [PubMed] [Google Scholar]
- 23.Eberhardt, W., el-S. Akool, J. Rebhan, S. Frank, K. F. Beck, R. Franzen, F. M. Hamada, and J. Pfeilschifter. 2002. Inhibition of cytokine-induced matrix metalloproteinase 9 expression by peroxisome proliferator-activated receptor α agonists is indirect and due to a NO-mediated reduction of mRNA stability. J. Biol. Chem. 277:33518-33528. [DOI] [PubMed] [Google Scholar]
- 24.Ehrenman, K., L. Long, B. J. Wagner, and G. Brewer. 1994. Characterization of cDNAs encoding the murine A+U-rich RNA-binding protein AUF1. Gene 149:315-319. [DOI] [PubMed] [Google Scholar]
- 25.Fan, X. C., and J. A. Steitz. 1998. Overexpression of HuR, a nuclear-cytoplasmic shuttling protein, increases the in vivo stability of ARE-containing mRNAs. EMBO J. 17:3448-3460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ford, L. P., J. Watson, J. D. Keene, and J. Wilusz. 1999. ELAV proteins stabilize deadenylated intermediates in a novel in vitro mRNA deadenylation/degradation system. Genes Dev. 13:188-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grzybowska, E. A., A. Wilczynska, and J. A. Siedlecki. 2001. Regulatory functions of 3′ UTRs. Biochem. Biophys. Res. Commun. 288:291-295. [DOI] [PubMed] [Google Scholar]
- 28.Henics, T., A. Sanfridson, B. J. Hamilton, E. Nagy, and W. F. Rigby. 1994. Enhanced stability of interleukin-2 mRNA in MLA 144 cells. Possible role of cytoplasmic AU-rich sequence-binding proteins. J. Biol. Chem. 269:5377-5383. [PubMed] [Google Scholar]
- 29.Keene, J. D. 1999. Why is Hu where? Shuttling of early-response-gene messenger RNA subsets. Proc. Natl. Acad. Sci. USA 96:5-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kunz, D., H. Mühl, G. Walker, and J. Pfeilschifter. 1994. Two distinct signalling pathways trigger the expression of inducible nitric oxide synthase in rat renal mesangial cells. Proc. Natl. Acad. Sci. USA 91:5387-5391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lander, H. M., P. Sehajpal, D. M. Levine, and A. Novogrodsky. 1993. Activation of human peripheral blood mononuclear cells by nitric oxide-generating compounds. J. Immunol. 150:1509-1516. [PubMed] [Google Scholar]
- 32.Lenz, O., S. J. Elliot, and W. G. Stetler-Stevenson. 2000. Matrix metalloproteinases in renal development and disease. J. Am. Soc. Nephrol. 11:574-581. [DOI] [PubMed] [Google Scholar]
- 33.Levy, A. P., N. S. Levy, and M. A. Goldberg. 1996. Post-transcriptional regulation of vascular endothelial growth factor by hypoxia. J. Biol. Chem. 271:2746-2753. [DOI] [PubMed] [Google Scholar]
- 34.Ma, W. J., S. Cheng, C. Campbell, A. Wright, and H. Furneaux. 1996. Cloning and characterization of HuR, a ubiquitously expressed Elav-like protein. J. Biol. Chem. 271:8144-8151. [DOI] [PubMed] [Google Scholar]
- 35.Malter, J. S. 1989. Identification of an AUUUA-specific messenger RNA binding protein. Science 246:664-666. [DOI] [PubMed] [Google Scholar]
- 36.Murrell, G. A., D. Jang, and R. J. Williams. 1995. Nitric oxide activates metalloprotease enzymes in articular cartilage. Biochem. Biophys. Res. Commun. 206:15-21. [DOI] [PubMed] [Google Scholar]
- 37.Nabors, L. B., G. Y. Gillespie, L. Harkins, and P. H. King. 2001. HuR, a RNA stability factor, is expressed in malignant brain tumors and binds to adenine- and uridine-rich elements within the 3′ untranslated regions of cytokine and angiogenic factor mRNAs. Cancer Res. 61:2154-2161. [PubMed] [Google Scholar]
- 38.Nagase, H., and J. F. Woessner, Jr. 1999. Matrix metalloproteinases. J. Biol. Chem. 274:21491-21494. [DOI] [PubMed] [Google Scholar]
- 39.Nair, A. P., S. Hahn, R. Banholzer, H. H. Hirsch, and C. Moroni. 1994. Cyclosporin A inhibits growth of autocrine tumour cell lines by destabilizing interleukin-3 mRNA. Nature 369:239-242. [DOI] [PubMed] [Google Scholar]
- 40.Peng, S. S., C. Y. Chen, N. Xu, and A. B. Shyu. 1998. RNA stabilization by the AU-rich element binding protein, HuR, an ELAV protein. EMBO J. 17:3461-3470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Perez-Sala, D., E. Cernuda-Morollon, M. Diaz-Cazorla, F. Rodriguez-Pascual, and S. Lamas. 2001. Posttranscriptional regulation of human iNOS by the NO/cGMP pathway. Am. J. Physiol. 280:F466-F473. [DOI] [PubMed] [Google Scholar]
- 42.Pfeilschifter, J., and H. Schwarzenbach. 1990. Interleukin 1 and tumor necrosis factor stimulate cGMP formation in rat renal mesangial cells. FEBS Lett. 273:185-187. [DOI] [PubMed] [Google Scholar]
- 43.Pfeilschifter, J., and K. Vosbeck. 1991. Transforming growth factor β 2 inhibits interleukin 1β- and tumour necrosis factor α-induction of nitric oxide synthase in rat renal mesangial cells. Biochem. Biophys. Res. Commun. 175:372-379. [DOI] [PubMed] [Google Scholar]
- 44.Pfeilschifter, J., W. Eberhardt, and A. Huwiler. 2001. Nitric oxide and mechanisms of redox signalling: matrix and matrix-metabolizing enzymes as prime nitric oxide targets. Eur. J. Pharmacol. 429:279-286. [DOI] [PubMed] [Google Scholar]
- 45.Pfeilschifter, J., K. F. Beck, W. Eberhardt, and A. Huwiler. 2002. Changing gears in the course of glomerulonephritis by shifting superoxide to nitric oxide-dominated chemistry. Kidney Int. 61:809-815. [DOI] [PubMed] [Google Scholar]
- 46.Pineda-Molina, E., and S. Lamas. 2001. Nitric oxide as a regulator of gene expression: studies with the transcription factor proteins cJun and p50. Biofactors 15:113-135. [DOI] [PubMed] [Google Scholar]
- 47.Rabbitts, P. H., A. Forster, M. A. Stinson, and T. H. Rabbitts. 1985. Truncation of exon 1 from the c-myc gene results in prolonged c-myc mRNA stability. EMBO J. 4:3727-3733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rodriguez-Pascual, F., M. Hausding, I. Ihrig-Biedert, H. Furneaux, A. P. Levy, U. Forstermann, and H. Kleinert. 2000. Complex contribution of the 3′-untranslated region to the expressional regulation of the human inducible nitric-oxide synthase gene. Involvement of the RNA-binding protein HuR. J. Biol. Chem. 275:26040-26049. [DOI] [PubMed] [Google Scholar]
- 49.Ross, J. 1995. mRNA stability in mammalian cells. Microbiol. Rev. 59:423-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schmidt, H. H., and U. Walter. 1994. NO at work. Cell 78:919-925. [DOI] [PubMed] [Google Scholar]
- 51.Stoeckle, M. Y. 1991. Post-transcriptional regulation of gro alpha, beta, gamma, and IL-8 mRNAs by IL-1β. Nucleic Acids Res. 19:917-920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Trachtman, H., S. Futterweit, P. Garg, K. Reddy, and P. C. Singhal. 1996. Nitric oxide stimulates the activity of a 72-kDa neutral matrix metalloproteinase in cultured rat mesangial cells. Biochem. Biophys. Res. Commun. 218:704-708. [DOI] [PubMed] [Google Scholar]
- 53.Villarete, L. H., and D. G. Remick. 1996. Transcriptional and post-transcriptional regulation of interleukin-8. Am. J. Pathol. 149:1685-1693. [PMC free article] [PubMed] [Google Scholar]
- 54.von Knethen, A., and B. Brüne. 2000. Superinduction of cyclooxygenase-2 by NO(∗) and agonist challenge involves transcriptional regulation mediated by AP-1 activation. Biochemistry 39:1532-1540. [DOI] [PubMed] [Google Scholar]
- 55.Walpen, S., K. F. Beck, W. Eberhardt, M. Apel, P. K. Chatterjee, G. M. Wray, C. Thiemermann, and J. Pfeilschifter. 2000. Downregulation of SPARC expression is mediated by nitric oxide in rat mesangial cells and during endotoxemia in the rat. J. Am. Soc. Nephrol. 11:468-476. [DOI] [PubMed] [Google Scholar]
- 56.Wang, W., M. C. Caldwell, S. Lin, H. Furneaux, and M. Gorospe. 2000. HuR regulates cyclin A and cyclin B1 mRNA stability during cell proliferation. EMBO J. 19:2340-2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang, W., J. Fan, X. Yang, S. Furer-Galban, I. Lopez de Silanes, C. von Kobbe, J. Guo, S. N. Georas, F. Foufelle, D. G. Hardie, D. Carling, and M. Gorospe. 2002. AMP-activated kinase regulates cytoplasmic HuR. Mol. Cell. Biol. 22:3425-3436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wickens, M., P. Anderson, and R. J. Jackson. 1997. Life and death in the cytoplasm: messages from the 3′ end. Curr. Opin. Genet. Dev. 7:220-232. [DOI] [PubMed] [Google Scholar]
- 59.Wilson, G. M., Y. Sun, J. Sellers, H. Lu, N. Penkar, G. Dillard, and G. Brewer. 1999. Regulation of AUF1 expression via conserved alternatively spliced elements in the 3′ untranslated region. Mol. Cell. Biol. 19:4056-4064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Winzen, R., M. Kracht, B. Ritter, A. Wilhelm, C. Y. Chen, A. B. Shyu, M. Muller, M. Gaestel, K. Resch, and H. Holtmann. 1999. The p38 MAP kinase pathway signals for cytokine-induced mRNA stabilization via MAP kinase-activated protein kinase 2 and an AU-rich region-targeted mechanism. EMBO J. 18:4969-4980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Woessner, J. F., Jr. 1998. The matrix metalloproteinase family, p. 1-13. In W. C. Parks and R. P. Mecham (ed.), Matrix metalloproteinases. Academic Press, San Diego, Calif.
- 62.Woessner, J. F., Jr. 1991. Matrix metalloproteinases and their inhibitors in connective tissue remodeling. FASEB J. 5:2145-2154. [PubMed] [Google Scholar]
- 63.Xu, K., A. M. Robida, and T. J. Murphy. 2000. Immediate-early MEK-1- dependent stabilization of rat smooth muscle cell cyclooxygenase-2 mRNA by G α(q)-coupled receptor signaling. J. Biol. Chem. 275:23012-23019. [DOI] [PubMed] [Google Scholar]
- 64.Xu, N., C.-Y. Chen, and A.-B. Shyu. 1997. Modulation of the fate of cytoplasmic mRNA by AU-rich elements: key sequence features controlling mRNA deadenylation and decay. Mol. Cell. Biol. 17:4611-4621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yokoo, T., and M. Kitamura. 1996. Dual regulation of IL-1β-mediated matrix metalloproteinase-9 expression in mesangial cells by NF-κB and AP-1. Am. J. Physiol. 270:123-130. [DOI] [PubMed] [Google Scholar]
- 66.Zaragoza, C., M. Balbin, C. Lopez-Otin, and S. Lamas. 2002. Nitric oxide regulates matrix metalloprotease-13 expression and activity in endothelium. Kidney Int. 61:804-808. [DOI] [PubMed] [Google Scholar]
- 67.Zhang, W., B. J. Wagner, K. Ehrenman, A. W. Schaefer, C. T. DeMaria, D. Crater, K. DeHaven, L. Long, and G. Brewer. 1993. Purification, characterization, and cDNA cloning of an AU-rich element RNA-binding protein, AUF1. Mol. Cell. Biol. 13:7652-7665. [DOI] [PMC free article] [PubMed] [Google Scholar]