Abstract
Phosphatidylinositol-3,4,5-trisphosphate (PIP3) has been proposed to modulate the odorant sensitivity of olfactory sensory neurons by inhibiting activation of cyclic nucleotide-gated (CNG) channels in the cilia. When applied to the intracellular face of excised patches, PIP3 has been shown to inhibit activation of heteromeric olfactory CNG channels, composed of CNGA2, CNGA4, and CNGB1b subunits, and homomeric CNGA2 channels. In contrast, we discovered that channels formed by CNGA3 subunits from cone photoreceptors were unaffected by PIP3. Using chimeric channels and a deletion mutant, we determined that residues 61–90 within the N terminus of CNGA2 are necessary for PIP3 regulation, and a biochemical “pulldown” assay suggests that PIP3 directly binds this region. The N terminus of CNGA2 contains a previously identified calcium–calmodulin (Ca2+/CaM)-binding domain (residues 68–81) that mediates Ca2+/CaM inhibition of homomeric CNGA2 channels but is functionally silent in heteromeric channels. We discovered, however, that this region is required for PIP3 regulation of both homomeric and heteromeric channels. Furthermore, PIP3 occluded the action of Ca2+/CaM on both homomeric and heteromeric channels, in part by blocking Ca2+/CaM binding. Our results establish the importance of the CNGA2 N terminus for PIP3 inhibition of olfactory CNG channels and suggest that PIP3 inhibits channel activation by disrupting an autoexcitatory interaction between the N and C termini of adjacent subunits. By dramatically suppressing channel currents, PIP3 may generate a shift in odorant sensitivity that does not require prior channel activity.
Keywords: lipid signaling, olfaction, phosphatidylinositide, sensory adaptation
Odorant binding to specialized receptors in the cilia of olfactory sensory neurons triggers an increase in intracellular cAMP (1–4), which directly opens cyclic nucleotide-gated (CNG) channels (5). Calcium influx through CNG channels activates an atypical chloride current (6–8), leading to depolarization of the cell membrane. The elevated calcium also causes rapid adaptation to odorants by triggering a calcium–calmodulin (Ca2+/CaM)-dependent decrease in the sensitivity of CNG channels to cAMP (9). Recent evidence suggests that phosphatidylinositol-3,4,5-trisphosphate (PIP3) also decreases the sensitivity of olfactory CNG channels and reduces the response of olfactory sensory neurons to complex odors, but the mechanism has yet to be elucidated (10, 11).
Ca2+/CaM inhibits homomeric CNGA2 channel activation by binding to a Baa-like motif in the N terminus (12–14), thereby disrupting an autostimulatory interaction with the C terminus of an adjacent subunit (15–17). Deletion of the Ca2+/CaM-binding domain (amino acids 68–81) in CNGA2 produces channels that are resistant to inhibition by Ca2+/CaM and exhibit dramatically reduced sensitivity to cyclic nucleotides due to the loss of the autostimulatory interaction. Native olfactory CNG channels are tetrameric assemblies of three different pore-forming subunits, CNGA2, CNGA4, and CNGB1b, in a 2:1:1 stoichiometry (18–20). Surprisingly, the N-terminal Ca2+/CaM-binding site on CNGA2 is functionally silent in heteromeric channels; instead, Ca2+/CaM exerts its inhibitory effect by binding to IQ-like motifs in the CNGA4 and CNGB1b subunits (21).
More recently, several lipids have been shown to regulate the activity of CNG channels. Activation of rod channels is reduced dramatically by application of diacylglycerol derivatives (22, 23), all-trans-retinal (24), and phosphatidylinositol-4,5-bisphosphate (PIP2) (25), and activation of olfactory CNG channels is inhibited by cholesterol depletion (26). Zhainazarov et al. (11) reported that PIP3 inhibits heterologously expressed CNGA2 homomeric channels or CNGA2/CNGA4 heteromeric channels to nearly the same extent as native channels in olfactory sensory neuron membranes. Interestingly, inhibition by PIP3 in many respects resembles that of Ca2+/CaM: in both cases, the apparent affinity of the channel for cAMP is reduced by at least 10-fold with no change in the single-channel conductance. Whereas the mechanism of Ca2+/CaM inhibition has been well characterized, the molecular mechanisms underlying PIP3 inhibition remain unknown. Furthermore, it is unclear how these two regulatory processes interact to modulate the odorant response.
Results
CNGA3 Channels Are Insensitive to PIP3.
To investigate the effects of PIP3 on CNG channel function, we expressed cloned channel subunits in HEK293 cells and exposed inside-out patches to varying concentrations of cyclic nucleotides. As shown in Fig. 1 A and D, exposure of patches containing homomeric CNGA2 channels to 10 μM dipalmitoyl-PIP3 produced, on average, a 17- and 13-fold shift in the apparent affinity for cGMP (n = 8) and cAMP (n = 12), respectively. In addition, dipalmitoyl-PIP3 (hereafter referred to as PIP3) decreased the efficacy of saturating cAMP by ≈70%, an effect typically ascribed to stabilization of closed-channel states. The decrease in cyclic nucleotide sensitivity generally occurred within 20 seconds of exposure to 10 μM PIP3; similar results were observed with concentrations of PIP3 as low as 1 μM, but the time course was generally slower (Fig. 1C). We were unable to reverse the effect of PIP3, even with wash times of >60 min, which we attribute to the high affinity of PIP3 for the hydrophobic membrane environment. Application of 50 μM dioctanoyl-PIP3 (a more water soluble analog with shorter acyl substituents) caused only a mild decrease in the apparent cAMP affinity (<2-fold), whereas 10 μM dioctanoyl-PIP3 and 10 μM IP4 had no effect (data not shown). In approximately half of the patches, PIP3 reduced the saturating cGMP current by 20–30%. This effect developed gradually with prolonged exposure to PIP3, suggesting that high concentrations of patch-resident PIP3 were required. We found no correlation between the size of the apparent affinity shift and the decrease in the maximum cGMP-activated current.
Fig. 1.
PIP3 inhibits CNGA2 channels but not CNGA3 channels. Representative traces from CNGA2 (A) and CNGA3 (B) before and after application of 10 μM PIP3. Currents were elicited by voltage steps to +50 and –50 mV in the presence of the indicated concentration of cyclic nucleotides. Data are representative of 12 patches for CNGA2 and 6 patches for CNGA3. (C) Two different patches containing CNGA2 channels activated by 1 mM cAMP and held at −50 mV were exposed to 10 μM or 1 μM PIP3 at 15 s (denoted by arrow). Data were normalized to the current level before PIP3 exposure. Representative CNGA2 (D) and CNGA3 (E) cyclic nucleotide dose–response relationships before (filled symbols) and after (open symbols) application of 10 μM PIP3. Hill parameters (K1/2, n) for CNGA2: (▾) cGMP = 1.6 μM, 2.2 before PIP3, and (▿) 21.5 μM, 2.1 after PIP3; (●) cAMP = 51.2 μM, 1.9 before PIP3, and (○) 1.4 mM, 1.5 after PIP3; for CNGA3: (▾) cGMP = 21.6 μM, 2.2 before PIP3, and (▿) 25.8 μM, 2.1 after PIP3; (●) cAMP = 1.8 mM, 1.3 before PIP3, and (○) 1.5 mM, 1.7 after PIP3. Open circles in E overlap and hide the filled circles. Data are representative of three separate experiments each for CNGA2 and CNGA3.
In contrast, application of 10 μM PIP3 had no effect on channels formed by CNGA3, the α subunit from cone photoreceptors (Fig. 1 B and E). Neither the apparent affinity for cGMP nor the maximum current elicited by saturating cGMP concentrations was altered significantly by even prolonged exposure to 10 μM PIP3 (up to 4 min). Similarly, the apparent affinity and efficacy of cAMP, a weak partial agonist for CNGA3, also were unaffected.
Molecular Determinants of PIP3 Regulation.
To identify channel regions involved in PIP3 regulation, we constructed chimeric channels by exchanging different regions of CNGA2 and CNGA3. These two subunits are ≈60% identical and 75% homologous, with most of their differences lying in the cytoplasmic N and C termini. Exchanging the pore domain and C terminus did not eliminate the PIP3 sensitivity of the parent channel; CNGA2 containing the CNGA3 pore and cyclic nucleotide-binding (CNB) domain (chimera 2233) still was inhibited by PIP3 (by ≈5-fold), and CNGA3 channels containing the CNGA2 pore and CNB domain (chimera 3322) were unaffected by PIP3 (Fig. 2A). We then assessed the PIP3 sensitivity of chimeric channels containing only the cytoplasmic N terminus of CNGA2, and we found that transplantation of this region onto CNGA3 was sufficient to confer PIP3 inhibition. Exposure of patches containing these chimeric channels (A2nA3) to 10 μM PIP3 caused a 5-fold decrease in their cGMP sensitivity, and a severe reduction in the current elicited by saturating cAMP (Fig. 2).
Fig. 2.
The N terminus of CNGA2 confers PIP3 sensitivity to CNGA3 channels. (A) Diagrams depicting CNGA2/CNGA3 chimeric subunits are shown on the left (see Methods for splice site locations). Portions of the channel sequence derived from CNGA2 are shown in black; portions derived from CNGA3 are shown in gray. Plotted adjacent is the mean K1/2 for cGMP before (●) and after (○) 10 μM PIP3. K1/2 values ± SD are as follows: A3, 18.6 ± 7 μM before PIP3 and 20.5 ± 8 μM after PIP3, three patches; 3322, 24.0 ± 9 μM before PIP3 and 24.6 ± 11 μM after PIP3, three patches; A2nA3, 9.3 ± 2.3 μM before and 43.5 ± 13.8 μM after, five patches; 2333, 10.6 ± 4 μM before and 64 ± 19 μM after, four patches; 2233, 12.0 ± 2.4 μM before and 57.3 ± 7 μM after, three patches; CNGA2, 1.8 ± 1 μM before and 23.9 ± 11 μM after, nine patches. (B) Representative cyclic nucleotide dose–response relationships, before (filled symbols) and after (open symbols) application of 10 μM PIP3, for channels containing A2nA3 subunits. Hill equation parameters (K1/2, n): (▾), cGMP = 6.8 μM, 2.1 before PIP3 and (▿) 31.8 μM, 2.4 after PIP3; (●), cAMP = 1.0 mM, 2.1 before PIP3 and (○) K1/2 = 2.1 mM, 2.9 after PIP3. Data are representative of four different patches.
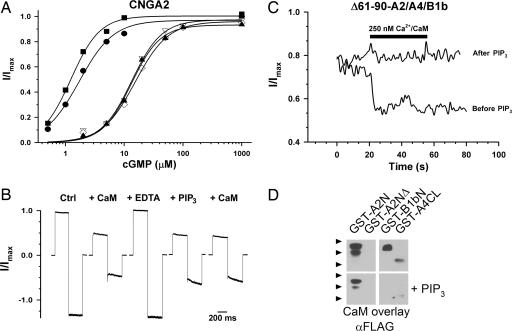
Calmodulin inhibition of CNGA2 channels resembles PIP3 inhibition and also relies on residues within the N terminus (residues 68–81). We therefore hypothesized that the two modulators share a common inhibitory mechanism or binding site. In support of this idea, we found that channels formed by CNGA2 subunits lacking residues 61–90 (Δ61–90-CNGA2) were virtually unaffected by application of 10 μM PIP3 (Fig. 3A). However, with prolonged PIP3 exposure, these channels, like WT CNGA2 channels, often exhibited a reduction in the maximum cGMP-activated current but no change in the maximum cAMP-activated current. Although difficult to explain based on current models of CNG channel activation, this effect was not investigated further.
Fig. 3.
PIP3 inhibits olfactory CNG channels through a direct interaction with the N terminus of CNGA2. (A) Shown are representative cyclic nucleotide dose–response relationships for Δ61–90-CNGA2, measured before (filled symbols) and after (open symbols) treatment with 10 μM PIP3. Hill equation parameters (K1/2, n): (▾) cGMP 22.6 μM, 2.8 before PIP3 and (▿) 26.9 μM, 2.2 after PIP3; (●) cAMP = 1.2 mM, 1.4 before PIP3 and (○) 917 μM, 1.6 after PIP3. Data are representative of three different experiments. (B) N-terminal regions of CNGA2 and CNGA3 were expressed as GST-fusion proteins and tested for PIP3 binding in vitro by using PIP3-agarose beads. Input proteins (Left and Right Lower) and bound proteins (Middle and Right Upper) were identified by immunoblotting with anti-GST antibodies. GST-Grp1PH, positive control pleckstrin homology domain (Echelon); GST-A2N, N-terminal cytoplasmic domain (amino acids 1–138) of rat CNGA2 (AF126808); GST-A2NΔ, amino acids 61–90 deleted; GST-A3N, N-terminal cytoplasmic domain (amino acids 1–164) of human CNGA3 (AF065314); GST-A3NΔ, amino acids 51–108 deleted. Data are representative of four different experiments. (C) Representative cGMP dose–response relationships before (filled symbols) and after (open symbols) application of 10 μM PIP3 to a patch containing wtCNGA2/A4/B1b channels. Hill equation parameters (K1/2, n): (▾) cGMP, 2.3 μM, 2.6 before PIP3 and (▿) 14.6 μM, 1.8 after PIP3; (●) cAMP, 6.4 μM, 2.8 before PIP3 and (○) 48.4 μM, 2.0 after PIP3. (D) Representative cGMP dose–response relationships before and after application of 10 μM PIP3 to a patch containing ▵61–90-CNGA2/A4/B1b channels. Hill equation parameters (K1/2, n): (▾) cGMP, 16.3 μM, 2.6 before PIP3 and (▿) 16.5 μM, 2.7 after PIP3; (●) cAMP, 70.4 μM, 1.4 before PIP3 and (○) 51.4 μM, 1.2 after PIP3. Data are representative of five patches for WT channels and three patches for deletion mutants.
Several mechanistic scenarios might explain the loss of PIP3 sensitivity in the Δ61–90-CNGA2 channels. First, PIP3 might interact directly with amino acids within this region. Alternatively, PIP3 might bind to adjacent sites and inhibit channels by disrupting the autostimulatory interaction between the N and C termini, which is already absent in the deletion mutant. To test for a direct interaction between PIP3 and the N terminus of CNGA2, we incubated a GST-tagged peptide containing the cytoplasmic N terminus of CNGA2 (GST-A2N) with PIP3-conjugated beads. The beads and bound protein were recovered by centrifugation and washed to reduce nonspecific binding. As shown in Fig. 3B, GST alone did not adhere to the PIP3 beads, but the high-affinity PIP3-binding domain from Grp1 (GST-Grp1PH) was enriched by the PIP3 beads. A fraction of the GST-A2N peptide also was recovered with the PIP3-conjugated beads. In contrast, a CNGA2 N-terminal peptide lacking residues 61–90 (GST-A2NΔ) did not adhere to the PIP3-coated beads. This biochemical result suggests that PIP3 directly binds to residues in the region between amino acids 61 and 90. Surprisingly, we found that the CNGA3 N terminus also adhered to PIP3-coated beads (Fig. 3B), even though PIP3 does not alter CNGA3 channel activity. When the Ca2+-CaM-binding site was removed from the CNGA3 N-terminal peptide (GST-A3NΔ), binding to PIP3 beads no longer was observed. Interestingly, previous biochemical experiments have established that the N terminus of CNGA3 binds Ca2+/CaM, but this binding is functionally silent (27).
Native-type olfactory CNG channels, formed by CNGA2, CNGA4, and CNGB1b subunits, were also strongly inhibited by PIP3. Heteromeric channels containing WT CNGA2 subunits exhibited a 5-fold average increase in their K1/2 for cGMP from 2.6 ± 0.6 μM to 15.7 ± 7.1 μM after brief exposures to 10 μM PIP3 (five patches; Fig. 3C). To test the functional relevance of the CNGA2 N-terminal PIP3-binding site in the context of the heteromeric channel, we expressed the Δ61–90-CNGA2 subunits along with CNGA4 and CNGB1b. We found that exposure to 10 μM PIP3 had no appreciable effect on the apparent cGMP affinity of these channels (K1/2 before PIP3, 14.7 ± 3.1 μM; K1/2 after, 19.9 ± 9.1 μM, three patches; Fig. 3D). Similar results were observed with cAMP for both WT and mutant channels (Fig. 3 C and D). These observations demonstrate that residues 61–90 of CNGA2 are necessary for PIP3 regulation of heteromeric olfactory CNG channels.
Interplay Between PIP3 and Ca2+/CaM Regulation.
Because our biochemical data imply that the PIP3-binding site overlaps that of Ca2+/CaM on CNGA2, we predicted that PIP3 might occlude the action of Ca2+/CaM on homomeric CNGA2 channels. As shown in Fig. 4A, CNGA2 channels exhibited a reversible decrease in their apparent affinity for cGMP after exposure to 250 nM Ca2+/CaM (K1/2 before Ca2+/CaM, 2.3 ± 1.2 μM; after Ca2+/CaM, 19.2 ± 5.3 μM; after EDTA wash, 1.6 ± 0.4 μM; four patches). Subsequently, a brief exposure to 10 μM PIP3 inhibited channel activation to an equal, or greater, extent (K1/2 = 29.9 ± 11.4 μM, four patches). Thereafter, a second exposure to Ca2+/CaM produced no further decrease in apparent affinity (K1/2 = 29.6 ± 14.7 μM, four patches). This finding suggests that PIP3 and Ca2+/CaM exert their effects through a common mechanism or that PIP3 inhibits Ca2+/CaM binding. To test the latter hypothesis, we measured Ca2+/CaM binding to the CNGA2 N terminus by using a calmodulin overlay assay (Fig. 4D). We found that the presence of 100 μM PIP3 reduced the binding of FLAG-tagged Ca2+/CaM to a GST-tagged CNGA2 N-terminal peptide by almost 70% under our assay conditions (67.3 ± 0.06%; n = 6). Thus, PIP3 appears to mimic and occlude Ca2+/CaM inhibition of homomeric CNGA2 channels, in part, by interfering with Ca2+/CaM binding to the CNGA2 N terminus.
Fig. 4.
PIP3 prevents calmodulin regulation of olfactory CNG channels. (A) CNGA2 cGMP dose–response relationships were measured before (●) and after (▿) application of 250 nM Ca2+/CaM. The patch then was washed with 0.5 mM EDTA (■) to remove the CaM. A 10 μM concentration of PIP3 (◇) caused a right shift that occluded a response to subsequent application of CaM (▴). K1/2 values: before CaM, 1.7 μM; after CaM, 13.3 μM; EDTA wash, 1.2 μM; after PIP3, 15.3 μM; PIP3 + CaM, 13.2 μM. Data are representative of four separate experiments. (B) Representative traces from an inside-out patch containing wtCNGA2/A4/B1b channels stepped to +50 mV and –50 mV and exposed to solutions containing either 500 nM Ca2+/CaM or 10 μM PIP3. Data are representative of three separate experiments. (C) Representative trace from an inside-out patch containing Δ61–90-CNGA2/A4/B1b channels before and after exposure to 10 μM PIP3. The current activated by 50 μM cGMP was measured at +50 mV every second and normalized to the current activated by 1 mM cGMP. Ca2+/CaM (250 nM) was added at 20 s. Data are representative of three separate experiments. (D) After blotting, GST-fusion proteins were probed in an overlay assay by using ≈50 nM FLAG-tagged CaM in 10 μM buffered calcium, either in the presence (below) or in the absence (above) of 100 μM PIP3. GST-A2N and GST-A2NΔ are as in Fig. 3B; GST-B1bN, proximal N-terminal region (amino acids 677–764) of bovine CNGB1; GST-A4CL, C-linker region (amino acids 271–339) of rat CNGA4. Bound CaM-FLAG was detected by using M2 anti-FLAG antibody. Arrowheads indicate approximate location of molecular mass markers: 49, 38, and 28 kDa. Corresponding blots above and below each other represent identical exposure times. Data are representative of six different experiments.
In heteromeric olfactory channels, the CNGA2 N-terminal CaM-binding site is functionally silent; instead, Ca2+/CaM exerts its inhibitory effects by binding to IQ-like motifs in the CNGA4 and CNGB1b subunits. Therefore, we expected that PIP3 and Ca2+/CaM inhibition would be additive. Surprisingly, we found that WT heteromeric channels were unaffected by application of Ca2+/CaM after exposure to 10 μM PIP3 (Fig. 4B). Exposure of heteromeric channels containing Δ61–90-CNGA2 to 10 μM PIP3 also occluded inhibition by Ca2+/CaM (Fig. 4C), even though PIP3 had no direct effect on the apparent affinity of the channels. Using calmodulin overlay assays (Fig. 4D), we found that the presence of 100 μM PIP3 inhibited the binding of FLAG-tagged calmodulin to peptides containing the IQ motifs from CNGA4 (86.1 ± 0.06%; n = 9) and CNGB1b (87.4 ± 0.08%; n = 7).
Discussion
We have shown that PIP3 dramatically shifts the cyclic nucleotide sensitivity of olfactory CNG channels by direct interaction with residues in the N terminus of CNGA2. This interaction mimics and occludes Ca2+/CaM regulation in homomeric CNGA2 channels. In heteromeric channels containing CNGA2, CNGA4, and CNGB1b, PIP3 and Ca2+/CaM produce distinct functional changes by binding to different subunits, yet PIP3 still blocks Ca2+/CaM regulation. Our findings reaffirm the regulatory significance of the CNGA2 N terminus in the heteromeric channel and indicate that each subunit in native olfactory CNG channels has a distinct and important role in channel regulation and trafficking (28–30).
Based on our observations, a possible mechanism for PIP3 inhibition of olfactory CNG channels can be inferred. In homomeric CNGA2 channels, removal of residues 61–90, exposure to Ca2+/CaM, and exposure to PIP3 produce nearly identical shifts in cyclic nucleotide sensitivity and in the maximum cAMP-activated current. The removal of residues 61–90 and regulation by Ca2+/CaM both are known to disrupt an autostimulatory interaction between the N and C termini of adjacent CNGA2 subunits (15, 17). Because PIP3 inhibition requires residues 61–90 and prevents Ca2+/CaM regulation, we postulate that PIP3 binding may anchor the N terminus of CNGA2 to the membrane surface, thereby disrupting the intersubunit autostimulatory interaction. This molecular mechanism resembles that recently proposed for prevention of K+ channel N-type inactivation by phosphoinositides: sequestering of the N-terminal domain at the cytoplasmic face of the membrane (31). Although the N terminus of CNGA3 can bind both PIP3 and Ca2+/CaM, these interactions appear to be functionally silent, most likely reflecting the absence of a pronounced autostimulatory interaction with the C terminus (27). Our data also suggest a possible mechanism for PIP3 inhibition of heteromeric olfactory channels. As we and others have observed, PIP3 inhibition of heteromeric channels causes a 10-fold shift in cyclic nucleotide sensitivity and a 2-fold reduction in the efficacy of cAMP (11). In contrast, Ca2+/CaM produces a larger shift in cyclic nucleotide sensitivity without decreasing the efficacy of cAMP (12). Taken together, the results suggest that autostimulatory subunit interactions still occur in heteromeric channels, and that PIP3 inhibits the heteromeric channel by interaction with CNGA2, whereas Ca2+/CaM inhibition requires interaction with CNGA4 or CNGB1b (21).
Phosphatidylinositides are known to regulate the activity of an ever-increasing number of ion channels. Binding of PIP2 to both Kir1 and Kir6 channels promotes channel activation by stabilizing the open state (32–34), and PIP3 has been proposed to activate both epithelial sodium channels and TRPC6 channels by direct binding (35, 36). Like PIP2-binding regions identified in other ion channels (37–39), the stretch of amino acids between residues 61–90 of CNGA2 contains multiple basic residues that may be important for the interaction with negatively charged phospholipids. Similar to PIP2 inhibition of TRPV1 channels (40), PIP3 inhibited activation of olfactory CNG channels. PIP2 and Ca2+/CaM have been shown to bind competitively and have antagonistic effects on the activity of many proteins, like RGS4 and MARCKS (41, 42). In contrast, PIP3 partially mimics and occludes Ca2+/CaM regulation of olfactory CNG channels. Our data suggest that for both homomeric and heteromeric olfactory CNG channels, PIP3 interferes with Ca2+/CaM binding to several channel subunits. Additionally, in homomeric channels, binding of PIP3 may induce a separation of the N and C termini, thereby preventing Ca2+/CaM from having any further effect on channel function.
Olfactory sensory adaptation is mediated by Ca2+/CaM-dependent channel inhibition and depends on activation of the odorant signaling pathway and the subsequent influx of Ca2+ through open CNG channels (9). In contrast, inhibition of olfactory signaling by PIP3 does not require prior channel activity, and channel regulation by PIP3 may serve to reduce overall olfactory sensitivity in the presence of complex odorant mixtures. One possible route for PIP3 synthesis is suggested by the recent discovery of purinergic receptors in olfactory sensory neuron membranes (43). These G protein-coupled receptors are activated by ATP and have been reported to stimulate PIP3 synthesis in astrocytes and C6 glioma cells (44, 45). In the olfactory epithelium, noxious stimuli or prolonged exposure to odorants may lead to the release of cellular ATP, causing activation of purinergic receptors and consequent production of PIP3. We speculate, therefore, that the stimulation of PIP3 synthesis by purinergic receptor activation may serve a neuroprotective role by preventing possibly toxic levels of calcium influx through CNG channels after excessive or noxious stimulation.
Materials and Methods
DNA and Mutagenesis.
WT bovine CNGA3, rat CNGA2, rat CNGA4, and rat CNGB1b, including the 5′ and 3′ untranslated regions, and all chimeras, were cloned into pcDNA3.0 (Invitrogen, Carlsbad, CA). A YFP-tagged CNGA2 was used (pEYFP-C1; Clontech, Mountain View, CA) and was indistinguishable from the native subunit. Construction of chimeric subunits has been described previously, with splice sites after S229 and I277 in CNGA2 and after V276 and I322 in CNGA3 (46). The A2nA3 chimera was constructed by using overlapping PCR, with residues 1–140 of CNGA2 spliced to residues 186–706 of CNGA3. DNAs encoding the N-terminal regions of rat CNGA2 (residues 1–138) and human CNGA3 (residues 1–164) were genetically fused with DNA-encoding GST (GE Healthcare Biosciences, Piscataway, NJ) as described in refs. 15 and 47. DNAs encoding the proximal N-terminal region of bovine CNGB1b (amino acids 677–764) and the C-linker region of rat CNGA4 (amino acids 271–339) were fused to GST to form CNGB1bN and GST-A4CL.
Cell Culture and Transfection.
HEK293 cells were maintained in DMEM (cellgro; Mediatech, Herndon, VA) containing 10% FBS (BioWhittaker Cambrex, East Rutherford, NJ) and 1% Pen-Strep (GIBCO Invitrogen, Carlsbad, CA), at 37°C in a humidified 95% O2/5% CO2 atmosphere. Cells were transfected with FuGENE 6 (Roche, Basel, Switzerland) or Effectene (Qiagen, Valencia, CA) according to the manufacturer's instructions. An unmodified pEGFP-N2 vector was cotransfected for identification during patch clamping.
Patch-Clamp Analysis.
HEK293 cells were lifted into room temperature normal media 18–72 h after transfection and were used for recording within 6 h. Cell aliquots were added to a bath solution containing 130 mM NaCl, 5 mM KCl, 20 mM Hepes, 0.5 mM EDTA, 5 mM MgCl2, and 2 mM CaCl2, pH 7.4. Pipettes were filled with a solution matching that in the bath, without MgCl2 and CaCl2, and had resistances ranging from 1.5 to 4.0 MΩ. After the formation of a GΩ seal, inside-out patches were excised and placed in front of a perfusion head controlled by a Biologic RSC-100 rapid solution changer (Molecular Kinetics, Pullman, WA). Patches were first washed in a solution containing 130 mM KCl, 5 mM NaCl, 20 mM Hepes, 0.5 mM EDTA (pH 7.4), and then exposed to varying concentrations of cAMP and cGMP (Sigma, St. Louis, MO) dissolved in the perfusion solution. Dose–response curves were generated by subtracting the current recorded at +50 mV in cyclic nucleotide-free solution from the current recorded in the presence of cyclic nucleotide at the same voltage. The leak-subtracted currents were normalized to the current elicited by 1 mM cGMP. Curves are fits to the Hill equation, I/Imax = Cn/[Cn + (K1/2)n], where C is the cyclic nucleotide concentration and n is the Hill coefficient. For Ca2+/CaM experiments, perfusion solutions typically contained 250 nM bovine brain calmodulin (Sigma), 2 mM NTA instead of EDTA, and 704 μM total CaCl2 (50 μM free Ca2+). All phosphatidylinositides were obtained from Matreya (Pleasant Gap, PA) as sodium salts and reconstituted with water to stock concentrations of 100 μM. The stock solutions were sonicated at low power for 30 min on ice and stored at −20°C. Perfusion solutions containing phosphatidylinositides were sonicated for an additional 30 min before use. Recordings were made with a CV201 headstage attached to an Axopatch 200A amplifier and a Digidata 1200 interface (Molecular Devices, Sunnyvale, CA). Currents were sampled at 10 kHz and digitally filtered at 1 kHz.
Biochemistry.
Expression of recombinant protein in bacteria and purification were carried out as described in refs. 15 and 47. Purified GST-fusion proteins were used for in vitro PIP3-binding assays in buffer containing 10 mM Hepes (pH 7.4), 150 mM NaCl, 5 mM EDTA and 0.25% Nonidet P-40. PIP3-agarose beads and the GST-Grp1PH positive-control protein were from Echelon Biosciences (Salt Lake City, UT). Fifty microliters of a 50% slurry of PIP3 beads and purified protein (2 μg/ml) were incubated in 0.5 ml of binding buffer for 2 h at 4°C with rocking. Beads were gently pelleted and washed five times with excess binding buffer; PIP3-interacting proteins were eluted with 1× NuPAGE sample buffer (Invitrogen). Protein samples then were separated under reducing conditions in 4–12% Bis-Tris gels and blotted onto nitrocellulose by using the NuPAGE transfer buffer system (Invitrogen). Blotted proteins were detected by using B-14 anti-GST monoclonal antibody (Santa Cruz Biotechnology, Santa Cruz, CA) at a dilution of 1:2,000 in 1% milk/500 mM NaCl/20 mM Tris·HCl (pH 7.5)/0.05% Tween 20, HRP-conjugated anti-mouse IgG secondary antibody, and the SuperSignal West Dura Extended Duration chemiluminescence substrate (Pierce Biotechnology, Rockford, IL).
CaM-overlay assays were carried out essentially as described in ref. 47. After blotting, GST-fusion proteins were probed for 1 h at room temperature with recombinant FLAG-tagged CaM (at ≈50 nM) in 10 mM Hepes (pH 7.4)/150 mM NaCl/0.5% Nonidet P-40/0.5% BSA/2 mM NTA/170 μM CaCl2 (10 μM free Ca2+), either with or without prior and concomitant incubation with 100 μM PIP3. Bound CaM was visualized by using M2 anti-FLAG antibody (Sigma), and the CaM signal was quantified by using Kodak (New Haven, CT) 1D Image Analysis software. Blots subsequently were stripped and reprobed with an antibody against GST to ensure that equivalent amounts of fusion protein were blotted in all lanes. Control Western blots for CaM demonstrated that incubation with PIP3 did not interfere with antibody binding or blot processing (data not shown). Inhibition of CaM binding is expressed as percentage reduction compared with control ± SEM.
Acknowledgments
We thank Dr. Martin Biel (Ludwig-Maximilians University, Munich, Germany) for providing the DNA-encoding CNGA3, CNGA4, and CNGB1b and Dr. William Zagotta (University of Washington) for the DNA-encoding CNGA2 and the GST-fusion peptides. This work was supported by the National Institutes of Health (NIH) Ruth Kirchstein National Predoctoral Research Service Award NS052103 (to J.D.B.) and NIH Grants EY009275 (to J.W.K.), EY12836 (to M.D.V.), and EY12837 (to R.L.B.).
Abbreviations
- CNG
cyclic nucleotide-gated
- PIP2
phosphatidylinositol-4,5-bisphosphate
- PIP3
phosphatidylinositol-3,4,5-trisphosphate
- Ca2+/CaM
calcium–calmodulin
Footnotes
Author contributions: J.D.B., J.W.K., M.D.V., and R.L.B. designed research; J.D.B. and E.D.R. performed research; J.D.B., M.D.V., and R.L.B. analyzed data; and J.D.B., J.R.M., J.W.K., M.D.V., and R.L.B. wrote the paper.
The authors declare no conflict of interest.
This article is a PNAS direct submission. K.-W.Y. is a guest editor invited by the Editorial Board.
References
- 1.Buck L, Axel R. Cell. 1991;65:175–187. doi: 10.1016/0092-8674(91)90418-x. [DOI] [PubMed] [Google Scholar]
- 2.Ronnett GV, Moon C. Annu Rev Physiol. 2002;64:189–222. doi: 10.1146/annurev.physiol.64.082701.102219. [DOI] [PubMed] [Google Scholar]
- 3.Ache BW, Young JM. Neuron. 2005;48:417–430. doi: 10.1016/j.neuron.2005.10.022. [DOI] [PubMed] [Google Scholar]
- 4.Axel R. Angew Chem Int Ed Engl. 2005;44:6110–6127. doi: 10.1002/anie.200501726. [DOI] [PubMed] [Google Scholar]
- 5.Kaupp UB, Seifert R. Physiol Rev. 2002;82:769–824. doi: 10.1152/physrev.00008.2002. [DOI] [PubMed] [Google Scholar]
- 6.Kleene SJ. Neuron. 1993;11:123–132. doi: 10.1016/0896-6273(93)90276-w. [DOI] [PubMed] [Google Scholar]
- 7.Kolesnikov SS, Kosolapov AV. Biochim Biophys Acta. 1993;1150:63–72. doi: 10.1016/0005-2736(93)90122-g. [DOI] [PubMed] [Google Scholar]
- 8.Reisert J, Lai J, Yau KW, Bradley J. Neuron. 2005;45:553–561. doi: 10.1016/j.neuron.2005.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bradley J, Reisert J, Frings S. Curr Opin Neurobiol. 2005;15:343–349. doi: 10.1016/j.conb.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 10.Spehr M, Wetzel CH, Hatt H, Ache BW. Neuron. 2002;33:731–739. doi: 10.1016/s0896-6273(02)00610-4. [DOI] [PubMed] [Google Scholar]
- 11.Zhainazarov AB, Spehr M, Wetzel CH, Hatt H, Ache BW. J Membr Biol. 2004;201:51–57. doi: 10.1007/s00232-004-0707-4. [DOI] [PubMed] [Google Scholar]
- 12.Chen TY, Yau K-W. Nature. 1994;368:545–548. doi: 10.1038/368545a0. [DOI] [PubMed] [Google Scholar]
- 13.Liu M, Chen TY, Ahamed B, Li J, Yau K-W. Science. 1994;266:1348–1354. doi: 10.1126/science.266.5189.1348. [DOI] [PubMed] [Google Scholar]
- 14.O'Neil KT, DeGrado WF. Trends Biochem Sci. 1990;15:59–64. doi: 10.1016/0968-0004(90)90177-d. [DOI] [PubMed] [Google Scholar]
- 15.Varnum MD, Zagotta WN. Science. 1997;278:110–113. doi: 10.1126/science.278.5335.110. [DOI] [PubMed] [Google Scholar]
- 16.Trudeau MC, Zagotta WN. J Biol Chem. 2003;278:18705–18708. doi: 10.1074/jbc.R300001200. [DOI] [PubMed] [Google Scholar]
- 17.Zheng J, Varnum MD, Zagotta WN. J Neurosci. 2003;23:8167–8175. doi: 10.1523/JNEUROSCI.23-22-08167.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sautter A, Zong X, Hofmann F, Biel M. Proc Natl Acad Sci USA. 1998;95:4696–4701. doi: 10.1073/pnas.95.8.4696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bonigk W, Bradley J, Muller F, Sesti F, Boekhoff I, Ronnett GV, Kaupp UB, Frings S. J Neurosci. 1999;19:5332–5347. doi: 10.1523/JNEUROSCI.19-13-05332.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zheng J, Zagotta WN. Neuron. 2004;42:411–421. doi: 10.1016/s0896-6273(04)00253-3. [DOI] [PubMed] [Google Scholar]
- 21.Bradley J, Bonigk W, Yau KW, Frings S. Nat Neurosci. 2004;7:705–710. doi: 10.1038/nn1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gordon SE, Downing-Park J, Tam B, Zimmerman AL. Biophys J. 1995;69:409–417. doi: 10.1016/S0006-3495(95)79913-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Crary JI, Dean DM, Nguitragool W, Kurshan PT, Zimmerman AL. J Gen Physiol. 2000;116:755–768. doi: 10.1085/jgp.116.6.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dean DM, Nguitragool W, Miri A, McCabe SL, Zimmerman AL. Proc Natl Acad Sci USA. 2002;99:8372–8377. doi: 10.1073/pnas.122681899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Womack KB, Gordon SE, He F, Wensel TG, Lu CC, Hilgemann DW. J Neurosci. 2000;20:2792–2799. doi: 10.1523/JNEUROSCI.20-08-02792.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brady JD, Rich TC, Le X, Stafford K, Fowler CJ, Lynch L, Karpen JW, Brown RL, Martens JR. Mol Pharmacol. 2004;65:503–511. doi: 10.1124/mol.65.3.503. [DOI] [PubMed] [Google Scholar]
- 27.Grunwald ME, Zhong H, Lai J, Yau K-W. Proc Natl Acad Sci USA. 1999;96:13444–13449. doi: 10.1073/pnas.96.23.13444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bradley J, Reuter D, Frings S. Science. 2001;294:2176–2178. doi: 10.1126/science.1063415. [DOI] [PubMed] [Google Scholar]
- 29.Munger SD, Lane AP, Zhong H, Leinders-Zufall T, Yau KW, Zufall F, Reed RR. Science. 2001;294:2172–2175. doi: 10.1126/science.1063224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jenkins P, Hurd TW, Zhang L, McEwen DP, Brown RL, Margolis B, Verhey KJ, Martens JR. Curr Biol. 2006;16:1211–1216. doi: 10.1016/j.cub.2006.04.034. [DOI] [PubMed] [Google Scholar]
- 31.Oliver D, Lien CC, Soom M, Baukrowitz T, Jonas P, Fakler B. Science. 2004;304:265–270. doi: 10.1126/science.1094113. [DOI] [PubMed] [Google Scholar]
- 32.Cukras CA, Jeliazkova I, Nichols CG. J Gen Physiol. 2002;119:581–591. doi: 10.1085/jgp.20028562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang CL, Feng S, Hilgemann DW. Nature. 1998;391:803–806. doi: 10.1038/35882. [DOI] [PubMed] [Google Scholar]
- 34.Logothetis DE, Zhang H. J Physiol (London) 1999;520:630. doi: 10.1111/j.1469-7793.1999.00630.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pochynyuk O, Staruschenko A, Tong Q, Medina J, Stockand JD. J Biol Chem. 2005;280:37565–37571. doi: 10.1074/jbc.M509071200. [DOI] [PubMed] [Google Scholar]
- 36.Tseng PH, Lin HP, Hu H, Wang C, Zhu MX, Chen CS. Biochemistry. 2004;43:11701–11708. doi: 10.1021/bi049349f. [DOI] [PubMed] [Google Scholar]
- 37.Dong K, Tang L, MacGregor GG, Hebert SC. J Biol Chem. 2002;277:49366–49373. doi: 10.1074/jbc.M208679200. [DOI] [PubMed] [Google Scholar]
- 38.Zhang H, He C, Yan X, Mirshahi T, Logothetis DE. Nat Cell Biol. 1999;1:183–188. doi: 10.1038/11103. [DOI] [PubMed] [Google Scholar]
- 39.Shyng SL, Cukras CA, Harwood J, Nichols CG. J Gen Physiol. 2000;116:599–608. doi: 10.1085/jgp.116.5.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Prescott ED, Julius D. Science. 2003;300:1284–1288. doi: 10.1126/science.1083646. [DOI] [PubMed] [Google Scholar]
- 41.Ishii M, Inanobe A, Kurachi Y. Proc Natl Acad Sci USA. 2002;99:4325–4330. doi: 10.1073/pnas.072073399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McLaughlin S, Murray D. Nature. 2005;438:605–611. doi: 10.1038/nature04398. [DOI] [PubMed] [Google Scholar]
- 43.Hegg CC, Greenwood D, Huang W, Han P, Lucero MT. J Neurosci. 2003;23:8291–8301. doi: 10.1523/JNEUROSCI.23-23-08291.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang M, Kong Q, Gonzalez FA, Sun G, Erb L, Seye C, Weisman GA. J Neurochem. 2005;95:630–640. doi: 10.1111/j.1471-4159.2005.03408.x. [DOI] [PubMed] [Google Scholar]
- 45.Czajkowski R, Banachewicz W, Ilnytska O, Drobot LB, Baranska J. Br J Pharmacol. 2004;141:497–507. doi: 10.1038/sj.bjp.0705639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brown RL, Lynch LL, Haley TL, Arsanjani R. J Gen Physiol. 2003;122:749–760. doi: 10.1085/jgp.200308823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Peng C, Rich ED, Thor CA, Varnum MD. J Biol Chem. 2003;278:24617–24623. doi: 10.1074/jbc.M301699200. [DOI] [PubMed] [Google Scholar]