Abstract
Mildly stressful early life experiences can potentially impact a broad range of social, cognitive, and physiological functions in humans, nonhuman primates, and rodents. Recent rodent studies favor a maternal-mediation hypothesis that considers maternal-care differences induced by neonatal stimulation as the cause of individual differences in offspring development. Using neonatal novelty exposure, a neonatal stimulation paradigm that dissociates maternal individual differences from a direct stimulation effect on the offspring, we investigated the effect of early exposures to novelty on a diverse range of psychological functions using several assessment paradigms. Pups that received brief neonatal novelty exposures away from the home environment showed enhancement in spatial working memory, social competition, and corticosterone response to surprise during adulthood compared with their home-staying siblings. These functional enhancements in novelty-exposed rats occurred despite evidence that maternal care was directed preferentially toward home-staying instead of novelty-exposed pups, indicating that greater maternal care is neither necessary nor sufficient for these early stimulation-induced functional enhancements. We suggest a unifying maternal-modulation hypothesis, which distinguishes itself from the maternal-mediation hypothesis in that (i) neonatal stimulation can have direct effects on pups, cumulatively leading to long-term improvement in adult offspring; and (ii) maternal behavior can attenuate or potentiate these effects, thereby decreasing or increasing this long-term functional improvement.
Keywords: hypothalamic–pituitary–adrenal (HPA) axis, maternal retrieval, neonatal handling, spatial learning, stress
The mother is an omnipresent figure in the life of rodent neonates. In rodent models that focus on understanding functional impairment, prolonged separation of neonates from their mother leads to long-term deficits relative to controls (1–3). In contrast, rodent models demonstrating functional enhancement reveal that brief separation of neonates from their mother and home environment leads to a range of advantages relative to controls (4–7). In both types of studies, maternal behavior is considered critical in mediating these effects. Complementary to these experimental manipulations, studies of naturally occurring maternal-care behaviors offer further evidence that maternal care is positively correlated with an impressive array of neuroendocrine, cellular, and molecular measures from adult offspring (8–10). Such relationships were considered as evidence that particular maternal-care behaviors are positive markers of offspring neural development (8).
Although these findings seem to lead to a compelling conclusion that the more maternal care offspring receive, the greater functional advantage they have during adulthood, it has long been noted in the literature on rodents that early stimulation of the mother and stimulation of pups are intrinsically confounded in neonatal handling studies because dams of handled pups are removed from the litter during the procedure (11–14). Because mothers of handled pups are likely to change their care behavior toward pups as a result of this experience, correlations between maternal care and changes in offspring behavioral and neuroendocrine measures (8) could be the result of a direct stimulation effect on pups, an indirect effect on pups due to stimulation-induced alterations in maternal care, or both. As such, a correlation between certain maternal-care behaviors and offspring measures cannot be used to rule out a direct effect of pup stimulation.
Thus far, the literature on rodents has offered no compelling data to rule out the alternative hypotheses that neonatal stimulation in rodents has a direct effect on pups, which then contributes to adult functional changes. As pointed out by Pryce and Feldon (12), evidence against the maternal-mediation hypothesis was also provided by its proponents (15), who showed that levels of hippocampal protein kinase A differed between handled and nonhandled pups within 4 h after handling, but this effect could not be induced by an increase in an around-the-clock maternal-care measure. To the contrary, stimulation afforded by the handling procedure must have exerted direct effects on pups because handling of the pups during the week immediately after early weaning on postnatal day 14 (P14) produced its hallmark effect on open-field activity in the total absence of the mother (16).
We approach the long-standing problem of confounded mother–pup stimulation by isolating the effects of pup stimulation from that of mother stimulation using a neonatal stimulation procedure called neonatal novelty exposure (11). This procedure employs a split-litter design in which, during the first 3 weeks of life, pups that are exposed to a novel environment and pups that remain in the familiar home environment are from the same litter and thus share the same mother. Therefore, maternal individual differences are shared between novelty-exposed and home-staying pups, and thus they cannot be the sole cause of a within-litter difference among pups. Here, in a 13-month longitudinal study, we examined the effect of this early stimulation procedure on both offspring physiology and behavior throughout adulthood and maternal-care behavior during the period of neonatal stimulation.
Results
Study 1: Effects of Neonatal Novelty Exposure on Offspring Development.
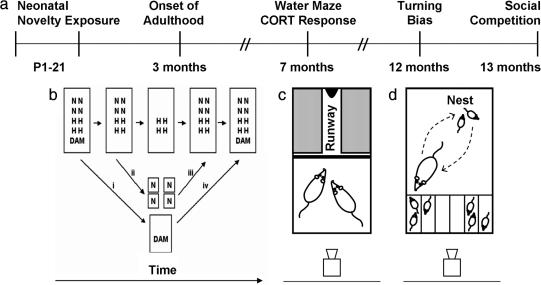
We followed the same set of male rats from birth to late adulthood, and we evaluated the effects of neonatal novelty exposure on a diverse range of adult functions (Fig. 1a). During P1–21, half of each litter was exposed to a relatively novel nonhome cage for 3 min daily (Novel), and the other half remained in the home cage (Home) (for details, see Fig. 1b). The duration that pups were separated from their dams was equalized between Novel and Home pups by removing the dam from and returning her to the litter before and after the differential treatment of pups. The amount of handling received from the experimenter was equalized between the two groups by matching the experimenter contact with Home pups to that received by Novel pups.
Fig. 1.
Experimental methods. (a) Time line of longitudinal study. (b) Sequential steps in neonatal novelty exposure. (i) Removal of dam from home cage. (ii) Transfer of Novel pups to individual nonhome cages. (iii) Return of Novel pups to home cage after 3-min exposure to nonhome cages. (iv) Return of dam to entire litter in home cage. Experimenter handling and duration of maternal separation were matched between Novel (N) and Home (H) pups. (c) Setup for social competition testing. After individual training, pairs of rats competed for access to a chocolate reward at the end of the runway. (d) Setup for observation of postnovelty exposure pup retrieval. The middle two compartments were used as buffer zones for better separation of Novel and Home pups.
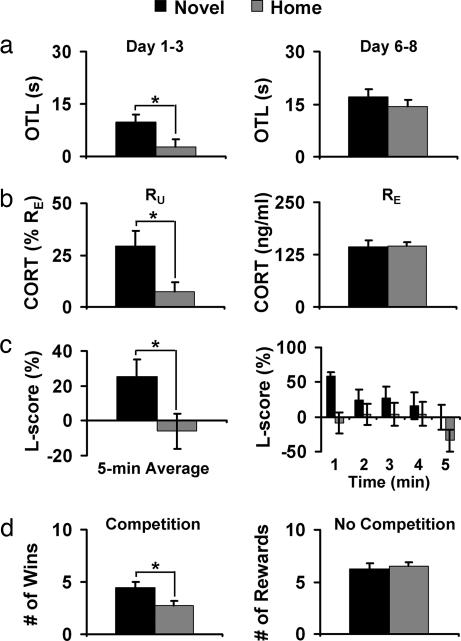
Six months later, spatial working memory was evaluated in the moving-platform version of the Morris water task when rats were 7 months old. In this task, rats must learn to locate a platform hidden under the surface of opaque water by using distal cues present in the testing room. A new platform location was used on each of the 8 testing days, and a total of four trials were given per day. Following procedures described by Tang (11), we measured working-memory function with a one-trial learning (OTL) score, defined as the swim latency difference between the first and second trials (T1 − T2). During the first 3 days of training, when the task was new to the rats, the average OTL score of Novel rats was significantly greater than that of Home rats [t (36) = 2.202, P = 0.031, d = 0.734; Fig. 2a Left], suggesting more rapid learning in Novel than in Home rats. Both groups reached indistinguishable levels of performance by the end of training (Fig. 2a Right), therefore ruling out sensory, motor, or motivational factors as potential confounds. These results indicate that rats that received brief neonatal exposures to a nonhome environment became adults that were better at spatial learning compared with home-staying controls. Meaney et al. (17) demonstrated that neonatal stimulation improves reference memory for a fixed-goal location among aged rats, and the present and an earlier study (11) extend this finding by showing that neonatal stimulation also improves juvenile and adult working memory for changing-goal locations.
Fig. 2.
Neonatal novelty exposure increased adult functionality. Black bars, Novel rats; gray bars, Home rats. (a) Novel rats showed greater OTL compared with Home rats when first exposed to the working memory version of the Morris water task (Left). Both groups performed at the same level at the end of testing, indicating a lack of differences in motivation and sensorimotor function (Right). Novel, n = 33; Home, n = 35. (b) Compared with Home rats, Novel rats showed a selective increase in CORT response to an unexpected 1-min exposure to an open field between T1 and T2 (RU; Left) but not in CORT response to an expected swim stressor (RE; Right). Novel, n = 17; Home, n = 14. (c) Novel rats showed a greater rightward turning bias than Home rats (Left), indexed by a greater lateralization score (L-score), a difference most prominent during the 1st min of testing (Right). Novel, n = 24; Home, n = 24. (d) Novel rats won more chocolate rewards than Home rats when competing against a conspecific (Left). Novel and Home rats did not differ in the number of chocolate rewards obtained when no competitor was present (Right). Novel, n = 24; Home, n = 24.
On the 2 days immediately after the above-described water task, we used this now-familiar task as a probe for determining the rats’ corticosterone (CORT) response to an expected stressor (RE), and we used a 1-min exposure to a novel open field between T1 and T2 as a probe for determining the rats’ ability to mount an additional response to an unexpected event (RU). Novel and Home rats did not differ in basal CORT concentration (in nanograms per milliliter: Novel, 88.38 ± 7.33; Home, 84.97 ± 9.30) or in CORT RE, indexed by the CORT increase after four trials of water task relative to basal CORT (Fig. 2b Right). Novel and Home rats, however, differed in CORT RU, indexed by an additional CORT response to the open-field exposure relative to that induced by the water task alone [t (29) = 2.479, P = 0.019, d = 0.921; Fig. 2b Left]. Furthermore, Novel rats exhibited an RU of 29%, which is significantly greater than zero [t (16) = 3.845, P = 0.001, d = 1.923], whereas Home rats exhibited an RU of only 8%, which is marginally significant [t (13) = 1.738, P = 0.106, d = 0.964]. These results indicate that neonatal novelty exposure selectively increased the ability of rats to mount an additional CORT response to an unexpected event but not to an expected stressor. Different from most measures reported in the literature that capture the recovery phase of the CORT response, RU most likely reflects the rising phase because samples were collected immediately after the last of the four swim trials and within 15 min of the unexpected open-field exposure. We interpret the greater RU among Novel rats as indicating a more effective hypothalamic–pituitary–adrenal (HPA) axis, capable of mounting fast and selective responses to environmental surprise or novelty, similar to the greater initial rise in CORT among handled rats after the termination of shock treatment compared with that among nonhandled rats (18).
Although stress is generally viewed as having a negative influence on cognitive function, several recent studies demonstrate that HPA activation can facilitate learning under conditions of arousal (19). For example, an elevation of circulating CORT correlates positively with memory for the platform location (20), and prevention of CORT action by blocking glucocorticoid receptors impairs spatial-memory performance (21). In our study, the ability of Novel rats to mount an additional CORT response to changes in the normal testing routine may have allowed them to encode the new platform location while coping with an expected event. In addition to their ability to maintain sensitivity to unexpected events against a background of expected stressors, Novel rats also exhibit reduced emotional reactivity measured in the open field (11, 22), enhanced HPA negative feedback control as indicated by a greater CORT suppression of hippocampal field potentials (23), and a reduced basal CORT concentration (13). Therefore, the HPA axis of Novel rats is similar to that of the handled rats or rats that received greater maternal care (4, 8, 17, 24, 25).
Functional brain asymmetry was evaluated by a spontaneous turning bias during a 5-min observation session in a nonhome cage when rats were 12 months old following procedures described by Tang et al. (13). Although total activity (sum of left and right turns and rears) did not differ between Novel and Home rats, Novel rats showed a greater rightward turning bias than Home rats as indicated by a greater L-score [t (46) = 2.200, P = 0.033, d = 0.635; Fig. 2c Left]. This difference was particularly apparent during the initial portion of the observation session (Fig. 2c Right). This pattern confirms the finding that neonatal novelty exposure modulates a dynamic aspect of functional brain asymmetry (26) in addition to the stable asymmetry of “handedness” (27). Perhaps more importantly, the fact that a simple sequence of early life stimulation can shape adult functional asymmetry may have significant implications for understanding and reshaping the trajectory of developmental disorders known to involve abnormal brain asymmetry (28).
The ability to compete against a conspecific for limited access to rewards was evaluated in a social competition task when rats were 13 months old. Rats were first individually trained to obtain a chocolate reward (Fig. 1c). On the last day of individual training, Novel and Home rats did not differ in the number of rewards obtained (Fig. 2d Right) or in latency to obtain the rewards (in seconds: Novel, 18.05 ± 4.20; Home, 18.63 ± 2.91), therefore ruling out motivation or ability to acquire the task as potential confounds. On the following 2 days, a Novel and a Home rat, both trained to obtain rewards, were tested simultaneously in the same cage. In the presence of this competitor, latency to obtain the reward was reduced to as low as 10% of that when no competitor was present (Novel, 2.08 ± 0.45; Home, 2.99 ± 0.28; Wilcoxon Z = 1.704, P = 0.088). Novel rats won the chocolate reward more often than Home rats [t (23) = 2.119, P = 0.045; d = 0.618; Fig. 2d Left]. These results demonstrate that when matched in motivation to consume the reward, novelty-exposed rats had a greater competitive ability than the home-staying rats to obtain limited food rewards in the presence of a conspecific, consistent with earlier reports on enhanced competitive ability among rodents receiving other forms of early stimulation (e.g., 29). To the extent that winning in a competitive situation reflects social dominance (e.g., 30), these findings suggest that mild neonatal stimulation may lead to an increase in an individual’s social dominance status. Furthermore, such early stimulation-induced changes in social dominance may be related to HPA function because dominant rats have been found to be less emotionally reactive in an open field (31) and have lower basal CORT levels (32) compared with subordinate rats.
Because genetic makeup did not differ systematically between Novel and Home pups before the neonatal stimulation, we conclude that these observed functional improvements were epigenetically induced, or in other words, triggered by brief exposures to a relatively novel nonhome environment. This epigenetic induction does not imply that novelty exposure effects were mediated by epigenetic factors alone because changes initially triggered by epigenetic influences can lead to later changes in gene expression (33). Nor does epigenetic induction imply epigenetic influence by a single event. Instead, neonatal novelty exposure represents only the initial critical trigger capable of setting off a chain of mediating and modulating events that jointly shape adult expression of functional differences. One of these events may be preferential maternal care toward the novelty-exposed pups.
Study 2: Effects of Neonatal Novelty Exposure on Maternal Care.
One of the most challenging aspects of conducting early stimulation studies is that no matter how carefully one isolates an experimental treatment (e.g., isolating novelty exposure from maternal stimulation), the process that produces long-term effects on adult function will inevitably involve other intervening variables. In the case of neonatal novelty exposure, Novel pups may behave differently than Home pups as a direct result of exposure to a nonhome environment, and this difference may trigger subsequent differential maternal care toward Novel and Home pups. Therefore, even with a split-litter design, preferential maternal care toward Novel pups remains a possible contributor to adult functional improvement.
To determine whether dams indeed show preferential maternal care toward Novel pups, one must be able to track separately pups of different group identity. Because pups tend to rest in a pile, with the dam assuming a position over them, discriminative maternal care indexed by the commonly used active-nursing measure cannot be observed separately for Novel and Home pups. Pup retrieval, a compound maternal-care behavior consisting of the dam picking up a pup with her mouth and carrying pups one-by-one to a particular area in the home cage (34), is a potential discriminative maternal-care behavior. Because it is readily induced by nest disturbance resulting from the neonatal novelty exposure procedure, it offers an ideal opportunity to measure preferential maternal care. For the same litters whose adult functions have been described above, dams and litters (n = 16 litters) were videotaped on P1–8 for the first 10 min of their reunion in the home cage after novelty exposure. To enable separate tracking, Novel and Home pups were returned to the home cage in different open-top compartments of a container (Fig. 1d).
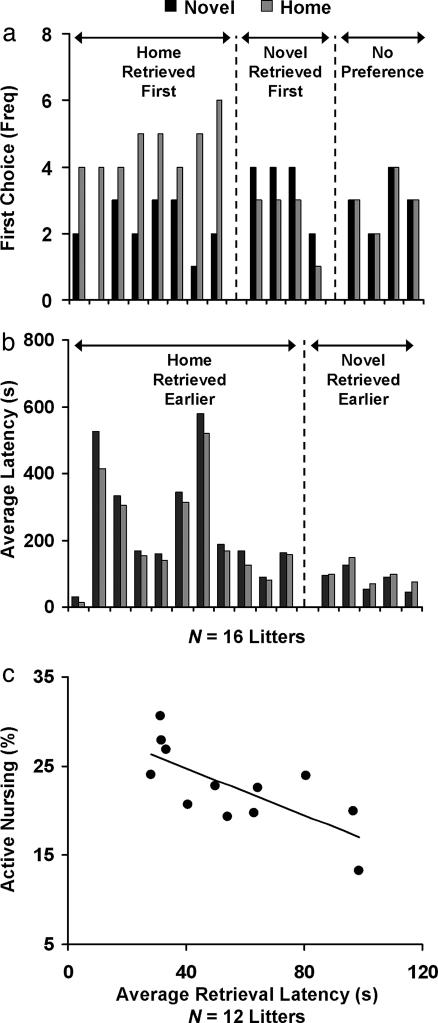
A comparison between the number of times Novel and Home pups were selected as the dams’ first choice across the 8 days revealed that first-retrieval choice differed significantly between Novel and Home pups, with Home pups, surprisingly, being the dams’ first choice more often than Novel pups (Wilcoxon Z = 1.992, P = 0.046; Fig. 3a). A comparison of average daily retrieval latency revealed a similar surprising pattern, with Home rats retrieved earlier than Novel pups (Wilcoxon Z = 1.913, P = 0.056; Fig. 3b). Both results are consistent with a previous finding that dams preferentially retrieved less-disturbed pups (35). These results indicate a lack of evidence for preferential maternal care toward Novel over Home pups, and, most importantly, they suggest that Novel pups were likely to have received a somewhat lower priority in maternal care.
Fig. 3.
Neonatal novelty exposure decreased maternal care toward novelty-exposed pups relative to home-staying siblings. (a) Home pups were more often the mother’s first-retrieval choice than Novel rats. (b) Home pups were retrieved with shorter latency than Novel pups. (c) Retrieval priority (inverse of latency) positively predicted active nursing.
To confirm that pup retrieval, a temporally restricted maternal-care behavior, is predictive of active nursing, a temporally distributed and more commonly used maternal-care behavior (36), we obtained both measures of retrieval latency and active nursing for each dam in a separate experiment (n = 12 litters). Consistent with the finding that more milk was found in the stomachs of pups that were retrieved earlier (37), we found a significant correlation between the two measures (r = −0.725, P = 0.008; Fig. 3c): the earlier pups were retrieved, the more active nursing they received throughout the day. Given this relationship, we infer that Novel pups, which were retrieved later, were unlikely to have received more active nursing throughout the day compared with Home pups.
Together, these results demonstrate that pup-retrieval measures are not only informative for active nursing but are also sufficiently sensitive to reveal preferential maternal care among offspring. Most importantly, the two retrieval measures offered converging evidence regarding the direction of maternal preference, both indicating preferential maternal care toward home-staying pups (see also Fig. 4 and Supporting Discussion: Variables Influencing Maternal Care, which are published as supporting information on the PNAS web site). This pattern of results is opposite that predicted by the hypothesis that neonatal novelty effects are mediated by greater maternal care toward Novel pups. Therefore, the data offered no evidence in support of maternal mediation of novelty-exposure effects. To the contrary, the fact that Novel rats showed improved functionality during adulthood despite receiving somewhat less maternal care during infancy makes it highly unlikely that greater maternal care is the sole mediator of the novelty-induced functional improvements in adult offspring.
Discussion
A large body of literature has explored the contribution of maternal care in mediating the early stimulation effect on offspring’s HPA axis development. Here we introduce neonatal novelty exposure, a recently developed neonatal stimulation paradigm, to dissociate two major sources of contribution to the psychological development of newborn rat pups: one from an indirect effect of maternal individual differences and the other from a direct effect of pup stimulation. In combination with this stimulation paradigm, we evaluated the impact of such stimulation on a diverse range of psychological and physiological functions later in life, including spatial working memory, social competitive ability, functional brain asymmetry, and selective hormonal responses to unexpected events. The most critical findings are that, in rodents, (i) functional enhancement during adulthood across diverse functional domains at both behavioral and neuroendocrine levels can be induced by repeated neonatal exposures to a relatively novel nonhome environment; and (ii) these functional improvements occurred in the absence of evidence of preferential maternal care and, more importantly, in the presence of direct evidence for lesser maternal care relative to home-staying siblings.
It is important to note that there is no question that in neonatal handling studies, mothers of handled pups provide greater amounts of care toward handled pups compared with nonhandled pups (8) and that pups receiving handling or greater maternal care showed changes in emotionality (24) and a cascade of changes within the HPA axis and related brain structures (38). Yet the interpretation of these findings needs to be modified because maternal care may have contributed to changes in offspring within only the specific context of neonatal handling (8), and such a finding does not necessarily generalize to all possible forms of neonatal stimulation, clearly not to neonatal novelty exposure. It remains to be examined by using the neonatal novelty exposure procedure whether the early stimulation-induced changes in neuroendocrine and associated cellular/molecular measures associated with handled rats (38) can be preserved despite a lack of greater maternal care. Furthermore, it would be important to investigate whether naturally occurring individual differences in maternal care are associated with offspring individual differences across diverse psychological functional domains.
Because neonatal handling increases blood CORT concentration in 2-day-old pups, detectable by pooling measures from several pups within a litter (39, 40), such a direct activation of the HPA axis may partially mediate the neonatal novelty exposure effect, leading to permanent modification of the adult HPA axis, which in turn can exert a long-term influence on social and cognitive functions. Although it would take extraordinary evidence to rule out the possibility that at least some aspects of the HPA axis are affected by neonatal stimulation, such evidence emerged in the name of a stress-nonresponsive period (SNRP) among rodent neonates (41). This construct led to a discounting, by some investigators, of the possibility that repeated mild activation of the HPA axis may partially contribute to neonatal stimulation effects. In the past 3 decades it has been quietly but firmly established that throughout the SNRP, aside from the adrenal gland, the rest of the HPA axis is capable of responding to certain mild stressors (for review, see ref. 41). With the logical obstacle of a general SNRP removed and given the findings of functional improvements in the absence of evidence for preferential maternal care, we favor the interpretation that early novelty exposure-induced functional improvements are most likely mediated, at least in part, by repeated HPA axis activation.
With this changing view on SNRP, a consistent picture can now be drawn across multiple mammalian species concerning the maternal-mediation hypothesis. Among rodents (present study; refs. 16 and 41), rabbits (42), and nonhuman primates (43), three mammals with varying levels of maternal care, mild stimulation consistently induced changes in the adult offspring without a correlated increase in maternal care, which means that greater maternal care is not necessary for the functional enhancement associated with early stimulation. Conversely, higher levels of maternal care were found in the absence of improved behavioral functionality (present work), more efficient stress responses (43–45), or better health (46). Because sufficiency can only be demonstrated when the increases in maternal care are always associated with offspring functional enhancement, these dissociations indicate that greater maternal care is insufficient for offspring functional enhancement. One exception to these dissociations is the case of spontaneously occurring individual differences in maternal care that were correlated with offspring measures (8). Because both background environmental stressors as part of the daily routine in the animal facility and social stressors afforded by the rats’ social environment may have provided necessary HPA activation for maternal behavior to exert a modulatory influence, such a correlation is at best consistent with a possible role of maternal care, but it cannot be taken as conclusive evidence for sufficiency. These considerations together lead to a logically inescapable conclusion that maternal care is neither sufficient nor necessary for mediating early stimulation effects on offspring development.
It is critical to point out that the present findings do not imply that maternal behavior is inconsequential, nor do they imply that any direct stimulation of the pups is sufficient for HPA axis development in offspring. To the contrary, this seemingly contradictory set of findings that make up the current literature may be reconciled by considering the contribution of both a stress activation of pups’ HPA axis and a maternal modulation of this stress response (39). Within this framework, it is assumed that neonatal stimulation by handling, novelty exposure, or naturally occurring background stressors repeatedly activates the HPA axis. The time course of these activations can be powerfully modulated by maternal behavior, with maternal presence or contact facilitating the recovery of the stress response (39, 47) or directly attenuating the stress response (48) and bad maternal behavior potentially retarding such recovery. Each and every stress response in principle can be made more or less manageable by setting the stimulation parameters or by way of maternal influence. When the mother’s behavior facilitates the recovery of an existing stress response, she can be viewed as an HPA attenuator; when her behavior retards such a recovery, she can be viewed as an HPA potentiator. We suggest that this account of neonatal stimulation be referred to as the maternal-modulation hypothesis, which is to be distinguished from the maternal-mediation hypothesis that excludes a direct stimulation effect or any other contributing factors.
This maternal-modulation hypothesis makes a testable prediction: neonatal novelty exposure-induced improvements in adult cognitive, social, and neuroendocrine functions should more likely be observed when the mother behaves as an HPA attenuator, and, conversely, functional impairments may result when the mother behaves as an HPA potentiator. This prediction appears to be confirmed by a recent study in which the magnitude and direction of neonatal novelty exposure were predicted by the variability of maternal-care behaviors immediately after the pups experienced the 3-min novelty exposures.‡‡ The more variable the maternal-care behavior is from day to day, i.e., the less reliably maternal care is provided, the less enhancement of cognitive and emotional functions will be induced in the offspring.
The outcome from a maternal modulation versus mediation debate can have significant implications for future research focus, and possibly also policy making, in the context of child development. If not careful, the conclusion of maternal mediation can be taken as evidence for an unsupported claim that other factors matter little or not at all for development. This conclusion can also lead to a false attribution of any other environmentally triggered effects to maternal mediation based simply on the mother’s omnipresence in an infant’s life. It is of great theoretical significance to determine whether maternal care is the dominant influence compared with other epigenetic factors, or alternatively, as one of many dynamically interacting factors that jointly shape infant development. Therefore, it is imperative that such a debate be based on findings that reveal other sources of environmental influence on development.
Methods
For additional details, see Supporting Methods, which is published as supporting information on the PNAS web site.
Study 1.
Animals.
Sixteen pregnant Long–Evans rats (Charles River, Wilmington, MA) arrived at the University of New Mexico Psychology Department vivarium before giving birth. After birth, litters were culled to eight or nine pups, keeping as many male pups as possible. A 12-h light/dark cycle was used with lights on at 0700.
Neonatal novelty exposure.
Details of the neonatal novelty exposure procedure are described elsewhere (11, 13). Briefly, half of each litter was assigned to the Novel group and the other half to the Home group. On P1–21, the dam was transferred to a holding cage, and Novel pups were placed individually in novel non-home cages lined with fresh bedding for 3 min (Fig. 1b). By using a split-litter design, the correlated stimulation of pups and dams inherent in the neonatal handling method was avoided.
Morris water task.
Spatial working memory was assessed in 33 Novel and 35 Home rats by using the moving platform version of the Morris water task. A new hidden-platform location was used on each of the 8 days of testing; four trials were given per day (11).
CORT response to expected and unexpected stressors.
CORT response to an unexpected versus expected stressor was assessed in 17 Novel and 14 Home rats. The concentration of circulating CORT was measured from blood samples obtained at three different time points: basal (CB), after an expected water maze stressor (CWM), and after an unexpected open-field exposure that was superimposed on the expected water maze stressor (COF+WM). The CORT response to the expected stressor (RE) was defined as CWM − CB. The CORT response to an unexpected event (RU) was defined as (COF+WM − CWM)/RE*100.
Turning asymmetry.
Spontaneous turning asymmetry was observed in 24 Novel and 24 Home rats following procedures described elsewhere (13). Frequencies of right turns (R), left turns (L), and rears were counted; L-score was defined as (R − L)/(R + L)*100. Positive and negative L-scores indicate rightward and leftward turning biases, respectively.
Social competition.
Social competition for chocolate rewards was assessed in 24 Novel and 24 Home rats. Food-restricted rats were individually trained to obtain chocolate drops located at the end of a narrow runway after removal of a divider from the cage (Fig. 1c); the number of rewards obtained and latency to obtain the rewards were recorded. Rats were then tested in pairs; the winner of a trial was defined as the rat that obtained the chocolate first.
Study 2.
Pup retrieval latency.
Using the same rats as in Study 1, we observed maternal pup retrieval for 10 min immediately after neonatal novelty exposure on P1–8 (Fig. 1d). Across days, pups’ locations within the container were counterbalanced between Novel and Home groups.
Relation of pup retrieval to active nursing.
For 15 separate litters, on P1–10, the nest was disturbed by removing both the dam and the entire litter of pups from the home cage for varying intervals of time (≤15 min). Pup retrieval was observed for 10 min after the return of dam and pups to the cage after the above-described procedure. Active nursing was observed daily from P1–10 during five 75-min observation periods around the clock. Active nursing was defined by the percentage of time spent on licking and grooming and arched-back nursing (36). An average active nursing score and average retrieval latency for each dam was computed across all observation days.
Supplementary Material
Acknowledgments
We thank Drs. Victor Denenberg, Jerome Kagan, and James Cutting for comments and A. Korzekwa and L. N. Rogers for assistance in recording maternal behavior.
Abbreviations
- CORT
corticosterone
- OTL
one-trial learning
- P
postnatal day
- RE
CORT response to expected stressor
- RU
CORT response to unexpected stressor
- L-score
lateralization score
- SNRP
stress-nonresponsive period.
Footnotes
The authors declare no conflict of interest.
Reeb, B. C., Sharifi, M., Romeo, R. D., McEwen, B. S., Tang, A. C. (2006) Soc. Neurosci. Abstr. 562.4.
References
- 1.Ladd CO, Huot RL, Thrivikraman KV, Nemeroff CB, Meaney MJ, Plotsky PM. Prog Brain Res. 2000;122:81–103. doi: 10.1016/s0079-6123(08)62132-9. [DOI] [PubMed] [Google Scholar]
- 2.Huot RL, Ladd CO, Plotsky PM. In: Encyclopedia of Stress. Fink G, editor. San Diego: Academic; 2000. pp. 699–707. [Google Scholar]
- 3.Lehmann J, Pryce CR, Bettschen D, Feldon J. Pharmacol Biochem Behav. 1999;64:705–715. doi: 10.1016/s0091-3057(99)00150-1. [DOI] [PubMed] [Google Scholar]
- 4.Denenberg VH. Psychol Rev. 1964;71:335–351. doi: 10.1037/h0042567. [DOI] [PubMed] [Google Scholar]
- 5.Levine S. Sci Am. 1960;202:81–86. [PubMed] [Google Scholar]
- 6.Meaney MJ, Diorio J, Francis D, Widdowson J, LaPlante P, Caldji C, Sharma S, Seckl JR, Plotsky PM. Dev Neurosci. 1996;18:49–72. doi: 10.1159/000111395. [DOI] [PubMed] [Google Scholar]
- 7.Levine S. In: Society, Stress, and Disease. Levi L, editor. London: Oxford Univ Press; 1975. pp. 43–50. [Google Scholar]
- 8.Liu D, Diorio J, Tannenbaum B, Caldji C, Francis D, Freedman A, Sharma S, Pearson D, Plotsky PM, Meaney MJ. Science. 1997;277:1659–1662. doi: 10.1126/science.277.5332.1659. [DOI] [PubMed] [Google Scholar]
- 9.Francis D, Diorio J, Liu D, Meaney MJ. Science. 1999;286:1155–1158. doi: 10.1126/science.286.5442.1155. [DOI] [PubMed] [Google Scholar]
- 10.Meaney MJ, Szyf M. Trends Neurosci. 2005;28:456–463. doi: 10.1016/j.tins.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 11.Tang AC. Learn Memory. 2001;8:257–264. doi: 10.1101/lm.43101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pryce CR, Feldon J. Neurosci Biobehav Rev. 2003;27:57–71. doi: 10.1016/s0149-7634(03)00009-5. [DOI] [PubMed] [Google Scholar]
- 13.Tang AC, Reeb BC, Romeo RD, McEwen BS. J Neurosci. 2003;23:8254–8260. doi: 10.1523/JNEUROSCI.23-23-08254.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meier GW, Schutzman LH. Dev Psychobiol. 1968;1:141–145. [Google Scholar]
- 15.Meaney MJ, Diorio J, Francis D, Weaver S, Yau J, Chapman K, Seckl JR. J Neurosci. 2000;20:3926–3935. doi: 10.1523/JNEUROSCI.20-10-03926.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Williams DI, Bailey GB, Lee MH. Behav Biol. 1975;13:505–509. doi: 10.1016/s0091-6773(75)91149-9. [DOI] [PubMed] [Google Scholar]
- 17.Meaney MJ, Aitken DH, van Berkel C, Bhatnagar S, Sapolsky RM. Science. 1988;239:766–768. doi: 10.1126/science.3340858. [DOI] [PubMed] [Google Scholar]
- 18.Haltmeyer GC, Denenberg VH, Zarrow MX. Physiol Behav. 1967;2:61–63. [Google Scholar]
- 19.Joels M, Pu Z, Wiegert O, Oitzl MS, Krugers HJ. Trends Cognit Sci. 2006;10:152–158. doi: 10.1016/j.tics.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 20.Sandi C, Loscertales M, Guaza C. Eur J Neurosci. 1997;9:637–642. doi: 10.1111/j.1460-9568.1997.tb01412.x. [DOI] [PubMed] [Google Scholar]
- 21.Oitzl MS, Reichardt HM, Joels M, de Kloet ER. Proc Natl Acad Sci USA. 2001;98:12790–12795. doi: 10.1073/pnas.231313998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tang AC, Nakazawa M, Reeb BC. Neuroreport. 2003;14:1553–1556. doi: 10.1097/00001756-200308260-00002. [DOI] [PubMed] [Google Scholar]
- 23.Zou B, Golarai G, Connor JA, Tang AC. Dev Brain Res. 2001;130:1–7. doi: 10.1016/s0165-3806(01)00173-0. [DOI] [PubMed] [Google Scholar]
- 24.Caldji C, Tannenbaum B, Sharma S, Francis D, Plotsky PM, Meaney MJ. Proc Natl Acad Sci USA. 1998;95:5335–5340. doi: 10.1073/pnas.95.9.5335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hess JL, Denenberg VH, Zarrow MX, Pfeifer WD. Physiol Behav. 1969;4:109–111. [Google Scholar]
- 26.Tang AC, Reeb BC. Dev Psychobiol. 2004;44:84–93. doi: 10.1002/dev.10158. [DOI] [PubMed] [Google Scholar]
- 27.Tang AC, Verstynen T. Behav Brain Res. 2002;131:1–7. doi: 10.1016/s0166-4328(01)00330-8. [DOI] [PubMed] [Google Scholar]
- 28.Davidson RJ. Psychophysiology. 2003;40:655–665. doi: 10.1111/1469-8986.00067. [DOI] [PubMed] [Google Scholar]
- 29.Mezei TC, Rosen J. Arch Gen Psychiatry. 1960;3:53–56. [Google Scholar]
- 30.Lore R, Flannelly K. Sci Am. 1977;236:106–111. doi: 10.1038/scientificamerican0577-106. [DOI] [PubMed] [Google Scholar]
- 31.Ruskin RS, Davis GG, DePeralta A. J Gen Psychol. 1975;92:53–58. doi: 10.1080/00221309.1975.9711327. [DOI] [PubMed] [Google Scholar]
- 32.Blanchard DC, Sakai RR, McEwen BS, Weiss SM, Blanchard RJ. Behav Brain Res. 1993;58:113–121. doi: 10.1016/0166-4328(93)90096-9. [DOI] [PubMed] [Google Scholar]
- 33.Weaver IC, Cervoni N, Champagne FA, D’Alessio AC, Sharma S, Seckl JR, Dymov S, Szyf M, Meaney MJ. Nat Neurosci. 2004;7:847–854. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- 34.Stern JM. Dev Psychobiol. 1997;31:19–37. doi: 10.1002/(sici)1098-2302(199707)31:1<19::aid-dev3>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 35.Young RD. Psychonom Sci. 1965;3:295–296. [Google Scholar]
- 36.Champagne FA, Francis DD, Mar A, Meaney MJ. Physiol Behav. 2003;79:359–371. doi: 10.1016/s0031-9384(03)00149-5. [DOI] [PubMed] [Google Scholar]
- 37.Thoman EB, Levine S. Physiol Behav. 1970;5:1417–1421. doi: 10.1016/0031-9384(70)90129-0. [DOI] [PubMed] [Google Scholar]
- 38.Meaney MJ. Annu Rev Neurosci. 2001;24:1161–1192. doi: 10.1146/annurev.neuro.24.1.1161. [DOI] [PubMed] [Google Scholar]
- 39.Smotherman WP. Dev Psychobiol. 1983;16:169–176. doi: 10.1002/dev.420160303. [DOI] [PubMed] [Google Scholar]
- 40.Denenberg VH, Brumaghim JT, Haltmeyer GC, Zarrow MX. Endocrinology. 1967;81:1047–1052. doi: 10.1210/endo-81-5-1047. [DOI] [PubMed] [Google Scholar]
- 41.Dallman MF. Endocrinology. 2000;141:1590–1592. doi: 10.1210/endo.141.5.7527. [DOI] [PubMed] [Google Scholar]
- 42.Denenberg VH. Dev Psychobiol. 1999;34:1–3. doi: 10.1002/(sici)1098-2302(199901)34:1<1::aid-dev2>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 43.Parker KJ, Buckmaster CL, Sundlass K, Schatzberg AF, Lyons DM. Proc Natl Acad Sci USA. 2006;103:3000–3005. doi: 10.1073/pnas.0506571103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Neumann ID, Wigger A, Kromer S, Frank E, Landgraf R, Bosch OJ. Neuroscience. 2005;132:867–877. doi: 10.1016/j.neuroscience.2005.01.034. [DOI] [PubMed] [Google Scholar]
- 45.Macri S, Mason GJ, Wurbel H. Eur J Neurosci. 2004;20:1017–1024. doi: 10.1111/j.1460-9568.2004.03541.x. [DOI] [PubMed] [Google Scholar]
- 46.Myers MM, Brunelli SA, Squire JM, Shindeldecker RD, Hofer MA. Dev Psychobiol. 1989;22:29–53. doi: 10.1002/dev.420220104. [DOI] [PubMed] [Google Scholar]
- 47.Stanton ME, Levine S. Dev Psychobiol. 1990;23:411–426. doi: 10.1002/dev.420230504. [DOI] [PubMed] [Google Scholar]
- 48.Moriceau S, Sullivan RM. Nat Neurosci. 2006;9:1004–1006. doi: 10.1038/nn1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.