Abstract
Objectives
Our objectives in this study were to expand a previously reported U.S. market basket survey using a larger sample size and to estimate levels of PBDE intake from food for the U.S. general population by sex and age.
Methods
We measured concentrations of 13 polybrominated diphenyl ether (PBDE) congeners in food in 62 food samples. In addition, we estimated levels of PBDE intake from food for the U.S. general population by age (birth through ≥60 years of age) and sex.
Results
In food samples, concentrations of total PBDEs varied from 7.9 pg/g (parts per trillion) in milk to 3,726 pg/g in canned sardines. Fish were highest in PBDEs (mean, 1,120 pg/g; median, 616 pg/g; range, 11.14–3,726 pg/g). This was followed by meat (mean, 383 pg/g; median, 190 pg/g; range, 39–1,426 pg/g) and dairy products (mean, 116 pg/g; median, 32.2 pg/g; range, 7.9–683 pg/g). However, using estimates for food consumption (excluding nursing infants), meat accounted for the highest U.S. dietary PBDE intake, followed by dairy and fish, with almost equal contributions. Adult females had lower dietary intake of PBDEs than did adult males, based on body weight. We estimated PBDE intake from food to be 307 ng/kg/day for nursing infants and from 2 ng/kg/day at 2–5 years of age for both males and females to 0.9 ng/kg/day in adult females.
Conclusion
Dietary exposure alone does not appear to account for the very high body burdens measured. The indoor environment (dust, air) may play an important role in PBDE body burdens in addition to food.
Keywords: age, dietary intake, market basket survey, PBDEs, polybrominated diphenyl ethers, sex
Polybrominated diphenyl ethers (PBDEs), persistent and bioaccumulative flame retardants, are of concern because they are ubiquitous in the United States, are potentially toxic, and have been found at rapidly rising levels in humans during the past few decades (Birnbaum and Staskal 2004; Hites 2004; Schecter et al. 2005b; Sjödin et al. 2004; Webster et al. 2005). The high level of PBDE contamination in the U.S. population and food is cause for concern because these compounds are chemically similar to polychlorinated biphenyls (PCBs) and have been shown in laboratory animal studies to be toxic in a number of ways. These include cancer in high-dose studies [National Toxicology Program (NTP) 1986], reproductive and developmental toxicity (Stoker et al. 2004), endocrine disruption (Hallgren and Darnerud 2002), and central nervous system effects (Eriksson et al. 2002; Viberg et al. 2003). PBDEs can be found in some textiles, electronics, (e.g., computers, televisions), plastics, and furniture such as sofas, chairs, and mattresses. Unlike dioxins and PCBs, these chemicals are primarily indoor pollutants and are found at high levels in household vacuum dust and other home and workplace environmental samples (Schecter et al. 2005a; Stapleton et al. 2005).
Very high levels of PBDEs have recently been found in the United States in mothers’ milk (Schecter et al. 2003, 2005b), blood (Mazdai et al. 2003; Morland et al. 2005; Schecter et al. 2004, 2005b; Sjödin et al. 2004), food (Schecter et al. 2004), and adipose tissue (Johnson-Restrepo et al. 2005; She et al. 2002). U.S. blood and milk concentrations were 10- to 20-fold higher than the levels found in Europe (Bocio et al. 2003; Meironyte et al. 1999; Norén and Meironyté 2000; Ohta et al. 2002). Although levels of dioxins, dibenzofurans, and PCBs in human tissues are declining, PBDEs have been increasing substantially in blood levels in the United States during the past two to three decades (Schecter et al. 2005b; Sjödin et al. 2004).
The penta-BDE and octa-BDE commercial PBDE mixtures are no longer being produced or sold in the United States, whereas deca-BDE continues to be manufactured and sold in the United States as well as worldwide. Furthermore, because these compounds are persistent in the environment, reservoir sources are likely to be present for substantial periods of time. These reservoir sources may continue to contaminate food and dust, both of which are believed to contribute substantially to human intake of these compounds (Birnbaum and Staskal 2004; Jones-Otazo et al. 2005; Webster et al. 2005).
Even though studies have begun to estimate PBDE intake from ingestion and inhalation, the amount and percent of intake from food in the U.S. general population have not been well characterized nor have the amounts of intake from dust ingestion and inhalation been well defined (Jones-Otazo et al. 2005; Stapleton et al. 2005; Webster et al. 2005). The present study expands and complements our previous U.S. market basket survey (Schecter et al. 2004) and also estimates dietary PBDE exposure by age and sex from birth through ≥60 years of age. The food sample size is approximately twice the size of the earlier study and includes previously unpublished congener data from that study (Schecter et al. 2004). For the first time, we characterize the U.S. population’s PBDE intake from food.
Methods
Food samples were purchased during 2003 and 2004 in Dallas, Texas, from three large supermarkets representing national chains. We chose commonly eaten food types and purchased national or store brands whenever possible. Items were frozen and shipped on dry ice to Eurofins-ERGO Laboratory for analysis. The laboratory measured 13 PBDE congeners (BDE-17, BDE-28, BDE-47, BDE-66, BDE-77, BDE-85, BDE-99, BDE-100, BDE-138, BDE-153, BDE-154, BDE-183, and BDE-209) in 62 food samples by gas chromatography-isotope dilution high resolution mass spectrometry. For quality control purposes, one laboratory blank and a quality control pool for each block of samples were run. Quantification was performed only if the sample level was at least twice the blank level. For the analysis of samples of major food types such as fish, meat, cooked egg, cheese, ice cream, and sausage, a total of 5–200 g of the sample was homogenized and mixed with sodium sulfate. Before column extraction, a mixture of internal 13C-labeled standards was added to each sample. A mixture of cyclohexane and dichloromethane was applied during column extraction of lipids. After solvent evaporation, gravimetric lipid determination was performed. Fish oil and human milk were used as quality control pools. Details of the analytical procedure have been described elsewhere (Päpke et al. 2004; Schecter et al. 2004). In the present study, we used half the limit of detection (LOD) to estimate levels of congeners below the LOD, whereas we previously calculated them as equal to zero (Schecter et al. 2004).
We used the mean PBDE concentrations in our food samples, in combination with other food consumption estimates, to estimate PBDE intake for several age and sex groups in the U.S. population. We assumed nursing to be the only source of food for nursing infants, and calculation of their intake was based on the assumption that the daily consumption of human milk is 800 g (Dewey et al. 1991; Kent et al. 1999; Neville et al. 1988); milk PBDE levels were primarily levels previously reported (Schecter AJ, unpublished data; Schecter et al. 2003, 2005b). To calculate PBDE intake relative to body weight, we estimated an average weight of 7 kg for nursing infants [Centers for Disease Control and Prevention (CDC) 2000]. For other population groups, we obtained the median amount consumed each day for each of several categories of food from the U.S. Department of Agriculture (USDA) dietary intake survey (USDA 1999a, 1999b) and from Smiciklas-Wright et al. (2003). These intake estimates were multiplied by the mean PBDE concentration of our sample foods in each category to obtain a PBDE intake estimate for a “typical” man or woman (one whose food intake is at the 50th percentile) for each age group.
Results
Tables 1–4 show the PBDE levels in U.S. food products reported on a whole weight basis. Of the 18 meat samples analyzed (Table 1), total PBDE levels varied from 39 ppt wet weight in a bacon sample to 1,426 ppt in one pork sausage sample. We observed considerable variation in levels, even between samples of the same type of meat, such as bacon, ground pork, and pork sausage. BDE-209 (deca-BDE), the major remaining congener in commercial production, was detected in 8 of the 18 meat samples and, when detected, ranged from 9.7 ppt in one ground beef sample to 245 ppt in ground turkey. Compared with PBDE levels in meat products in Spain (10–172 ppt) and Japan (6.25–63.6 ppt), PBDE levels were higher in the United States (Bocio et al. 2003; Ohta et al. 2002). Of the 24 fish samples analyzed (Table 2), total PBDE concentration ranged from 11 to 3,726 ppt. These values, although somewhat higher, are comparable with PBDE concentrations in fish of 88–1,019 ppt in Spain and 17.7–1,720 ppt in Japan (Bocio et al. 2003; Ohta et al. 2002). As with meat, we found considerable variation between samples of the same type of fish. Farm grown salmon and other fish tend to have higher PBDE concentrations than wild fish. Unfortunately, it is not always clear from the store label whether salmon or other fish were farm grown, because mislabeling appears to be common (Burros 2005). We found BDE-209 in 10 of the 24 fish samples, ranging from 4.9 ppt in a canned tuna sample to 1,269 ppt in a catfish sample. Of 15 dairy products analyzed (Table 3), the total PBDE concentration varied from 7.9 ppt in both whole and nonfat cows’ milk, to 683 ppt in cream cheese. We measured BDE-209 in 7 of the 15 dairy samples, with concentrations ranging from 9.1 ppt in lowfat yogurt to 481 ppt in cream cheese. Again, this was considerably higher than concentrations in these foods in Spain (10–48 ppt) (Bocio et al. 2003). Of the five types of miscellaneous food products analyzed (Table 4), eggs were lowest at 85 ppt and calf liver highest at 2,835 ppt total PBDEs. BDE-209 was found in all of the miscellaneous samples except margarine and varied from 10.3 ppt in eggs to 288 ppt in calf liver.
Table 1.
PBDE levels (pg/g wet weight) in 18 U.S. meat samples.
| Sample | Lipid (%) | BDE-17 | BDE-28 | BDE-47 | BDE-66 | BDE-77 | BDE-85 | BDE-99 | BDE-100 | BDE-138 | BDE-153 | BDE-154 | BDE-183 | BDE-209 | Total PBDEsa |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bacon A | 52.3 | ND (5.2) | ND (7.1) | ND (78.8) | ND (5.2) | ND (5.2) | ND (5.2) | ND (28.8) | ND (6.8) | ND (5.2) | ND (5.2) | ND (5.2) | ND (5.2) | ND (166.6) | 165 |
| Bacon B | 43.4 | ND (0.4) | ND (2.1) | ND (19.9) | ND (0.4) | ND (0.2) | NA | ND (15.6) | ND (2.8) | ND (0.4) | ND (1.1) | ND (0.9) | ND (1.7) | ND (32.8) | 39b |
| Bacon C | 35.3 | 0.7 | ND (2.0) | 30.1 | NA | NA | 1.4 | 16.8 | 4.8 | ND (0.7) | 4.5 | 2.8 | 14.3 | 28.4 | 105b |
| Beef (ground) A | 30.7 | ND (3.1) | 59.7 | 87.5 | ND (3.1) | ND (3.1) | ND (3.1) | 35.5 | 6.2 | ND (3.1) | 6.8 | 4.6 | ND (4.2) | ND (95.7) | 258 |
| Beef (ground) B | 13.6 | 0.2 | ND (0.7) | 23.4 | 0.5 | NA | NA | 32.3 | 4.5 | 0.4 | 4.7 | 2.5 | NA | 9.7 | 79b |
| Beef tenderloin | 13.7 | ND (1.4) | ND (1.5) | 35.1 | ND (1.4) | NA | 1.7 | 40.3 | 6.9 | ND (1.4) | 4.9 | 3.7 | 3.8 | ND (11.1) | 105 |
| Chicken breast | 4.9 | ND (0.04) | 0.5 | 60.5 | NA | NA | NA | 128 | 17.1 | 2.2 | 12.0 | 10.8 | 3.2 | 48.5 | 283b |
| Duck | 75.1 | ND (0.5) | ND (3.0) | 286 | 2.7 | ND (0.3) | 15.2 | 609 | 122 | 7.3 | 52.3 | 42.9 | 31.6 | 113 | 1,283b |
| Ground chicken | 7.3 | ND (0.7) | ND (1.5) | 11.0 | ND (0.7) | NA | ND (0.7) | 18.9 | 4.6 | ND (0.7) | 4.1 | 2.6 | 5.8 | 80 | 129 |
| Ground lamb | 19.7 | ND (2.0) | ND (2.1) | ND (23.0) | ND (2.0) | ND (2.0) | 3.2 | 56.8 | 16.8 | ND (2.0) | 9.6 | 6.3 | ND (2.0) | ND (150.6) | 186 |
| Ground pork | 21.5 | ND (2.2) | ND (3.5) | 53.8 | ND (2.2) | NA | 3.1 | 74.2 | 12.9 | 4.3 | 18.7 | 15.0 | 19.9 | ND (31.3) | 221 |
| Ground turkey | 11.1 | 0.2 | ND (0.5) | 98 | 0.8 | ND (0.1) | NA | 217 | 54.4 | 3.9 | 32.9 | 24.1 | 36.8 | 245 | 713b |
| Pork | 8.9 | 0.1 | ND (0.5) | 6.9 | NA | NA | NA | 16.3 | 1.8 | 0.2 | 1.0 | 1.2 | 1.3 | 11.7 | 41b |
| Pork sausage A | 23.7 | ND (1.3) | ND (6.9) | 387 | ND (1.0) | ND (0.3) | 16.8 | 688 | 74.5 | 5.6 | 81.6 | 55.3 | 14.6 | 49.7 | 1,378b |
| Pork sausage B | 24.4 | ND (2.4) | ND (3.4) | 39.4 | ND (2.4) | ND (2.4) | 2.6 | 71.6 | 8.3 | ND (2.4) | 22.0 | 13.7 | 10.7 | ND (139) | 244 |
| Sausage A | 26.2 | ND (2.6) | ND (5.5) | ND (34.8) | ND (2.6) | NA | 3.1 | 40.1 | 6.4 | ND (2.6) | 5.9 | 4.9 | 6.9 | ND (51.0) | 1,426 |
| Sausage B | 28.5 | ND (2.9) | ND (3.2) | 94.1 | ND (3.5) | ND (2.9) | ND (2.9) | 43.7 | 8.3 | ND (2.9) | 8.5 | 9.2 | ND (2.9) | ND (41.7) | 195 |
| Wieners | 32.9 | ND (0.3) | ND (1.5) | 386 | 1.4 | ND (0.2) | 11.1 | 703 | 53.9 | 7.2 | 106 | 49.8 | 14.3 | ND (28.7) | 1,348b |
| Mean | 26.3 | 0.76 | 4.59 | 93.2 | 1.19 | 0.83 | 4.93 | 157 | 22.7 | 2.33 | 21.1 | 14 | 10.1 | 53.3 | 383 |
| Median | 24.1 | 0.66 | 1.03 | 39.4 | 1.08 | 0.57 | 2.62 | 42 | 7.57 | 1.37 | 7.68 | 5.63 | 5.83 | 38.1 | 190 |
| Minimum | 4.87 | 0.02 | 0.24 | 6.93 | 0.21 | 0.06 | 0.36 | 7.79 | 1.39 | 0.16 | 0.53 | 0.44 | 0.86 | 5.53 | 39 |
| Maximum | 75.1 | 2.62 | 59.7 | 387 | 2.74 | 2.62 | 16.8 | 703 | 121 | 7.28 | 106 | 55.3 | 36.8 | 245 | 1,426 |
Abbreviations: NA, not available; ND, not detected. LODs are shown in parentheses. Total PBDE levels and statistics for each congener were calculated by assuming that nondetected concentrations were one-half the LOD; for calculations, these were treated as zero.
Totals rounded to the nearest whole number for hundreds and to the nearest decimal place for tens.
Data from Schecter et al. (2004).
Table 4.
PBDE levels (pg/g wet weight) of five U.S. miscellaneous food samples.
| Sample | Lipid (%) | BDE-17 | BDE-28 | BDE-47 | BDE-66 | BDE-77 | BDE-85 | BDE-99 | BDE-100 | BDE-138 | BDE-153 | BDE-154 | BDE-183 | BDE-209 | Total PBDEsa |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chicken eggs (6) | 11.5 | 0.14 | 0.20 | 22.5 | 0.24 | 0.03 | 1.52 | 36.6 | 5.93 | 0.58 | 3.63 | 2.56 | 0.68 | 10.32 | 85b |
| Butter | 78.3 | 0.4 | 1.3 | 165 | 1.2 | NA | NA | 172 | 40.4 | 4.5 | 16.8 | 12.6 | 5.3 | 66.2 | 485b |
| Calf liver | 6.4 | 0.1 | 0.4 | 9.0 | NA | ND (0.6) | 0.5 | 10.5 | 1.6 | ND (0.2) | 2.9 | 1.8 | 6.2 | 81.6 | 115b |
| Chicken liver | 6.4 | 0.3 | 1.1 | 687 | 5.3 | NA | 27.2 | 1,258 | 261 | 17.9 | 148 | 130 | 11.5 | 288 | 2,835b |
| Margarine | 83.3 | ND (0.7) | ND (2) | ND (12) | ND (2.3) | ND (1) | ND (1.1) | ND (7.2) | ND (2) | ND (1.4) | 0.9 | ND (0.9) | ND (2.6) | ND (142) | 88b |
Abbreviations: NA, not available; ND, not detected. LODs are shown in parentheses. Total PBDE levels and statistics for each congener were calculated by assuming that nondetected concentrations were one-half the LOD; for calculations, these were treated as zero.
Totals were rounded to the nearest whole number for hundreds and to the nearest decimal place for tens.
Data from Schecter et al. (2004).
Table 2.
PBDE levels (pg/g wet weight) of 24 U.S. fish samples.
| Sample | Lipid (%) | BDE-17 | BDE-28 | BDE-47 | BDE-66 | BDE-77 | BDE-85 | BDE-99 | BDE-100 | BDE-138 | BDE-153 | BDE-154 | BDE-183 | BDE-209 | Total PBDEsa |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Canned tuna A | 0.3 | 0.1 | 0.6 | 5.1 | 0.2 | NA | 0.2 | 3.2 | 0.6 | ND (0.0) | 0.3 | 0.2 | 1.1 | 4.9 | 16.6 |
| Canned tuna B | 0.5 | ND (0.1) | 0.2 | 2.1 | 0.2 | NA | ND (0.1) | 1.1 | 0.4 | ND (0.1) | 0.2 | 0.3 | 2.1 | 8.8 | 15.5 |
| Catfish A | 11.1 | 4.6 | 6.4 | 372 | 4.3 | NA | NA | 589 | 116 | 5.1 | 37.1 | 39.6 | 7.3 | 1269 | 2,450b |
| Catfish B | 5.3 | 4.6 | 5.1 | 438 | 13.5 | ND (0.1) | 41.6 | 834 | 102 | 7.9 | 49.9 | 45.8 | 4.9 | ND (15.9) | 1,547b |
| Catfish C | 5.2 | 2.2 | 3.7 | 137 | 0.7 | ND (0.5) | 11.7 | 184 | 39.5 | ND (2.7) | 15.8 | 15.2 | ND (1.6) | ND (49.4) | 437 |
| Catfish fillet (farm) | 5.7 | 1.1 | 3.7 | 197 | 6.3 | NA | 16.4 | 282 | 53.0 | ND (4.1) | 18.4 | 21.3 | 3.8 | 22.7 | 627 |
| Halibut | 0.2 | 0.6 | 4.1 | 76.6 | 2.8 | NA | ND (0.1) | 10.6 | 12.4 | ND (0.1) | 1.1 | 2.6 | 1.8 | 11.4 | 124 |
| Herring | 9.1 | 4.1 | 56.3 | 2,072 | 69.4 | 3.6 | ND (0.9) | 267 | 221 | ND (0.9) | 29.3 | 69.9 | 2.5 | ND (26.4) | 2,809 |
| Mahi mahi | 0.5 | 0.6 | ND (2.0) | 24.1 | 2.0 | NA | 0.6 | 13.0 | 5.1 | ND (0.8) | 1.4 | 4.9 | 4.3 | ND (16.6) | 66 |
| Salmon A | 8.0 | 79.2 | 92.6 | 1,222 | 30.6 | ND (0.2) | NA | 93.2 | 348 | ND (0.2) | 27.7 | 98.8 | 1.4 | ND (9.0) | 1,999b |
| Salmon B | 13.9 | 118 | 142 | 2,081 | 59.1 | ND (0.1) | NA | 147 | 353 | ND (0.2) | 36.6 | 142 | ND (1.2) | ND (7.0) | 3,082b |
| Salmon C | 10.3 | 18.4 | 49.4 | 1,103 | 35.3 | ND (0.1) | ND (0.1) | 239 | 217 | ND (0.1) | 18.3 | 45.1 | ND (1.3) | ND (11.2) | 1,732b |
| Salmon D | 6.3 | 1.4 | 5.2 | 94.7 | 5.2 | ND (0.9) | ND (0.6) | 15.4 | 7.1 | ND (0.6) | 1.4 | 5.0 | ND (0.8) | ND (9.1) | 141 |
| Salmon E | 12.3 | 1.7 | 20.4 | 356 | ND (2.1) | ND (1.2) | ND (1.2) | 84.4 | 84.2 | ND (1.2) | 10.1 | 29.8 | ND (1.4) | ND (29.2) | 605 |
| Salmon fillet (farm) A | 7.4 | 11.1 | 50.5 | 1,000 | 63.1 | NA | 7.9 | 410 | 210 | ND (1.4) | 37.4 | 104 | 3.7 | 20.5 | 1,919 |
| Salmon fillet (farm) B | 6.9 | 2.3 | 27.9 | 517 | 24.3 | NA | ND (0.7) | 168 | 115 | ND (0.7) | 16.0 | 35.8 | 1.7 | 681 | 1,590 |
| Sardines | 9.6 | 3.3 | 53.6 | 2,748 | 85.6 | ND (5.0) | ND (1.0) | 358 | 257 | ND (1.0) | 51.9 | 139 | ND (3.2) | ND (51.4) | 3,726 |
| Shark | 0.4 | 1.1 | 29.8 | 784 | 29.5 | 0.3 | NA | 57.8 | 608 | 0.4 | 112 | 291 | 2.0 | 5.4 | 1,920b |
| Shrimp | 0.6 | 0.3 | 3.6 | 75.6 | NA | NA | NA | 9.4 | 14.3 | ND (0.1) | 1.2 | 2.6 | 0.2 | ND (1.3) | 108b |
| Tilapia | 1.0 | ND (0.1) | ND (0.7) | 5.9 | NA | NA | 0.1 | 1.3 | 0.6 | ND (0.1) | 0.2 | 0.5 | ND (0.2) | ND (4.0) | 11b |
| Trout A | 4.2 | 4.8 | 22.2 | 320 | NA | NA | ND (0.2) | 79.8 | 66.5 | 0.2 | 11.8 | 26.3 | 4.4 | ND (26.7) | 549b |
| Trout B | 10.1 | 4.3 | 49.3 | 826 | ND (5.6) | ND (1.0) | ND (1.0) | 128 | 198 | ND (1.0) | 24.7 | 61.3 | 2.5 | ND (42.9) | 1,319 |
| Tuna | 0.2 | ND (0.1) | ND (1.0) | 16.6 | 0.7 | NA | ND (0.0) | ND (4.6) | 2.9 | ND (0.1) | ND (0.4) | ND (1.0) | 0.5 | 23.4 | 48 |
| Wild perch | 1.2 | ND (0.1) | 0.7 | 10.2 | 0.4 | NA | ND (0.1) | 2.3 | 2.1 | ND (0.1) | 0.7 | 2.4 | 0.6 | 5.9 | 25 |
| Mean | 5.43 | 11.01 | 26.19 | 603 | 20.8 | 0.78 | 4.29 | 166 | 126 | 0.89 | 21 | 49.3 | 2.08 | 91.8 | 1,120 |
| Median | 5.52 | 1.97 | 5.77 | 338 | 5.23 | 0.30 | 0.35 | 88.8 | 75.3 | 0.33 | 15.9 | 28. | 1.68 | 10.1 | 616 |
| Minimum | 0.15 | 0.03 | 0.20 | 2.11 | 0.18 | 0.06 | 0.02 | 1.15 | 0.43 | 0.02 | 0.21 | 0.21 | 0.12 | 0.63 | 11.14 |
| Maximum | 13.9 | 118 | 142 | 2,748 | 85.6 | 3.60 | 41.6 | 834 | 608 | 7.94 | 112 | 291 | 7.32 | 1,269 | 3,726 |
Abbreviations: NA, not available; ND, not detected. LODs are shown in parentheses. Total PBDE levels and statistics for each congener were calculated by assuming that nondetected concentrations were one-half the LOD; for calculations, these were treated as zero.
Totals were rounded to the nearest whole number for hundreds and to the nearest decimal place for tens.
Data from Schecter et al. (2004).
Table 3.
PBDE levels (pg/g wet weight) of 15 U.S. dairy product samples.
| Sample | Lipid (%) | BDE-17 | BDE-28 | BDE-47 | BDE-66 | BDE-77 | BDE-85 | BDE-99 | BDE-100 | BDE-138 | BDE-153 | BDE-154 | BDE-183 | BDE-209 | Total PBDEsa |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| American cheese A | 19.0 | ND (1.9) | ND (1.9) | 45.5 | ND (1.9) | NA | ND (1.9) | 34.9 | 5.91 | ND (1.9) | 3.67 | 2.67 | 2.09 | 14.6 | 114 |
| American cheese B | 11.6 | ND (1.2) | ND (1.9) | 28.2 | 0.80 | NA | ND (1.2) | 23.1 | 4.14 | ND (1.2) | 2.37 | 1.67 | ND (1.2) | 17.5 | 81.1 |
| Gouda cheese | 26.2 | ND (2.6) | ND (3.0) | 75.6 | ND (2.6) | NA | 5.52 | 57.4 | 12.2 | ND (2.6) | 8.27 | 4.76 | 1.6 | ND (22.7) | 182 |
| Cottage cheese A | 4.7 | ND (0.5) | ND (1.5) | 13.6 | ND (0.5) | NA | ND (0.5) | 14 | 2.63 | ND (0.5) | 1.39 | 1.01 | ND (0.9) | ND (13.5) | 41.5 |
| Cottage cheese B | 1.7 | ND (0.2) | ND (1.3) | ND (6.9) | ND (0.2) | ND (0.2) | ND (0.2) | 2.24 | ND (0.4) | ND (0.2) | 0.39 | 0.27 | ND (0.3) | ND (4.2) | 9.8 |
| Cream cheese | 39.2 | 0.4 | ND (1.8) | 97.8 | 1.57 | ND (0.2) | NA | 77.1 | 12.2 | NA | 5.96 | 2.84 | ND (5.6) | 481.4 | 683b |
| Milk (cow’s) | 3.2 | ND (0.3) | ND (0.3) | ND (5.6) | ND (0.5) | ND (0.3) | ND (0.3) | 1.58 | 0.23 | ND (0.32) | ND (0.3) | 0.22 | ND (0.3) | ND (3.5) | 7.9 |
| Evaporated milk A | 6.6 | ND (0.1) | ND (0.9) | 15.8 | NA | NA | NA | 8.47 | 1.89 | ND (0.2) | 1.35 | 0.44 | 0.22 | ND (1.9) | 29.7b |
| Evaporated milk B | 6.3 | ND (0.1) | ND (0.9) | 11.9 | NA | NA | NA | 12.6 | 2.27 | 0.20 | 1.91 | 0.80 | ND (0.1) | ND (1.9) | 31.1b |
| Goat milk | 6.7 | 0.20 | 2.56 | 105 | 1.82 | ND (0.07) | NA | 97.9 | 27.3 | NA | 29 | 8.27 | 12.22 | 5.67 | 290b |
| Nonfat milk | 0 | ND (0.0) | ND (0.6) | ND (3.8) | ND (0.1) | NA | ND (0.1) | ND (2.5) | ND (0.8) | ND (0.1) | ND (0.1) | ND (0.2) | ND (0.1) | ND (7.5) | 7.9b |
| Infant formula A | 3.4 | ND (0.0) | ND (0.5) | ND (3.1) | ND (0.1) | NA | NA | 12.3 | 1.10 | 0.27 | 1.41 | 1.08 | 0.20 | 14 | 32.2b |
| Infant formula B | 3.2 | ND (0.1) | ND (1.2) | ND (7.7) | ND (0.1) | NA | NA | ND (5.1) | ND (1.5) | ND (0.3) | ND (0.5) | ND (0.3) | 0.40 | 16.5 | 25.4 |
| Lowfat yogurt | 1.3 | 0.2 | 0.9 | 9.05 | 0.24 | ND (0.02) | NA | 7.78 | 1.39 | 0.05 | 0.97 | 0.40 | 1.44 | 9.08 | 31.6b |
| Ice cream | 19.9 | ND (0.2) | ND (0.8) | 60.5 | ND (1.0) | ND (0.4) | NA | 63.7 | 9.45 | ND (0.4) | 6.91 | 3.41 | 5.47 | ND (41.2) | 171b |
| Mean | 10.2 | 0.29 | 0.79 | 31.8 | 0.61 | 0.10 | 1.08 | 27.8 | 5.48 | 0.33 | 4.27 | 1.87 | 1.86 | 40.5 | 116 |
| Median | 6.3 | 0.16 | 0.63 | 13.6 | 0.24 | 0.10 | 0.24 | 12.6 | 2.27 | 0.20 | 1.41 | 1.01 | 0.45 | 9.08 | 32.2 |
| Minimum | 0.0 | 0.02 | 0.16 | 1.54 | 0.03 | 0.01 | 0.05 | 1.26 | 0.22 | 0.04 | 0.06 | 0.08 | 0.05 | 0.94 | 7.91 |
| Max | 39.2 | 1.31 | 2.56 | 105 | 1.82 | 0.20 | 5.52 | 97.9 | 27.3 | 1.31 | 29.0 | 8.27 | 12.2 | 481 | 683 |
Abbreviations: NA, not available; ND, not detected. LODs are shown in parentheses. Total PBDE levels and statistics for each congener were calculated by assuming that nondetected concentrations were one-half the LOD; for calculations, these were treated as zero.
Totals were rounded to the nearest whole number for hundreds and to the nearest decimal place for tens.
Data from Schecter et al. (2004).
When PBDE concentrations are expressed on a lipid basis, fish still contain the highest levels, followed by meat and dairy products. Table 5 shows the PBDE levels for the various food types analyzed, on a lipid basis as well as on a wet weight basis. Although these lipid-normalized values reflect animal or fish body burdens of PBDEs, they are not useful in calculating dietary intake.
Table 5.
PBDE concentrations (pg/g wet weight) in the survey items.
| Lipid-based
|
Wet weight/whole weight
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| No. of samples | Minimum | Mean | Median | Maximum | Minimum | Mean | Median | Maximum | |
| Human milka | 62 | 6,000 | 66,000 | 32,000 | 419,000 | 31 | 1,916 | 968 | 21,359 |
| Meat | |||||||||
| Poultry | 4 | 1,708 | 3,919 | 3,771 | 6,423 | 129 | 602 | 498 | 1,283 |
| Beef | 3 | 581 | 729 | 766 | 840 | 79 | 147 | 105 | 258 |
| Pork | 2 | 461 | 744 | 744 | 1,028 | 41 | 131 | 131 | 221 |
| Bacon | 3 | 90 | 234 | 298 | 316 | 39 | 103 | 105 | 165 |
| Processed meat | 5 | 684 | 3,408 | 4,097 | 5,814 | 195 | 918 | 1,348 | 1,426 |
| Dairy | |||||||||
| Ice cream | 2 | 859 | 1,645 | 1,645 | 2,431 | 31.6 | 101.3 | 101.3 | 171 |
| Milk | 2 | NA | NA | NA | NA | 7.9 | 7.9 | 7.9 | 7.9 |
| Cheese | 6 | 577 | 866 | 697 | 883 | 9.8 | 185 | 97.6 | 683 |
| Eggs | 1 | NA | 739 | 739 | NA | NA | 85 | 85 | NA |
| Fat | |||||||||
| Margarine | 1 | NA | 106 | 106 | NA | NA | 88 | 88 | NA |
| Butter | 1 | NA | 619 | 619 | NA | NA | 485 | 485 | NA |
| Fish | 24 | 1,100 | 37,319 | 17,408 | 480,000 | 11 | 1,119 | 616 | 3,726 |
NA, not available.
Data from Schecter AJ (unpublished data) and Schecter et al. (2005b).
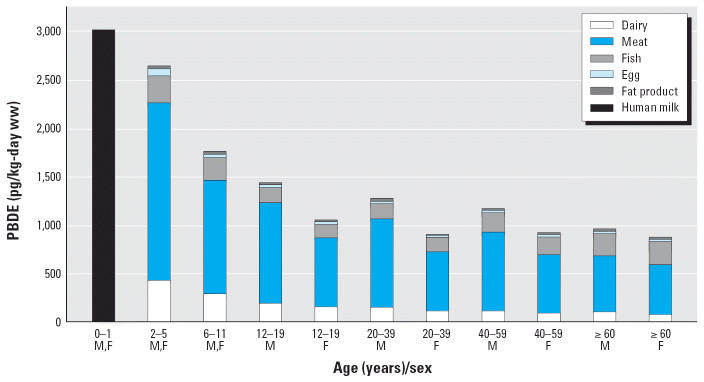
Table 6 and Figure 1 present estimates of dietary intake of PBDEs subdivided by food types for the U.S. population. In all groups > 1 year of age, total PBDE intake from meat is significantly higher than from any other food. As shown in Table 6, the highest dietary intake values of PBDEs are in nursing infants (307 ng/kg body weight per day, which compares to 1.0 or 0.9 ng/kg/day at ≥60 years of age for men and women, respectively; these are much higher than Swedish values of 0.63 and 0.58 ng/kg/day, respectively (Lind et al. 2002).
Table 6.
Daily PBDE dietary intake from food sources (pg/kg, or parts per quadrillion bw).
| Age (years)/sex/bw
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Food | 0–1 M and F (5 kg) | 2–5 M and F (16 kg) | 6–11 M and F (29 kg) | 12–19 M (55 kg) | 12–19 F (49 kg) | 20–39 M (70 kg) | 20–39 F (60 kg) | 40–59 M (70 kg) | 40–59 F (60 kg) | ≥60 M (70 kg) | ≥60 F (60 kg) |
| Dairy | |||||||||||
| Ice cream and ice milk | 0 | 63 | 59 | 33 | 35 | 25 | 17 | 27 | 20 | 30 | 22 |
| Milk | 0 | 191 | 113 | 61 | 42 | 29 | 27 | 29 | 25 | 30 | 28 |
| Total cheese | 0 | 173 | 121 | 118 | 83 | 90 | 62 | 56 | 46 | 37 | 28 |
| Total dairy | 427 | 293 | 212 | 160 | 144 | 106 | 112 | 91 | 97 | 78 | |
| Meat | |||||||||||
| Poultry | 0 | 790 | 477 | 449 | 344 | 413 | 331 | 396 | 331 | 275 | 291 |
| Beef | 0 | 211 | 177 | 187 | 126 | 168 | 93 | 134 | 88 | 95 | 74 |
| Pork | 0 | 52 | 36 | 37 | 23 | 34 | 22 | 34 | 22 | 32 | 22 |
| Bacon | 0 | 6 | 4 | 4 | 2 | 3 | 2 | 3 | 3 | 4 | 3 |
| Processed meat | 0 | 780 | 476 | 334 | 215 | 300 | 164 | 244 | 153 | 178 | 120 |
| Total meat | 1,839 | 1,170 | 1,011 | 710 | 918 | 612 | 811 | 597 | 584 | 510 | |
| Fish | |||||||||||
| Total fish | 0 | 280 | 232 | 163 | 137 | 160 | 149 | 208 | 187 | 224 | 243 |
| Eggs | |||||||||||
| Total eggs | 0 | 69 | 38 | 31 | 24 | 27 | 21 | 30 | 23 | 32 | 26 |
| Fat | |||||||||||
| Margarine | 0 | 6 | 6 | 3 | 4 | 3 | 3 | 4 | 4 | 5 | 4 |
| Butter | 0 | 30 | 17 | 9 | 10 | 14 | 8 | 7 | 8 | 14 | 8 |
| Total fat products | 36 | 23 | 12 | 14 | 17 | 11 | 11 | 12 | 19 | 12 | |
| Human milk | 306,560 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Sum PBDE intake per body weight (pg/kg-day ww) | 306,560 | 2,652 | 1,755 | 1,429 | 1,045 | 1,264 | 900 | 1,172 | 912 | 957 | 869 |
Abbreviations: bw, body weight; F, female; M, male; ww, wet weight.
Figure 1.
Daily PBDE dietary intake of U.S. population by age and food group (pg/kg body weight) as shown in Table 5. Abbreviations: F, female; M, male.
Discussion
This larger U.S. market basket survey confirms that PBDE contamination levels in U.S. food are currently higher than previously reported in other countries (Bocio et al. 2003; Huwe and Larsen 2005; Ohta et al. 2002). Fish are highest in PBDE contamination on a whole weight basis, followed by dairy products and meat. Meat is the major source of PBDEs in the U.S. diet after nursing ends, followed by dairy products and fish, unlike some other countries where fish intake predominates (Bocio et al. 2003; Darnerud et al. 2001; Ohta et al. 2002). Men, with larger daily intakes of food, have a larger dietary intake of PBDEs than do women.
A large variation of PBDE levels exist, even for the same type of food (Huwe and Larsen 2005). Although the present study is the largest PBDE food survey in the United States to date to the best of our knowledge, we cannot claim that these new data are a representative sampling of the U.S. diet. Like other published surveys from other countries, the sample size needs to be increased and the samples need to be representative of the diet(s) of the country. Until this is done, uncertainty in estimates of food levels will exist, and as a result, intake estimates will be somewhat imprecise.
The comparatively higher PBDE levels in food cannot however be the only explanation for the 10- to 20-fold higher levels in blood and milk from the U.S. general population compared with European and Canadian levels (Bocio et al. 2003; Mazdai et al. 2003; Meironyte et al. 1999; Morland et al. 2005; Norén and Meironyté 2000; Ohta et al. 2002). Our total daily PBDE intake from dietary sources for adults is only 0.9–1.2 ng/kg body weight, which compares to Spain’s 1.2–1.4 ng/kg/day (Bocio et al 2003) and the United Kingdom’s approximately 1.5 ng/kg/day, assuming an average adult weight of 70 kg (Harrad et al. 2004), but is higher than Sweden’s 0.58–0.63 ng/kg/day (Lind et al. 2002). Although there is a great deal of uncertainty on half-lives of PBDEs, assuming a maximum half-life of 2 years (Geyer et al. 2004; Thuresson et al. 2006) and an American body composition of approximately 25% adipose tissue, PBDE intake from food would lead to a steady-state body burden of < 10 ppb lipid. Given that the median lipid-adjusted levels in the United States from recent blood, milk, and adipose specimens exceed 30 ppb lipid, and that those of the top 5% of the population are 10–100 times greater, it appears unlikely that diet is the sole or even major source of exposure to PBDEs. This is in direct contrast to the situation with PCBs and dioxins in which > 95% of the exposure of the general population comes from food (U.S. Environmental Protection Agency 2004). This suggests that other routes of intake might be more significant for PBDEs than is the case for dioxins and PCBs.
The trends in dietary intake of PBDEs show a decreased intake per kilogram of body weight with age, with the highest dietary intake during nursing in the first year of life, 307 ng/kg body weight. This is due to the high level of PBDEs in human milk (median, 1,056 pg/g wet weight), assuming that human milk was the only food consumed. Children 2–5 years of age have higher PBDE dietary intake per kilogram of body weight than do older persons because of higher food intake per kilogram of body weight.
PBDE congeners 47, 99, 100, 153, and 154, and in some cases 209, are major contributors in both food concentration and dietary intake estimates. This reflects the previously reported findings on the congener distribution in human blood (Schecter et al. 2005b).
As is true for dioxins and PCBs, human breast milk is a major source of daily exposure to PBDEs for infants. Based on lactational exposure to dioxins, the body burden of the infant does not exceed 3–5 times that of the mother, in spite of the 50–100 times greater daily intake (Abraham et al. 1996; Lorber and Phillips 2002). A similar situation may exist for PBDE exposure of nursing infants, in which case human milk can be a significant route of exposure for babies. We join Wu et al. (2005) in suggesting that, as well as food, routes of exposure such as house dust ingestion and inhalation are likely important pathways of PBDE intake for children as well as adults.
Extrapolating from rodent studies, MacDonald (2005) hypothesized that health risks are possible for more highly exposed persons in the U.S. general population. Although the health effects of the levels we report are not clear, it seems reasonable from a public health standpoint to reduce the levels of these chemicals in the environment.
Footnotes
This study was partially funded by a grant from the CS Foundation, Warsh Mott Legacy.
The information in this document has been subjected to review by the National Health and Environmental Effects Research Laboratory, U.S. Environmental Protection Agency, and approved for publication. Approval does not signify that the contents reflect the views of the agency, nor does mention of trade names or commercial products constitute endorsement or recommendation for use.
References
- Abraham K, Knoll A, Ende M, Papke O, Helge H. Intake, fecal excretion, and body burden of polychlorinated dibenzo-p-dioxins and dibenzofurans in breast-fed and formula-fed infants. Pediatr Res. 1996;40:671–679. doi: 10.1203/00006450-199611000-00005. [DOI] [PubMed] [Google Scholar]
- Birnbaum LS, Staskal DF. Brominated flame retardants: cause for concern? Environ Health Perspect. 2004;112:9–17. doi: 10.1289/ehp.6559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bocio A, Llobet JM, Domingo JL, Corbella J, Teixido A, Casas C. Polybrominated diphenyl ethers (PBDEs) in foodstuffs: human exposure through the diet. J Agric Food Chem. 2003;51(10):3191–3195. doi: 10.1021/jf0340916. [DOI] [PubMed] [Google Scholar]
- Burros M. 2005. Stores says wild salmon, but tests say farm bred. New York Times , 10 April: A1.
- CDC (Centers for Disease Control and Prevention) 2000. Clinical Growth Charts. http://www.cdc.gov/nchs/about/major/nhanes/growthcharts/clinical_charts.htm [accessed 26 January 2006].
- Darnerud PO, Eriksen GS, Jóhannesson T, Larsen PB, Viluksela M. Polybrominated diphenyl ethers: occurrence, dietary exposure, and toxicology. Environ Health Perspect. 2001;109(suppl 1):49–68. doi: 10.1289/ehp.01109s149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewey KG, Heinig MJ, Nommsen LA, Lonnerdal B. Maternal versus infant factors related to breast milk intake and residual milk volume: the DARLING study. Pediatrics. 1991;87:829–837. [PubMed] [Google Scholar]
- Eriksson P, Viberg H, Jakobsson E, Orn U, Fredriksson A. A brominated flame retardant, 2,2’,4,4’,5-pentabromo-diphenyl ether: uptake, retention, and induction of neuro-behavioral alterations in mice during a critical phase of neonatal brain development. Toxicol Sci. 2002;67(1):98–103. doi: 10.1093/toxsci/67.1.98. [DOI] [PubMed] [Google Scholar]
- Geyer HJ, Schramm K-W, Darnerud PO, Aune M, Feicht EA, Fried KW, et al. Terminal elimination half-lives of the brominated flame retardants TBBPA, HBCD, and lower brominated PBDEs in humans. Organohalogen Compounds. 2004;66:3867–3872. [Google Scholar]
- Hallgren S, Darnerud PO. Polybrominated diphenyl ethers (PBDEs), polychlorinated biphenyls (PCBs) and chlorinated paraffins (CPs) in rats-testing interactions and mechanisms for thyroid hormone effects. Toxicology. 2002;177(2-3):227–243. doi: 10.1016/s0300-483x(02)00222-6. [DOI] [PubMed] [Google Scholar]
- Harrad S, Wijesekera R, Hunter S, Halliwell C, Baker R. Preliminary assessment of U.K. human dietary and inhalation exposure of polybrominated diphenyl ethers. Environ Sci Technol. 2004;38(8):2345–2350. doi: 10.1021/es0301121. [DOI] [PubMed] [Google Scholar]
- Hites RA. Polybrominated diphenyl ethers in the environment and in people: a meta-analysis of concentrations. Environ Sci Technol. 2004;38(4):945–956. doi: 10.1021/es035082g. [DOI] [PubMed] [Google Scholar]
- Huwe JK, Larsen GL. Polychlorinated dioxins, furans, and polybrominated diphenyl ethers in a U.S. meat market basket and estimates of dietary intake. Environ Sci Technol. 2005;39(15):5606–5611. doi: 10.1021/es050638g. [DOI] [PubMed] [Google Scholar]
- Johnson-Restrepo B, Kannan K, Rapaport DP, Rodan B. Polybrominated diphenyl ethers and polychlorinated biphenyls in human adipose tissue from New York. Environ Sci Technol. 2005;39(14):5177–5182. doi: 10.1021/es050399x. [DOI] [PubMed] [Google Scholar]
- Jones-Otazo HA, Clarke JP, Diamond ML, Archbold JA, Ferguson G, Harner T, et al. Is house dust the missing exposure pathway for PBDEs? An analysis of the urban fate and human exposure to PBDEs. Environ Sci Technol. 2005;39(14):5121–5130. doi: 10.1021/es048267b. [DOI] [PubMed] [Google Scholar]
- Kent JC, Mitoulas L, Cox DB, Owens RA, Hartmann PE. Breast volume and milk production during extended lactation in women. Exp Physiol. 1999;84(2):435–447. [PubMed] [Google Scholar]
- Lind Y, Aune M, Atuma S, Becker W, Bjerselius R, Glynn A, et al. Food intake of the brominated flame retardants PBDEs and HBCD in Sweden. Organohalogen Compounds. 2002;58:181–184. [Google Scholar]
- Lorber M, Phillips L. Infant exposure to dioxin-like compounds in breast milk. Environ Health Perspect. 2002;110:A325–332. doi: 10.1289/ehp.021100325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald TA. Polybrominated diphenylether levels among United States residents: daily intake and risk of harm to the developing brain and reproductive organs. Integr Environ Assess Manag. 2005;1(4):343–354. [PubMed] [Google Scholar]
- Mazdai A, Dodder NG, Abernathy MP, Hites RA, Bigsby RM. Polybrominated diphenyl ethers in maternal and fetal blood samples. Environ Health Perspect. 2003;111:1249–1252. doi: 10.1289/ehp.6146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meironyte D, Noren K, Bergman A. Analysis of polybrominated diphenyl ethers in Swedish human milk. A time-related trend study, 1972–1997. J Toxicol Environ Health A. 1999;58(6):329–341. doi: 10.1080/009841099157197. [DOI] [PubMed] [Google Scholar]
- Morland KB, Landrigan PJ, Sjödin A, Gobeille AK, Jones RS, McGahee EE, et al. Body burdens of polybrominated diphenyl ethers among urban anglers. Environ Health Perspect. 2005;113:1689–1692. doi: 10.1289/ehp.8138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neville MC, Keller R, Seacat J, Lutes V, Neifert M, Casey C, et al. Studies in human lactation: milk volumes in lactating women during the onset of lactation and full lactation. Am J Clin Nutr. 1988;48(6):1375–1386. doi: 10.1093/ajcn/48.6.1375. [DOI] [PubMed] [Google Scholar]
- Norén K, Meironyté D. Certain organochlorine and organobromine contaminants in Swedish human milk in perspective of the past 20–30 years. Chemosphere. 2000;40(9–11):1111–1123. doi: 10.1016/s0045-6535(99)00360-4. [DOI] [PubMed] [Google Scholar]
- NTP. 1986. Toxicology and Carcinogenesis Studies of Decabromodiphenyl Oxide (CAS No 1163-19-5) in F344/N Rats and B6C3F1 Mice (Feed Studies). Technical Report 309. Research Triangle Park, NC:National Toxicology Program. Available: http://ntp.niehs.nih.gov/ntp/htdocs/LT_rpts/tr309.pdf [accessed 5 June 2006]. [PubMed]
- Ohta S, Ishizuka D, Nishimura H, Nakao T, Aozasa O, Shimidzu Y, et al. Comparison of polybrominated diphenyl ethers in fish, vegetables, and meats and levels in human milk of nursing women in Japan. Chemosphere. 2002;46(5):689–696. doi: 10.1016/s0045-6535(01)00233-8. [DOI] [PubMed] [Google Scholar]
- Päpke O, Fürst P, Herrmann T. Determination of poly-brominated diphenylethers (PBDEs) in biological tissues with special emphasis on QC/CA measures. Talanta. 2004;63:1203–1211. doi: 10.1016/j.talanta.2004.05.033. [DOI] [PubMed] [Google Scholar]
- Schecter A, Päpke O, Joseph JE, Tung KC. Polybrominated diphenyl ethers (PBDEs) in U.S. computers and domestic carpet vacuuming: possible sources of human exposure. J Toxicol Environ Health A. 2005a;68(7):501–513. doi: 10.1080/15287390590909715. [DOI] [PubMed] [Google Scholar]
- Schecter A, Päpke O, Tung KC, Joseph J, Harris TR, Dahlgren J. Polybrominated diphenyl ether flame retardants in the U.S. population: current levels, temporal trends, and comparison with dioxins, dibenzofurans, and polychlorinated biphenyls. J Occup Environ Med. 2005b;47(3):199–211. doi: 10.1097/01.jom.0000158704.27536.d2. [DOI] [PubMed] [Google Scholar]
- Schecter A, Päpke O, Tung KC, Staskal D, Birnbaum L. Polybrominated diphenyl ethers contamination of United States food. Environ Sci Technol. 2004;38(20):5306–5311. doi: 10.1021/es0490830. [DOI] [PubMed] [Google Scholar]
- Schecter A, Pavuk M, Päpke O, Ryan JJ, Birnbaum L, Rosen R. Polybrominated diphenyl ethers (PBDEs) in U.S. mothers’ milk. Environ Health Perspect. 2003;111:1723–1729. doi: 10.1289/ehp.6466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- She J, Petreas M, Winkler J, Visita P, McKinney M, Kopec D. PBDEs in the San Francisco Bay area: measurements in harbor seal blubber and human breast adipose tissue. Chemosphere. 2002;46(5):697–707. doi: 10.1016/s0045-6535(01)00234-x. [DOI] [PubMed] [Google Scholar]
- Sjödin A, Jones RS, Focant JF, Lapeza C, Wang RY, McGahe E, III, et al. Retrospective time-trend study of polybrominated diphenyl ether and polybrominated and polychlorinated biphenyl levels in human serum from the United States. Environ Health Perspect. 2004;112:654–658. doi: 10.1289/ehp.112-1241957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smiciklas-Wright H, Mitchell DC, Mickle SJ, Goldman JD, Cook A. Foods commonly eaten in the United States, 1989–1991 and 1994–1996: are portion sizes changing? J Am Diet Assoc. 2003;103(1):41–47. doi: 10.1053/jada.2003.50000. [DOI] [PubMed] [Google Scholar]
- Stapleton HM, Dodder NG, Offenberg JH, Schantz MM, Wise SA. Polybrominated diphenyl ethers in house dust and clothes dryer lint. Environ Sci Technol. 2005;39(4):925–931. doi: 10.1021/es0486824. [DOI] [PubMed] [Google Scholar]
- Stoker TE, Laws SC, Crofton KM, Hedge JM, Ferrell JM, Cooper RL. Assessment of DE-71, a commercial poly-brominated diphenyl ether (PBDE) mixture, in the EDSP male and female pubertal protocols. Toxicol Sci. 2004;78(1):144–155. doi: 10.1093/toxsci/kfh029. [DOI] [PubMed] [Google Scholar]
- Thuresson K, Höglund P, Hagmar L, Sjödin A, Bergman Å, Jakobsson K. Apparent half-lives of hepta- to decabrominated diphenyl ethers in human serum as determined in occupationally exposed workers. Environ Health Perspect. 2006;114:176–181. doi: 10.1289/ehp.8350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- USDA 1999a. Food and Nutrient Intakes by Children 1994–96, 1998, Table Set 17. Beltsville, MD:U.S. Department of Agriculture. Available: http://www.ars.usda.gov/SP2UserFiles/Place/12355000/pdf/scs_all.PDF [accessed 13 January 2006].
- USDA 1999b. Data Tables: Results from USDA’s 1994–96 Continuing Survey of Food Intakes by Individuals and 1994–96 Diet and Health Knowledge Survey, Table Set 10. Beltsville, MD:U.S. Department of Agriculture. Available: http://www.ars.usda.gov/SP2UserFiles/Place/12355000/pdf/Csfii3yr.PDF [accessed 13 January 2006].
- U.S. Environmental Protection Agency 2004. Exposure and Human Health Reassessment of 2,3,7,8-Tetrachlorodibenzo-p-Dioxin (TCDD) and Related Compounds National Academy Sciences (NAS) Review Draft. http://www.epa.gov/ncea/pdfs/dioxin/nas-review/ [accessed 1 June 2006].
- Viberg H, Fredriksson A, Jakobsson E, Orn U, Eriksson P. Neurobehavioral derangements in adult mice receiving decabrominated diphenyl ether (PBDE 209) during a defined period of neonatal brain development. Toxicol Sci. 2003;76(1):112–120. doi: 10.1093/toxsci/kfg210. [DOI] [PubMed] [Google Scholar]
- Webster T, Vieira V, Schecter A. Estimating human exposure to PBDE-47 via air, food and dust using Monte Carlo Methods. Organohalogen Compounds. 2005;67:505–508. [Google Scholar]
- Wu N, Webster T, Hermann T, Paepke O, Tickner J, Hale R, et al. Associations of PBDE levels in breast milk with diet and indoor dust concentrations. Organohalogen Compounds. 2005;67:654–657. [Google Scholar]