Abstract
Plants with the C4 photosynthetic pathway dominate today's tropical savannahs and grasslands, and account for some 30% of global terrestrial carbon fixation. Their success stems from a physiological CO2-concentrating pump, which leads to high photosynthetic efficiency in warm climates and low atmospheric CO2 concentrations. Remarkably, their dominance of tropical environments was achieved in only the past 10 million years (Myr), less than 3% of the time that terrestrial plants have existed on Earth. We critically review the proposal that declining atmospheric CO2 triggered this tropical revolution via its effects on the photosynthetic efficiency of leaves. Our synthesis of the latest geological evidence from South Asia and North America suggests that this emphasis is misplaced. Instead, we find important roles for regional climate change and fire in South Asia, but no obvious environmental trigger for C4 success in North America. CO2-starvation is implicated in the origins of C4 plants 25–32 Myr ago, raising the possibility that the pathway evolved under more extreme atmospheric conditions experienced 10 times earlier. However, our geochemical analyses provide no evidence of the C4 mechanism at this time, although possible ancestral components of the C4 pathway are identified in ancient plant lineages. We suggest that future research must redress the substantial imbalance between experimental investigations and analyses of the geological record.
Keywords: atmospheric CO2 concentration, C4 plants, plant evolution, stable carbon isotopes
1. Introduction
Photosynthetic CO2-fixation has provided the carbon for life on Earth for at least the past 2.7 billion years (Gyr). More than 99% of the history of this ancient process has been dominated by C3 photosynthesis (figure 1), so-called because its first products are carboxylic acids formed of three linked carbon atoms. But between 25 and 32 million years (Myr) ago, a revolutionary innovation evolved in tropical grasses in the form of a solar-powered carbon dioxide pump based on four-carbon acids (‘C4 photosynthesis’), which boosts photosynthesis in hot conditions. It works by pumping CO2 from the mesophyll into a specialized ring of bundle sheath cells centred around leaf veins, where an extremely localized version of C3 photosynthesis operates, bathed in high CO2 concentrations (figure 1; Hatch 1971). Although, this specialized ‘Kranz’ anatomy is the norm in the vast majority of C4 plants, it is not essential, and certain desert species have evolved an alternative form of the pathway in which all elements are packed into single cells (Voznesenskaya et al. 2001). A variation on the single-celled carbon concentrating mechanism is called Crassulacean acid metabolism (CAM) and is more widely adopted in drought tolerant species. It operates by temporally separating the activities of a C4-like pump mechanism and C3 photosynthesis.
Figure 1.
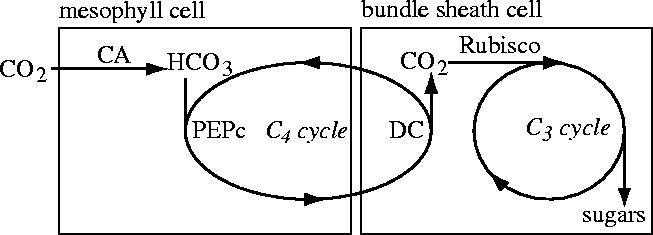
Simple schematic diagram of the C4 photosynthetic pathway showing compartmentalization of the different enzyme systems involved, and the connection between CO2-pumping by the C4 cycle and CO2-fixation by the C3 cycle. Abbreviations: CA, carbonic anhydrase; HCO3, bicarbonate; PEPc, phosphoenolpyruvate carboxylase; DC, decarboxylase enzyme(s); Rubisco, ribulose-1,5-bisphosphate carboxylase/oxygenase.
The C4 photosynthetic pathway is a major evolutionary success, accounting for some 20–30% of terrestrial CO2-fixation on Earth (Lloyd & Farquhar 1994) and 30% of global agricultural grain production (Steffen et al. 2004). Plants utilizing this pathway dominate tropical grasslands and savannahs, and rank among the world's most important crops, including sugarcane (Saccharum officinarum), maize (Zea mays) and sorghum (Sorghum bicolor). And the C4 revolution is not confined to grasses. In one of the most striking examples of convergent evolution in plants (Conway-Morris 2003), a C4 carbon-concentrating mechanism has originated in more than 40 independent evolutionary groups (Sage 2004). Paradoxically, its multiple origins belie a complex trait that requires the coordinated expression of at least 20–30 unlinked genes to operate efficiently (Wyrich et al. 1998; Furumoto et al. 2000).
The C4 and CAM mechanisms probably arose as a ‘fix’ for an intrinsic inefficiency in Rubisco (ribulose-1,5-bisphosphate carboxylase/oxygenase), the enzyme catalysing CO2-fixation in every plant. This ancient enzyme originated in ‘greenhouse’ conditions when the Earth's atmosphere contained CO2 at up to 100 times today's level (Rye et al. 1995) and negligible amounts of O2 (Bekker et al. 2004). A CO2-fixing enzyme in this atmosphere had little requirement for high CO2-affinity, and gained no advantage from distinguishing between CO2 and O2 molecules. However, the ensuing long-term decline in atmospheric CO2 subsequently exposed an important problem; Rubisco fixes O2 as well as CO2, at a rate depending on the CO2 : O2 ratio. In today's low CO2 : O2 atmosphere, O2-fixation in C3 plants wastes captured solar energy and causes a net loss of CO2 via the photorespiration pathway.
C4 plants overcome this problem using a coupled enzyme system with a much higher affinity than Rubisco for CO2 (figure 1), first dissolving the gas by using carbonic anhydrase (CA) to form bicarbonate , and then fixing it using phosphoenolpyruvate carboxylase (PEPc). The four-carbon products of this fixation diffuse into bundle sheath cells, where CO2 is released by decarboxylase enzymes (DC, figure 1) and reaches concentrations of 3–8 times those in C3 photosynthetic cells (reviewed by Kanai & Edwards 1999). Rubisco in C4 plants, therefore, experiences a saturating CO2 environment similar to that of C3 plants growing in ancient ‘greenhouse’ atmospheres, and photorespiration is minimized (Osmond 1971). Through this mechanism, C4 plants achieve a substantial photosynthetic advantage over their C3 contemporaries with falling atmospheric CO2 and at high temperatures (figure 2; Björkman 1971), as Rubisco becomes increasingly unable to distinguish O2 from CO2. However, the C4 mechanism carries a major cost; its dependence on light to energize the CO2-pump lowers photosynthetic efficiency relative to the C3 type, especially in conditions when photorespiration is naturally suppressed, such as high CO2 and cool temperatures (figure 2).
Figure 2.
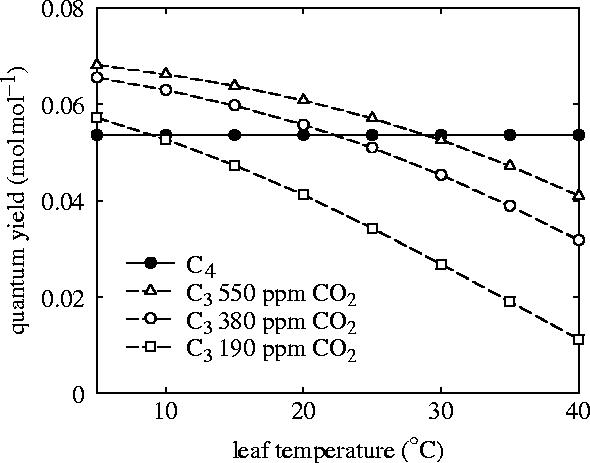
Modelled interaction between temperature and CO2 on the photosynthetic quantum yields (maximum light-use efficiencies) of C3 and C4 plants. Notice that the temperature at which C3 and C4 quantum yields cross over declines with falling atmospheric CO2 concentration.
The use of an energy-dependent system to alleviate photorespiration in C4 plants leads to a trade-off, with beneficial results for photosynthetic light-use efficiency at high temperatures and a decline in efficiency at low temperatures (figure 2). The point at which costs match benefits is termed the ‘crossover temperature’ and decreases with CO2, as photorespiration becomes increasingly problematic for C3 plants (figure 2). This simple physiological contrast between photosynthetic types was first quantified 30 years ago (Ehleringer & Björkman 1977), and has been invoked subsequently as the ‘quantum yield hypothesis’ to explain biogeographical patterns of C4 ecological (Ehleringer et al. 1997) and evolutionary success (Ehleringer et al. 1991; Cerling et al. 1997). Along geographic temperature gradients, the crossover point matches the mean growing season temperature where C4 grasslands are replaced by C3 types (Ehleringer et al. 1997), both from the equator towards cooler climates and on mountains, where the air cools with altitude (reviewed by Sage et al. 1999). In warmer climates, the C4 type has a photosynthetic advantage, while the C3 type benefits at cooler temperatures.
C4 plants have clearly evolved an effective solution for the inherent kinetic inefficiency of Rubisco. However, the mechanisms translating this physiological advantage into evolutionary and ecological success remain unclear, and are major unresolved questions in biology. Here, we review recent advances in our understanding of C4 plant evolution, integrating recent developments across the geological, ecological, physiological and molecular sciences. We begin with the origins of the C4 photosynthetic pathway, reconciling geological evidence with molecular data on the evolutionary history of plant lineages. Next, we investigate the remarkable picture built-up from isotopic analyses of fossil remains, demonstrating rapid global expansion of C4-dominated ecosystems, and consider its likely cause. Finally, we present a theoretical analysis of the possible selection pressures for a C4-type of carbon concentrating mechanism resulting from the unusual atmospheric composition of the Permo-Carboniferous 300 Myr ago (Ma). We investigate the possibility that the necessary environmental and physiological prerequisites were in place for the C4 mechanism to have evolved 300 Myr earlier than is currently accepted, through a detailed isotopic analysis of fossil plant remains and preliminary physiological measurements of evolutionarily ancient plant lineages.
2. Origins of C4 plants
The evolutionary origins of C4 plants may be elucidated using fossil evidence of Kranz anatomy and an important difference in the stable carbon isotope composition (δ13C) of C3 and C4 plants. Rubisco discriminates strongly against the heavy isotope of carbon (13C), relative to its more abundant form (12C). In contrast, the dissolution of CO2 to form bicarbonate and its subsequent fixation by the CA–PEPc enzyme system slightly favours the heavier isotope (Farquhar 1983). This physiological difference translates into a marked contrast in the δ13C of C3 and C4 plant tissues, which persists long after they have been eaten and incorporated into the bones or teeth of herbivores, or decomposed into biomarkers within geological sediments (Cerling 1999). Analysis of δ13C in geological materials, therefore, provides an important opportunity for reconstructing changes in C4 plant abundance on evolutionary time-scales. However, interpreting these data requires care because shifts in the δ13C of atmospheric CO2 cause parallel changes in plant tissues.
Kranz anatomy can be identified in well-preserved fossil leaf fragments dating to the Late Miocene, 5–12 Ma. A petrified grass from the Ricardo Formation (12.5 Ma) of California is currently the earliest undisputed C4 plant, and additionally characterized by a typical C4 δ13C signature (Nambudiri et al. 1978). A second early example of Kranz anatomy is reported for a silicified grass from the Ogallala Formation (5–7 Ma) in Kansas (Thomasson et al. 1986). Several older C4 grass species are claimed from the Fort Ternan locality in Kenya (14 Ma) on the basis of cuticle morphology (Dugas & Retallack 1993), but internal leaf anatomy is not preserved, and their photosynthetic type remains in question (Cerling 1999). These fossils highlight a major difficulty in identifying fossil plants on the basis of anatomical features; in most cases these are simply not preserved, an issue especially acute for C4 plants, which inhabit seasonally dry environments where fossilization is unlikely. Direct evidence of Kranz anatomy is therefore rare, and the origins of C4 photosynthesis must be inferred by integrating data from stable carbon isotope analyses and investigations of plant molecular genetics.
Despite intriguing isotopic evidence from the Cretaceous (90 Ma, Kuypers et al. 1999), general consensus currently places the earliest origins of C4 photosynthesis within the family Poaceae (grasses) (Kellogg 2000). Grasses first appear in the fossil record as pollen in the Palaeocene (55–60 Ma), with additional, more equivocal records of grass-like pollen in the latest Cretaceous (70 Ma; Jacobs et al. 1999). Estimates of when C4 photosynthesis arose in this group come from molecular genetic techniques, in which evolutionary histories are retraced by comparing differences in DNA sequences between species. Since most DNA mutations are selectively neutral, with no effect on Darwinian fitness, they accumulate over time by ‘genetic drift’ and therefore indicate evolutionary distance between species. By using the number of mutations between species and a mutation rate calibrated using fossils, the ‘molecular clock’ technique dates the appearance of C4 photosynthesis in the grass sub-family Panicoideae at 25–32 Ma (Gaut & Doebley 1997). However, phylogenetic data are unable to distinguish with confidence whether this event was followed by multiple reversions back to the C3 type, up to six further C4 origination events, or the persistence of genes allowing flipping between types (Kellogg 2000; Duvall et al. 2001, 2003; Guissani et al. 2001). Molecular reconstructions also confirm three additional, and independent, origination events occurring earlier than 25 Ma, within the cluster of related grass lineages Aristidoideae, Eriachneae and Chloridoideae, although these are not precisely dated at present (Kellogg 2000, 2001). Each origination co-opted a slightly different set of biochemical pathways to achieve a functional C4 cycle (Sinha & Kellogg 1996), and followed an adaptation to open habitats in grass groups whose ancestors were confined to forest shade habitats, like today's bamboos (Kellogg 2001). However, the location of this evolutionary innovation remains mysterious, because high diversity and ancient origins in the grasses make geographic centres of C4 evolution hard to pinpoint (Sage 2004).
C4 photosynthesis also proliferated within the Cyperaceae (sedges), and numerous families of Eudicots, including the Asteraceae (daisies), Brassicaceae (cabbages), Euphorbiaceae, but especially the Chenopodiaceae and related Amaranthaceae, where 13 independent origination events are postulated (Kadereit et al. 2003). Molecular genetic evidence points to 37 independent evolutionary origins for C4 photosynthesis outside the grasses (Sage 2004), starting as early as 14–21 Ma in the Chenopodiaceae (Kadereit et al. 2003). Biogeographical analysis of diversity in these groups suggest diverse centres of C4 origin located across the world, in southern Texas-central Mexico, central Asia, sub-tropical Africa and sub-tropical South America (Sage 2004).
Molecular genetics, therefore, predicts that the carbon isotope signature of C4 grasses should be picked up from the Oligocene (23–35 Ma) onwards, with C4 Eudicots contributing soon afterwards (from 14 to 21 Ma). The signal is faint, but present in carbonates from fossil soils (palaeosols) dating to 23 Ma in the southern Great Plains of North America (Fox & Koch 2003). After ruling out a number of potential biases, reconstructions for this region suggest that C4 plants made up 12–34% of the biomass from 23 to 7 Ma in the Late Miocene (Fox & Koch 2003), and grew in open woodland ecosystems (Strömberg 2004). Similar claims from East Africa of a persistent, but relatively low, abundance of C4 plants in a savannah or woodland ecosystem from 15 to 7 Ma are disputed, on the grounds of conflicting evidence from mammalian teeth and possible misidentification of palaeosols (Cerling 1999). What is clear, however, is that prior to 9 Ma there is no isotopic signature of a C4-dominated ecosystem anywhere in the world. Data from Africa, South America, the Indian sub-continent, and China all show ecosystems comprised entirely of C3 plants, although a low C4 presence is difficult to exclude against a varying C3 δ13C background (Cerling et al. 1997). For up to 25 Myr from their inferred origins, C4 plants were rare or absent from the tropics. The C4 revolution was a long-time coming.
3. CO2 and the rise of C4 plants to ecological dominance
C4 plants came to dominate terrestrial ecosystems abruptly in the Late Miocene (5–8 Ma). A revolutionary expansion of C4 plants is identified by major shifts in δ13C (figure 3) across the southern US Great Plains, Argentina, Bolivia, India, Pakistan, Nepal and Kenya (reviewed by Cerling et al. 1997). Isotopic changes are recorded in palaeosol carbonate, organic matter, and in the diets of large mammals and flightless birds via tooth, bone and egg shell δ13C (figure 3; reviewed by Cerling et al. 1997; Cerling 1999). The revolution continued into the Pliocene (2–5 Ma), with later C4 expansions in the northern US Great Plains (Cerling et al. 1997), China (Ding & Yang 2000), Chad (Zazzo et al. 2000), and across East Africa (Levin et al. 2004). It transformed ecosystems from the tropical to the warm temperate climate zones across four continents, with C4 biomass increasing from near zero to more than 80% of vegetation in just 2–4 Myr (figure 3).
Figure 3.
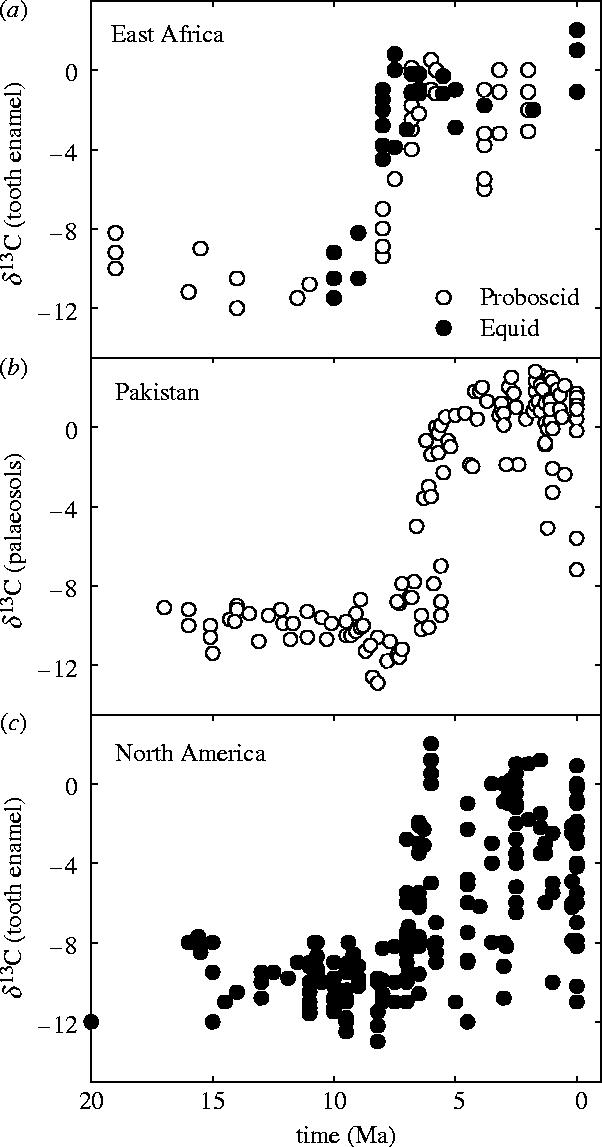
Increase in δ13C from palaeosols and tooth enamel showing apparent synchronicity in the transition to C4-dominated terrestrial ecosystems across continents. Data for (a) from Cerling et al. (1997), (b) from Quade & Cerling (1995) and (c) from Passey et al. (2002).
The near-synchronous expansion of C4 plants across diverse geographical regions in the Late Miocene points towards a global trigger for the phenomenon, and led Ehleringer, Cerling and co-workers (Ehleringer et al. 1991; Cerling et al. 1997) to propose the net decline in atmospheric CO2 over the last 150 Myr as the mechanism. According to this proposal, falling CO2 gradually lowered the crossover temperature of C3 plants (figure 2) until it fell below tropical temperatures, making C4 photosynthesis progressively more advantageous and allowing C4 plants to achieve ecological dominance in ever-cooler climates (Ehleringer et al. 1991; Cerling et al. 1997). Circumstantial evidence for this decline in crossover temperature with CO2 comes from a slight asynchrony in the timing of C4 expansion, with dominance achieved first in hot, equatorial Kenya, followed by the southern Great Plains and Pakistan (20–37°N), and finally the northern Great Plains (40–43°N; Cerling et al. 1997). Further indirect support is provided by the inverse relationship between tropical C4 plant abundance from δ13C records during recent ice age (glacial) cycles (Ehleringer et al. 1997), and fluctuations of atmospheric CO2 from 180 p.p.m during glacial intervals to 280 p.p.m during interglacial periods (Petit et al. 1999).
The CO2 starvation mechanism rapidly achieved widespread acceptance, but geological data are now beginning to challenge its proposed role in C4 success (Keeley & Rundel 2003). Palaeo-CO2 reconstructions from three independent proxies indicate low CO2 concentrations for at least 15 Myr before the Late Miocene expansion of C4 grasslands (figure 4; Pagani et al. 1999; Pearson & Palmer 2000; Royer et al. 2001). The inferred levels of CO2 vary between 180 and 320 p.p.m (figure 4), and correspond to crossover temperatures of less than 10–22 °C (figure 2), well within tropical temperature limits. According to the CO2 hypothesis, the C4 mechanism would, therefore, have presented a substantial photosynthetic advantage in the tropics as early as the Oligocene (23 Ma). Critically, we note that CO2 was not declining during the Late Miocene period of ecological change, and one proxy even indicates an increase at 8 Ma (figure 4; Pagani et al. 1999). Further evidence from the last glacial cycle also challenges the primary role of CO2 as a driver of ancient C4 successes, with uncoupling of C4 plant abundance from CO2 being attributed to lower summer rainfall (Huang et al. 2001; Scott 2002).
Figure 4.
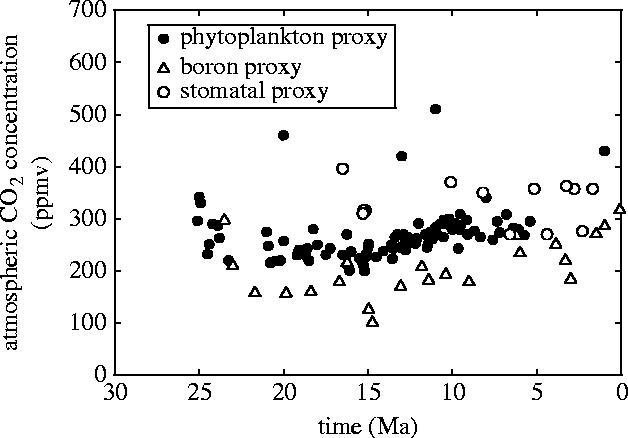
Atmospheric CO2 trends over the past 35 Myr, as reconstructed from three different proxies (data from Pagani et al. 1999; Pearson & Palmer 2000; Royer et al. 2001).
On the basis of our assessment, we reject the hypothesis of CO2-starvation as the proximate driver of Miocene C4 expansions. However, we still see C4 physiology as an adaptation to low CO2 atmospheres, because it only provides a photosynthetic advantage at tropical temperatures of less than 30 °C when CO2 concentrations are lower than 500 p.p.m (figure 2; Ehleringer et al. 1997). Consequently, although declining CO2 was not the direct trigger for Miocene expansions of C4 plants, a decrease in its concentration was a necessary pre-condition for this widespread C4 success (Sage 2001). Three independent palaeo-CO2 proxies all show a drop in CO2 from a high point of greater than 1000 p.p.m in the mid-Cretaceous to its Early Miocene low (Royer et al. 2004), and a new record based on phytoplankton δ13C now points to a sharp drop in CO2 from between 1000 and 1500 p.p.m to around 300 p.p.m during the Oligocene (23–35 Ma; Pagani et al. 2005), coincident with continental ice-sheet initiation on Antarctica (Zachos et al. 2001). This decrease corresponds to a fall in crossover temperature from greater than 40 to 17–21 °C (figure 2), and matches almost exactly with the dates of C4 grass origins suggested by molecular clocks.
Declining CO2 in the Oligocene may, therefore, have selected for the first C4 plants (Keeley & Rundel 2003), but verification of this proposal must await the publication of further molecular clock dates and more palaeoclimate evidence. When did C4 photosynthesis originate in the grass lineages Aristidoideae, Eriachneae and Chloridoideae? How closely do these dates coincide with the drop in palaeo-CO2 concentrations, and what other environmental factors changed during the Oligocene, when major upheaval occurred in the climate system? CO2 may yet have a part to play in C4 evolution, but current evidence suggests that it was not directly responsible for the rise of C4 plants to dominance in the Miocene and Pliocene (figure 4).
4. Alternative mechanisms for C4 success
Explaining the Late Miocene expansion of C4 grasslands requires mechanisms other than CO2. In seeking these, we begin by considering the environmental and biotic factors promoting dominance by C4 plants in modern ecosystems. All are regional rather than global in their sphere of influence, and stem from continental climate change or the interaction between disturbance and grassland ecosystem development. To investigate the relative merits of these explanations, we examine evidence in the geological record for the mechanistic drivers of C4 success in the Indian subcontinent and North America.
(a) Competition, climate and disturbance
Two primary factors control the distribution of C4-dominated ecosystems in the modern world; the limits of forest distribution set by rainfall and disturbance, and geographical gradients of temperature and rainfall. Since C4 plants are predominantly herbaceous, only rarely achieving the stature of shrubs or small trees (Sage 2001), they are rapidly overtaken in height by forest trees or savannah shrubs and suppressed by shading. Most C4 plants cannot tolerate shading of sunlight to below 25% of its open sky value, possibly because of anatomical (Ogle 2003), phylogenetic, environmental or physiological constraints (reviewed by Sage & Pearcy 2000). Closed forest, therefore, precludes ecosystem dominance by C4 plants, but is replaced by open woodland, savannah or grassland in seasonally arid climates because of water-shortage and disturbance by fire (Bond et al. 2005). The latter is especially common in monsoonal climates, where the dry season is followed by a high incidence of lightning strikes accompanying convective rainfall (Keeley & Rundel 2003), and favours C4 grasses with their ability to grow more rapidly than C3 grasses in hot, open conditions after a recent burn (Knapp & Medino 1999).
Herbivores may also tip the balance between forest and grasslands, and can be classified on the basis of their foodstuff; specialist grazers eat only grasses, while dedicated browsers prefer forbs, shrubs and trees, avoiding the intense dental wear caused by the silica bodies (phytoliths) in grass leaves. Browsing and other damage to woody vegetation by large herbivores promotes grasses, through an important interaction with fire in savannah ecosystems (Bond et al. 2005). However, grazing can permit forest recovery through the removal of grass biomass, which lowers the amount of fuel for fires and decreases their intensity (Briggs et al. 2002). Shifts to seasonal climates, fire and herbivory may therefore all be implicated in the loss of forest vegetation that was a critical prerequisite for Miocene C4 success.
Creation of open woodland, savannah or grassland removes light-limitations on C4 plants, and their occurrence then depends on temperature, with the relative abundance of C4 relative to C3 grasses increasing with minimum summer temperatures (reviewed Sage et al. 1999). However, seasonally arid conditions mean that the effects of temperature on grassland distribution are mediated strongly by the amount of precipitation and timing of rainfall events, which constrain the length of the growing season through soil moisture availability.
C4 grasses typically dominate regions receiving rainfall from monsoonal weather systems during hot summer conditions, when the suppression of photorespiration provides a photosynthetic advantage over the C3 pathway (figure 2). Their CO2-concentrating mechanism also allows C4 leaves to achieve higher photosynthetic rates at lower stomatal conductances than in C3 species, thereby conserving water in hot conditions when evaporative demand is high (reviewed Long 1999). This efficient use of water throughout the life cycle of C4 plants must offer a major selective advantage over C3 species in open, seasonally dry habitats. In contrast, C3 grasses tend to populate regions where rains fall during cool winter weather (reviewed by Sage et al. 1999), a period when C4 species are susceptible to damage caused by chilling (reviewed Long 1983). Together, these interacting physiological responses to rainfall and temperature are the likely cause of the strong correlations between the total representation of C4 species in a flora, annual or summer rainfall, and minimum or mean summer temperature at the continental scale (e.g. Hattersley 1983). However, the direction of these correlations differs significantly between C4 physiological sub-types (Hattersley 1992) and phylogenetic groups (Taub 2000), for reasons that are poorly understood.
Modern plant biogeography points to important roles for temperature, rainfall patterns and disturbance in controlling C4 plant distributions, although the precise interactions between these variables remain unclear. In seeking the primary cause of Miocene C4 expansion, we must therefore examine both the geological history of climatic- and disturbance-mediated loss of forest cover, and the development of seasonal palaeoclimate regimes.
(b) Indian subcontinent
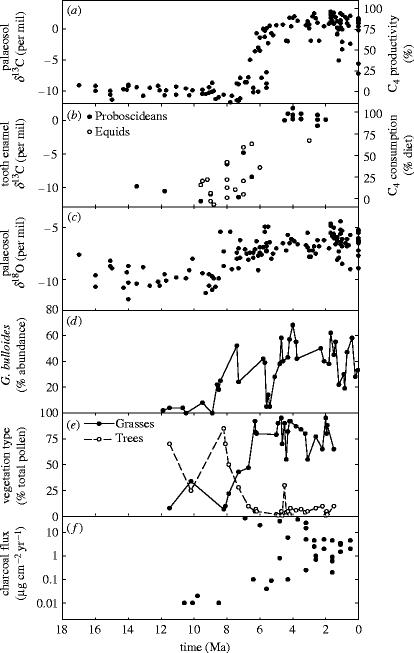
The Siwalik formation stretches through Pakistan, northwest India and Nepal, and contains one of the best-studied geological records of C4 success in the Late Miocene. Its fossils and sediments richly document the vegetation type, fauna, photosynthetic type and climate of the Himalayan foothills and Ganges floodplain from the Early Miocene through the Pliocene, with Pacific Ocean sediments offering complimentary evidence of fire frequency (figure 5).
Figure 5.
Geological evidence from the Indian subcontinent of ecosystem dynamics and climate change through the Late Miocene and Pliocene: (a) δ13C of palaeosol carbonates (data from Quade & Cerling 1995) and inferred C4 plant productivity (following Fox & Koch 2003), (b) δ13C of tooth enamel from Equids (horses) and Proboscideans (elephant-like mammals) (data from Quade & Cerling 1995) and the inferred proportion of diet comprised of C4 plant biomass (following Passey et al. 2002), (c) δ18O of palaeosol carbonates (data source as (a)), (d) abundance of Globigerinoides bulloides in the Arabian Sea (data from Zhisheng et al. 2001), (e) vegetation type inferred from pollen abundance (data from Hoorn et al. 2000), and (f) charcoal flux to North Pacific sediments (data from Keeley & Rundel 2003).
Measurements of δ13C from palaeosol carbonates in the Siwalik formation and organic sediments in the Bay of Bengal securely date the timing of the C4 revolution in this region (figure 5a). C4 plants began to increase in abundance from 7.7 Ma in Pakistan (figure 5a), 7.0 Ma in Nepal and the Bengal Fan, and 6.0 Ma in northern India. This increase gathered pace rapidly, resulting in the complete replacement of C3 vegetation across the region and ecosystem dominance by C4 plants by 5.5 Ma (reviewed by Quade et al. 1995). Timing of the transition is paralleled by an increase in the δ13C values of herbivore tooth enamel (figure 5b), demonstrating major changes in the diet of mammals with the shift in vegetation type (Cerling et al. 1997).
Expansion of C4 grasslands in the Indian subcontinent has been attributed to the initiation or intensification of the Indian monsoon (Quade et al. 1995). This climatic mechanism is supported by geological evidence from the stable oxygen isotope ratio (δ18O) of palaeosol carbonates, which show significant increases from 8.5 to 6.0 Ma, arriving ahead of the shift in δ13C by 0.5–1.0 Ma (figure 5c). The δ18O of soil carbonates depends on complex interactions between temperature, the δ18O of rainwater and the rate of evaporation from the soil surface. The Miocene increase in δ18O could, therefore, result from warming, a larger proportion of rainfall in the summer rather than winter and/or a more southerly source of water in rainfall, all consistent with the initiation or a major intensification of the Indian monsoon. This interpretation is further supported by significant increases in the abundance of marine organisms such as Globigerinoides bulloides at 8.5 Ma (figure 5d), indicative of greater monsoon-driven upwelling in the Arabian Sea, and by the initiation of dust deposition in China (evidence reviewed by Zhisheng et al. 2001).
Climate model simulations demonstrate that sudden uplift of the Tibetan Plateau at ca 8 Ma could be responsible for the onset of the Indian monsoon (Zhisheng et al. 2001). However, the timing of this mechanism is disputed, and new palaeo-elevation estimates suggest that uplift was largely complete by 15 Ma (Spicer et al. 2003; Currie et al. 2005), pointing to a more subtle interaction between tectonic activity and climate. Moreover, δ18O data from the Himalayan foreland now suggest constant monsoon intensity in this area from 10.7 Ma, with a general decline in rainfall from ca 8 Ma (Dettman et al. 2001). Whatever drove the change, 8.5 Ma was marked by a rapid transition to a drier climate, with most evidence pointing to a shift from year-round moist conditions to a summer-wet, winter-dry monsoon climate.
The transition to a monsoonal climate had major impacts on vegetation in the Indian subcontinent. Prior to this climatic shift, in the Early to Middle Miocene (8–18 Ma), plant fossils and pollen from the Lower Siwalik indicate a landscape comprising wet tropical evergreen forests in the lowlands, and moist deciduous forest with patches of pines at higher altitudes (figure 5e; Hoorn et al. 2000). Pollen demonstrates that grasses were present (figure 5e) and probably confined to gaps or forest margins, but palaeosol isotopes cannot resolve their photosynthetic type against the background signal from forest vegetation (Jacobs et al. 1999; Hoorn et al. 2000). These interpretations of Middle Miocene vegetation are confirmed by the presence of mouse deer and lorises, small mammals confined to dense forest today (reviewed by Jacobs et al. 1999).
Monsoonal conditions caused major changes in vegetation, characterized by a significant increase in grass and shrub pollen through sediments from the Late Miocene (6.5–8 Ma), accompanied by a decline in forest plant groups (figure 5e). This indicates a gradual transition from forest to open savannah that began at higher altitudes and spread down to the floodplain (reviewed by Jacobs et al. 1999; Hoorn et al. 2000). It was accompanied by the appearance of savannah and grassland large mammal taxa, including giraffes, grazing ungulates and hippopotamus (reviewed by Cerling et al. 1997; Jacobs et al. 1999). Complimentary pollen records show the complete loss of woody vegetation at this time, and the consequent establishment of C4 grasslands on both the Ganges floodplain and Himalayan foothills (figure 5a,e).
Ecological expansion of C4 grasslands and the immigration of large mammals to the northern Indian subcontinent followed the rapid loss of C3 woody vegetation from 8 to 5.5 Ma. As the winter drought intensified under monsoonal conditions and forest cover declined, fire also seems to have played a major part in the transition to grassland. Cores of sediment drilled from the deep-ocean floor in the northwest Pacific indicate a greater than 1000-fold increase in charcoal abundance during the Late Miocene (figure 5f). The charcoal probably originated in South Asia and includes charred grass cuticle fragments (Keeley & Rundel 2003, 2005). It suggests dramatic increases in fire frequency, intensity or extent accompanying the Indian monsoon, greatly amplifying the negative effects of climate change on tree cover (discussed by Keeley & Rundel 2003). Our review of terrestrial and marine geological evidence, therefore, implicates the initiation/intensification of the Indian monsoon, drought-mediated loss of forest cover, and a re-enforcing feedback promoted by fire as the reasons for C4 expansion rather than any change in CO2 concentration (figure 5c–f).
(c) North America
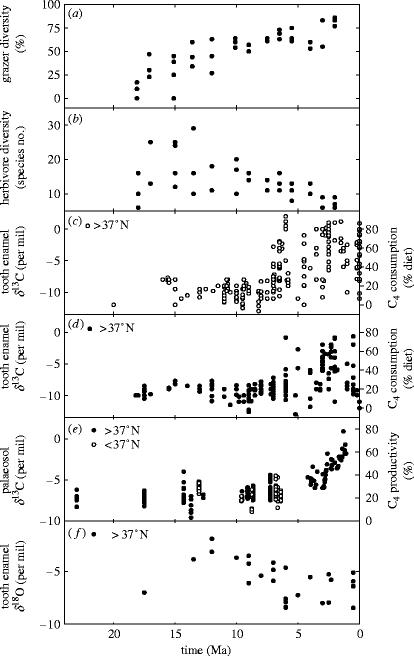
Investigations in the Great Plains region of North America have provided abundant records of fossil teeth and soils (palaeosols) for the Miocene and Pliocene offering unrivalled information on the coevolution of herbivore diet and C4 plants. However, the emerging picture from North American fossils is more complex than for the Indian subcontinent, and the role of climate is less clear. In the Great Plains, the evolutionary emergence of grazers, the appearance of species with a specialized diet of C4 plants, and the rise of these plants to dominance in Great Plains ecosystems occurred asynchronously (Fox & Koch 2003).
Replacement of closed forest by woodland or savannah ecosystems on the Great Plains began in the Early Miocene (15–23 Ma; reviewed by Jacobs et al. 1999), some 15–Myr earlier than the equivalent change on the Indian subcontinent (§4b). It was accompanied by a period of continuous diversification in the grasses, as they adapted to the new open habitats (Jacobs et al. 1999; Kellogg 2001), and the coevolution of grazing mammals. By 15 Ma ca 25% of mammals were typically hypsodont grazers, with tooth morphology indicating a mixed diet for 35% and browsing diet for 40% of the remaining species (figure 6a). By the height of the Middle Miocene Climatic Optimum (15–17 Ma; Zachos et al. 2001), these communities were more diverse than those of modern East Africa (figure 6b; Janis et al. 2000).
Figure 6.
Geological evidence from the Great Plains of North America of ecosystem dynamics and climate change through the Late Miocene and Pliocene. Data sources are: (a) and (b) Janis et al. (2000); (c) Cerling et al. (1997), Latorre et al. (1997) and Passey et al. (2002), (d) Cerling et al. (1997) and Passey et al. (2002), (e) Fox & Koch (2003), and (f) MacFadden et al. (1999) and Passey et al. (2002).
Analyses of δ13C from tooth fossils of the Middle Miocene indicates that the new species of specialized grazers subsisted primarily on C3 grasses at sites across the Great Plains (figure 6c,d). A slight positive bias in these δ13C values can be interpreted as either a drought response in the C3 food plants or a diet composed of up to 25% C4 grasses (Passey et al. 2002). The latter interpretation is consistent with δ13C evidence from palaeosols (figure 6e), suggesting a 12–34% contribution by C4 grasses to plant productivity (Fox & Koch 2003), and analyses based on the taxonomic identification of fossil phytoliths, indicating the dominance of C3 grasses with a lesser C4 component (Strömberg 2004). The scenario of savannah ecosystems populated by C3 grasses contrasts with the situation in the Indian sub-continent, where the creation of open woodland and savannah starting at 8.5 Ma permitted the immediate dominance of ecosystems by C4 grasses. Clearly, the loss of woody vegetation in North America that began sooner, at 23 Ma, did not occur at a time when conditions favoured the dominance of C4 grasses.
δ13C values unequivocally indicating a diet composed of C4 plants are first detected 6.6 Ma in horse teeth from the Coffee Ranch formation in Texas (figure 6c; Passey et al. 2002), 9 Myr after the peak of mammalian diversity (figure 6b; Janis et al. 2000), and more than 2 Myr after the equivalent change in Pakistan (figure 5b). The transition to a predominantly C4 diet was complete in some Texan animals by 6 Ma, but others continued to feed on C3 plants or had a mixed diet (figure 6c; Cerling et al. 1997). The cause of such a long delay in the evolution of grazers like the modern bison, with their ability to subsist on a specialized C4 diet, remains unclear (Passey et al. 2002).
Comparison of the transition from C3-based to C4-dominated diets (figure 6c,d) and the equivalent change in regional plant productivity (figure 6e) is complex because the region spans a wide latitudinal (climatic) gradient. Data from fossil teeth indicate that the dietary transition occurred more rapidly in the southern Great Plains (figure 6c), being followed more slowly in the northern part of the region (figure 6d). This observation suggests an interaction with temperature, where C4 plants rose to dominance earlier in warmer climates (Cerling et al. 1997). Palaeosol δ13C data from the latest Miocene and Pliocene are not yet available for the southern Great Plains (figure 6e), but values from the northern part of the region (figure 6e) match the late rise in δ13C of fossil teeth (figure 6d). Some indication of a delay between the origin of C4-based diets and increasing C4 plant productivity requires further investigation. It is also interesting to note that Late Miocene horses co-occurring with thermophile tortoises had a higher proportion of C4 grasses in their diets (Passey et al. 2002), perhaps suggesting heterogeneity in the Great Plains regional climate, and a greater abundance of C4 grasses in warmer areas (Passey et al. 2002). However, while these arguments are entirely reasonable, they cannot be the full explanation. It is unclear, for example, why C4 plants only come to dominate North American ecosystems in the globally cool Pliocene, instead of during the Middle Miocene Climatic Optimum (15–17 Ma) when global temperatures were warmer. Part of the answer would seem to lie with the role played by regional climatic warming or changing patterns of precipitation.
Our understanding of regional climate change accompanying ecological transitions in diet and vegetation is incomplete at present, but some insights have been obtained from analyses of δ18O from tooth enamel. δ18O values decline in the Middle to Late Miocene (13–5 Ma), and remain relatively stable from the Pliocene onwards (less than 5 Ma; figure 6f), most likely reflecting a combination of physiological and behavioural changes in the animals and climatic change (Passey et al. 2002). These patterns are also seen in palaeosol carbonates during the Late Miocene and Pliocene, once the effects of latitude have been removed by statistical procedures (Fox & Koch 2004). Decreases in tooth and palaeosol δ18O during the Late Miocene are consistent with the global cooling trend shown for the same period by δ18O in deep-sea sediments (Zachos et al. 2001). However, the stabilization of these terrestrial values in the Pliocene appears to be at odds with the ocean records, which suggest increased ice volume and continued global cooling over the past 5 Myr. Relative stability in the δ18O of North American mammal tissues therefore points to regional warming set against a background of global cooling, or climatic change involving a warming of rainfall, caused either by an increase in summer rains or switch to a more southerly source of rainwater. Although speculative, this idea is consistent with the climatic change expected to accompany the narrowing and closure of the ocean straits between North and South America during the latest Miocene and Pliocene. Ocean–atmosphere model simulations suggest that an open sea passage through Central America led to a collapse of the North Atlantic thermohaline circulation (Maier-Reimer et al. 1990). Narrowing and closure of these straits would have reinstated circulation (Mikolajewicz and Crowley 1997), an event associated in climate model simulations with warming and increased humidity over the Great Plains (Vellinga and Wood 2002). Perhaps an increase in summer moisture drove a Late Miocene transition from arid C3 grassland with a minor C4 component, to a more mesic C4 prairie ecosystem (Keeley & Rundel 2005)?
5. Are C4 plants TEN-times older than we think?
The origin of C4 plants during the Oligocene is consistent with the C4 photosynthetic pathway evolving as an adaptation to CO2 starvation (§3), with climate playing an important part in determining the relative performance of C3 and C4 plants in the field (§4). From these observations, the question arises as to whether plants experienced environmental conditions driving the selection of a C4 carbon-concentrating mechanism earlier in their evolutionary history (Beerling 2005). In this section, we assess the theoretical potential for variations in atmospheric composition and climate to select for the C4 photosynthetic pathway in the Late Palaeozoic and Mesozoic (65–365 Ma), and report results from an isotope-based survey of fossil plants, designed to search for evidence of its early origination.
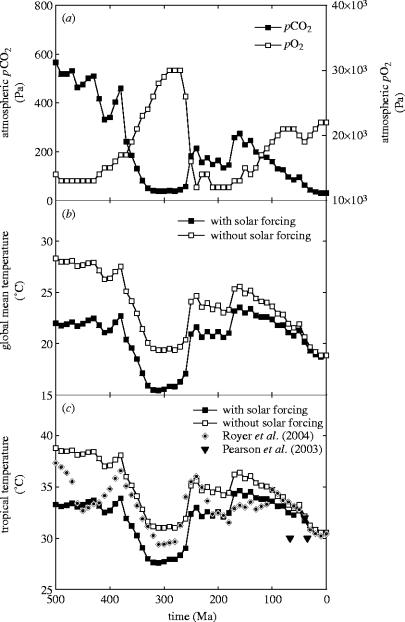
Geochemical models of Earth's atmospheric evolution clearly indicate large variations in atmospheric CO2 and O2 throughout the 470 Myr interval that plants have colonized the continents (figure 7a; Berner 2005). Model predictions of CO2 variations are generally well-supported by independent evidence based on analyses of a wide range of fossil materials, including soils, leaves of land plants, and the isotopic composition of molecular biomarkers of marine phytoplankton metabolism (Crowley & Berner 2001; Berner 2005). Corresponding variations in atmospheric O2 occurred largely because, on multimillion-year time-scales, the two cycles are coupled through the burial of organic carbon both on land and in the oceans (Berner 2005). The models show a striking decrease in atmospheric CO2 and an increase in O2 during the Late Palaeozoic (figure 7a; 320–500 Ma), which, through different mechanisms, resulted from the rise of vascular land plants and the spread of rooted forests (Berner 2005). Evolutionary trends towards increased vegetation activity accelerated the extent and rate of silicate rock weathering, the primary sink for CO2 in the long-term, and caused a massive increase in sedimentary organic carbon burial, most obviously manifested as the formation of extensive Carboniferous coal deposits (Berner 2005).
Figure 7.
Variations in: (a) the partial pressure of atmospheric CO2 and O2 over the past 500 Myr of the Phanerozoic, (b) calculated changes in global mean surface temperature, and (c) calculated changes in the tropics. Also shown in (c) are estimates of tropical temperatures from oxygen isotope ratios of fossil foraminifera shells (Pearson et al. 2001) and calculations from marine calcium carbonate fossils (Veizer et al. 2000) corrected for the CO2-effects on ocean pH (Royer et al. 2004).
Identifying times favouring C4 over C3 photosynthesis during the Phanerozoic (the past 570 Myr) requires consideration of the influence of both atmospheric composition and climate. We calculated global changes in climate through the Phanerozoic to provide a basis for this assessment, by modifying a simple zero-dimensional model of planetary energy balance that collapses latitude, altitude and longitude to give a single global mean value for a given atmospheric CO2 concentration (figure 7b; Beerling & Woodward 2001). The calculations include the direct effects of CO2, via the atmospheric greenhouse effect, and the effect of the estimated ca 5% increase in the Sun's output over the past 500 Myr (Caldeira & Kasting 1992). Calculated changes in global mean surface temperature strongly track the concentration of atmospheric CO2 (figure 7a,b), and are sensitive to changes in solar forcing, especially in the early part of the Palaeozoic, when the global climate is 5 °C cooler than expected from a simple consideration of the greenhouse effect alone (figure 7b). The drastic decline in atmospheric CO2 caused by the rise of vascular land plants leads to a pronounced global climatic cooling (320–500 Ma) as evidenced by the widespread Permo-Carboniferous continental glaciation (Crowell 1999; Royer et al. 2004).
However, C4 plants are usually distributed in sub-tropical or tropical climates, where temperatures exceed the crossover temperature for photosynthetic efficiency (figure 2). We approximated long-term trends in tropical land surface temperature (30°N to 30°S) over the past 500–Myr using a conversion factor based on results from general circulation models of the climate system at different times in Earth history and a range of atmospheric CO2 concentrations (figure 7c; Otto-Bliesner 1995; Valdes & Crowley 1998; Otto-Bliesner et al. 2000; Beerling & Woodward 2001). The approach is necessarily approximate, but predicted temperatures can be compared with those obtained by analysis of shallow marine calcium carbonate fossils (Veizer et al. 2000), after correction for the effects of seawater pH (Royer et al. 2004), and the oxygen isotope composition of foraminifera from the tropical oceans during past warm climate intervals (Pearson et al. 2001). Comparison of observed and predicted trends in tropical land surface temperatures shows a reasonable degree of agreement and provides support for the approach (figure 7c).
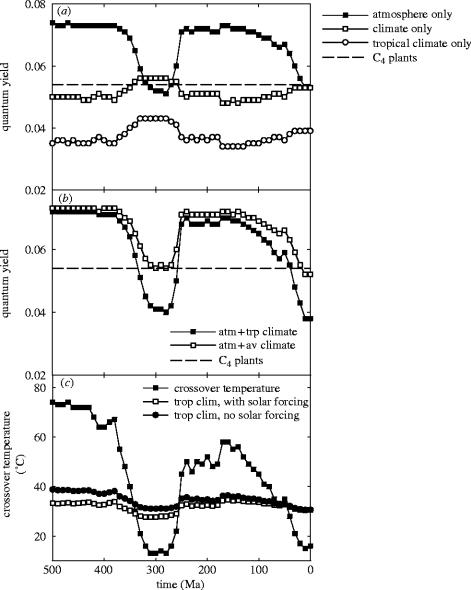
We determined the likely effects of these changes in atmospheric composition (figure 7a) and climate (figure 7c) on C3 and C4 plant performance using the quantum yield approach (figure 2; Ehleringer et al. 1991, 1997; Cerling et al. 1997). Long-term variations in atmospheric composition produced a marked drop in the predicted quantum yield of C3 plants during the Permo-Carboniferous (245–365 Ma) that was only partially offset by the effects of a cooler climate (figure 8a). Further analyses of the combined influence of variations in atmospheric composition and tropical climate revealed the Permo-Carboniferous and Late Tertiary (less than 50 Ma) to be the only times throughout the entire ca 470 Myr history of terrestrial plant life when C3 quantum yield dropped below that of C4 plants (figure 8b). Calculation of the crossover temperature (figure 2) reinforces this view. The temperatures at which C4 plants attain higher quantum yields than their C3 counterparts far exceed the highly conserved high temperature limits for photosynthesis (40–50 °C; Larcher 1994) for most of the Phanerozoic except during the Permo-Carboniferous and the Tertiary (figure 8c). This result is quite robust, being insensitive to uncertainties in our approach to calculating tropical temperatures (figure 8c). Since C4 photosynthesis did actually arise in the Tertiary (§2), these results and those from modelling photosynthesis (Beerling 2005) suggest the Permo-Carboniferous as a time when environmental conditions could have strongly promoted the evolutionary selection of C4 plants.
Figure 8.
Calculated changes in: (a) quantum yield of C3 and C4 plants through the past 500 Myr of the Phanerozoic, (b) the effects of CO2 in combination with either tropical climate (filled squares) or global mean climate (open squares) on quantum yield, and (c) the calculated crossover temperature for C4 and C4 plants in comparison with the tropical climate (with and without the effects of solar forcing) shown in figure 7.
To identify possible geographic regions of C4 plant occurrence in the Permo-Carboniferous we extended our analysis to the global scale (Beerling & Woodward 2001). We achieved this aim using a process-based generalized dynamic global vegetation model forced with two contrasting climates of the Late Carboniferous (ca 305 Ma), simulated by different general circulation models (GCMs); the UK Universities Global Atmospheric Modelling Programme (UGAMP) and the National Centre for Atmospheric Research (NCAR) GCM (Valdes & Crowley 1998; Otto-Bliesner & Shields, unpublished). The global simulations are made assuming the C4 photosynthetic pathway had evolved, predicting regions where C4 plants dominated the vegetation.
Our simulations indicate consistent tropical regions where C4 plants could have persisted, regardless of the underlying land surface climatology (figure 9). The simulated presence of C4-dominated vegetation in the high latitudes is also indicated with the warmer, drier high latitude climate of the UGAMP GCM but not the with the much colder NCAR GCM climate (figure 9). It is possible that an increased frequency of ancient wildfires due to the high O2 content of the atmosphere would have allowed C4 plant distributions to expand considerably through the removal of forest cover (section 4; Beerling & Woodward 2001). However, although a high O2 atmosphere may have increased the probability of ignition of a fuel source (Watson et al. 1978), there is no evidence that it enhanced the spread of fire (Wildman et al. 2004). Indeed, Wildman et al. (2004) reported from their thermochemistry and flame-spread experiments that, with moisture contents typical of forest floor fuel sources, there was no sustained burning in an atmosphere of between 21 and 35% O2.
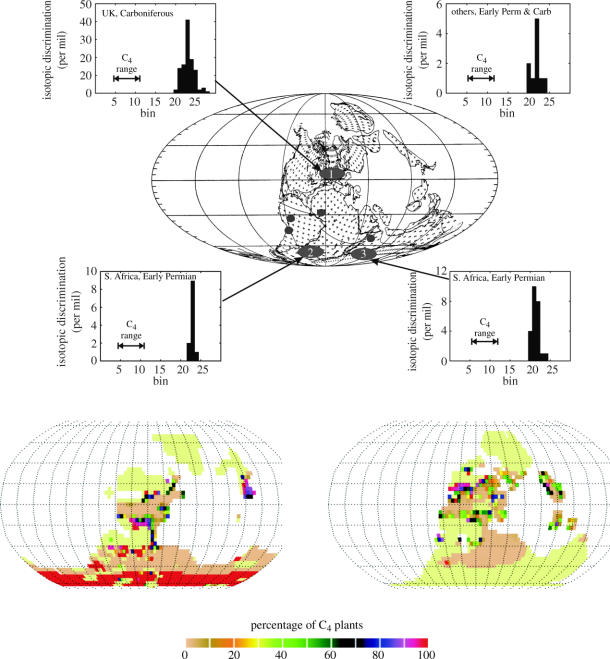
Figure 9.
Simulated global distribution of C4 plants using a generalized dynamic global vegetation model (Beerling & Woodward 2001) and either the land surface climatology from the UGAMP (lower left map) or NCAR (lower right map) GCMs. Central picture shows the palaeogeography of the Late Carboniferous (Westphalian; Scotese & McKerrow 1990) and the main regions/localities from which fossil plants were sampled for stable carbon isotope analysis (see appendix A for details). The frequency histograms depict the calculated values of carbon isotope discrimination and, for comparison, the values expected if plants operated with the C4 photosynthetic pathway.
To examine the possibility of C4 plant occurrence this far back in time, we conducted an isotopic survey of Late Carboniferous and Early Permian plant fossils from three key regions (Beerling 2005), encompassing the tropics and the high southern latitudes (appendix A). Plant materials were sampled from the collections of the British Geological Survey, Keyworth, and the Natural History Museum, London, and supplemented with Early Permian plant remains from the northern Prince Charles Mountains, East Antarctica (McLoughlin & Drinnan 1997), kindly supplied by Stephen McLoughlin (University of Melbourne, Australia). From these sources, we sampled and analysed foliage and stem material of Late Carboniferous lycopsids, the dominant plant group in the tropics (DiMichele et al. 2001), as well as a number of other herbaceous and woody plant groups (figure 9, group 1; appendix A). We also sampled the foliage and bark of the short woody plant Glossopteris which dominated the high southern latitudes in the Early Permian (Chaloner & Lacey 1973), from two different regions, Antarctica and South Africa (groups 2 and 3, figure 9; appendix A). The isotopic survey was completed by a small number of samples from a scattered set of localities encompassing areas (Peru, India, Brazil, Russia and Ghana) of predicted C4 occurrence. Results are expressed in terms of isotopic discrimination (Δ) to remove the effects of the δ13C of atmospheric CO2, which were estimated using a smoothed marine carbonate δ13C curve (Veizer et al. 1999), assuming equilibrium between these reservoirs.
We find no isotopic evidence of C4 photosynthesis in any Late Carboniferous or Early Permian fossil plants remains, irrespective of location (figure 9). This is in agreement with previous reports of no clear C4 isotopic signature in fossil plants pre-dating the Tertiary (Bocherens et al. 1994; Beerling 2005). Two studies have interpreted spurious isotopic values as indicative of C4 or CAM plants (Wright & Vanstone 1991; Jones 1994). Wright & Vanstone (1991) sampled thin clay layers between thick marine limestone sequences, interpreted as exposed surfaces where the calcification of soils into rhizolith crusts or root mats had occurred. However, it is doubtful whether these clays, and the postulated soil carbonates associated with them, formed in a manner suitable for faithfully preserving δ13C, and the claim was unsupported by any anatomical information (Cerling 1999). The study of isotopic charcoal remains of putative gymnosperms and lycopods from Donegal, Ireland requires further investigation to better resolve the nature of the anomalous values (Jones 1994).
We conclude that the atmospheric composition of the Late Carboniferous and Early Permian would have provided a powerful selective pressure for the evolution of C4 photosynthesis, yet isotopic surveys of fossil plants have so far failed to detect any evidence of the pathway at this time. We recognize, of course, that more exhaustive isotopic surveys may yet reveal the presence of the C4 photosynthetic pathway in the Permo-Carboniferous. However, the lack of evidence for its existence leads us to ask in §7 more fundamentally whether an essential physiological precursor of C4 photosynthesis is absent in ancient plant lineages dating from the Permo-Carboniferous.
6. Are C4-like enzyme systems present in all plants?
The realization that some modern C3 plants actually operate with components of the C4 photosynthetic pathway (Hibberd & Quick 2002) suggests a hypothesis for explaining the absence of the syndrome in Permo-Carboniferous taxa; if ancient plant lineages lack this component pathway, they may lack an essential pre-condition for evolving C4 photosynthesis.
Hibberd & Quick (2002) reported that tobacco (Nicotiana tabacum) and celery (Apium graveolens), both typical C3 plants, use C4-type enzymes to strip CO2 from four-carbon organic acids dissolved in the xylem sap. The authors fed these species with the C4 organic acid malate and reported its assimilation into sugars by the green photosynthetic cells located within the stems. The pathway they described requires C4-type decarboxylase enzymes to produce CO2 from malate, and Rubisco to fix it into sugars. Both were present in the vascular bundles of tobacco and celery, with the decarboxylases at much higher levels than in leaf cells. The system appears to help the plants conserve carbon, by converting malate produced by respiration in the roots into sucrose and starch for growth (Raven 2002).
Perhaps a pathway for recapturing respired CO2 that would have otherwise been lost to the atmosphere represents a first step in C4 plant evolution (Monson 1999)? It is possible then that early ferns and gymnosperms, the two groups that predominated in the Late Palaeozoic, lacked the necessary anatomy or physiology to evolve even this first step towards the C4 photosynthetic pathway (Raven 2002). To investigate this possibility, experiments were conducted with two species of plants from ancient lineages of ferns (Osmunda regalis) and gymnosperms (Ginkgo biloba; Palmer & Quick, unpublished). Following the methodology of Hibberd & Quick (2002), 14C labelled malic acid was fed in a 0.1 mM malate carrier solution to cut petioles of O. regalis and G. biloba for 30 min in the light. Plants were then flash frozen, heated in 70% ethanol to remove soluble sugars and exposed to X-ray film.
The results of this preliminary investigation (figure 10; Palmer & Quick, unpublished data) reveal an accumulation of 14C in insoluble material within cells associated with the vascular tissue. These data are consistent with the idea that CO2 can be removed from malate in the xylem transpiration stream and fixed by nearby photosynthetic cells. Further support for this possibility was obtained by imaging the distribution of chlorophyll in the photosynthetic cells of thin sections of O. regalis and G. biloba petioles. Under fluorescence microscopy, abundant chlorophyll was detected preferentially surrounding the xylem and phloem tissue in the petioles of both species (Fletcher & Quick, unpublished data). Although activities of the decarboxylase enzymes within the petioles of these two species still need to be assayed, these preliminary datasets would seem to support earlier speculation that photosynthesis in vascular tissue was common in the past (Raven 2002).
Figure 10.
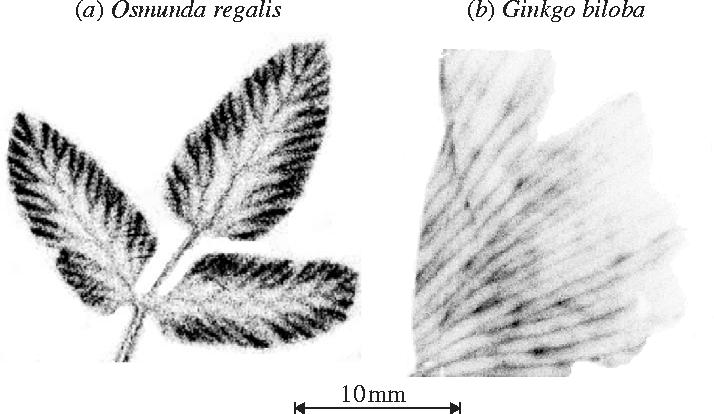
Photographic evidence showing the incorporation of 14C-labelled malate into insoluble compounds in leaves of (a) Osmunda regalis and (b) Ginkgo biloba (Palmer & Quick, unpublished data). Note the 14C activity concentrated around vascular tissues.
7. Future directions
This review has identified several key uncertainties in our understanding of C4 plant evolution that point to future research priorities. Extensive isotopic evidence from fossil materials clearly indicates major changes in the proportion of plants with the C4 photosynthetic pathway over the past 30 Myr of the Tertiary. Up until now these regional-scale patterns of C4/C3 vegetation dynamics across the Earth's surface have been interpreted largely on the basis of the quantum yield model, in conjunction with inferred changes in the global environment. However, our review indicates that neither the switch to C4 dominated ecosystems in North America nor on the Indian sub-continent are adequately explained in this way, with evidence instead pointing to important roles for the pattern of seasonal rainfall. A clear example of how misleading the quantum yield model can be comes from CO2 enrichment experiments in a tallgrass prairie community, which show an increase in abundance of C4 over C3 grasses via a feedback involving plant water relations (Owensby et al. 1999). New approaches are, therefore, required to understand what actually happened in the past and why. We suggest that a significant change in direction is required with new foci on experiments with C3 and C4 plants grown under manipulated environmental conditions, and on approaches for better quantifying the role of disturbance from sedimentary records.
Experiments are required to investigate how the differential physiological responses of C3 and C4 species to climate and CO2 translate into ecological success. Crucially, the emphasis must shift from models centred on physiology towards ecology, focusing on the processes linking leaf physiology with differential growth, resource-use, and survival responses in C3 and C4 plants. This approach will lead to a better understanding of competition, reproductive fitness and survivorship, factors directly responsible for evolutionary and ecological success. We currently know little about the differences between C3 and C4 species in their seasonal responses of photosynthesis and the timing of growth and reproduction in regions with contrasting climatic regimes. Recent experimental work has highlighted important interactions between high CO2 responses in C3 and C4 plants and the efficiency of carbon, water and nutrient-use. However, it is unclear how these responses change under CO2-starvation, and how they interact with plant tolerance of extreme temperatures and drought. To comprehensively address these uncertainties, a programme of experiments must aim to establish the key processes by linking controlled environment, common garden and field-based approaches.
Detailed studies in the Indian sub-continent and charcoal abundance in sediment cores from the Pacific Ocean suggest an association between the occurrence of fire and the expansion of ecosystems dominated by C4 plants. A key priority for future research must be to investigate the strength of this likely interaction, through more detailed isotopic and morphological examination of combustion products in well-dated, high-resolution ocean sediment cores. Careful selection of ocean cores with footprints originating from contrasting continental sources must first identify whether the coevolution of C4 ecosystems and fire is a general phenomenon. The issue of whether increasing fire frequency is specifically associated with the rise of C4 grasslands per se, or with the replacement of forest by grasslands in general also needs to be addressed. A test of this issue can be achieved by comparison of the evidence for fire history in the Indian subcontinent and North America, with their contrasting histories of grassland evolution.
Further back in time, beyond the Tertiary, it is still far from clear why unusual atmospheric conditions did not select for C4 photosynthesis. Current ideas concern the evolution of high vein densities in plants experiencing drought from water deficit or salinity as a prerequisite for C4 Kranz anatomy (Sage 2001). Whether these anatomical properties are confined to certain groups of flowering plants is unclear. A major direction for future research must, therefore, be identification of the developmental genetic and environmental controls over vascular tissue production in leaves, and the extent to which these can operate in non-flowering plants.
Acknowledgements
We gratefully acknowledge funding from the Royal Society and thank Bette Otto-Bliesner and Christine Shields for providing the NCAR Carboniferous climate, Paul Kenrick, Bill Chaloner and Mike Howe for access to the fossil collections of the Natural History Museum, London and the British Geological Survey, respectively, and Stephen McLoughlin (University of Melbourne, Australia) for providing the Antarctic fossil plant samples. We also thank Ben Palmer, Ben Fletcher and Paul Quick for their analysis of stem CO2 fixation in ancient plant groups, Richard Leegood and Ian Woodward for discussion and advice, Emily Wythe and Maria Jarman for helpful comments and assistance.
Appendix A
Carbon isotope composition (δ13C) and isotopic discrimination (Δ13C) of Carboniferous and Permian fossil plants.
| fossil specimen | specimen number | locality | age | material analysed | δ13C (‰) | Δ13C (‰) |
|---|---|---|---|---|---|---|
| A. British Geological Survey collections, Keyworth | ||||||
| Lycopsids | ||||||
| Bothrodendron minutifolium | RC2872 | South Yorkshire, UK | Westphalian B | foliage | −22.5 | 20.9 |
| B. minutifolium | 51813 | Kent, UK | Westphalian | foliage | −23.0 | 21.4 |
| Cyperites eileitus | 4168 | South Yorkshire, UK | Westphalian B | foliage | −22.4 | 20.8 |
| Lepidostrobophyllum alatum | RC4838 | Somerset, UK | Westphalian D | foliage | −22.1 | 20.5 |
| L. intermedium | RC3134 | Staffordshire, UK | Westphalian | foliage | −21.9 | 20.2 |
| Lepidophyllum sp. | 6127 | Lancashire, UK | Westphalian | foliage | −22.0 | 20.3 |
| Lepidodendron gracile | 5284 | UK | Westphalian | foliage | −22.3 | 20.7 |
| L. lanceolatum | JP218 | Monmouthshire, UK | Westphalian | foliage | −22.9 | 21.2 |
| L. lycopodioides | 18239 | UK | Westphalian | foliage | −22.2 | 20.6 |
| Sigillariophyllum bicarinatelm | 4907 | South Yorkshire, UK | Westphalian B | foliage | −23.7 | 22.1 |
| Sigillariostrobus rhombractiatus | 1177 | South Yorkshire, UK | Westphalian B | foliage | −22.0 | 20.3 |
| Lepidodendron aculeatum | 2640 | South Yorkshire, UK | Westphalian A | bark | −23.4 | 21.8 |
| 4033 | South Yorkshire, UK | Westphalian A | bark | −23.8 | 22.2 | |
| 4034 | South Yorkshire, UK | Westphalian A | bark | −24.8 | 23.3 | |
| 7105 | South Yorkshire, UK | Westphalian A | bark | −25.1 | 23.6 | |
| 3821 | South Yorkshire, UK | Westphalian A | bark | −23.7 | 22.1 | |
| 2729 | South Yorkshire, UK | Westphalian A/B | bark | −23.5 | 21.9 | |
| 2643 | South Yorkshire, UK | Westphalian A/B | bark | −24.4 | 22.9 | |
| 3892 | South Yorkshire, UK | Westphalian C | bark | −24.9 | 23.4 | |
| 1009 | South Yorkshire, UK | Westphalian C | bark | −26.0 | 24.5 | |
| 2482 | Ayrshire, UK | Westphalian A | bark | −27.7 | 26.3 | |
| 2481 | Warwickshire, UK | Westphalian A/B | bark | −24.1 | 22.5 | |
| 1765 | Somerset, UK | Westphalian C/D | bark | −26.1 | 24.6 | |
| 5686 | Trane Coll., UK | Westphalian | bark | −27.7 | 26.3 | |
| L. berwickense | 2775 | Berwickshire, UK | Westphalian | bark | −24.2 | 22.6 |
| L. camertion | 3246 | Somerset, UK | Westphalian C | bark | −24.4 | 22.9 |
| L. canobianum | 3127 | Dumfrieshire, UK | Carboniferous limestone series | bark | −23.7 | 22.1 |
| L. feistmantel | 4946 | South Yorkshire, UK | Westphalian B | bark | −24.3 | 22.8 |
| L. fusiforme | 4984 | South Yorkshire, UK | Westphalian B | bark | −24.0 | 22.4 |
| L. gaudryi | 5157 | South Yorkshire, UK | Westphalian B | bark | −27.4 | 26.0 |
| 4877 | Lanarkshire, UK | Westphalian | bark | −24.3 | 22.8 | |
| L. jaraczwskii | 4878 | South Yorkshire, UK | Westphalian A/B | bark | −23.7 | 22.1 |
| 1414 | Gartness Colliery, Aidrie, UK | Westphalian A | bark | −24.6 | 23.1 | |
| 4616 | Lancashire, UK | Westphalian A | bark | −23.4 | 21.8 | |
| L. jaschei | 4288 | Herbertshire, UK | Scottish Carboniferous limestone series | bark | −24.8 | 23.3 |
| 4289 | Herbertshire, UK | Scottish Carboniferous limestone series | bark | −24.6 | 23.1 | |
| 2459 | Stirlingshire, UK | Scottish Carboniferous limestone series | bark | −24.1 | 22.5 | |
| 3043 | Stirlingshire, UK | Scottish Carboniferous limestone series | bark | −24.0 | 22.4 | |
| 2452 | Stirlingshire, UK | Scottish Carboniferous limestone series | bark | −24.0 | 22.4 | |
| L. lansburgi | 1549 | Ayrshire, UK | Westphalian A | internal stem | −26.0 | 24.5 |
| L. lycopodoides | 1468 | South Yorkshire, UK | Westphalian A/B | bark | −22.8 | 21.2 |
| 2717 | South Yorkshire, UK | Westphalian A/B | bark | −22.0 | 20.3 | |
| 2787 | Low Moor, Yorkshire, UK | Westphalian A/B | bark | −23.9 | 22.3 | |
| 1868 | Cadedy Coll., Yorkshire, UK | Westphalian | bark | −24.1 | 22.5 | |
| 1869 | Cadedy Coll., Yorkshire, UK | Westphalian | bark | −26.0 | 24.5 | |
| L. mannabachense | 2474 | Yorkshire, UK | Westphalian A/B | bark | −25.3 | 23.8 |
| 2473 | South Yorkshire, UK | Westphalian A/B | bark | −24.5 | 23.0 | |
| L. nathorsti | 5751 | Berwickshire, UK | Westphalian | bark | −24.8 | 23.3 |
| 2957 | Berwickshire, UK | Westphalian | bark | −24.4 | 22.9 | |
| 2954 | Berwickshire, UK | Westphalian | bark | −23.2 | 21.6 | |
| L. obovatum | 4868 | South Yorkshire, UK | Westphalian A | bark | −24.6 | 23.1 |
| 2641 | South Yorkshire, UK | Westphalian A | bark | −24.3 | 22.8 | |
| 2644 | South Yorkshire, UK | Westphalian A/B | bark | −24.5 | 23.0 | |
| 3362 | Lancashire, UK | Westphalian | bark | −24.4 | 22.9 | |
| 3367 | Lancashire, UK | Westphalian | bark | −24.0 | 22.4 | |
| 3366 | Lancashire, UK | Westphalian | bark | −25.1 | 23.6 | |
| 6141 | Lancashire, UK | Westphalian | bark | −23.6 | 22.0 | |
| 2475 | Lancashire, UK | Westphalian | bark | −24.0 | 22.4 | |
| 2469 | Northumberland, UK | Westphalian A | bark | −26.0 | 24.5 | |
| 2470 | Northumberland, UK | Westphalian A | bark | −23.4 | 21.8 | |
| L. ophiurus | 413 | Staffordshire, UK | Westphalian A/B | internal stem | −24.2 | 22.6 |
| L. peachii | 2466 | Durham, UK | Westphalian | bark | −24.4 | 22.9 |
| L. rimosum | 3894 | South Yorkshire, UK | Westphalian C | bark | −25.5 | 24.0 |
| 5155 | South Yorkshire, UK | Westphalian C | bark | −25.7 | 24.2 | |
| 4894 | South Yorkshire, UK | Westphalian A/B | bark | −23.9 | 22.3 | |
| L. rhodeanum | 2494 | Grange Colliery, UK | Carboniferous Limestone series | bark | −24.3 | 22.8 |
| L. simile | 4893 | South Yorkshire, UK | Westphalian A/B | bark | −23.6 | 22.0 |
| 4892 | South Yorkshire, UK | Westphalian A/B | bark | −25.9 | 24.4 | |
| 4728 | South Yorkshire, UK | Westphalian | bark | −25.3 | 23.8 | |
| 6372 | Staffordshire, UK | Westphalian | bark | −26.5 | 25.1 | |
| 6106 | Devon, UK | Westphalian | bark | −23.2 | 21.6 | |
| L. spitsbergense | 6509 | Spitsbergen | Westphalian | bark | −25.9 | 24.4 |
| L. tijoui | 3946 | Holland | Westphalian | bark | −22.3 | 20.7 |
| L. veltheimianum | 29 | Lothian, UK | Scottish Carboniferous limestone series | bark | −24.7 | 23.2 |
| 3482 | Lothian, UK | Scottish Carboniferous limestone series | bark | −23.7 | 22.1 | |
| 4828 | Manor Powis Colliery, UK | Carboniferous limestone series | bark | −25.5 | 24.0 | |
| 60 | Grange Colliery, UK | Carboniferous limestone series | bark | −25.1 | 23.6 | |
| 82 | Grange Colliery, UK | Carboniferous limestone series | internal stem | −28.3 | 27.0 | |
| 71 | Grange Colliery, UK | Carboniferous limestone series | stem scars | −24.2 | 22.6 | |
| 61 | Grange Colliery, UK | Carboniferous limestone series | stem scars | −24.8 | 23.3 | |
| 88 | BurghLee Pit, UK | Carboniferous limestone series | internal stem | −23.5 | 21.9 | |
| 65 | Rafstoch Quarry, Stirling, UK | Carboniferous limestone series | bark | −25.3 | 23.8 | |
| 67 | Rafstoch Quarry, Stirling, UK | Carboniferous limestone series | bark | −24.2 | 22.6 | |
| 68 | Rafstoch Quarry, Stirling, UK | Carboniferous limestone series | bark | −25.9 | 24.4 | |
| 2624 | Rafstoch Quarry, Stirling, UK | Carboniferous limestone series | bark | −24.0 | 22.4 | |
| 48 | Rafstoch Quarry, Stirling, UK | Carboniferous limestone series | bark | −25.5 | 24.0 | |
| 46 | Northumberland, UK | Westphalian | bark | −24.7 | 23.2 | |
| L. wedekindi | 2157 | South Yorkshire, UK | Westphalian B | bark | −25.7 | 24.2 |
| Lepidocystis vesicularis | 1363 | Pennsylvania, USA | Westphalian A | bark | −23.8 | 22.2 |
| Lyginoendron landsburgii | 3220 | Ayrshire, UK | Westphalian | bark | −22.7 | 21.1 |
| Sphenopsids | ||||||
| Sphenophyllum emarginatum | 76043 | Staffordshire, UK | Westphalian | foliage | −27.2 | 25.8 |
| S. majus | RC2848 | South Yorkshire, UK | Westphalian B | foliage | −24.4 | 22.9 |
| Horsetails | ||||||
| Annularia fratens | 88 | UK | Westphalian | foliage | −22.7 | 21.1 |
| A. radiata | 4275 | Limburg, Holland | Westphalian | foliage | −23.1 | 21.5 |
| A. stellata | 75930 | Somerset, UK | Westphalian D | foliage | −24.3 | 22.8 |
| free sporing plants with gymnospermous wood | ||||||
| Archaeopteris reussi | RC2823 | Staffordshire, UK | Westphalian | foliage | −24.1 | 22.5 |
| Seed ferns | ||||||
| Alethopteris grandiniodes | 75930 | Somerset, UK | Westphalian D | foliage | −23.3 | 21.7 |
| A. lonchitica | RC2137 | Cumbria, UK | Westphalian | foliage | −23.8 | 22.2 |
| A. rubescens | 250 | Somerset, UK | Westphalian D | foliage | −22.3 | 20.7 |
| Aulacotheca dixiana | 76261 | UK | Westphalian | foliage | −23.0 | 21.4 |
| Neuropteris acuminata | RC2858 | South Yorkshire, UK | Westphalian B | foliage | −22.4 | 20.8 |
| N. flexuosa | 14254 | Gloucstershire, UK | Westphalian | foliage | −24.0 | 22.4 |
| N. macrophylla | 14272 | Somerset, UK | Westphalian D | foliage | −23.4 | 21.8 |
| N. tenuifolia | 14231 | UK | Westphalian | foliage | −23.3 | 21.7 |
| Neuropteris spp. | RC2976 | Somerset, UK | Westphalian D | foliage | −21.5 | 19.8 |
| Sphenopteris affinis | 5190 | Lothian, UK | Westphalian | foliage | −23.6 | 22.0 |
| S. obtusifolia | RC3335 | Staffordshire, UK | Westphalian | foliage | −25.3 | 23.8 |
| Tree ferns | ||||||
| Acitheca polymorpha | RC3080 | Somerset, UK | Westphalian D | foliage | −23.0 | 21.4 |
| Pecopteris arborescens | GSD3241 | Somerset, UK | Westphalian D | foliage | −21.5 | 19.8 |
| Coniferophytes | ||||||
| Cordaites principalis | RC2881 | South Yorkshire, UK | Westphalian B | foliage | −21.6 | 19.9 |
| B. Natural History Museum, London | ||||||
| Lycopsods | ||||||
| Brasilodendron pedoanus | v-230 | Rio Grande, Brazil | Permian | bark | −21.6 | 19.5 |
| B. pedoanus | v230 g | Rio Grande, Brazil | Permian | bark | −21.3 | 19.2 |
| Paikhoia | v-53069 | Russia | Permian | bark | −23.6 | 21.6 |
| Lepidodendropsis lissonia | v-25932 | Peru | Lower Carboniferous | bark | −23.0 | 20.5 |
| L. sekoidiensis | v-57018 | Ghana, West Africa | Lower Carboniferous | bark | −23.1 | 20.6 |
| Glossopterids | ||||||
| Glossopteris sp. | v-13459 | Buckley Island, Antarctica | Permian | foliage | −25.6 | 23.7 |
| Glossopteris sp. | v-52409 | Theron Mts, Antarctica | Permian | foliage | −23.0 | 21.0 |
| Glossopteris sp. | v-61704 | Antarctica | Permian | stem | −23.4 | 21.4 |
| Glossopteris sp. | — | Estcourt, South Africa | Permian | foliage | −23.5 | 21.5 |
| Glossopteris sp. | v-3132 | South Africa | Permian | foliage | −24.4 | 22.4 |
| Glossopteris sp. | v-7348 | South Africa | Permian | foliage | −24.1 | 22.1 |
| Glossopteris sp. | v-7126 | Nagpur, India | Permian | foliage | −24.8 | 22.9 |
| Glossopteris (hormonia?) | — | Tanzania, southern Africa | Permian | stem | −24.1 | 22.1 |
| Lidgettonia africana | v-34639 | South Africa | Permian | foliage | −23.3 | 21.3 |
| Lidgettonia | v-34637 | South Africa | Permian | stem | −24.4 | 22.4 |
| Lidgettonia | v-34637 | South Africa | Permian | foliage | −23.9 | 21.9 |
| Schzamann gondiscum | v-12948 | Zimbabwe, southern Africa | Permian | foliage | −24.6 | 22.7 |
| S. gondiscum | v-12948 | Zimbabwe, southern Africa | Permian | foliage | −24.5 | 22.6 |
| Vertibraria indica | v-24436 | Australia | Permian | stem | −24.9 | 23.0 |
| Vertebratia | v-7597 | Zimbabwe, southern Africa | Permian | stem | −23.9 | 21.9 |
| Gymnosperm woods | ||||||
| Anarcticoxylon preisty | v-13490 | Priestley Glacier, Antarctica | Permian | wood | −23.3 | 21.3 |
| Dadoxylon | v-20451 | Antarctica | Permian | wood | −22.7 | 20.7 |
| Dadoxylon | v-20451 | Antarctica | Permian | wood | −22.8 | 20.8 |
| Dadoxylon | v-20450 | South Victoria Island, Antarctica | Permian | wood | −22.9 | 20.9 |
| Gangamopteris sp. | v-61712 | East Antarctica | Permian | wood | −24.0 | 22.0 |
| unidentified sp. | v-20451 | Victoria Land, Antarctica | Permian | stem | −22.7 | 20.7 |
| unidentified sp. | v-52411 | Theron Mts, Antarctica | Permian | stem | −22.9 | 20.9 |
| unidentified sp. | v-13464 | Buckley Island, Antarctica | Permian | wood | −22.9 | 20.9 |
| unidentified sp. | v-11272 | Antarctica | Permian | stem | −23.0 | 21.0 |
| unidentified sp. | v-61728 | Droning Maud Land, E. Antarctica | Permian | stem | −23.6 | 21.6 |
| C. collections of S. McLoughlin | ||||||
| Glossopteris sp. | PCM 1-B | Prince Charles Mountains | Permian | leaf mats | −22.0 | 19.9 |
| Glossopteris sp. | PCM 2-B | Prince Charles Mountains | Permian | leaf mats | −23.1 | 21.1 |
| Australoxyon mondii | PCM 1-C | Prince Charles Mountains | Permian | wood | −21.4 | 19.3 |
| Glossopteris sp./A. mondii | PCM 15-B | Prince Charles Mountains | Permian | leaves and wood | −21.6 | 19.5 |
| Glossopteris sp./A. mondii | PCM 9-A | Prince Charles Mountains | Permian | leaves and wood | −22.2 | 20.1 |
| charcoal | PCM 1-A | Prince Charles Mountains | Permian | wood | −22.9 | 20.9 |
| charcoal | PCM 15-A | Prince Charles Mountains | Permian | wood | −21.5 | 19.4 |
| charcoal | PCM 2-A | Prince Charles Mountains | Permian | wood | −21.3 | 19.2 |
| Glossopteris sp./A. mondii | HV6-133 | Homevale Station, Bowen Basin | Permian | leaves and wood | −22.3 | 20.3 |
| Araucarioxylon sp. | HV6-154 | Homevale Station, Bowen Basin | Permian | wood | −23.4 | 21.4 |
| Glossopteris sp./Araucarioxylon | HV2-140 | Homevale Station, Bowen Basin | Permian | leaves and wood | −23.4 | 21.4 |
References
- Beerling D.J. Evolutionary responses of land plants to atmospheric CO2. In: Ehleringer J.R, Cerling T.E, Dearing D.M, editors. A history of atmospheric CO2 and its effects on plants, animals and ecosystems. Ecological studies. vol. 177. Springer; Berlin: 2005. pp. 114–132. [Google Scholar]
- Beerling D.J, Woodward F.I. Modelling the first 400 million years. Cambridge University Press; Cambridge, UK: 2001. Vegetation and the terrestrial carbon cycle. [Google Scholar]
- Bekker A, Holland H.D, Wang P.L, Rumble D, Stein H.J, Hannah J.L, Coetzee L.L, Beukes N.J. Dating the rise of atmospheric oxygen. Nature. 2004;427:117–120. doi: 10.1038/nature02260. doi:10.1038/nature02260 [DOI] [PubMed] [Google Scholar]
- Berner R.A. CO2 and O2. Oxford University Press; Oxford, UK: 2005. The Phanerozoic carbon cycle. [Google Scholar]
- Björkman O. Comparative photosynthetic CO2 exchange in higher plants. In: Hatch M.D, Osmond C.B, Slayter R.O, editors. Photosynthesis and photorespiration. Academic Press; San Diego, CA: 1971. pp. 18–32. [Google Scholar]
- Bocherens H, Friis E.M, Mariotti A, Pedersen K.R. Carbon isotopic abundances in Mesozoic and Cenozoic fossil plants. Palaeontological implications. Lethaia. 1994;26:347–358. [Google Scholar]
- Bond W.J, Woodward F.I, Midgley G.F. The global distribution of ecosystems in a world without fire. New Phytol. 2005;165:525–538. doi: 10.1111/j.1469-8137.2004.01252.x. doi:10.1111/j.1469-8137.2004.01252.x [DOI] [PubMed] [Google Scholar]
- Briggs J.M, Knapp A.K, Brock B.L. Expansion of woody plants in a tallgrass prairie: a fifteen-year study of fire and fire-grazing interactions. Am. Midl. Nat. 2002;147:287–294. [Google Scholar]
- Caldeira K, Kasting J.F. The life-span of the biosphere. Nature. 1992;360:721–723. doi: 10.1038/360721a0. doi:10.1038/360721a0 [DOI] [PubMed] [Google Scholar]
- Cerling T.E. Palaeorecords of C4 plants and ecosystems. In: Sage R.F, Monson R.k, editors. C4 plant biology. Academic Press; San Diego, CA: 1999. pp. 445–469. [Google Scholar]
- Cerling T.E, Harris J.M, MacFadden B.J, Leakey M.G, Quade J, Eisenmann V, Ehleringer J.R. Global vegetation change through the Miocene/Pliocene boundary. Nature. 1997;389:153–158. doi:10.1038/38229 [Google Scholar]
- Chaloner W.G, Lacey W.S. The distribution of late Palaeozoic floras. Spec. Pap. Palaeontol. 1973;12:271–289. [Google Scholar]
- Conway-Morris S. Inevitable humans in a lonely universe. Cambridge University Press; Cambridge, UK: 2003. Life's solution. [Google Scholar]
- Crowell J.C. Pre-Mesozoic ice ages: their bearing on understanding the climate system. vol. 192. Geological Society of America Memoir; Boulder, Colorado: 1999. [Google Scholar]
- Crowley T.J, Berner R.A. CO2 and climate change. Science. 2001;292:870–872. doi: 10.1126/science.1061664. doi:10.1126/science.1061664 [DOI] [PubMed] [Google Scholar]
- Currie B.S, Rowley D.B, Tabor N.J. Middle Miocene paleoaltimetry of southern Tibet: implications for the role of mantle thickening and delamination in the Himalayan orogen. Geology. 2005;33:181–184. doi:10.1130/G21170.1 [Google Scholar]
- Dettman D.L, Kohn M.J, Quade J, Ryerson F.J, Ojha T.P, Hamidullah S. Seasonal stable isotope evidence for a strong Asian monsoon throughout the past 10.7 m.y. Geology. 2001;29:31–34. doi:10.1130/0091-7613(2001)029<0031:SSIEFA>2.0.CO;2 [Google Scholar]
- DiMichele W.A, Pfefferkorn H.W, Gastaldo R.A. Response of late Carboniferous and early Permian plant communities to climate change. Ann. Rev. Earth Planet. Sci. 2001;29:461–487. doi:10.1146/annurev.earth.29.1.461 [Google Scholar]
- Ding Z.L, Yang S.L. C3/C4 vegetation evolution over the last 7.0 Myr in the Chinese Loess Plateau: evidence from pedogenic carbonate δ13C. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2000;160:291–299. doi:10.1016/S0031-0182(00)00076-6 [Google Scholar]
- Dugas D.P, Retallack G.J. Middle Miocene fossil grasses from Fort Ternan. Kenya. J. Paleon. 1993;67:113–128. [Google Scholar]
- Duvall M.R, Noll J.D, Minn A.H. Phylogenetics of Paniceae (Poaceae) Am. J. Bot. 2001;88:1988–1992. [PubMed] [Google Scholar]
- Duvall M.R, Saar D.E, Grayburn W.S, Holbrook G.P. Complex transitions between C3 and C4 photosynthesis during the evolution of Paniceae: a phylogenetic case study emphasizing the position of Steinchisma hians (Poaceae), a C3-C4 intermediate. Int. J. Plant Sci. 2003;164:949–958. doi:10.1086/378657 [Google Scholar]
- Ehleringer J, Björkman O. Quantum yields for CO2 uptake in C3 and C4 plants. Dependence on temperature, CO2, and O2 concentration. Plant Physiol. 1977;59:86–90. doi: 10.1104/pp.59.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehleringer J.R, Sage R.F, Flanagan L.B, Pearcy R.W. Climate change and the evolution of C4 photosynthesis. TREE. 1991;6:95–99. doi: 10.1016/0169-5347(91)90183-X. [DOI] [PubMed] [Google Scholar]
- Ehleringer J.R, Cerling T.E, Helliker B.R. C4 photosynthesis, atmospheric CO2, and climate. Oecologia. 1997;112:285–299. doi: 10.1007/s004420050311. doi:10.1007/s004420050311 [DOI] [PubMed] [Google Scholar]
- Farquhar G.D. On the nature of carbon isotope discrimination in C4 species. Aus. J. Plant Physiol. 1983;10:205–226. [Google Scholar]
- Fox D.L, Koch P.L. Tertiary history of C4 biomass in the Great Plains, USA. Geology. 2003;31:809–812. doi:10.1130/G19580.1 [Google Scholar]
- Fox D.L, Koch P.L. Carbon and oxygen isotope variability in Neogene paleosol carbonates: constraints on the evolution of the C4-grasslands of the Great Plains, USA. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2004;207:S305–S329. doi:10.1016/S0031-0182(04)00045-8 [Google Scholar]
- Furumoto T, Hata S, Izui K. Isolation and characterization of cDNAs for differentially accumulated transcripts between mesophyll cells and bundle sheath strands of maize leaves. Plant Cell Physiol. 2000;41:1200–1209. doi: 10.1093/pcp/pcd047. doi:10.1093/pcp/pcd047 [DOI] [PubMed] [Google Scholar]
- Gaut B.S, Doebley J.F. DNA sequence evidence for the segmental allotetraploid origin of maize. Proc. Natl Acad. Sci. USA. 1997;94:6809–6814. doi: 10.1073/pnas.94.13.6809. doi:10.1073/pnas.94.13.6809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guissani L.M, Cota-Sánchez J.H, Zuloaga F.O, Kellogg E.A. A molecular phylogeny of the grass subfamily Panicoideae (Poaceae) shows multiple origins of C4 photosynthesis. Am. J. Bot. 2001;88:1993–2012. [PubMed] [Google Scholar]
- Hatch M.D. Mechanism and function of the C4 pathway of photosynthesis. In: Hatch M.D, Osmond C.B, Slayter R.O, editors. Photosynthesis and photorespiration. Academic Press; San Diego, CA: 1971. pp. 139–152. [Google Scholar]
- Hattersley P.W. The distribution of C3 and C4 grasses in Australia in relation to climate. Oecologia. 1983;57:113–128. doi: 10.1007/BF00379569. doi:10.1007/BF00379569 [DOI] [PubMed] [Google Scholar]
- Hattersley P.W. C4 photosynthetic pathway variation in grasses (Poaceae): its significance for arid and semi-arid lands. In: Chapman G.P, editor. Desertified grasslands: their biology and management. Academic Press; London: 1992. pp. 181–212. [Google Scholar]
- Hibberd J.M, Quick W.P. Characteristics of C4 photosynthesis in stems and petioles of C3 flowering plants. Nature. 2002;415:451–454. doi: 10.1038/415451a. doi:10.1038/415451a [DOI] [PubMed] [Google Scholar]
- Hoorn C, Ohja T, Quade J. Palynological evidence for vegetation development and climatic change in the Sub-Himalayan Zone (Neogene,Central Nepal) Palaeogeogr. Palaeoclimatol. Palaeoecol. 2000;163:133–161. doi:10.1016/S0031-0182(00)00149-8 [Google Scholar]
- Huang Y, Street-Perrott F.A, Metcalfe S.E, Brenner M, Moreland M, Freeman K.H. Climate change as the dominant control on glacial-interglacial variations in C3 and C4 plant abundance. Science. 2001;293:1647–1651. doi: 10.1126/science.1060143. doi:10.1126/science.1060143 [DOI] [PubMed] [Google Scholar]
- Jacobs B.F, Kingston J.D, Jacobs L.L. The origin of grass-dominated ecosystems. Ann. Missouri Bot. Gard. 1999;86:590–643. [Google Scholar]
- Janis C.M, Damouth J, Theodor J.M. Miocene ungulates and terrestrial primary productivity: where have all the browsers gone? Proc. Natl Acad. Sci. USA. 2000;97:7899–7904. doi: 10.1073/pnas.97.14.7899. doi:10.1073/pnas.97.14.7899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones T.P. 13C enriched lower Carboniferous fossil plants from Donegal. Ireland: carbon isotope constraints on taphonomy, diagenesis and palaeoenvironments. Rev. Pal. Pal. 1994;81:53–64. [Google Scholar]
- Kadereit G, Borsch T, Weising K, Freitag H. Phylogeny of Amaranthaceae and Chenopodiaceae and the evolution of C4 photosynthesis. Int. J. Plant Sci. 2003;164:959–986. doi:10.1086/378649 [Google Scholar]
- Kanai R, Edwards G.E. The biochemistry of C4 photosynthesis. In: Sage R.F, Monson R.K, editors. C4 plant biology. Academic Press; San Diego, CA: 1999. pp. 49–87. [Google Scholar]
- Keeley J.E, Rundel P.W. Evolution of CAM and C4 carbon-concentrating mechanisms. Int. J. Plant. Sci. 2003;164:S55–S77. doi:10.1086/374192 [Google Scholar]
- Keeley J.E, Rundel P.W. Fire and the Miocene expansion of C4 grasslands. Ecol Lett. 2005;8:683–690. doi:10.1111/j.1461-0248.2005.00767.x [Google Scholar]
- Kellogg E.A. The grasses: a case study in macroevolution. Ann. Rev. Ecol. Syst. 2000;31:217–238. doi:10.1146/annurev.ecolsys.31.1.217 [Google Scholar]
- Kellogg E.A. Evolutionary history of the grasses. Plant Physiol. 2001;125:1198–1205. doi: 10.1104/pp.125.3.1198. doi:10.1104/pp.125.3.1198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp A.K, Medino E. Success of C4 photosynthesis in the field: lessons from communities dominated by C4 plants. In: Sage R.F, Monson R.K, editors. C4 plant biology. Academic Press; San Diego, CA: 1999. pp. 251–283. [Google Scholar]
- Kuypers M.M. M, Pancost R.D, Damsté J.S. S. A large and abrupt fall in atmospheric CO2 concentration during Cretaceous times. Nature. 1999;399:342–345. doi:10.1038/20659 [Google Scholar]
- Larcher W. Photosynthesis as a tool for indicating temperature stress events. In: Schulze E.D, Caldwell M.M, editors. Ecophysiology of photosynthesis. Springer; Berlin: 1994. pp. 261–277. [Google Scholar]
- Latorre C, Quade J, McIntosh W.C. The expansion of C4 grasses and global change in the late Miocene: stable isotope evidence from the Americas. Earth Planet. Sci. Lett. 1997;146:83–96. doi:10.1016/S0012-821X(96)00231-2 [Google Scholar]
- Levin N.E, Quade J, Simpson S.W, Semaw S, Rogers M. Isotopic evidence for Plio-Pleistocene environmental change at Gona, Ethiopia. Earth Planet. Sci. Lett. 2004;219:93–110. doi:10.1016/S0012-821X(03)00707-6 [Google Scholar]
- Lloyd J, Farquhar G.D. 13C discrimination during CO2 assimilation by the terrestrial biosphere. Oecologia. 1994;99:201–215. doi: 10.1007/BF00627732. doi:10.1007/BF00627732 [DOI] [PubMed] [Google Scholar]
- Long S.P. C4 photosynthesis at low temperatures. Plant Cell Environ. 1983;6:345–363. [Google Scholar]
- Long S.P. Environmental responses. In: Sage R.F, Monson R.K, editors. C4 plant biology. Academic Press; San Diego, CA: 1999. pp. 215–249. [Google Scholar]
- MacFadden B.J, Cerling T.E, Harris J.M, Prado J. Ancient latitudinal gradients of C3/C4 grasses interpreted from stable isotopes of New World Pleistocene horse (Equus) teeth. Global Ecol. Biogeog. 1999;8:137–149. [Google Scholar]
- Maier-Reimer E, Mikolajewicz U, Crowley T.J. Ocean general circulation model sensitivity experiment with an open central American isthmus. Paleoceanography. 1990;5:349–366. [Google Scholar]
- McLoughlin S, Drinnan A.N. Revised stratigraphy of the Permian Bainmedart Coal Measures, northern Prince Charles Mountains, East Antarctica. Geol. Mag. 1997;134:335–353. doi:10.1017/S0016756897006870 [Google Scholar]
- Mikolajewicz U, Crowley T.J. Response of a coupled ocean/energy balance model to restricted flow through the central America isthmus. Paleoceanography. 1997;12:429–441. doi:10.1029/96PA03542 [Google Scholar]
- Monson R.K. The origins of C4 genes and evolutionary pattern in the C4 metabolic phenotype. In: Sage R.F, Monson R.K, editors. C4 plant biology. Academic Press; San Diego, CA: 1999. pp. 377–410. [Google Scholar]
- Nambuduri E.M.V, Tidwell W.D, Smith B.N, Hebbert N.P. A C4 plant from the Pilocene. Nature. 1978;276:816–817. doi:10.1038/276816a0 [Google Scholar]
- Ogle K. Implications of interveinal distance for quantum yield in C4 grasses: a modeling and meta-analysis. Oecologia. 2003;136:532–542. doi: 10.1007/s00442-003-1308-2. doi:10.1007/s00442-003-1308-2 [DOI] [PubMed] [Google Scholar]
- Osmond C.B. The absence of photorespiration in C4 plants: real or apparent? In: Hatch M.D, Osmond C.B, Slayter R.O, editors. Photosynthesis and photorespiration. Academic Press; San Diego, CA: 1971. pp. 472–482. [Google Scholar]
- Otto-Bliesner B.L. Continental drift, runoff, and weathering feedbacks: implications from climate model experiments. J. Geophys. Res. 1995;100:11537–11548. doi:10.1029/95JD00591 [Google Scholar]
- Otto-Bliesner B.L, Brady E.C, Shields C. Late Cretaceous ocean: coupled simulations with the National Centre for Atmospheric Research climate system model. J. Geophys. Res. 2000;107:4019. doi:10.1029/2001JD000821 [Google Scholar]
- Owensby C.E, Ham J.M, Knapp A.K, Auen L.M. Biomass production and species composition change in a tallgrass prairie ecosystem after long-term exposure to elevated atmospheric CO2. Global Change Biol. 1999;5:497–506. doi:10.1046/j.1365-2486.1999.00245.x [Google Scholar]
- Pagani M, Freeman K.H, Arthur M.A. Late Miocene atmospheric CO2 concentrations and the expansion of C4 grasses. Science. 1999;285:876–879. doi: 10.1126/science.285.5429.876. doi:10.1126/science.285.5429.876 [DOI] [PubMed] [Google Scholar]
- Pagani M, Zachos J, Freeman K.H, Tipple B, Boharty S. Marked decline in atmospheric carbon dioxide concentrations during the Paleogene. Science. 2005;309:600–603. doi: 10.1126/science.1110063. doi:10.1126/science.1110063 [DOI] [PubMed] [Google Scholar]
- Passey B.H, Cerling T, Perkins M, Voorhies M, Harris J, Tucker S. Environmental change in the Great Plains: an isotopic record from fossil horses. J. Geol. 2002;110:123–140. doi:10.1086/338280 [Google Scholar]
- Pearson P.N, Palmer M.R. Atmospheric carbon dioxide concentrations over the past 60 million years. Nature. 2000;406:695–699. doi: 10.1038/35021000. doi:10.1038/35021000 [DOI] [PubMed] [Google Scholar]
- Pearson P.N, Ditchfield P.W, Singano J, Harcourt-Brown K.G, Nicholas C.J, Olsson R.K, Shackleton N.J, Hall M.A. Warm tropical sea surface temperatures in the Late Cretaceous and Eocene epochs. Nature. 2001;413:481–487. doi: 10.1038/35097000. doi:10.1038/35097000 [DOI] [PubMed] [Google Scholar]
- Petit J.R, et al. Climate and atmospheric history of the past 420,000 years from the Vostok ice core, Antarctica. Nature. 1999;399:429–436. doi:10.1038/20859 [Google Scholar]
- Quade J, Cerling T.E. Stable isotopes in paleosols and the expansion of C4 grasses in the late Miocene of Northern Pakistan. Palaeogeogr. Palaeoclimatol. Palaeoecol. 1995;115:91–116. doi:10.1016/0031-0182(94)00108-K [Google Scholar]
- Quade J, Cater J.M. L, Ojha T.P, Adam J, Harrison T.M. Late Miocene environmental change in Nepal and the northern Indian subcontinent: stable isotope evidence from paleosols. GSA Bulletin. 1995;107:1381–1397. [Google Scholar]
- Raven J.A. Evolutionary options. Nature. 2002;415:375–376. doi: 10.1038/415375a. doi:10.1038/415375a [DOI] [PubMed] [Google Scholar]
- Royer D.L, Wing S.L, Beerling D.J, Jolley D.W, Koch P.L, Hickey L.J, Berner R.A. Paleobotanical evidence for near present-day levels of atmospheric CO2 during part of the Tertiary. Science. 2001;292:2310–2313. doi: 10.1126/science.292.5525.2310. doi:10.1126/science.292.5525.2310 [DOI] [PubMed] [Google Scholar]
- Royer D.L, Berner R.A, Montañez I.P, Tabor N.J, Beerling D.J. CO2 as a primary driver of Phanerozoic climate. GSA Today. 2004;14:4–10. doi:10.1130/1052-5173(2004)014<4:CAAPDO>2.0.CO;2 [Google Scholar]
- Rye R, Kuo P.H, Holland H.D. Atmospheric carbon dioxide concentrations before 2.2 billion years ago. Nature. 1995;378:603–605. doi: 10.1038/378603a0. doi:10.1038/378603a0 [DOI] [PubMed] [Google Scholar]
- Sage R.F. Environmental and evolutionary preconditions for the origin and diversification of the C4 photosynthesis syndrome. Plant Biol. 2001;3:202–213. doi:10.1055/s-2001-15206 [Google Scholar]
- Sage R.F. The evolution of C4 photosynthesis. New Phytol. 2004;161:341–370. doi: 10.1111/j.1469-8137.2004.00974.x. doi:10.1111/j.1469-8137.2004.00974.x [DOI] [PubMed] [Google Scholar]
- Sage R.F, Pearcy R.W. The physiological ecology of C4 photosynthesis. In: Leegood R.C, Sharkey T.D, von Caemmerer S, editors. Photosynthesis: physiology and metabolism. Kluwer; The Netherlands: 2000. pp. 497–532. [Google Scholar]
- Sage R.F, Wedin D.A, Li M. The biogeography of C4 photosynthesis: patterns and controlling factors. In: Sage R.F, Monson R.K, editors. C4 plant biology. Academic Press; San Diego, CA: 1999. pp. 313–373. [Google Scholar]
- Scotese C.R, McKerrow W.S. Revised world maps and introduction. In: McKerrow W.S, Scotese C.R, editors. Palaeozoic palaeogeography and biogeography. Geological Society Memoir No. 12. Geological Society of London; London: 1990. pp. 1–21. [Google Scholar]
- Scott L. Grassland development under glacial and interglacial conditions in southern Africa: review of pollen, phytolith and isotope evidence. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2002;177:47–57. doi:10.1016/S0031-0182(01)00351-0 [Google Scholar]
- Sinha N.R, Kellogg E.A. Parallelism and diversity in multiple origins of C4 photosynthesis in grasses. Am. J. Bot. 1996;83:1458–1470. [Google Scholar]
- Spicer R.A, Harris N.B. W, Widdowson M, Herman A, Guo S, Valdes P.J, Wolfe J.A, Kelly S.P. Constant elevation of southern Tibet over the past 15 million years. Nature. 2003;421:622–624. doi: 10.1038/nature01356. doi:10.1038/nature01356 [DOI] [PubMed] [Google Scholar]
- Steffen W, et al. A planet under pressure. Springer; Berlin: 2004. Global change and the Earth System. [Google Scholar]
- Strömberg C.A. E. Using phytolith assemblages to reconstruct the origin and spread of grass-dominated habitats in the great plains of North America during the late Eocene to early Miocene. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2004;207:239–275. doi:10.1016/S0031-0182(04)00043-4 [Google Scholar]
- Taub D.R. Climate and the US distribution of C4 grass subfamilies and decarboxylation variants of C4 photosynthesis. Am. J. Bot. 2000;87:1211–1215. [PubMed] [Google Scholar]
- Thomasson J.R, Nelson M.E, Zakrzewski R.J. A fossil grass (Gramineae: Chloridoideae) from the Miocene with Kranz anatomy. Science. 1986;233:876–878. doi: 10.1126/science.233.4766.876. [DOI] [PubMed] [Google Scholar]
- Valdes P.J, Crowley T.J. A climate model intercomparison for the Carboniferous. Palaeoclimates: Data Modelling. 1998;2:219–238. [Google Scholar]
- Veizer J, et al. 87Sr/86Sr, δ13C and δ18O evolution of Phanerozoic seawater. Chem. Geol. 1999;161:59–88. doi:10.1016/S0009-2541(99)00081-9 [Google Scholar]
- Veizer J, Godderis Y, François L.M. Evidence for the decoupling of atmospheric CO2 and global climate during the Phanerozoic eon. Nature. 2000;408:698–701. doi: 10.1038/35047044. doi:10.1038/35047044 [DOI] [PubMed] [Google Scholar]
- Vellinga M, Wood R.A. Global climatic impacts of a collapse of the Atlantic thermohaline circulation. Climatic Change. 2002;54:251–267. doi:10.1023/A:1016168827653 [Google Scholar]
- Voznesenskaya E.V, Franceschi V.R, Kiirats O, Freitag H, Edwards G.E. Kranz anatomy is not essential for terrestrial C4 plant photosynthesis. Nature. 2001;414:543–546. doi: 10.1038/35107073. doi:10.1038/35107073 [DOI] [PubMed] [Google Scholar]
- Watson A.J, Lovelock J.E, Margulis L. Methanogenesis, fires and the regulation of atmospheric oxygen. Biosystems. 1978;10:293–298. doi: 10.1016/0303-2647(78)90012-6. doi:10.1016/0303-2647(78)90012-6 [DOI] [PubMed] [Google Scholar]
- Wildman R.A, Hickey L.J, Dickinson M.B, Berner R.A, Robinson J.M, Dietrich M, Essenhigh R.H, Wildman C.B. Burning of forest materials under late Palaeozoic high O2 levels. Geology. 2004;32:457–460. doi:10.1130/G20255.1 [Google Scholar]
- Wright V.P, Vanstone S.D. Assessing the carbon dioxide content of ancient atmospheres using palaeocalcretes: theoretical and empirical constraints. J. Geol. Soc. 1991;148:945–947. [Google Scholar]
- Wyrich R, Dressen U, Brockmann S, Streubel M, Chang C, Qiang D, Paterson A.H, Westhoff P. The molecular basis of C4 photosynthesis in sorghum: isolation, characterization and RFLP mapping of mesophyll- and bundle-sheath-specific cDNAs obtained by differential screening. Plant Mol. Biol. 1998;37:319–335. doi: 10.1023/a:1005900118292. doi:10.1023/A:1005900118292 [DOI] [PubMed] [Google Scholar]
- Zachos J, Pagani M, Sloan L, Thomas E, Billups K. Trends, rhythms, and aberrations in global climate 65 Ma to present. Science. 2001;292:686–693. doi: 10.1126/science.1059412. doi:10.1126/science.1059412 [DOI] [PubMed] [Google Scholar]
- Zazzo A, Bocherens H, Brunet M, Beauvilian A, Billiou D, Taisso Mackaye H, Vignaud P, Mariotti A. Herbivore paleodiet and paleoenvironmental changes in Chad during the Pliocene using stable carbon isotope ratios of tooth enamel carbonate. Paleobiology. 2000;26:294–309. [Google Scholar]
- Zhisheng A, Kutzbach J.E, Prell W.L, Porter S.C. Evolution of Asian monsoons and phased uplift of the Himalaya–Tibetan plateau since Late Miocene times. Nature. 2001;411:62–66. doi: 10.1038/35075035. doi:10.1038/35075035 [DOI] [PubMed] [Google Scholar]