Abstract
Glutamate dehydrogenase (GDH) may be a stress-responsive enzyme, as GDH exhibits considerable thermal stability, and de novo synthesis of the α-GDH subunit is induced by exogenous ammonium and senescence. NaCl treatment induces reactive oxygen species (ROS), intracellular ammonia, expression of tobacco (Nicotiana tabacum cv Xanthi) gdh-NAD;A1 encoding the α-subunit of GDH, increase in immunoreactive α-polypeptide, assembly of the anionic isoenzymes, and in vitro GDH aminating activity in tissues from hypergeous plant organs. In vivo aminating GDH activity was confirmed by gas chromatorgraphy–mass spectrometry monitoring of 15N-Glu, 15N-Gln, and 15N-Pro in the presence of methionine sulfoximine and amino oxyacetic acid, inhibitors of Gln synthetase and transaminases, respectively. Along with upregulation of α-GDH by NaCl, isocitrate dehydrogenase genes, which provide 2-oxoglutarate, are also induced. Treatment with menadione also elicits a severalfold increase in ROS and immunoreactive α-polypeptide and GDH activity. This suggests that ROS participate in the signaling pathway for GDH expression and protease activation, which contribute to intracellular hyperammonia. Ammonium ions also mimic the effects of salinity in induction of gdh-NAD;A1 expression. These results, confirmed in tobacco and grape (Vitis vinifera cv Sultanina) tissues, support the hypothesis that the salinity-generated ROS signal induces α-GDH subunit expression, and the anionic iso-GDHs assimilate ammonia, acting as antistress enzymes in ammonia detoxification and production of Glu for Pro synthesis.
INTRODUCTION
Most biotic and abiotic stresses signal the generation of reactive oxygen species (ROS), particularly O2·− and H2O2, both extra- and intracellularly, by deregulating electron transport in chloroplasts and mitochondria and by activation of the plasma membrane–bound NADPH oxidases, the cell wall–bound NAD(P)H oxidase-peroxidase, and possibly the amine oxidases (Apel and Hirt, 2004; Mittler et al., 2004; Papadakis and Roubelakis-Angelakis, 2005; Paschalidis and Roubelakis-Angelakis, 2005b). ROS have been viewed as toxic by-products of cellular metabolism; however, a growing body of evidence suggests that they function as signaling molecules in eukaryotes, leading to specific downstream responses (Mittler et al., 2004 and references therein). The activation of two of the Arabidopsis thaliana mitogen-activated protein kinases, MPK3 and MPK6, is essential for signal transduction in response to H2O2 (Kovtun et al., 2000). The OXI1 kinase acts as a ROS sensor in plants to activate MPK3 and MPK6 (Rentel and Knight, 2004), and OMTK1 plays a mitogen-activated protein kinase scaffolding role and activates H2O2-induced cell death in plants (Nakagami et al., 2004). Hydrogen peroxide produced directly or by superoxide dismutase (SOD) induces expression of antioxidant genes, such as ascorbate peroxidase and catalase (CAT), which act toward detoxification of ROS (Apel and Hirt, 2004 and references therein). Efficient scavenging of ROS plays a significant role in the osmotic stress tolerance of plants, although the exact contribution in the response to abiotic stresses remains unknown (Hasegawa et al., 2000). Furthermore, there is substantial evidence that the expression and functioning of many proteins depend on redox state (Foyer and Noctor, 2005).
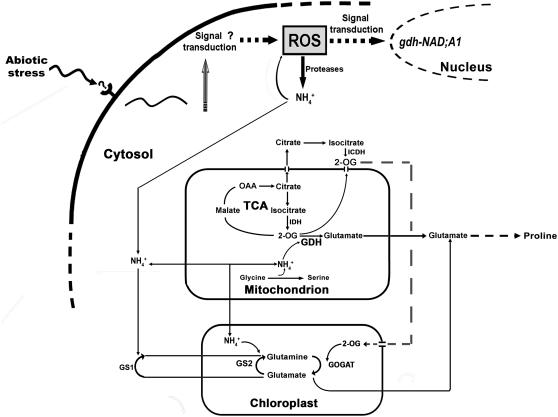
Under stress conditions, such as salinity, increased proteolytic activity results in increased intracellular hyperammonia and toxicity if not efficiently removed (Lutts et al., 1999). Ammonium ions are incorporated into Gln and Glu by glutamine synthetase/glutamate synthase (GS/GOGAT; EC 6.3.1.2/EC 1.4.7.1, respectively; Lea and Miflin, 1974; Figure 1). In addition, glutamate dehydrogenase (GDH) (EC 1.4.1.2) catalyzes the reductive amination of 2-oxoglutarate (2OG) and the oxidative deamination of Glu in vitro and is abundant in plant tissues (Loulakakis and Roubelakis-Angelakis, 1990a, 1990b; Melo-Oliveira et al., 1996; Turano et al., 1997; Restivo, 2004; Figure 1). The GDH holoenzyme has a hexameric structure consisting of two subunit polypeptides, α and β, with small but distinct differences in their molecular mass and charge. The two subunits are associated in an ordered ratio so that isoenzymes 1 and 7 are homohexamers of the β- and α-subunits, respectively (Loulakakis and Roubelakis-Angelakis, 1991, 1992; Turano et al., 1997). The two GDH polypeptides are encoded by distinctive genes (for review, see Purnell et al., 2005). The exact physiological roles of GDH in plant carbon and nitrogen metabolism and remobilization in plants remain largely speculative (reviewed in Miflin and Habash, 2002; Stitt et al., 2002; Dubois et al., 2003). Intracellular hyperammonia due to either exogenous ammonium (Loulakakis and Roubelakis-Angelakis, 1992; Restivo, 2004; Tercé-Laforgue et al., 2004a), senescence-induced high proteolytic activities (Masclaux et al., 2000; Loulakakis et al., 2002; Tercé-Laforgue et al., 2004b), or abiotic stress (Lutts et al., 1999; Hoai et al., 2003) results in increased aminating GDH activity in vitro. Early in the season, in planta, fully developed and physiologically active leaves in the basal part of the shoot exhibit high GOGAT expression, protein accumulation, and activity, whereas GDH concentration and activity are low. Later during development, GS and GOGAT decrease in the senescing leaves, accompanied by expression of gdh-NAD;A1, GDH protein accumulation, and increased in vitro aminating activity in tissues from senescing leaves (Masclaux et al., 2000; Loulakakis et al., 2002; Tercé-Laforgue et al., 2004a, 2004b). In salt-tolerant rice (Oryza sativa) cultivars, aminating GDH activity increases with increasing salt stress, whereas it decreases in the salt-sensitive ones (Kumar et al., 2000), and in pea (Pisum sativum) plants, an ammonium-tolerant plant, the aminating GDH activity in roots is high (Lasa et al., 2002).
Figure 1.
Potential Role of GDH under Stress Conditions.
Abiotic stresses result in ROS generation, inducing protease activity, increases in intracellular ammonium ions, and expression of gdh-NAD;A1, which participates, along with the GS/GOGAT pathway, in NH4+ assimilation. The supply of 2OG for Glu synthesis is provided by isocitrate deamination catalyzed by I(C)DHs. Glu synthesis under stress conditions by GDH is shifted to Pro synthesis. The enzymes involved in this metabolic scheme include the following: GS1, cytosolic glutamine synthetase; GS2, chloroplastic glutamine synthetase; IDH, mitochondrial isocitrate dehydrogenase; and ICDH, cytosolic isocitrate dehydrogenase.
A plausible hypothesis tested in this work is that GDH is a stress-responsive protein that may reflect an additional/alternative route to the GS/GOGAT pathway for ammonia assimilation under intracellular hyperammonia conditions. This hypothesis was extensively tested under salinity using whole plants and various in vitro models, such as cells, calluses, and leaf discs, from two plant species, tobacco (Nicotiana tabacum cv Xanthi) and grapevine (Vitis vinifera cv Sultanina). ROS generated by salt, menadione (Hassan and Fridovich, 1979), and ammonium ions induced the expression of the gene encoding the α-subunit of GDH (gdh-NAD;A1), the accumulation of immunoreactive GDH-α-subunit, the anionic GDH isoenzymes, and, in vitro, aminating GDH activities. This effect was reversed in the presence of ascorbate, suggesting that ROS act as the primary signals for gdh-NAD;A1 expression. Gas chromatography–mass spectrometry (GC-MS) analysis of the incorporation of 15NH4+ into 15N-Glu, 15N-Gln, and 15N-Pro in the presence of methionine sulfoximine (MSX), an inhibitor of GS, and amino oxyacetic acid (AOA), an inhibitor of transaminases, supports the aminating role of the anionic GDH isoenzymes and the shift of Glu toward Pro synthesis. The increased aminating activity of GDH in the presence of salt was accompanied by upregulation of the genes encoding for isocitrate dehydrogenases [I(C)DHs; EC NAD: 1.1.1.41; NADP: 1.1.1.42], which provide 2OG (Figure 1). Overall, these results firmly support the antistress physiological role and the aminating activity of the anionic isoenzymes of GDH, since gene expression, protein accumulation, and in vivo aminating activity were induced by salinity in a dose–response and time-response manner.
RESULTS
Addition of NaCl to Suspension Cells and Leaf Discs Resulted in Accumulation of ROS, DNA Fragmentation, and Induction of Programmed Cell Death
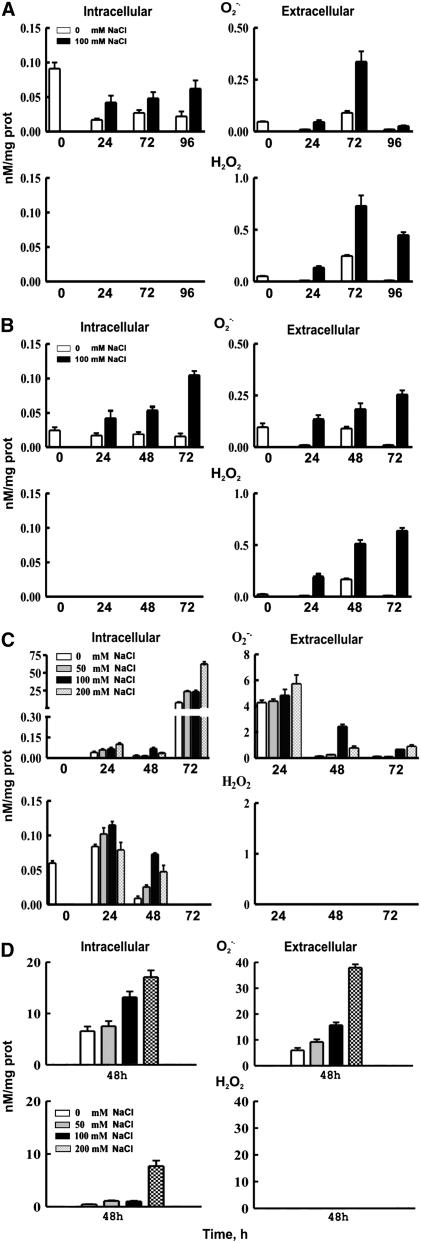
The intracellular and extracellular accumulation of O2·− and H2O2 was followed over a 96-h period with lucigenin- and luminol-dependent chemiluminescence assays, respectively, in grapevine suspension cells and leaf discs and tobacco Bright Yellow 2 (BY-2) cells and leaf discs supplemented with 100 mM NaCl. In grapevine cell suspensions, O2·− accumulated both intra- and extracellularly (Figure 2A; P = 0.05). The intracellular O2·− increased during the entire culture period; at 96 h, the content was fourfold greater compared with zero time and 2.8-fold greater compared with the control. The extracellular O2·− reached a peak at 72 h, which was sevenfold higher than at zero time (P = 0.05). On the other hand, H2O2 accumulated only extracellularly at 24, 48, and 72 h (Figure 2A; P = 0.05); at 72 h, it was 14.6-fold greater compared with zero time and threefold greater compared with the control. The accumulation of ROS at 50 mM NaCl showed a similar profile, whereas 200 mM NaCl resulted in significant reduction of cell viability (see Supplemental Figure 1 online). Accumulation of ROS in BY-2 tobacco suspension cells cultured in the presence of NaCl followed similar trends (Figure 2B).
Figure 2.
NaCl Induction and Accumulation of ROS in Grapevine and Tobacco.
(A) Generation of ROS in V. vinifera cv Sultanina suspension cells.
(B) Generation of ROS in N. tabacum BY-2 suspension cells.
(C) Generation of ROS in V. vinifera cv Sultanina leaf discs.
(D) Generation of ROS in N. tabacum leaf discs.
The cells were treated with 100 mM NaCl and the leaf discs with 0, 50, 100, and 200 mm NaCl for 72 h. ROS were determined 24 h after treatment as described in Methods. Data are the means of three replicates out of three independent experiments, and bars represent ±se.
In addition, the accumulation of O2·− and H2O2, intracellularly and extracellularly, in the culture medium of grapevine leaf discs supplemented with 0, 50, 100, and 200 mm NaCl was followed over a 72-h period (Figure 2C). Superoxide ions accumulated intracellularly, with maximum levels at 72 h, and extracellularly, with the maximum at 24 h (Figure 2C; P = 0.05). Hydrogen peroxide accumulated intracellularly, with maximum values at 24 h (Figure 2C; P = 0.05), whereas it was below detection limits in the culture medium. In tobacco leaf discs treated with NaCl for 24 h, O2·− accumulated in a dose–response manner, both intra- and extracellularly, while H2O2 accumulated only intracellularly (Figure 2D; P = 0.05). These results confirm that the different model systems respond to NaCl in a similar manner with respect to the generation and accumulation of ROS. The lack of appreciable intracellular amounts of H2O2 in cell suspensions but not in leaf discs can be attributed to the diffusion of H2O2 into the culture medium. Although CAT increased in leaf discs after 48 h, it was not sufficient to scavenge the generated H2O2 (see Supplemental Figure 2 online).
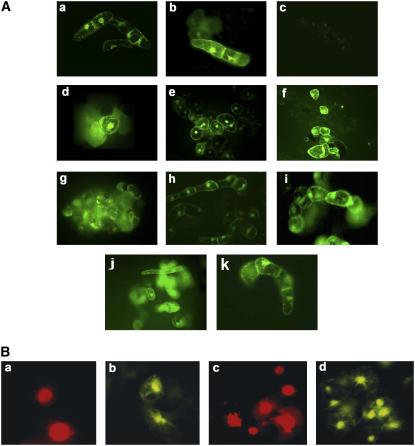
For further confirmation, in situ ROS in tobacco BY-2 (Figure 3A) and grapevine cells (Figure 3A) were detected after treatment with 200 mM NaCl using the highly sensitive, cell-permeable probe 2′,7′-dichlorodihydrofluorescein diacetate (DCFH-DA) according to Schopher et al. (2001). Salt-treated BY-2 and grapevine cell cultures accumulated ROS as early as 2 h after treatment (Figures 3Ab and 3Af). Treatment of BY-2 cells with 200 mM NaCl resulted in DNA fragmentation, a symptom of programmed cell death (PCD), as assessed by the TdT-mediated dUTP nick-end labeling (TUNEL) method, which labels free 3′-OH groups of DNA; 24 h after treatment, ∼40% of the cells were stained positively in the TUNEL assay (Figures 3Bc and 3Bd).
Figure 3.
Effect of NaCl, Menadione, NH4+, and MSX on ROS Accumulation and Induction of PCD in Tobacco BY-2 and Grapevine Suspension Cells.
(A) In situ ROS detection in control grapevine cells (a), grapevine cells treated with 200 mM NaCl for 2 h (b), grapevine cells treated with 200 mM NaCl and 10 mM ascorbate for 2 h (c), grapevine cells treated with 100 μM menadione for 2 h (d), control tobacco BY-2 cells for 2 h (e), tobacco BY-2 cells treated with 200 mM NaCl for 2 h (f), tobacco BY-2 cells treated with 100 μM menadione for 2 h (g), grapevine cells treated with 10 mM NH4+ for 2 h (h), grapevine cells treated with 10 mM NH4+ for 24 h (i), grapevine cells treated with 200 mM NaCl for 24 h (j), and grapevine cells treated with MSX for 2 h (k).
(B) Induction of the PCD syndrome: cells treated with 200 mM NaCl ([a] and [b]) and 50 μM menadione ([c] and [d]) after 24 h, propidium iodide ([a] and [c]), and the TUNEL assay ([b] and [d]).
In situ ROS detection and TUNEL assay were performed as described in Methods.
NaCl Resulted in Increased Intracellular Ammonium Ions, Expression of gdh-NAD;A1, Increased GDH Immunoreactive Protein and α-Subunit Polypeptide, Assembly of the Anionic Iso-GDHs, and Increased GDH in Vitro Aminating Activity
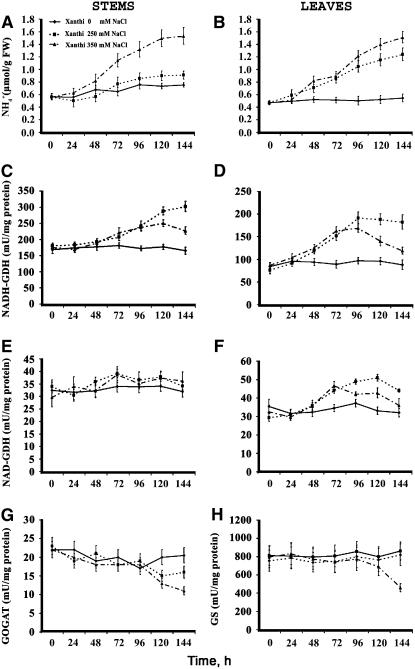
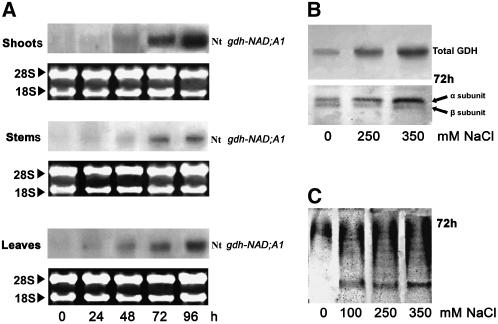
Culture of N. tabacum cv Xanthi plants in the presence of 0, 250, and 350 mM NaCl resulted in a gradual increase (up to threefold) of intracellular ammonium ions in shoots (see Supplemental Figure 3 online), stems, and leaves (Figures 4A and 4B) during a 6-d period. Concomitantly, the Nt gdh-NAD;A1 transcript (Figure 5A; at 250 mM NaCl and 72 h in shoots, stems, and leaves), the immunoreactive total GDH protein due to the α-GDH polypeptide (Figure 5B; at 250 mM NaCl and 72 h in shoots), the anionic iso-GDHs (Figure 5C; at 250 mM NaCl and 72 h in shoots), and the in vitro aminating GDH activity increased significantly in shoots (see Supplemental Figure 3 online), stems, and leaves (Figures 4C and 4D). The in vitro GDH deaminating activity in stems remained unchanged, while an increase (50%) was observed in leaves (Figures 4E and 4F). The abundance of Nt gdh-NAD;A1 transcript and GDH protein increased more than fourfold under NaCl treatment at 96 h in shoots as revealed by densitometric analysis (Figure 5B). The specific activities of GS/GOGAT did not change except at 144 h when they decreased in the leaves of plants treated with 350 mM NaCl (Figures 4G and 4H).
Figure 4.
Ammonium Ion Content and in Vitro Aminating and Deaminating Activities of GDH in Tobacco Plants Grown under Salinity Conditions.
(A) and (B) Endogenous free NH4+ content in stems and leaves of tobacco plants, respectively.
(C) and (D) In vitro aminating–specific activities of GDH (NADH-GDH) in stems and leaves, respectively.
(E) and (F) In vitro deaminating–specific activities of GDH (NAD-GDH) in stem and leaves, respectively.
(G) and (H) In vitro–specific activities of GOGAT and GS in leaves, respectively.
The plants were treated with 0, 250, and 350 mM NaCl for 6 d as described in Methods. Data are the means of three replicates out of four independent experiments, and bars represent ±se.
Figure 5.
Salinity-Induced Expression of GDH in Tobacco Plants.
(A) Abundance of Nt gdh-NAD;A1 transcript encoding for the α-subunit of GDH in shoots, stems, and leaves of N. tabacum cv Xanthi.
(B) Immunoreactive GDH protein and subunit polypeptides in tobacco shoots.
(C) Isoenzymic profile of GDH; assembly of the anionic iso-GDHs in tobacco shoots.
The plants were treated with 0, 250, and 350 mm NaCl for 6 d as described in Methods.
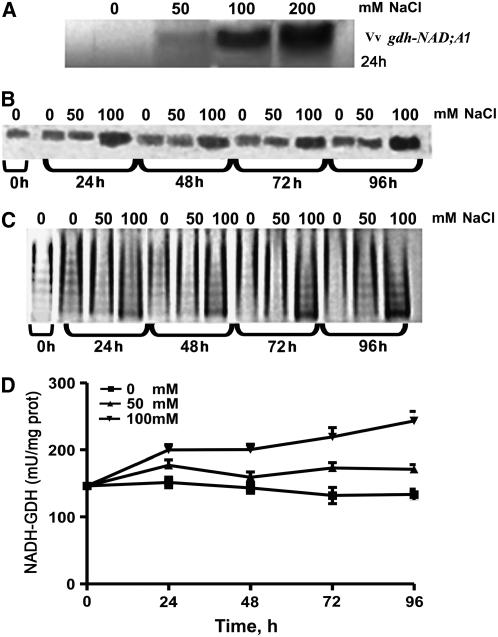
To assess the validity of the in planta results, several in vitro plant model systems were also used, such as grapevine suspension cells, calluses, and leaf discs as well as tobacco BY-2 cells and leaf discs. In grapevine suspension cells, NaCl induced expression of Vv gdh-NAD;A1, increase in immunoreactive GDH, assembly of the anionic isoenzymes, and increase in the in vitro aminating enzyme activity in a dose–response manner (Figures 6A to 6D, respectively), in accordance with the in planta results (Figures 4 and 5). Vv gdh-NAD;A1 transcript was 4.4-, 9.5-, and 11.8-fold more abundant at 50, 100, and 200 mM NaCl, respectively, at 24 h (Figure 6A). The immunoreactive GDH protein was maximum (3.7-fold) at 100 mM and 96 h (Figure 6B) compared with the control. The intensity of the anionic isoenzymes of GDH was evident at 24 h and continued increasing thereafter (Figure 6C). The GDH aminating–specific activity in vitro increased in a dose–response manner throughout the culture period, with the maximum value (1.8-fold greater compared with the control) at 100 mM and 96 h (Figure 6D). Similar results were obtained with tobacco BY-2 cells (see Supplemental Figure 4 online). Furthermore, results from tobacco leaf discs treated with NaCl (Figures 7A to 7C) were comparable to those obtained with tobacco plants (Figures 4 and 5), grapevine cells (Figure 6) and leaf discs (see Supplemental Figure 5 online), and tobacco BY-2 suspension cells (see Supplemental Figure 4 online) with respect to Vv gdh-NAD;A1 transcript, GDH immunoreactive protein, and GDH aminating–specific activity. These results validate the use of in vitro models, such as suspension cells, leaf discs, and calluses, to study molecular and biochemical responses of plants to abiotic stresses, such as salinity.
Figure 6.
Salinity-Induced Expression of GDH in Grapevine Cells.
(A) Abundance of Vv gdh-NAD;A1 transcript encoding for the α-subunit of GDH in V. vinifera suspension cells.
(B) Immunoreactive GDH protein.
(C) Isoenzymic profile of GDH; assembly of the anionic iso-GDHs.
(D) In vitro aminating GDH-specific activities.
The cells were treated with 0, 50, and 100 mm NaCl for 96 h as described in Methods. Data are the means of three replicates out of four independent experiments, and bars represent ±se.
Figure 7.
Salinity-Induced Expression of GDH in Tobacco Leaf Discs.
(A) Abundance of Nt gdh-NAD;A1 transcript encoding for the α-subunit of GDH in leaf discs of N. tabacum cv Xanthi.
(B) Immunoreactive GDH protein.
(C) In vitro aminating GDH-specific activity.
Leaf discs were treated with 0, 50, 100, and 200 mM NaCl for 72 h as described in Methods. Data are the means of three replicates out of three independent experiments, and bars represent ±se.
Menadione-Induced Accumulation of ROS, Increased Proteolytic Activity, Expression of gdh-NAD;A1, and Increase in GDH Aminating–Specific Activity in Cell Suspensions
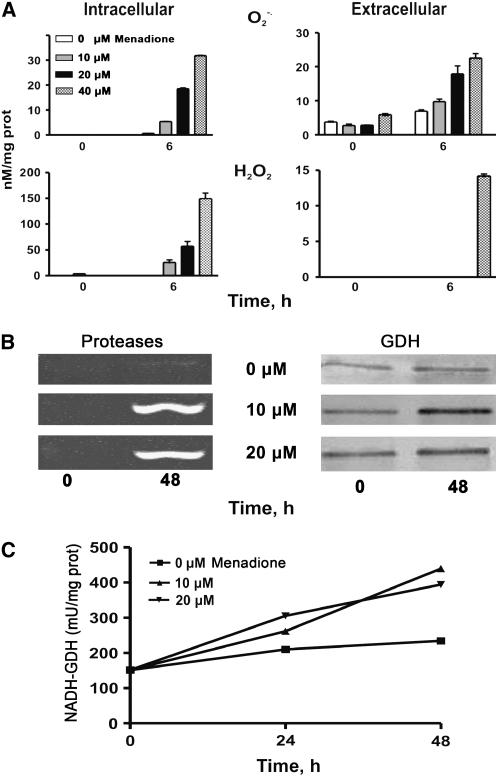
To further test whether in fact ROS signal the expression of the gdh-NAD;A1 gene, menadione was applied to grapevine suspension cell cultures. This compound is a vitamin known to uncouple electron transport in the mitochondria, resulting in the generation of O2·− (Hassan and Fridovich, 1979). In turn, O2·− is disproportionated by SOD, generating H2O2. At concentrations of 50, 100, and 200 μM menadione, the generated ROS were lethal for the suspension cells; the cells turned brown, and their viability decreased dramatically. A concentration of 100 μM menadione induced ROS accumulation (2 h after treatment) (Figure 3Ad), and concentrations of 10, 20, and 40 μM induced ROS accumulation in a dose–response manner, with a maximum at 6 h (Figure 8A). At 6 h after menadione treatment, the intracellular O2·− increased 1.3-, 2.5-, and 3.1-fold at 10, 20, and 40 μM menadione, respectively, and the extracellular O2·− increased 8.8-, 29.4-, and 49.2-fold, respectively, at the same menadione concentrations (Figure 8A). The increase in the intracellular H2O2 was analogous, but extracellularly it increased only at the highest menadione concentration (40 μm; Figure 8A). In situ detection of ROS with the DCFH-DA probe confirmed ROS accumulation in both grapevine and tobacco BY-2 cells (Figures 3Ad and 3Ag). In addition, menadione resulted in increased protease activity (Figure 8B, left).
Figure 8.
Effect of Menadione on ROS Generation and on the Expression of Proteases and GDH in Grapevine Cells.
(A) Intra- and extracellular generation and accumulation of O2·− and H2O2 in cells treated with 0 to 40 μM menadione.
(B) Expression of proteases (left) and immunoreactive GDH protein (right) in cells treated with 0 (top), 10 (middle), and 20 μM menadione (bottom).
(C) Aminating GDH-specific activity.
Bars represent ±se from four replicates.
Concomitantly, menadione resulted in increased GDH immunoreactive protein (Figure 8B, right) and aminating-specific activity in a dose–response manner (Figure 8C). The GDH immunoreactive protein increased 2.8- and 1.9-fold at 10 and 20 μM menadione, respectively, as revealed by densitometric analysis. Concentrations of 10 and 20 μM menadione resulted in 1.2- and 1.9-fold increases, respectively, in GDH-specific activity after 24 h, and in 2- and 2.6-fold increases at 48 h (Figure 8C). Addition of 10 mM ascorbate, a ROS scavenger, to grapevine cell cultures scavenged ROS completely (Figure 3Ac) and prevented the induction of GDH (see Supplemental Figure 6 online).
The Increased in Vitro GDH Aminating Activity Induced by NaCl was Reflected in the Incorporation of 15N-Ammonium into 15N-Glu, 15N-Gln, and 15N-Pro in the Presence of MSX and AOA
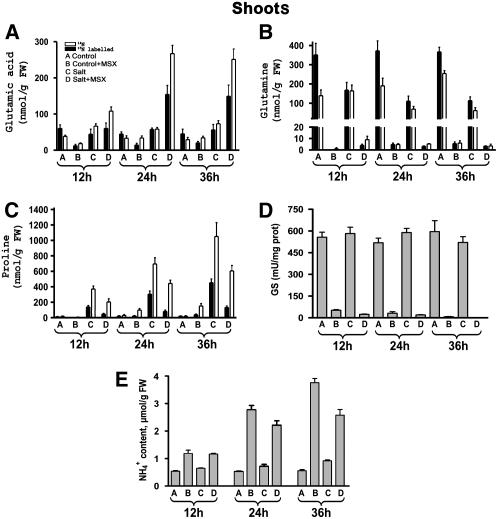
To confirm the validity of in vitro measurements of aminating activity of GDH, tobacco plants were supplied with 15NH4+, and GC-MS analyses of 15N-Glu, 15N-Gln, and 15N-Pro were performed at 12, 24, and 36 h (Figures 9A to 9C). Because expression of gdh-NAD;A1 was evident 24 h following salt treatment (Figures 5 to 7), plants were treated with 250 mM NaCl for 24 h prior to the addition of 15NH4+. 15N-Glu in the shoots of control and salt-treated plants in the presence of MSX at 36 h accounted for 45 and 250%, respectively, of 15N-Glu levels without MSX (Figure 9A). The corresponding nonlabeled Glu fractions accounted for 117 and 342%, respectively. Labeled and nonlabeled Gln were negligible in MSX-treated plants (Figure 4B), in agreement with the drastic reduction of GS (Figure 9D; P = 0.01). Treatment with 250 mM NaCl resulted in a time-dependent accumulation of 15N-Pro (Figure 9C, treatment C), reaching a fourfold increase after 36 h, compared with control plants (Figure 9C, treatment C). In the presence of MSX, 15N-Pro accumulation occurred at a lower rate, explaining the higher level of 15N-Glu (Figure 9C, treatment D; P = 0.01). Similar trends were observed for the nonlabeled Pro. Ammonium content in the shoots was consistent with the results obtained by the in vivo studies. Shoots from salt-treated plants contained higher NH4+ content (P = 0.05), increasing by 38% at 24 h and 66% at 36 h compared with control plants (Figure 9E). Salt-treated plants with MSX contained lower NH4+ content (P = 0.05), which corresponds to a 20 and 32% decrease at 24 and 36 h, respectively, compared with those treated with MSX (Figure 9E).
Figure 9.
GC-MS Analysis of the Fate of 15NH4 in Shoots of N. tabacum cv Xanthi Plants Grown in the Presence of NaCl.
Fractions of labeled and nonlabeled Glu (A), Gln (B), Pro (C), GS activity (D), and ammonium ion (E) accumulation in plants grown in 250 mM NaCl at 12, 24, and 36 h after 15NH4 application. Amino acids were extracted and characterized by GC-MS as described in Methods. Data are the means of four replicates, and bars represent ±se. FW, fresh weight.
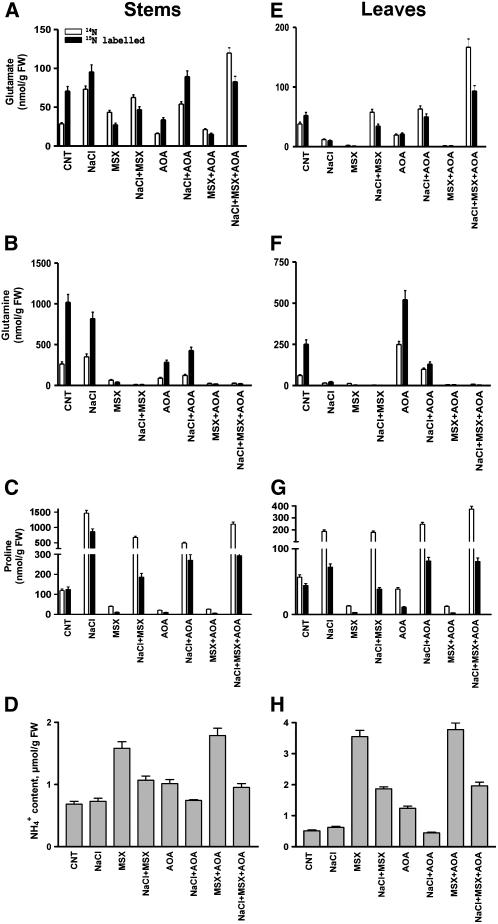
Additionally, we performed GC-MS analyses of stems and leaves separately from salt-treated tobacco plants in which 15NH4+ was applied 24 h after salt treatment (Figure 10). The residual 15N-Glu in stems and leaves of MSX-treated control plants was 38 and 5%, respectively, compared with control plants, 47 and 40% in the presence of AOA, and 21 and 3.5% in the presence of MSX plus AOA. In the stems of salt-treated plants plus MSX, 15N-Glu was 48% of that in salt-treated plants, while 15N-Glu was not affected by either AOA or MSX plus AOA; in addition, the nonlabeled Glu increased 1.6-fold more in the presence of inhibitors (plus salt) than without them (Figure 10A). Compared with salt-treated plants without inhibitors, the leaves of salt-treated plants with either MSX or AOA had higher values of 15N-Glu (P = 0.01), corresponding to 1.5- and 2.1-fold increases, respectively, whereas MSX plus AOA resulted in a 4.2-fold increase. The corresponding increases in nonlabeled Glu were 2.3- (+MSX), 2.5- (+AOA), and 6.9-fold (MSX+AOA). In the stems and leaves of MSX-treated plants, both 15N-Gln and nonlabeled Gln showed negligible values, whereas in AOA-treated plants 15N-Gln was 29 and 228% in stems and leaves, respectively, compared with controls. 15N-Pro in the control plants was low, whereas in salt-treated plants (250 mM), it increased significantly in the stems (8.1-fold) and in the leaves (4.5-fold). Copresence of inhibitors resulted in a 15N-Pro decrease (Figure 10C; P = 0.01). In the leaves of salt-treated plants plus MSX, 15N-Pro was 48% of the control (salt-treated plants), and it maintained at the same levels in AOA and MSX plus AOA treatments (Figure 10G; P = 0.01). By contrast, the amount of nonlabeled Pro increased 1.9-fold in the presence of MSX plus AOA when compared with salt-treated plants without inhibitors (Figure 10G). The effects of MSX, AOA, or a combination in the NaCl-treated plants further support the notion that when GS activity is diminished (see Supplemental Figure 7 online), a significant residual aminating activity is present, attributable to the aminating activity of the anionic-GDH isoenzymes. Overall, GDH in the stems exhibited greater in vivo aminating activity (Figure 10; P = 0.01), consistent with the localization of GDH in the companion cells of the phloem (Dubois et al., 2003). Furthermore, the endogenous levels of ammonium ions in the stems and leaves of NaCl-treated tobacco plants in the presence of MSX or AOA (Figures 10D and 10H), in combination with the GC-MS results (Figures 10A, 10E, 10C, and 10G), further support the NaCl-induced aminating activity of the anionic GDH isoenzymes.
Figure 10.
Fate of 15NH4 in Stems and Leaves of N. tabacum cv Xanthi Plants Grown in the Presence of NaCl.
Fractions of labeled (closed bars, 15N) and nonlabeled (open bars, 14N) Glu ([A] and [E]), Gln ([B] and [F]), Pro ([C] and [G]), and ammonium ion ([D] and [H]) accumulation in plants grown in 250 mM NaCl for 24 h with and without inhibitors (MSX and/or AOA). Note that in this experiment the plants were exposed for 24 h to salt treatment before supplying 15NH4. Amino acids were extracted and characterized by GC-MS as described in Methods. Data are the means of four replicates, and bars represent ±se.
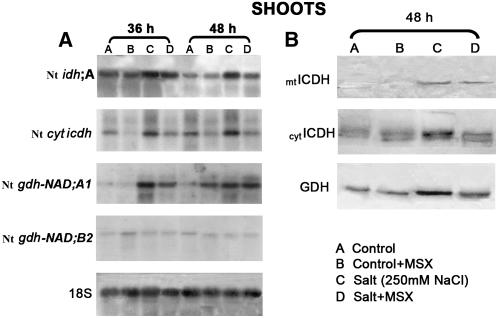
The Salt-Induced Increase in gdh-NAD;A1 Transcript Was Accompanied by Increase in Nt idh;A and Nt cyticdh Transcripts and Immunoreactive mtICDH and cytICDH Protein
I(C)DHs supply the cell with 2OG to form Glu (Figure 1; Lancien et al., 1999). It was of interest to test whether or not the increase in the transcript of Nt gdh-NAD;A1 and immunoreactive GDH protein brought about by NaCl (Figures 5A and 5B) was accompanied by similar trends in the transcript(s) and protein of I(C)DH. Thus, the abundance of Nt idh;A and Nt cyticdh transcripts (Gálvez et al., 1994) and the levels of immunoreactive mtICDH and cytICDH proteins were examined in the shoots of tobacco seedlings 36 and 48 h after salt treatment (250 mM NaCl) and with and without MSX. RNA gel blot analysis revealed that the increase in the Nt gdh-NAD;A1 transcript in the shoots of salt-treated plants (with and without MSX) at 36 h (Figure 11) was accompanied by a concomitant increase in the transcript of Nt idh;A (2.5- and 2-fold at 36 h and 6- and 2.2-fold at 48 h; Figure 11A) but not in the plants treated only with MSX (Figure 11A). With regard to Nt cyticdh, a 2.5- and 3-fold increase was observed only in the shoots of salt-treated plants at 36 and 48 h, respectively (Figure 11A). Protein gel blot analysis showed an increase in the levels of mtICDH at 48 h in salt-treated plants in the presence and absence of MSX (Figure 11B). Similarly, a 5.2- and 2-fold increase in the levels of cytICDH immunoreactive protein was found in salt-treated plants with and without MSX, respectively (Figure 11B).
Figure 11.
Effect of NaCl in the Presence/Absence of MSX on the Expression of gdh and icdh Genes in Tobacco Shoots.
(A) Expression of NAD-dependent Nt idh;A, cytocolic NADP-dependent Nt cytICDH, Nt gdh-NAD;A1 encoding for the α-subunit of GDH, and Nt gdh-NAD;B2 encoding for the β-subunit of GDH.
(B) Immunoreactive mtICDH, cytICDH, and GDH protein.
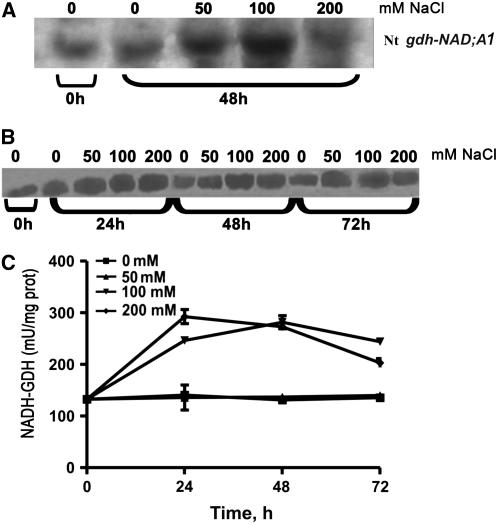
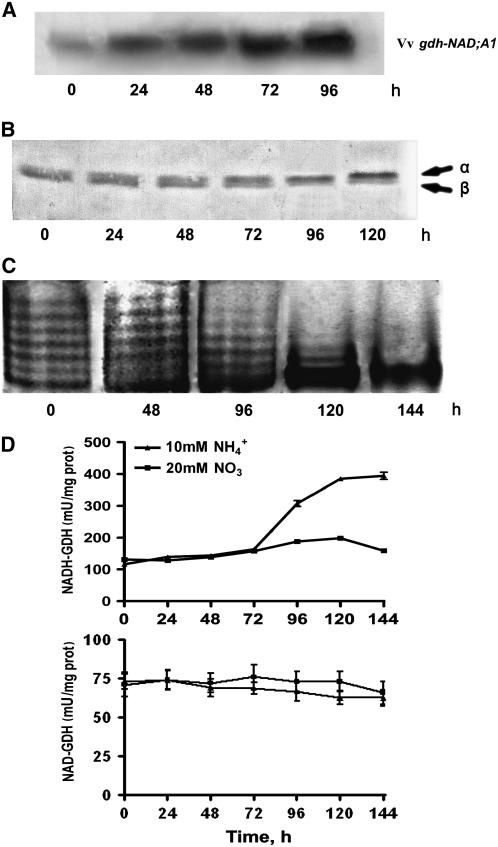
Exogenous Ammonium Ions Mirror the Effects of Salinity in Inducing the Expression of Vv gdh-NAD;A1, Increase in α-GDH Immunoreactive Protein, Assembly of the Anionic Iso-GDHs, and Increase in in Vitro GDH Aminating Activity
The response of V. vinifera suspension cells cultured in the presence of ammonium ions as a sole nitrogen source mirrored the effect of salinity and confirmed our previous results with grapevine calluses grown in the presence of ammonium ions as sole nitrogen source, in which the increase in aminating GDH activity was due to de novo synthesis of the α-GDH polypeptide and the assembly of the anionic GDH isoenzymes (Loulakakis and Roubelakis-Angelakis, 1992). Ammonium treatment resulted in induction of Vv gdh-NAD;A1 transcript, which continued throughout the entire culture period (Figure 12A); the respective increase was 3.5-, 4.5-, 7-, and 12-fold at 24, 48, 72, and 96 h, respectively. The immunoreactive GDH protein, corresponding to the α-subunit polypeptide, also increased 2-, 2.4-, and 3.5-fold at 72, 96, and 120 h (Figure 12B); after 48 h, the anionic GDH isoenzymes prevailed (Figure 12C), whereas the in vitro GDH aminating–specific activity reached a twofold increase at 144 h (Figure 12D, top). By contrast, the in vitro deaminating GDH-specific activity was not affected (Figure 12D, bottom).
Figure 12.
Effect of Exogenous Ammonium, as a Sole Nitrogen Source, on GDH Expression in V. vinifera Suspension Cells.
(A) Abundance of Nt gdh-NAD;A1 transcript encoding for the α-subunit of GDH.
(B) Immunoreactive α-GDH protein.
(C) Isoenzymic profile of GDH; assembly of the anionic iso-GDHs.
(D) In vitro aminating–specific activity GDH.
Suspension cells were treated with 10 mM NH4Cl or 20 mM KNO3 for 144 h as described in Methods. Bars represent ±se of three replicates out of three independent experiments.
In ammonium-treated cells, the levels of generated ROS were lower at 2 h compared with salt-treated ones (Figures 3Ah and 3Af). Ammonium treated cells for 24 h accumulated similar levels to those treated with salt at 2 h (Figures 3Ai and 3Af). At the same time (24 h), salt treated cells continued to induce further ROS accumulation, compared with ammonium treatment (Figures 3Aj and 3Ai). In addition, the increase in GDH immunoreactive protein and in vitro GDH aminating–specific activity was evident 72 and 96 h, respectively, after the treatment with ammonium (Figure 12), which is 48 h later than the salt response (Figure 6). Treatment of suspension cells with MSX resulted in lower accumulation of ROS (Figure 3Ai) compared with salt-treated cells, which is consistent with the lack of induction of GDH aminating–specific activity and immunoreactive protein (Figures 11A and 11B).
DISCUSSION
ROS generation is a central response of plant cells to stress. At low levels they are scavenged by enzymatic and nonenzymatic scavengers, such as CAT, SOD, ascorbate peroxidase, the Halliwell-Assada cycle enzymes, or numerous antioxidant molecules present in plant cells. Among them, Pro is a potent ROS scavenger associated with prevention of apoptotic-like PCD in Colletotrichum trifolii (Chen and Dickman, 2005). In addition, polyamines contribute to the homeostasis of ROS by inhibiting NAD(P)H-oxidase, preventing the induction of PCD (Papadakis and Roubelakis-Angelakis, 2005) and generating H2O2 via the catalytic action of polyamine oxidase (Paschalidis and Roubelakis-Angelakis, 2005b). ROS participate in the redox status of the cell, and redox signals are key regulators of responses to various stress conditions. For example, H2O2 induces the synthesis of heat shock proteins (Foyer et al., 1997; Foyer and Noctor, 2005). Moreover, ROS regulate the action of secondary messengers, such as Ca2+ (Foyer and Noctor, 2005). If not efficiently scavenged, ROS can have a detrimental effect(s) on metabolism and eventually on growth and development of cells through their ability to initiate reaction cascades, resulting in the production of toxic chemical species, such as hydroxyl radicals and lipid peroxides (Baier and Dietz, 2005; Rhoads et al., 2006). High levels of H2O2 trigger a distinct local sequence of events in gene expression that leads inevitably to PCD (Pastori and Foyer, 2002; Figure 3B).
ROS and the redox state of the cell participate in the signal network for the induction of expression of genes encoding effector proteins functioning toward increased stress tolerance (Mittler et al., 2004). Salt stress induces ROS generation in all in vitro model systems tested in this work, in agreement with other studies (Apel and Hirt, 2004 and references therein). Long-term treatment with ammonium ions in tobacco cells also elicited ROS generation, although to a lower extent compared with salt treatment (Figures 3Ai and 3Aj), and ammonium ions generated by Gln transamination induced the generation of O2·− in mammalian cells by upregulating the expression of the NADPH oxidase components (Pithon-Curi et al., 2002). Menadione, an elicitor of ROS, also increased proteases (Figure 8). Treatment of cells with H2O2 induced protease activity putatively responsible for the degradation of oxidatively damaged proteins and increased ammonia in the mitochondria (Sweetlove et al., 2001). Also, excessive intracellular accumulation of salt resulted in expression of proteases (Figure 8), leading to extensive proteolysis and deamination, which contributed to intracellular hyperammonia in rice (Lutts et al., 1999; Hoai et al., 2003) and Arabidopsis (Wong et al., 2004), as in stems and leaves of tobacco plants, grown under salt stress (Figures 4A and 4B).
Overall, in the in vitro plant models that were treated with either salt, ammonium ions, or menadione, ROS accumulation was accompanied by increased gdh-NAD;A1 transcript, immunoreactive GDH protein, anionic GDH isoenzymes, and in vitro GDH aminating activity, thus establishing a role for GDH in ROS sensing/responses. Cells treated with either ammonium or MSX exhibited lower ROS accumulation compared with salt-treated cells. In MSX-treated control plants, although ammonium accumulated, gdh-NAD;A1 was not induced (Figure 11), in contrast with the effect of exogenous ammonium in tobacco cell cultures (Figure 12). In salt-treated plants, gdh-NAD;A1 was induced with a 36-h lag phase, which was the duration of MSX monitoring, since a longer time resulted in damaged plant tissues. Therefore, in the MSX-treated plants, ammonium accumulation during the 36-h follow-up period was probably not sufficient to induce the threshold of ROS necessary for induction of gdh-NAD;A1. In addition, lower intracellular ammonia titers, intracellular ammonia compartmentation or translocation, and the source of ammonia (exogenous or photorespiratory) might differentiate the response of gdh-NAD;A1 in MSX-treated plants. Photorespiratory-produced ammonia in mitochondria is effectively reassimilated in the chloroplast by the action of the chloroplastic GS2, since in gs2 mutants of barley (Hordeum vulgare) (Lea and Forde, 1994), the cytosolic GS1 could not compensate for the loss of chloroplastic GS2. Furthermore, mitochondria possess an efficient defense antioxidant system that may prevent ROS accumulation and/or diffusion in the cytosol and, therefore, gdh-NAD;A1 expression (Rhoads et al., 2006). Recently, it was suggested that GDH in phloem companion cells may play a dual role in the cytosol when ammonium concentration increases above a certain threshold or in the mitochondria when mineral nitrogen availability is low (Tercé-Laforgue et al., 2004a). It can be inferred therefore that exogenous ammonium and its accumulation in the cytosol results in higher levels of ROS generation effective in the induction of gdh-NAD;A1. If ammonium were the principal signal and not ROS for gdh-NAD;A1 gene induction, then the response in salt-treated cells/calluses/leaf discs/plants would follow the timing of ammonium rather than that of ROS, which was not the case. For ammonium to induce gdh-NAD;A1, it has to reach an intracellular threshold before being able to generate sufficient levels of ROS to act as a signal. These results allow the proposition that ROS participate in the signaling pathway, and either alternatively or additively, they result in proteolysis contributing to intracellular hyperammonia.
Although GS/GOGAT are the main enzymes mediating the assimilation of ammonium in plant cells (Lea and Miflin, 1974; Figure 1), their activities in salt-treated tobacco shoots (stems and leaves) remained largely unchanged in contrast with the aminating activity of GDH, which increased. When photorespiratory ammonium accumulates as a result of impaired GOGAT activity (Ferrario-Mery et al., 2002) or when GS becomes limiting (Harrison et al., 2003), alternative metabolic pathways may be activated to avoid ammonium accumulation or that may be important in controlling the flux of nitrogen.
Because the in vitro aminating activities do not always reflect the in vivo function of the enzyme (Dubois et al., 2003; Maslaux-Daubresse et al., 2006; our unpublished data), GC-MS analyses were performed for monitoring the fate of 15NH4+. The aminating role of the anionic GDH isoenzymes acting toward ammonia detoxification was supported by the incorporation of 15NH4+ into 15N-Glu in salt-treated tobacco plants in the presence of MSX or AOA. In the presence of salt, 15N-Glu synthesis was significantly enhanced, and the sum of 15N-Glu plus 15N-Pro in the presence of MSX firmly supports the idea that the anionic isoenzymes of GDH, which consist mostly of the stress-induced α-subunit, do function in ammonia assimilation. Although the use of inhibitors may lead to conflicting findings, the relatively low in vivo amination of 15NH4+ found in MSX control plants eliminates the possibility of an artificial diversion of carbon and nitrogen to GDH. The reaction catalyzed by GDH could be one of the alternative pathways that may be induced when the ammonium concentration reaches a certain threshold (Dubois et al., 2003), and our results support this view.
The fact that the 15N-Glu produced in salt-treated plants was shifted toward 15N-Pro synthesis further supports the idea that these molecular responses are induced as a reaction to stress. The biosynthetic pathway of Pro from Glu is mediated by the enzymes P5CS and P5CR (Yoshiba et al., 1997). Pro accumulation in stressed plants has been associated with enhanced tolerance to conditions of abiotic stress (Nanjo et al., 1999a, 1999b). It also plays an important role in the protection of complex II in the mitochondria of roots of salt-treated plants (Hamilton and Heckathorn, 2001). Recent findings reveal that Pro functions as an antioxidant and inhibitor of PCD (Chen and Dickman, 2005). In addition to Pro synthesis, the ammonia detoxification effect of GDH links the entrance of Glu into the Krebs-Henseleit (urea) cycle and to polyamine biosynthesis (Paschalidis et al., 2001). Under hyperammonia, Glu is shifted to synthesize Orn, which is recycled via arginase as part of the urea cycle (Paschalidis and Roubelakis-Angelakis, 2005a) and may be the substrate for Pro synthesis. Both Orn and Arg are used for the biosynthesis of polyamines (Paschalidis and Roubelakis-Angelakis, 2005a, 2005b), which may also play a role in the protection of plants against abiotic stress (Capell et al., 2004).
As the induction of gdh-NAD;A1 by salt is accompanied by increased aminating GDH activity, it is expected that the genes encoding the enzymes that mediate 2OG synthesis should be upregulated as well. Two types of I(C)DH have been identified in plants that use different cofactors (Hodges, 2002). The first is IDH, a tricarboxylic acid cycle enzyme localized in the mitochondria of eukaryotic cells that use NAD. The second is ICDH, consisting of several isoenzymes, localized in the cytosol (Gálvez et al., 1996), the chloroplasts (Gálvez et al., 1994), the mitochondria (Gálvez et al., 1998), and the peroxisomes (Corpas et al., 1999). Both enzymes are potential candidates for supplying 2OG for the amination reactions of GOGAT and GDH. In rice plants treated with ammonium ions, IDH increased concomitantly with GOGAT, suggesting that it could serve to provide 2OG (Abiko et al., 2005). The increase in the Nt gdh-NAD;A1 transcript in the shoots of salt-treated plants was accompanied by a concomitant increase in the transcripts of Nt idh;A and Nt cyticdh and in the levels of mtICDH and cytICDH immunoreactive proteins. These results suggest that, in the presence of salt, both enzymes could supply 2OG for Glu synthesis by GDH.
Quantitative genetic approaches strongly suggest that the reaction catalyzed by NAD(H)-GDH is of major importance in the control of plant growth and productivity (Dubois et al., 2003). Compared with wild-type plants, transgenic tobacco overexpressing bacterial gdh (α-subunit of GDH) showed increased tolerance to herbicides and to toxic levels of ammonia as well as higher biomass (Ameziane et al., 2000). Plants transformed with the α- and β-subunits of GDH from Chlorella sorokiniana showed increased growth rates and improved stress tolerance (Schmidt and Miller, 1999), whereas a maize (Zea mays) GDH null mutant was cold temperature sensitive (Pryor, 1990). Fruits from transgenic tomato (Solanum lycopersicum) plants carrying a gene for NADP-GDH from Aspergillus nidulans contained twofold to threefold more free amino acids and twofold more Glu (Kisaka and Kida, 2003), which has been proposed as a signaling molecule in plant growth and environmental response (Glevarec et al., 2004). This idea is strongly supported by the fact that plants possess functional homologues of animal Glu receptors (Lam et al., 1998; Galili et al., 2001). Thus, consistent with its stress-related and signaling role, Glu content might be highly regulated and maintained at a constant level in plants (Glevarec et al., 2004).
Finally, the large amount of GDH activity in phloem companion cells and in the soluble fraction (Paczek et al., 2002; Dubois et al., 2003; Tercé-Laforgue et al., 2004a) opens new perspectives for elucidating the role of GDH in stress response. For instance, under abiotic stress conditions, such as salinity, the sorting of the protein to the mitochondria might be inhibited to allow its accumulation in the cytosol (Ficarelli et al., 1999).
Our results strongly support the hypothesis that plant cells cope with stress conditions by generating ROS, which signal the expression of the α-subunit of GDH, the assembly of the anionic iso-GDHs, and an increase in GDH aminating activity. As a result, GDH detoxifies the high intracellular ammonia that is generated by the proteolytic and deaminating activities. These data further reinforce the stress-related function of the anionic isoenzymes of GDH, suggest different physiological roles for the seven GDH isoenzymes, and present a new strategy that plant cells could employ to cope with abiotic stress. Studies of transgenic plants overexpressing gdh-NAD;A1 and gdh-NAD;B2 (Purnell et al., 2005) could contribute further to our understanding of the molecular mechanisms and signaling pathways of stress response in plants.
METHODS
Plant Material and Treatments
Nicotiana tabacum cv Xanthi seeds were surface-sterilized by immersion in 10% (v/v) NaClO for 10 min with gentle shaking. After washing thoroughly with sterile water, the seeds were placed in sterile half-strength Murashige and Skoog (MS) liquid medium (Murashige and Skoog, 1962). Plants were grown in a growth chamber at a 16/8-h photoperiod, 25°C, and 75% RH. Two weeks after emergence, seedlings were transferred in half-strength MS medium without sucrose and allowed to grow for another 2 weeks before the salt treatments (0, 250, and 350 mM NaCl).
Leaf discs (1 disc of 0.1 g mL−1) from glasshouse-grown Vitis vinifera cv Sultanina or tobacco plants were prepared according to Papadakis and Roubelakis-Angelakis (2005). Leaf discs were immersed in NaCl (0 to 200 mM) under continuous light (100 μmol photons m−2 s−1) for up to 72 h. Calluses derived from shoot explants of in vitro–grown grapevine plants were used for the establishment of a suspension cell culture as previously described (Primikirios and Roubelakis-Angelakis, 1999). Calluses were grown for three generations on MS medium containing 20 mM nitrate as the sole nitrogen source. TBY-2 cells (derived from N. tabacum cv BY-2) were cultured as previously described (de Pinto et al., 2000). Calluses, cell suspension cultures of grapevine, or BY-2 cells were treated with NaCl (0 to 200 mM) to monitor the NaCl effect or with 10 mM NH3Cl to study the effect of ammonium ions. Furthermore, cell suspension cultures were treated with 0, 10, 20, and 40 μM menadione for up to 48 h to monitor the effect of ROS.
Protein Extraction, Enzyme Assays, and Electrophoresis
Total proteins were extracted from cells, calluses, and leaf discs as previously described (Primikirios and Roubelakis-Angelakis, 1999; Papadakis et al. 2005). In brief, 0.2 M Tris-HCl, pH 8.0, 5 mM DTT, 0.5 mM PMSF, 10 μM leupeptin, 10% (w/v) glycerol, and 0.25% (w/v) Triton X-100 was used in the presence of 20% (w/v) polyvinylpolypyrrolidone. The samples were homogenized using a Polytron (Ultra Turrax T25, probe S15 N 10G; LabX) at a speed of 20,000 rpm. The ratio of sample:extraction buffer was 1:3 for cells and 1:4 for other tissues. The homogenates were centrifuged at 12,000 rpm for 30 min, and the supernatants were divided into aliquots and frozen at −80°C. All work was done at 4°C.
In vitro enzyme activity of GDH was determined as described previously (Loulakakis and Roubelakis-Angelakis, 1990a), and values are presented as units ± se. One unit of GDH is defined as the reduction of 1 μmol NADH per min at 30°C. Analytical and preparative SDS-PAGE of protein extracts was performed as described by Loulakakis and Roubelakis-Angelakis (1992). Transfer of electrophoretically resolved proteins onto nitrocellulose filters (0.2-μm pore size; Schleicher and Schuell) was performed according to Loulakakis and Roubelakis-Angelakis (1990b). GDH isoenzymes were localized in nondenaturating polyacrylamide gels using the in situ staining technique as analytically described by Loulakakis and Roubelakis-Angelakis (1991).
In vitro activity of NADH-GOGAT was assayed using NADH as the electron donor by determining the formation of Glu in the reaction as described by Matoh and Takahashi (1982). One unit of enzyme activity was defined as the amount catalyzing the formation of 1.0 μmol of Glu per minute at 30°C. GS activity was determined as described by Loulakakis and Roubelakis-Angelakis (1996). One unit of enzyme was defined as the amount that catalyzed the formation of 1.0 μmol of α-glutamyl hydroxamate per minute at 30°C.
For I(C)DH detection, 100 μg of crude extract protein was analyzed in 10% SDS-PAGE. cytICDH and mtICDH primary antibodies were used according to Gálvez et al. (1994).
RNA Gel Blot Analysis
Extraction of RNA from plant tissues was as described by Loulakakis et al. (1996). Total RNA (15 μg) was fractionated in 1% agarose-formaldehyde gel, blotted onto Hybond-N+ membrane (Amersham Pharmacia Biotech), and then hybridized with radiolabeled probes, under high-stringency conditions. Prehybridization, hybridization, and washing conditions were performed as described by Church and Gilbert (1984). Probes were labeled using a random hexamer oligonucleotide system (RadPrime DNA Labeling System; Invitrogen). The full-length cDNAs of Vv gdh-NAD;A1 (Syntichaki et al., 1996) and Nt gdh-NAD;B2 (accession number AY366370) from grapevine were used as probes. Moreover, the catalytic NAD-dependent Nt idh (accession number X96727) and the cytocolic NADP-dependent Nt cyticdh (accession number X77944) isocitrate dehydrogenase clones from N. tabacum were used (Gálvez et al., 1996). Hybridization and washing conditions were stringent to avoid cross-reaction with other subunits.
Protease Activity Assay
For protease activity assays, 20 μg of total protein were separated in 10% acrylamide gel containing 1 mg mL−1 gelatin (Lockwood et al., 1987). Electrophoresis was performed at 10 mA constant current for 2 h at 4°C. After electrophoresis, the resolving gel was incubated overnight at 37°C in 40 mM Tris-HCl, pH 8.6, and 0.2% Triton X-100. The gel was stained in Coomassie Brilliant Blue R 250 and destained, and the protease activity appeared as white bands.
Ammonia Extraction and Determination
Tissues were ground in liquid nitrogen and extracted in 10 volumes of 10 mM ice-cold formic acid. Free ammonium was determined fluorometrically according to Husted et al. (2000) following derivatization at 63°C with 3 mM O-phthalaldehyde and 10 mM 2-mercaptoethanol (in 0.2 M Tris-HCl, pH 6.8) and quantification in an HPLC system (HP 1100; Hewlett-Packard). Ammoniun concentration was also determined as the amount of NADH oxidized via the aminating reaction of GDH at 340 nm at room temperature in a total reaction volume of 0.5 mL consisting of 100 mM Tris, pH 8.0, 15 mM 2OG, 1 mM CaCl2, 1 mM NADH, and 2 units of GDH (1 mg protein equals 46 units; Sigma-Aldrich). Control assays were conducted by omitting either 2OG or GDH. The ammonium was quantified by preparing a standard curve with ammonium nitrate.
Chemiluminescence Assay for H2O2 and O2.−
The production of O2.− and H2O2 from V. vinifera cv Sultanina cell cultures was determined by the chemiluminescence assay of luminol and lucigenin, respectively, in cells and in the culture medium as already described (Papadakis and Roubelakis-Angelakis, 1999). Three independent experiments, with three replicates each, were performed. One-way analysis of variance was used for statistical analysis.
Incorporation of 15NH4+ into Nitrogenous Compounds in Tobacco Plants Grown in the Presence of NaCl
N. tabacum cv Xanthi plants (30 d after emergence) were used for the in vivo 15N assimilation analysis. Two sets of experiments were performed to confirm whether GDH is involved in the amination of 2OG to Glu.
In the first experiment, tobacco plants were transferred in distilled water and 250 mM NaCl (without nutrients) for 24 h before the addition of 15NH4+. Then the plants were transferred to half-strength MS solution with 20 mM 15NH3Cl, as a sole nitrogen source, and the incorporation of 15NH4+ into Glu, Gln, and Pro was assessed at 12, 24, and 36 h. For GS inhibition, plants were sprayed with 1.5 mM MSX solution of the potential GS inhibitor, and roots were immersed in the same solution for 10 min.
A second set of experiments was performed with a similar design to that described previously to study the response of different organs. Twenty-four hours following the addition of 15NH4+, the tobacco plants were separated into stems and leaves, and the enrichment of Glu, Gln, and Pro in isotopic nitrogen was determined by GC-MS. In addition to MSX, 1 μM AOA, an inhibitor of transaminases, was added to ensure that transaminations of Glu did not occur.
In all experiments, the plants were harvested and kept at −80°C. Tissue was extracted with 0.01 N HCl and 100 μL of the extraction solution and dried at 90°C under gas nitrogen. Subsequently, 100 μL of acetonitrile and 100 μL of N-methyl-N-(tert-butyldimethylsilyl)-trifluoroacetamide were added to the residue. The mixture was sonicated for 30 s, heated at 70°C for 30 min, and filtered. One-chlorohexadecane was added as internal standard, and 1 μL of the resulting solution was administered to GC-MS for amino acid determination (Sobolevsky et al., 2003). GC-MS analyses were performed using a Hewlett-Packard 5971A mass-selective detector with the appropriate data system. A Hewlett-Packard model 5890 gas chromatograph, equipped with a Grob-type split-splitless injector, was directly coupled with the fused-silica capillary column (HP-5 MS with 0.25-mm film, 30 m × 0.25 mm i.d.) to the ion source. Helium was used as the carrier gas with a back pressure of 0.8 Atm. The injector temperature was 280°C, and the oven temperature program started at 70°C, staying there 2 min, and then increased until 290°C with 5°C/min rate. At 290°C, it stayed for 10 min. Two independent experiments, with two replicates each, were performed. One-way analysis of variance was used for statistical analysis.
Epifluorescence Cytochemical Localization of H2O2
In situ localization of H2O2 was performed using the highly sensitive, cell-permeable probe DCFH-DA (Molecular Probes) according to Schopher et al. (2001). Cells were harvested, after centrifugation at 3000 rpm, and were incubated in 1 mL buffer (20 mM K-phosphate, pH 6.0, containing 50 μM DCHF-diacetate and 3 μg mL−1 horseradish peroxidase; Sigma-Aldrich) for 10 min at 25°C in darkness. An aliquot of cells (0.1 mL) was removed, washed in the same buffer, and visualized immediately. Pictures were taken with an epifluorescence microscope (Nikon Eclipse E800) with excitation filter EX 450-490 and emission filter BA 520 using a Sony DXC-950P camera.
Detection of DNA Fragmentation
Cell suspensions, cultured for 18 h in 200 mM NaCl or 40 μM menadione, were analyzed for nuclear DNA fragmentation as previously described (Papadakis and Roubelakis-Angelakis, 2005). Total cellular DNA was stained with propidium iodide (1 μg mL−1), and the free 3′-OH groups in DNA were labeled by the TUNEL method using an in situ cell death detection kit (Boehringer Mannheim) as instructed by the manufacturer. DNA was visualized with a Nikon Eclipse 800 fluorescence microscope.
Preparation of Figures
The Coomassie blue–stained polyacrylamide gels were photographed using a Kodak DC120 digital camera, and the densitometry was performed with Kodak Digital Science 1D image analysis software. Protein and RNA gel blots were scanned with an HP ScanJet 6100 scanner (Hewlett Packard).
Accession Numbers
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers AY366369 (Nt gdh-NAD;A1), X86924 (Vv gdh-NAD;A1), AY366370 (Nt gdh-NAD;B2), X77944 (Nt cytICDH), and X96727 (Nt idh;A).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure 1. Viability of Grapevine Cells Grown in 0, 50, 100, and 200 mM NaCl for 24 h.
Supplemental Figure 2. Salinity Induced the Expression of Catalase in Grapevine Leaf Discs Exposed to Salt Stress.
Supplemental Figure 3. Ammonium Content and in Vitro Aminating and Deaminating Activities of GDH and in Vitro–Specific Activities of GS and GOGAT in Tobacco Plants Grown under Salinity Conditions.
Supplemental Figure 4. Salinity Induced the Expression of GDH in BY-2 Cells Grown under Salinity Conditions.
Supplemental Figure 5. Salinity Induced the Expression of GDH in Grapevine Leaf Discs.
Supplemental Figure 6. Addition of Ascorbate (10 μM) Prevents Increase in in Vitro Aminating–Specific Activity of GDH in Grapevine Cells Exposed to Salinity Treatment (200 mM NaCl) for 24 h.
Supplemental Figure 7. In Vitro–Specific Activities of GS in Stems and Leaves of Tobacco Plants Grown in the Presence of MSX ± AOA for 24 h.
Supplementary Material
Acknowledgments
We thank L. Panagis and A. Dermon (University of Crete) for their advice and assistance in performing the TUNEL assay and the in situ staining of ROS. We also thank M. Hodges (University of Paris) for kindly offering I(C)DH clones and antibodies, M.C. de Pinto (Universita degli Studi di Bari, Italy) for kindly providing the BY-2 cells, and G. Iamine (USDA, Beltsville, MD) for providing the antibody against catalase. The project is cofunded by the European Social Fund and National resources, projects Herakleitos and Pythagoras, and was implemented in the frame of COST858.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantcell.org) is: Kalliopi A. Roubelakis-Angelakis (poproube@biology.uoc.gr).
Online version contains Web-only data.
References
- Abiko, T., Obara, M., Ushioda, A., Hayakawa, T., Hodges, M., and Yamaya, T. (2005). Localization of NAD-isocitrate dehydrogenase and glutamate dehydrogenase in rice roots: Candidates for providing carbon skeletons to NADH-glutamate synthase. Plant Cell Physiol. 46 1724–1734. [DOI] [PubMed] [Google Scholar]
- Ameziane, R.K., Bernhard, R.B., and Lightfoot, D. (2000). Expression of the bacterial gdhA gene encoding NADPH glutamate dehydrogenase in tobacco affects plant growth and development. Plant Soil 221 47–57. [Google Scholar]
- Apel, K., and Hirt, H. (2004). Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 55 373–399. [DOI] [PubMed] [Google Scholar]
- Baier, M., and Dietz, K.J. (2005). Chloroplasts as source and target of cellular redox regulation: A discussion on chloroplast redox signals in the context of plant physiology. J. Exp. Bot. 56 1449–1462. [DOI] [PubMed] [Google Scholar]
- Capell, T., Bassie, L., and Christou, P. (2004). Modulation of the polyamine biosynthetic pathway in transgenic rice confers tolerance to drought stress. Proc. Natl. Acad. Sci. USA 101 9909–9914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, C., and Dickman, M.B. (2005). Proline suppresses apoptosis in the fungal pathogen Colletotrichum trifolii. Proc. Natl. Acad. Sci. USA 102 3459–3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church, G.M., and Gilbert, W. (1984). Genomic sequencing. Proc. Natl. Acad. Sci. USA 81 1991–1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corpas, F.J., Barroso, J.B., Sandalio, L.M., Palma, J.M., Lupianez, J.A., and del Rio, L.A. (1999). Peroxisomal NADP-dependent isocitrate dehydrogenase. Characterization and activity regulation during natural senescence. Plant Physiol. 121 921–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Pinto, M.C., Tommasi, F., and de Gara, L. (2000). Enzymes of the ascorbate biosynthesis and ascorbate-glutathione cycle in cultured cells of tobacco Bright Yellow 2. Plant Physiol. Biochem. 38 541–550. [Google Scholar]
- Dubois, F., Tercé-Laforgue, T., Gonzalez-Moro, M.B., Estavillo, J.M., Sangwan, R., Gallais, A., and Hirel, B. (2003). Glutamate dehydrogenase in plants: Is there a new story for an old enzyme? Plant Physiol. Biochem. 41 565–576. [Google Scholar]
- Ferrario-Mery, S., Hodges, M., Hirel, B., and Foyer, C.H. (2002). Photorespiration-dependent increases in phosphoenolpyruvate carboxylase, isocitrate dehydrogenase and glutamate dehydrogenase in transformed tobacco plants deficient in ferredoxin-dependent glutamine-a-ketoglutarate aminotransferase. Planta 214 877–886. [DOI] [PubMed] [Google Scholar]
- Ficarelli, A., Tassi, F., and Restivo, F.M. (1999). Isolation and characterization of two cDNA clones encoding for glutamate dehydrogenase in Nicotiana plumbaginifolia. Plant Cell Physiol. 40 339–342. [DOI] [PubMed] [Google Scholar]
- Foyer, C.H., Lopez-Delgando, H., Dat, J.E., and Scott, I.M. (1997). Hydrogen peroxide- and glutathione-associated mechanisms of acclimatory stress tolerance and signaling. Physiol. Plant. 100 241–254. [Google Scholar]
- Foyer, C.H., and Noctor, G. (2005). Redox homeostasis and antioxidant signaling: A metabolic interface between stress perception and physiological responses Plant Cell 17 1866–1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galili, G., Tang, G.L., Zhu, X.H., and Gakiere, B. (2001). Lysine catabolism: A stress and development super-regulated metabolic pathway. Curr. Opin. Plant Biol. 4 261–266. [DOI] [PubMed] [Google Scholar]
- Gálvez, S., Bismuth, E., Sarda, C., and Gadal, P. (1994). Purification and characterization of chloroplastic NADP-isocitrate dehydrogenase from mixotrophic tobacco cells. Comparison with the cytosolic isoenzyme. Plant Physiol. 105 593–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gálvez, S., Hodges, M., Decottignies, P., Bismuth, E., Lancien, M., Sangwan, R.S., Dubois, F., LeMarechal, P., Cretin, C., and Gadal, P. (1996). Identification of a tobacco cDNA encoding a cytosolic NADP isocitrate dehydrogenase. Plant Mol. Biol. 30 307–320. [DOI] [PubMed] [Google Scholar]
- Gálvez, S., Roche, O., Bismuth, E., Brown, S., Gadal, P., and Hodges, M. (1998). Mitochondrial localization of a NADP-dependent isocitrate dehydrogenase isoenzyme by using the green fluorescent protein as a marker. Proc. Natl. Acad. Sci. USA 95 7813–7818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glevarec, G., Bouton, S., Jaspard, E., Riou, M.-T., Cliquet, J.-B., Suzuki, A., and Limami, A.M. (2004). Respective roles of the glutamine synthetase/glutamate synthase cycle and glutamate dehydrogenase in ammonium and amino acid metabolism during germination and post-germinative growth in the model legume Medicago truncatula. Planta 219 286–297. [DOI] [PubMed] [Google Scholar]
- Hamilton, E.W., and Heckathorn, S.A. (2001). Mitochondrial adaptations to NaCl. Complex I is protected by anti-oxidants and small heat shock proteins, whereas complex II is protected by proline and betaine. Plant Physiol. 126 1266–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison, J., Pou de Crescenzo, M.A., Sené, O., and Hirel, B. (2003). Does lowering glutamine synthetase activity in nodules modify nitrogen metabolism and growth of Lotus japonicus? Plant Physiol. 133 253–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa, P.M., Bressan, S.A., Zhu, J.K., and Bohnert, H.J. (2000). Plant cellular and molecular responses to high salinity. Annu. Rev. Plant Physiol. Plant Mol. Biol. 51 463–499. [DOI] [PubMed] [Google Scholar]
- Hassan, H.M., and Fridovich, I. (1979). Intracellular production of superoxide radicals and of hydrogen peroxide by redox active compounds. Arch. Biochem. Biophys. 196 385–395. [DOI] [PubMed] [Google Scholar]
- Hoai, N.T.T., Shim, I.S., Kobayashi, K., and Usui, K. (2003). Accumulation of some nitrogen compounds in response to salt stress and their relationships with salt tolerance in rice (Oryza sativa) seedlings. Plant Growth Regul. 41 159–164. [Google Scholar]
- Hodges, M. (2002). Enzyme redundancy and the importance of 2-oxoglutarate in plant ammonium assimilation. J. Exp. Bot. 53 905–916. [DOI] [PubMed] [Google Scholar]
- Husted, S., Hebbern, C.A., Mattsson, M., and Schjoerring, J.K. (2000). A critical experimental evaluation of methods for determination of NH4+ in plant tissue, xylem sap and apoplastic fluid. Physiol. Plant. 109 167–179. [Google Scholar]
- Kisaka, H., and Kida, T. (2003). Transgenic tomato plant carrying a gene for NADP-dependent glutamate dehydrogenase (gdhA) from Aspergillus nidulans. Plant Sci. 164 35–42. [Google Scholar]
- Kovtun, Y., Chiu, W.L., Tena, G., and Sheen, J. (2000). Functional analysis of oxidative stress-activated mitogen-activated protein kinase cascade in plants. Proc. Natl. Acad. Sci. USA 97 2940–2945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar, R.G., Shah, K., and Dubey, R.S. (2000). Salinity induced behavioral changes in malate dehydrogenase and glutamate dehydrogenase activities in rice seedlings of differing salt tolerance. Plant Sci. 156 23–34. [DOI] [PubMed] [Google Scholar]
- Lam, H.M., Chiu, J., Hsieh, M.H., Meisel, L., Oliveira, I.C., Shin, M., and Coruzzi, G. (1998). Glutamate-receptor genes in plants. Nature 396 125–126. [DOI] [PubMed] [Google Scholar]
- Lancien, M., Ferrario-Mery, S., Roux, Y., Bismuth, E., Masclaux, C., Hirel, B., Gadal, P., and Hodges, M. (1999). Simultaneous expression of NAD-dependent isocitrate dehydrogenase and other Krebs cycle genes after nitrate resupply to short-term nitrogen-starved tobacco. Plant Physiol. 120 717–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasa, B., Frechilla, S., Aparicio-Tejo, P.M., and Lamsfus, C. (2002). Role of glutamate dehydrogenase and phosphoenolpyruvate carboxylase activity in ammonium nutrition tolerance in roots. Plant Physiol. Biochem. 40 969–976. [Google Scholar]
- Lea, P.J., and Forde, B.G. (1994). The use of mutants and transgenic plants to study amino acid metabolism. Plant Cell Environ. 17 541–556. [Google Scholar]
- Lea, P.J., and Miflin, B.J. (1974). An alternative route for nitrogen assimilation in plants. Nature 251 680–685. [DOI] [PubMed] [Google Scholar]
- Lockwood, B., Scott, K.I., Bremner, A.F., and Coombs, G.H. (1987). The use of a highly sensitive electrophoretic method to compare the proteinases of trichomonads. Mol. Biochem. Parasitol. 24 89–95. [DOI] [PubMed] [Google Scholar]
- Loulakakis, K.A., Primikirios, N.I., Nikolantonakis, M.A., and Roubelakis-Angelakis, K.A. (2002). Immunocharacterization of Vitis vinifera L. ferredoxin-dependent glutamate synthase and its spatial and temporal changes during leaf development. Planta 215 630–638. [DOI] [PubMed] [Google Scholar]
- Loulakakis, K.C., and Roubelakis-Angelakis, K.A. (1990. a). Intracellular localization and properties of NADH-glutamate dehydrogenase from Vitis vinifera L.: Purification and characterization of the major isoenzyme. J. Exp. Bot. 41 1223–1230. [Google Scholar]
- Loulakakis, K.C., and Roubelakis-Angelakis, K.A. (1990. b). Immunocharacterization of NADH-glutamate dehydrogenase from Vitis vinifera. Plant Physiol. 94 109–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loulakakis, K.C., and Roubelakis-Angelakis, K.A. (1991). Plant NAD(H) glutamate dehydrogenase consists of two subunit polypeptides and their participation in the seven isoenzymes occurs in an ordered ratio. Plant Physiol. 97 104–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loulakakis, K.C., and Roubelakis-Angelakis, K.A. (1992). Ammonium-induced increase in NADH-glutamate dehydrogenase activity is caused by de novo synthesis of the α-subunit. Planta 187 322–327. [DOI] [PubMed] [Google Scholar]
- Loulakakis, K.A., and Roubelakis-Angelakis, K.A. (1996). Characterization of Vitis vinifera L. glutamine synthetase and molecular cloning of cDNAs for the cytosolic enzyme. Plant Mol. Biol. 31 983–992. [DOI] [PubMed] [Google Scholar]
- Loulakakis, K.C., Roubelakis-Angelakis, K.A., and Kanellis, A.K. (1996). Isolation of functional RNA from grapevine tissues poor in nucleic acid content. Am. J. Enol. Vitic. 47 181–185. [Google Scholar]
- Lutts, S., Majerus, V., and Kinet, J.M. (1999). NaCl effects on proline metabolism in rice (Oryza sativa) seedlings. Physiol. Plant. 105 450–458. [Google Scholar]
- Masclaux, C., Valadier, M.H., Brugière, N., Morot-Gaudry, J.F., and Hirel, B. (2000). Characterization of the sink/source transition in tobacco (Nicotiana tabacum L.) shoots in relation to nitrogen management and leaf senescence. Planta 211 510–518. [DOI] [PubMed] [Google Scholar]
- Masclaux-Daubresse, C., Reisdorf-Cren, M., Pageau, K., Lelandais, M., Grandjean, O., Kronenberger, J., Valadier, M.-H., Feraud, M., Jouglet, T., and Suzuki, A. (2006). Glutamine synthetase-glutamate synthase pathway and glutamate dehydrogenase play distinct roles in the sink-source nitrogen cycle in tobacco. Plant Physiol. 140 444–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matoh, T., and Takahashi, E. (1982). Changes in the activities of ferredoxin- and NADH-glutamate synthase during seedling development of peas. Planta 154 289–294. [DOI] [PubMed] [Google Scholar]
- Melo-Oliveira, R., Cinha-Oliveria, I., and Coruzzi, G.M. (1996). Arabidopsis mutant analysis and gene regulation define a non-redundant role for glutamate dehydrogenase in nitrogen assimilation. Proc. Natl. Acad. Sci. USA 96 4718–4723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miflin, B.J., and Habash, D.Z. (2002). The role of glutamine synthetase and glutamate dehydrogenase in nitrogen assimilation and possibilities for improvement in the nitrogen utilization of crops. J. Exp. Bot. 53 979–987. [DOI] [PubMed] [Google Scholar]
- Mittler, R., Vanderauwere, S., Gollery, M., and van Breusegem, F. (2004). Reactive oxygen gene network of plants. Trends Plant Sci. 9 490–498. [DOI] [PubMed] [Google Scholar]
- Murashige, T., and Skoog, F. (1962). A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol. Plant. 15 473–497. [Google Scholar]
- Nakagami, H., Kiegerl, S., and Hirt, H. (2004). OMTK1, a novel MAPKKK channels oxidative stress signaling through direct MAPK interaction. J. Biol. Chem. 279 26959–26966. [DOI] [PubMed] [Google Scholar]
- Nanjo, T., Kobayashi, M., Yoshiba, Y., Kakubari, Y., Yamaguchi-Shinozaki, K., and Shinozaki, K. (1999. a). Antisense suppression of proline degradation improves tolerance to freezing and salinity in Arabidopsis thaliana. FEBS Lett. 461 205–210. [DOI] [PubMed] [Google Scholar]
- Nanjo, T., Kobayashi, M., Yoshiba, Y., Sanada, Y., Wada, K., Tsukaya, H., Kakubari, Y., Yamaguchi-Shinozaki, K., and Shinozaki, K. (1999. b). Biological functions of proline in morphogenesis and osmotolerance revealed in antisense transgenic Arabidopsis thaliana. Plant J. 18 185–193. [DOI] [PubMed] [Google Scholar]
- Paczek, V., Dubois, F., Sangwan, R., Morot-Gaudry, J.F., Roubelakis-Angelakis, K.A., and Hirel, B. (2002). Cellular and subcellular localisation of glutamine synthetase and glutamate dehydrogenase in grapes gives new insights on the regulation of C and N metabolism. Planta 216 245–254. [DOI] [PubMed] [Google Scholar]
- Papadakis, A.K., Paschalidis, K.A., and Roubelakis-Angelakis, K.A. (2005). Biosynthesis profile and endogenous titers of polyamines differ in totipotent and recalcitrant plant protoplasts. Physiol. Plant. 125 10–20. [Google Scholar]
- Papadakis, A.K., and Roubelakis-Angelakis, K.A. (1999). The generation of active oxygen species differs in Nicotiana and Vitis plant protoplasts. Plant Physiol. 121 197–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadakis, A.K., and Roubelakis-Angelakis, K.A. (2005). Polyamines inhibit NADPH oxidase-mediated superoxides generation and putrescine prevents programmed cell death syndrome induced by the polyamine oxidase generated hydrogen peroxide. Planta 220 826–837. [DOI] [PubMed] [Google Scholar]
- Paschalidis, K.A., Aziz, A., Geny, L., Primikirios, N.I., and Roubelakis-Angelakis, K.A. (2001). Polyamines in grapevine. In Molecular Biology and Biotechnology of the Grapevine, K.A. Roubelakis-Angelakis, ed (Dordrecht, The Netherlands: Kluwer Academic Publishers), pp. 109–152.
- Paschalidis, K.A., and Roubelakis-Angelakis, K.A. (2005. a). Spatial and temporal distribution of polyamine levels and polyamine anabolism in different organs/tissues of the tobacco plant: Correlations with age, cell division/expansion, and differentiation. Plant Physiol. 138 142–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paschalidis, K.A., and Roubelakis-Angelakis, K.A. (2005. b). Sites and regulation of polyamine catabolism in the tobacco plant: Correlations with cell division/expansion, cell-cycle progression, and vascular development. Plant Physiol. 138 2174–2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastori, G.M., and Foyer, C.H. (2002). Common components, networks and pathways of cross-tolerance to stress. The central role of redox and abscissic acid-mediated control. Plant Physiol. 129 460–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pithon-Curi, T.C., Levada, A.C., and Lopes, L.R. (2002). Glutamine plays a role in superoxide production and the expression of p47phox, p22phox and gp91phox in rat neutrophils. Clin. Sci. 103 403–408. [DOI] [PubMed] [Google Scholar]
- Primikirios, N.I., and Roubelakis-Angelakis, K.A. (1999). Characterization and expression of arginine decarboxylase in Vitis vinifera L. Planta 208 574–582. [DOI] [PubMed] [Google Scholar]
- Pryor, A.J. (1990). A maize glutamic dehydrogenase null mutant is cold temperature sensitive. Maydica 35 367–372. [Google Scholar]
- Purnell, M.P., Skopelitis, D.S., Roubelakis-Angelakis, K.A., and Botella, J.R. (2005). Modulation of higher-plant NAD(H)-dependent glutamate dehydrogenase activity in transgenic tobacco via alteration of beta subunit levels. Planta 222 167–180. [DOI] [PubMed] [Google Scholar]
- Rentel, M.C., and Knight, M.R. (2004). Oxidative stress-induced calcium signaling in Arabidopsis. Plant Physiol. 135 1471–1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Restivo, F.M. (2004). Molecular cloning of glutamate dehydrogenase genes of Nicotiana plumbaginifolia: Structure analysis and regulation of their expression by physiological and stress condition. Plant Sci. 166 971–982. [Google Scholar]
- Rhoads, D.M., Umbach, A.L., Subbaiah, C.C., and Siedow, J.N. (2006). Mitochondrial reactive oxygen species. Contribution to oxidative stress and interorganellar signaling. Plant Physiol. 141 357–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt, R.R., and Miller, P. (1999). Polypeptides and polynucleotides relating to the α and β subunits of a glutamate dehydrogenase and methods of use. United States Patent number 5879941.
- Schopher, P., Plachy, C., and Frahry, G. (2001). Release of reactive oxygen intermediates (superoxide radicals, hydrogen peroxide, and hydroxyl radicals) and peroxidase in germinating radish seeds controlled by light, gibberellin, and abscisic acid. Plant Physiol. 125 1591–1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobolevsky, T.G., Revelsky, A.I., Miller, B., Oriedo, V., Chernetsova, E.S., and Revelsky, I.A. (2003). Comparison of silylation and esterification/acylation procedures in GC-MS analysis of amino acids. J. Sep. Sci. 26 1474–1478. [Google Scholar]
- Stitt, M., Muller, C., Matt, P., Gibon, Y., Carillo, P., Morcuende, R., Scheible, W.R., and Krapp, A. (2002). Steps towards an integrated view of nitrogen metabolism. J. Exp. Bot. 53 959–970. [DOI] [PubMed] [Google Scholar]
- Sweetlove, L.J., Mowday, B., Hebestreit, H.F., Leaver, C.J., and Millar, A.H. (2001). Nucleoside diphosphate kinase III is localized to the inter-membrane space in plant mitochondria. FEBS Lett. 508 272–276. [DOI] [PubMed] [Google Scholar]
- Syntichaki, K.M., Loulakakis, K.A., and Roubelakis-Angelakis, K.A. (1996). The amino acid sequence similarity of plant glutamate dehydrogenase with the extremophilic archaeal enzyme conforms to its stress related function. Gene 168 87–92. [DOI] [PubMed] [Google Scholar]
- Tercé-Laforgue, T., Dubois, F., Ferrario-Mery, S., de Crecenzo, M.A.P., Sangwan, R., and Hirel, B. (2004. a). Glutamate dehydrogenase of tobacco is mainly induced in the cytosol of phloem companion cells when ammonia is provided either externally or released during photorespiration. Plant Physiol. 136 4308–4317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tercé-Laforgue, T., Mack, G., and Hirel, B. (2004. b). New insights towards the function of glutamate dehydrogenase revealed during source-sink transition of tobacco (Nicotiana tabacum) plants grown under different nitrogen regimes. Physiol. Plant. 120 220–228. [DOI] [PubMed] [Google Scholar]
- Turano, F.J., Thakkar, S.S., Fang, T., and Weisemann, J.M. (1997). Characterization and expression of NAD(H)-dependent glutamate dehydrogenase genes in Arabidopsis. Plant Physiol. 113 1329–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong, H.-K., Chan, H.-K., Coruzzi, G.M., and Lam, H.-M. (2004). Correlation of ASN2 gene expression with ammonium metabolism in Arabidopsis. Plant Physiol. 134 332–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshiba, Y., Kiyosue, T., Nakashima, K., Yamaguchi-Shinozaki, K., and Shinozaki, K. (1997). Regulation of levels of proline as an osmolyte in plants under water stress. Plant Cell Physiol. 38 1095–1102. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.