Abstract
Reelin (Reln) is a glycoprotein that in postnatal and adult mammalian brain is believed to be secreted from telencephalic GABAergic interneurons and cerebellar glutamatergic granule neurons into the extracellular matrix. To address the question of whether Reln neurosecretion occurs via a regulated or a constitutive process, we exposed postnatal rat cerebellar granule neurons (CGNs) maintained in culture for 7–9 days to: (i) 100 μM N-methyl-d-aspartate (NMDA) in a Mg+2-free medium to stimulate NMDA-selective glutamate receptors and Ca2+-dependent neurotransmitter release, (ii) 50 mM KCl to depolarize the cells and elicit Ca2+-dependent exocytosis, (iii) 10–100 μM nicotine to activate excocytosis by nicotinic receptors present in these cells, (iv) 10 μM 1,2,3,4-tetrahydro-6-nitro-2,3-dioxo-benzo[f]quinoxaline-7-sulfonamide in combination with 10 μM dizocilpine to block α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid- and NMDA-preferring glutamate receptors activated by endogenously released glutamate, or (v) EGTA (5 mM) to virtually eliminate extracellular Ca2+ and block Ca2+-dependent exocytosis. Although, CGNs express and secrete Reln (measured by quantitative immunoblotting), none of the above-mentioned conditions that control regulated exocytosis alters the stores or the rate of Reln release. In contrast, application of either: (i) a Reln antisense oligonucleotide (5′-GCAATGTGCAGGGAAATG-3′) (10 μM) that reduces Reln biosynthesis or (ii) brefeldin A (5 × 10−5 M), an inhibitor of the traffic of proteins between the endoplasmic reticulum and the Golgi network, sharply curtail the rate of Reln secretion. Because, in subcellular fractionation studies, we have shown that Reln is not contained in synaptic vesicles, these data suggest that Reln secretion from CGNs does not require Ca2+-dependent exocytosis, but probably is related to a Reln pool stored in Golgi secretory vesicles mediating a constitutive secretory pathway.
Keywords: antisense, cerebellar granule cells, regulated secretion, constitutive secretion
Reelin (Reln) is an extracellular matrix (ECM) glycoprotein of approximately 400 kDa that is operative during mammalian corticogenesis in regulating lamination, alignment, and final positioning of pyramidal neurons in the telencephalon and in promoting dendritic sprouting and positioning of Purkinje cells in the cerebellum (1–5). In embryonic brain, this protein is secreted by transient pioneer neurons located in the marginal zone of the developing cortex (Cajal-Retzius cells), and in the cerebellum by pioneer granule neurons of the external granule layer. The extracellular secretion of Reln does not occur in reelerrl/orl mice (4), in which a mutation of the Reln gene occurs in a region of positively charged amino acids near the carboxyl terminus, or in COS-7 cells transfected with a Reln construct in which 133 aa at the carboxyl terminus are deleted (6). These studies indicate that this sequence plays an important role in the regulation of Reln secretion.
In the adult telencephalon, even though the Cajal-Retzius cells have virtually disappeared, Reln is still present in the ECM, presumably secreted by GABAergic interneurons. In the adult cerebellum Reln is secreted in the ECM by glutamatergic granule cells, after they are positioned in the internal granule cell layer (6–9). Although the role of Reln has been extensively studied during embryonic development of the mammalian brain, much less is known regarding the function of this protein in the adult brain.
The chemical structure of Reln includes motifs typically present in other ECM proteins, such as a F-spondin-like sequence in proximity of the amino terminus and eight repetitive cysteine-rich epidermal growth factor-like motifs in the central domain of the molecule (3–5). Probably in the mammalian brain, once secreted, Reln binds to cell surface receptors for ECM proteins located on the dendritic spines of either Purkinje cells or pyramidal neurons (7, 8, 10–12). It is generally accepted that in brain, the signaling pathway activated by Reln brings about tyrosine phosphorylation of a specific cytosolic adaptor protein termed mouse disabled-1 (Dab1), which is expressed in neurons that are located postsynaptically to Reln-secreting neurons (for a review see ref. 12). The mode of Reln transduction at the surface of the target cells now is being investigated by several groups (10, 11, 13, 14).
In vitro Reln secretion has been demonstrated by immunoprecipitation from the supernatant of embryonic brain explants, and it has been suggested that Reln after release is processed by extracellular metalloproteinases into smaller molecular weight proteins (15). The mechanisms regulating Reln destiny after secretion from adult brain neurons remain to be addressed. Here, we sought to begin clarifying the molecular mechanisms of Reln secretion from adult neurons by studying Reln storage and secretion in rat cerebellar granule neurons (CGNs) in culture. We selected 7–8 days postnatal CGN maintained up to 21 days in vitro (DIV) as a model for these studies. CGNs, after their migration and positioning in the internal granule cell layer, express large amounts of Reln during postnatal and adult life (7, 8). In mammals, rodents included, during postnatal cerebellar maturation, the Purkinje cell dendritic spines are an important putative target for Reln released from CGN into ECM (5, 7, 16). Postnatal rat CGNs in primary culture express many of the properties of mature glutamatergic CGN, including the receptors for γ-aminobutyric acid (GABA) (GABAA, GABAB) (17, 18), glutamate [N-methyl-d-aspartate (NMDA), α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid, and metabotropic] (19–22), and acetylcholine (nicotinic and muscarinic) (23, 24). Using this model, we addressed the question of whether Reln neurosecretion is mediated by excitatory neurotransmitter-induced depolarization and activation of Ca2+-dependent exocytosis or by a Ca2+-independent constitutive process. The expression of multiple excitatory and inhibitory neurotransmitter receptors by CGNs makes these neurons a suitable model for evaluating which mechanism is operative in Reln secretion.
Materials and Methods
Cultures of CGN.
These cultures were prepared from 7- to 8-day-old Sprague–Dawley rats (25) and were maintained in neurobasal medium supplemented with B27 (GIBCO/BRL), KCl (25 mM total), 2 mM glutamine, and 100 μg/ml gentamicin as described by Brewer (26). Two milliliters of this suspension, containing 1 × 106 cells/ml, was plated in a 35-mm dish and incubated at 37°C in 95% air with 5% CO2 up to 21 DIV.
On the day of the experiment and before drug treatment, the cells were washed three times with the B27/neurobasal medium.
Quantitative Analysis of mRNAs Encoding Reln or Nicotinic Acetylcholine (Ach) Receptor Subunits.
Quantification of Reln mRNA and nicotinic Ach receptor α4, α7, and β2 mRNAs was carried out by competitive reverse transcription–PCR (7, 27).
G-10 Anti-Reln mAb.
This antibody, directed against the amino terminus of Reln amino acid residues 164–496, was a generous gift of A.M. Goffinet, (University of Namur Medical School, Brussels, Belgium) and was prepared by using the H-fusion protein (Reln 164–496) as an antigen (28). This antibody fails to immunoreact with protein blots obtained from brain extracts of Reeler mice or from extracts of saline-perfused livers or kidneys dissected from wild-type mice. The immunoreactivity of this antibody is eliminated by preadsorption with Reln H-protein.
Immunocytochemistry.
CGNs cultured for 7 DIV were fixed with 4% paraformaldehyde in PBS for 1 hr at 4°C, permeabilized in 0.25% Triton X-100/Tris-buffered saline, and immunostained as described (20).
Western Blot Analysis of Reln and β-Actin Proteins.
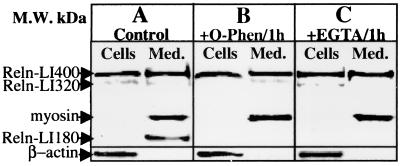
CGNs cultured in 35-mm dishes were lysed with 150 μl of 2× SDS/PAGE buffer. The culture media were concentrated 10-fold by using a 100-kDa cut-off filter (Centricon-100, Millipore) without loss of Reln (≈ 100% recovery). The extracted Reln protein and other immunoreactive products were separated by 7.5% SDS/PAGE and blotted overnight onto Hybond ECL nitrocellulose membranes (Amersham Pharmacia). The overnight blotting allows a satisfactory transfer of different molecular weight immunoreactive proteins. The membrane blots were blocked for 1 hr at room temperature with 2% nonfat dry milk in PBS (10 mM PBS, pH 7.4) and then reacted with G-10 anti-Reln mAb, diluted 1:10,000, for 6 hr at 25°C. The immune complexes were detected by using a goat anti-mouse IgG, peroxidase-conjugated, and preadsorbed with rat serum proteins (Sigma), at 1:1,000 for 1 hr and the ECL Plus Chemiluminescence Western Blotting kit (Amersham Pharmacia). Reln-like immunoreactivity in CGN extracts or culture medium (see Fig. 1A) can be resolved into two distinct molecular forms by this method: one form of approximately 400 kDa (presumably full-length Reln, and referred to here as Reln-LI), the most abundant form detected in CGNs, and a second of approximately 180 kDa, virtually absent in CGNs, but abundantly present in the culture medium. The 180-kDa immunoreactive protein presumably represents a Reln metabolite or processing product. In addition, we detected a minor immunoreactive band of ≈320 kDa in CGNs and their respective culture media (Fig. 1).
Figure 1.
Reln expression in CGN and their culture medium. Typical Reln-LI Western blot analysis of proteins extracted from CGN at 7 DIV (Cells) or culture medium (Med.). (A) Basal culture conditions. (B) Treatment with 1 mM 1,10-phenanthroline (O-Phen)/1 hr or (C) with 5 mM EGTA/1 hr. The cells (≈2 × 106) were lysed with 150 μl of 2× concentrated gel loading buffer (see Materials and Methods). The medium (2 ml) was concentrated to 0.2 ml on a 100-kDa cut-off filter (see Materials and Methods); 40 μl of cells or 20 μl of medium were run on a 7.5% SDS/PAGE. Indicated by arrows are Reln (≈ 400-kDa immunopositive band) and the other Reln immunopositive protein fragments, whose molecular mass was calculated by using prestained markers (205 to 7 kDa). In each cell extract, β-actin (44 kDa) immunoreactive intensity was used as a control of cell density and blotting efficiency. In the medium, because of the absence of β-actin, the efficiency of Reln-LI blot transferring was estimated by measurements of the optical density of a prestained myosin marker (205 kDa) added to the medium extract. For practical purposes, the myosin band was overlaid on the immunochemifluorescent blot. Note that O-Phen/1 hr or EGTA/1 hr failed to change the density of the 400-kDa Reln-LI band but significantly decreased the 180-kDa immunopositive band. Similar results were obtained in three different experiments.
The intensity of β-actin immunofluorescence was determined on the same blot with a monoclonal β-actin antibody (1:1,000) (Clone AC-15, Sigma), and was used as an indirect measurement of the cell density.
Quantitative Analysis of Reln.
The intensities of the 400-kDa Reln-LI band and the band generated by Reln H-protein (the fusion protein used to prepare the G-10 antibody) were analyzed on nitrocellulose membrane blots by chemiluminescent detection using the Blue Fluorescence/Chemifluorescence Storm system (Molecular Dynamics) with imagequant analysis software.
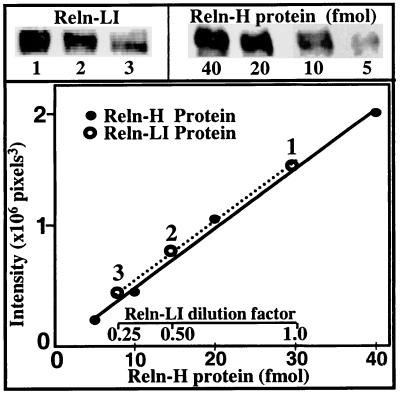
As shown in Fig. 2, the plot of the amount of Reln H-protein against the intensity of the immunoreactive signal is linear from 5 to 40 fmol, and the slope of this linear relationship is identical for Reln extracted from cells or medium.
Figure 2.
Linearity of Western blot chemifluorescence intensity of serial dilutions of Reln H-protein standard (●) and 400-kDa Reln-LI protein (○) obtained from CGN extracts probed with the G-10 antibody. Experimental conditions are as described in Fig. 1. Given the linear range of assay for Reln H-protein, between 5 and 40 fmol, and considering that the volume applied to the gel was 40 μl, the final concentration of Reln H-protein standard solution was between 125 and 1,000 pM. Thus, the volume of the cell extract was adjusted to obtain similar Reln concentrations.
The reliability of the assay procedure, determined in triplicate cell extracts or culture media, had an intra-assay and an inter-assay accuracy of 95% (ANOVA). Specifically, the intra-assay [F = 0.97 (F 95%= 9.55, F 99%= 30.8, df = 5)] and the inter-assay [F = 0.52 (F 95%= 9.55, F 99% = 30.8, df = 5)] ratio of variances failed to reach level of significance at P < 0.05. The relative quantity of Reln-LI was estimated in arbitrary units, defined as fmol of Reln-H-protein equivalents (RHE).
Glutamate Assay.
Glutamate content in CGNs and in their incubation media was measured after derivatization with orthophtalaldehyde (29). Aminovaleric acid was used as an internal standard. Glutamate was separated from the other amino acids by HPLC using a C18 column (25). NMDA does not interfere with the measurement of glutamate in this system.
Reln Antisense Oligodeoxynucleotides (ODNs).
The Reln phosphorothioate antisense ODNs (Reln-aODNs) used in our experiments consisted of an 18-bp sequence (5′-GCAATGTGCAGGGAAATG-3′) complementary to nucleotides 453–471 of the mus musculus Reln cDNA sequence (GenBank accession no. U24703). In parallel, some culture dishes were treated with the corresponding mismatched ODNs (Reln-mODNs) containing the same bases as Reln-aODNs in a random order (5′-ACCTTGTGACCCATCTCT-3′).
Subcellular Location of Reln.
Synaptosomes and synaptic vesicles were prepared from rat (Sprague–Dawley) brain homogenates by differential centrifugation, according to the method used by Ferrarese et al. (30). The purity and quality of the subcellular fractionations were verified by electron microscopic analysis (30). Reln content was estimated with the immunoblot assay described above.
Results
Reln-LI in CGNs and Culture Media.
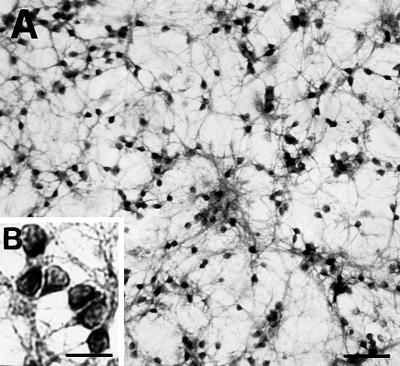
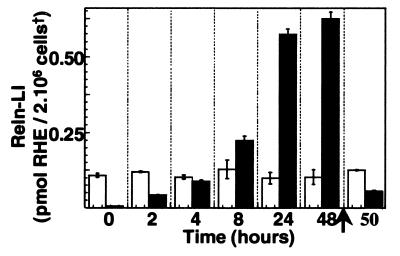
CGN cultures, 4–21 DIV, express 20–30 attomol Reln mRNA per μg total RNA. Virtually every CGN in culture (7 DIV) expressed Reln when stained with Reln G-10 antibodies (Fig. 3). The average of Reln-LI content of each culture was around 120 fmol of RHE/2.106 cells (Fig. 4).
Figure 3.
Reln expression in CGNs at 7 DIV. Reln staining with the G-10 antibody shows immunopositive CGN, whereas the few glial cells present in the culture are not stained. (A) Objective ×2.5. (Scale bar = 50 μm.) (B, Inset) A magnification (objective ×40) of CGN. (Scale bar = 25 μm.) No staining was detected when G-10 antibody was omitted.
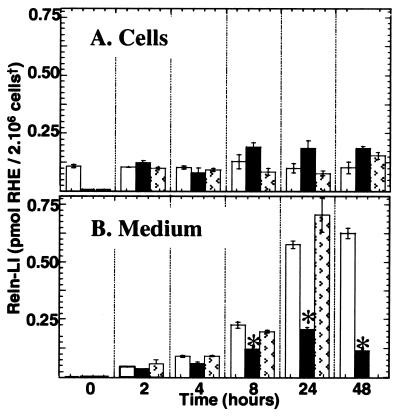
Figure 4.
Time-dependent accumulation of Reln-LI in the medium of CGNs at 7 DIV. Open bars represent Reln-LI content in 2 × 106 CGN, and filled bars represent Reln-LI content in the medium of the same cells. Reln quantification was carried out as described in Figs. 1 and 2. Bars represent the means ± SEM of three experiments. The arrow indicates that after 48 hr the cells are washed with fresh medium and Reln-LI is measured in the cells and the medium after the application of new medium. Note that the content of Reln-LI in cells and medium is similar to that measured 2 hr after the first change of the medium. The accumulation of Reln-LI in the medium during the first 24 hr is linear with time (correlation coefficient = 0.99, P < 0.01). †, Cell density was normalized in each sample by correcting for β-actin immuno-chemifluorescence intensity.
Reln is secreted in the culture medium under basal conditions (Fig. 1) at an average rate of about 25 fmol/hr per culture, approaching a steady state of 570 ± 35 fmol/culture, in 24 hr (Fig. 4) when Reln-LI content in the culture medium is 4.75-fold that of the CGN. Interestingly, after 24 hr, when Reln-LI in the medium approached a steady-state level, a marked increase of small molecular-mass Reln immunoreactive metabolites (i.e., the 180-kDa band) appeared in the medium (data not shown). Moreover, we also observed that if after 48 hr of incubation (9 DIV), the cells were washed and then cultured with fresh medium, the initial rate (1–2 hr) of Reln secretion was similar to that observed at 7 DIV (Fig. 4). The virtual removal of extracellular Ca2+ by exposing the cultures to 5 mM EGTA for 15 min or 1 hr (Fig. 1C) or the blockade of metalloproteases by application of 1 mM 1,10-phenanthroline for 1 hr did not result in appreciable changes in the Reln-LI content of either the CGNs or the medium (Fig. 1B). However, both treatments appeared to reduce the content of the 180-kDa Reln metabolite in the medium (Fig. 1). Intra- or inter-experimental differences caused by cell density or extraction procedures were normalized by using β-actin as a reference standard (see Materials and Methods). Because β-actin was not present in the medium, efficacy of the blot transfer was established by measuring the density of the 205-kDa prestained myosin band in the same blot. Only blots in which prestained myosin was completely transferred were used for the quantitative measurement of Reln in the medium.
Mechanisms of Reln Secretion.
Studies of the subcellular localization of Reln in homogenates of rat brain have shown that Reln is not compartmentalized in synaptic vesicles (data not shown). In Table 1, we show that at 9 DIV in CGNs, secretion of Reln-LI into the culture medium remained virtually unchanged after a 15-min application of 100 μM NMDA in the absence of Mg2+, a condition that increases endogenous glutamate release (Table 1). As established in previous studies from our laboratory (19), NMDA addition under the conditions used in this experiment increases free cytosolic Ca2+, associated with a promptly reversible swelling of CGNs. Similarly, as with NMDA, a 15-min application of a depolarizing concentration (50 mM) of KCl (31) fails to increase Reln release (Table 1).
Table 1.
NMDA- and high potassium-induced depolarization release glutamate but not Reln-LI from CGNs at 9 DIV
| Groups |
Reln-LI, fmol/2.106 cells
|
Glutamate, nmol/2.106 cells
|
||
|---|---|---|---|---|
| Cells | Medium | Cells | Medium | |
| Control | 120 ± 10 | 22 ± 4.7 | 13 ± 0.83 | 1.4 ± 0.18 |
| NMDA | 137 ± 24 | 19 ± 3.0 | 12 ± 1.40 | 3.0 ± 0.29* |
| KCl50 | 90 ± 20 | 20 ± 10 | nd | nd |
Locke's solution containing 5 mM KCl (control) or 50 mM KCl or Mg2+-free Locke's solution (replacing an equal amount of NaCl) containing 100 μM NMDA was applied to the cells for 15 min. Reln-LI was measured in cell extracts or medium as described in Fig. 1. Glutamate was measured as described in Materials and Methods. Each value is the mean ± SEM of at least three experiments. ∗, P = 0.0031 (Student's t test). Because a linear relationship exists between the quantity of β-actin and the number of cells plated in the culture dish, the values of Reln-LI and glutamate in each sample were normalized for β-actin and expressed for 2 × 106 cells. nd: not determined.
CGNs express mRNAs encoding several nicotinic Ach receptor subunits (α4 mRNA = 33 ± 4.6 amol/μg RNA, n = 5; α7 = 72 ± 5.5 amol/μg RNA, n = 3; and β2 = 141 ± 13 amol/μg RNA, n = 3; see also ref. 29). This finding prompted us to test whether nicotine could increase Reln release. However, the application of 10 or 100 μM nicotine for 15 min or 1 hr failed to elicit Reln-LI release (ratio of Reln-LI in medium to Reln-LI in cells: vehicle = 0.33 ± 0.17, nicotine 10 μM = 0.28 ± 0.09, nicotine 100 μM = 0.36 ± 0.06; n = 3, P = 0.896).
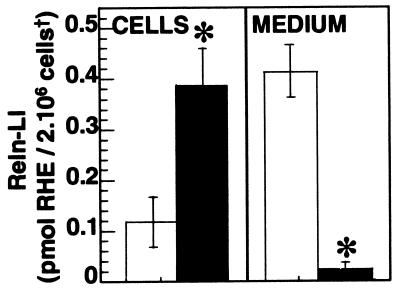
To define the mechanisms regulating spontaneous Reln secretion, we have used brefeldin A, an in vivo blocker of membrane export of proteins out of the endoplasmic reticulum (32, 33). Fig. 5 shows that Reln secretion from CGNs in culture is virtually abolished by the presence of 5 × 10−5 M brefeldin A for 24 hr. This is inferred from the lack of increase of Reln-LI in the medium and from the 3.3-fold increase in the content of Reln-LI in the CGNs. No differences in β-actin content were observed in brefeldin A-treated CGNs compared with control CGN cultures.
Figure 5.
Brefeldin A increases Reln-LI in the CGN but drastically reduces Reln-LI in the medium. Treatment of CGNs at 7 DIV with 5 × 10−5 M brefeldin A (filled bars) for 24 hr results in the inhibition of Reln-LI in the medium and an increase of Reln-LI in the CGNs. No difference in β-actin content was observed. Each bar represents the average of four experiments ± SEM. * = P < 0.01 (Student's t test) when Reln-LI values for brefeldin A-treated cells (filled bar) or medium were compared with vehicle-treated (open bar) cells or medium, respectively. †, Cell density was estimated in each sample by normalizing for β-actin immuno-chemifluorescence intensity (see Materials and Methods).
Because CGNs in culture release glutamate in the medium under basal conditions (see Table 1) we also tested whether protracted (48 hr) blockade of glutamate action at NMDA and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors by a simultaneous application of dizocilpine (10 μM) and 1,2,3,4-tetrahydro-6-nitro-2,3-dioxo-benzo[f]quinoxaline-7-sulfonamide (NBQX) (10 μM) could influence basal Reln secretion. However, the percentages of Reln release in vehicle or dizocilpine plus NBQX-treated CGN cultures were not significantly different (ratio of Reln-LI in medium to Reln-LI in cells: vehicle = 4.0 ± 0.60; dizocilpine + NBQX = 5.6 ± 1.5; n = 3, P = 0.374).
Antisense Experiments.
The addition of Reln-aODNs at a dose of 10 μM, but not a corresponding addition of Reln-mODNs, to 7 DIV CGN reduced the culture medium content of Reln-LI significantly within 8 hr (Fig. 6B). Importantly, the Reln-LI CGN steady-state level remained constant (around 120 fmol/2.106 cells) for at least 48 hr after a single application of Reln-aODNs (Fig. 6A). In contrast, repeated additions of 10 μM Reln-aODNs at 0, 24, and 48 hr resulted, at 72 hr, in the virtual disappearance of Reln-LI from CGNs (Fig. 7) and their respective media. In these CGN cultures, the levels of β-actin were not changed, which suggests that the Reln-LI disappearance was not caused by an artifact related to CGN detachment from the culture dish.
Figure 6.
Reln-aODNs inhibit the accumulation of Reln-LI in media of CGNs. Seven DIV cells were treated for the time indicated with 10 μM Reln-aODNs (filled bars) (5′-GCAATGTGCAGGGAAATG-3′) or 10 μM mODNs (5′-ACCTTGTGACCCATCTCT-3′) (patterned bars) or vehicle (open bars). * = P < 0.01 (one-way ANOVA followed by Bonferoni analysis) when the amount of Reln-LI in Reln-aODN-treated cells is compared with time-matched Reln-mODN- or vehicle-treated CGN. Data are the means ± SEM from three different experiments. †, Cell density was estimated in each sample by normalizing for β-actin immuno-chemifluorescence intensity.
Figure 7.
Inhibition of Reln expression in CGN by aODNs. Reln-aODNs (10 μM) (filled bar), Reln-mODNs (10 μM) (patterned bar), or vehicle (open bar) were applied to CGNs at 4 DIV at 0, 24, and 48 hr, and Reln-LI was determined at 72 hr after the beginning of the treatment. Each value is the mean ± SEM of at least three different experiments. †, Cell density was estimated in each sample by normalizing for β-actin immuno-chemifluorescence intensity.
Discussion
In these studies, we have addressed the following questions: (a) is the Reln expressed by neurons released via a depolarization-secretion coupling mechanism?; (b) is neuronal Reln released by a constitutive secretory mechanism that is independent of depolarization elicited by occupancy of glutamate or nicotinic Ach receptors?; (c) is neuronal Reln stored in synaptic vesicles of the neurotransmitter-regulated secretory pathway or in vesicles of the constitutive secretory pathway?; and d) is neuronal Reln cleaved by proteolytic enzymes in the intracellular or the extracellular compartment?
To quantitatively determine Reln in CGN and in the culture medium, we developed an assay based on the separation of Reln-LI 400 kDa by Western blot and on the comparison of the chemifluorescence intensity produced by the reaction of serial dilutions of this Reln-LI band with the anti-Reln G-10 mAb with a chemiluminescent standard curve generated by reacting various concentrations of Reln H-protein with the same antibody. This method is reliable and sensitive (can detect in CGN extracts RHE in 0.1 nM concentration range) and allows the quantification of Reln expressed in either the extracts of 2 × 106 CGN or in the culture media (see Fig. 2). In our laboratory, this assay also is used for the measurement of Reln expressed in human plasma containing only trace amounts of small molecular-weight products of Reln (34) or in human spinal fluid containing small amounts of Reln and large amounts of small molecular-weight products of Reln.
The intracellular pool of Reln in 7 DIV CGNs [approximately 120 fmol of RHE/2.106 cells (see Fig. 4)] approaches a steady-state level after few days of culture, and a similar steady state also is found in different CGN preparations (compare data in Figs. 4 and 5). Under basal culture conditions, the steady-state concentrations of Reln in CGNs probably is maintained by an equilibrium between synthesis and secretion. Because Reln accumulates in the medium almost linearly for 24 hr and exceeds the intracellular content by 4.75-fold after 24 or 48 hr, we suggest that in our conditions Reln release rate depends on synthesis.
A single application of aODNs to the medium reduced the accumulation of Reln-LI in the medium between 8 and 48 hr without decreasing the intracellular content of Reln-LI during this time. One interpretation of these data is that this concentration of Reln-aODNs blocks Reln biosynthesis in CGNs incompletely, leaving a reserve capacity of Reln biosynthesis sufficient to maintain intracellular steady-state levels. Indeed, three repeated applications of Reln-aODNs during a 72-hr period resulted in virtually a complete disappearance of Reln from CGNs and the medium. These data further support the view that Reln secretion from CGNs depends on its synthesis rate and not on a stimulus-regulated Ca2+-dependent exocytosis because the intracellular Reln pool is small and presumably is not located in synaptic vesicles, as we failed to detect Reln in those organelles. This conclusion is supported by experiments with brefeldin A, a drug that completely disassembles the Golgi complex by blocking membrane export out of the endoplasmic reticulum and inhibiting vesicle formation, without blocking protein synthesis. When CGNs are treated with brefeldin A, the decrease in Reln secretion is accompanied by an accumulation of Reln in the cells (Fig. 5). Thus, taken together these data strongly suggest that the Reln release rate is not regulated by depolarization, but strictly depends on its synthesis rate. This relationship is similar to that reported for the secretion of other ECM proteins (35).
In a recent study Lambert et al. (15) reported that in embryonic mouse brain explants Reln secreted extracellularly is cleaved into smaller molecular weight processing products by the action of metalloproteinases, which presumably are located at the neuronal cell surface. We have tested whether the amount of Reln in the intracellular pool or that released into the medium could be altered by reducing its rate of cleavage by applying 1,10-phenanthroline (1 mM), a metalloprotease inhibitor. We found that the amounts of CGN Reln and Reln released during the first hour of incubation were not affected by the presence of 1,10-phenanthroline in a dose that is sufficient to block the metalloproteases, as judged by the reduction of Reln cleavage products in the medium (Fig. 1). Higher concentrations of this inhibitor or a longer incubation period could not be tested because of the direct toxicity of 1,10-phenantroline on the neurons in culture. However, neither our data nor those of Lambert et al. (15) are an adequate model of Reln destiny after secretion from neurons in brain. In fact, in adult mammalian brain, ECM Reln is bound to integrin receptors (10) and thereby probably is protected from metabolism by metalloproteinases.
An interesting observation in our study is that the content of Reln-LI in the medium increases almost linearly for 24 hr after the medium is replaced, but the accumulation rate decreases and Reln-LI level approaches a steady state within 24 and 48 hr. This steady state may result from Reln reaching a concentration sufficient for the catalytic action of proteolytic enzymes so that the rate of Reln destruction in the medium balances the rate of Reln release from CGN. This hypothesis is supported by: (a) lack of evidence of CGN neurotoxicity up to 21 DIV, (b) Reln-LI accumulates in the medium at a rate virtually identical to that observed after the first application of fresh medium, if after 48 hr, the culture medium is replaced with fresh medium (Fig. 4), and (c) Reln-LI metabolites (i.e., the 180-kDa Reln immunoreactive protein) (Fig. 1) are virtually absent in the cell compartment but are abundant in the medium and increase with increased incubation time.
The functional significance of the small molecular-mass forms of Reln (180 kDa and others) in the extracellular space cannot be studied until we develop a method to measure the trophic action of Reln. Because these smaller molecular weight Reln fragments lack the carboxyl-terminal tail of Reln, they cannot be secreted and therefore are generated once secretion takes place. Perhaps one can use cerebellar slices of reeler−/− mice to measure the dendritic proliferation of Purkinje cells, presumably stimulated by either Reln or a putative processing product. If this bioassay is successful, one then can assess whether cleavage of extracellular Reln is required to terminate the action of Reln or whether this process is necessary for its biological activity. Thus, in addition to establishing a Reln bioassay, it is also important to prepare antibodies directed against additional parts (including the COOH terminus) of the Reln molecule to establish the structure and the relative abundance of the various Reln metabolic products and to assess their putative biological significance.
Acknowledgments
We thank Dr Andre M. Goffinet, Neurobiology Unit, University of Namur Medical School, Brussels, Belgium for constructive criticisms and suggestions in the preparation of the manuscript. This work was supported by a postdoctoral fellowship from Elf-Aquitaine/Sanofi to P.N.L., National Institutes of Health Grant 56500 to E.C., and National Institutes of Health Grant 49486 to A.G.
Abbreviations
- Reln
Reelin
- CGN
cerebellar granule neurons
- Reln-LI
full-length Reln
- ECM
extracellular matrix
- ODN
oligodeoxynucleotide
- NMDA
N-methyl-d-aspartate
- DIV
days in vitro
- Ach
acetylcholine
- RHE
Reln-H-protein equivalents
- Reln-aODN
Reln phosphorothioate antisense ODN
- mODN
mismatched ODN
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.050589597.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.050589597
References
- 1.D'Arcangelo G, Miao C G, Chen S C, Soares H D, Morgan J J, Curran T. Nature (London) 1995;374:719–723. doi: 10.1038/374719a0. [DOI] [PubMed] [Google Scholar]
- 2.Curran T, D'Arcangelo G. Brain Dev Brain Res Rev. 1998;26:285–294. doi: 10.1016/s0165-0173(97)00035-0. [DOI] [PubMed] [Google Scholar]
- 3.D'Arcangelo G, Curran T. BioEssays. 1998;20:235–244. doi: 10.1002/(SICI)1521-1878(199803)20:3<235::AID-BIES7>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 4.Lambert de Rouvroit C, Goffinet A M. Adv Anat Embryol Cell Biol. 1998;150:1–106. [PubMed] [Google Scholar]
- 5.Miyata T, Nakajima K, Mikoshiba K, Ogawa M J. Neuroscience. 1997;17:3599–3609. doi: 10.1523/JNEUROSCI.17-10-03599.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.D'Arcangelo G, Nakajima K, Miyata T, Ogawa M, Mikoshiba K, Curran T. J Neurosci. 1997;17:23–31. doi: 10.1523/JNEUROSCI.17-01-00023.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pesold C, Impagnatiello F, Pisu M G, Uzunov D P, Costa E, Guidotti A, Caruncho H J. Proc Natl Acad Sci USA. 1998;95:3221–3226. doi: 10.1073/pnas.95.6.3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pesold C, Liu W S, Guidotti A, Costa E, Caruncho H J. Proc Natl Acad Sci USA. 1999;96:3217–3222. doi: 10.1073/pnas.96.6.3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Impagnatiello F, Guidotti A, Pesold C, Dwivedi Y, Caruncho H J, Pisu M G, Uzunov D, Smalheiser N, Davis J, Pandey G, et al. Proc Natl Acad Sci USA. 1998;95:15718–15723. doi: 10.1073/pnas.95.26.15718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rodriguez, M., Pesold, C., Liu, W. S., Kriho, V., Guidotti, A., Pappas, G. D. & Costa, E. (March 21, 2000) Proc. Natl. Acad Sci, USA. 10.1073/pnas.050589797. http://www.pnas.org/cgi/doi/10.1073/pnas.050589797 [DOI] [PMC free article] [PubMed]
- 11.Senzaki K, Ogawa M, Yagi T. Cell. 1999;99:635–647. doi: 10.1016/s0092-8674(00)81552-4. [DOI] [PubMed] [Google Scholar]
- 12.Rice D S, Curran T. Genes Dev. 1999;13:2758–2773. doi: 10.1101/gad.13.21.2758. [DOI] [PubMed] [Google Scholar]
- 13.Hiesberger T, Trommsdorff M, Howell B W, Goffinet A, Mumby M C, Cooper J A, Herz J. Neuron. 1999;24:481–489. doi: 10.1016/s0896-6273(00)80861-2. [DOI] [PubMed] [Google Scholar]
- 14.D'Arcangelo G, Homayouni R, Keshvara L, Rice D S, Sheldon M, Curran T. Neuron. 1999;24:471–479. doi: 10.1016/s0896-6273(00)80860-0. [DOI] [PubMed] [Google Scholar]
- 15.Lambert de Rouvroit C, de Bergeyck V, Cortvrindt C, Bar I, Eeckhout Y, Goffinet A M. Exp Neurol. 1999;156:214–217. doi: 10.1006/exnr.1998.7007. [DOI] [PubMed] [Google Scholar]
- 16.Hadj-Sahraoui N, Frederic F, Delhaye-Bouchaud N, Mariani J. J Neurogenet. 1996;11:45–58. doi: 10.3109/01677069609107062. [DOI] [PubMed] [Google Scholar]
- 17.Zheng T M, Zhu W J, Puia G, Vicini S, Grayson D R, Costa E, Caruncho H J. Proc Natl Acad Sci USA. 1994;91:10952–10956. doi: 10.1073/pnas.91.23.10952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wojcik W J, Neff N H. Mol Pharmacol. 1984;25:24–28. [PubMed] [Google Scholar]
- 19.Kiedrowski L, Costa E, Wroblewski J T. Mol Pharmacol. 1992;41:779–784. [PubMed] [Google Scholar]
- 20.Longone P, Impagnatiello F, Mienville J M, Costa E, Guidotti A. J Mol Neurosci. 1998;11:23–41. doi: 10.1385/JMN:11:1:23. [DOI] [PubMed] [Google Scholar]
- 21.Wroblewski J T, Nicoletti F, Fadda E, Costa E. Proc Natl Acad Sci USA. 1987;84:5068–5072. doi: 10.1073/pnas.84.14.5068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Santi M R, Ikonomovic S, Wroblewski J T, Grayson D R. J Neurochem. 1994;63:1207–1217. doi: 10.1046/j.1471-4159.1994.63041207.x. [DOI] [PubMed] [Google Scholar]
- 23.Didier M, Berman S A, Lindstrom J, Bursztajn S. Brain Res Mol Brain Res. 1995;30:17–28. doi: 10.1016/0169-328x(94)00266-h. [DOI] [PubMed] [Google Scholar]
- 24.Holopainen I, Wojcik W J. J Pharmacol Exp Ther. 1993;264:423–430. [PubMed] [Google Scholar]
- 25.Vaccarino F M, Alho H, Santi M R, Guidotti A. J Neurosci. 1987;7:65–76. doi: 10.1523/JNEUROSCI.07-01-00065.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brewer G J. J Neurosci Res. 1995;42:674–683. doi: 10.1002/jnr.490420510. [DOI] [PubMed] [Google Scholar]
- 27.Auta J, Longone P, Guidotti A, Costa E. J Mol Neurosci. 1999;12:1–19. doi: 10.1385/JMN:13:1-2:31. [DOI] [PubMed] [Google Scholar]
- 28.de Bergeyck V, Naerhuyzen B, Goffinet A M, Lambert de Rouvroit C. J Neurosci Methods. 1998;82:17–24. doi: 10.1016/s0165-0270(98)00024-7. [DOI] [PubMed] [Google Scholar]
- 29.Rowley H L, Martin K F, Marsden C A. J Neurosci Methods. 1995;57:93–99. doi: 10.1016/0165-0270(94)00132-z. [DOI] [PubMed] [Google Scholar]
- 30.Ferrarese C, Vaccarino F, Alho H, Mellstrom B, Costa E, Guidotti A. J Neurochem. 1987;48:1093–1102. doi: 10.1111/j.1471-4159.1987.tb05632.x. [DOI] [PubMed] [Google Scholar]
- 31.Levi G, Patrizio M, Gallo V. J Neurochem. 1991;56:199–206. doi: 10.1111/j.1471-4159.1991.tb02581.x. [DOI] [PubMed] [Google Scholar]
- 32.Miller S G, Carnell L, Moore H H. J Cell Biol. 1992;118:267–283. doi: 10.1083/jcb.118.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sciaky N, Presley J, Smith C, Zaal K J, Cole N, Moreira J E, Terasaki M, Siggia E, Lippincott-Schwartz J. J Cell Biol. 1997;139:1137–1155. doi: 10.1083/jcb.139.5.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smalheiser N R, Costa E, Guidotti A, Impagnatiello F, Auta J, Lacor P, Kriho V, Pappas G E. Proc Natl Acad Sci USA. 2000;97:1281–1286. doi: 10.1073/pnas.97.3.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alberts B, Bray D, Lewis J, Raff M, Roberts K, Watson J D, editors. Molecular Biology of the Cell. New York: Garland; 1994. pp. 626–627. [Google Scholar]