Abstract
Cerebellar granule neurons (CGNs) are one of the most populous cells in the mammalian brain. They express an outwardly rectifying potassium current, termed a “standing-outward” K+ current, or IKSO, which does not inactivate. It is active at the resting potential of CGNs, and blocking IKSO leads to cell depolarization. IKSO is blocked by Ba2+ ions and is regulated by activation of muscarinic M3 receptors, but it is insensitive to the classical broad-spectrum potassium channel blocking drugs 4-aminopyridine and tetraethylammonium ions. The molecular nature of this important current has yet to be established, but in this study, we provide strong evidence to suggest that IKSO is the functional correlate of the recently identified two-pore domain potassium channel TASK-1. We show that IKSO has no threshold for activation by voltage and that it is blocked by small extracellular acidifications. Both of these are properties that are diagnostic of TASK-1 channels. In addition, we show that TASK-1 currents expressed in Xenopus oocytes are inhibited after activation of endogenous M3 muscarinic receptors. Finally, we demonstrate that mRNA for TASK-1 is found in CGNs and that TASK-1 protein is expressed in CGN membranes. This description of a functional two-pore domain potassium channel in the mammalian central nervous system indicates its physiological importance in controlling cell excitability and how agents that modify its activity, such as agonists at G protein-coupled receptors and hydrogen ions, can profoundly alter both the neuron's resting potential and its excitability.
On the basis of sequence similarities, the pore-forming α-subunits of K channels have been grouped into superfamilies. The two principal superfamilies are the inward rectifier or 2TM (for two transmembrane domains) superfamily and the voltage-gated or 6TM superfamily. Most electrophysiologically characterized native K channels are encoded by genes from one or the other of these two superfamilies, although in most cases, the exact subunit configuration that underlies each native current is not clear (1–3). During the last 4 years, a third major superfamily of K channels, the two-pore domain potassium channel family (2-PK) (4), has emerged, initially identified from yeast K channel sequencing (4, 5) and now identified in mammalian cells (6–14).
In mammals, six functional members of the 2-PK family with 4TM domains (or TWIK family) have been identified to date. These are TWIK-1, TWIK-2, TREK-1, TASK-1, TASK-2, and TRAAK (6–14). Despite a relatively low sequence similarity and different functional properties, these 2-PK channels all produce quasiinstantaneous and noninactivating currents (although TASK-2 currents have relatively slow activation kinetics; ref. 13). The 2-PK channels are presently classified into three distinct functional subfamilies (13). TASK-1 and TASK-2 are sensitive to small variations in external pH close to physiological pH; only TASK-1 is expressed in the nervous system to any significant degree (6, 9, 13). TREK-1 and TRAAK are arachidonic acid-activated, mechanosensitive K channels (7, 8, 11, 12), whereas TWIK-1 and TWIK-2 are weakly inward rectifying K channels (10, 14). To date, no functional correlates of 2-PK channels have been identified in the mammalian nervous system.
Cerebellar granule neurons (CGNs) express an outwardly rectifying potassium current, termed a “standing-outward” K+ current or IKSO, (15). IKSO does not inactivate and is active at the resting potential of CGNs; blocking IKSO leads to cell depolarization. IKSO is insensitive to the classical broad-spectrum potassium channel blockers 4-aminopyridine and tetraethylammonium, but it is blocked by Ba2+ ions and regulated by activation of G protein coupled receptors, such as muscarinic M3 receptors. In this study, we provide evidence to suggest that IKSO is the functional correlate of the 2-PK channel TASK-1.
Materials and Methods
CGNs.
CGNs were isolated from 6- to 9-day-old Sprague–Dawley rats and cultured as described (15), and cerebellar slices (250 μm thick) were prepared from 3- to 5-week-old male mice (TO strain) as described (16).
Oocytes.
Oocytes were injected with cRNA transcribed from rTASK-1 cDNA, which was a kind gift from S. Yost (University of California at San Francisco). RNA (2–10 ng) was injected manually into oocytes that were kept for 3 days before recordings.
Electrophysiological Recordings.
For cultured cells, currents were recorded in the whole-cell perforated patch-clamp configuration with amphotericin B (240 μg/ml) as the permeabilizing agent from CGNs between 7 and 10 days in culture. Recording solutions have been described (15), and all experiments were performed at room temperature. Solutions were applied by bath perfusion at a rate of 4–5 ml⋅min−1, and complete exchange of the bath solution occurred within 30–40 s. For slices, perforated patch recordings were made from freshly prepared slices with recording conditions and solutions as described (16). For oocyte recordings, standard two-electrode voltage clamp measurements were performed at room temperature.
Reverse Transcriptase–PCR (RT-PCR).
Total RNA was prepared from cell suspensions of CGNs from 10-day-old cultures with the RNeasy miniprep kit (Qiagen, Chatsworth, CA). Samples of RNA were DNase treated and reverse transcribed by using Moloney murine leukemia virus-reverse transcriptase (Promega) and random hexamer primers. PCR was performed with Taq DNA polymerase (Promega) for 30 cycles. Two sets of primer pairs for TASK-1 were used: 5′-CACCGTCATCACCACAATCG (F1) with 5′-TGCTCTGCATCACGCTTCTC (R1) and 5′-AGTACGTGGCCTTCAGCTTC (F2) with 5′-TGGAGTACTGCAGCTTCTCG (R2). Actin primers were 5′-TTGTAACGAACTGGGACGATATGG with 5′-GATCTTGATCTTCATGGTGCTAGG. PCR products were gel extracted and purified, and their identities were confirmed by sequencing.
Antibody Labeling.
Anti-TASK-1, a polyclonal antibody raised in rabbit against a highly purified peptide (TASK 252–269) corresponding to residues 252–269 of human TASK-1 was obtained from both Alomone Labs (Jerusalem) and Chemicon and used at a dilution of 1:100. This epitope is specific for TASK-1 and is highly conserved in mouse and rat TASK-1. Staining was abolished by preabsorption of the antibody with 10 μg/ml of the peptide. Glial cells were labeled in the same CGN cultures with a mixture of mouse monoclonal antibodies against glial fibrillary acidic protein (GFAP; clones 4A11, 1B4, and 2E1; PharMingen; dilution 1:100). Primary antibodies were detected with FITC- and Cy3-labeled secondary antibodies, respectively, and visualized with an Olympus Fluoview laser scanning confocal microscope (New Hyde Park, NY).
Results
It has been suggested that IKSO in CGNs may result from expression of ether-a-go-go channels (such as eag-1; refs. 17 and 18) or be related to the M current (ref. 19; now thought to be formed from heteromultimers of KCNQ2 and KCNQ3, ref. 20). However, these channels are members of the 6TM family of K channels and have definable thresholds for voltage activation (19, 21). By contrast, a key diagnostic feature of members of the 2-PK channel family is that they are open at all potentials. To help determine the molecular nature of IKSO, we have examined the steady-state characteristics of the current over a range of potentials to determine whether it has a defined activation threshold.
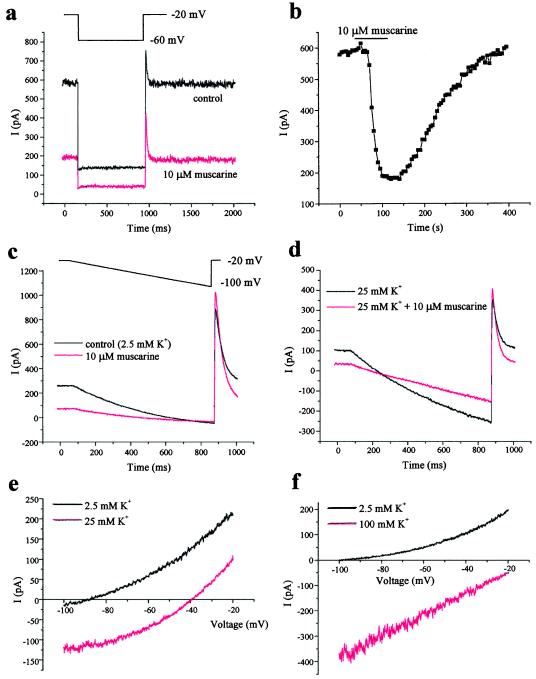
The basic features of IKSO seen in CGNs from both rats and mice are shown in Fig. 1a. IKSO can be seen as a noninactivating current when cells are held at −20 mV, which is reduced in amplitude when the cell is hyperpolarized to −60 mV. The current is rapidly and reversibly inhibited by 10 μM muscarine (Fig. 1 a and b; 68 ± 2%, n = 51) acting on muscarinic acetylcholine receptors. Current-voltage relations were obtained by hyperpolarizing the cell in ramp waveforms as shown in Fig. 1c. After step voltage changes, IKSO currents have been shown to reach steady-state with a time constant of 0.5 ms (see ref. 15); therefore, the ramp was sufficiently slow (10 ms/mV) to allow IKSO to reach steady-state at each potential. To ensure that IKSO was studied in isolation, we have exploited the sensitivity to muscarine. The control current-voltage relation for IKSO was obtained by subtracting currents evoked by hyperpolarizing ramps obtained in the presence of muscarine from those in its absence (Fig. 1e). The current-voltage relation was outwardly rectifying, and the reversal potential (−90 ± 4 mV, n = 4) was close to the predicted K+ equilibrium potential (−98 mV) when extracellular [K+] was 2.5 mM. The protocol was then repeated when the extracellular [K+] was raised to 25 mM (Fig. 1 d and e). This process revealed two things. First, the current must be a selective K+ current, because its reversal potential was shifted in a depolarizing direction to −39 ± 1 mV (n = 4) virtually identical to that predicted by the Nernst equation (−40 mV). Second, IKSO showed no deactivation (did not switch off) over the whole range of potentials tested, and therefore, it has no threshold for activation between these potentials. When [K+] was raised further to 100 mM (Fig. 1f), the reversal potential was shifted further, and the current-voltage relationship linearized, exactly as predicted by the Goldman–Hodgkin–Katz equation and in exactly the way seen for certain cloned 2-PK channels such as TASK-1 (6, 9).
Figure 1.
IKSO in rat cultured CGNs is active at all potentials and is inhibited by muscarine. (a) In perforated patch recordings, IKSO is seen as a noninactivating current at −20 mV and is instantaneously reduced in amplitude when cells are stepped to −60 mV for 800 ms once every 6 s. The current is inhibited by muscarine (10 μM). (b) Block and washout of block by muscarine is illustrated by measuring the standing-outward current at −20 mV. (c) Cells were held at −20 mV and then hyperpolarized to −100 mV by means of a ramp waveform at 0.1 mV/ms over 800 ms once every 6 s in the presence and absence of 10 μM muscarine. (d) Cells were treated as described in c, except in raised external [K+] (25 mM). (e) The muscarine-sensitive current during ramps is obtained by subtraction and shown in control and 25 mM [K+]. (f) Cells were treated as described in e, except in control and 100 mM external [K+].
Of the six functional mammalian 2-PK domain channels cloned thus far, only two, TREK-1 and TASK-1, show a number of striking similarities to IKSO. IKSO is insensitive to 4-aminopyridine (10 mM) and tetraethylammonium (5 mM) but blocked by Ba2+ (see below), quinidine (100 μM; 43 ± 4%, n = 6), and Na+ substitution by N-methyl-d-glucamine in the external solution (55 ± 4%, n = 7), all of which are features shared by TASK-1 and TREK-1 (6, 7, 9, 12). However, the two main diagnostic features of TASK-1 (in contrast to TREK-1) are its sensitivity to small changes in external pH and its lack of enhancement by arachidonic acid (13).
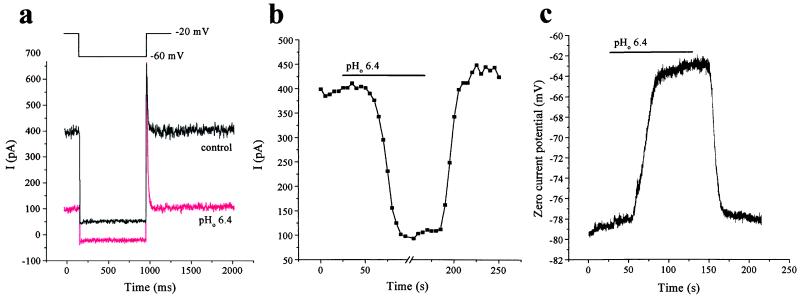
Treatment of CGNs with arachidonic acid (10 μM) did not enhance IKSO. This result argues against the idea that IKSO current is caused by expression of TREK-1 (12). Indeed, arachidonic acid caused a transient inhibition of IKSO (10.6 ± 2.2%, n = 9), but this inhibition was not maintained (steady-state inhibition was 0.2 ± 1.8%, n = 9), a result consistent with previous observations of TASK-1 (12). Exposure to extracellular solutions of pH 6.4 inhibited IKSO by 77 ± 3% (n = 10) compared with control currents measured at −20 mV in pH 7.4 solution (Fig. 2 a and b). Changing to an external solution of pH 6.9 gave only 31 ± 4% (n = 4) inhibition, whereas pH 7.9 slightly increased IKSO (7 ± 3%, n = 5) compared with control values. These results are very similar to the effects of changing external pH on TASK-1 currents expressed in Xenopus oocytes (6, 9). In our own experiments on heterologously expressed TASK-1 (see Fig. 3a), reducing external pH from 7.2 to 6.2 inhibited TASK-1 currents by 72 ± 7% (n = 3), whereas raising it from 7.2 to 8.2 enhanced TASK-1 currents by 25 ± 9% (n = 3). Ramp voltage protocols showed that the degree of inhibition of IKSO by acidification of the external solution was relatively independent of voltage. In current-clamp recordings, exposure to pH 6.4 solution depolarized cells by around 19 mV (from −78 ± 4 to −59 ± 3 mV, n = 5; Fig. 2c). The input resistance of CGNs around the resting potential of the cells (−70 to −90 mV) substantially increased from 477 ± 56 MΩ in normal external solution to 1,517 ± 367 MΩ (n = 5) at pH 6.4, indicating that external acidification enhances the excitability of the neurons by block of this resting conductance. Thus, block of IKSO by extracellular acidification will both depolarize CGNs directly and enhance the effects of other depolarizing inputs, such as glutamate acting on ionotropic glutamate receptors.
Figure 2.
IKSO in rat cultured CGNs is inhibited by extracellular acidification. (a) IKSO current recorded as in Fig. 1a is inhibited by changing external pH from 7.4 to 6.4. (b) Block and washout of block by pH 6.4 external solution (control pH 7.4) is illustrated by measuring the standing-outward current at −20 mV. (c) Current-clamp recordings show depolarization of a CGN after extracellular acidification.
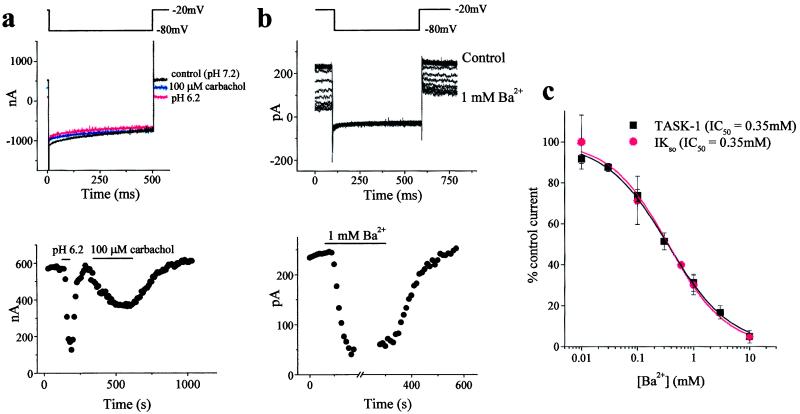
Figure 3.
TASK-1 expressed in oocytes shares properties of IKSO that is present in cerebellar slices. (a) TASK-1 currents expressed in oocytes and recorded by stepping to −80 mV for 500 ms once every 10 s from a holding potential of −20 mV have the same current profile as IKSO and are blocked by extracellular acidification and by carbachol. Block by extracellular acidification and carbachol (100 μM) is illustrated (Lower) by measuring the standing-outward current at −20 mV. (b) IKSO, recorded from CGNs in mouse cerebellar slices, was determined by the same voltage protocol described for a. Block by 1 mM Ba2+ is illustrated (Lower) by measuring the standing-outward current at −20 mV. (c) Ba2+ sensitivity of TASK-1 in oocytes and IKSO in cerebellar slices.
It is important to determine whether IKSO seen in cultured neonatal CGNs is also present in cells from more mature animals maintained as closely as possible to conditions in vivo. Observations on cerebellar slices (250 μm thick) from 3- to 5-week-old mice (where cerebellar synaptogenesis is almost complete; ref. 22) are illustrated in Fig. 3b. As with neonatal CGNs in culture (see ref. 15 and Fig. 1), CGNs in acutely isolated slices possess a rapidly activating, noninactivating potassium current with an amplitude of 179 ± 15 pA (n = 41) at −20 mV. As with IKSO in cultured CGNs, IKSO in cerebellar slices is blocked by Ba2+ ions; in the slice experiment, the IC50 was 0.35 mM (Fig. 3c).
The currents resulting from expression of TASK-1 have been studied previously by holding cells at negative potentials and applying step or ramp depolarizations (6, 9). Herein, we show that by holding TASK-1 expressing oocytes at a potential of −20 mV, the currents evoked show no appreciable inactivation over a time scale of seconds between hyperpolarizing steps to −80 mV. Hyperpolarizing steps to −80 mV reduced TASK-1 currents in proportion to the reduction in driving force, exactly as is seen for IKSO in neurons. Xenopus oocytes possess endogenous muscarinic receptors thought to be primarily of the M3 subtype (23, 24). Activation of these receptors by carbachol (100 μM) produced fully reversible 52 ± 6% (n = 4) inhibition of TASK-1 currents and an associated decrease in membrane conductance (Fig. 3a). In addition to their pH and carbachol sensitivity, TASK-1 currents were also inhibited by Ba2+ ions with an IC50 value of 0.35 mM, identical to the inhibition found for IKSO in CGNs in mouse cerebellar slices (Fig. 3c).
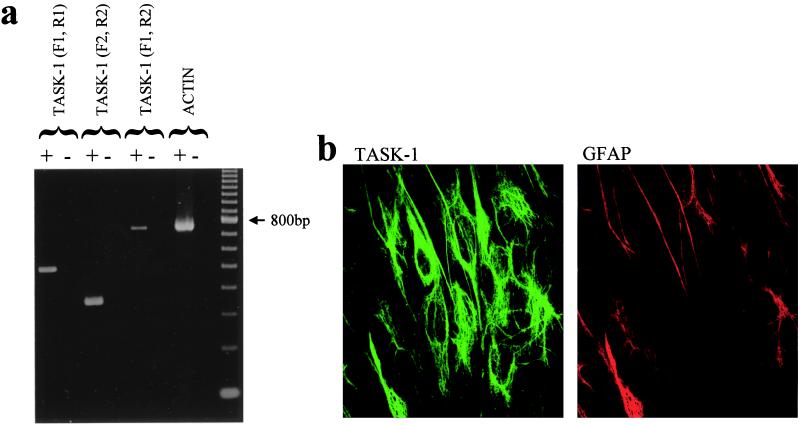
RT-PCR experiments with two specific primer pairs for TASK-1 indicated that the mRNA for TASK-1 is present in our CGN cultures (Fig. 4a). Furthermore, by using specific anti-TASK-1 antibodies, TASK-1 protein was seen to be expressed in the cytoplasm and surface membrane of CGNs in 7-day-old cultures (Fig. 4b). In contrast, negligible labeling for TASK-1 was seen in CGNs cultured for only 1 day, which is consistent with our previous observation of a lack of any measurable IKSO in such cells (15). Double-labeling with antibodies against GFAP indicated that TASK-1 protein was also expressed by glial cells in culture (Fig. 4b) and in adult rat astrocytes in situ (not shown).
Figure 4.
TASK-1 mRNA is present in CGN cultures and TASK-1 protein is expressed in the membrane of CGNs. (a) RT-PCR experiments identify the presence of mRNA for TASK-1 in CGNs cultures. (b) TASK-1 antibody (green) and GFAP (red) labeling in CGN cultures in the same field of view. TASK-1 protein appears to be expressed in both the cytoplasm and surface membrane of CGNs. Note also the TASK-1 staining in GFAP-labeled glial cells.
Discussion
In this study, we have shown that the noninactivating K current in CGNs, IKSO, has all the properties predicted for a background K channel belonging to the 2-PK family. Furthermore, its lack of a defined voltage threshold for activation, its regulation by muscarinic receptor activation, and its exquisite sensitivity to changes in the pH of the extracellular solution strongly suggest that IKSO is the functional correlate of the 2-PK channel TASK-1. Earlier in situ hybridization studies of Duprat et al. (6) showed that TASK-1 mRNA was present in the granule cell layer of the cerebellum. We have extended these observations with RT-PCR and a selective TASK-1 antibody, and we now show that TASK-1 is expressed in CGNs themselves and that the protein is found in the plasma membrane of these cells. Furthermore, protein expression is correlated with the magnitude of IKSO.
The sensitivity of IKSO to small changes in extracellular pH is of particular physiological importance, because extracellular acidification will both depolarize CGNs and increase their excitability after block of IKSO. Substantial decreases in extracellular pH (0.6 units) are known to accompany increases in extracellular glutamate levels with ischemia and are also seen during epileptic seizures (25). Furthermore, transient acid shifts occur during the release of neurotransmitters from storage vesicles (25), which are also highly acidic. These transient acidic shifts are known to facilitate excitatory transmission in the central nervous system (26).
Remarkably, of more than 80 known potassium channel α-subunits identified to date from the sequence of the nematode Caenorhabditis elegans, over 50 belong to this emerging family of K channels (27). It is likely, therefore, that the very few 2-PK channels identified thus far in mammalian neurons are the forerunners of a large extended family. It has been suggested recently that they may also represent an important target protein for general anaesthetic agents, which can enhance the activity of certain 2-PK channels thereby decreasing neuronal excitability (28, 29). This functional demonstration of a mammalian 2-PK channel in neurons shows the channel's likely importance in controlling cell excitability and also suggests a mechanism for how agents that modify its activity, such as muscarinic receptor agonists and hydrogen ions, can profoundly alter both the neuron's resting potential and its excitability.
Acknowledgments
This work was supported by the Medical Research Council, the Wellcome Trust, and the National Institutes of Health. Thanks to Mark Farrant and Steve Brickley for comments on an earlier version of the manuscript, Annette Dolphin for access to her molecular laboratory, Dianne Dewey for help with the immunohistochemistry, David Boyd for providing some CGN cultures, and Spencer Yost for rTASK cDNA.
Abbreviations
- CGN
cerebellar granule neuron
- 2-PK
two-pore domain potassium
- IKSO
standing-outward K+ current
- TM
transmembrane domain
- RT-PCR
reverse transcriptase–PCR
- GFAP
glial fibrillary acidic protein
Note Added in Proof
Since the original submission of our work, we have become aware of a study showing functional expression of TASK-1 in hypoglossal motoneurons (30).
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.050012597.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.050012597
References
- 1.Chandy K G, Gutman G A. In: Handbook of Receptors and Channels. North R A, editor. Boca Raton, FL: CRC; 1995. pp. 1–71. [Google Scholar]
- 2.Mathie A, Wooltorton J R A, Watkins C S. Gen Pharmacol. 1998;30:13–24. doi: 10.1016/s0306-3623(97)00034-7. [DOI] [PubMed] [Google Scholar]
- 3.Robertson B. Trends Pharmacol Sci. 1997;18:474–483. doi: 10.1016/s0165-6147(97)01140-1. [DOI] [PubMed] [Google Scholar]
- 4.Goldstein S A N, Wang K W, Ilan N, Pausch M H. J Mol Med. 1998;76:13–20. doi: 10.1007/s001090050186. [DOI] [PubMed] [Google Scholar]
- 5.Ketchum K A, Joiner W J, Sellers A J, Kaczmarek L K, Goldstein S A N. Nature (London) 1995;376:690–695. doi: 10.1038/376690a0. [DOI] [PubMed] [Google Scholar]
- 6.Duprat F, Lesage F, Fink M, Reyes R, Heurteaux C, Lazdunski M. EMBO J. 1997;16:5464–5471. doi: 10.1093/emboj/16.17.5464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fink M, Duprat F, Lesage F, Reyes R, Romey G, Heurteaux C, Lazdunski M. EMBO J. 1996;15:6854–6862. [PMC free article] [PubMed] [Google Scholar]
- 8.Fink M, Lesage F, Duprat F, Heurteaux C, Reyes R, Fosset M, Lazdunski M. EMBO J. 1998;17:3297–3308. doi: 10.1093/emboj/17.12.3297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leonoudakis D, Gray A T, Winegar B D, Kindler C H, Harada M, Taylor D N, Chavez R A, Forsayeth J R, Yost C S. J Neurosci. 1998;18:868–877. doi: 10.1523/JNEUROSCI.18-03-00868.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lesage F, Guillemare E, Fink M, Duprat F, Lazdunski M, Romey G, Barhanin J. EMBO J. 1996;15:1004–1011. [PMC free article] [PubMed] [Google Scholar]
- 11.Maingret F, Fosset M, Lesage F, Lazdunski M, Honore E. J Biol Chem. 1999;274:1381–1387. doi: 10.1074/jbc.274.3.1381. [DOI] [PubMed] [Google Scholar]
- 12.Patel A J, Honore E, Maingret F, Lesage F, Fink M, Duprat F, Lazdunski M. EMBO J. 1998;17:4283–4290. doi: 10.1093/emboj/17.15.4283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reyes R, Duprat F, Lesage F, Fink M, Salinas M, Farman N, Lazdunski M. J Biol Chem. 1998;273:30863–30869. doi: 10.1074/jbc.273.47.30863. [DOI] [PubMed] [Google Scholar]
- 14.Chavez R A, Gray A T, Zhao B B, Kindler C H, Mazurek M J, Mehta Y, Forsayeth J R, Yost C S. J Biol Chem. 1999;274:7887–7892. doi: 10.1074/jbc.274.12.7887. [DOI] [PubMed] [Google Scholar]
- 15.Watkins C S, Mathie A. J Physiol. 1996;491:401–412. doi: 10.1113/jphysiol.1996.sp021224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Southan A, Robertson B. J Neurosci. 1998;18:948–955. doi: 10.1523/JNEUROSCI.18-03-00948.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marrion N V. Trends Neurosci. 1997;20:243–244. doi: 10.1016/s0166-2236(97)86998-6. [DOI] [PubMed] [Google Scholar]
- 18.Hoshi N, Takahashi H, Shahidullah M, Yokoyama S, Higashida H. J Biol Chem. 1998;273:23080–23085. doi: 10.1074/jbc.273.36.23080. [DOI] [PubMed] [Google Scholar]
- 19.Brown D A. In: Ion channels. Narahashi T, editor. New York: Plenum; 1988. pp. 54–94. [Google Scholar]
- 20.Wang H S, Pan Z, Shi W, Brown B S, Wymore R S, Cohen I S, Dixon J E, McKinnon D. Science. 1998;282:1890–1893. doi: 10.1126/science.282.5395.1890. [DOI] [PubMed] [Google Scholar]
- 21.Stansfeld C E, Roper J, Ludwig J, Weseloh R M, Marsh S J, Brown D A, Pongs O. Proc Natl Acad Sci USA. 1996;93:9910–9914. doi: 10.1073/pnas.93.18.9910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Larramendi L M. In: Neurobiology of Cerebellar Evolution and Development. Llinas R, editor. Chicago: Am. Med. Assoc.; 1969. pp. 803–843. [Google Scholar]
- 23.Kusano K, Miledi R, Stinnakre J. J Physiol. 1982;328:143–170. doi: 10.1113/jphysiol.1982.sp014257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Davidson A, Mengod G, Matusleibovitch N, Oron Y. FEBS Lett. 1991;284:252–256. doi: 10.1016/0014-5793(91)80697-2. [DOI] [PubMed] [Google Scholar]
- 25.Chesler M. Prog Neurobiol. 1990;34:401–427. doi: 10.1016/0301-0082(90)90034-e. [DOI] [PubMed] [Google Scholar]
- 26.Yanovsky Y, Reymann K, Haas H L. Eur J Neurosci. 1995;7:2017–2020. doi: 10.1111/j.1460-9568.1995.tb00624.x. [DOI] [PubMed] [Google Scholar]
- 27.Bargmann C I. Science. 1998;282:2028–2033. doi: 10.1126/science.282.5396.2028. [DOI] [PubMed] [Google Scholar]
- 28.Patel A J, Honore E, Lesage F, Fink M, Romey G, Lazdunski M. Nat Neurosci. 1999;2:422–426. doi: 10.1038/8084. [DOI] [PubMed] [Google Scholar]
- 29.Franks N P, Lieb W R. Nat Neurosci. 1999;2:395–396. doi: 10.1038/8054. [DOI] [PubMed] [Google Scholar]
- 30.Talley E M, Lei Q, Sirois J E, Bayliss D A. Neuron. 2000;25:399–410. doi: 10.1016/s0896-6273(00)80903-4. [DOI] [PubMed] [Google Scholar]