Abstract
The cloned vanilloid receptor VR1 has attracted recent attention as a molecular integrator of painful stimuli on primary sensory neurons. The existence of vanilloid-sensitive neurons in the brain is, however, controversial. In this study, we have used an antibody and a complementary RNA probe to explore the distribution of neurons that express VR1 in rat and in certain areas of human brain. In the rat, we observed VR1-expressing neurons throughout the whole neuroaxis, including all cortical areas (in layers 3 and 5), several members of the limbic system (e.g., hippocampus, central amygdala, and both medial and lateral habenula), striatum, hypothalamus, centromedian and paraventricular thalamic nuclei, substantia nigra, reticular formation, locus coeruleus, cerebellum, and inferior olive. VR1-immunopositive cells also were found in the third and fifth layers of human parietal cortex. Reverse transcription–PCR performed with rat VR1-specific primers verified the expression of VR1 mRNA in cortex, hippocampus, and hypothalamus. In the central nervous system, neonatal capsaicin treatment depleted VR1 mRNA from the spinal nucleus of the trigeminal nerve, but not from other areas such as the inferior olive. The finding that VR1 is expressed not only in primary sensory neurons but also in several brain nuclei is of great importance in that it places VRs in a much broader perspective than pain perception. VRs in the brain (and putative endogenous vanilloids) may be involved in the control of emotions, learning, and satiety, just to name a few exciting possibilities.
Capsaicin, the pungent principle in hot pepper, has long been used as a neuro-pharmacological tool to identify sensitive neurons and their contribution to health and disease. In 1969, capsaicin was shown to induce characteristic ultrastructural changes (swollen mitochondria) in sensitive neurons (1). Using this morphological criterion and observations based on the neurotoxic action of capsaicin (2, 3) to define capsaicin sensitivity, three major divisions of capsaicin-sensitive neurons were described: (i) small-diameter neurons with C-fibers as well as some Aδ-neurons in sensory (dorsal root and trigeminal) ganglia; (ii) a peptidergic subpopulation of neurons in nodose ganglia; and (iii) cells in the anterior hypothalamus (1). Capsaicin-sensitive primary sensory neurons transmit nociceptive information from the periphery into the central nervous system (CNS) (4–6). The peripherial terminals of these neurons are sites of release for a variety of proinflammatory neuropeptides that initiate the cascade of neurogenic inflammation (7). Spinal and trigeminal capsaicin-sensitive fibers are believed to participate in local vasoregulation of mucosal protection (8). Finally, capsaicin-sensitive neurons in the hypothalamus were suggested to mediate the well-known hypothermic action of capsaicin (9).
The existence of a capsaicin receptor was first predicted based on the strict structure-activity relations for capsaicin-like bioactivity (10). Specific binding of [3H]resiniferatoxin (RTX), an ultrapotent capsaicin analog provided the first unequivocal biochemical proof that capsaicin receptors exist (11). To reflect the structural similarity between capsaicin and RTX, their common recognition site was renamed as the vanilloid receptor (VR) (5). [3H]RTX binding confirmed the presence of VRs on primary sensory and nodose ganglion neurons (5). Also, a relatively low level of specific [3H]RTX binding sites were described in several brain nuclei (12), suggesting that the tissue distribution of VRs might be much broader than thought previously. This concept is entirely in accord with the pharmacological actions of capsaicin microinjected into the rat brain (6).
In 1997, the first VR, termed VR1, was cloned from a rat dorsal root ganglia (DRG) cDNA library (13). This receptor now is recognized as a common molecular target for protons, noxious heat, and vanilloids (4–6, 13). Using Northern blotting or in situ hybridization (see Fig. 7, which is published as supplemental material on the PNAS web site, www.pnas.org), the presence of mRNA encoding VR1 was shown in sensory and nodose ganglia (13, 14). By PCR detection, VR1 mRNA also seems to be present in the rat brain (15). Although Northern blot analysis failed to show VR1 mRNA in the brain (13), this discrepancy may be caused by the difference in sensitivity between PCR and Northern blot hybridization. Consequently, the existence of VRs in the brain remained elusive. Therefore, we decided to perform immunocytochemistry using well-characterized antibodies (16) and in situ hybridization histochemistry (ISHH) using a specific RNA probe to localize VR1-expressing cells in the CNS of the rat. Additionally, we confirmed the expression of VR1 mRNA in CNS tissues by reverse transcription–PCR (RT-PCR).
Methods
Tissues.
Adult male Sprague–Dawley rats were used in the experiments to determine the distribution of VR1-like immunoreactivity and its mRNA. Two kinds of tissue samples were used. First, fresh frozen tissues were mounted on tissue holders and 12-μm sections were cut in a cryostat. Second, animals were perfused through the ascending aorta under pentobarbital anesthesia with buffered paraformaldehyde, cryoprotected, and cut on a cryostat. A few of the perfused brains were removed and postfixed for 3 h in the same fixative, and 50-μm-thick vibratome sections also were cut.
Human material was obtained 4–6 h postmortem. The tissues were frozen on dry ice then 12-μm-thin sections were cut and stored at −80°C until used.
For immunohistochemistry in both frozen and vibratome sections, the avidin-biotin (ABC) technique and the indirect fluorescence approach were used. Two previously characterized antibodies (one raised in rabbit and one in guinea pig) were used to detect VR1-like immunoreactivity (16). The specificity of antisera was determined by absorption controls with the cognate peptide and by staining VR1-transfected mammalian (HEK293) cells. For both techniques, the slides were incubated with the primary antibodies overnight at 4°C, and then a secondary antibody was added. For the ABC technique, the secondary antibody was a biotinylated anti-rabbit or anti-guinea-pig IgG (Vector Laboratories) used at a 1:1,000 dilution at room temperature for 1 h. For indirect fluorescent staining, affinity-purified Fab fragments of anti-rabbit, anti-mouse, and anti-guinea pig IgG were used preconjugated to either CY3, Alexa-546 (red), or FITC (green) fluorochromes at a 1:1,000 and 1:100 dilution, respectively, for 1 h at room temperature. For double stainings, the incubations were performed consecutively with the first primary antibody, first secondary antibody, second primary antibody (antityrosine-hydroxylase mAb manufactured by Boehringer Mannheim; used at 1:1,000), and second secondary antibody. The sections then were stained with a chromosomal stain (4′,6-diamidino-2-phenylindole; Sigma) and viewed through a Leitz Dialux 20 fluorescent microscope using the appropriate filter sets.
Controls included immunostaining without the primary antibodies and with the antisera that were previously preabsorbed with the immunizing peptide (absorption controls). Another control was performed by using both antibodies in the same section and performing double immunofluorescent labeling to demonstrate that both antibodies recognize the same cells. Controls of the double stainings included incubating the sections with the first primary antibody and adding the second secondary antibody to make sure of no cross-reaction between the secondary and primary antibodies.
In situ hybridization was performed by using standard methods. A radiolabeled antisense probe was generated with SP6 RNA polymerase and 35S-UTP using a plasmid (pGT rVR1), as a template, that contained nucleotides 1779–2704 of the rat VR1 cDNA (13). A sense probe also was generated with T7 polymerase and used as a control in adjacent brain series. For more technical details, see our web page: http://intramural.nimh.nih.gov/lcmr/snge/Protocols/ISHH/ISHH.html.
Capsaicin Treatment of Newborn Rats.
Rats were injected with capsaicin (50 mg/kg dissolved in 10% ethanol, 10% Tween 20, 80% saline) or solvent (n = 6) on postnatal day 2 as originally described by Jancsó and coworkers (2). After 6 weeks, the capsaicin-treated rats were challenged with a 0.01% capsaicin solution applied to the cornea, and six rats were selected that responded with two or fewer protective eye-wiping movements. Rats were decapitated, the brains and DRGs were taken, and adjacent series of sections were cut in a cryostat from the whole brain and the ganglia. These sections then were hybridized by using the riboprobe for VR1. Optical density of the ISHH signal was measured directly from the slides by using the Scion image program (National Institutes of Health). Data from measurements of the DRG, spinal trigeminal nuclei, and the inferior olives were statistically evaluated by t test analysis. Results are expressed as mean ± SEM. The slides were viewed with a Leitz Dialux 20 microscope, and then pictures were taken.
RT-PCR Assays.
Tissues were rapidly dissected from male Sprague–Dawley rats (200–250 g), and total RNA was isolated by the method of Chomczynski and Sacchi (17). Samples were examined by gel electrophoresis to ensure that the RNA was not degraded. RT was performed as follows: 5 μg total RNA was digested with 1 unit of RQ1 DNase I (Promega) for 15 min at 37°C. The products of this reaction were extracted with phenol/chloroform, precipitated, and recovered by centrifugation. The pellet was resuspended in water, 0.5 μg random primers (Life Technologies, Bethesda, MD), and 1 μl RNasin (Promega) in a final volume of 9 μl. This sample was heated to 75°C for 10 min and slow-cooled to 3°C to allow primers to anneal. Then, 4 μl of 5× First Strand Synthesis Buffer (Life Technologies), 4 μl 10 mM dNTPs, 2 μl 0.1 M DTT, and 1 μl Superscript II (Life Technologies) were added to the sample. The reaction was incubated at 42°C for 60 min and 7°C for 10 min. The products were recovered by precipitation with 5 μl 10 mM ammonium acetate and 50 μl absolute ethanol followed by centrifugation. The resulting pellet was resuspended in 10 μl of sterile water, and 1–2 μl was analyzed by PCR. A reaction without RNA was run as a negative control in every experiment. PCR was performed by using the GeneAmp XL PCR kit (Perkin–Elmer as directed by the manufacturer with 10 pmol of each primer per reaction. Specific rat VR1 primers were primer 1 (5′-GACATGCCACCCAGCAGG) and primer 2 (5′-TCAATTCCCACACACCTCCC) corresponding to nucleotides 2491–2508 and 2755–2735, respectively, of the published rVR1 cDNA sequence (13). Specific rat stretch inhibitable channel (SIC) primers were primer 3 (5′-TCACCTCCGAGGTAGTGTGG) and primer 4 (5′-GATCAAGTGGTGGTCCCC) corresponding to nucleotides 1993–1974 and 1787–1805, respectively, of the published rSIC nucleotide sequence (18). PCR conditions were 94°C, 30 sec; 55°C, 30 sec; 69°C, 3 min for 30 cycles. Reactions without any template were included as negative controls. PCRs were analyzed by electrophoresis in agarose (1.6%) gels for the presence of specific rVR1 or rSIC bands. In some experiments, these bands were excised from the gel, subcloned, and analyzed by nucleotide sequencing to verify their identity.
Results
Immunohistochemistry showed neurons with VR1-like immunopositivity in DRG (Fig. 1 C and D) and in several areas in the rat brain. The antibodies raised in guinea pig and the rabbit showed identical staining patterns (Fig. 1 A and B). In all cortical areas, many neurons were stained in layers 3 and 5 (not shown). Several areas of the limbic system contained positive cells, including the hippocampus and the dentate gyrus, the fimbrial, dorsal and lateral septal nucleus, the central amygdala, both medial and lateral habenula, the mammillary nuclei, and the interpeduncular nucleus (not shown). Many small neurons in the striatum showed a weak immunoreactivity (not shown). In the hypothalamus, there were immunopositive cells in the suprachiasmatic nucleus (not shown). Also, numerous weakly immunostained cells were detected in the anterior hypothalamic nuclei, the paraventricular nuclei among the magnocellular hypothalamic neurons, the dorsomedial hypothalamic nucleus, and the arcuate nuclei (not shown). There was a weak staining in the centromedian and paraventricular thalamic nuclei (not shown). In the mesencephalon, the pars compacta of the substantia nigra showed a distinct immunolabeling. A few cells in the dorsal raphe nucleus and the reticular formation (not shown) were also positive. In the lower brainstem, the cerebellum, the locus coeruleus, the cochlear nuclei, cells in the nucleus of the solitary tract, and the nucleus of the trigeminal nerve contained cells with VR1-like immunoreactivity (not shown). The inferior olive also showed strong immunostaining for VR1 (Fig. 1 A and B).
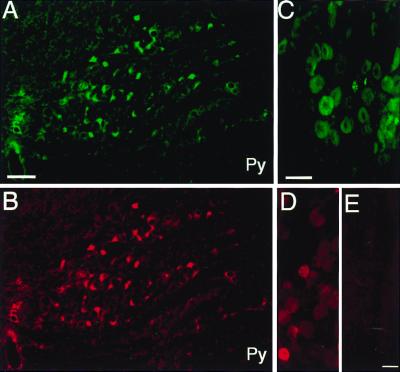
Figure 1.
Immunohistochemistry controls. Immunostaining is shown in the inferior olive by using an antibody to VR1 receptor raised in guinea pig (A) and rabbit (B) in adjacent sections. Note that the cells immunopositive with both antibodies are identical. (C and D) Immunostaining using the same antibodies (C, guinea pig; D, rabbit) in the DRG. (E) A section of DRG immunostained with the rabbit antibody that had been previously preabsorbed with the immunizing peptide (negative control). Py, pyramidal tract. (Scale: 100 μm, A and B; 50 μm, C–E.)
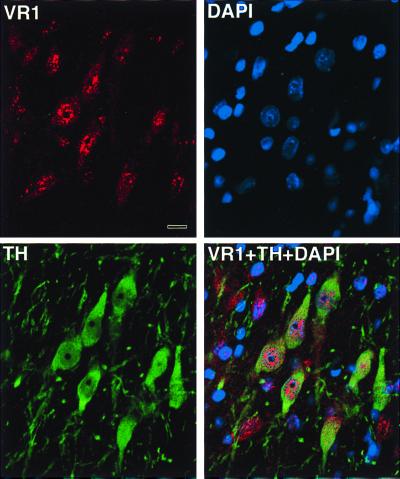
Double labeling immunofluorescence showed a complete overlap between VR1-positive and tyrosine hydroxylase (TH)-positive cells in the substantia nigra by using rabbit (anti-VR1) and mouse (anti-TH) mAbs (Fig. 2).
Figure 2.
Fluorescent images taken through a confocal microscope. Section thickness: 1 μm. Rat substantia nigra immunostained with the anti-VR1 antibody raised in rabbit and then anti-rabbit IgG-Alexa 546 dye was applied as a secondary antibody. Many of the substantia nigra neurons show a punctate-like cytoplasmic as well as nuclear staining. A monoclonal mouse anti-TH antibody (Boehringer Mannheim) was used to mark the dopaminergic neurons in the same area. FITC was used as a secondary antibody-green fluorescence and 4′,6-diamidino-2-phenylindole (DAPI) (blue) was used as a nuclear marker viewed through the UV filter. Finally, a digital overlay of the three images demonstrates that all substantia nigra TH immunopositive neurons are also VR1 positive and shows that some of the immunostaining is nuclear. Note that there are some VR1 immunopositive cells that are not TH positive. (Scale: 10 μm.)
There were no labeled cells present after immunostaining with the antisera that were previously preabsorbed with the immunizing peptides (Fig. 1E). Also, no labeling was found when the primary antibody was not applied (not shown). When the guinea pig (anti-VR1C) and rabbit (anti-VR1N) antibodies were applied together on the same sections the two stainings showed a complete overlap in all regions examined (Fig. 1 A and B).
In the human tissues examined we found VR1-immunopositive cells in the DRG (Fig. 3A).
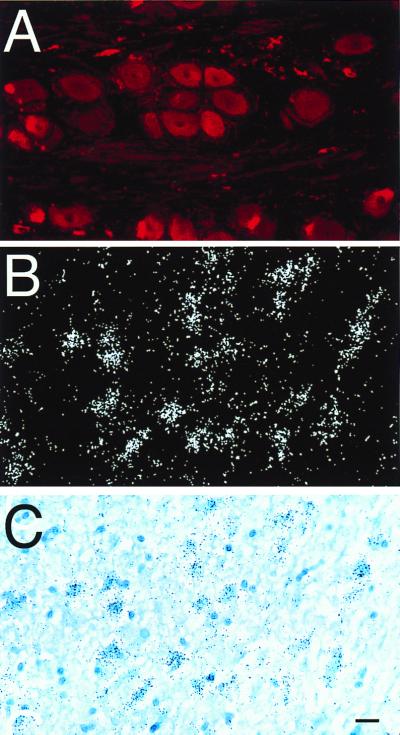
Figure 3.
(A) Immunostaining of a human DRG using the rabbit anti-VR1 antibody and CY3 as a fluorochrome. (B) A section of human parietal cortex hybridized with a probe recognizing the VR1 mRNA. (C) The identical field illuminated in bright field. Note the large number of neurons expressing the receptor. (Scale: 25 μm, A; 50 mm, B and C.)
ISHH showed the presence of VR1 mRNA in many CNS regions. The strongest signals were detected in the following areas: all cortical areas including the olfactory cortex (Fig. 4 A and B), septum (Fig. 4 C and D), hippocampus and dentate gyrus (Fig. 4 E and F), substantia nigra (zona compacta) (Fig. 4 J and K), cerebellum and locus coeruleus (Fig. 4 H and I), and inferior olive (Fig. 4 L and M). A weaker but clear signal was detected in the habenula, central amygdala, several hypothalamic nuclei, midline thalamic nuclei, solitary tract nucleus, and the nucleus of the spinal trigeminal tract (not shown). There were scattered positive cells in the reticular formation (not shown). The sense RNA probe failed to detect any structures in the corresponding areas including the hippocampus (Fig. 4G), confirming the specificity of the labeling by the antisense probe.
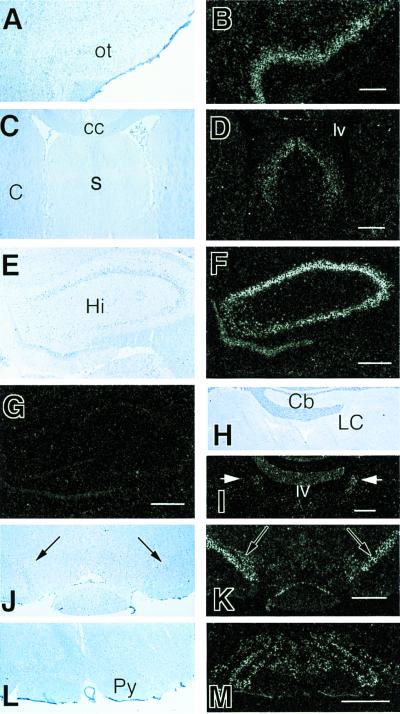
Figure 4.
ISHH in the rat CNS using a riboprobe complementary to the VR1 mRNA. We found high to medium levels of mRNA being expressed in neurons of several areas including the olfactory cortex (A) bright-field and (B) dark-field illumination of the same field; the lateral and dorsal septal nuclei (C) bright-field and (D) dark-field illumination; hippocampal pyramidal cells and dentate gyrus (E) bright field and (F) dark field; in the locus coeruleus (arrows) (H) bright field and (I) dark field; in the substantia nigra (J) bright field and (K) dark field, and in the inferior olive (L) bright-field and (M) dark-field illumination. (G) A hippocampal area similar to E and F that was hybridized with a sense probe and shows no signal. ot, olfactory tubercle; cc, corpus callosum; s, septum; lv, lateral ventricle; C, caudate; Cb, cerebellum; Hi, hippocampus; LC, locus coeruleus; Py, pyramidal tract. (Scale: 1 mm, A–D, L, and M; 800 μm, E–G, J, and K; 500 μm, H and I).
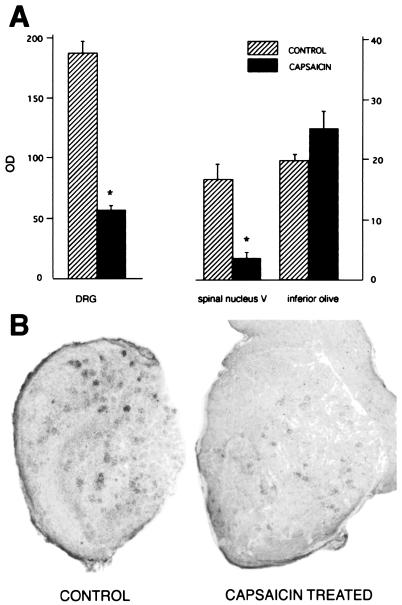
In most areas examined such as the inferior olive (Fig. 5A), there were no clear differences in the expression and/or distribution of the VR1 receptor mRNA between the brains of rats given neonatally capsaicin or solvent only. The sole exception was the nucleus of the spinal trigeminal nerve, where the number of grains per cell as well as the number of labeled cells were 70% lower (P < 0.001) in the treated rats than in the normal (saline injected) controls (Fig. 5A). As expected, DRG neurons showed similar changes after neonatal capsaicin treatment (Fig. 5 A and B).
Figure 5.
Effect of the single neonatal capsaicin treatment on VR1 receptor mRNA levels [OD, optical density in the DRG, the spinal trigeminal nucleus (spinal nucleus V), and the inferior olive in adult rats]. Data are expressed as mean (n = 8–10) ± SEM, and P < 0.001 (*) versus capsaicin-treated and control groups. Although there is a significant drop (70–80%) in the amount of mRNA in the DRG and the nucleus of the spinal trigeminal tract, there is no change in the inferior olive. After in situ hybridization of the DRG sections a significant drop in the number of positive cells as well as in the total amount of silver grains over cells in control vs. capsaicin-treated rats is shown.
In good agreement with the rat data, we also observed a number of cell bodies containing VR1 mRNA in the human parietal cortical third and fifth layers (Fig. 3 B and C).
RT-PCR Assay.
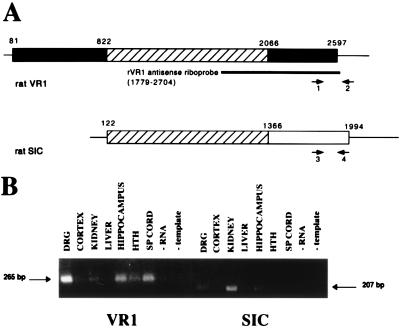
To validate that VR1 was expressed in CNS tissues RT-PCR was performed with VR1-specific primers. The expected 265-bp PCR product was detected in DRG, hippocampus, spinal cord, hypothalamus, cerebral cortex, and kidney (Fig. 6B). Nucleotide sequence analysis of VR1 PCR products from DRG and hypothalamus revealed that these bands are a fragment of rVR1. Because the recently identified SIC sequence (18) contains a region of identity to VR1 (Fig. 6A), we also performed RT-PCR with SIC-specific primers. The expected 207-bp PCR product was detected in kidney, DRG, cerebral cortex, hippocampus, hypothalamus, and spinal cord. Nucleotide sequence analysis of SIC PCR products from kidney and hippocampus indicates that these bands are a fragment of rSIC. No PCR product was observed for either primer sets in liver. Ribonuclease protection assays indicate that the rVR1 antisense riboprobe (nucleotides 1779–2704) detects both VR1 and SIC RNAs (data not shown).
Figure 6.
Sequence comparison and RT-PCR analysis of VR1 and SIC RNA expression in several rat tissues. (A) A schematic nucleotide sequence comparison of rat VR1 and SIC is shown. Cross-hatched boxes correspond to the region of sequence identity between VR1 and SIC. Other boxes represent gene-specific, protein coding sequences; lines represent untranslated sequences. Oligonucleotide primers for rat VR1 (primers 1 and 2) and rat SIC (primers 3 and 4) were designed to amplify gene-specific fragments as shown. The antisense riboprobe used for ISHH is shown for reference. (B) RT-PCR analysis was performed by using VR1-specific primers or SIC-specific primers to examine expression in DRG, cerebral cortex, kidney, liver, hippocampus, hypothalamus (HTH), spinal cord, negative RT control (no RNA), and negative PCR control (no template). The example shown is representative of at least two independent experiments.
Discussion
Although the presence of vanilloid (capsaicin) receptors is firmly established in dorsal root, trigeminal, as well as nodose ganglia (4–6), the existence of vanilloid-sensitive intrinsic neurons in the brain has been hotly debated with the sole exception of the anterior hypothalamus, an area linked to the hypothermic action of capsaicin three decades ago (9). In favor of the existence of vanilloid-sensitive neurons in the CNS, four lines of evidence usually are mentioned: (i) capsaicin evokes various biological effects when microinjected into different brain areas (1); (ii) systemic capsaicin treatment leads to extensive argyrophylia (a capability to bind silver salts) through the whole neuroaxis of the rat (19); (iii) there is a low to moderate level of specific [3H]RTX binding to membranes obtained from various CNS areas (12); and (iv) RT-PCR detects VR1 mRNA widely in the brain (15). Those who oppose the existence of vanilloid-sensitive structures in the CNS cite the following negative findings: (i) with the exception of the area postrema/nucleus of the solitary tract, a central termination site for nodose ganglion neurons, [3H]RTX autoradiography fails to detect any specific labeling on rat brain sections (20), and (ii) Northern blot hybridization performed with total RNA isolated from whole brain did not detect mRNA encoding VR1 (13).
These results were confirmed by RT-PCR, which shows that VR1 is expressed throughout the rat CNS, including cortex, hypothalamus, and hippocampus. We also found evidence that the recently cloned SIC mRNA is expressed in these tissues. Because VR1 and SIC share a large region of sequence identity (1,244 nt in the region coding for the transmembrane domains; Fig. 6A), the coexpression of VR1 and SIC in several tissues raises the possibility that they are splice variants of a single gene. The VR1 riboprobe used for ISHH analysis in this study contains a portion of the VR1/SIC region of identity (nucleotides 1799–2066 of the rat VR1 gene; Fig. 6A), and, therefore, may hybridize with both VR1 and SIC RNAs. However, immunohistochemical analysis with VR1-specific antisera supports the assertion that VR1 is expressed in brain regions that stain positive by in situ hybridization.
A number of previous studies implied the existence of VRs in the cortex. In 1977, Jancsó and Wolleman (21) reported that capsaicin stimulated adenylate cyclase activity in the rat cerebral cortex in vitro. In 1995, neonatal capsaicin treatment was found to reduce the density of nicotinic acetylcholine receptors (specific [3H]nicotine binding sites) in the cerebral cortex of the rat by 35% (22) and depress the metabolic activity of the somatosensory cortex as determined by 2-deoxy-glucose uptake (23). In the adult rat, capsaicin administration was shown to induce the immediate early gene NGFI-A in the cerebral cortex (24).
The presence of VR1-expressing neurons in the limbic system is not unexpected either. Capsaicin was shown to block Ca2+ channels in isolated trigeminal and hippocampal neurons with similar potencies (25). Other groups also have detected mRNA for VR1 in the hippocampus by RT-PCR (15). Somewhat paradoxically, capsaicin treatment increased substance P (SP) content in the amygdala of spontaneously hypertensive rats (26). Moreover, capsaicin induced the expression of the immediate early genes c-fos, c-jun, junB, and junD in the amygdala (27). In the habenula, capsaicin was reported to cause discernible changes in deep electroencephalogram activity (28) and to cause profound neurotoxicity as detected by argyrophylia (19). This neurodegenation occurred in other areas of the limbic system, too, including the mammillary nuclei and the interpeduncular nucleus (19).
The striatum receives massive input both from the cortex and the amygdala, two areas where we detect VR1-expressing neurons. Apparently, some neurons in the striatum also express VR1. This is in accord with the presence of mRNA for VR1 in the striatum as detected by RT-PCR (15). Furthermore, our finding is in keeping with previous observations that capsaicin, when microinjected into the striatum, causes peripheral vasodilatation and a subsequent hypothermia response (29) and, when given systemically, increases mRNA for NGFI-A in the striatum (24).
The presence of capsaicin-responsive structures in the preoptic area and in other areas of the hypothalamus was first postulated by Szolcsányi and colleagues in the early 1970s (9, 30). Later, the existence of such neurons was repeatedly confirmed by other groups (28, 31, 32). These capsaicin-sensitive neurons are thought to carry 5-hydroxytryptamine receptors (32). The vanilloid-sensitive terminals in the hypothalamus may be glutamatergic because capsaicin was shown to evoke glutamate release from slices of hypothalamus (15). A subpopulation of hypothalamus neurons, like primary sensory neurons, expresses SP (33). Capsaicin was shown to deplete SP from primary sensory neurons by Jessell and coworkers in 1978 (34). However, capsaicin effects on the SP content of hypothalamus neurons are controversial. For instance, capsaicin-treated mice show isolation-induced aggressive behavior, and a correlation between SP depletion by capsaicin in the hypothalamus and offensive upright posture was demonstrated (35). By contrast, capsaicin seems to enhance SP expression the preoptic area of spontaneously hypertensive rats (26). Nonetheless, Helke and colleagues (36) failed to detect any changes in SP-like immunoreactivity in the hypothalamus of capsaicin-treated rats. A depletion by capsaicin in the β-endorphin content of the hypothalamus also was described (37), an effect which might be mediated through the VR1 receptors in the arcuate nucleus.
In 1976, the high concentration of SP in the substantia nigra was first reported (38) and the search for capsaicin-sensitive neurons in the basal ganglia began. Enhanced locomotor activity in rats after bilateral intranigral capsaicin injection and a concomitant decrease in the cataleptic action of fluphenazine was reported (39) as well as a peripheral vasodilatation response and subsequent hypothermia (29). Until now, it was not known which subpopulation of nigral neurons expresses VRs. Neonatal capsaicin treatment does not deplete SP from the striatum, nor does it alter the expression of the opioid peptides dynorphin and met5-enkephalin (40). With regard to monoaminergic systems, increased (41) or decreased 5-hydroxytryptamine levels (39), or no change at all (42), because of capsaicin treatment were reported in the very same year. The complete overlap between TH- and VR1-immunoreactive neurons in the nigra now finally identifies the vanilloid-sensitive nigral cells as monoaminergic.
From the dorsal raphe, serotoninergic fibers reach both the striatum and the substantia nigra. Capsaicin was reported to change the electroencephalogram activity in the dorsal raphe (28) and to elicit heat loss (43) after microinjection. As the source of the brain's major noradrenergic system, the locus coeruleus is of particular interest. The locus coeruleus is believed to play an important role in sleep, attention, and memory formation (44). Capsaicin was shown to excite locus coeruleus neurons directly and this action was suggested to be involved in the analgesic activity of this compound (43). It is not unlikely that neurons in the locus coeruleus are responsible for the well-known, transient, anesthesia-like condition that follows capsaicin treatment. Given these observations, it is of great importance that our results firmly establish the presence of VR1 both in the dorsal raphe and the locus coeruleus.
The presence of VR1-expressing neurons in the inferior olive and the cerebellum is intriguing. Previous studies with RT-PCR already demonstrated VR1 mRNA in the cerebellum (15). Recent reports suggest that in addition to its well-known role in motor coordination, the cerebellum also might participate in autonomic control (45), in cognitive functions as well as affective behavior (46). The role of the inferior olive in the operation of the cerebellum remains enigmatic. The role of VR1 in cochlear nuclei or the olfactory bulb is also elusive. On the other hand, it is easy to visualize how VR1-expressing neurons in the reticular formation may mediate some vanilloid actions on autonomic regulation (47).
Neonatal capsaicin treatment kills vanilloid-sensitive neurons in sensory ganglia (2). As a matter of fact, depletion by neonatal capsaicin treatment has been used extensively as a criterion to identify vanilloid-sensitive structures (6). Our finding that neonatal capsaicin administration does not change the density of VR1 in most brain areas provides an explanation as to why vanilloid-responsive cells in the CNS remained largely overlooked. The mechanism(s) by which capsaicin given to newborn rats kills neurons is poorly understood (5). Some neurons may die immediately because of direct excitotoxicity; however, most neurons seem to perish in a delayed fashion (5). The majority of these neurons may be rescued by exogenous nerve growth factor (48). We speculate that capsaicin does not kill VR1-expressing neurons in the brain because these cells do not depend on any neurotrophic factor for survival that capsaicin may deplete.
We conclude that VR1-expressing neurons exist in several areas of the brain. These neurons may participate in various as-yet-unsuspected vanilloid-sensitive pathways and may be regulated by endogenous vanilloids. Furthermore, recent evidence suggest that VR1 might be a potential target for cannabis as well as endogenous cannabinoids (i.e., anandamide) (49, 50). Thus, these findings place VR1 in a much broader context than perception of noxious stimuli.
Supplementary Material
Abbreviations
- VR
vanilloid receptor
- CNS
central nervous system
- RTX
resiniferatoxin
- DRG
dorsal root ganglia
- ISHH
in situ hybridization histochemistry
- RT-PCR
reverse transcription–PCR
- SIC
stretch inhibitable channel
- TH
tyrosine hydroxylase
- SP
substance P
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.060496197.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.060496197
References
- 1.Joo F, Szolcsányi J, Jancsó-Gábor A. Life Sci. 1969;8:621–626. doi: 10.1016/0024-3205(69)90023-x. [DOI] [PubMed] [Google Scholar]
- 2.Jancsó G, Király E, Jancsó-Gábor A. Nature (London) 1977;270:741–743. doi: 10.1038/270741a0. [DOI] [PubMed] [Google Scholar]
- 3.Jancsó G, Király E. J Comp Neurol. 1980;190:781–792. doi: 10.1002/cne.901900409. [DOI] [PubMed] [Google Scholar]
- 4.Wood J D. Capsaicin in the Study of Pain. San Diego: Academic; 1993. [Google Scholar]
- 5.Szallasi A, Blumberg P M. Pharmacol Rev. 1999;51:159–212. [PubMed] [Google Scholar]
- 6.Holzer P. Pharmacol Rev. 1991;43:143–201. [PubMed] [Google Scholar]
- 7.Geppetti P, Holzer P. Neurogenic Inflammation. Boca Raton, FL: CRC; 1996. [Google Scholar]
- 8.Holzer P. Am J Physiol. 1998;275:G8–G13. doi: 10.1152/ajpgi.1998.275.1.G8. [DOI] [PubMed] [Google Scholar]
- 9.Jancsó-Gábor A, Szolcsányi J, Jancsó N. J Physiol (London) 1970;206:495–507. doi: 10.1113/jphysiol.1970.sp009027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Szolcsányi J, Jancsó-Gábor A. Arzneim-Forsch. 1975;25:1877–1881. [PubMed] [Google Scholar]
- 11.Szallasi A, Blumberg P M. Brain Res. 1990;524:106–111. doi: 10.1016/0006-8993(90)90498-z. [DOI] [PubMed] [Google Scholar]
- 12.Ács G, Palkovits M, Blumberg P M. Life Sci. 1996;59:1899–1908. doi: 10.1016/s0024-3205(96)00537-1. [DOI] [PubMed] [Google Scholar]
- 13.Caterina M J, Schumacher M A, Tominaga M, Rosen T A, Levine J D, Julius D. Nature (London) 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- 14.Tominaga M, Caterina M J, Malmberg A B, Rosen T A, Gilbert H, Skinner K, Raumann B E, Basbaum A I, Julius D. Neuron. 1998;21:531–543. doi: 10.1016/s0896-6273(00)80564-4. [DOI] [PubMed] [Google Scholar]
- 15.Sasamura T, Sasaki M, Tohda C, Kuraishi Y. NeuroReport. 1998;9:2045–2048. doi: 10.1097/00001756-199806220-00025. [DOI] [PubMed] [Google Scholar]
- 16.Guo A, Vulchanova L, Wang J, Li X, Elde R. Eur J Neurosci. 1999;11:946–958. doi: 10.1046/j.1460-9568.1999.00503.x. [DOI] [PubMed] [Google Scholar]
- 17.Chomczynski P, Sacchi N. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 18.Suzuki M, Sato J, Kutsuwada K, Ooki G, Imai M. J Biol Chem. 1999;274:6330–6335. doi: 10.1074/jbc.274.10.6330. [DOI] [PubMed] [Google Scholar]
- 19.Ritter S, Dinh T T. In: Capsaicin in the Study of Pain. Wood J N, editor. San Diego: Academic; 1993. pp. 105–138. [Google Scholar]
- 20.Szallasi A, Nilsson S, Farkas-Szallasi T, Blumberg P M, Hokfelt T, Lundberg J M. Brain Res. 1995;703:175–183. doi: 10.1016/0006-8993(95)01094-7. [DOI] [PubMed] [Google Scholar]
- 21.Jancsó G, Wollemann M. Brain Res. 1977;123:323–329. doi: 10.1016/0006-8993(77)90483-8. [DOI] [PubMed] [Google Scholar]
- 22.Roberts R G, Stevenson J E, Westerman R A, Pennefather J. NeuroReport. 1995;6:1578–1582. doi: 10.1097/00001756-199507310-00028. [DOI] [PubMed] [Google Scholar]
- 23.Wu C C, Gonzalez M F. Brain Res Dev Brain Res. 1995;87:62–68. doi: 10.1016/0165-3806(95)00056-j. [DOI] [PubMed] [Google Scholar]
- 24.Honkaniemi J, Kononen J, Kainu T, Pyykonen I, Pelto-Huikko M. Brain Res Mol Brain Res. 1994;25:234–241. doi: 10.1016/0169-328x(94)90158-9. [DOI] [PubMed] [Google Scholar]
- 25.Kopanitsa M V, Panchenko V A, Magura E I, Lishko P V, Krishtal O A. NeuroReport. 1995;6:2338–2340. doi: 10.1097/00001756-199511270-00016. [DOI] [PubMed] [Google Scholar]
- 26.Virus R M, McManus D Q, Gebhart G F. Eur J Pharmacol. 1982;81:67–73. doi: 10.1016/0014-2999(82)90602-1. [DOI] [PubMed] [Google Scholar]
- 27.Honkaniemi J, Kainu T, Ceccatelli S, Rechardt L, Hökfelt T, Pelto-Huikko M. NeuroReport. 1992;3:849–852. doi: 10.1097/00001756-199210000-00007. [DOI] [PubMed] [Google Scholar]
- 28.Rabe L S, Buck S H, Moreno L, Burks T F, Dafny N. Brain Res Bull. 1980;5:755–758. doi: 10.1016/0361-9230(80)90216-6. [DOI] [PubMed] [Google Scholar]
- 29.Hajós M, Engberg G, Nissbrandt H, Magnusson T, Carlsson A. J Neural Transm. 1988;74:129–139. doi: 10.1007/BF01244779. [DOI] [PubMed] [Google Scholar]
- 30.Szolcsányi J, Joo F, Jancsó-Gábor A. Nature (London) 1971;229:116–117. doi: 10.1038/229116a0. [DOI] [PubMed] [Google Scholar]
- 31.Benedek G, Obal F, Jr, Lelkes Z, Obal F. Acta Physiol Acad Sci Hung. 1982;60:27–35. [PubMed] [Google Scholar]
- 32.Hori T, Shibata M, Kiyohara T, Nakashima T, Asami A. Neuropharmacology. 1988;27:135–142. doi: 10.1016/0028-3908(88)90162-1. [DOI] [PubMed] [Google Scholar]
- 33.Larsen P J. J Comp Neurol. 1992;316:287–313. doi: 10.1002/cne.903160304. [DOI] [PubMed] [Google Scholar]
- 34.Jessell T M, Iversen L L, Cuello A C. Brain Res. 1978;152:183–188. doi: 10.1016/0006-8993(78)90146-4. [DOI] [PubMed] [Google Scholar]
- 35.Bigi S, De Acetis L, De Simone R, Aloe L, Alleva E. Behav Neurosci. 1993;107:363–369. doi: 10.1037//0735-7044.107.2.363. [DOI] [PubMed] [Google Scholar]
- 36.Helke C J, DiMicco J A, Jacobowitz D M, Kopin I J. Brain Res. 1981;222:428–431. doi: 10.1016/0006-8993(81)91049-0. [DOI] [PubMed] [Google Scholar]
- 37.Panerai A E, Martini A, Locatelli V, Mantegazza P. Pharmacol Res Commun. 1983;15:825–832. doi: 10.1016/s0031-6989(83)80090-3. [DOI] [PubMed] [Google Scholar]
- 38.Brownstein M J, Mroz E A, Kizer J S, Palkovits M, Leeman S E. Brain Res. 1976;116:299–305. doi: 10.1016/0006-8993(76)90907-0. [DOI] [PubMed] [Google Scholar]
- 39.Dawbarn D, Harmar A J, Pycock C J. Neuropharmacology. 1981;20:341–346. doi: 10.1016/0028-3908(81)90006-x. [DOI] [PubMed] [Google Scholar]
- 40.Sivam S P, Krause J E. J Neurochem. 1992;59:2278–2284. doi: 10.1111/j.1471-4159.1992.tb10121.x. [DOI] [PubMed] [Google Scholar]
- 41.Holzer P, Saria A, Skofitsch G, Lembeck F. Life Sci. 1981;29:1099–1105. doi: 10.1016/0024-3205(81)90197-1. [DOI] [PubMed] [Google Scholar]
- 42.Jancsó G, Hökfelt T, Lundberg J M, Király E, Halász N, Nilsson G, Terenius L, Rehfeld J, Steinbusch H, Verhofstad A, et al. J Neurocytol. 1981;10:963–980. doi: 10.1007/BF01258524. [DOI] [PubMed] [Google Scholar]
- 43.Hajós M, Jancsó G, Engberg G. Acta Physiol Scand. 1987;129:415–420. doi: 10.1111/j.1748-1716.1987.tb08086.x. [DOI] [PubMed] [Google Scholar]
- 44.Aston-Jones G, Shipley M T, Chouvet G, Ennis M, van Bockstaele E, Pieribone V, Shiekhattar R, Akaoka H, Drolet G, Astier B, et al. Prog Brain Res. 1991;88:47–75. doi: 10.1016/s0079-6123(08)63799-1. [DOI] [PubMed] [Google Scholar]
- 45.Paton J F, Spyer K M. J Physiol (London) 1990;427:533–552. doi: 10.1113/jphysiol.1990.sp018186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bloedel J R, Bracha V. Int Rev Neurobiol. 1997;41:613–634. doi: 10.1016/s0074-7742(08)60373-6. [DOI] [PubMed] [Google Scholar]
- 47.Koulchitsky S V, Azev O A, Gourine A V, Kulchitsky V A. Neurosci Lett. 1994;182:129–132. doi: 10.1016/0304-3940(94)90780-3. [DOI] [PubMed] [Google Scholar]
- 48.Otten U, Lorez H P, Businger F. Nature (London) 1983;301:515–517. doi: 10.1038/301515a0. [DOI] [PubMed] [Google Scholar]
- 49.Zygmunt P M, Petersson J, Andersson D A, Chuang H, Sorgard M, Di Marzo V, Julius D, Hogestatt E D. Nature (London) 1999;400:452–457. doi: 10.1038/22761. [DOI] [PubMed] [Google Scholar]
- 50.Melck D, Bisogno T, De Petrocellis L, Chuang H, Julius D, Bifulco M, Di Marzo V. Biochem Biophys Res Commun. 1999;262:275–284. doi: 10.1006/bbrc.1999.1105. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.