Abstract
Practicing movements results in improvement in performance and in plasticity of the motor cortex. To identify the underlying mechanisms, we studied use-dependent plasticity in human subjects premedicated with drugs that influence synaptic plasticity. Use-dependent plasticity was reduced substantially by dextromethorphan (an N-methyl-d-aspartate receptor blocker) and by lorazepam [a γ-aminobutyric acid (GABA) type A receptor-positive allosteric modulator]. These results identify N-methyl-d-aspartate receptor activation and GABAergic inhibition as mechanisms operating in use-dependent plasticity in intact human motor cortex and point to similarities in the mechanisms underlying this form of plasticity and long-term potentiation.
The functional organization of the motor system, including the primary motor cortex (1–5), is modified by use, and it has been suggested that use-dependent plasticity may play a major role in the recovery of function after stroke (3, 6). Identifying the underlying neural mechanisms may contribute to the design of rationally founded strategies to enhance these plastic changes when they play a compensatory and beneficial role (6). Plasticity of the motor cortex has been studied in slices of rat brain (7). It has been demonstrated that synaptic efficacy is modifiable in an activity-dependent manner, resulting in long-term potentiation (LTP) (7). Use-dependent plasticity, involving cortical reorganization within the thumb representation, has been demonstrated in the human motor cortex (4). A short period of training, consisting of simple, voluntary, repetitive thumb movements in a specific direction, elicits reorganization of the cortical representation of the thumb that encodes the kinematic details of the practiced movement (4). Similarly, relatively brief training periods involving synchronous movements of the thumb and upper arm (8) and the thumb and foot (9) elicit a medial expansion of the thumb representation (8, 9).
In the current study, we hypothesized that pharmacological manipulation that interferes with synaptic plasticity (10, 11) would block reorganization within the cortical thumb representation, thereby identifying the mechanisms underlying use-dependent plasticity in the intact human motor cortex. Specifically, we tested the effects on use-dependent plasticity of (i) lorazepam (LZ), a drug that enhances γ-aminobutyric acid type A (GABAA) receptor function by acting as a positive allosteric modulator (12) and that blocks the induction of LTP (10); (ii) dextromethorphan (DM), a drug that blocks N-methyl-d-aspartate (NMDA) receptors (11, 13), required for LTP in the motor cortex (7, 11), and experience-dependent plasticity in the somatosensory cortex (14); and (iii) lamotrigine (LG), a drug that modifies the gating of voltage-activated Na+ and Ca2+ channels (15) without affecting LTP induction (16, 17).
Methods
Subjects.
The study protocol was approved by the Institutional Review Boards of the National Institute of Neurological Disorders and Stroke. Subjects gave their written informed consent for the study.
Stimulation and Recording.
Subjects were seated in a chair firmly connected to a frame that kept the head steady and the stimulating coil in a constant position with respect to the head. Head and coil stability was monitored with a three-dimensional laser system. Each subject's right forearm was immobilized in a molded armrest with the four long fingers supported and the thumb entirely unconstrained. Thumb movements were recorded with a two-dimensional accelerometer mounted on the proximal phalanx of the thumb (4). The directions of transcranial magnetic stimulation (TMS)-evoked and of voluntary thumb movements were calculated from the first-peak acceleration vector.
Surface electromyographic activity was recorded from the extensor pollicis brevis and its antagonist muscle, flexor pollicis brevis. TMS was delivered from a custom-built magnetoelectric stimulator (Cadwell Laboratories, Kennewick, WA) through a figure eight-shaped magnetic coil (wing diameter, 7.0 cm). As described (18), TMS predominantly activates corticospinal neurons transsynaptically. Stimuli were delivered to the optimal scalp position for eliciting isolated thumb movements. Motor threshold, a measure of neuronal excitability (19), was defined as the minimum TMS intensity that evoked a motor evoked potential (MEP) of at least 50 μV in 5 of 10 trials at rest. The motor threshold, the intensity of TMS required to elicit mild thumb movements in a consistent direction, and the MEP amplitudes evoked by these stimulus intensities did not differ across conditions. Trials with background activity were discarded from analysis.
Experimental Setup.
Baseline.
Before training, 60 TMS stimuli were delivered at 0.1 Hz to the optimal scalp position to elicit thumb movements. Subjects occasionally realized that the thumb had moved, but could not determine in which direction. In these trials, the baseline direction was defined as the mean angle of TMS-evoked movements that fell in the predominant direction (Fig. 1).
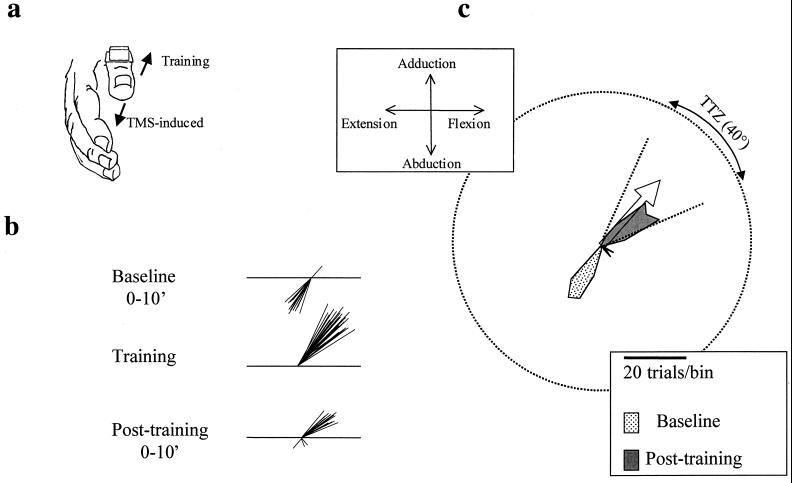
Figure 1.
(a) Acceleration signals were recorded in the horizontal (extension and flexion) and vertical (abduction and adduction) axes of thumb movements. The direction of TMS-evoked or voluntary movement was derived from the first-peak acceleration in the two major axes of the movement. (b) Schematic diagram of the directional change of first-peak-acceleration vector of movements evoked by TMS after 30 min of training. At baseline, TMS evoked predominantly extension and abduction thumb movements. Therefore, training consisted of repetitive, stereotyped, brisk thumb movements in a flexion and adduction direction. Posttraining, the direction of TMS-evoked thumb movements changed from the baseline direction to the trained direction. (c) Circular frequency histogram from one representative subject. Baseline TMS-induced movement directions are predominantly a combination of extension and abduction. The open arrow indicates the mean training direction at the center of the training target zone (TTZ). The scale shows the number of TMS-evoked movements that fall in each 10° bin (see Methods). TMS-induced movement directions after training fell largely within the TTZ, close to a 180° change from the baseline direction. Circular frequency histograms in the following figures are constructed in the same way.
Training.
After identifying the baseline direction, subjects practiced voluntary brisk thumb movements in a direction opposite to baseline for 30 min at 1 Hz. After each movement, the thumb returned to the start position by relaxation, as confirmed by electromyograph. Acceleration and electromyographic signals of 180 representative training movements were sampled at 1 kHz. Accuracy and consistency of training were monitored on-line by the investigators. If necessary, the subject was encouraged to perform better. Additionally, we measured the angular difference between training and baseline directions, the dispersion of training movement directions, and the magnitude of the first-peak accelerations of these movements, which did not differ across conditions (Table 1).
Table 1.
Kinematics of training movements in different conditions
| Condition | Peak acceleration, g | Angular deviation, degrees | Dispersion, r |
|---|---|---|---|
| Control | 0.35 ± 0.06 | 175.0 ± 1.14 | 0.97 ± 0.01 |
| LG | 0.40 ± 0.07 | 158.8 ± 9.24 | 0.99 ± 0.01 |
| LZ | 0.39 ± 0.07 | 169.4 ± 3.90 | 0.97 ± 0.01 |
| DM | 0.35 ± 0.06 | 176.0 ± 1.14 | 0.99 ± 0.01 |
Results are expressed as mean ± SE for five subjects.
Posttraining.
At the end of the training period, TMS was reapplied to the motor cortex at 0.1 Hz for 30 min. To describe the training effects on TMS-evoked movement directions, we defined a training target zone (TTZ) as a window of ±20° centered on the training direction. The TTZ is depicted in Fig. 1c. Our measure was the increase in the proportion of TMS-evoked movements within the TTZ posttraining (Figs. 1, 2, and 3b). Because, by design, the training was in the direction opposite to the baseline direction, the proportion of TMS-evoked movements within the TTZ before training was very small. Therefore, our principal measure was the degree to which training increased the proportion of evoked movements falling within the TTZ. Posttraining TMS-evoked movement directions were grouped in 10-min intervals of 60 trials each.
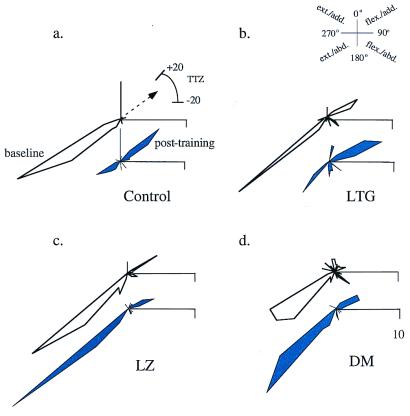
Figure 2.
Drug effects on directional distribution of TMS-evoked movements in a single subject. Directions of TMS-evoked movements are shown in pairs of circular histograms, baseline (Upper) and posttraining (Lower). Frequencies are plotted on the same scale. Directions are grouped in bins of 10°. Mean training angle (arrow) and TTZ for all conditions are shown in a. In the control (a) and LG (b) condition, TMS-evoked movements at baseline were mainly in the extension/abduction (ext./abd.) direction (Inset). Posttraining, the majority of TMS-evoked movements occurred in TTZ, in the flexion/adduction (flex./add.) direction. LZ (c) and DM (d) blocked the training effect. TMS-evoked movements remained in the ext./abd. direction after training.
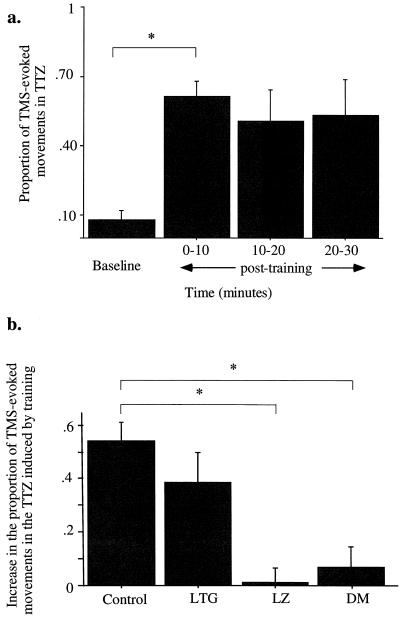
Figure 3.
Drug effects on TMS-evoked movements in TTZ in five subjects. (a) Control condition. Proportion of TMS-evoked movements that occurred in TTZ at baseline and 0–10, 10–20, and 20–30 min after the training was completed (mean ± SE). Compared with baseline, the number of movements that occurred in TTZ increased significantly after training (0–10 min) and remained high for at least 30 min. (b) Posttraining (0–10 min) condition. Increase in the proportion of movements falling in the TTZ in the control and drug conditions (mean ± SE). LZ and DM blocked the increase in proportions seen in the control condition. *, P < 0.025.
Inclusion Criteria.
Of 11 subjects who were naïve to the experimental procedure, 5 (2 women and 3 men, 24–44 years old) fulfilled strict inclusion criteria in a separate session before the study (inclusion experiment). (i) The ability of TMS to elicit isolated thumb movements in the absence of movement of any other digits, wrist, or arm; (ii) consistent (reproducible) direction of TMS-evoked movements at baseline; and (iii) posttraining TMS-evoked movement directions that matched the training direction.
Experimental Design.
All subjects completed four sessions that were separated by at least 72 h, a control session (drug-naïve) and one for each of the three drugs LZ, LG, and DM, in a double-blind, counterbalanced design. A single oral dose of LZ (0.038 mg/kg) (20, 21) and LG (300 mg) (22) was administered 2 h before testing, whereas a single oral dose of DM (2 mg/kg) was given 3 h before testing (23, 24). In human subjects, LZ at this dose previously was shown to occupy brain benzodiazepine receptors sufficiently to produce functional potentiation of GABAA receptors (21). The dose of LG chosen was similar to the one commonly used as an antiepileptic (25, 26). In humans, DM at the dose used results in brain concentrations (23) similar to those that induce NMDA receptor block in vitro (11, 27). Transient side effects included mild sedation (LZ, DM, and LG), mild nausea and blurry vision (LG), and slight incoordination (DM). None of these effects affected training performance (see Experimental Setup). Three subjects participated in additional sessions that determined that drug administration in the absence of motor training did not elicit consistent directional changes in TMS-evoked movement directions.
Statistical Analysis.
A distribution-free multiple-comparison procedure based on Friedman rank sums (28) was used to compare increases in proportions of TMS-evoked movements in the TTZ after training in the three drug and control conditions. A significance level of 0.025 was chosen for each of the two applications of the Friedman procedure (within and between conditions). Separate one-way ANOVA was used to assess the effect of drugs on motor thresholds, stimulus intensities, and MEP amplitudes. All data are expressed as mean ± SE.
Results
At the beginning of each experimental session, we determined the direction of the majority of TMS-evoked movements (baseline direction, see Methods and Fig. 1). Next, each subject practiced repetitive, voluntary thumb movements in the direction opposite to baseline (training direction) for 30 min (Table 1). After practice, we calculated the increase in the proportion of TMS-evoked movements that fell within a ±20o window centered on the training direction (TTZ, see Methods).
In the control (drug-naïve) condition (Figs. 2a and 3b), the proportion of TMS-evoked movements that fell in the TTZ after training increased by 0.54 ± 0.07 in reference to baseline (P = 0.003). The effect lasted for at least 30 min (Fig. 3a). In the LG condition, this proportion increased by 0.39 ± 0.11 (P = 0.018; Figs. 2b and 3b). In contrast, in the LZ (which enhances GABAA receptor activity) and DM (which blocks NMDA receptor activity) conditions, the increase in the proportion of TMS-evoked movements falling in the TTZ after training were 0.01 ± 0.06 and 0.07 ± 0.11, respectively (Figs. 2 c and d and 3b). Compared with the control condition, the increased proportion of TMS-evoked movements falling in the TTZ after training were significantly different in the LZ (P = 0.006) and the DM (P = 0.019) but not in the LG condition. Therefore, both LZ and DM blocked the training-induced shift in TMS-evoked movement directions toward the TTZ.
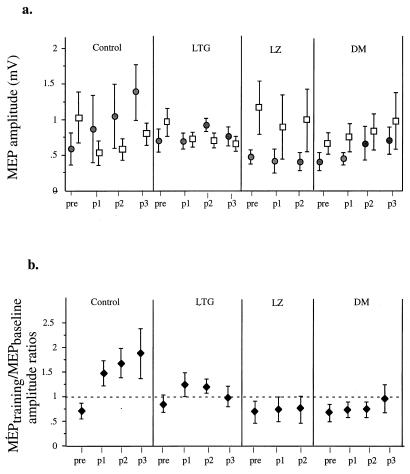
In addition to TMS-evoked movement directions, we recorded motor potentials (MEPs) evoked by TMS in muscles mediating movements in the training (MEPtraining) and in the baseline (MEPbaseline) directions. Before training, MEPtraining amplitudes were smaller than MEPbaseline amplitudes in all conditions (Fig. 4a). Therefore, the MEPtraining/MEPbaseline amplitude ratio was <1 (Fig. 4b). In the control condition, which showed the most pronounced increase of TMS-evoked movements in TTZ, the MEPtraining amplitudes after training increased, whereas the MEPbaseline amplitudes decreased relative to the baseline period (Fig. 4 a and b). Therefore, training evoked a relatively specific increase in cortical excitability for muscles mediating movements in the training direction and a decrease in cortical excitability for muscles mediating movements in the baseline direction. This effect lasted for at least 30 min (Fig. 4b), as did the shift in TMS-evoked movement directions (Fig. 3a). Results obtained in this control session closely resembled those that were obtained in the inclusion experiment (see Methods). In both cases, training resulted in significant directional changes in TMS-evoked movements (Watson U2 test (29) <0.01 in each case). In the LG condition, training led to similar but less marked changes in MEPtraining/MEPbaseline amplitude ratios (Fig. 4b). On the other hand, DM and LZ blocked the change in MEPtraining/MEPbaseline amplitude ratios seen in the control and LG conditions (Fig. 4b), corresponding to the persistence of the TMS-evoked movements in the baseline direction.
Figure 4.
Drug effects on MEP amplitudes in five subjects. (a) Amplitudes for MEPbaseline (□) and MEPtraining (●) at baseline (pre) and posttraining p1 (0–10 min), p2 (10–20 min), and p3 (20–30 min). For the LZ condition, only p1 and p2 are shown, because one subject did not complete p3. At baseline, MEPbaseline amplitude exceeded that of MEPtraining in all conditions. (b) MEPtraining/MEPbaseline amplitude ratios (mean ± SE) at baseline (pre) and posttraining (p1, p2, and p3). In the control and LG conditions, training resulted in MEPtraining/MEPbaseline amplitude ratios (♦) >1. By contrast, LZ and DM blocked these training-induced changes, resulting in MEPtraining/MEPbaseline amplitude ratios <1.
Discussion
The consistency of training kinematics (Table 1), motor threshold (see Methods), and baseline MEP amplitudes (Fig. 4a) across conditions and the differential effects of training on the excitability of muscles with different functions (Fig. 4 a and b) indicate that these results are not likely due to nonspecific global changes in cortical or subcortical excitability or attentional differences related to the use of each drug, including side effects. Additionally, in the absence of motor training, these drugs did not elicit consistent directional changes. Because there is evidence that this form of use-dependent plasticity occurs in the motor cortex (4) and that both LZ and DM influence cortical excitability at suprasegmental sites (30, 31), the reported differences in TMS-induced movement directions and MEP amplitudes likely reflect differential excitability changes within the thumb representation of the motor cortex. The net effect of such changes in excitability could be training-induced strengthening of the intracortical neuronal ensembles generating outputs in the training direction. Such a mechanism previously has been proposed for a different form of plasticity, the reorganization of cortical motor maps across representational boundaries (5, 7). The possibility that DM and/or LZ might block that form of plasticity or act also at subcortical sites in this paradigm remains to be tested.
The finding that DM blocks the training-induced directional changes in thumb movements suggests that NMDA receptor activation is necessary for the manifestation of use-dependent plasticity in humans. Additionally, our results demonstrate a substantial reduction of use-dependent plasticity by LZ, a drug that enhances GABAA receptor-mediated inhibition (12) and blocks activity-dependent plasticity, such as LTP (10). Taken together, our results favor the involvement of an activity-dependent LTP-like mechanism. The evidence in favor of this view includes the findings that LTP in the motor cortex requires activation of NMDA receptors (13), that down-regulation of GABA facilitates LTP after tetanic stimulation in motor cortex slices (7, 13), and that LG, a drug that does not affect LTP induction (16, 17), had no discernable effect on use-dependent plasticity in our experimental paradigm. These findings point to a similarity between mechanisms of LTP, which is widely held to underlie learning and memory and use-dependent plasticity of the motor system.
Acknowledgments
We thank our subjects for their participation in the study; M. Hallett, M. Rogawski, and J. Grafman for their comments; N. Dang and G. Dold for technical support; B. Knebel for recommendations regarding the administration of drugs; and D. G. Schoenberg for skillful editing. This work was supported partially by a grant from the Office of Alternative Medicine, National Institutes of Health.
Abbreviations
- LTP
long-term potentiation
- LZ
lorazepam
- DM
dextromethorphan
- NMDA
N-methyl-d-aspartate
- LG
lamotrigine
- TMS
transcranial magnetic stimulation
- MEP
motor evoked potential
- TTZ
training target zone
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.050350297.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.050350297
References
- 1.Pascual-Leone A, Grafman J, Hallet M. Science. 1994;263:1287–1289. doi: 10.1126/science.8122113. [DOI] [PubMed] [Google Scholar]
- 2.Karni A, Meyer G, Jezzard P, Adams M M, Turner R, Ungerleider L G. Nature (London) 1995;377:155–158. doi: 10.1038/377155a0. [DOI] [PubMed] [Google Scholar]
- 3.Nudo J R, Wise B M, SiFuentes F, Milliken G W. Science. 1996;272:1791–1794. doi: 10.1126/science.272.5269.1791. [DOI] [PubMed] [Google Scholar]
- 4.Classen J, Liepert A, Wise S P, Hallett M, Cohen L G. J Neurophysiol. 1998;79:1117–1123. doi: 10.1152/jn.1998.79.2.1117. [DOI] [PubMed] [Google Scholar]
- 5.Rioult-Pedotti M-S, Friedman D, Hess G, Donoghue J P. Nat Neurosci. 1998;1:230–234. doi: 10.1038/678. [DOI] [PubMed] [Google Scholar]
- 6.Bütefisch C M, Hummelsheim H, Denzler P, Mauritz K-H. J Neurol Sci. 1995;130:59–68. doi: 10.1016/0022-510x(95)00003-k. [DOI] [PubMed] [Google Scholar]
- 7.Hess G, Donoghue J P. J Neurophysiol. 1994;71:2543–2547. doi: 10.1152/jn.1994.71.6.2543. [DOI] [PubMed] [Google Scholar]
- 8.Cohen L G, Gerloff C, Faiz L, Uenishi N, Hallett M. Soc Neurosci Abstr. 1996;22:1452. [Google Scholar]
- 9.Liepert J, Terborg C, Weiller C. Exp Brain Res. 1999;125:435–439. doi: 10.1007/s002210050700. [DOI] [PubMed] [Google Scholar]
- 10.Riches I P, Brown M W. Neurosci Lett Suppl. 1986;24:S42. [Google Scholar]
- 11.Wong B Y, Coulter D A, Choi D W, Prince D A. Neurosci Lett. 1988;85:261–266. doi: 10.1016/0304-3940(88)90362-x. [DOI] [PubMed] [Google Scholar]
- 12.Macdonald R L, Kelly K M. Epilepsia. 1995;36, Suppl. 2:S2–S12. doi: 10.1111/j.1528-1157.1995.tb05996.x. [DOI] [PubMed] [Google Scholar]
- 13.Hess G, Aizenman C, Donoghue J P. J Neurophysiol. 1996;75:1765–1778. doi: 10.1152/jn.1996.75.5.1765. [DOI] [PubMed] [Google Scholar]
- 14.Rema V, Armstrong-James M, Ebner F F. J Neurosci. 1998;18:10196–10206. doi: 10.1523/JNEUROSCI.18-23-10196.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lees G, Leach M J. Brain Res. 1993;612:190–199. doi: 10.1016/0006-8993(93)91660-k. [DOI] [PubMed] [Google Scholar]
- 16.Xiong Z Q, Stringer J L. Epilepsy Res. 1997;27:187–194. doi: 10.1016/s0920-1211(97)00022-3. [DOI] [PubMed] [Google Scholar]
- 17.Otsuki K, Morimoto K, Sato K, Yamada N, Kuroda S. Epilepsy Res. 1998;31:101–112. doi: 10.1016/s0920-1211(98)00018-7. [DOI] [PubMed] [Google Scholar]
- 18.Day B L, Thompson P D, Dick J P, Nakashima K, Marsden C D. Neurosci Lett. 1987;75:101–106. doi: 10.1016/0304-3940(87)90083-8. [DOI] [PubMed] [Google Scholar]
- 19.Mavroudakis N, Caroyer J M, Brunko E, Zegers de Beyl D. Electroencephalogr Clin Neurophysiol. 1994;93:428–433. doi: 10.1016/0168-5597(94)90149-x. [DOI] [PubMed] [Google Scholar]
- 20.Legrand F, Vidailhet P, Danion J-M, Grange D, Giersch A, van der Linden M, Imbs J-L. Psychopharmacology. 1995;118:475–479. doi: 10.1007/BF02245949. [DOI] [PubMed] [Google Scholar]
- 21.Sybirska E, Seibyl J P, Bremner J D, Baldwin R M, al-Tikriti M S, Bradberry C, Malison R T, Zea-Ponce Y, Zoghbi S, During M, et al. Neuropharmacology. 1993;32:671–680. doi: 10.1016/0028-3908(93)90080-m. [DOI] [PubMed] [Google Scholar]
- 22.Hussein Z, Posner J. Br J Clin Pharmacol. 1997;43:457–465. doi: 10.1046/j.1365-2125.1997.00594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Steinberg G K, Bell T E, Yenari M A. J Neurosurg. 1996;84:860–866. doi: 10.3171/jns.1996.84.5.0860. [DOI] [PubMed] [Google Scholar]
- 24.Silvasti M, Karttunen P, Tukiainen H, Kokkonen P, Hanninen U, Nykanen S. Int J Clin Pharmacol Ther Toxicol. 1987;25:493–497. [PubMed] [Google Scholar]
- 25.Jawad S, Richens A, Goodwin G, Yuen W C. Epilepsia. 1989;30:356–363. doi: 10.1111/j.1528-1157.1989.tb05309.x. [DOI] [PubMed] [Google Scholar]
- 26.Jawad S, Oxley J, Yuen W C, Richens A. Br J Clin Pharmacol. 1986;22:191–193. doi: 10.1111/j.1365-2125.1986.tb05249.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Apland J P, Braitman D J. Brain Res. 1990;529:277–285. doi: 10.1016/0006-8993(90)90838-3. [DOI] [PubMed] [Google Scholar]
- 28.Miller R G., Jr . Simultaneous Statistical Inference. New York: Springer; 1985. [Google Scholar]
- 29.Batschelet E. Circular Statistics in Biology. London: Academic; 1981. [Google Scholar]
- 30.Ziemann U, Chen R, Cohen L G, Hallett M. Neurology. 1998;51:1320–1324. doi: 10.1212/wnl.51.5.1320. [DOI] [PubMed] [Google Scholar]
- 31.Ziemann U, Lonnecker S, Steinhoff B J, Paulus W. Exp Brain Res. 1996;109:127–135. doi: 10.1007/BF00228633. [DOI] [PubMed] [Google Scholar]