Abstract
We show that seed-specific overexpression of the sunflower (Helianthus annuus) HaHSFA9 heat stress transcription factor (HSF) in tobacco (Nicotiana tabacum) enhances the accumulation of heat shock proteins (HSPs). Among these proteins were HSP101 and a subset of the small HSPs, including proteins that accumulate only during embryogenesis in the absence of thermal stress. Levels of late embryogenesis abundant proteins or seed oligosaccharides, however, were not affected. In the transgenic seeds, a high basal thermotolerance persisted during the early hours of imbibition. Transgenic seeds also showed significantly improved resistance to controlled deterioration in a stable and transgene-dependent manner. Furthermore, overexpression of HaHSFA9 did not have detrimental effects on plant growth or development, including seed morphology and total seed yield. Our results agree with previous work tentatively associating HSP gene expression with phenotypes important for seed longevity. These findings might have implications for improving seed longevity in economically important crops.
Mature seeds of most plants (the so-called orthodox seeds) withstand extreme desiccation and temperature conditions, but only when preserved in the very dry state reached during zygotic embryogenesis (typical moisture content fresh weight [MCFW] ≤ 5%). The industrial conservation of seeds is detrimentally influenced by unintended rehydration and temperature increases (McDonald, 1999; Halmer, 2000), which also leads to stress-induced damage and to inefficient germination. Germination efficiency and seed longevity involve the expression of multiple genes. Attempts to identify such genes have involved the use of mutants (Ooms et al., 1993; Clerkx et al., 2004a; Sattler et al., 2004), or analyses of allelic variation in model plants (Clerkx et al., 2004b) and crops (Miura et al., 2002). These studies revealed the genetic complexity of these traits, as well as the regulatory genes (Ooms et al., 1993; Clerkx et al., 2004a) and enzymatic activities (Sattler et al., 2004) involved. To date, only genes that reduce seed longevity have been described, including mutants in Arabidopsis (Arabidopsis thaliana) with pleiotropic defects in developing seeds and during germination. However, there is considerable variation in seed longevity among accessions of Arabidopsis (Clerkx et al., 2004b). Therefore, natural genetic diversity for seed longevity exists, and this diversity could be exploited to improve longevity. Alternatively, and toward this aim, key transcription factors with specific and multiple effects on the genes involved in longevity are good candidates to be tested in transgenic approaches. Among the genes with potential roles in seed longevity are those coding for small heat shock proteins (sHSPs; Scharf et al., 2001) since they contribute to different processes that have been associated with seed longevity (Wehmeyer and Vierling, 2000; Sun et al., 2002; Tsvetkova et al., 2002), such as thermotolerance, tolerance to embryo desiccation, membrane stabilization, and oxidative stress resistance. Furthermore, mutants with reduced seed longevity also show impaired expression of sHSP genes in embryos (Wehmeyer and Vierling, 2000; Sun et al., 2002). Previously, we have shown the heat stress transcription factor (HSF) HaHSFA9 to be specifically involved in the developmental regulation of sHSP genes in sunflower (Helianthus annuus) embryos (Almoguera et al., 2002).
Here, we have tested the effects of seed-specific overexpression of HaHSFA9 in transgenic tobacco (Nicotiana tabacum) under the control of the promoter and additional 5′- and 3′-flanking sequences from HaDS10G1 (DS10), which is an unusual late embryogenesis abundant (LEA) gene (of group 1 [Wise 2003]) expressed in sunflower seeds from mid-maturation. The DS10 promoter is not only highly efficient in seeds but also seed specific except for a marginal expression in pollen (Prieto-Dapena et al., 1999; Rousselin et al., 2002; P. Prieto-Dapena, unpublished data). We found that the commonly used cauliflower mosaic virus 35S (CaMV35S) promoter confers constitutive expression levels that, in seeds, are 2 orders of magnitude lower than when the DS10 promoter is used. Using the DS10 promoter, we observed specific changes in gene expression induced by overexpression of HaHSFA9. These changes resulted in an increase of seed longevity, as determined by a controlled deterioration test (CDT; McDonald, 1999; Halmer, 2000; Clerkx et al., 2004b). The observed phenotypes were stable over at least two generations, and they segregated with the DS10:HaHSFA9 transgene. Adverse effects on plant growth, morphology, or seed production were not observed. The specific, and harmless, effects of HaHSFA9 overexpression represent a novel example of genetically improved resistance to CDT of seeds. We discuss how the observed effects involve the temporal extension of the high thermotolerance of mature dry seeds to the early stages of seed imbibition. The identification of HaHSFA9 as a transcription factor with positive effects on resistance to CDT opens new possibilities for improving seed longevity.
RESULTS
Overexpression of HaHSFA9 in Transgenic Tobacco Plants
Transgenic tobacco plants expressing the cDNA encoding HaHSFA9 under the control of the DS10 promoter (DS10:A9 plants) were produced by Agrobacterium-mediated gene transfer. Twenty-two independent DS10:A9 primary plants (T0) were obtained. Seed-specific expression of HaHSFA9 in the transgenic plants was confirmed by northern-blot analyses (Supplemental Fig. S1). Detection of the HaHSFA9 protein using the available antibodies (Almoguera et al., 2002) was not possible due to the presence of cross-reacting proteins in tobacco. However, we could observe transgene-induced changes in the expression of tobacco HSP genes, allowing the selection and analysis of transgenic seeds in subsequent generations.
DS10:A9 Transgenic Tobacco Plants Show Greater Abundance of Specific HSPs in Seeds
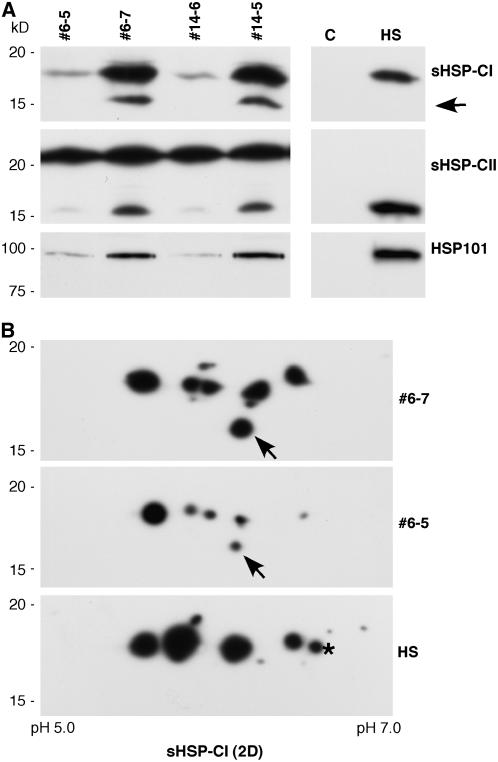
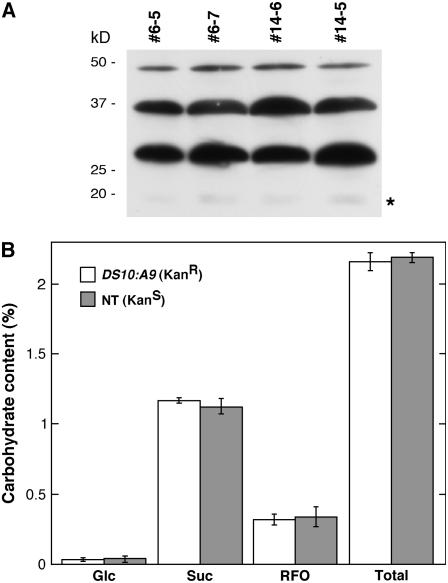
The DS10:A9 plants did not show ectopic HSP expression in seedlings 3 to 4 weeks after germination (data not shown). However, HaHSFA9 induced the overexpression of different HSP genes in mature seeds, including CI and CII sHSP genes (that encode two classes of cytosol-localized proteins; Scharf et al., 2001) and HSP101 (Queitsch et al., 2000). The transgene dependence of such an effect is illustrated with seeds from two different homozygous lines compared to their respective nontransgenic siblings (Fig. 1). We further show that HaHSFA9 caused the overexpression of the genes encoding all CI proteins also present in the nontransgenic mature seeds under control conditions (Fig. 1B). Comparison with heat-stressed samples from seedlings indicated the presence in seeds of specific sHSPs that were absent or barely detected after heat stress. These seed-specific polypeptides were up-regulated in seeds of the DS10:A9 plants, as exemplified for one CI sHSP (Fig. 1, arrow). We also found that the transgene induced the accumulation of most of the CII sHSPs present in seeds. However, in this case the accumulation changes induced by HaHSFA9 were not as extensive as shown for the CI sHSPs, and the levels of the CII seed-specific polypeptides were unchanged in the transgenic seeds (Supplemental Fig. S2, spots labeled s). The effects of HaHSFA9 on gene expression were very specific. As such, the DS10:A9 transgene did not affect the levels of dehydrin proteins or the total amount or composition of soluble sugars in seeds (Fig. 2). We did not find any significant difference among transgenic and nontransgenic seeds for sugar contents or for any of the carbohydrate content estimates (F < 1.304, P > 0.25, 1 and 14 degrees of freedom [df], for all possible a posteriori comparisons). HPLC analyses confirmed the enzymatic determination of sugar composition and showed that raffinose was the sole raffinose family oligosaccharide (RFO; raffinose, stachyose, and verbascose) detected in mature tobacco seeds. Using HPLC, we determined that the Suc content in transgenic seeds averaged 1.38% ± 0.1% (sugar contents expressed as percentage of total seed fresh weight). A similar amount of Suc was measured in nontransgenic seeds (1.36% ± 0.2%). The raffinose content in transgenic seeds was 0.33% ± 0.03%, with a similar abundance of this sugar also observed in nontransgenic seeds (0.29% ± 0.03%). Glc was at a concentration undetectable by our HPLC assay.
Figure 1.
Effects of the DS10:A9 transgene on HSP accumulation in seeds. A, Protein samples from mature seeds of T1 plants (second-generation seeds) were analyzed after 1D electrophoresis. Examples from sibling homozygous lines with (#6-7, #14-5) or without (#6-5, #14-6) the transgene are depicted. Also shown are samples from nontransgenic seedlings under control conditions (C) and after a heat stress for 3 h at 40°C (HS). The primary antibody used for western detection of each HSP class is indicated on the right. B, Western analysis using CI sHSP antibodies after 2D electrophoresis of the same samples. The asterisk marks a heat stress-specific polypeptide not detectable in transgenic seeds. The pH range for IEF is indicated at the bottom. The arrows indicate a seed-specific polypeptide resolved by 1D and 2D gels. Molecular mass standards are on the left.
Figure 2.
Dehydrin protein accumulation and soluble sugar content are unaltered in DS10:A9 seeds. A, Samples analyzed after 1D electrophoresis. Results are shown for the same samples used to analyze HSP accumulation in Figure 1A. The asterisk marks a faint nonspecific band that was detected. All other bands were identified as dehydrins in western blots probed with the dehydrin antibody together with an excess of the antigenic dehydrin peptide (Close et al., 1993). B, Enzymatic determination of the Glc, Suc, and total raffinose oligosaccharide (RFO) content in seeds. The total content of soluble sugars (Total) is also depicted. In each case, we show the average content following two measurements. Samples from three different homozygous plants (#6-7, #14-5, and #23-8) were used along with their corresponding nontransgenic siblings (#6-5, #14-6, and #23-4). The carbohydrate content is expressed as the percentage (w/w) of total seed fresh weight, and error bars represent se.
Additional analyses using specific probes for LEA-protein (Wise, 2003) mRNAs of groups 2 (dehydrins), 1, 3, or 4 did not reveal transgene effects on their regulation. Total Pro content in seeds was also unaltered (data not shown).
Persistence of Basal Thermotolerance in DS10:A9 Seeds
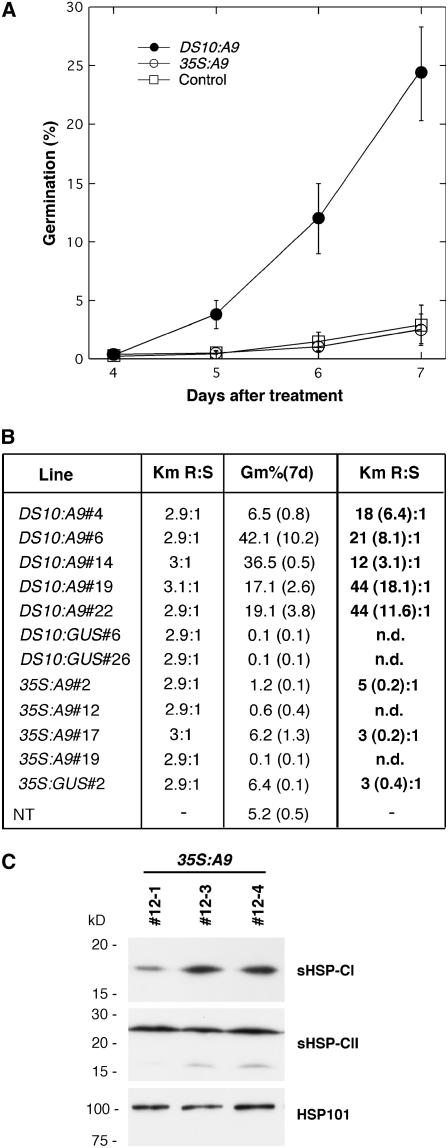
We first investigated whether the specific effects of HaHSFA9 on gene expression modified the basal thermotolerance of imbibing seeds from T0 lines. As negative controls, we used seeds from nontransgenic plants, from lines with different, unrelated transgenes, or from 35S:A9 lines. All control lines contained the same marker gene (conferring kanamycin resistance). The persistence of basal thermotolerance was assayed by determining germination percentages after the high-temperature treatments (4 h at 50°C). In the dried state reached upon natural seed maturation, 100% of the seeds from the control lines resisted the 50°C treatments (the same was true for the DS10:A9 seeds; data not shown). The difference between both kinds of seeds was revealed after a short rehydration, which raised MCFW to 41.3% ± 0.4%. Control seeds lose their thermotolerance but the DS10:A9 seeds retain it substantially. Mendelian segregation analyses strongly suggested that the thermotolerant phenotype was linked to the DS10:A9 transgene (Fig. 3). The analyses with seeds from the T0 plants indicated a clear effect of the DS10:A9 transgene. The percentage of germination of control seeds was reduced from 95% to 100% (observed before treatment) to 0% to 6% (Fig. 3B). This reduction was observed 4 to 7 d after transferring the treated seeds to germination conditions following the 50°C treatment. In contrast, the DS10:A9 seeds resisted the treatment and germinated much better, reaching average germination of 24% ± 5% after 7 d (Fig. 3A). The observed differences were significant (F = 6.99, P = 0.015, 1 and 11 df). Resistance to kanamycin in transgenic seeds was evaluated before and after the 50°C treatments (with the seeds that completed germination). Figure 3B shows that values close to the expected 3:1 ratio, between antibiotic resistance and sensitivity, were observed before the treatment both for control and DS10:A9 seeds. However, segregation ratios up to 44:1 were observed with the DS10:A9 seeds but not with transgenic control seeds (e.g. DS10:β-glucuronidase [GUS]) that survived the treatment. The results in Figure 3 also show that seeds from the 35S:A9 lines have similar thermotolerant phenotypes as the rest of the control lines (Fig. 3, A and B). The expression of HaHSFA9 from the 35S promoter caused minor effects on HSP accumulation only observed after transgene homozygosis (Fig. 3C). These effects were much smaller than observed for the DS10:A9 lines (compare Fig. 3C with Fig. 1A).
Figure 3.
Germination following basal thermotolerance assays is linked to DS10:A9 transgene inheritance in first-generation transgenic seeds. A, Germination percentage observed at various times after a 50°C treatment for 4 h. Each time point represents average values obtained for DS10:A9, 35S:A9, and other control (NT, 35S:GUS, and DS10:GUS) seeds from the lines listed in B. Such lines contain each transgene in heterozygosis and integrated at a different single locus. The experiments were repeated at least twice. B, Table summarizing the germination percentage 7 d after transfer to germination conditions for the individual lines [Gm%(7 d)]. The transgene segregation data obtained before and after treatment at 50°C also are summarized. The segregation is expressed as the ratio (R:S) between seedling numbers that were resistant (R) or sensitive (S) to kanamycin (Km). The numbers and lettering in bold correspond to the transgene segregation after the 50°C treatment. The numbers in parentheses represent the se for the germination data. Segregation was not determined (n.d.) when there were insufficient germinated seeds. C, Minor effects of the 35S:A9 transgene on HSP accumulation in seeds. Examples from sibling homozygous lines with (35S:A9#12-3, 35S:A9#12-4) or without (35S:A9#12-1) the transgene are presented. The rest of the symbols are as in Figure 1A.
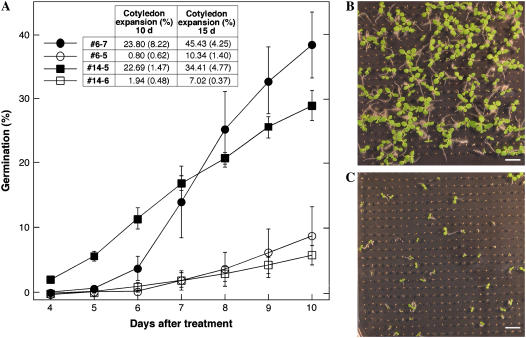
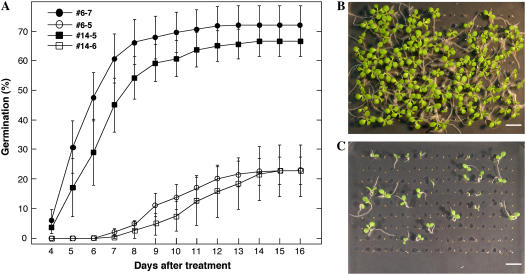
The persistent-thermotolerant phenotype was confirmed after transgene segregation in the subsequent generation. This was demonstrated with the same material used for the gene expression analyses (compare Figs. 1 and 4). Furthermore, the levels of HSP accumulation observed for transgenic and nontransgenic seeds were unaltered by the 50°C treatments. Therefore, these treatments did not induce additional HSP accumulation in either seed type (Supplemental Fig. S3). Seeds carrying the transgene resisted the 50°C treatments (Fig. 4), but sibling seeds without the transgene did not as previously observed with control seeds (see also Fig. 3A). We found highly significant differences among transgenic and control plants for germination percentage (F = 44.93, P < 0.0001, 1 and 66 df) and cotyledon expansion (F = 45.32, P < 0.0001, 1 and 11 df). Additional analyses indicated that the seeds that did not complete germination after the treatments were dead; as for example, these seeds did not stain using tetrazolium (data not shown). Among the seeds that survived the 50°C treatments, transgenic seeds completed germination earlier than their respective nontransgenic siblings. This indicates a faster recovery after damage induced by the treatment, which is consistent with other seedling establishment parameters, such as the higher percentage of cotyledon expansion observed for the transgenic seeds (Fig. 4A). Representative results for two of the lines analyzed are detailed in Figure 4, B and C. A high proportion of the transgenic seeds had completed germination 15 d after the 50°C treatments, whereas most sibling nontransgenic seeds did not complete germination. Because of the difference in germination timing, the transgenic seedlings were larger than the scarce nontransgenic siblings that completed germination (Fig. 4, compare B and C).
Figure 4.
Persistence of basal thermotolerance in imbibed second-generation transgenic DS10:A9 seeds. A, The germination percentage observed at various times after the 50°C treatment and the percentage of expanded cotyledons observed at the two time points analyzed are represented. Samples were seeds from the same lines as in Figure 1, and transgenic lines contained the transgene in homozygosis and integrated at a different single locus. Each transgenic sample is compared to the corresponding nontransgenic sibling sample. The error bars or numbers represent the se (inset table, in parentheses). B and C, Pictures are examples of the differences in germination and growth observed with the transgenic #6-7 (B) and nontransgenic #6-5 (C) seeds, 15 d after the 50°C treatment. Scale bars = 1 cm.
Resistance to Controlled Deterioration of DS10:A9 Seeds and Lack of Adverse Phenotypic Effects Caused by Overexpression of HaHSFA9
Resistance of seeds to a CDT has been successfully used for the rapid evaluation and prediction of seed longevity (Powell, 1995; TeKrony, 1995; McDonald, 1999; Halmer, 2000; Clerkx et al., 2004a, 2004b; Sattler et al., 2004). CDT is achieved after the exposure of rehydrated seeds to high temperatures, which leads to rapid decline of seed vigor and loss of viability (determined as a negative effect on seed germinability). Conceptually, CDT resembles assays for basal thermotolerance performed with imbibing seeds. The difference between them is the temporal separation between seed rehydration and the incubation at high temperatures. That separation is not required for basal thermotolerance assays, but it is usual in CDT performed with small-sized seeds (Powell, 1995; McDonald, 1999; for details, see “Materials and Methods”).
Seeds from the same lines analyzed for basal thermotolerance in Figure 4 were subjected to CDT suited for tobacco seeds, under conditions similar to those reported in the literature for other small-sized seeds (Powell, 1995; McDonald, 1999), in particular, for seeds of other Solanaceae (such as tomato [Lycopersicon esculentum]), which are quite resistant to CDT (Argerich et al., 1989). The seed MCFW was raised to 28.1% ± 0.5% by controlled addition of water, and rehydrated seeds were sealed in plastic bags and subsequently incubated at 50°C (for details, see “Materials and Methods”). We found that CDT at 45°C did not substantially deteriorate even the seeds from the nontransgenic sibling lines (data not shown). Compared to our conditions for the basal thermotolerance assays (Fig. 4), a lower MCFW was reached after CDT. In addition, our CDT protocol avoided imbibitional damage at 50°C. Therefore, longer treatments at 50°C were necessary to reduce the germination percentage of nontransgenic seeds after CDT. Examples of results of CDT for 2 d at 50°C are given in Figure 5. Germination was analyzed as in Figure 4, but we extended the duration of the experiment until 16 d after CDT, following the recommendations of the International Seed Testing Association for the germination of tobacco seeds (International Seed Testing Association, 1999). The results in Figure 5 demonstrate differences in the germination percentage after CDT, which was significantly higher for the seed of transgenic lines (up to 72% ± 6.4%, 16 d after CDT) than for nontransgenic lines (22.8% ± 4.6%, 16 d after CDT). Statistical analyses confirmed significant differences between the germination percentages of transgenic and nontransgenic seeds during the whole time span of the experiment (4–16 d after CDT [F = 68.77, P < 0.0001, 1 and 240 df; repeated-measures ANOVA]). CDT had a milder effect on seed germination percentages than the treatments used for the basal thermotolerance assays, and the difference between the transgenic and sibling nontransgenic lines was therefore more evident (compare the results of Figs. 4 and 5 and the statistics for the data in each figure). Therefore, resistance to CDT is also associated with inheritance of the DS10:A9 transgene.
Figure 5.
Resistance to CDT of second-generation transgenic DS10:A9 seeds. A, The germination percentage observed at various times after CDT for 48 h at 50°C is represented. The samples were seeds from the same lines as in Figures 1 and 4. Each transgenic sample is compared to the corresponding nontransgenic sibling sample. Pictures in B and C and symbols are as in Figure 4.
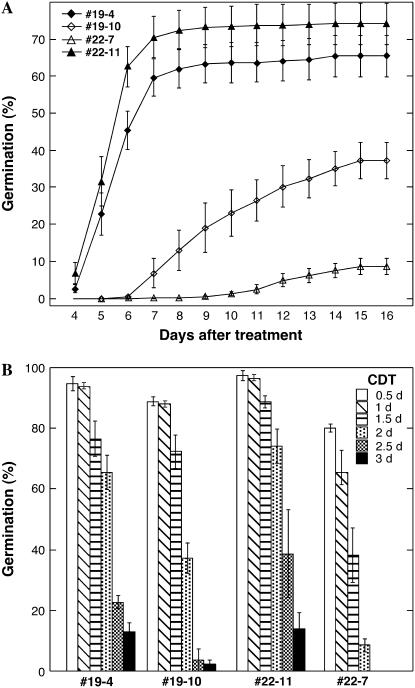
The longevity of seeds from transgenic and sibling nontransgenic lines was estimated by performing CDT in which we exposed seeds at 50°C for different times between 0.5 and 3 d. This allowed us to determine their respective LD50 (the number of days of CDT required to decline to 50% germination), a single parameter related to longevity. Additional homozygous transgenic (DS10:A9#19-4, DS10:A9#22-11) and sibling nontransgenic lines (DS10:A9#19-10, DS10:A9#22-7) were used. These lines showed similar results as the previously analyzed lines after CDT for 2 d (for them, transgenic and nontransgenic lines also differed significantly, F = 96.99, P < 0.0001, 1 and 228 df, repeated-measures ANOVA; compare Figs. 5 and 6A). LD50 estimates from the germination data in Figure 6B confirmed that seeds from each line increasingly deteriorated as a function of the duration of CDT; the nontransgenic seeds reaching 50% lethality significantly earlier than their respective transgenic siblings were: DS10:A9#19-4, LD50 = 2.11 ± 0.15 d; DS10:A9#22-11, LD50 = 2.33 ± 0.37 d; DS10:A9#19-10, LD50 = 1.78 ± 0.09 d; and DS10:A9#22-7, LD50 = 1.25 ± 0.30 d. The germination percentages shown in Figure 6B were significantly different for both pairs of lines (DS10:A9#19-4 versus DS10:A9#19-10, F = 14.67, P = 0.0003; DS10:A9#22-11 versus DS10:A9#22-7, F = 122.3, P < 0.0001; a posteriori contrasts).
Figure 6.
Increased longevity (LD50) in transgenic DS10:A9 seeds. A, Resistance to CDT of the DS10:A9 seeds used for LD50 determinations. CDT conditions and symbols are as in Figure 5. B, Seeds from the lines analyzed in A were subjected to CDT for the times listed at top right (0.5–3 d at 50°C). Germination percentage was scored 16 d after transferring the treated seeds to normal germination conditions. These results were used for the LD50 determinations mentioned in “Results.”
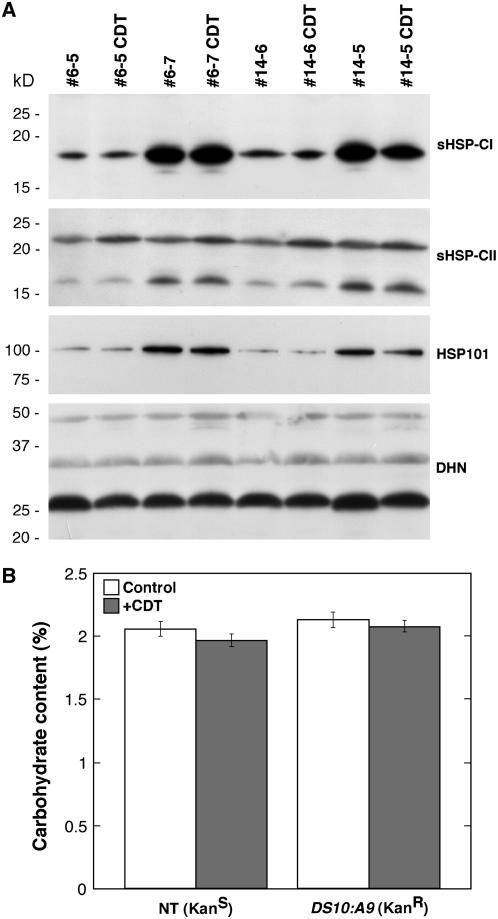
The expression changes induced by overexpression of HaHSFA9, as a higher accumulation of specific CI sHSPs, persisted after CDT for 2 d at 50°C. The same was true for the HaHSFA9-induced accumulation of CII sHSPs and HSP101. In addition, this CDT treatment did not induce new HSPs in either transgenic or sibling nontransgenic seeds. Furthermore, the CDT did not result in differences between the total sugar contents (or the dehydrin accumulation patterns) of the transgenic and nontransgenic seeds (Fig. 7).
Figure 7.
A, Top, Persistence of higher CI sHSP accumulation in the DS10:A9 transgenic seeds after CDT and lack of heat induction by CDT. A, Middle, Same result for CII sHSP and HSP101. A, Bottom, CDT does not induce changes in dehydrin protein (DHN) content. B, Unchanged total sugar contents after CDT. Results for samples from sibling homozygous lines with (#6-7, #14-5) or without (#6-5, #14-6) the transgene are shown. Conditions and symbols for the western and carbohydrate assays, respectively, are as in the legends of Figures 1A and 2B.
Plants carrying the DS10:A9 transgene did not show differences in either reproductive or vegetative growth, from wild-type or control plants (data not shown). In particular, no differences in seed yield, size, or morphology were observed when we compared sibling lines with or without resistance to CDT (Supplemental Fig. S4).
DISCUSSION
Plants show two conditions of tolerance to high temperature, basal and acquired thermotolerance, which appear in the absence of and after heat stress treatment at a sublethal temperature, respectively. The natural high basal thermotolerance of mature seeds is lost shortly after rehydration. For example, this happens upon seed imbibition (during germination) or if seeds are accidentally rehydrated during (or after) storage. Imbibed seeds are therefore thermosensitive because they lose basal thermotolerance (as shown for the control seeds in Figs. 3A and 4). Our results with the DS10:A9 seeds imply the temporal extension of basal thermotolerance to thermosensitive stages of seed germination. Basal thermotolerance persisted, to a significant extent, after the controlled rehydration of the DS10:A9 seeds (Fig. 4). This correlated with major, and specific, increases in HSP accumulation (for example, see Fig. 1). In contrast, we could detect only minor HSP accumulation and deterioration-resistance modifications in seeds when HaHSFA9 was expressed under the CaMV35S promoter (Fig. 3). The thermotolerant phenotype of the rehydrated DS10:A9 seeds requires gene expression modifications during embryogenesis that must persist in seeds within the early hours of imbibition. Our results demonstrate that this novel phenotype requires the very high expression level of HaHSFA9 conferred in seeds by the DS10 gene regulatory sequences. We should note that increases in basal thermotolerance have been previously described in transgenic plants overexpressing different HSFs, but the phenotype was observed in vegetative tissues well after seed germination (i.e. Prändl et al., 1998; Mishra et al., 2002). In these previous reports, the persistence of thermotolerance in imbibed seeds was not analyzed. Because these studies employed the CaMV35S promoter, our own observations with the CaMV35S:A9 seeds suggest that we would not expect significant seed thermotolerance modifications to occur (Fig. 3).
The specific effects of HaHSFA9 could also be required for the persistence of basal thermotolerance in germinating seeds. In plants, sHSPs and HSP101 contribute to basal and acquired thermotolerance (Queitsch et al., 2000; Sun et al., 2002). Our analyses indicate that HaHSFA9-up-regulated HSPs are involved in the persistent-thermotolerant phenotype (compare Figs. 1 and 4). Two-dimensional (2D) immunoblots showed that HaHSFA9 mainly induced the accumulation to higher levels of a subset of the CI sHSPs (Fig. 1). This included all proteins from that class expressed in seeds, some of which are exclusively present in seeds. HaHSFA9 also increased, although to lower levels, the accumulation of HSP101 and that of some CII-sHSP polypeptides (see Fig. 1 and Supplemental Fig. S2). In contrast, the accumulation of dehydrin proteins was not increased (Fig. 2). Recent data implicate plant HSFs in the transcriptional control of genes coding for enzymes involved in RFO biosynthesis (Busch et al., 2005), but the total soluble sugar or RFO content was not changed in the DS10:A9 seeds (Fig. 2). Taken together, these results demonstrate the specificity of HaHSFA9. This transcription factor selectively up-regulated putative components of seed longevity, as different HSP genes (Wehmeyer and Vierling, 2000; Sun et al., 2002; Tsvetkova et al., 2002) but not dehydrins (Ooms et al., 1993; Buitink et al., 2002) or sugars, including RFOs (Ooms et al., 1993; Buitink et al., 2002; Clerkx et al., 2004b). The seed-specific sHSP 2D spots that are up-regulated by HaHSFA9 could represent proteins encoded by genes that are noninducible or barely inducible by heat stress. This would agree with observations for CI sHSP genes in crops such as sunflower (Carranco et al., 1997) and rice (Oryza sativa; Guan et al., 2004), where such genes exist and could perform seed-specific functions. For example, the sHSP genes that are up-regulated by HaHSFA9 might contribute to the desiccation tolerance of mature seeds. In agreement with this suggestion, it is worth mentioning previous reports on maturing seeds indicating a strong association between longevity and the ability of seeds to tolerate desiccation (Ellis and Hong, 1994; Hay and Probert, 1995; Bruggink et al., 1999). In addition, Arabidopsis mutants that at the same time show decreased seed longevity and low accumulation levels of seed sHSPs produce seeds that are desiccation intolerant (Wehmeyer and Vierling, 2000). The suggested relevance of the sHSPs in the extended thermotolerance of the DS10:A9 seeds is also supported by two previous reports showing that the overexpression of a single sHSP gene resulted in increased thermotolerance of carrot (Daucus carota) cells (Malik et al., 1999) or of tobacco plants (Sanmiya et al., 2004). The involvement of proteins different from HSPs is also likely because high temperatures cause complex effects. This includes protein denaturation, alterations of membrane fluidity and of cellular metabolism. Indeed, several non-HSP genes are essential for thermotolerance at different stages, as revealed by mutants of Arabidopsis (Larkindale et al., 2005). Therefore, we cannot exclude the occurrence of additional gene expression changes mediated by HaHSFA9 overexpression. Such changes could contribute to the thermotolerant (Fig. 4) and CDT-resistant phenotypes (Figs. 5 and 6; see below), in addition to the HSP accumulation changes experimentally observed and linked to both phenotypes. The detected changes (for example, Figs. 1 and 2) would agree with a precedent study showing no relation between quantitative trait loci for RFO content and seed storability (Bentsink et al., 2000). Our results also conform with work demonstrating correlation between seed longevity (LD50) and a quantitative trait locus for tolerance to heat- and salt-stressed germination (Clerkx et al., 2004b). Furthermore, Bettey and Finch-Savage (1998) showed that a rapid aging treatment that reduced the germination performance of Brassica oleracea seeds also reduced the amount of HSP17.6 (a CI sHSP). In the same study, dehydrins did not show a positive correlation with seed performance.
We also demonstrate that HaHSFA9 induces a novel seed deterioration-resistant phenotype. The CDT conditions for tobacco seeds required adaptation to the high CDT resistance already showed by the nontransgenic material. Such resistance has precedents in other small-sized seeds from Solanaceae, such as tomato (Argerich et al., 1989). Our conditions to achieve substantial deterioration of tobacco seeds in a reasonable time are not very different from those generally used for less resistant seeds (45°C, MCFW up to 24%; Powell, 1995). Furthermore at the temperature used in our experiments (50°C), the absence of a heat stress response (Fig. 7) allowed us to separate the contribution to CDT of basal and acquired thermotolerance. Under the standard CDT conditions, there are precedents for association between resistance to controlled deterioration procedures, improved seed longevity (shelf-life), and better field emergence under stress conditions (Powell, 1995; for review, see McDonald, 1999). Despite this, we should add caution to any inference from our results of improved seed shelf life because, as in other cases, longevity predictions based on results on CDT experiments might be controversial (for review, see McDonald, 1999). However even if similar predictions have not been performed with tobacco seeds or with small-sized seeds from Solanaceae, it is worth mentioning that tomato seeds are highly resistant to CDT (Argerich et al., 1989). Tomato seeds also have been independently shown to have a high relative storability index (for review, see Copeland and McDonald, 2001).
Our results provide a novel example of increased resistance to controlled deterioration of seeds. We have identified a master gene (encoding the HaHSFA9 transcription factor) that positively affects the resistance to CDT. This could pave the way toward the improvement of seed longevity by genetic transformation. Since HaHSFA9 has orthologs in other dicot and monocot plants (Almoguera et al., 2002; Kotak et al., 2004) and there is a conserved developmental regulation of seed sHSP genes (Carranco et al., 1999; Almoguera et al., 2002), functionally equivalent transcription factors can be useful tools for improving CDT resistance in seeds of different crops. The modified seeds would have an advantage under conditions of accidental rehydration during storage. Seeds wetted to MCFW between 18% and 20% would increase seed respiration and temperature, with the consequent decrease of germinability. The enhanced expression of HaHSFA9 (or of ortholog factors) could act as a safeguard against the accidental loss of basal thermotolerance upon uncontrolled rehydration of seeds. Moreover, the quality of developing seed is critically affected by high temperature stress in field conditions. Additional advantages of the overexpression of HSFA9 in seeds from mid-maturation might include improved seed germinability in the field under high soil temperatures and the reduction of the negative effects of high temperatures that occur at all stages of grain filling (Maestri et al., 2002).
MATERIALS AND METHODS
Overexpression of HaHSFA9 in Transgenic Plants
For constitutive overexpression of HaHSFA9 in transgenic plants (35S:A9 lines), the cDNA containing the complete open reading frame and the untranslated 5′- and 3′-flanking sequences was obtained from plasmid p35S:HSFA9 (Almoguera et al., 2002). A fragment containing the cDNA and the CaMV35S sequences was subcloned into the plasmid pBI101.1 by replacing the GUS coding region between the SphI and SacI sites.
For seed-specific overexpression of HaHSFA9 (DS10:A9 lines), the complete cDNA was obtained from the plasmid pSKHSFA9-F (Almoguera et al., 2002). First, the cDNA was inserted into the EcoRI site of pSKDS10EC1 (Rousselin et al., 2002). Subsequently, a fragment containing the DS10-5′:HaHSFA9:DS10-3′ sequence was inserted between the SalI and SacI sites of the binary vector pBIN19. Constructs with transgenes were transferred into the Agrobacterium tumefaciens strain LBA4404 and used for leaf disc transformation (Carranco et al., 1999) of tobacco (Nicotiana tabacum cv Xanthi). The T0 plants were regenerated and maintained as described previously (Carranco et al., 1999). Transgenic plants were selected on medium with kanamycin (300 μg mL−1) and different lines containing single transgene integration events in heterozygosis were obtained. This was confirmed by the 3:1 segregation observed for kanamycin resistance. Homozygous and sibling seeds without the transgene were obtained in the subsequent generation. Homozygosis was confirmed by the lack of segregation of antibiotic resistance and the absence of the transgene was inferred by sensitivity to kanamycin. Standard PCR was used to detect the presence of HaHSFA9, linked to the different promoter sequences, in leaf samples from the regenerated transgenic plants or their progeny. For PCR analyses of the 35S:A9 lines, the primers used were 5′-CATCTCTTCAGACAAAT-3′ (HaHSFA9) and 5′-ACTATCCTTCGCAAGACCCTTCC-3′ (CaMV35S). Annealing was at 51°C for 1 min. The DS10:A9 lines were analyzed using primers 5′-CATCTCTTCAGACAAAT-3′ and 5′-CCACCACGTCATCATACCAC-3′, which amplify a DNA fragment of 326 bp. The PCR conditions were as follows: 30 cycles of 1 min at 94°C, 1 min at 50°C, and 1 min at 72°C, plus a final step at 72°C for 5 min.
Protein Electrophoresis and Western-Blot Analysis
Gel electrophoresis and the conditions for western-blot analysis were as described previously (Almoguera et al., 2002) with minor modifications. Proteins were extracted using the phenol buffer method (Lehmann et al., 1995) and the protein samples were resuspended in 2D sample buffer (9 m urea, 2% [w/v] CHAPS, and 1% [w/v] dithiothreitol). For one-dimensional (1D) gels, the buffer conditions were adjusted by the addition of an equal volume of 2× Laemmli buffer and 45 μg of total protein was loaded per lane. SDS-PAGE gels were 15% (w/v) (for CI and CII sHSPs), 12.5% (w/v) (for dehydrins), or 8% (w/v) polyacrylamide (for HSP101). For 2D gels, ampholytes (0.2% [w/v] Bio-Lyte 3-10 buffer; Bio-Rad) were added to the resuspended protein samples, 140 μg or 300 μg for seedling and seed samples, respectively. Isoelectrofocusing (IEF) was performed using the Protean IEF cell system (Bio-Rad), and IEF strips (ReadyStrip IPG strips, 7 cm, pH 5–8; Bio-Rad) were subjected to active rehydration for 12 to 16 h at 50 V and 20°C. The IEF program was as follows: 15 min at 250 V, followed by a slow voltage rise to 4,000 V for 2 h and a final rise to 20,000 V. The strips were used to separate the proteins in the second dimension on 15% (w/v) polyacrylamide SDS gels (Almoguera et al., 2002). For western detection, the samples were transferred to Hybond-P membranes (Amersham Biosciences) after electrophoresis. The membranes were stained with Ponceau S and incubated with primary antibodies at the following dilutions: anti-sHSP CI (Coca et al., 1994), 1/1,000; anti-sHSP CII (Coca et al., 1994), 1/2,000; anti-HSP101 (Queitsch et al., 2000), 1/5,000; or anti-DHN (Close et al., 1993), 1/1,000. The secondary antibody (anti-rabbit IgG, peroxidase linked) was used at a dilution of 1/20,000. The immunoreaction was detected by using the ECL Plus system (Amersham Biosciences).
Basal Thermotolerance Assays and Controlled Deterioration of Seeds
Tobacco plants were grown under controlled environment, as described previously (Carranco et al., 1999). Mature seeds were harvested at 35 DPA and stored with silica gel at 4°C in sealed containers until use. Seeds produced and stored in this way consistently showed germination percentages ≥99% over the time span of the studies carried out here.
For basal thermotolerance assays, dry seeds were imbibed in water under controlled conditions and then subjected to a short incubation at 50°C. Assays were performed on three to four replicates, each involving 30 mg of dry seeds (≈400–500 seeds) per plant line. The MCFW of dry mature seeds was 4.8% ± 0.2%, expressed as the percentage mass fraction of water of the total tissue mass (fresh weight). Rehydration was achieved by the sequential incubation of the seeds in 1 mL of the sterilizing solution (1% [v/v] sodium hypochlorite for 20 min) and 1 mL of sterile water (40 min) at 25°C. The MCFW of seeds after rehydration was 28.1% ± 0.7%. The rehydrated seeds were then incubated for 4 h at 50°C in a water bath, in direct contact with 1 mL of sterile water. At this step, the MCFW of seeds increased to 41.3% ± 0.4%. After this treatment, germination was scored by placing individual seeds at defined points in grids drawn on petri dishes with 1× Murashige and Skoog basal medium (MS salts; Duchefa Biochemie; 3% [w/v] Suc and 0.8% [w/v] phytoagar). The dishes were incubated at 25°C (day) and 20°C (night) in a 16-h photoperiod at 100 μmol m−2 s−1 and photographed at different times after deterioration. For scoring the germination percentage, only the seedlings that kept growing after radicle protrusion during the experiment were considered. For the transgene segregation analyses performed with seeds from heterozygous T0 lines, the seedlings were transplanted to Murashige and Skoog medium with 300 μg mL−1 kanamycin ≈ 15 d after germination. Seedlings that remained green 3 weeks after transplanting were scored as positive for transgene inheritance.
CDTs were performed on four to six experimental replicates, each involving 10 mg of dry seeds per plant line. Seeds were rehydrated before their exposure to high temperatures for different time lengths. MCFW was raised in controlled conditions by adding 20 μL of water to the seeds in Eppendorf tubes. After 2 h at 25°C, the excess of water was removed by briefly placing the rehydrated seeds on filter paper. The seeds were then placed in a single layer and sealed in plastic bags. Caution was taken to remove as much air as possible before sealing the seeds in the bags. Deterioration was achieved by incubation of the bags with the rehydrated seeds at 50°C in prewarmed sealed boxes (12 × 7.5 × 6 cm) containing eight wet paper towels (with 50 mL of water) placed within a water bath. The incubation at 50°C was usually performed for 48 h (conditions found to be the most discriminating CDT between transgenic and nontransgenic material). MCFW of seeds was 28.1% ± 0.5% and 27.9% ± 0.5%, determined immediately before or after CDT, respectively. Different 50°C treatments were performed to estimate seed longevity as a single parameter (LD50; as, for example, in Clerkx et al., 2004b).
Soluble Carbohydrate and RFO Contents
Total soluble extracts were prepared from mature seeds. Samples of 30 mg were homogenized in 3 mL of 80% (v/v) ethanol and incubated for 20 min at 90°C. The insoluble residue was removed by centrifuging at 5,000g for 10 min, and the extracts were air-dried to completely remove the ethanol. The total carbohydrate content was determined by the phenol-sulfuric acid method (Dubois et al., 1956). The total Glc, Suc, and RFO contents were measured using the raffinose series oligosaccharide assay from Megazyme. We followed the manufacturer's instructions except that the quantities of reagents were scaled down starting from ≈200 μg of soluble sugars (in ≈200 μL of Milli-Q water). We also determined the Suc and raffinose contents by HPLC. Samples (≈40 μg of sugars in 40 μL of Milli-Q water) were filtered by centrifugation through 0.22-μm Ultrafree-MC filters (Millipore) before HPLC injection. We used a Waters HPLC system (600E + 700 Satellite WISP) equipped with a Luna NH2 100A column (15 × 0.46 cm, 5 μm) and a light-scattering detection system (LSD Sedex 45 SEDERE). The mobile phase was a gradient of acetonitrile and water, and the flow rate was 1 mL min−1 under operating conditions at room temperature. The carbohydrate standards were from Sigma.
Statistical Analysis
We tested for differences between the transgenic and control groups of sibling plants by means of ANOVA. For comparisons involving temporal responses (e.g. germination percentage and cotyledon expansion in the basal thermotolerance and CDT assays), we used a repeated-measures ANOVA. This adequately controls for temporal variation in the response of the individual plants of different lines, the experimental subjects for which the dependent variable is measured at repeated time intervals. In the experimental design, the individual plants were nested within each treatment group, with sibling plants included in both groups. Germination data of the CDT were used for probit regression on a time scale (days) to estimate LD50. These data were further analyzed using general linear models, and P values were adjusted (a posteriori contrasts) for multiple comparisons between the same data set. Unless stated otherwise, means ± se are reported. We used SAS and JMP (SAS Institute, 1990) statistical packages to perform the analyses.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Seed-specific overexpression of HaHSFA9 mRNAs from the DS10 promoter in transgenic tobacco.
Supplemental Figure S2. Two-dimensional western analyses using CII sHSP antibodies in DS10:A9 seeds.
Supplemental Figure S3. Persistence of higher HSP accumulation after the basal thermotolerance assays and lack of response to heat in the imbibed DS10:A9 seeds.
Supplemental Figure S4. The seed yield and seed morphology were unaltered in the DS10:A9 lines.
Supplementary Material
Acknowledgments
We thank Elizabeth Vierling and Timothy Close for providing the anti-HSP101 and anti-DHN antibodies, respectively. We are indebted to Pedro Jordano for performing the statistical analyses. We are grateful to José Manuel Pardo, Angela Nieto, and Andrés Aguilera for suggestions on the manuscript.
This work was supported by the Spanish Ministry of Education and Science (grant nos. BIO02–1463 and BIO05–0949). We also received partial support from the Andalusian Regional Government (“Junta de Andalucía”; grant no. CVI148).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Juan Jordano (fraga@cica.es).
The online version of this article contains Web-only data.
The online version of this article contains Web-only data.
References
- Almoguera C, Rojas A, Díaz-Martín J, Prieto-Dapena P, Carranco R, Jordano J (2002) A seed-specific heat-shock transcription factor involved in developmental regulation during embryogenesis in sunflower. J Biol Chem 277: 43866–43872 [DOI] [PubMed] [Google Scholar]
- Argerich CA, Bradford KJ, Tarquis AM (1989) The effects of priming and ageing on resistance to deterioration in tomato seeds. J Exp Bot 40: 593–598 [Google Scholar]
- Bentsink L, Alonso Blanco C, Vreugdenhil D, Tesnier K, Groot SP, Koornneef M (2000) Genetic analysis of seed-soluble oligosaccharides in relation to seed storability of Arabidopsis. Plant Physiol 124: 1595–1604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettey M, Finch Savage WE (1998) Stress protein content of mature Brassica seeds and their germination performance. Seed Sci Res 8: 347–355 [Google Scholar]
- Bruggink GT, Ooms JJJ, van der Toorn P (1999) Induction of longevity in primed seeds. Seed Sci Res 9: 49–53 [Google Scholar]
- Buitink J, Hoekstra FA, Leprince O (2002) Biochemistry and biophysics of tolerance systems. In M Black, HW Pritchard, eds, Desiccation and Survival in Plants: Drying without Dying. CAB International, Oxon, UK, pp 293–318
- Busch W, Wunderlich M, Schöffl F (2005) Identification of novel heat shock factor-dependent genes and biochemical pathways in Arabidopsis thaliana. Plant J 41: 1–14 [DOI] [PubMed] [Google Scholar]
- Carranco R, Almoguera C, Jordano J (1997) A plant small heat shock protein gene expressed during zygotic embryogenesis but noninducible by heat stress. J Biol Chem 272: 27470–27475 [DOI] [PubMed] [Google Scholar]
- Carranco R, Almoguera C, Jordano J (1999) An imperfect heat shock element and different upstream sequences are required for the seed-specific expression of a small heat shock protein gene. Plant Physiol 121: 723–730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clerkx EJM, Blankestijn-De Vries H, Ruys GJ, Groot SPC, Koornneef M (2004. a) Genetic differences in seed longevity of various Arabidopsis mutants. Physiol Plant 121: 448–461 [Google Scholar]
- Clerkx EJM, El-Lithy ME, Vierling E, Ruys GJ, Blankestijn-De Vries H, Groot SPC, Vreugdenhil D, Koornneef M (2004. b) Analysis of natural allelic variation of Arabidopsis seed germination and seed longevity traits between the accessions Landsberg erecta and Shakdara, using a new recombinant inbred line population. Plant Physiol 135: 432–443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Close TJ, Fenton RD, Moonan F (1993) A view of plant dehydrins using antibodies specific to the carboxy terminal peptide. Plant Mol Biol 23: 279–286 [DOI] [PubMed] [Google Scholar]
- Coca MA, Almoguera C, Jordano J (1994) Expression of sunflower low-molecular-weight heat-shock proteins during embryogenesis and persistence after germination: localization and possible functional implications. Plant Mol Biol 25: 479–492 [DOI] [PubMed] [Google Scholar]
- Copeland LO, McDonald MB (2001) Seed storage and deterioration. In Principles of Seed Science and Technology, Ed 4. Kluwer Academic Publishers, Boston, pp 192–230
- Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28: 350–356 [Google Scholar]
- Ellis RH, Hong TD (1994) Desiccation tolerance and potential longevity of developing seeds of rice (Oryza sativa L.). Ann Bot (Lond) 73: 501–506 [Google Scholar]
- Guan JC, Jinn TL, Yeh CH, Feng SP, Chen YM, Lin CY (2004) Characterization of the genomic structures and selective expression profiles of nine class I small heat shock protein genes clustered on two chromosomes in rice (Oryza sativa L.). Plant Mol Biol 56: 795–809 [DOI] [PubMed] [Google Scholar]
- Halmer P (2000) Commercial seed treatment technology. In M Black, JD Bewley, eds, Seed Technology and Its Biological Basis. Sheffield Academic Press, Sheffield, England, pp 257–286
- Hay FR, Probert RJ (1995) Seed maturity and the effects of different drying conditions on desiccation tolerance and seed longevity in Foxglove (Digitalis purpurea L.). Ann Bot (Lond) 76: 639–647 [Google Scholar]
- International Seed Testing Association (1999) International rules for seed testing. Seed Sci Technol (Suppl) 27: 1–333 [Google Scholar]
- Kotak S, Port M, Ganguli A, Bicker F, von Koskull-Döring P (2004) Characterization of C-terminal domains of Arabidopsis heat stress transcription factors (Hsfs) and identification of a new signature combination of plant class A Hsfs with AHA and NES motifs essential for activator function and intracellular localization. Plant J 39: 98–112 [DOI] [PubMed] [Google Scholar]
- Larkindale J, Hall JD, Knight MR, Vierling E (2005) Heat stress phenotypes of Arabidopsis mutants implicate multiple signaling pathways in the acquisition of thermotolerance. Plant Physiol 138: 882–897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann J, Atzorn R, Brückner C, Reinbothe S, Leopold J, Wasternack C, Parthier B (1995) Accumulation of jasmonate, abscisic acid, specific transcripts and proteins in osmotically stressed leaf segments. Planta 197: 156–162 [Google Scholar]
- Maestri E, Klueva N, Perrotta C, Gulli M, Nguyen HT, Marmiroli N (2002) Molecular genetics of heat tolerance and heat shock proteins in cereals. Plant Mol Biol 48: 667–681 [DOI] [PubMed] [Google Scholar]
- Malik MK, Slovin JP, Hwang CH, Zimmerman JL (1999) Modified expression of a carrot small heat shock protein gene, Hsp17.7, results in increased or decreased thermotolerance. Plant J 20: 89–99 [DOI] [PubMed] [Google Scholar]
- McDonald MB (1999) Seed deterioration: physiology, repair and assessment. Seed Sci Technol 27: 177–237 [Google Scholar]
- Mishra SK, Tripp J, Winkelhaus S, Tschiersch B, Theres K, Nover L, Scharf KD (2002) In the complex family of heat stress transcription factors, HsfA1 has a unique role as master regulator of thermotolerance in tomato. Genes Dev 16: 1555–1567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura K, Lin Y, Yano M, Nagamine T (2002) Mapping quantitative trait loci controlling seed longevity in rice (Oryza sativa L.). Theor Appl Genet 104: 981–986 [DOI] [PubMed] [Google Scholar]
- Ooms JJJ, Leon-Kloosterziel KM, Bartels D, Koornneef M, Karssen CM (1993) Acquisition of desiccation tolerance and longevity in seeds of Arabidopsis thaliana (a comparative study using abscisic acid-insensitive abi3 mutants). Plant Physiol 102: 1185–1191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell AA (1995) The controlled deterioration test. In HA van de Venter, ed, Seed Vigour Testing Seminar. International Seed Testing Association, Zurich, pp 73–87
- Prändl R, Hinderhofer K, Eggers-Schumacher G, Schöffl F (1998) HSF3, a new heat shock factor from Arabidopsis thaliana, derepresses the heat shock response and confers thermotolerance when overexpressed in transgenic plants. Mol Gen Genet 258: 269–278 [DOI] [PubMed] [Google Scholar]
- Prieto-Dapena P, Almoguera C, Rojas A, Jordano J (1999) Seed-specific expression patterns and regulation by ABI3 of an unusual late embryogenesis-abundant gene in sunflower. Plant Mol Biol 39: 615–627 [DOI] [PubMed] [Google Scholar]
- Queitsch C, Hong SW, Vierling E, Lindquist S (2000) Heat shock protein 101 plays a crucial role in thermotolerance in Arabidopsis. Plant Cell 12: 479–492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousselin P, Molinier J, Himber C, Schontz D, Prieto-Dapena P, Jordano J, Martini N, Weber S, Horn R, Ganssmann M, et al (2002) Modification of sunflower oil quality by seed-specific expression of a heterologous Δ9-stearoyl-(acyl carrier protein) desaturase gene. Plant Breed 121: 108–116 [Google Scholar]
- Sanmiya K, Suzuki K, Egawa Y, Shono M (2004) Mitochondrial small heat-shock protein enhances thermotolerance in tobacco plants. FEBS Lett 557: 265–268 [DOI] [PubMed] [Google Scholar]
- SAS Institute (1990) SAS/STAT User's Guide, Version 6, Ed 4, Vol 1. SAS Institute, Cary, NC
- Sattler SE, Gilliland LU, Magallanes-Lundback M, Pollard M, DellaPenna D (2004) Vitamin E is essential for seed longevity and for preventing lipid peroxidation during germination. Plant Cell 16: 1419–1432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharf KD, Siddique M, Vierling E (2001) The expanding family of Arabidopsis thaliana small heat stress proteins and a new family of proteins containing alpha-crystallin domains (Acd proteins). Cell Stress Chaperones 6: 225–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun W, Van Montagu M, Verbruggen N (2002) Small heat shock proteins and stress tolerance in plants. Biochim Biophys Acta 1577: 1–9 [DOI] [PubMed] [Google Scholar]
- TeKrony DM (1995) Accelerated aging. In HA van de Venter, ed, Seed Vigour Testing Seminar. International Seed Testing Association, Zurich, pp 53–73
- Tsvetkova NM, Horváth I, Török Z, Wolkers WF, Balogi Z, Shigapova N, Crowe LM, Tablin F, Vierling E, Crowe JH, et al (2002) Small heat-shock proteins regulate membrane lipid polymorphism. Proc Natl Acad Sci USA 99: 13504–13509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehmeyer N, Vierling E (2000) The expression of small heat shock proteins in seeds responds to discrete developmental signals and suggests a general protective role in desiccation tolerance. Plant Physiol 122: 1099–1108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise MJ (2003) LEAping to conclusions: a computational reanalysis of late embryogenesis abundant proteins and their possible roles. BMC Bioinformatics 4: 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.