Abstract
For a number of mammalian ion channels, trafficking to the plasma membrane was found to be controlled by intrinsic sequence motifs. Among these sequences are diacidic motifs that function as endoplasmic reticulum (ER) export signals. So far it is unclear if similar motifs also exist in plant ion channels. In this study we analyzed the function of four diacidic DXE/DXD motifs of the plant K+ channel KAT1. Mutation of the first diacidic DXE motif resulted in a strong reduction of the KAT1 conductance in both guard cell protoplasts and HEK293 cells (human embryonic kidney cells). Confocal fluorescence microscopy of guard cells expressing the mutated KAT1 fused to green fluorescent protein revealed localization of the mutated channel only in intracellular structures around the nucleus. These structures could be identified as part of the ER via coexpression of KAT1 fused to yellow fluorescent protein with an ER-retained protein (HDEL) fused to cyan fluorescent protein. Block of vesicle formation from the ER by overexpression of the small GTP-binding protein Sar1 fixed in its GDP-bound form led to retention of wild-type KAT1 in similar parts of the ER. Mutation of the three other diacidic motifs had no effect. Together, the results demonstrate that one diacidic motif of KAT1 is essential for ER export of the functional channel in both guard cell protoplasts and HEK293 cells. This suggests that trafficking of plant plasma membrane ion channels is controlled via a conserved mechanism.
Plasma membrane (PM) K+ channels are crucial for cellular ion homeostasis, osmotic regulation, and excitability of cells. Their correct functioning depends not only on the control of their activity in the PM but also on the regulation of their number in the PM. Ion channels are transported to the PM along the secretory pathway via endoplasmic reticulum (ER) and Golgi. Until recently, PM proteins, including ion channels, were considered to leave the ER by default (Wieland et al., 1987). However, studies on trafficking of PM ion channels in mammalian cells revealed that transport from the ER to the Golgi apparatus is highly regulated and PM channel density may be adjusted by controlling their export from the ER (Ma et al., 2001; Wang et al., 2004). Among the few motifs identified as ER export signals in ion channels are the diacidic D/E-X-D/E motifs, which have also been shown to function as ER export signals in other PM proteins in yeast and animal cells (Nishimura and Balch, 1997; Votsmeier and Gallwitz, 2001). Mutation of these diacidic motifs resulted in a strong reduction of proteins in the PM and an accumulation in the ER.
ER export motifs are probably critical for enrichment of cargo proteins into coat protein complex II (COPII) vesicles, which are responsible for transport of proteins to the Golgi. A good candidate for the interaction of cargo proteins with the COPII coat complex is the coat protein Sec24, which can bind to a variety of ER export motifs (Bickford et al., 2004).
Homologs of the COPII coat proteins have also been identified in plants (Bar-Peled and Raikhel, 1997; Movafeghi et al., 1999; Contreras et al., 2004; Yang et al., 2005). However, knowledge about the molecular mechanism of COPII-mediated transport from the ER in plants is still limited (for review, see Aniento et al., 2006). The small GTP-binding protein Sar1, which has been shown to be crucial for formation of COPII in yeast and animal cells, has also been found to play an important role in ER-to-Golgi trafficking in plants (Takeuchi et al., 2000; Phillipson et al., 2001; daSilva et al., 2004). Investigation on the trafficking of the PM H+-ATPase from Arabidopsis (Arabidopsis thaliana) has demonstrated that transport to the PM is not by default but requires cytosolic domains (Lefebvre et al., 2004). However, the motif responsible for targeting of the H+-ATPase to the PM was not identified. ER export signals in plants have so far only been identified for Golgi proteins (Contreras et al., 2004; Hanton et al., 2005; Yuasa et al., 2005). Recently, Hanton et al. (2005) demonstrated that transport of Golgi-localized membrane proteins out of the ER was reduced to about 60% by mutation of a diacidic DXE motif.
Previous studies on trafficking of the plant K+ channel KAT1 from Arabidopsis revealed that the channel underlies a constitutive and pressure-driven turnover (Hurst et al., 2004; Meckel et al., 2004). Clusters of KAT1 were found to be inserted and retrieved into and from the PM during constitutive and pressure-driven exocytosis and endocytosis of small vesicles. Here, we investigate the role of diacidic motifs in trafficking of KAT1 using patch-clamp measurements and confocal fluorescence microscopy. The former allowed us to analyze the functioning of the channel in its target compartment PM while the latter provided information on the subcellular localization of the channel. Amino acid sequence analysis predicts four diacidic motifs in the cytosolic C terminus of KAT1: (I), DAE(394–396) located inside the putative cyclic nucleotide-binding domain (cNBD); (II), DTE(555–557); and two more inside the so-called KHA domain [(III), DLD(662–664); and (IV), DGD(668–670)], which is possibly involved in channel tetramerization (Daram et al., 1997) or channel clustering (Ehrhardt et al., 1997). Here, we show that ER export was strongly dependent on the first diacidic motif. Mutation of this motif nearly completely abolished the transport of functional KAT1 to the PM and resulted in the accumulation of the channel in the ER. These results demonstrate that ER export can act as a site of regulation of ion channel trafficking in plants.
RESULTS
Mutation of a Diacidic Motif Reduces the Number of Active KAT1 Channels in the PM of Guard Cell Protoplasts
Sequence analysis of the plant K+ inward rectifier KAT1 from Arabidopsis revealed two diacidic DXE motifs and two diacidic DXD motifs in the cytosolic carboxy-terminal tail of the channel. To determine the role of these diacidic motifs, we constructed four fusion constructs between green fluorescent protein (GFP) and KAT1 mutants. In each of these mutants were the acidic amino acids Asp and Glu of one DXE or DXD motif substituted by Ala. The resulting mutants, KAT1(I)∷GFP [DAE(394–396) mutated to AAA], KAT1(II)∷GFP [DTE(555–557) mutated to ATA], KAT1(III)∷GFP [DLD(662–664) mutated to ALA], KAT1(IV)∷GFP [DGD(668–670) mutated to AGA], and wild-type KAT1 fused to GFP (KAT1∷GFP), were transiently expressed in guard cell protoplasts. Transfected cells were analyzed by whole-cell patch-clamp measurements.
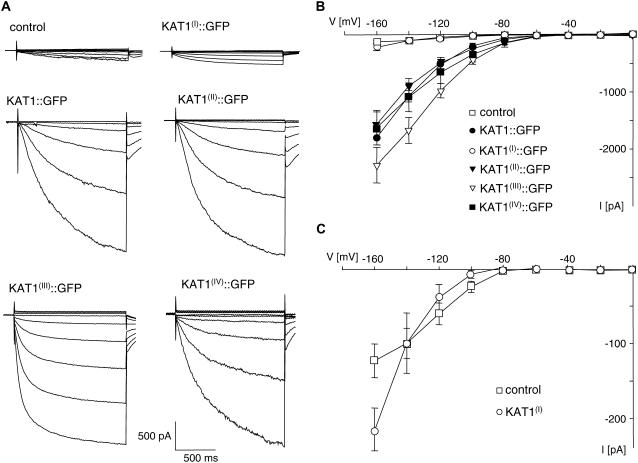
At voltages more negative than 280 mV, control and transfected guard cell protoplasts exhibit a time- and voltage-dependent inward conductance (Fig. 1). In KAT1∷GFP-expressing protoplasts, the average time-dependent inward current at −160 mV exceeded those of untransfected protoplasts by a factor of about 10 (Fig. 2), as demonstrated before (Hurst et al., 2004). This large inward conductance corresponds to K+ influx through KAT1. A similar increase in inward conductance was recorded from guard cell protoplasts expressing the mutated channels KAT1(II)∷GFP, KAT1(III)∷GFP, and KAT1(IV)∷GFP (Figs. 1, A and B, and 2). The time-dependent activation of K+ inward currents and the current-voltage relation of KAT1(II)∷GFP- and KAT1(IV)∷GFP-expressing protoplasts resembled that of KAT1∷GFP (Fig. 1, A and B). In protoplasts expressing KAT1(III)∷GFP, activation of the K+ inward current occurred already at −80 mV and was faster than the time-dependent activation of wild-type KAT1 and of the other two mutants described above (Fig. 1, A and B). This suggests that the voltage-dependent activation of KAT1 is affected by mutation of the diacidic motif (III). However, the focus of this investigation is on trafficking of KAT1, which seems not to be affected by the mutation.
Figure 1.
Mutation of the diacidic motif (I) of KAT1 reduces inward conductance of transfected guard cell protoplasts. A, Current response to voltage steps from a holding voltage of 255 mV to test voltages of 0 to 2160 mV in 20-mV increments of untransfected guard cell protoplasts (control) and protoplasts transfected with either GFP fusion constructs of wild-type KAT1 or one of the four mutants of KAT1 as indicated above the traces. B, Current-voltage relationship of mean steady-state current minus instantaneous current recorded from untransfected guard cell protoplasts (control, n = 15) and protoplasts transfected with KAT1∷GFP (n = 5), KAT1(I)∷GFP (n = 5), KAT1(II)∷GFP (n = 5), KAT1(III)∷GFP (n = 8), or KAT1(IV)∷GFP (n = 7). C, Current-voltage relationship of mean steady-state current minus instantaneous current recorded from untransfected guard cell protoplasts (control, n = 15) and protoplasts transfected with KAT1(I)∷GFP (n = 5). Error bars correspond to mean ± SEM.
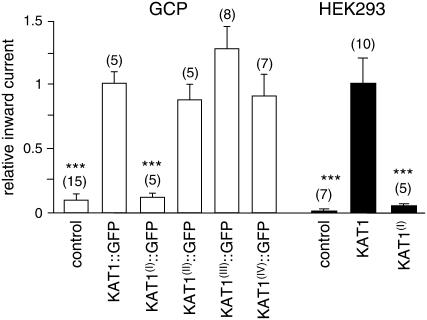
Figure 2.
Comparison of average steady-state inward currents. Currents were measured at 2160 mV (guard cell protoplasts [GCP]) or 2140 mV (HEK293 cells) in untransfected cells (control) and cells expressing KAT1 or KAT1 mutants (as indicated below bars). Average steady-state currents were normalized to the current recorded in cells expressing wild-type KAT1. Numbers of independent experiments are given in parentheses. Error bars correspond to mean ± SEM. Asterisks indicate significant differences compared to cells transfected with wild-type KAT1 (***, P < 0.005; Student's t test).
Measurements of protoplasts expressing KAT1(I)∷GFP revealed a completely different picture. The average time-dependent inward current at −160 mV was much lower than the one recorded from KAT1∷GFP-transfected protoplasts and was similar to untransfected control protoplasts (Fig. 2). This suggests that KAT1(I)∷GFP is inactive or not incorporated into the PM. However, in contrast to the endogenous K+ inward rectifier that exhibits saturation of the conductance at voltages negative of −140 mV, the inward conductance in protoplasts transfected with KAT1(I)∷GFP revealed no saturation in the voltage range analyzed (Fig. 1C). The current-voltage relation of KAT1(I)∷GFP-expressing protoplasts was very similar to the current-voltage relation of wild-type KAT1 and the other KAT1 mutants (Fig. 1, B and C). This implies that KAT1(I)∷GFP is indeed incorporated and active in the PM of guard cells, albeit to a very low extent. Due to the large variability in endogenous K+ inward conductance, the additional KAT1(I)∷GFP conductance is not seen as a significant increase in the average inward current.
Together, the results demonstrate that mutation of the diacidic motif (I) largely reduced the number of active KAT1 channels in the PM of guard cells, while mutation of the three other diacidic motifs had no effect on the KAT1 conductance in transfected guard cell protoplasts.
Mutation of a Diacidic Motif Results in ER Retention of KAT1
The results described above imply that mutation of the diacidic motif (I) of KAT1 strongly affects the number of active channels in the PM. This can in principle result from an inhibition of channels in the PM or from a reduced incorporation of channels into the PM. The latter explanation seems more likely as diacidic motifs have been shown to act as ER export signals (Barlowe, 2003). To investigate whether the reduction in the number of channels in the PM of KAT1(I)∷GFP-expressing guard cell protoplasts results indeed from retention of the channel in the ER, we compared the subcellular distribution of wild-type KAT1 and KAT1 mutants fused to GFP or yellow fluorescent protein (YFP) in transfected guard cells using confocal laser scanning microscopy.
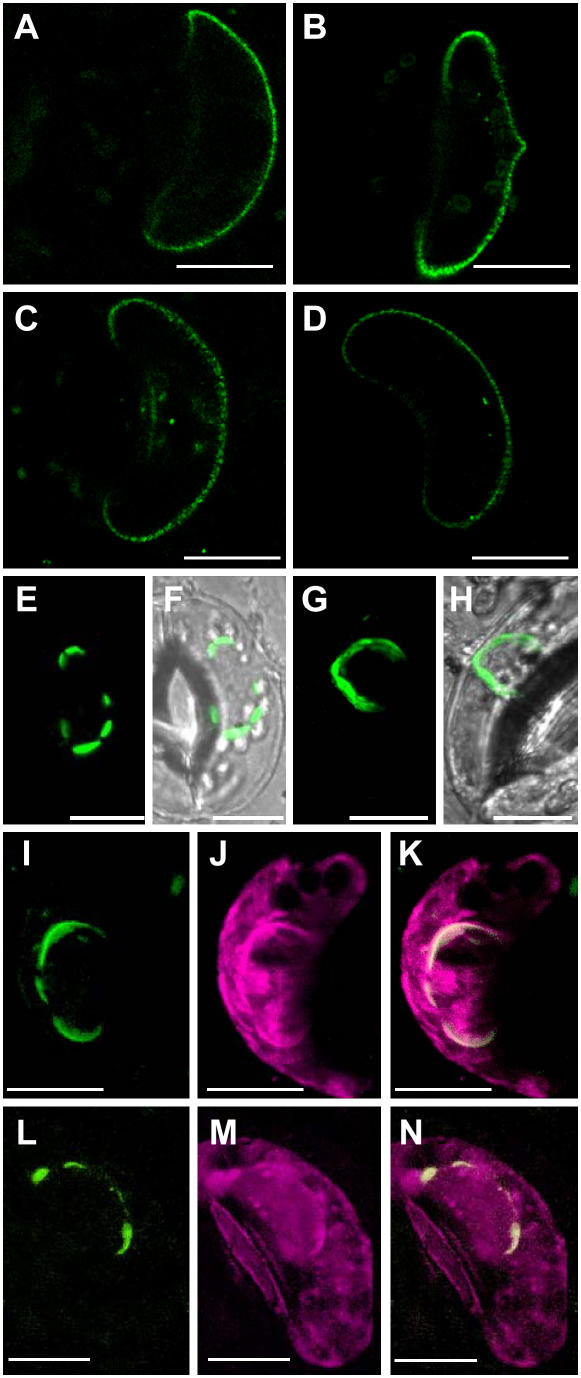
In guard cells expressing KAT1∷GFP or KAT1(II)∷GFP, KAT1(III)∷GFP and KAT1(IV)∷GFP fluorescence was mainly found in the PM (Fig. 3, A–D). In addition to the bright labeling of the PM, some guard cells also exhibit staining of intracellular compartments mainly around the nucleus (data not shown). Expression of KAT1(I)∷GFP led to a completely different staining pattern. None of the guard cells transfected with this mutant displayed labeling of the PM. Instead, only intracellular compartments, mainly around the nucleus, were brightly labeled by GFP (Fig. 3, E and F). A comparable staining pattern was observed in guard cells cotransfected with wild-type KAT1∷GFP and Sar1[T39N], a mutant of the small GTP-binding protein Sar1 fixed in the GDP-bound form (Fig. 3, G and H). Sar1 has been shown to be essential for the formation of COPII vesicles and, thus, for export of proteins from the ER. Block of Sar1 in its GDP-bound form led to the inhibition of ER export in tobacco (Nicotiana spp.) and Arabidopsis cultured cells (Takeuchi et al., 2000). The fluorescent intracellular structures shown in Figure 3, E to H, therefore, most likely correspond to part of the ER. This was confirmed by colocalization studies of KAT1∷YFP or KAT1(I)∷YFP and the ER retention signal HDEL fused to secretory cyan fluorescent protein (CFP) as an ER marker. Figure 3, I to N, shows that all structures labeled by KAT1(I)∷YFP (Fig. 3, I–K) or KAT1∷YFP expressed in the presence of the GDP-fixed Sar1 mutant (Fig. 3, L–N) were also stained by CFP∷HDEL. The largest part of the CFP∷HDEL-labeled ER did not show any KAT1(I)∷YFP or KAT1∷YFP staining. In particular, KAT1(I)∷YFP or KAT1∷YFP was never localized to the cortical ER. Together, the results demonstrate that inhibition of ER export of KAT1 is associated with the accumulation of the channel in restricted areas of the ER, mainly around the nucleus.
Figure 3.
Mutation of the diacidic motif (I) of KAT1 results in ER retention. A to D, Projection of two optical sections through the equatorial region of guard cells expressing KAT1∷GFP (A), KAT1(II)∷GFP (B), KAT1(III)∷GFP (C), or KAT1(IV)∷GFP (D). E to H, Overlay of transparency and fluorescent projection of four optical sections through the equatorial region of a guard cell expressing KAT1(I)∷GFP (E and F) or wild-type KAT1∷GFP and GDP-fixed Sar1 (G and H). I to K, Projection of four optical sections through the equatorial region of a guard cell cotransfected with KAT1(I)∷YFP and CFP∷HDEL; KAT1(I)∷YFP fluorescence is shown in I, CFP∷HDEL fluorescence is shown in J, and K represents overlay of both images. L to N, Projection of four optical sections through the equatorial region of a guard cell cotransfected with GDP-fixed Sar1, KAT1∷YFP, and CFP∷HDEL; KAT1∷YFP fluorescence is shown in L, CFP∷HDEL fluorescence is shown in M, and N represents overlay of both images. Scale bars correspond to 10 μm.
The localization of KAT1(I)∷YFP was not time dependent. Even 48 h after transfection, neither the current of KAT1(I)∷GFP-transfected guard cell protoplasts nor the staining pattern was any different compared to measurements carried out 15 h after transfection. This implies that the distribution of channels reached a steady state soon after start of expression and did not change over time.
In conclusion, the localization studies imply that the reduced K+ inward conductance observed in KAT1(I)∷GFP-transfected protoplasts results from the inhibition of ER export and consequent decrease in the number of KAT1(I)∷GFP in the PM. The diacidic motif (I) thus most likely functions as an ER export signal.
Mutation of a Diacidic Motif Also Affects KAT1 Conductance in HEK293 Cells
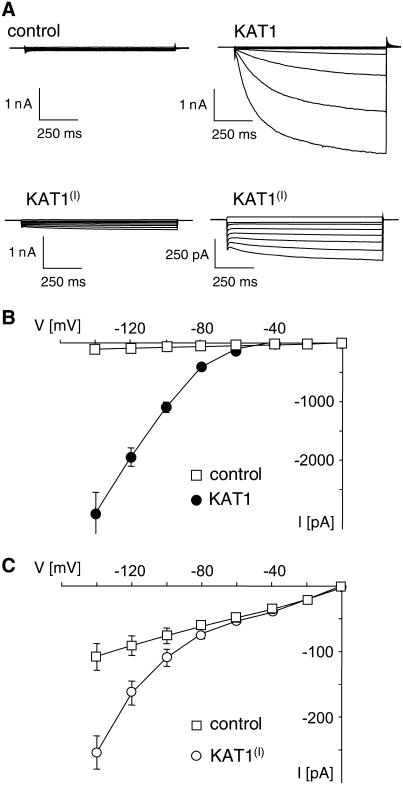
To investigate whether the function of the first diacidic motif of KAT1 as an ER export signal is conserved among the plant and animal kingdom, we analyzed HEK293 cells (human embryonic kidney cells) transfected with wild-type or mutant KAT1. HEK293 cells exhibit only a low PM conductance at negative voltages and no endogenous time-activated K+ inward current (Fig. 4). They therefore provide an excellent system to study the K+ inward rectifier KAT1. HEK293 cells expressing wild-type KAT1 showed large time-dependent inward currents at voltages more negative than 280 mV (Fig. 4, A and B), as demonstrated before (Hertel et al., 2005). The inward currents displayed the typical activation kinetic and voltage dependence recorded from wild-type KAT1-expressing guard cell protoplasts (Hurst et al., 2004). Measurements of HEK293 cells transfected with KAT1(I) showed a completely different current response that was at first glance similar to the one recorded from untransfected cells (Fig. 4A). However, a blow up of the current traces clearly revealed a time- and voltage-dependent activation of an inward current that was never observed in untransfected HEK293 cells (Fig. 4A). The activation kinetic of this current qualitatively matches the kinetics of the current recorded from wild-type KAT1-transfected cells. All transfected cells exhibit similar voltage dependence with an increase in current at voltages negative of −80 mV (Fig. 4, B and C). However, in KAT1(I)-expressing cells, the current at 2140 mV was reduced to only about 5% of the current recorded in wild-type KAT1-expressing cells (Fig. 2). This demonstrates that KAT1(I) is inserted into the PM of HEK293 cells, albeit to a lower extent.
Figure 4.
Mutation of the diacidic motif (I) of KAT1 reduces inward conductance of transfected HEK293 cells. A, Current response to voltage steps from a holding voltage of 210 mV to test voltages of 0 to 2140 mV in 20-mV increments of untransfected HEK293 cells (control) and of HEK293 cells transfected with wild-type KAT1 or KAT1(I) as indicated above the traces. B, Current-voltage relationship of mean steady-state current minus instantaneous current recorded from untransfected HEK293 cells (control, n = 7) and HEK293 cells transfected with wild-type KAT1 (n = 10). C, Current-voltage relationship of mean steady-state current including instantaneous current recorded from untransfected HEK293 cells (control, n = 7) and HEK293 cells transfected with KAT1(I) (n = 5). Error bars correspond to mean ± SEM.
Together, these results imply that mutation of the diacidic motif (I) of KAT1 affects ER export in HEK293 and in guard cells, suggesting that the mechanism of ER export is conserved among plant and animal cells.
DISCUSSION
The Diacidic Motif (I) of KAT1 Is Essential for Efficient ER Export
In a number of proteins, diacidic motifs have been shown to be crucial for efficient transport of these proteins from the ER to their target compartments (Nishimura and Balch, 1997; Votsmeier and Gallwitz, 2001; Ma et al., 2001; Wang et al., 2004; Hanton et al., 2005). In the cytosolic C terminus of the plant K+ channel KAT1, four diacidic motifs have been identified. Patch-clamp analysis of guard cell protoplasts and HEK293 cells revealed that mutation of only one [diacidic motif (I)] out of the four diacidic motifs of KAT1 dramatically reduced the inward conductance of transfected cells. The reduction in inward conductance could be quantified in HEK293 cells, which do not exhibit any endogenous inward conductance. In HEK293 cells expressing the mutant channel KAT1(I), the inward conductance was reduced to only about 5% compared to wild-type KAT1-transfected cells. The reduced current recorded from cells expressing KAT1(I) showed a qualitatively similar kinetic and voltage dependence as wild-type KAT1. This implies that the lower inward conductance of KAT1(I)-transfected cells results from a reduced number of functional channels in the PM, suggesting that transport but not function of the channel to the PM is inhibited.
This was confirmed by confocal images of KAT1(I)∷GFP-expressing guard cells, which showed a bright staining of intracellular compartments mainly around the nucleus without any detectable staining of the PM. Coexpression studies with the ER marker CFP∷HDEL confirmed that these intracellular structures correspond to ER, demonstrating that the largest amount of the mutated channel is indeed retained in the ER. The fluorescence of the remaining channels that still reached the PM was too low to be detected by confocal laser scanning microscopy.
In principle, the retention of KAT1(I)∷GFP in the ER could result from misfolding of the mutated protein, which is kept in the ER for subsequent degradation. However, the fact that in guard cell protoplasts as well as in HEK293 cells functional KAT1(I) channels with similar voltage dependence and time-dependent activation kinetics as wild-type KAT1 can be detected in the PM argues against this hypothesis. Our results rather implicate that the observed retention of KAT1(I)∷GFP in the ER is due to a reduction of ER export of fully functional KAT1 channels. This is consistent with our analysis of wild-type KAT1-expressing guard cells where ER export has been blocked by coexpression of the GDP-fixed Sar1 mutant. These cells showed the same staining pattern as KAT1(I)∷GFP-expressing cells. We therefore conclude that the diacidic motif (I) of KAT1 acts as an ER export signal in both HEK293 and guard cells. This also suggests that the mechanism of ER export is conserved among plant and animal cells.
ER Retention of KAT1 Is Very Efficient and May Be Restricted to Certain Areas
Recently, a diacidic motif has been shown to affect ER export of two plant Golgi-localized membrane proteins (Hanton et al., 2005). Using imaging of transfected tobacco leaves, Hanton et al. (2005) demonstrated that mutation of a diacidic motif led to a reduction of the Golgi localization of these proteins by about 40%. The authors therefore suggest that factors other than diacidic motifs also influence the ER export of these proteins. In our studies, mutation of the diacidic motif (I) nearly completely blocked transport of the channel to the PM without affecting its functioning. This implicates that efficient transport of KAT1 from the ER to the Golgi is highly dependent on the first diacidic motif. Similar results have been described for the function of diacidic motifs in trafficking of PM transporters in mammalian cells (Ma et al., 2001; Wang et al., 2004). Investigation of trafficking of mammalian PM transporters suggests that binding of scaffold proteins, such as PDZ domain-containing proteins, can change the relative effectiveness of ER export signals and thus allow regulation of the number of transporters in the PM (Ma and Jan, 2002). A tight regulation of the protein density is of particular importance for PM ion channels, as small changes in the number of channels can have a pronounced effect on the conductance and thus function of the cell. Most likely the diacidic motif (I) of KAT1 is part of such a regulatory mechanism that controls the density of this channel in the PM of plant cells.
Analysis of the localization of KAT1(I)∷GFP revealed that the channel is mainly retained in the ER around the nucleus. Similar results were found for guard cells transfected with KAT1∷GFP when ER export was blocked by coexpression of GDP-fixed Sar1. This distinct localization of ER-retained KAT1 was also found in epidermal cells (data not shown). As the outer nuclear membrane is continuous with the ER, it is not unexpected that proteins that are retained in the ER can also be found in the nuclear envelope. However, previous investigations on proteins retained in the ER revealed localization of these proteins in the nuclear envelope only in addition to the distribution throughout the rest of the ER (Herman et al., 1990; Batoko et al., 2000). The reasons for retention of KAT1 in restricted areas around the nucleus remain to be identified.
Function of the Diacidic Motif Is Position Dependent
From the four diacidic motifs found in KAT1, only mutation of the motif (I) had an effect on both the KAT1 conductance and the cellular localization of the mutated channel. This implies that the position of the diacidic motif in the protein is important for its functioning. Position dependence of the function of diacidic motifs has also been described for yeast PM protein Sys1p (Votsmeier and Gallwitz, 2001) and for the plant Golgi-localized membrane proteins GONST1 and CASP (Hanton et al., 2005).
The diacidic motif (I) of KAT1 is located in the putative cNBD. Recent investigations on disruption of the cNBD in the animal K+ channels HCN and HERG implied that highly conserved regions in the cNBD are generally critical for ion channel trafficking (Akhavan et al., 2005) This is supported by the fact that the diacidic ER export motif of the CFTR channel is also located within the cNBD (Wang et al., 2004). Using structural models of the cNBD of the CFTR channel, Wang et al. (2004) demonstrated the ability of the loop containing the diacidic motif to insert directly into the diacidic code-binding pocket of the COPII coat complex Sec23/24. In plant cells the cNBD was found to be required for efficient transport of a H+-ATPase from Nicotiana plumbaginifolia to the PM even though ER export motifs have so far not been identified (Lefebvre et al., 2004). An alignment of KAT1 with other related plant K+ inward rectifiers revealed that the diacidic motif (I) and the diacidic motif (IV) are highly conserved among these channels (Fig. 5). However, only the diacidic motif (I), which is located in the cNBD, was found to affect ER export, suggesting that the cNBD is per se important for ER export of plant channels. Together, the results demonstrate that a diacidic motif of KAT1 acts as an ER export signal in plant and animal cells, probably via a conserved mechanism.
Figure 5.
Alignment of amino acid sequences of plant K+ inward rectifier. Comparison of amino acid sequences of the potential diacidic ER export signals found in the C-terminal region of KAT1 (gray boxes) with the corresponding region of other closely related plant K+ inward rectifying channels is shown. The GenBank accession numbers of the aligned channels sequences are as follows: KAT1, M86990; KAT2, AJ288900; KST1, X79779; KPT1, AJ244623; SIRK, AF359522; and KMT1p, AAF81250.
MATERIALS AND METHODS
Vectors for KAT1 Expression
For expression of KAT1∷GFP fusion protein in guard cells, we used the cDNA of kat1 cloned into the pAVA393 expression vector in frame with mGFP5 or YFP under the control of two strong 35S promoters as described previously by Hurst et al. (2004).
Expression of KAT1 in the mammalian cell line HEK293 (human embryonic kidney cells) was obtained with kat1 cDNA cloned into pCB6 (accession no. ATCC37274) eukaryotic expression vector at the NcoI restriction site under control of a cytomegalovirus 35S promoter. HEK293 cells were cotransfected with the pEGFP-N2 vector (CLONTECH) to express cytosolic GFP as a transfection control.
Mutagenesis of Putative ER Export Motifs in kat1 cDNA
Mutations in the DXE and DXD motifs of channel protein were created by PCR-based, site-directed mutagenesis (QuikChange site-directed mutagenesis kit; Stratagene) and confirmed by sequencing. The expression vectors pAVA393-kat1 and pCB6-kat1, both containing the kat1 cDNA as described above, served as templates. The plasmids were cloned into Escherichia coli/DH5α and isolated with Qiagen high-speed Midi-Kit for cell transfection.
Transfection of Intact Guard Cells via Particle Delivery and Isolation of Protoplasts
Vicia faba L. cv Bunyan were grown under controlled climate conditions with 18°C, 70% relative humidity, and a 14/10-h photoperiod at 350 to 400 μmol photons m−2 s−1. Transfection of intact guard cells via particle delivery was performed as described earlier (Hurst et al., 2004).
Cotransfection was performed via coating of gold with equal molar amounts of each plasmid DNA to give a total amount of 15 μg of DNA. Guard cell protoplasts were prepared from transfected leaves after overnight incubation at room temperature as described previously (Homann, 1998).
Cultivation and Transfection of Mammalian Cell Line HEK293
HEK293 cells were grown at 37°C and 5% CO2. For transient expression of KAT1 and KAT1 mutants, HEK293 cells were transfected with 0.75 μg of each pCB6-kat1 and pEGFP-N2 vector using the liposomal transfection reagent Metafectene (Biontex) according to manufacturer's instructions.
Patch-Clamp Measurements
HEK293 Cells
Experiments were performed on cells incubated at 37°C in 5% CO2 for 2 to 3 d after transfection (for details, see Hertel et al., 2005). Cells were bathed in a solution containing the following: 20 mm KCl, 1.8 mm CaCl2, 1 mm MgCl2, and 5 mm HEPES at pH 7.4. Choline chloride was used to adjust the osmolarity to 300 mOsmol/kg. Patch pipettes contained the following: 130 mm K+-gluconate, 10 mm NaCl, 5 mm HEPES, 0.1 mm Na2GTP, 0.1 μm CaCl2, 2 mm MgCl2, 5 mm Na2Phosphocreatin, and 2 mm K2ATP at pH 7.4 with an osmolarity of approximately 330 mOsmol/kg.
Guard Cell Protoplasts
Guard cell protoplasts were bathed in 10 mm KCl, 10 mm CaCl2, and 5 mm MES, pH 6.0/KOH. The osmolarity was adjusted to 520 mOsmol/kg with Sorbitol. Patch pipettes were filled with 150 mm K+-gluconate, 10 mm KCl, 2 mm MgCl2, 2 mm EGTA, 10 mm HEPES, and 2 mm K2ATP, pH 7.8/KOH. Osmolarity was adjusted to 560 mOsmol/kg with Sorbitol.
In both cell systems, measurements were performed in a standard whole-cell patch-clamp experiment as described in detail previously (Homann and Thiel, 2002; Hurst et al., 2004; Hertel et al., 2005).
Confocal Laser Scanning Microscopy
Confocal microscopic analysis of transfected turgid guard cells was performed after overnight incubation as described earlier using a confocal laser scanning microscope (Leica TCS SP; for details, see Meckel et al., 2004). For excitation of fluorescent proteins, the following lines of a 25-mW argon ion laser were used: 488 nm for GFP, 458 nm for CFP, and 514 nm for YFP. Fluorescence was detected at 505 to 535 nm for GFP, 465 to 490 nm for CFP, and 600 to 650 nm for YFP. Images were processed using the Leica Confocal Software 2.00. For the microscopic analysis, guard cells were bathed in 0.1 mm CaCl2, 10 mm MES, 45 mm KCl, pH 6.1/KOH.
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers M86990, AJ288900, X79779, AJ244623, AF359522, and AAF81250.
Acknowledgments
We thank Prof. G. Thiel for discussion and very helpful comments on the manuscript. We are grateful to B. Kost (Heidelberg) and C. Ritzenthaler (Strasbourg, France) for providing us with CFP∷HDEL and the GDP-fixed Sar1, respectively. We also thank J. Hewing for help with image processing.
This work was supported by the Deutsche Forschungsgemeinschaft (grant no. SPP 1108 HO–2046/3–2 to U.H.).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Ulrike Homann (homann-u@bio.tu-darmstadt.de).
References
- Akhavan A, Atanasiu R, Noguchi T, Han W, Holder N, Shrier A (2005) Identification of the cyclic-nucleotide-binding domain as a conserved determinant of ion-channel cell-surface localization. J Cell Sci 118: 2803–2812 [DOI] [PubMed] [Google Scholar]
- Aniento F, Matsuoka K, Robinson DG (2006) ER-to-Golgi pathway: the COPII pathway. In DG Robinson, ed, The Plant Endoplasmic Reticulum-Plant Cell Monographs, Vol 4. Springer, Heidelberg (in press)
- Barlowe C (2003) Signals for COPII-dependent export from the ER: What's the ticket out? Trends Cell Biol 13: 295–300 [DOI] [PubMed] [Google Scholar]
- Bar-Peled M, Raikhel NV (1997) Characterization of AtSEC12 and AtSAR1. Proteins likely involved in endoplasmic reticulum and Golgi transport. Plant Physiol 114: 315–324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batoko H, Zheng HQ, Hawes C, Moore I (2000) A rab1 GTPase is required for transport between the endoplasmic reticulum and golgi apparatus and for normal golgi movement in plants. Plant Cell 12: 2201–2218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickford LC, Mossessova E, Goldberg J (2004) A structural view of the COPII vesicle coat. Curr Opin Struct Biol 14: 147–153 [DOI] [PubMed] [Google Scholar]
- Contreras I, Yang Y, Robinson DG, Aniento F (2004) Sorting signals in the cytosolic tail of plant p24 proteins involved in the interaction with the COPII coat. Plant Cell Physiol 45: 1779–1786 [DOI] [PubMed] [Google Scholar]
- Daram P, Urbach S, Gaymard F, Sentenac H, Cherel I (1997) Tetramerization of the AKT1 plant potassium channel involves its C-terminal cytoplasmic domain. EMBO J 16: 3455–3463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- daSilva LL, Snapp EL, Denecke J, Lippincott-Schwartz J, Hawes C, Brandizzi F (2004) Endoplasmic reticulum export sites and Golgi bodies behave as single mobile secretory units in plant cells. Plant Cell 16: 1753–1771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrhardt T, Zimmermann S, Müller-Röber B (1997) Association of plant K+(in) channels is mediated by conserved C-termini and does not affect subunit assembly. FEBS Lett 409: 166–170 [DOI] [PubMed] [Google Scholar]
- Hanton SL, Renna L, Bortolotti LE, Chatre L, Stefano G, Brandizzi F (2005) Diacidic motifs influence the export of transmembrane proteins from the endoplasmic reticulum in plant cells. Plant Cell 17: 3081–3093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman EM, Tague BW, Hoffman LM, Kjemtrup SE, Chrispeels MJ (1990) Retention of phytohemagglutinin with carboxy-terminal tetrapeptide KDEL in the nuclear envelope and the endoplasmic reticulum. Planta 182: 305–312 [DOI] [PubMed] [Google Scholar]
- Hertel B, Horváth F, Wodala B, Hurst AC, Moroni A, Thiel G (2005) KAT1 inactivates at sub-millimolar concentrations of external potassium. J Exp Bot 56: 3103–3110 [DOI] [PubMed] [Google Scholar]
- Homann U (1998) Fusion and fission of plasma-membrane material accommodates for osmotically induced changes in the surface area of guard-cell protoplasts. Planta 206: 495–499 [Google Scholar]
- Homann U, Thiel G (2002) The number of K+ channels in the plasma membrane of guard cell protoplasts changes in parallel with the surface area. Proc Natl Acad Sci USA 99: 10215–10220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurst AC, Meckel T, Tayefeh S, Thiel G, Homann U (2004) Trafficking of the plant potassium inward rectifier KAT1 in guard cell protoplasts of Vicia faba. Plant J 37: 391–397 [DOI] [PubMed] [Google Scholar]
- Lefebvre B, Batoko H, Duby G, Boutry M (2004) Targeting of a Nicotiana plumbaginifolia H+-ATPase to the plasma membrane is not by default and requires cytosolic structural determinants. Plant Cell 16: 1772–1789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma D, Jan LY (2002) ER transport signals and trafficking of potassium channels and receptors. Curr Opin Neurobiol 12: 287–292 [DOI] [PubMed] [Google Scholar]
- Ma D, Zerangue N, Lin Y, Collins A, Yu M, Jan Y, Jan L (2001) Role of ER export signals in controlling surface potassium channel numbers. Science 291: 316–319 [DOI] [PubMed] [Google Scholar]
- Meckel T, Hurst AC, Thiel G, Homann U (2004) Endocytosis against high turgor: intact guard cells of Vicia faba constitutively endocytose fluorescently labelled plasma membrane and GFP-tagged K+-channel KAT1. Plant J 39: 182–193 [DOI] [PubMed] [Google Scholar]
- Movafeghi A, Happel N, Pimpl P, Tai GH, Robinson DG (1999) Arabidopsis Sec21p and Sec23p homologs. Probable coat proteins of plant COP-coated vesicles. Plant Physiol 199: 1437–1446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura N, Balch WE (1997) A di-acidic signal required for selective export from the endoplasmic reticulum. Science 277: 556–558 [DOI] [PubMed] [Google Scholar]
- Phillipson BA, Pimpl P, daSilva LL, Crofts AJ, Taylor JP, Movafeghi A, Robinson DG, Denecke J (2001) Secretory bulk flow of soluble proteins is efficient and COPII dependent. Plant Cell 13: 2005–2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi M, Ueda T, Sato K, Abe H, Nagata T, Nakano A (2000) A dominant negative mutant of sar1 GTPase inhibits protein transport from the endoplasmic reticulum to the Golgi apparatus in tobacco and Arabidopsis cultured cells. Plant J 23: 517–525 [DOI] [PubMed] [Google Scholar]
- Votsmeier C, Gallwitz D (2001) An acidic sequence of a putative yeast Golgi membrane protein binds COPII and facilitates ER export. EMBO J 20: 6742–6750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Matteson J, An Y, Moyer B, Yoo JS, Bannykh S, Wilson IA, Riordan JR, Balch WE (2004) COPII-dependent export of cystic fibrosis transmembrane conductance regulator from the ER uses a di-acidic exit code. J Cell Biol 167: 65–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieland F, Gleason M, Serafini T, Rothman J (1987) The rate of bulk flow from the endoplasmic reticulum to the cell surface. Cell 50: 289–300 [DOI] [PubMed] [Google Scholar]
- Yang Y-D, Elamawi R, Bubeck J, Pepperkok R, Ritzenthaler C, Robinson DG (2005) Dynamics of COPII vesicles and the Golgi apparatus in cultured Nicotiana tabacum BY-2 cells provides evidence for transient association of Golgi stacks with endoplasmic reticulum exit sites. Plant Cell 17: 1343–1359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuasa K, Toyooka K, Fukuda H, Matsuoka K (2005) Membrane-anchored prolyl hydroxylase with an export signal from the endoplasmic reticulum. Plant J 41: 81–94 [DOI] [PubMed] [Google Scholar]