Abstract
The molecular nature of store-operated Ca2+-selective channels has remained an enigma, due largely to the continued inability to convincingly demonstrate Ca2+-selective store-operated currents resulting from exogenous expression of known genes. Recent findings have implicated two proteins, Stim1 and Orai1, as having essential roles in store-operated Ca2+ entry across the plasma membrane. However, transient overexpression of these proteins on their own results in little or no increase in store operated entry. Here we demonstrate dramatic synergism between these two mediators; co-transfection of HEK293 cells with Stim1 and Orai1 results in an approximate 20-fold increase in store-operated Ca2+ entry and Ca2+-selective current. This demonstrates that these two proteins are limiting for both the signaling and permeation mechanisms for Ca2+-selective store-operated Ca2+ entry. There are three mammalian homologs of Orai1, and in expression experiments they all produced or augmented store-operated Ca2+ entry with efficacies in the order Orai1 > Orai2 > Orai3. Stim1 apparently initiates the signaling process by acting as a Ca2+ sensor in the endoplasmic reticulum. This results in rearrangement of Stim1 within the cell and migration toward the plasma membrane to regulate in some manner Orai1 located in the plasma membrane. However, we demonstrate that Stim1 does not incorporate in the surface membrane, and thus likely regulates or interacts with Orai1 at sites of close apposition between the plasma membrane and an intracellular Stim1-containing organelle.
Store-operated Ca2+ (SOC) influx is the major mechanism for Ca2+ entry in many non-excitable cell types. Despite more than two decades of research, little is known about the activation mechanism for the channels responsible for this type of Ca2+ entry. Recently, based primarily on RNAi screens from either Drosophila or mammalian cells, two proteins have been identified as essential components in SOC influx: Stim1 (1;2), and Orai1 (3;4). Stim1 is thought to act as a sensor for Ca2+ in the endoplasmic reticulum, or in that compartment of the endoplasmic reticulum responsible for signaling to store-operated channels. Zhang et al (5) proposed a mechanism for Stim1 mediated SOC influx by which Stim1, normally an ER membrane resident protein, is transported to and inserted into the plasma membrane upon Ca2+ store depletion. However, others have suggested that Stim1 may re-localize near the plasma membrane without inserting into the membrane upon Ca2+-store depletion (2). Mammalian cells may also express a homolog of Stim1, Stim2 (1;2;6), although currently its function in SOC entry is uncertain. Orai1 was first identified by Feske et al. through a combined RNAi screen and analysis of gene mutations in patients suffering from severe combined immunodeficiency (3). The protein appears to be resident in the plasma membrane (3;4) and its molecular function has not yet been defined. However, Vig et al. suggest that Orai1 (which they designated as CRACM1) could function in the plasma membrane either as a component of the calcium-release-activated calcium (CRAC) channel, or as a regulator of CRAC channels (4).
In the current study, we have sought to determine if Stim1 and Orai1 functionally interact by co-expressing them in HEK293 cells. Surprisingly, we find that the combination of Stim1 and Orai1 results in a substantial increase in SOC entry, suggesting that these proteins are limiting for, and may actually comprise both the activation as well as permeation mechanism for SOC influx. This is to our knowledge the first demonstration of an Icrac-like current produced by the ectopic expression of known genes. In addition to Orai1, Orai2 expression with Stim1 also enhanced SOC entry in these cells, however to a lesser extent. Although Orai3 alone or with Stim1 showed no elevation in current or Ca2+ entry, it did rescue the knockdown of Orai1 in HEK293 cells. In addition, we have investigated the movements and distribution of Stim1 and conclude that this protein translocates to the vicinity of the plasma membrane, where it presumably interacts with and activates Orai1, but Stim1 does not incorporate into the plasma membrane.
Methods
Cell Culture
HEK293 cells, obtained from ATCC, were cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% heat-inactivated fetal bovine serum and 2 mM glutamine and maintained in a humidified 95% air, 5% CO2 incubator at 37° C. In preparation for cDNA or siRNA transfection, cells were transferred to 6-well plates and allowed to grow to ∼90% confluence. In preparation for Ca2+ measurements, cells were transferred onto 30 mm round glass coverslips (#1 thickness) as a 0.5 ml cell suspension (∼400,000 cells/ml) and allowed to attach for a period of 12 h. Additional DMEM was then added to the coverslip, and the cells maintained in culture for an additional 12-36 h before use in Ca2+ measurements.
Plasmids
Full length Stim1, Orai1, Orai2 and Orai3 cDNA plasmids were purchased from Origene in the pCMV6-XL5 (Stim1 and Orai2) and pCMV-XL4 (Orai1 and Orai3) vectors. The following mutations were made in both the native Stim1 plasmid from Origene as well as Stim1 with the yellow enhanced fluorescent protein fused to the N-terminus, obtained from Tobias Meyer, Stanford University. Single amino acid mutations to the putative EF-hand of Stim1 (D76A, D76N and E87Q) as well as a multi amino acid mutant (D76N,D78N) were made by site-directed mutagenesis with the QuikChange Site-Directed Mutagenesis Kit (Stratagene). The mutations were all sequence confirmed. The KIR-GFP plasmid was obtained from Deborah Burshtyn, University of Alberta.
siRNA Knockdown
HEK293 cells were plated in a 6-well plate on Day 1. On Day 2, cells were transfected with siRNA (100 nM) against Stim1 (Dharmacon, USA) or Orai1 (Invitrogen) using Metafectene (Biontex Laboratories GmbH, Martinsried/Planegg, Germany) 7 μl per well), and including siGLO (Dharmacon, USA) as a marker. The sequence of the siRNA against Stim1 was: agaaggagcuagaaucucac; for Orai1 it was: cccuucggccugaucuuuaucgucu. After a 6 hr incubation period, the medium bathing the cells was replaced with complete DMEM and maintained in culture. On Day 3, siRNA treated cells were transfected with cDNA for Stim1 tagged with EYFP, or EYFP alone, as described below. On Day 4, cells were transferred to 30 mm glass coverslips in preparation for Ca2+ measurements as described above, which were performed on Day 5 or 6.
cDNA transfection
HEK293 cells were plated in a 6-well plate on Day 1. On Day 2, cells were transfected using Lipofectamine 2000 (Invitrogen ; 2 μl per well) with cDNA (0.5 μg/well) for EYFP, Orai1, Orai2, Orai3 or EYFP tagged Stim1 (a gift from Dr. Tobias Meyer) and mutant forms of Stim1. The latter constructs have a mutations in the putative EF-Hand region of the Stim1 molecule (described in Results). After a 6 hr incubation period, the medium bathing the cells was replaced with complete DMEM and maintained in culture. On Day 3, cDNA treated cells were transferred to 30 mm glass coverslips in preparation for Ca2+ measurements as described above, which were performed on Day 4 or 5. In general, the concentration of plasmid used was 0.5 μg/well, except for EYFP (0.1 μg/well) Higher concentrations of plasmid (2.0 μg/well) were used in some of the experiments with Orai2 and 3, as indicated in Results.
Single Cell Ca2+ Measurements
Fluorescence measurements were made in HEK293 cells loaded with the Ca2+ sensitive dye, fura-5F, as described previously (2). Briefly, cells plated on 30 mm round coverslips and mounted in a Teflon chamber were incubated in DMEM with 1 μM acetoxymethyl ester of fura-5F (Fura-5F/AM, Molecular Probes, USA) at 37° C in the dark for 25 min. For [Ca2+]i measurements, cells were bathed in HEPES-buffered salt solution (HBSS: NaCl 120; KCl 5.4; Mg2SO4 0.8; HEPES 20; CaCl2 1.8 and glucose 10 mM, with pH 7.4 adjusted by NaOH) at room temperature. Nominally Ca2+-free solutions were HBSS with no added CaCl2. Fluorescence images of the cells were recorded and analyzed with a digital fluorescence imaging system (InCyt Im2, Intracellular Imaging Inc., Cincinnati, OH). Changes in intracellular Ca2+ are represented by and expressed as the ratio of fura-5F fluorescence due to excitation at 340 nm and 380 nm (F340/F380). Before starting the experiment, regions of interests identifying transfected cells expressing the EYFP fluorescence tag were created by observing cells at a 530 nm emission wavelength and illuminated with 477 nm excitation light. Typically, 20 to 30 cells were monitored per experiment. In all cases, ratio values have been corrected for contributions by autofluorescence, which is measured after treating cells with 10 μM Ionomycin and 20 mM MnCl2.
Electrophysiology
Whole-cell currents were investigated at room temperature (20-25°C) in HEK293 cells using the patch-clamp technique in the whole-cell configuration. The standard extracellular recording solution contained (mM): 140 NaCl, 3 mM KCl, 1.2 MgCl2, 0-10 mM CaCl2, 10 glucose, and 10 HEPES (pH to 7.4 with NaOH). Divalent free solution (DVF) contained the same as above, except Ca2+ and Mg2+ were eliminated and 0.1 mM EGTA was added. Nominally Ca2+ free (NCF) external solution was also the same as above, except no Ca2+ was added. Fire-polished pipettes fabricated from borosilicate glass capillaries (WPI, Sarasota, FL) with 3-5 MΩ resistance were filled with (in mM): 145 Cs-methanesulfonate, 10 BAPTA, 10 HEPES, and 8 MgCl2 (pH to 7.2 with CsOH). In some experiments, 100 μM inositol 1,4,5-trisphosphate (IP3) was directly added to the intracellular pipette solution, and 1 μM gadolinium (Gd3+) or 30 μM 2-aminoethoxydiphenylborane (2APB) was added to the extracellular recording solution. External solution changes were made using a multibarrel perfusion pencil (Automate Scientific) placed adjacent to the cell under investigation. Voltage ramps (−100 mV to +100 mV) of 250 ms were recorded every two seconds immediately after gaining access to the cell, and the currents were normalized based on cell capacitance (mean = 14.4 ± 1.2 pF). Leak currents were subtracted by taking an initial ramp current before the store-operated currents developed and subtracting this from all subsequent ramp currents. Access resistance was typically between 5-10 MΩ. The currents were acquired using pCLAMP-9.2 (Axon Instruments) and analyzed using Clampfit (Axon Instruments) and Origin 6 (Microcal) software.
Confocal Microscopy
For experiments examining the intracellular distribution of Stim1, HEK293 cells, expressing EYFP-Stim1 or EYFP-Stim1 EF hand mutants, were prepared for confocal microscopy in a similar way as described for Ca2+ measurements. Cells plated on 30 mm round coverslips and mounted in a Teflon chamber were placed on the stage of a Zeiss LSM 510 confocal
microscope equipped with a 40 × water immersion objective (N.A. 1.2). Images of EYFP were obtained with 488 nm excitation light from an Argon ion Laser (Lasos T812 M24), and emission fluorescence selected with a 505-550 nm bandpass filter. Confocal images were collected with a pinhole set at 1 Airy unit (∼0.9 μm image thickness).
For experiments examining surface expression of Stim1, HEK293 cells that had been transfected with cDNA encoding EYFP-Stim1, EYFP-Stim1-D76A, or EYFP-Stim1 in combination with Orai1 were plated onto glass coverslips and allowed to attach overnight. Cells were washed with Ca2+-free PBS prior to addition of either PBS containing 1.8 mM CaCl2 or Ca2+-free PBS with 2 μM thapsigargin. Following a 15 minute incubation period, cells were fixed by the addition of para-formaldehyde to a final concentration of 1.6%. Cells were incubated 10 minutes at room temperature and then washed with FACS buffer. Permeabilized cells were washed once for 3 minutes with FACS buffer supplemented with 0.1% triton-x 100, followed by additional washes with FACS buffer to remove residual detergent. Cells were then stained with anti-EYFP antibody conjugated to Alexa 647 (Molecular Probes, Eugene, OR). Following 3 wash steps, coverslips were mounted onto slides using ProLong Gold Anti-fade mounting reagent (Molecular Probes, Eugene, OR). The mounting agent was allowed to set overnight prior to analysis on a Zeiss LSM 510 UV Meta confocal microscope. Background for antibody staining was determined by setting the gain setting just below the point where fluorescence in the 647 nm channel was visible in HEK293 cells transfected with EYFP cDNA. For intact, EYFP-Stim1 transfected samples, the gain was left at this level throughout the analysis. For permeabilized samples, the gain was adjusted downward until the image was just below the saturation point for all pixels.
Total Internal Reflection Fluorescence Microscopy (TIRFM)
TIRFM was carried out using an Olympus (Melville, NY) IX2-RFAEVA-2 illumination system mounted on an Olympus IX71 inverted microscope as previously described (7). Illumination was provided by a 488-nm argon ion laser (Melles Griot, Carlsbad, CA) directed through a fiber optic cable, and emitted fluorescence passed through a D525/50m filter (Chroma) before being captured by a Photometrics Cascade 512F cooled CCD (Roper Scientific, Tucson, AZ). Laser illumination was not toxic to cells, because HEK293 cells loaded with the Ca2+ dye Fluo-3 did not exhibit aberrant Ca2+ release or signs of degeneration when monitored with this system (data not shown).
Materials
Thapsigargin was purchased from Alexis (San Diego, CA), fura-5F/AM from Molecular Probes (Eugene, OR).
Results
Co-expression of Stim1 and Orai1 results in a synergistic and robust increase in SOC entry
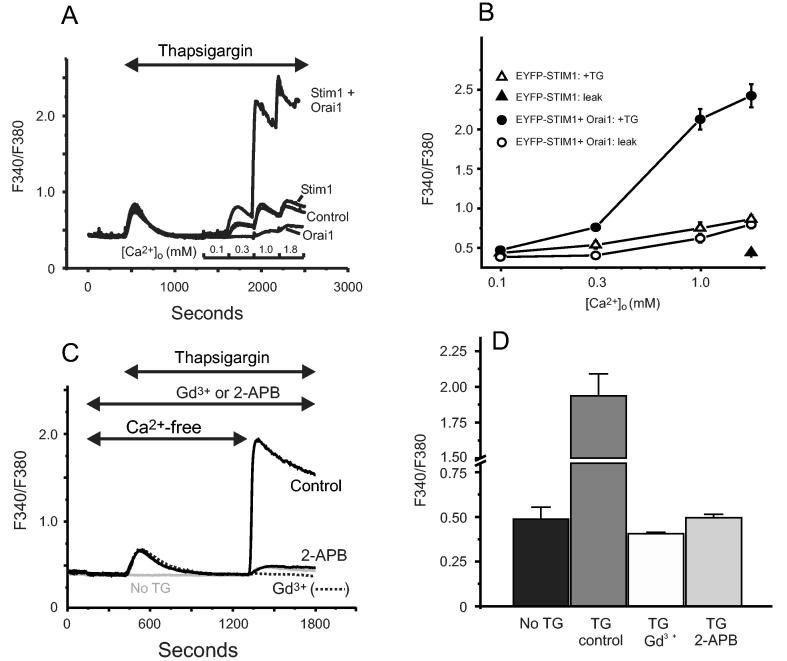
Figure 1 shows the results of experiments in which HEK293 cells were transiently transfected with EYFP-Stim1, Orai1, or both. Successfully transfected cells were identified by the fluorescence from EYFP-Stim1, or by co-transfected EYFP for control cells or cells transfected only with Orai1. SOC entry was assessed by examining the magnitude of the [Ca2+]i signal upon reintroduction of Ca2+ to cells previously treated with the sarcoplasmic-endoplasmic reticulum ATPase inhibitor, thapsigargin, in the absence of extracellular Ca2+. Graded additions of extracellular Ca2+ resulted in a graded elevation in [Ca2+]i. Overexpression of EYFP-Stim1 had little if any effect on Ca2+ entry assessed in this way. Surprisingly, overexpression of Orai1 significantly inhibited SOC entry. More importantly, co-expression of both EYFP-Stim1 with Orai1 resulted in a substantial increase in store-operated Ca2+ entry (Figure 1). Similar synergism between expression of Stim1 and Orai1 was observed in experiments utilizing Ba2+ as a surrogate for Ca2+ (not shown). The large Ca2+ entry resulting from co-expression of EYFP-Stim1 and Orai1 was blocked by 1 μM Gd3+ or 30 μM 2APB, which is the expected pharmacological profile of store-operated channels (8). Finally, co-expression of Stim2 with Orai1 resulted in responses resembling those with Orai1 alone, i.e., inhibition of entry (not shown).
Figure 1.
Characterization of thapsigargin-induced Ca2+ entry in HEK293 cells transfected with Orai1 and Stim1. (A) HEK293 cells transfected with combinations of EYFP (control), Stim1-EYFP and Orai1 were loaded with the fluorescent Ca2+ indicator fura-5F. Before treatment with thapsigargin (2 μM), the HBSS bathing the cells was switched from one containing 1.8 mM Ca2+ to one that was nominally Ca2+-free. After the thapsigargin-induced [Ca2+]i transient had returned to basal levels, the extracellular Ca2+ concentration was sequentially elevated (in mM; 0.1, 0.3, 1.0, 1.8), each for a 5 minute period. The experiment shown is representative of 3 similar experiments, and each trace is an average of data from 20-30 cells. (B) Summarized means ± SEM of peak [Ca2+]i levels at each Ca2+ concentration from three experiments, 20-30 cells per coverslip, for the protocol represented in (A). Filled circles show responses to Ca2+ concentrations in thapsigargin-treated cells transfected with EYFP-Stim1 and Orai1. The control data are for EYFP-Stim1 transfected cells (no Orai1, open triangles) Also shown are data for leak (no thapsigargin) in EYFP-Stim1 transfected (filled triangle) and EYFP-Stim1 + Orai1 transfected (open circles) cells. (C) Ca2+ entry in EYFP-Stim1-expressing cells is blocked by 1 μM Gd3+ or 30 μM 2APB. The data shown in C contains averaged traces from 3 experiments, with each experiment an average of data from 20 - 30 cells. (D) Summarized means ± SEM of peak [Ca2+]i levels from three experiments, 20-30 cells per coverslip, for the protocol represented in (C).
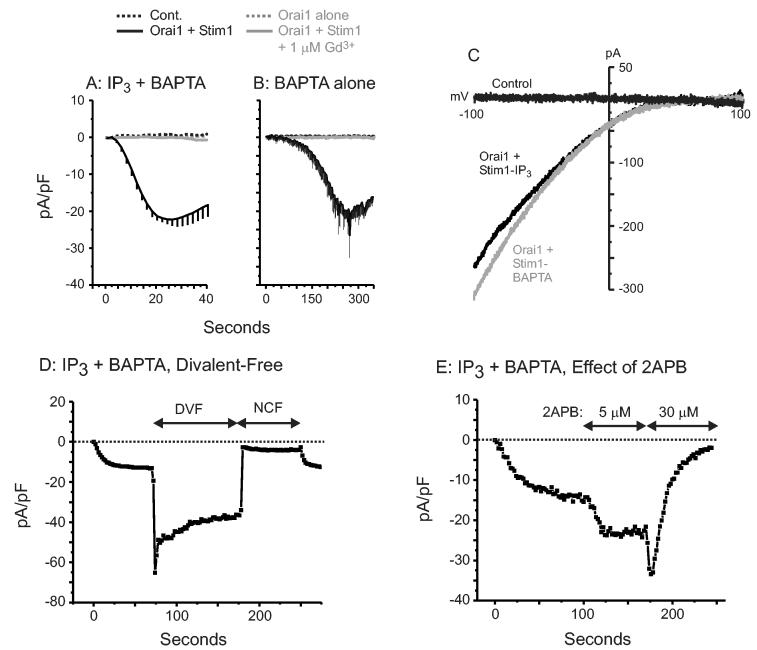
We examined whole-cell currents in HEK293 cells transfected with these same constructs. In our hands, wild-type HEK293 cells do not reproducibly show detectable store-operated currents, and in other laboratories, these currents have been described as very small and are inconsistently detectable (4;9;10). When we broke into HEK293 cells with pipettes containing 10 mM BAPTA and 100 μM IP3, we saw no consistent development of current in control cells (Figure 2a) or in cells transfected with either EYFP-Stim1 (not shown) or Orai1 alone. However, in cells co-transfected with EYFP-Stim1 and Orai1, we observed very large inward currents, of the order of 300 - 500 pA with 10 mM Ca2+ outside (Figure 2A,B). The currents developed rapidly with BAPTA and IP3 in the pipette (Figure 2A), and after a delay when stores were depleted passively with BAPTA alone (Figure 2B). These currents showed strong inward rectification and reversed at +50 mV, as expected for a calcium-selective channel (Figure 2C). The current was fully blocked by 1 μM Gd3+ (Figure 2), which at this concentration is believed to block only store-operated channels (11;12). A hallmark of Icrac is that rapid removal of extracellular divalent cations causes a transient increase in current due to initial removal of Ca2+ block (13) followed by a process of depotentiation involving loss of Ca2+ from external modulatory sites (14). As shown in Figure 2D, the large currents observed in Orai1 + Stim1 expressing cells showed similar behavior upon removal of extracellular divalent cations. Finally, the IP3-activated current was transiently augmented by 5 μM 2APB and was completely blocked by 30 μM of this drug, which is a pharmacological hallmark of Icrac (15) (Figure 2E).
Figure 2.
IP3- and BAPTA-mediated whole-cell currents in isolated HEK293 cells co-expressing Stim1 and Orai1. (A) Average time course of SOC current development after activation with 100 μM inositol 1,4,5-trisphosphate (IP3) and 10 mM BAPTA in the pipette solution in HEK293 cells expressing YFP alone (Control: black dashed trace), Orai1 (Orai1 alone: grey dashed trace), Orai1 and Stim1 (Orai1 + Stim1: black trace), or Orai1 and Stim1 under the presence of 1 μM Gd3+ (Orai1 + Stim1 + 1 μM Gd3+: grey trace). External solution contained 10 mM Ca2+ in these experiments to keep experimental conditions consistent between YFP alone and overexpressing cells. (B) Same as (A), except the internal stores were passively depleted with only 10 mM BAPTA in the intracellular pipette solution. (C) Current-voltage relationship recorded from HEK293 cells expressing only YFP (Control: black trace), Orai1 and Stim1 depleted of their internal Ca2+ stores by the addition of 100 μM IP3 and 10 mM BAPTA (Orai1 + Stim1-IP3: black trace), or 10 mM BAPTA alone (Orai1 + Stim1-BAPTA: grey trace). (D) Representative time course of the transient divalent-free potentiation of Orai1 + Stim1 currents, followed by the inhibitory effect of nominally Ca2+ free conditions (NCF) on the current recorded at −100 mV (n=7). (E) Typical time course showing both the potentiation at low (5 μM) and inhibition at high (30 μM) concentration of 2APB (n=3). Error bars indicate mean ± SEM (n=7 for Orai1 + Stim1 in A, n=5 for Orai1 + Stim1 in B, all others n=5).
Although we did not consistently observe store-operated currents in our untransfected HEK293 cells, based on the value reported by Vig et al. (4) of 0.5 pA/pF, the large Icrac-like currents observed with co-expression of Stim1 and Orai1 suggest an increase in current density of at least a factor of 20.
Effects of expression of Orai2 and Orai3
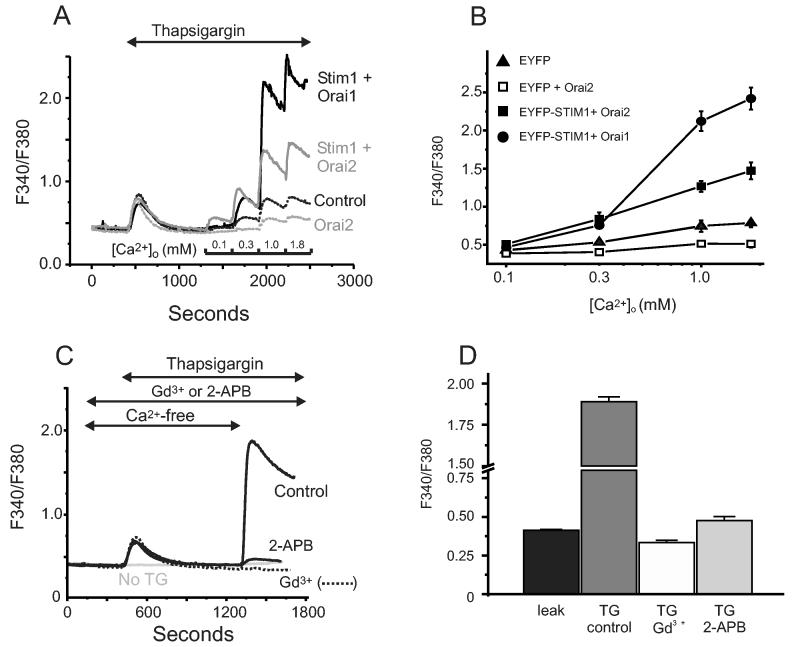
In addition to Orai1, mammalian cells express two additional homologous genes, Orai2 and Orai3 (3). To determine if these genes also encoded components of an Icrac-like entry mechanism, we co-expressed each of these with Stim1 in the same manner as for Orai1. With this protocol, Orai2, like Orai1, inhibits Ca2+ entry on its own, and substantially augments thapsigargin-activated Ca2+ entry when co-expressed with Stim1 (Figure 3A, B); however, the increase in [Ca2+]i was consistently less than seen in the Orai1 experiments (Orai1 data are included in Figure 3A for comparison). As for Orai1, this entry is blocked by 1 μM Gd3+ or 30 μM 2APB (Figure 3C, D).
Figure 3.
Overexpression of Orai2 and Stim1 augments thapsigargin-induced Ca2+ entry. (A) HEK293 cells transfected with combinations of EYFP (control), EYFP-Stim and Orai2 were loaded with the fluorescent Ca2+ indicator fura-5F. Before treatment with thapsigargin (2 μM), the HBSS bathing the cells was switched from one containing 1.8 mM Ca2+ to one that was nominally Ca2+-free. After the thapsigargin-induced [Ca2+]i transient had returned to basal levels, the extracellular Ca2+ concentration was sequentially elevated (in mM; 0.1, 0.3, 1.0, 1.8), each for a 5 minute period. The experiment shown is representative of 3 similar experiments, and each trace is an average of data from 20-30 cells. For comparison, data for EYFP-Stim1 + Orai1 (from Figure 1) are included for comparison. (B) Summarized means ± SEM of peak [Ca2+]i levels at each Ca2+ concentration from three coverslips, 20-30 cells per coverslip, for the protocol represented in (A). (C) Ca2+ entry in EYFP-Stim1-expressing cells is blocked by 1 μM Gd3+ or 30 μM 2APB. For this experiment, to increase the Ca2+ entry response, 2.0 μg/well of plasmid encoding Orai2 was used, where as for the experiments in (A) and (B), the same amount of plasmid was used as for Orai1, 0.5 μg per well. The data shown contains averaged traces from 3 experiments, with each experiment an average of data from 20 - 30 cells. (D) Summarized means ± SEM of peak [Ca2+]i levels from three separate experiments for the protocol represented in (C).
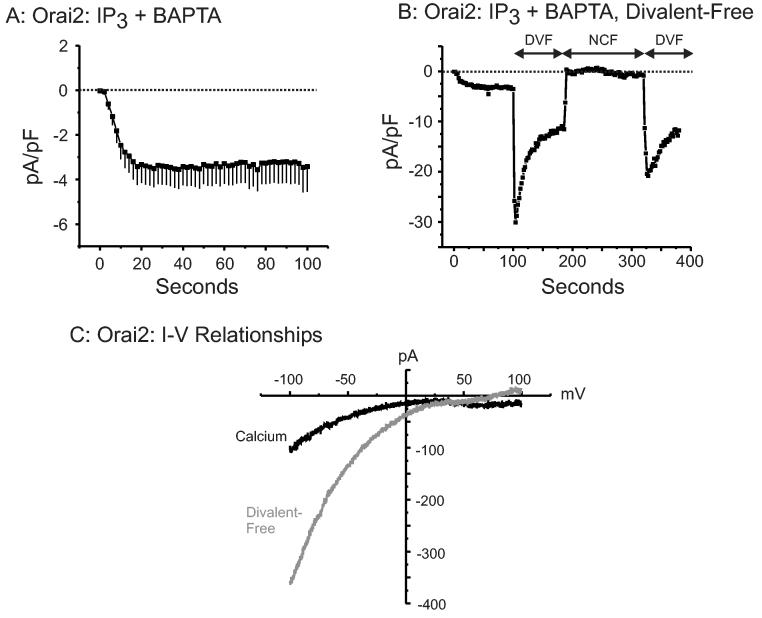
In the experiments in Figure 3A, cells were transfected with 0.5 μg/well of the Orai2 containing plasmid, a similar concentration to that used for Orai1 experiments. In patch-clamp experiments, we found that currents were inconsistently observed with cells transfected in this manner. We thus increased the concentration of Orai2 plasmid to 2.0 μg/well, which resulted in larger [Ca2+]i increases (this is actually the concentration used for experiments in Figure 3C) and consistently observed Icrac-like currents. Figure 4 shows that overexpression of Orai2 and Stim1 results in currents somewhat smaller than for Orai1, although with similar properties. The currents were transiently increased in divalent-free solutions, and subsequently underwent depotentiation (Figure 4B). The IV relationships for the Orai2 + Stim1 currents showed strong inward rectification, typical of Icrac-like currents (Figure 4C). No such currents were observed following transfection with Orai2 alone (not shown).
Figure 4.
Whole cell currents in cells expressing EYFP-Stim1 and Orai2. (A) Average time course of current development at −100 mV for HEK293 cells overexpressing both Orai2 and Stim1 (n=10, ± SEM). Currents were activated by store depletion using IP3 and BAPTA in the pipette, and were recorded under the presence of 2 mM Ca2+ in the bathing solution. (B) Representative time course (n=9) of current development under the presence of 2 mM Ca2+ followed by the addition of divalent-free solution (0.1 mM EGTA) (DVF) and nominally Ca2+ free external solution (NCF). (C) Current-Voltage relationship taken from the same recording seen in (B) showing the inwardly rectifying Orai2 + Stim1 currents that are transiently potentiated by switching to DVF conditions.
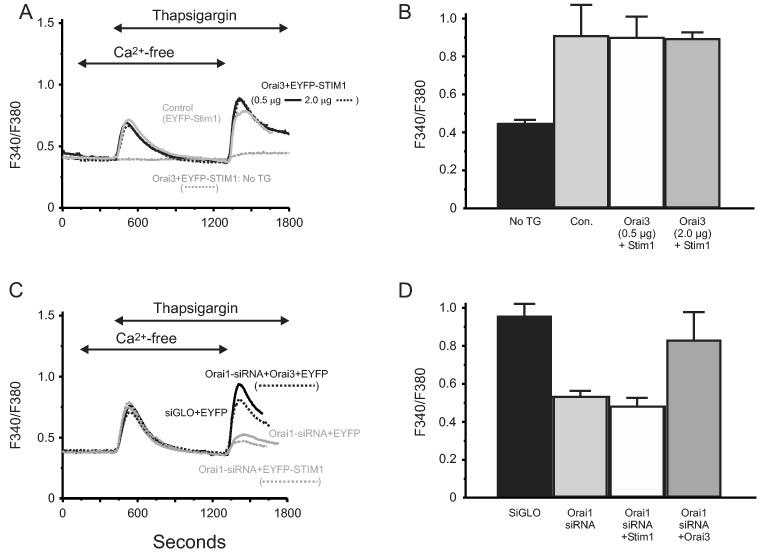
Expression of Orai3 alone failed to suppress SOC entry (not shown) and co-expression of Stim1 and Orai3 failed to produce increased thapsigargin-induced Ca2+ entry, or store-operated currents, even with 2.0 μg/well of plasmid (Figure 5A, B). The failure of Orai3 could mean that this particular protein has some function other than regulation or mediation of Ca2+ entry. Alternatively, it could be poorly expressed, or only function in conjunction with other players, for example in complexes with other Orai family members. However, because Orai3 alone did not suppress Ca2+ entry, we were able to obtain evidence that Orai3 can function in store-operated Ca2+ entry in RNAi rescue experiments. As shown in Figure 5C and D, knockdown of Orai1 by RNAi results in substantial abrogation of thapsigargin-activated Ca2+ entry, in confirmation of previous reports (3;4;16). When we expressed Orai3 in cells following Orai1 knockdown, thapsigargin-activated Ca2+ entry was essentially restored to the control level (Figure 5C, D). We still did not see significant Ca2+ currents in these cells, consistent with the idea that entry was returned only to the control level. The ability of Orai3 to rescue Ca2+ entry following knockdown of Orai1 may indicate that Orai3 is expressed at a more modest (perhaps physiological) level than Orai1, or that it can function together with limited remaining Orai1. Additional experiments will be needed to distinguish among these, or other possibilities. Nonetheless, the clear augmentation of entry by Orai3 in this particular situation demonstrates that this gene product can also play a role in the generation or regulation of store-operated Ca2+ entry.
Figure 5.
Orai3 rescues thapsigargin-activated Ca2+ entry after RNAi knockdown of Orai1. (A) HEK293 cells transfected with combinations of Stim1-EYFP (control) and Orai3 plus Stim1-EYFP. In the latter condition, the concentration of Orai3 cDNA used was either 0.5 μg or 2 μg per well of a 6-well plate. Transfected cells were loaded with the fluorescent calcium indicator fura-5F. Before treatment with thapsigargin (2 μM), the HBSS bathing the cells was switched from one containing 1 mM Ca2+ to one that was nominally Ca2+-free. After the thapsigargin-induced [Ca2+]i transient had returned to basal levels, the extracellular calcium concentration was elevated to 1 mM. The traces shown contain averaged traces from 3 similar experiments, and each experiment is an average of data from 20-30 cells. (B) Means ± SEM of the peak increase upon Ca2+ addition for the experiments in (A). (C) As described in Methods, HEK293 cells were transfected with Orai1-siRNA and siGLO. Two days post transfection, a subset of these cells were subsequently transfected with cDNA for EYFP, Stim1-EYFP or Orai3 plus EYFP. The concentration of Orai3 cDNA used in these experiments was 2 μg per well of a 6-well plate. 48 hours after cDNA transfection, cells attached to coverslips were then loaded with fura-5F and treated with thapsigargin in the absence and then the presence of 1.8 mM extracellular Ca2+. The traces shown contain averaged traces from 3 similar experiments, and each experiment is an average of data from 20-30 cells. (D) Means ± SEM of the peak increase upon Ca2+ addition for all three experiments in (C).
In summary, we find that in expression experiments, all three Orai genes can generate or augment store-operated Ca2+ entry with efficacies in the order Orai1 > Orai2 > Orai3. How this apparent rank order relates to their function under conditions of physiological expression will require additional study.
Stim1 movements in response to Ca2+ store depletion
We next sought to address the question of how Stim1 might regulate the complex leading to channel activation. Two ideas have been suggested; according to Liou et al. (2), Stim1 redistributes within the cell, approaching the plasma membrane, but does not incorporate into the plasma membrane. A contrasting idea put forth by Zhang et al. (5) suggests that Stim1 actually traffics to, and is incorporated into the plasma membrane where it interacts with key players in the store-operated Ca2+ entry pathway. Also, Spassova et al. (17) presented evidence for a role of plasma membrane Stim1, but did not address the question of whether Stim1 moves into the plasma membrane upon store depletion, or is present there constitutively.
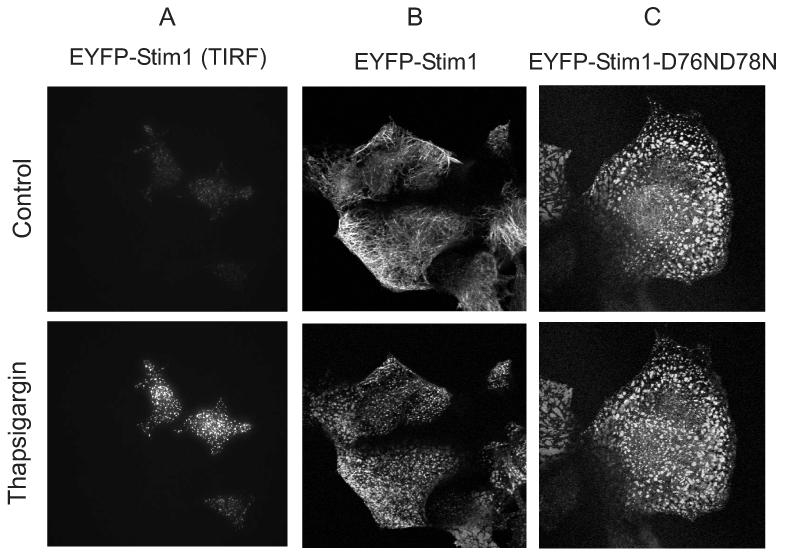
By use of total internal reflected fluorescence (TIRF) microscopy with HEK293 cells transfected with EYFP-Stim1, we confirmed the observation made by Zhang et al (5) and by Liou et al (2) that Stim1 does move to the vicinity of the plasma membrane following depletion of Ca2+ stores with thapsigargin (Figure 6A). We also carried out confocal imaging studies with this construct; consistent with previous findings (2), EYFP-Stim1 reorganizes from an apparent fibrillar or tubular form into discrete punctae in response to depletion of Ca2+ stores with thapsigargin (Figure 6B).
Figure 6.
Cellular movements of the Ca2+ sensor, Stim1. A: A field of several HEK293 cells expressing EYFP-Stim1 was imaged by TIRFM just prior to (upper panel) and 6.5 min after (lower panel) depletion of intracellular Ca2+ stores with thapsigargin (2 μM) in nominally Ca2+ free extracellular medium (n = greater than 10 coverslips). B,C: A field of several HEK293 cells expressing EYFP-Stim1 (B) or EYFP-Stim1-D76N, D78N (C) was imaged by fluorescence confocal microscopy just prior to (upper panel) and 10 min after (lower panel) depletion of intracellular Ca2+ stores with thapsigargin (2 μM) in HBSS containing 1.8 mM Ca2+. For B and C, data are representative of 3 independent experiments.
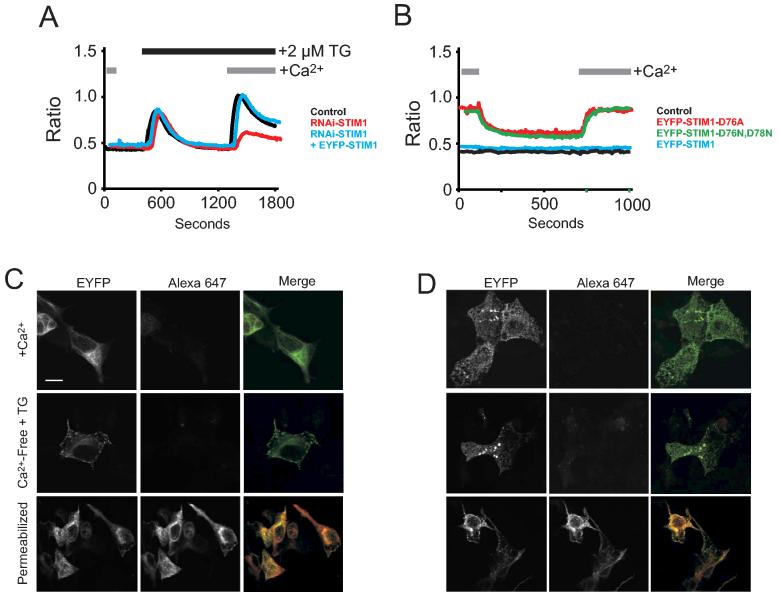
We prepared several mutant versions of Stim1 targeting acidic residues in the EF hand domain: D76A (previously reported in (2)), E87Q (previously reported in (5)), D76N, D78N (D76A, D78A was previously reported in (5)), and D76N. All with the exception of D76N produced constitutive punctate patterns (D76N, D78N in Figure 6C), and all produced constitutive Ca2+ entry (not shown, and see D76A and D76N, D78N in Figure 7B), again with the exception of D76N which had dominant negative activity (not shown). In single cell Ca2+ imaging experiments we determined that the EYFP-Stim1 construct can fully rescue Ca2+ entry in cells which have been treated with siRNA directed against Stim1 (Figure 7A), and that the EYFP-Stim1-D76A and D76N, D76N mutants activate constitutive Ca2+ entry (Figure 7B).
Figure 7.
EYFP-Stim1 or EYFP-Stim1-D76A are not detected at the cell surface by confocal microscopy. (A) HEK293 cells were transfected with siRNA directed against Stim1. Two days post transfection, a subset of these cells was transfected with EYFP or EYFP-Stim1. 24 hours later, cells were loaded with Fura-5F and their ability to activate SOC influx was assessed by single-cell Ca2+ imaging. Shown are average traces from at least 20-30 cells per trace. (B) HEK293 cells were transfected with EYFP, EYFP-Stim1, EYFP-Stim1-D76A, or EYFP-STIM1-D76N,D78N. Cells were loaded with Fura-5F/AM and their intracellular Ca2+ content was assessed by single-cell Ca2+ imaging. Initially, cells were kept in buffer containing 1.8 mM CaCl2, cells were then switched to buffer nominally free of Ca2+, and finally the buffer was exchanged again with buffer containing 1.8 mM CaCl2. Shown are average traces from at least 20-30 cells per trace. (C) and (D) HEK293 cells were transfected with N-terminally tagged EYFP-Stim1 (C) or EYFP-Stim1-D76A (D). Before fixation and permeabilization with Triton X-100 (bottom panels only), a subset of cells was treated with thapsigargin for 15 minutes in Ca2+ free PBS to deplete intracellular Ca2+ stores. Cells were then stained with anti EYFP Alexa 647-conjugated antibody. Cells were then analyzed by laser scanning confocal microscopy to detect EYFP and/or Alexa 647.
We next examined both EYFP fluorescence as well as staining of the cells by an antibody (tagged with a fluorophore, Alexa 647) directed against the N-terminal EYFP, which would be extracellular and accessible to the antibody if the protein were inserted into the plasma membrane (18). However, we found that in fixed cells with intact plasma membranes (i.e. no detergent treatment following fixation) antibody binding was not detected before or after depletion of intracellular Ca2+ stores, with either wild-type or the D76A versions of Stim1 (Figure 7C and D). This was not due to failure of the antibody to recognize the epitope as cells which were permeabilized with Triton X-100 prior to antibody staining showed co-localization of the antibody (red) with EYFP-Stim1 (green) (Figure 7C and D).
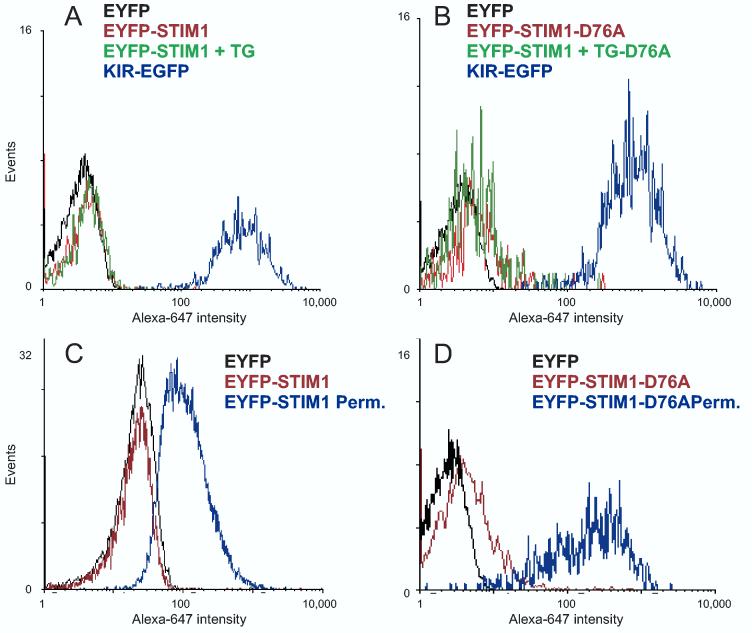
We next performed flow cytometry with fixed, intact Jurkat cells which had been transfected with EYFP-Stim1 and stained with anti-EYFP Alexa 647. This technique allows the separation of viable cells from non-viable cells based on forward and side-scatter properties. 99.8% of Jurkat cells that had been transfected with the NK cell receptor KIR, which is expressed with GFP fused with its extracellular domain, (19) stained positive with the antibody (Figure 8A). In comparison, only 2.25% (± 0.23%) of Jurkat cells that were transfected with EYFP-Stim1 stained with the antibody, and those cells were only weakly stained (Figure 8A). Importantly, in Jurkat cells that were treated with thapsigargin in nominally Ca2+ free buffer for 15 minutes in order to deplete Ca2+ stores prior to staining only 2.8% (± 0.5%) of cells fell into the antibody positive gate (Figure 8A). Additionally, the mean fluorescence intensity for these cells is not significantly different from cells transfected with cytoplasmic GFP and which fall into the positive gate (p=0.459 and 0.352 respectively), indicating that these few cells are likely falsely positive. We did observe a somewhat higher percentage of cells that stained with the antibody when transfected with a dominant active mutant version of EYFP-Stim1 (D76A) (2) that results in activation of constitutive Ca2+ entry (2) (Figure 8B). However, the average number of positive cells was still very low, only 17.05 % (± 4.35 %) for resting cells and 20.5 % (± 1.8 %) for TG treated cells, while virtually all cells transfected with this construct exhibit constitutive Ca2+ entry. Finally, permeabilization of the transfected Jurkat cells reveals a substantial staining of both EYFP-Stim1 and EYFP-Stim1-D76A (Figure 8C and D).
Figure 8.
Extracellular EYFP-Stim1 is not detected by flow cytometry. (A) Jurkat cells were transfected with EYFP, N-terminal tagged EYFP-Stim1, or KIR-EGFP, an NK cell receptor with GFP fused to the extracellular domain. The next day, EYFP-Stim1 cells were either left untreated in Ca2+ containing buffer or treated with thapsigargin for 15 minutes in the absence of Ca2+. All cells were then gently fixed on ice with 0.25% para-formaldehyde to prevent additional biological changes. Cells were then stained with anti-EYFP Alexa 647 and analyzed by flow cytometry. Histograms shown are gated on the EGFP/EYFP+ portion of the viable cells. (B) Jurkat cells were transfected with EYFP, EYFP-Stim1-D76A or KIR-EGFP and treated as in (A). (C) and (D) Jurkat cells expressing either EYFP-Stim1 (C), or EYFP-Stim1-D76A (D) were either left intact or permeabilized with Triton X-100 prior to antibody staining and analysis by flow cytometry.
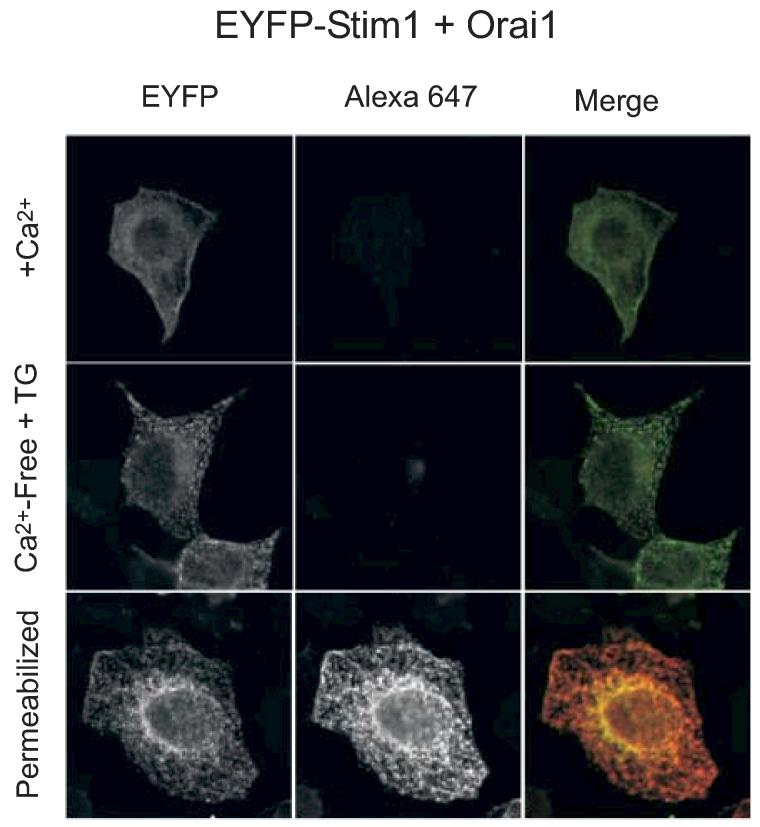
We considered the possibility that only a small fraction of EYFP-Stim1 might need to move to the surface, and this might be difficult to detect against the large background of intracellular fluorescently tagged molecules. In addition, it is apparent from the data in Figure 7A that knockdown of Stim1 by RNAi is incomplete and thus a small but sufficient complement of surface Stim1 might remain in these cells. Thus we co-transfected cells with the combination of EYFP-Stim1 and Orai1 and attempted to detect surface-expressed EYFP-Stim1 as in Figure 7. We reasoned that if plasma membrane Stim1 is a necessary component or signal for SOC entry, then the huge increases in SOC entry and current density that result from overexpression of EYFP-Stim1 and Orai1 should be accompanied by a correspondingly significant increase in the levels of EYFP-Stim1 detectable in the plasma membrane. However, the results were indistinguishable from those in Figure 8; again we failed to observe any antibody labeling of the surface of EYFP-Stim1 + Orai1 cotransfected cells (Figure 9).
Figure 9.
When co-expressed with Orai1, EYFP-Stim1 is not detected at the cell surface by confocal microscopy. HEK293 cells transfected with EYFP-Stim1 and Orai1 cDNA were incubated in PBS with 1.8 mM Ca2+ (top and bottom panels), or in Ca2+-free PBS containing 2 μM thapsigargin for 15 minutes prior to fixation and permeabilization with triton X-100 (bottom panels only). Cells were stained with anti-EYFP Alexa 647 conjugated antibody and analyzed by laser scanning confocal microscopy to detect EYFP and/or Alexa 647.
Discussion
Since the inception of the concept of capacitative Ca2+ entry (20), research has focused on two basic questions: the signaling mechanism linking the depletion of intracellular Ca2+ stores to plasma membrane Ca2+ channels, and the identity (or identities) of the store-operated channels (21;22). Various hypotheses have been put forth for the signaling mechanism, including a diffusible messenger, or “calcium influx factor” (CIF) (23;24), and a protein-protein interaction mechanism involving coupling of endoplasmic reticulum IP3 receptors to plasma membrane Ca2+ channels, sometimes referred to as a “conformational coupling” model (25;26). Perhaps the leading candidates for the channels themselves have been members of the TRP superfamily (27;28). However, despite evidence implicating TRPs from knockdown or knockout experiments, and despite clear demonstrations that TRPs can, under specific experimental conditions, be activated by Ca2+ store depletion, in no instance has a current been generated by expression of TRPs that resembles the archetypical SOC current, Icrac. Thus, it is possible that SOC currents that have distinct properties from Icrac may involve channels composed of TRP subunits.
We found little or no effect of overexpression of Stim1 on store-operated Ca2+ entry, and, surprisingly, inhibition of entry by overexpressing Orai1 or Orai2. This latter finding was not investigated in detail in the current study; we speculate that it may reflect the same phenomenon seen with overexpression of TRP channels whereby overexpression of one member of a complex of more than two proteins reduces the likelihood of correctly assembling a functional signaling unit (29). In this instance, the other two components could be either two Stim1 molecules, Stim1 and another as yet unidentified component, or other structural components of the signaling complex (30;31).
Remarkably, Stim1 and Orai1 (or Orai2) appeared to interact synergistically such that a large current with Icrac-like properties was generated by over expression of the two proteins together. While this paper was in review, reports by Peinelt et al. (32) and Soboloff et al. (33) appeared describing similar, large currents in HEK 293 cells, and a report by Zhang et al. (16) described a similar synergism between Stim and Orai in Drosophila. The evidence that the N-terminal EF hand of Stim1 serves as the sensor for endoplasmic reticulum luminal Ca2+ is strong (2;5;17). We cannot at this stage definitively say that Stim1 acts directly on Orai1, or acts through intermediate effectors, for example through generation of a Ca2+ influx factor (24). However, the size of the store-operated Ca2+-selective current obtained with these two proteins argues that additional stoichiometric components may not be necessary; thus, these two proteins may be capable of reconstituting both the activation and permeation mechanisms of Ca2+-selective store-operated channels. Although neither Stim1 nor Orai1 show any obvious sequence homology to known ion channels, Icrac itself has biophysical properties quite different from other known ion channels (13;22;34). Further studies will be needed to definitively confirm or refute the hypothesis that any or all of the Orai proteins can function as the major pore-forming constituent of Ca2+-selective store-operated channels such as Icrac. If this is not so, however, it would imply that HEK293 cells constitutively express a huge excess of normally non-functional CRAC channels. Nonetheless, despite almost a decade of attempts to produce store-operated currents by expression of, for example, TRP genes, to our knowledge the combination of Stim1 and Orai1 represents the first successful reconstitution of an Icrac-like current by ectopic expression of known genes3.
There are two other potential homologs of Orai1 in the mammalian genome, Orai2 and Orai3 (3). The homology between the sequences of these proteins and Orai1 suggests they may have similar physiological functions. As a first step to understanding their function, we expressed these proteins with and without Stim1, as with Orai1. Orai2 behaved qualitatively similarly to Orai1; when expressed alone entry was inhibited, and when expressed with Stim1, large entry and currents were obtained. In this particular assay, Orai2 dependent currents were smaller than those obtained with Orai1. With Orai3, we saw no increased Ca2+ signals or currents when expressed with Stim1. However, Orai3 was able to rescue Ca2+ entry in cells in which Orai1 was knocked down by RNAi. Thus we conclude that all three Orai homologs are capable of constituting or regulating store-operated channels. This raises the interesting possibility that native SOC channels may involve combinations of Orai proteins. Future work will be necessary to determine their respective roles in specific physiological environments.
Zhang et al (5) utilized surface biotinylation of Stim1 to determine that it is inserted into the plasma membrane following Ca2+ store depletion. This technique relies on covalent attachment of cell impermeant biotin to proteins located on the cell surface. Following cell lysis, the biotinylated proteins are separated from other cellular proteins by immobilizing them onto streptavidin coated agarose beads and washing away non-bound proteins. This technique has been widely used to identify proteins that have access to the extracellular space. However, an important caveat for this technique is that dead or dying cells that have compromised plasma membrane integrity will be permeable to the biotin and thus a portion of biotinylated proteins in the extract can be intracellular proteins from these cells. This could be particularly problematic when the proportion of total protein believed to lie on the surface is very small (as appears to be the case here). In addition, it is possible that proteins that are not actually within the plasma membrane, but bind tightly to plasma membrane proteins can be detected with this technique (for example, see (35)). To circumvent these potential problems, we used surface labeling of HEK293 cells, as well as flow cytometric analysis of surface labeling of Jurkat T-cells; the latter technique permits gating on physiologically intact cells only on the basis of light scattering properties. With both assays, we failed to observe significant localization of Stim1 in the plasma membrane, and we did not observe any movement of immunodetectable Stim1 to the surface in response to Ca2+ store depletion. Since we are examining the distribution of a transfected fusion protein, we cannot definitively rule out the possible presence of a small constitutive quantity of native Stim1 in the plasma membrane, as suggested by Spassova et al. (17). However, our data indicate that stimulated transfer of Stim1 from endoplasmic reticulum into the plasma membrane in response to Ca2+ store depletion does not occur, and that the plasma membrane insertion model for Stim1 is incorrect. We have demonstrated that EYFP-Stim1, which can rescue Ca2+ entry in Stim1 siRNA treated cells, and EYFP-Stim1(D76A), which produces constitutive Ca2+ entry, are undetectable at the cell surface of intact cells by immunofluorescence microscopy and only weakly detectable in a small percentage of cells by flow cytometry. In the case of the D76A mutant of Stim1, Ca2+ entry is activated in the apparent absence of store depletion such that even if native Stim1 were capable of responding differently from the EYFP fusion, there is no stimulus for its activation (i.e., no store depletion). Furthermore, the fact that we observed no EYFP-Stim1 in the plasma membrane of HEK293 cells under conditions whereby the quantity of functional channels would appear to have been dramatically increased calls into question any significant role for plasma membrane Stim1, whether constitutive or otherwise. These data support a model for re-localization of Stim1 within the ER following Ca2+ store depletion, as suggested by Liou et al. (2). This is an important distinction because it rules out the possibility of Stim1 as a subunit of the store-operated channel, and suggests that the C-terminus of Stim1 likely interacts with an intracellular target, perhaps Orai1, which either regulates or forms the store-operated Ca2+ channel.
Acknowledgements
The EYFP-Stim1 construct was generously provided by Tobias Meyer, Stanford, University. Drs. David Armstrong and Stephen Shears read the manuscript and provided helpful comments. This work was supported by funds from the Intramural Program of NIEHS and the NIH.
Footnotes
The abbreviations are: SOC, store-operated Ca2+; EYFP, enhanced yellow fluorescent protein; DMEM, Dulbecco's minimal essential medium; siRNA, small inhibitory RNA; IP3, inositol 1,4,5-trisphosphate; Icrac, calcium-release-activated calcium current; TRP, transient receptor potential.
Reference List
- 1.Roos J, DiGregorio PJ, Yeromin AV, Ohlsen K, Lioudyno M, Zhang S, Safrina O, Kozak JA, Wagner SL, Cahalan MD, Velicelebi G, Stauderman KA. J. Cell Biol. 2005;169:435–445. doi: 10.1083/jcb.200502019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liou J, Kim ML, Heo WD, Jones JT, Myers JW, Ferrell JE, Jr., Meyer T. Curr. Biol. 2005;15:1235–1241. doi: 10.1016/j.cub.2005.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Feske S, Gwack Y, Prakriya M, Srikanth S, Puppel SH, Tanasa B, Hogan PG, Lewis RS, Daly M, Rao A. Nature. 2006 doi: 10.1038/nature04702. advanced online publication. [DOI] [PubMed] [Google Scholar]
- 4.Vig M, Peinelt C, Beck A, Koomoa DL, Rabah D, Koblan-Huberson M, Kraft S, Turner H, Fleig A, Penner R, Kinet JP. Science. 2006;312:1220–1223. doi: 10.1126/science.1127883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang SL, Yu Y, Roos J, Kozak JA, Deerinck TJ, Ellisman MH, Stauderman KA, Cahalan MD. Nature. 2005;437:902–905. doi: 10.1038/nature04147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Williams RT, Manji SS, Parker NJ, Hancock MS, Van SL, Eid JP, Senior PV, Kazenwadel JS, Shandala T, Saint R, Smith PJ, Dziadek MA. Biochem. J. 2001;357:673–685. doi: 10.1042/0264-6021:3570673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smyth JT, Lemonnier L, Vazquez G, Bird GS, Putney JW., Jr. J. Biol. Chem. 2005;281:11712–11720. doi: 10.1074/jbc.M510541200. [DOI] [PubMed] [Google Scholar]
- 8.Putney JW., Jr. Mol. Interventions. 2001;1:84–94. [PubMed] [Google Scholar]
- 9.Bugaj V, Alexeenko V, Zubov A, Glushankova L, Nikolaev A, Wang Z, Kaznacheyeva E, Bezprozvanny I, Mozhayeva GN. J. Biol. Chem. 2005 doi: 10.1074/jbc.M500192200. M500192200. [DOI] [PubMed] [Google Scholar]
- 10.Fasolato C, Nilius B. Pflüg. Arch. 1998;436:69–74. doi: 10.1007/s004240050605. [DOI] [PubMed] [Google Scholar]
- 11.Broad LM, Cannon TR, Taylor CW. J. Physiol. (Lond.) 1999;517:121–134. doi: 10.1111/j.1469-7793.1999.0121z.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Luo D, Broad LM, Bird GS, Putney JW., Jr. J Biol. Chem. 2001;276:20186–20189. doi: 10.1074/jbc.M100327200. [DOI] [PubMed] [Google Scholar]
- 13.Hoth M, Penner R. J. Physiol. (Lond.) 1993;465:359–386. doi: 10.1113/jphysiol.1993.sp019681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zweifach A, Lewis RS. J. Gen. Physiol. 1996;107:597–610. doi: 10.1085/jgp.107.5.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Prakriya M, Lewis RS. J. Physiol. (Lond.) 2001;536:3–19. doi: 10.1111/j.1469-7793.2001.t01-1-00003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang SL, Yeromin AV, Zhang XH, Yu Y, Safrina O, Penna A, Roos J, Stauderman KA, Cahalan MD. Proc. Natl. Acad. Sci U. S. A. 2006;103:9357–9362. doi: 10.1073/pnas.0603161103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Spassova M, Soboloff J, He L-P, Xu W, Dziadek M, Gill DL. Proc. Nat. Acad. Sci. USA. 2006;103:4040–4045. doi: 10.1073/pnas.0510050103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Manji SS, Parker NJ, Williams RT, Van SL, Pearson RB, Dziadek M, Smith PJ. Biochim. Biophys. Acta. 2000;1481:147–155. doi: 10.1016/s0167-4838(00)00105-9. [DOI] [PubMed] [Google Scholar]
- 19.Borszcz PD, Peterson M, Standeven L, Kirwan S, Sandusky M, Shaw A, Long EO, Burshtyn DN. Eur. J. Immunol. 2003;33:1084–1093. doi: 10.1002/eji.200323582. [DOI] [PubMed] [Google Scholar]
- 20.Putney JW., Jr. Cell Calcium. 1986;7:1–12. doi: 10.1016/0143-4160(86)90026-6. [DOI] [PubMed] [Google Scholar]
- 21.Putney JW., Jr. Capacitative Calcium Entry. Landes Biomedical Publishing; Austin, TX: 1997. [Google Scholar]
- 22.Parekh AB, Penner R. Physiol. Rev. 1997;77:901–930. doi: 10.1152/physrev.1997.77.4.901. [DOI] [PubMed] [Google Scholar]
- 23.Randriamampita C, Tsien RY. Nature. 1993;364:809–814. doi: 10.1038/364809a0. [DOI] [PubMed] [Google Scholar]
- 24.Bolotina VM, Csutora P. Trends Biochem. Sci. 2005;30:378–387. doi: 10.1016/j.tibs.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 25.Irvine RF. FEBS Letters. 1990;263:5–9. doi: 10.1016/0014-5793(90)80692-c. [DOI] [PubMed] [Google Scholar]
- 26.Berridge MJ. Biochem. J. 1995;312:1–11. doi: 10.1042/bj3120001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Birnbaumer L, Zhu X, Jiang M, Boulay G, Peyton M, Vannier B, Brown D, Platano D, Sadeghi H, Stefani E, Birnbaumer M. Proc. Nat. Acad. Sci. USA. 1996;93:15195–15202. doi: 10.1073/pnas.93.26.15195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Putney JW, Jr., McKay RR. Bioessays. 1999;21:38–46. doi: 10.1002/(SICI)1521-1878(199901)21:1<38::AID-BIES5>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 29.Putney JW., Jr. Trends Cell Biol. 2004;14:282–286. doi: 10.1016/j.tcb.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 30.Levchenko A, Bruck J, Sternberg PW. Proceedings of the National Academy of Sciences. 2000;97:5818–5823. doi: 10.1073/pnas.97.11.5818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Burack WR, Shaw AS. Current Opinion in Cell Biology. 2000;12:211–216. doi: 10.1016/s0955-0674(99)00078-2. [DOI] [PubMed] [Google Scholar]
- 32.Peinelt C, Vig M, Koomoa DL, Beck A, Nadler MJ, Koblan-Huberson M, Lis A, Fleig A, Penner R, Kinet JP. Nat. Cell Biol. 2006 doi: 10.1038/ncb1435. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Soboloff J, Spassova MA, Tang XD, Hewavitharana T, Xu W, Gill DL. J Biol. Chem. 2006 doi: 10.1074/jbc.C600126200. in press. [DOI] [PubMed] [Google Scholar]
- 34.Parekh AB, Putney JW., Jr. Physiol Rev. 2005;85:757–810. doi: 10.1152/physrev.00057.2003. [DOI] [PubMed] [Google Scholar]
- 35.Yuan JP, Kiselyov K, Shin DM, Chen J, Shcheynikov N, Kang SH, Dehoff MH, Schwarz MK, Seeburg PH, Muallem S, Worley PF. Cell. 2003;114:777–789. doi: 10.1016/s0092-8674(03)00716-5. [DOI] [PubMed] [Google Scholar]
- 36.Yue L, Peng J-B, Hediger MA, Clapham DE. Nature. 2001;410:705–709. doi: 10.1038/35070596. [DOI] [PubMed] [Google Scholar]
- 37.Voets T, Prenen J, Fleig A, Vennekens R, Watanabe H, Hoenderop JGJ, Bindels RJM, Droogmans G, Penner R, Nilius B. J. Biol. Chem. 2001;276:47767–47770. doi: 10.1074/jbc.C100607200. [DOI] [PubMed] [Google Scholar]