Abstract
Maternal effects occur when offspring phenotype is influenced by environmental factors experienced by the mother. Mothers are predicted to invest differentially in offspring in ways that will maximize offspring fitness depending on the environment she expects them to encounter. Here, we test for maternal effects in response to mate attractiveness on offspring developmental traits in the zebra finch Taeniopygia guttata. We controlled for parental genetic quality by manipulating male attractiveness using coloured leg rings and by randomly assigning mating pairs. The potential confounding effect of differential nestling care was controlled for by cross-fostering clutches and by allowing for variance due to foster father attractiveness in general linear models. We found a difference in egg mass investment between attractiveness groups and, importantly, we found that all of the offspring traits we measured varied with the attractiveness of the father. This provides strong evidence for maternal effects in response to mate attractiveness. Furthermore, due to the experiment design, we can conclude that these effects were mediated by differential investment of egg resources and not due to genetic differences or differences in nestling care.
Keywords: maternal effects, mate attractiveness, egg resources, offspring development, zebra finch, Taeniopygia guttata
1. Introduction
Maternal effects occur when offspring phenotype or performance is influenced by environmental factors experienced by the mother. Maternal effects have been recorded across taxa and may be a maternal strategy to optimize offspring fitness according to the environment the mother predicts her offspring will encounter (Mousseau & Fox 1998). For example, in the crustacean Daphnia pulicaria whether parthenogenetic or resting eggs are produced depends on the food and photoperiod experienced by the mother (Alekseev & Lampert 2001), and in the bulb mite Rhizoglyphus robini polyandrous females produce sons with lower reproductive success than sons of monandrous females, due to maternal effects (Kozielska et al. 2004).
Interestingly, mate attractiveness could also, potentially, be an environmental factor influencing offspring phenotype non-genetically, through maternal effects, even where the mate does not contribute resources to the female. Studies of avian species have shown that, when paired to attractive males, females provision nestlings more often (Burley 1988) and they lay larger eggs (Cunningham & Russell 2000), larger clutches (Petrie & Williams 1993; Balzer & Williams 1998; Uller et al. 2005) and deposit more androgens (Gil et al. 1999, 2004) and immunoglobulins (Saino et al. 2002) into egg yolks. If such differential investment results in fitness consequences for offspring, then there are clear implications for using good genes arguments alone in interpreting correlations between male attractiveness and offspring fitness when maternal effects have not been controlled for experimentally.
It is intuitive that different levels of egg resources may influence offspring fitness traits. For example, in birds yolk immunoglobulins may improve a hatchling's immune response (Saino et al. 2002), and yolk androgens such as testosterone (T) may have effects on offspring hatching, neck musculature, begging rate, growth rate, competitiveness, immune function and mortality (reviewed in Groothuis et al. 2005).
Thus, we might expect male attractiveness to affect offspring fitness not only through male ‘good genes’ effects, but also through maternal effects acting via egg resources. Clearly, in order to test this, any overriding effects of parental genetic quality on offspring fitness must be controlled for. This can be achieved by: (i) experimentally manipulating perceived male attractiveness and (ii) preventing assortative mating, i.e. mating between attractive males and high quality females. In addition, the link between the genetic parents and nestling care must be broken, e.g. by cross-fostering, so that there is no overriding effect on offspring fitness of parental differential investment in offspring provisioning in response to mate attractiveness or due to parental quality.
Here, we test for mate attractiveness-dependent maternal effects on offspring developmental traits by using the zebra finch Taeniopygia guttata, a species that allows for the above experimental requirements to be applied and where males do not provide food resources to females at any stage of reproduction.
Because zebra finches breed readily in captivity, we were able to allocate pairs and breed them in individual cages, thereby avoiding the confounding factor of assortative mating. Also, since they accept any similar-looking eggs or hatchlings in their nest, we could cross-foster clutches. Furthermore, studies of both captive-bred and wild-caught zebra finches have demonstrated that females prefer males with red leg rings, whereas males with green rings are the least attractive and preference for ring colour appears to over-ride all other male secondary sexual characteristics (Burley et al. 1982; Burley 1988; Hunt et al. 1997). We have confirmed this preference in the zebra finch population at St Andrews University used in this study (Williamson 2005). Importantly, therefore, we could easily manipulate male attractiveness using coloured leg rings, thereby disassociating perceived attractiveness from real genetic quality. Moreover, we allowed for variance due to foster male attractiveness, female condition and laying or hatching order by entering these variables into general linear models (GLMs).
By experimentally controlling for, and statistically taking into account the above confounding effects, we can predict that any correlations between the father's attractiveness and offspring developmental traits are most likely due to differential provisioning of egg resources by the mother in response to mate attractiveness. To investigate the possibility that such maternal effects are mediated by maternally derived yolk T, we analyse T in one egg of each clutch. We predict that there will be differences in offspring development due to differential investment of egg resources by the mother in response to manipulation of her mate's attractiveness. However, we cannot predict in what direction, because the fitness consequences are likely to be context-dependent (Mousseau & Fox 1998).
2. Material and methods
(a) Breeding set-up
All zebra finches were wild-type, sourced from captive-bred populations of UK universities. No birds had prior experience of red or green rings and none had bred in the six months before the study. Before breeding males were housed separately from females.
We manipulated mate attractiveness by randomly assigning males with red or green leg rings. Males were then paired with females in individual cages to produce eggs and rear chicks. A total of 36 breeding pairs were used, 18 from each treatment. Since female size and condition could affect egg resources, we recorded the mass of each female at pairing and confirmed there was no difference in mass between females allocated to red- or green-ringed males (F1,31=2.70, p=0.1104).
Birds were provided daily with mixed seed (foreign finch mix by Haith's, Cleethorpes, Lincolnshire, UK), oystershell grit, cuttlebone and fresh drinking water containing calcium and vitamin supplement ad libitum. This was supplemented with reconstituted Haith's egg biscuit and fresh spinach twice weekly. Birds were maintained on a 14 h light : 10 h dark lighting schedule with full-spectrum artificial lights, since ultraviolet light is important for correct colour discrimination and mate choice in zebra finches (Bennett et al. 1996; Hunt et al. 1997).
Egg laying order was recorded by marking each egg on the morning of laying and each egg was also weighed at this time. Gil et al. (1999) found that females mated to attractive (red-ringed) males invested more yolk T. Therefore, in order to investigate whether maternally derived yolk T was part of the mechanism of any maternal effects found in this study, we removed the second egg of each clutch, replaced it with a dummy egg and froze it at −20 °C to await hormone analysis. The second egg was chosen because, first, all females laid at least two eggs, thereby ensuring that all nests had a yolk androgen estimate. Second, the second egg lies closer to the middle of the mean clutch size (of 5.6 eggs) than the 1st egg, so that it could better be used to estimate the androgen levels in the rest of the clutch (see below).
We cross-fostered whole clutches between nests of similar clutch size (±1 egg) and similar dates (±3 days) of clutch initiation and in equal numbers between and within attractiveness treatment groups.
Chick hatching order was recorded by checking nests four times daily and marking new hatchlings with a non-toxic marker.
(b) Offspring parameters
We estimated begging rates before chicks' eyes opened, at 4 and 5 days old using a similar technique to Cotton et al. (1999). Before each begging trial we attempted to minimize the effect of different hunger levels by feeding each chick to satiation with reconstituted Haith's egg biscuit, using a plastic 1 ml pipette tip. We considered the chick satiated if it did not respond (open its mouth or beg) when we tapped its bill lightly with a pipette tip. Each chick was then left on a heated towel for exactly 1 h, at which time the begging trial began. We tapped the bill of the chick and recorded the amount of time it begged. When it stopped begging, we allowed 3 sec to elapse before tapping its bill again and measuring the next begging response. This continued until the allotted trial time of exactly 3 min. For analysis we used the total time spent begging over the 3 min trial period, averaged over the 2 days (i.e. at 4 and 5 days old).
We estimated chick size at 5 days old by measuring the right tarsus. We estimated skeletal growth rate of each chick by also measuring the right tarsus at age 15 days (to encompass the rapid and linear growth phase) and then calculating the amount of growth in millimetres between 5 and 15 days old. Final tarsus measurements were taken at 100 days old, after the birds were considered fully mature.
Nestling zebra finches have black bills while adults have red bills, so we estimated the rate of maturity by measuring the proportion of red on the upper mandible at age 50 days. For each bird we took three photographs of the bill from above, left and right using a Fuji Finepix digital camera. Using Sigma Scan, we measured the proportion of red on the upper mandible for each photograph and used the mean (arcsine-transformed) for analysis. We estimated maturity rates for offspring that were wild-type only.
(c) Yolk androgen extraction and assay
Androgens were extracted and assayed as described in Rutstein et al. (2004a, 2005) and Gilbert et al. (2005). This followed the methodology of a commercially available assay kit (Amersham Pharmacia Biotech), used by Gil et al. (1999). Briefly, total androgens were extracted from yolk using diethyl ether and from lipids using ethanol. Half of each extract was oxidized and further extracted with ether, which removes T, but leaves 5α-dihydrotestosterone (DHT). The extraction recovery of total androgens (T and DHT) was 75.6±9.0% s.e. and the extraction recovery of DHT only was 59.8±0.9% s.e. We conducted radioimmunoassays of total androgens (T and DHT) on the unoxidized samples and DHT only assays on the oxidized samples. The cross-reactivity between the T-specific antibody and DHT was 46% (see also Nash et al. 2000), so we estimated the T content of each sample as total−0.46DHT). The intra-assay coefficient of variation was 4.3±0.3% (mean±s.e.) for T and 6.3±0.6% for DHT. The inter-assay coefficient of variation was 17.0±2.2% for both.
Because RIAs were conducted on extracts from only the 2nd egg of each clutch, we used the negative relationship between yolk androgen content and egg laying order (using data from Gil et al. 1999) to estimate androgen content in the rest of the clutch. These estimates were then used to explore whether yolk androgen concentration might correlate with nestling development.
(d) Statistical analysis
GLMs were conducted using the SAS System for Windows v. 8. Since there were several offspring per nest, nest identity was entered as a random factor to allow for between and within nest effects in the analyses of offspring developmental traits (Grafen & Hails 2002). Explanatory variables entered into the GLMs initially were: father's ring colour, foster father's ring colour, mother's mass, estimated yolk T concentration, egg mass, laying order of the egg within the clutch (or hatching order of the chick within the brood), brood size at age 5 days (or clutch size) and offspring sex. Initially, the two-way interactions included in the GLMs were between all possible combinations of father's ring colour, foster father's ring colour and mother's mass. Then, separately, we entered two-way interactions between the first three (father, mother and foster father properties) variables and the last five (properties of the individual) variables. We also added chick body size at 5 days old to the models as a potential proximate influence on the developmental traits measured. Body size at 5 days old was not entered into models of chick growth rate due to autocorrelation, since it was used in the calculation of growth rate.
We analysed reproductive output and offspring survival at the nest level, i.e. each nest was one sample point. Variables initially entered into GLMs of clutch and brood sizes were father's attractiveness, foster father's attractiveness, mother's mass, and interactions thereof. For GLMs of offspring survival we additionally entered brood size and estimated yolk T concentration.
For all analyses, a backwards step-wise procedure was carried out, such that variables with p>0.1 were sequentially dropped from the model, with the least significant interaction terms removed first. Hence, we do not present statistical outputs for insignificant variables that were dropped from the models.
3. Results
(a) Egg mass
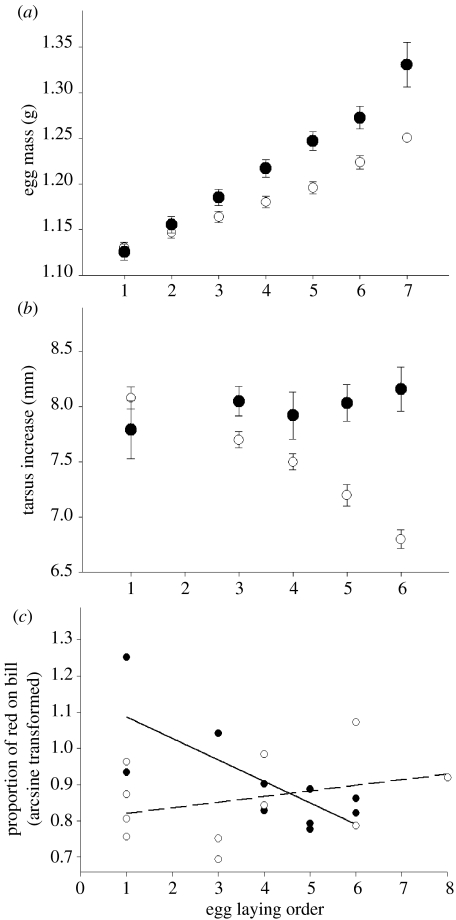
A significant interaction between laying order and male attractiveness (F1,128=4.71, p=0.0318) showed that, although egg mass increased with laying order for both male attractiveness groups, this was greater for clutches laid by females mated to attractive (red-ringed) males (figure 1). There was also a marginally significant trend for heavier females to lay larger eggs (F1,128=3.69, p=0.0569).
Figure 1.
Interactions between egg laying order and father attractiveness for (a) egg mass, (b) growth rate (tarsus increase between 5 and 15 days old) and (c) maturation rate (proportion of red on the upper mandible at 50 days old). Black symbols refer to offspring of attractive (red-ringed) males and open symbols refer to offspring of unattractive (green-ringed) males. Symbols represent: (a) and (b) the fitted means with standard error bars from the GLM for (c) data from individual offspring.
(b) Skeletal growth rate
An interaction between male attractiveness and laying order (F1,26=9.39, p=0.0050) showed that for offspring with unattractive (green-ringed) fathers, tarsus growth rate declined with laying order, but was relatively unchanged for offspring with attractive (red-ringed) fathers (figure 1). In addition, chicks from larger broods grew faster (F1,26=4.73; p=0.0389). Foster father's ring colour, mother's mass, estimated yolk T concentration, egg mass and offspring sex were eliminated from the model (p>0.1).
(c) Maturation rate
There was a significant interaction between male attractiveness treatment and egg laying order on the proportion of red on the bill at 50 days (F1,8=9.72, p=0.014; figure 1). Maturity rate declined with laying order for offspring of attractive fathers (F1,4=10.7, p=0.031), whereas those of unattractive fathers matured at a rate independent of laying order (F1,4=1.2, p=0.335). Foster father's ring colour, mother's mass, estimated yolk T concentration, egg mass, body size at 5 days old, brood size at 5 days old and offspring sex were eliminated from the model (p>0.1). It should be pointed out that the sample sizes for this analysis were quite small and, as a result, this finding may not be robust.
(d) Begging duration
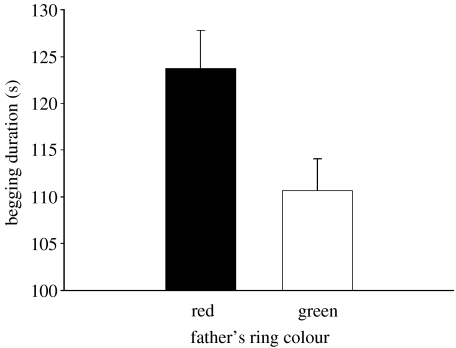
Offspring aged 3 and 4 days old begged for longer if they had an attractive, rather than an unattractive, father (F1,35=6.04, p=0.0191; figure 2). Foster father's ring colour, mother's mass, estimated yolk T concentration, egg mass, laying order of the egg, body size at 5 days old, brood size at 5 days old and offspring sex were eliminated from the model (p>0.1).
Figure 2.
During 3 min begging trials at 4 and 5 days old offspring begged for significantly longer if their original fathers were attractive (black bars) rather than unattractive (open bars). Bars represent the least-squares means with standard error bars from the GLM.
(e) Body size
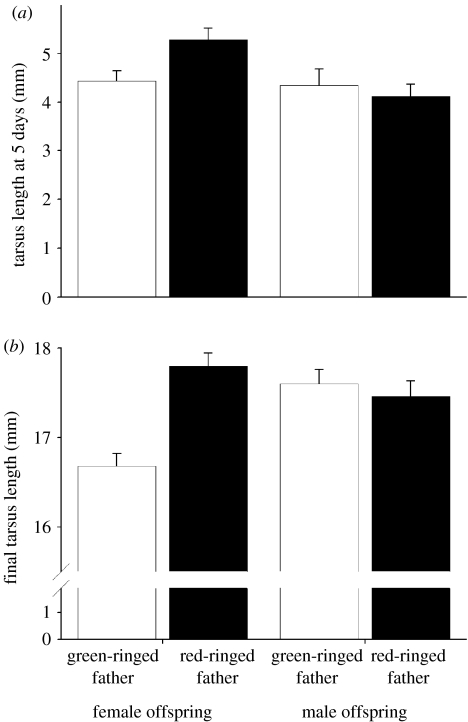
Father's attractiveness and offspring sex showed a highly significant interaction for both body size at 5 days old (F1,30=7.98, p=0.0083; figure 3) and final body size at 100 days old (F1,27=16.14, p=0.0004; figure 3). Female offspring with attractive fathers were bigger than females with unattractive fathers at 5 days (F1,28=10.08, p=0.0036) and 100 days old (F1,12=23.81, p=0.0004). Male offspring showed no difference in final tarsus length with their fathers' attractiveness at 5 days (F1,20=0.71, p=0.4102) or 100 days old (F1,9=0.15, p=0.7099). Foster father's ring colour, mother's mass, estimated yolk T concentration, egg mass, laying order of the egg and brood size at age 5 days were eliminated from the models (p>0.1) and body size at 5 days old was also not a predictor of final body size (p>0.1).
Figure 3.
Female offspring (left-hand bars) of attractive fathers (black bars) had larger body size at (a) 5 days old and at (b) 100 days old than female offspring of unattractive fathers (open bars). The size of male offspring (right-hand bars) did not differ according to the attractiveness of their fathers at 5 or 100 days old. Bars represent the least-squares means with standard error bars from the GLM.
(f) Reproductive output and offspring survival
There were no significant predictors of any measures of reproductive output, i.e. clutch size, initial brood size (number hatched) and measures of survival to adulthood (these were the number (logged) of young per nest, the proportion (arcsine-transformed) of eggs laid per nest, and the proportion (arcsine-transformed) of hatchlings per nest that survived to adulthood). All the explanatory variables (father's attractiveness, foster father's attractiveness, mother's mass, clutch size, estimated yolk T concentration and their interactions) were eliminated from the GLMs due to insignificance (F1,22<1.0, p>0.1).
4. Discussion
We tested for maternal effects, mediated by mate attractiveness, on offspring developmental traits in the zebra finch. First, we found that the increased investment in egg mass with laying order exhibited by females was greater if they were paired to attractive as compared with unattractive males (see also Rutstein et al. 2004a). Then, having accounted for egg mass, estimated yolk T, laying order, offspring sex, brood size, mother's mass and foster father's attractiveness in the GLMs, we found that many of the offspring traits we measured varied with the perceived attractiveness of their father. Offspring of attractive fathers spent more time begging; growth rate declined with laying order in offspring of unattractive but not attractive fathers; maturation rate declined with laying order for offspring of attractive but not unattractive fathers; and female (but not male) offspring were larger at 5 days and 100 days old if they had attractive rather than unattractive fathers. Proximately, some of these traits may be interdependent; for example, begging rates may influence growth rates which may affect final body size, although we found no effect of egg mass or chick size at 5 days old on these traits. However, ultimately, all these traits were influenced by the perceived attractiveness (colour ring) of the father.
These results show strong evidence for maternal effects in response to mate attractiveness. Furthermore, they are important because the differences in offspring development between attractive and unattractive fathers were most likely due to differences in how the mother allocated resources to different eggs. Since the experiment did not allow assortative mating between high-quality males and females, and males were allocated colour rings and females randomly, the results were unlikely to be due to any overriding genetic differences between fathers or mothers. Also, because we cross-fostered clutches and included the foster father attractiveness in the models, the observed effects of father attractiveness on offspring were unlikely to be due to differences in post-hatching nestling care by the foster parents. Our finding of a sex-dependent effect on body size is interesting because, although zebra finches do not alter the primary sex ratio in response to mate attractiveness (Rutstein et al. 2004a, 2005), there is a mate attractiveness-dependent sex ratio bias at fledging or adulthood (Rutstein et al. 2005). Our result provides further evidence of a sex bias in offspring development in response to mate attractiveness that may stem from sex-biased maternal allocation of egg resources or sex-biased intrinsic responses to the same conditions (e.g. Martins 2004).
We found no effect of mate attractiveness on clutch size or fledging success, which might suggest that females may not have simply invested more egg resources for attractive mates and less egg resources for unattractive mates, as would be predicted from differential allocation theory (Burley 1986). Instead, it may be that females allocate resources depending on the needs or value of individual offspring depending on the environment they are predicted to encounter (Mousseau & Fox 1998). In this case, the predicted environment is dependent on mate attractiveness because mate attractiveness could (under natural conditions) influence the offspring's genotype and other factors such as food provisioning and protection. This could help explain the difference in investment in egg mass with laying order between attractiveness treatments. Egg mass tends to increase with laying order in zebra finches (e.g. Rutstein et al. 2004a,b), and we found this increase was more pronounced in the attractive treatment group. This would be predicted from brood reduction theory where, under poor conditions (such as with unattractive mates) but not good conditions, the last hatched chicks (from last laid eggs) die, which reduces the size of the brood so that the early hatched chicks are more likely to survive. Conversely, with good quality parents (such as with attractive mates) that should be able to raise the entire brood, we predict relatively high investment at the end of a clutch to offset the inherent disadvantage of hatching last in order to maximize survival of late-hatched chicks.
The variables that we found positively correlated with father attractiveness are generally considered to be positive fitness-related traits. For example, females that grow to be large as adults generally lay larger eggs (this study; Rutstein et al. 2004a,b) and a positive relationship is often found between egg size and early chick growth and mortality (Williams 1994; Christians 2002; Rutstein et al. 2004b). However, it is important to note that there are usually trade-offs associated both with maternal investment of resources and with offspring developmental traits (Metcalf & Monaghan 2003; Groothuis et al. 2005). For example, chicks that beg more may acquire more food, but this may increase the likelihood of nest predation or bear an energetic cost (e.g. impairing growth; Kilner 2001). Fast growth or development rates are beneficial for rapid fledging, which may reduce the likelihood of nest predation, but may bear costs later in life such as reduced longevity (Metcalf & Monaghan 2003; Hall et al. 2004). In addition, the fitness consequences of some developmental traits may be context-dependent (e.g. Mousseau & Fox 1998). For example, we could speculate that chicks could benefit from begging more if having attractive fathers means better protection from predators or more food availability, whereas begging less could benefit a chick if having an unattractive father means a higher predation risk or that energy spent begging is wasted due to lack of available food. Since mothers may allocate egg resources in order to maximize offspring fitness in whatever environment (including genetic) they are predicted to encounter, then it is possible that offspring of attractive fathers that beg more, grow faster and become larger are predicted by the mother to be able to cope with the costs associated with these traits.
Which egg resources could produce the differences in offspring traits observed in this study? One candidate is yolk androgens such as T. One study found that female zebra finches invested more yolk T when paired to attractive (red-ringed) males (Gil et al. 1999), and female canaries exposed to ‘sexy’ song phrases invest more yolk T than control females exposed to ‘un-sexy’ song (Gil et al. 2004). Such increased yolk T could affect the offspring, since several studies have found that experimentally increased yolk T levels can have various effects on the development of the chicks (Groothuis et al. 2005), such as increased hatching muscle growth (Lipar & Ketterson 2000), increased begging behaviour, growth and dominance (Eising et al. 2001; von Engelhardt 2004) and decreased hatchability and survival (Sockman & Schwabl 2000). However, we found no effects of natural yolk T concentration (but note we used estimated values) on the offspring traits we measured, which may suggest that yolk T alone may not have had a measurable effect on the traits we measured. Instead, other hormones or other egg resources may have played a role in shaping offspring development, for example, maternally derived yolk antioxidants and immunoglobulins can be differentially invested with respect to male attractiveness and could affect offspring development, immune function and survival (see Saino et al. 2002; Williamson et al. in press).
Our results also suggested an effect of maternal quality on offspring, which would be expected since egg resources can be limited by female condition. Heavier females laid larger eggs than did light females; and brood size, which often reflects female quality or condition (Rutstein et al. 2004b), was positively correlated with offspring growth rate. Egg resources can also be constrained when females are mated to poor quality males (Gowaty & Buschhaus 1998; Cunningham & Russell 2000). However, this could not be true in this study because mate attractiveness was experimentally manipulated and did not reflect the male's true quality.
To conclude, we have shown that the father's attractiveness, via maternal effects, influences several aspects of offspring development. Furthermore, by manipulating male attractiveness, randomly assigning breeding pairs, cross-fostering clutches and using GLMs to account for variance of other factors, we have eliminated any genetic effects of the male and these effects must be mediated by egg resources allocated differentially by the female. Since our results clearly indicate strong maternal effects on offspring traits in response to mate attractiveness, acting via the egg, future studies on male genetic effects on offspring must take such maternal effects into account.
Acknowledgments
This research was funded by a BBSRC grant awarded to J.A.G. and N.H. We are grateful to Isobel Maynard for bird husbandry, Monique Mackenzie for statistical advice and Andrew Beckerman and two anonymous referees for helpful comments on the manuscript.
References
- Alekseev V, Lampert W. Maternal control of resting-egg production in Daphnia. Nature. 2001;414:899–901. doi: 10.1038/414899a. doi:10.1038/414899a [DOI] [PubMed] [Google Scholar]
- Balzer A.L, Williams T.D. Do female zebra finches vary in primary reproductive effort in relation to mate attractiveness? Behaviour. 1998;135:297–309. [Google Scholar]
- Bennett A.T.D, Cuthill I.C, Partridge J.C, Maier E.J. Ultravioet vision and mate choice in zebra finches. Nature. 1996;380:433–435. doi:10.1038/380433a0 [Google Scholar]
- Burley N. Sexual selection for aesthetic traits in species with biparental care. Am. Nat. 1986;127:415–445. doi:10.1086/284493 [Google Scholar]
- Burley N. The differential-allocation hypothesis—an experimental test. Am. Nat. 1988;132:611–628. doi:10.1086/284877 [Google Scholar]
- Burley N, Krantzberg G, Radman P. Influence of color-banding on the consepecific preferences of zebra finches. Anim. Behav. 1982;30:444–445. [Google Scholar]
- Christians J.K. Avian egg size: variation within species and inflexibility within individuals. Biol. Rev. 2002;77:1–26. doi: 10.1017/s1464793101005784. [DOI] [PubMed] [Google Scholar]
- Cotton P.A, Wright J, Kacelnik A. Chick begging strategies in relation to brood hierarchies and hatching asynchrony. Am. Nat. 1999;153:412–420. doi: 10.1086/303178. doi:10.1086/303178 [DOI] [PubMed] [Google Scholar]
- Cunningham E.J.A, Russell A.F. Egg investment is influenced by male attractiveness in the mallard. Nature. 2000;404:74–76. doi: 10.1038/35003565. doi:10.1038/35003565 [DOI] [PubMed] [Google Scholar]
- Eising C.M, Eikenaar C, Schwabl H, Groothuis G.G. Maternal androgens in black-headed gull (Larus ridibundus) eggs: consequences for chick development. Proc. R. Soc. B. 2001;268:839–846. doi: 10.1098/rspb.2001.1594. doi:10.1098/rspb.2001.1594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil D, Graves J.A, Hazon N, Wells A. Male attractiveness and differential testosterone investment in zebra finch eggs. Science. 1999;268:126–128. doi: 10.1126/science.286.5437.126. doi:10.1126/science.286.5437.126 [DOI] [PubMed] [Google Scholar]
- Gil D, Leboucher G, Lacroix A, Cue R, Kreutzer M. Female canaries produce eggs with greater amounts of testosterone when exposed to preferred male song. Horm. Behav. 2004;45:64–70. doi: 10.1016/j.yhbeh.2003.08.005. doi:10.1016/j.yhbeh.2003.08.005 [DOI] [PubMed] [Google Scholar]
- Gilbert L, Rutstein A.N, Hazon N, Graves J.A. Sex-biased investment in yolk androgens depends on female quality and laying order in zebra finches (Taeniopygia guttata) Naturwissenschaften. 2005;92:178–181. doi: 10.1007/s00114-004-0603-z. doi:10.1007/s00114-004-0603-z [DOI] [PubMed] [Google Scholar]
- Gowaty P.A, Buschhaus N. Ultimate causation of aggressive and forced copulation in birds: female resistance, the CODE hypothesis and social monogamy. Am. Zool. 1998;38:207–225. [Google Scholar]
- Grafen A, Hails R. Oxford University Press; Oxford: 2002. Modern statistics for the life sciences. ISBN 0-19-925231-9. [Google Scholar]
- Groothuis T.G.G, Müller W, von Engelhardt N, Carere C, Eising C. Maternal hormones as a tool to adjust offspring phenotype in avian species. Neurosci. Biobehav. Rev. 2005;29:329–352. doi: 10.1016/j.neubiorev.2004.12.002. doi:10/1016/j.neubiorev.2004.12.002 [DOI] [PubMed] [Google Scholar]
- Hall M.E, Nasir L, Daunt F, Gault E.A, Croxall J.P, Wanless S, Monaghan P. Telomere loss in relation to age and early environment in long-lived birds. Proc. R. Soc. B. 2004;271:1571–1576. doi: 10.1098/rspb.2004.2768. doi:10.1098/rspb.2004.2691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt S, Cuthill I.C, Swaddle J.P, Bennett A.T.D. Ultravioelt vision and band-colour preferences in female zebra finches, Taeniopygia guttata. Anim. Behav. 1997;54:1383–1392. doi: 10.1006/anbe.1997.0540. doi:10.1006/anbe.1997.0540 [DOI] [PubMed] [Google Scholar]
- Kilner R.M. A growth of begging in captive canary chicks. Proc. Natl Acad. Sci. USA. 2001;98:11 394–11 398. doi: 10.1073/pnas.191221798. doi:10.1073/pnas.191221798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozielska M, Krzeminska A, Radwan J. Good genes and the maternal effects of polyandry on offspring reproductive success in the bulb mite. Proc. R. Soc. B. 2004;271:165–170. doi: 10.1098/rspb.2003.2585. doi:10.1098/rspb.2003.2585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipar J.L, Ketterson E.D. Maternally derived yolk testosterone enhances the development of the hatching muscle in the red-winged blackbird Agelaius phoeniceus. Proc. R. Soc. B. 2000;267:2005–2010. doi: 10.1098/rspb.2000.1242. doi:10.1098/rspb.2000.1242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins T.L.F. Sex-specific growth in zebra finch nestlings; a possible mechanism for sex ratio adjustment. Behav. Ecol. 2004;15:174–180. doi:10.1093/beheco/arg094 [Google Scholar]
- Metcalf N.B, Monaghan P. Growth versus lifespan: perspectives from evolutionary ecology. Exp. Gerontol. 2003;38:935–940. doi: 10.1016/s0531-5565(03)00159-1. doi:10.1016/S0531-5565(03)00159-1 [DOI] [PubMed] [Google Scholar]
- Mousseau T.A, Fox C.W. The adaptive significance of maternal effects. Trends Ecol. Evol. 1998;13:403–407. doi: 10.1016/s0169-5347(98)01472-4. doi:10.1016/S0169-5347(98)01472-4 [DOI] [PubMed] [Google Scholar]
- Nash J.P, Cuisset B.D, Bhattacharyya S, Suter H.C, Le Menn F, Kime D.E. An enzyme linked immunosorbant assay (ELISA) for testosterone, estradiol, and 17,20 beta-dihydroxy-4-pregenen-3-one using acetylcholinesterase as tracer: application to measurement of diet patterns in rainbow trout (Oncorhynchus mykiss) Fish Physiol. Biochem. 2000;22:355–363. doi:10.1023/A:1007850014021 [Google Scholar]
- Petrie M, Williams W.A. Peahens lay more eggs for peacocks with larger trains. Proc. R. Soc. B. 1993;251:127–131. [Google Scholar]
- Rutstein A.N, Gilbert L, Slater P.J.B, Graves J.A. Mate attractiveness and primary resource allocation in the zebra finch. Anim. Behav. 2004a;68:1087–1094. doi:10.1016/j.anbehav.2004.02.011 [Google Scholar]
- Rutstein A.N, Slater P.J.B, Graves J.A. Diet quality and resource allocation in the zebra finch. Proc. R. Soc. B. 2004b;271:286–289. doi: 10.1098/rsbl.2003.0154. doi:10.1098/rsbl.2003.0154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutstein A.N, Gilbert L, Slater P.J.B, Graves J.A. Sex-specific patterns of yolk androgen allocation depend on maternal diet in the zebra finch. Behav. Ecol. 2005;16:62–69. doi:10.1093/beheco/arh123 [Google Scholar]
- Saino N, Ferrari R.P, Martinelli R, Romano M, Rubolini D, Møller A.P. Early maternal effects mediated by immunity depend on sexual ornamentation of the male partner. Proc. R. Soc. B. 2002;269:1005–1009. doi: 10.1098/rspb.2002.1992. doi:10.1098/rspb.2002.1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sockman K.W, Schwabl H. Yolk androgens reduce offspring survival. Proc. R. Soc. B. 2000;267:1451–1456. doi: 10.1098/rspb.2000.1163. doi:10.1098/rspb.2000.1163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uller T, Eklöf J, Andersson S. Female egg investment in relation to male sexual traits and the potential for transgenerational effects in sexual selection. Behav. Ecol. Sociobiol. 2005;57:584–590. doi:10.1007/s00265-004-0886-2 [Google Scholar]
- von Engelhardt, N. 2004 Proximate control of avian sex allocation: a study on zebra finches. Ph.D. thesis, University of Groningen.
- Williams T.D. Intraspecific variation in egg size and egg composition in birds: effects on offspring fitness. Biol. Rev. 1994;68:35–59. doi: 10.1111/j.1469-185x.1994.tb01485.x. [DOI] [PubMed] [Google Scholar]
- Williamson, K. A. 2005 Mothers have favourites: egg composition, mate attractiveness and maternal effects in the zebra finch. Ph.D. thesis, St Andrews University.
- Williamson, K. A., Surai, P. F. & Graves, J. A. In press. Yolk antioxidants and mate attractiveness in the zebra finch. Funct. Ecol